Abstract
Epoxyeicosatrienoic acids, hydrolyzed by soluble epoxide hydrolase (sEH), have multiple biological functions, including the regulation of vascular tone, renal tubular transport, and being anti-inflammatory. Inhibitors of sEH have been demonstrated to be antihypertensive and renal protective. To elucidate the role of sEH in glomerulonephritis, we first determined the expression of sEH in human kidney by examining biopsies from 153 patients with a variety of glomerulonephritis, including minimal-change, membranous, and IgA nephropathy. Immunohistochemical staining of frozen kidney biopsy samples revealed sEH preferentially expressed in the renal proximal tubular cells, and its expression increased in all patients with glomerulonephritis. The level of sEH in the cortex was positively correlated with proteinuria and negatively with serum albumin level. To investigate the role of sEH in proteinuria-induced renal damage, we incubated purified urine protein from patients with rat renal proximal tubular epithelial cells in vitro. The level of sEH was elevated, as were monocyte chemoattractant protein 1 and the process of tubular epithelial-to-mesenchymal transition, characterized with increased α-smooth muscle actin (α-SMA) and decreased E-cadherin. These effects were attenuated by administration of a potent sEH inhibitor and mimicked with adenovirus-mediated sEH overexpression. In adriamycin-induced nephropathic mice, sEH inhibitor did not ameliorate proteinuria or level of serum albumin but reduced the long-term elevated serum creatinine level, interstitial inflammation, fibrosis, and α-SMA expression. Thus upregulation of sEH in proximal tubular cells in chronic proteinuric kidney diseases may mediate proteinuria-induced renal damage; sEH inhibition by increasing renal eicosanoid levels could prevent the progression of chronic proteinuric kidney diseases.
Keywords: glomerulonephritis, soluble epoxide hydrolase, proteinuria, inflammation, epithelial-mesenchymal transition
epoxyeicosatrienoic acids (EETs) are cytochrome P-450 (CYP) metabolites of arachidonic acid (AA) with potent biological effects, such as vasorelaxation, promotion of sodium excretion and profibrinolytic activities, as well as anti-inflammatory and antiproliferative effects (3, 9, 19, 31). Several regioisomeric EETs (5,6-, 8,9-, 11,12-, 14,15-) have been implicated as endothelium-derived hyperpolarizing factors and potently dilate numerous vascular beds through their activation of calcium-dependent K+ (KCa) channels in vascular smooth muscle cells (3, 31, 40). EETs may prevent vascular inflammation by a mechanism involving inhibition of adhesion molecule expression and modulate the growth of vascular smooth muscle cells in vitro and in vivo (9, 19, 32). Soluble epoxide hydrolase (sEH) converts EETs into their corresponding less-biological dihydroxyeicosatrienoic acids. The activity of sEH is therefore thought to be a major determinant of EET bioavailability (6, 14). Genetic deletion of sEH, as well as pharmacological inhibition, increases plasma EET levels and potentiates their effects (22, 23); thus sEH inhibition has antihypertensive and anti-inflammatory effects. Indeed, sEH inhibition may reduce hypertension, myocardial infarct size, cerebral infarction, vascular remodeling and atherosclerosis, and even tobacco smoke-induced airway inflammation (5, 13, 30).
The kidney produces all four regioisomeric EETs (32), and both EET-related CYPs (2C8, 2C9, and 2J2 in human) and sEH expression in the kidney are high. sEH activity is primarily located in the cytosol and peroxisomes of proximal tubular cells (8). One important progression factor of chronic renal disease is hypertension. The antihypertensive effect of sEH inhibition has been demonstrated in numerous rat and mouse models, such as spontaneously hypertensive rats, angiotensin II (AngII)-induced hypertension, and deoxycorticosterone acetate-salt-induced and salt-sensitive hypertension (2, 15, 16, 20, 25, 39). The antihypertensive effect was believed to be mediated by decreased vascular resistance and enhanced renal Na+ excretion, which resemble biological effects of EETs and enhance renal blood flow by dilating afferent preglomerular arterioles (12).
Beyond vasodilatation, EETs also function as endogenous anti-inflammatory, antiproliferative agents that might protect blood vessels against inflammation and sclerosis (6, 21). Glomerular inflammation and sclerosis are primary pathological features of glomerulonephritis, and proteinuria is one of main symptoms and a major progression factor for glomerulonephritis. EETs have effects on renal blood flow, glomerular filtration rate, and urinary sodium excretion rate (12, 29). sEH inhibition protects the kidney against inflammatory components of hypertension by attenuating glomerular macrophage filtration rate, diminution of mesangial proliferation, and renal arteriolar intimal thickening (25). Thus it is logical to assume that sEH inhibition is a strategy to prevent progression of glomerulonephritis (12). Accordingly, sEH inhibition was reported to attenuate albuminuria and GFR in salt- or AngII-induced hypertensive or diabetic nephropathy (11, 16, 39).
A major limitation of these animal models is that their hypertensive background and high inflammatory activity does not necessarily reflect chronic glomerulonephritis in humans, which is dominated by sclerotic and fibrotic processes and characterized by a progressive nature. In human glomerulonephritis, proteinuria is more frequent and appears earlier than hypertension. Subsequently, progressive chronic renal failure is a consequence of glomerulosclerosis and interstitial fibrosis. Relatively little is known about CYP-catalyzed AA metabolism and the biological effects of the resulting eicosanoids in human kidney. sEH expression and cellular localization in kidney, as well as the role of sEH in glomerulonephritis, especially the relation with proteinuria, hypertension, and renal function, is not well studied. In this study, we investigated the renal expression of sEH in various forms of glomerulonephritis in humans and the relation of sEH and clinical characters. sEH was upregulated in proximal tubular cells in patients with glomerulonephritis, which was associated with proteinuria but not hypertension. Furthermore, we studied the role of the sEH in proteinuria-induced cell injury in rat proximal tubular epithelial cells (RPTECs) in vitro and in an adriamycin (ADR)-induced nephropathy mouse model in vivo. The upregulation of sEH in proximal tubular cells in chronic proteinuric kidney diseases may mediate the proteinuria-induced renal damage; sEH inhibition by increasing renal eicosanoid levels could be translated to therapies for preventing the progression of chronic proteinuric kidney diseases to end-organ damage.
MATERIALS AND METHODS
Patients.
All procedures and use of anonymous tissue were according to guidelines of the ethics committee of Peking University Third Hospital. We collected renal biopsy specimens and clinical data from 153 inpatients who were admitted to the Nephrology Department of Peking University Third Hospital from May 2008 to March 2009. All patients gave informed consent to be in this study. The pathological diagnoses were minimal change nephropathy (MCD, n = 28), membranous nephropathy (MN, n = 50), and IgA nephropathy (IgAN, n = 75). Renal normal tissue specimens from the cortex apart from tumor tissue of patients with renal carcinoma (n = 10, age and sex matched) served as controls. Clinical parameters such as edema, blood pressure, serum albumin level, 24-h proteinuria, and serum creatinine level were measured 2 to 4 days before biopsy.
Reagents.
DMEM/F12 medium was from GIBCO, and fetal bovine serum (FBS) was from Hyclone. All other cell culture supplements, including recombinant human epidermal growth factor (EGF), insulin, and transferrin, were from Sigma (St. Louis, MO). Antibodies against sEH, CYP2C9, Tamm-Horsfall protein (THP), β-actin, α-smooth muscle actin (α-SMA), and GAPDH were from Sigma, Cayman, and Abcam, respectively. sEH selective inhibitor 1-(1-methylsulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)-urea (TUPS) was kindly provided by Drs. Paul D. Jones and Bruce D. Hammock (University of California, Davis), as described (2, 34). The adenoviral vector encoding full-length human sEH (Ad-sEH) was created in our laboratory as previously reported (2). Polymyxin B-immobilized columns for removal of endotoxin were from Detoxi-Gel (Pierce Chemical, Rockford, IL).
Immunohistochemistry.
Frozen slides of biopsy sections from patients were heat pretreated and blocked with 10% goat serum in PBS, then incubated with primary antibody, including anti-sEH (1:100), anti-CYP2C9 (1:100), or anti-THP and horseradish peroxidase-conjugated secondary antibody, then counterstained with diaminobenzidine tetrahydrochloride, dehydrated, and mounted. The staining of sEH or CYP2C9 was scored semiquantitatively by estimating the percentage of cortical tubules expressing the responding protein per field. The staining was presented as + for 0–25%; ++ for 25–50%; +++ for 50–75%, and ++++ for 75–100%. Immunohistochemistry staining was scored blindly by two nephropathologists, independently.
Extraction of urinary protein.
Three urine samples from patients with MCD, MN, or IgAN, who had not received glucocorticoid or immunodepressant treatment, were collected and pooled. Urinary proteins were isolated and purified by ammonium sulfate precipitation. The protein components were analyzed by 10% SDS-PAGE and Coomassie blue staining (26). The extracted protein liquid was freeze-dried to protein powder. Before being added to cell cultures, powder was resolved by culture medium, endotoxin was removed by use of polymyxin B-immobilized columns, and powder was filtered through a 0.22-mm cell culture filter.
Cell culture.
RPTECs (NRK-52E, American Type Culture Collection, Rockville, MD) were cultured in DMEM/F12 medium containing 1.2 g/l sodium bicarbonate, 1 μg/l endothelial growth factor, 5 mg/l insulin, 5 mg/l transferrin, 4 mg/l dexamethasone, antibiotics, glutamine, and 5% FBS. RPTECs were exposed to different concentrations of urinary proteins with or without TUPS for different times, then infected with recombinant adenoviral vectors at the indicated multiplicity and incubated for 48 h before experiments.
Western blot analysis.
RPTECs and renal cortex tissues from patients or animals were lysed, and cellular proteins were extracted and underwent Western blot analysis with the primary antibodies anti-sEH (1:1,000), anti-β-actin (1:1,000), anti-α-SMA (1:2,000), and anti-GAPDH (1:1,000) and quantification by use of Scion Image (Scion, Frederick, MD).
Real-time PCR.
Total RNA was isolated from cells with TRIzol reagent (Invitrogen). The isolated RNA was converted into cDNA and underwent quantitative RT-PCR by the Brilliant SYBR green QPCR system with GAPDH as an internal control. The primer sequences were for E-cadherin, 5′-TCGGTGCCCGTATTGC-3′ and 5′-GAATGCCCTCGTTGGTC-3′; TGF-β1, 5′-G-AGGCGGTGCTCGCTTTGT-3′ and 5′-TGTTGCGGTCCACCATTAGC-3′; collagen I, 5′-CCTGCGCCTGATGTCCACCG-3′ and 5′-ATGATGGGCAGGCGGGAGGT-3′; collagen III, 5′-CCCCAGGACCTACTGGCGCA-3′ and 5′-TTCCTGCGGTTCCAGGGGGT-3′; fibronectin, 5′-TGACTCGCTTTGACTTCACCAC-3′ and 5′-TCTCCTTCCTCGCTCAG TTCGT-3′; monocyte chemoattractant protein 1 (MCP-1), 5′-CAGCCGACTCATTGGGATCA-3′ and 5′-CTATGCAGGTCTC TGTCACGCTTC-3′; and GAPDH, 5′-AATGCATCCTGCACCACC-3′ and 5′-ATGCCAGT GAGCTTCCCG-3′.
Animal experiments.
All animal experimental protocols were approved by the Peking University Institutional Animal Care and Use Committee. Male BALB/c mice were kept in a 12-h light:12-h dark cycle and had free access to standard tap water for a 2-day adaptation. We divided 48 male mice into 2 groups for treatment (n = 24 each): ADR, tail-vein injection of 10 mg/kg ADR once; control, equivalent volume of saline. Each half of each group (n = 12) received by oral gavage the sEH inhibitor TUPS, 1 mg·kg−1·day−1 for 2 or 6 wk. Mice were housed in metabolic cages at weekly intervals to collect 24-h urine. After 2 or 6 wk of ADR administration, mice were killed, blood was collected, and kidneys were harvested. Histological examination was assessed by Masson, pulmonary artery smooth muscle, and α-SMA immunohistochemical staining on 2-μm-thick paraffin sections of kidney. Serum albumin and creatinine levels were measured by use of the commercial kits (albumin kit from BioSino Beijing, China; creatinine kit from Yantai, Ausbio Laboratories, Beijing, China) according to the manufacturers' instructions. Urinary protein concentration was expressed as protein (mg)/creatinine (mg).
Statistical analysis.
Categorical variables were analyzed by χ2 tests and continuous variables by one-way ANOVA (homogeneity of variance) or nonparametric t-test (heterogeneity of variance). Spearman rank correlation was used for correlation analysis. Statistical analysis involved use of SPSS 10.0 (SPSS, Chicago, IL). Data are expressed as means ± SD. A two-sided P < 0.05 was considered statistically significant.
RESULTS
sEH expression and clinical characteristics of patients with glomerulonephritis.
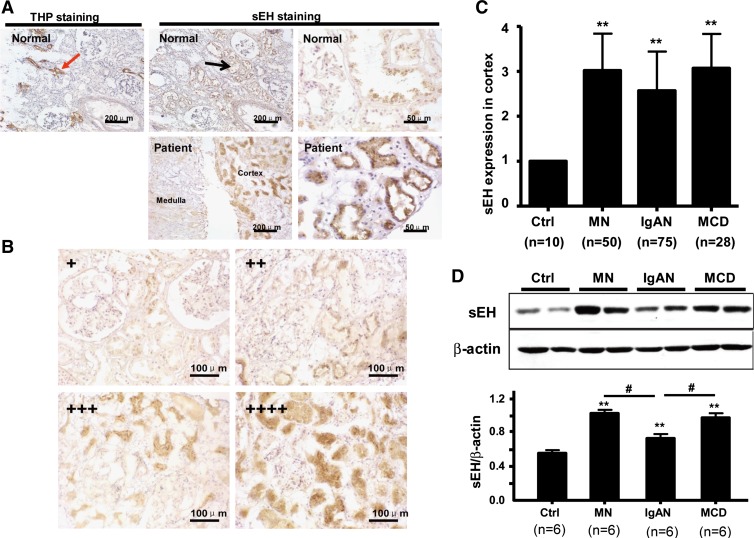
To detect the expression of sEH in kidneys of patients with renal diseases, we collected renal biopsy specimens from 153 patients with glomerulonephritis. Among them, the pathological diagnoses were MCD (n = 28), MN (n = 50), and IgAN (n = 75). Normal renal tissue specimens apart from tumor tissue of patients with renal carcinoma (n = 10) served as controls. In both controls and renal disease tissue, sEH protein expression was found mainly in proximal tubules but not in glomeruli or in THP-stained distal tubules (Fig. 1A). According to the percentage and degree of positive-stained tubules, the immunohistochemistry results of sEH expression were classified from + to ++++ (Fig. 1B). Tubular sEH level was higher in tissues from patients than in those from control subjects (2.82 ± 0.85 fold, P < 0.01). As shown in Table 1, the levels of sEH in cases and control agreed with level of 24-h proteinuria, serum albumin level, incidence of edema, and renal function, but not incidence of hypertension, age, or sex.
Fig. 1.
Soluble epoxide hydrolase (sEH) expression in proximal tubules of patients with glomerulonephritis. A: immunohistochemical staining for sEH in frozen biopsy sections of normal and diseased kidney. Localization of sEH is labeled. Sections were stained with Tamm-Horsfall protein (THP), a marker of distal tubules, as a control. B: immunohistochemical staining for sEH in frozen biopsy sections from kidneys with glomerulonephritis (×400). sEH staining was scored semiquantitatively by estimating the percentage of cortical tubules expressing sEH per field. +, 0∼25%; ++, 25∼50%; +++, 50∼75% and ++++, 75∼100%. C: mean sEH expression in patients with different glomerulonephritis was calculated according to the score in B. D: Western blot analysis of sEH in renal cortex of biopsy samples. β-Actin was a normalization control. Results are representative or means ± SD from at least 6 patients in each group (**P < 0.01 vs. Ctrl; #P < 0.05 vs. IgAN). Ctrl, control; MCD, minimal change nephropathy; MN, membranous nephropathy; IgAN, IgA nephropathy.
Table 1.
Clinical characteristics of patients with different tubular sEH level
| sEH Expression in Renal Cortex of All Patients |
||||
|---|---|---|---|---|
| Clinical Information | + | ++ | +++ | ++++ |
| Sex, male/female | 6/5 | 20/21 | 37/30 | 17/17 |
| Age, yr | 31.3 ± 12.1 | 40.1 ± 13.4 | 36.2 ± 16.3 | 39.8 ± 15.5 |
| 24-h proteinuria, g/d* | 2.7 ± 3.0 | 3.3 ± 2.8 | 4.8 ± 4.6 | 5.9 ± 4.0 |
| Serum albumin level, U/l* | 38.6 ± 8.8 | 33.5 ± 8.2 | 31.1 ± 8.9 | 27.5 ± 7.5 |
| Incidence of edema* | 45.5% | 53.7% | 62.7% | 82.4% |
| Hypertension | 27.3% | 41.5% | 32.8% | 32.4% |
| Number of high Cr/normal* | 5/6 | 16/25 | 18/49 | 6/28 |
| Incidence of renal failure* | 45.5% | 39.0% | 26.9% | 17.6% |
Data are means ± SD or number or percentage. sEH, soluble epoxide hydrolase.
P < 0.01, among different level of tubular sEH.
The correlation analysis revealed that the extent of renal sEH expression in diseased tissue was positively correlated with 24-h proteinuria (r = 0.571, P = 0.001) and incidence of edema (r = 0.446, P = 0.001) and negatively with levels of serum albumin (r = −0.514, P = 0.001) and serum creatinine but not incidence of renal failure (r = −0.265, P = 0.063) or hypertension (r = −0.201, P = 0.124).
sEH expression and clinical characteristics in different glomerulonephritis.
The expression of sEH was significantly increased in patients with all three types of glomerulonephritis: MCD (3.12 ± 0.78), MN (3.02 ± 0.82), and IgAN (2.65 ± 0.89) (Fig. 1C). Similarly, Western blot analysis revealed a similar pattern of increased sEH protein expression in patients with different glomerulonephritis, with the expression of sEH greater in MCD and MN than in IgAN (Fig. 1D).
All patients with MCD, MN, or IgAN showed increased proteinuria, low serum albumin level, and edema (Table 2). However, proteinuria and edema was more severe and serum albumin level, hypertension, and renal failure were lower in patients with MCD and MN than IgAN (P < 0.05). Therefore, the expression of sEH in proximal tubules was related to the loss of protein in urine.
Table 2.
sEH expression and clinical parameters in normal and diseased patients
| Patients |
||||||
|---|---|---|---|---|---|---|
| Normal | Total | MCD | MN | IgAN | ||
| sEH expression in cortex | + | 10 | 11 | 1 | 1 | 9 |
| ++ | 0 | 41 | 4 | 13 | 24 | |
| +++ | 0 | 67 | 15 | 20 | 32 | |
| ++++ | 0 | 34 | 8 | 16 | 10 | |
| sEH expression | 1.00 (n = 10) | 2.8 ± 0.9* (n = 153) | 3.1 ± 0.8*† (n = 28) | 3.0 ± 0.8*† (n = 50) | 2.6 ± 0.9* (n = 75) | |
| 24-h proteinuria, g/d | <0.15 | 4.0 ± 0.3 | 6.9 ± 3.8† | 5.5 ± 4.0† | 3.9 ± 3.9 | |
| Serum albumin level, U/l | 46 ± 6.4 | 31.3 ± 8.1 | 22.8 ± 4.9† | 27.9 ± 5.7† | 35.6 ± 9.1 | |
| Incidence of edema, % | 0.00 | 59.2 | 96.0† | 87.4† | 33.8 | |
Data are expressed as means ± SD. MCD, minimal change nephropathy; MN, membranous nephropathy; IgAN, IgA nephropathy.
P < 0.01 vs. normal group.
P < 0.05 vs. IgAN.
Proteinuria increased the expression of sEH in tubular epithelial cells in vitro.
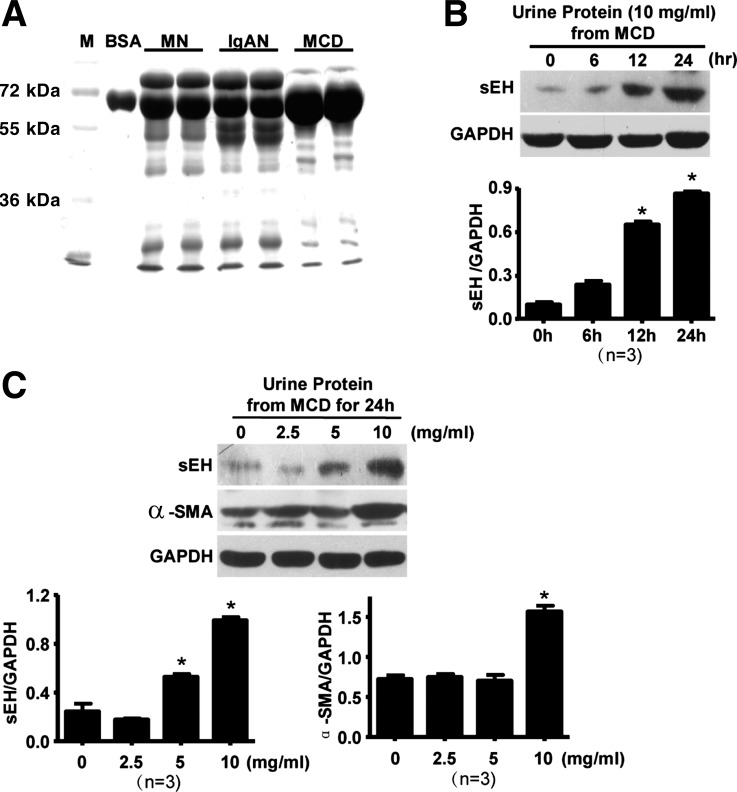
To investigate the role of proteinuria in sEH expression in renal diseases, we extracted proteins from urinary samples of patients with MCD, MN, and IgAN. Protein electrophoresis results showed the primary bands being albumin, of ∼62 kDa, especially from MCD patients (Fig. 2A). Exposure of urinary proteins from patients with MCD to cultured RPTECs could time and dose dependently upregulate sEH (Fig. 2, B and C). Urine proteins from patients with MN or IgAN had a similar effect (data not shown). Interestingly, the expression of α-SMA, a marker of the process of epithelial-mesenchymal transition (EMT), was simultaneously upregulated with sEH in RPTECs with urine proteins from patients with MCD. The level of sEH began to increase at 12 h, and the expression of α-SMA increased significantly at 24 h, which indicates sEH was upregulated earlier than α-SMA (Fig. 2C).
Fig. 2.
Proteinuria in patients with induced sEH expression in cultured rat proximal tubular epithelial cells (RPTECs). A: protein from patients' urine was extracted and analyzed by 10% SDS-PAGE and Coomassie blue staining. Bovine serum album (BSA) was a control. RPTECs were treated with 10 mg/ml proteins from patients with MCD for different times (B) or with different concentrations of urine proteins from patients with MCD for 24 h (C). Western blot analysis of sEH, α-smooth muscle actin (α-SMA), and GAPDH proteins. Expression was normalized to that of GAPDH. Results are representative or means ± SD from at least 3 independent experiments (*P < 0.05 vs. 0 h).
sEH inhibition attenuated EMT and inflammatory reaction in RPTECs.
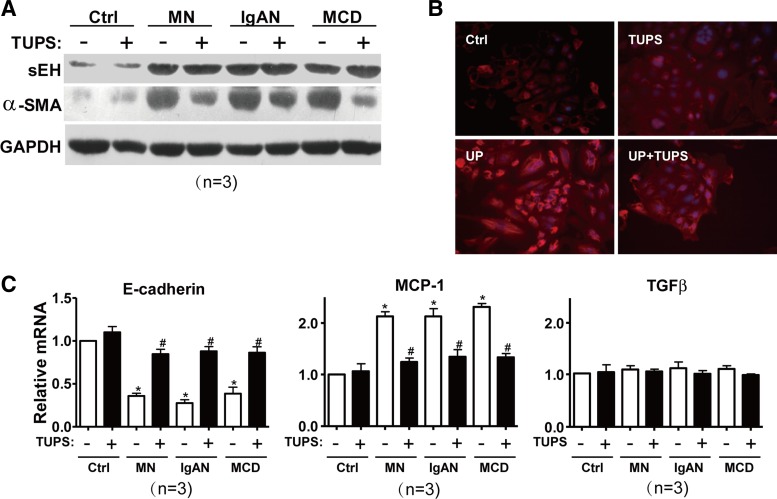
We previously reported that TUPS could significantly attenuate AngII-induced cardiac hypertrophy in vitro and in vivo (2). To decipher whether α-SMA upregulation and EMT of RPTECs was due to sEH upregulation, cells were administrated a selective sEH inhibitor TUPS, before treatment with urine proteins from different patients. Cell survivability was not affected by either urine proteins from patients or TUPS, as measured by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assays (data not shown). Western blot analysis revealed that TUPS did not affect the increased expression of sEH by urine proteins (Fig. 3A); inhibition of sEH markedly reduced proteinuria-induced α-SMA upregulation. This result was further supported by confocal microscopy of immunostaining with anti-α-SMA antibody in RPTECs (Fig. 3B). Furthermore, real-time PCR demonstrated that the urinary proteins from patients increased the mRNA level of MCP-1 and decreased that of E-cadherin, which was reversed by pretreatment with TUPS (Fig. 3C). The mRNA level of TGF-β1 in RPTECs was not altered by either treatment.
Fig. 3.
Effect of sEH inhibition on proteinuria-induced EMT and inflammatory reaction in RPTECs. RPTECs were treated with different urinary proteins with or without 1-(1-methylsulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)-urea (TUPS, 1.0 μM) for 24 h. A: Western blot analysis of sEH, α-SMA, and GAPDH proteins. B: confocal microscopy images of α-SMA in monolayer cells. Cell nuclei were stained by Hoechst (×400). UP, urinary proteins. C: real-time RT-PCR quantification of mRNA levels of E-cadherin, monocyte chemoattractant protein 1 (MCP-1), and TGF-β1. Data are means ± SD of relative mRNA normalized to that of GAPDH from at least 3 independent experiments (*P < 0.05 vs. Ctrl; #P < 0.05 vs. TUPS− in each group).
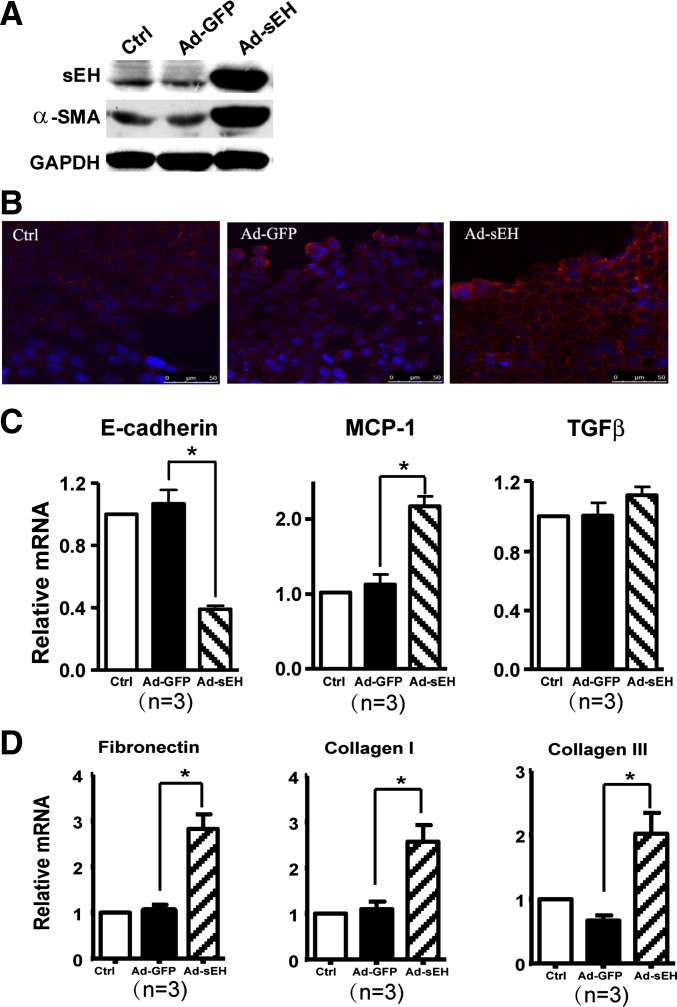
We studied next whether sEH was sufficient to induce EMT and an inflammatory effect by adenoviral overexpression of sEH in cultured RPTECs. The levels of protein of sEH were higher in Ad-sEH-infected cells than untreated or adenovirus encoding green fluorescent protein-infected controls (Fig. 4A). Noticeably, the expression of α-SMA and MCP-1 was increased in cells overexpressing sEH (Fig. 4, A–C). In contrast, the mRNA level of E-cadherin was decreased in Ad-sEH-infected RPTECs, which indicates the reduction of the cell junction (Fig. 4C) and suggests that sEH functions as a mediator of the proinflammatory effect of proteinuria in humans and is sufficient for inducing EMT in epithelial cells. Furthermore, the mRNA levels of fibrosis markers, including collagen I, collagen III, and fibronectin, were also increased in Ad-sEH-infected RPTECs (Fig. 4D).
Fig. 4.
Role of sEH overexpression in cultured RPTECs. Confluent RPTECs were infected with recombinant adenovirus encoding sEH (Ad-sEH) or adenovirus encoding green fluorescent protein (Ad-GFP) for 48 h. A: Western blot analysis of sEH, α-SMA and GAPDH proteins. B: confocal microscopy of α-SMA in monolayer cells. Cell nuclei were stained by Hoechst (400×). C: real-time RT-PCR quantification of mRNA levels of E-cadherin, MCP-1, and TGF-β1. D: real-time RT-PCR quantification of mRNA levels of collagen I, collagen III, and fibronectin. Data are means ± SD of the relative mRNA normalized to that of GAPDH from at least 3 independent experiments (*P < 0.05).
sEH inhibition improved parameters in ADR-induced nephropathy in mice.
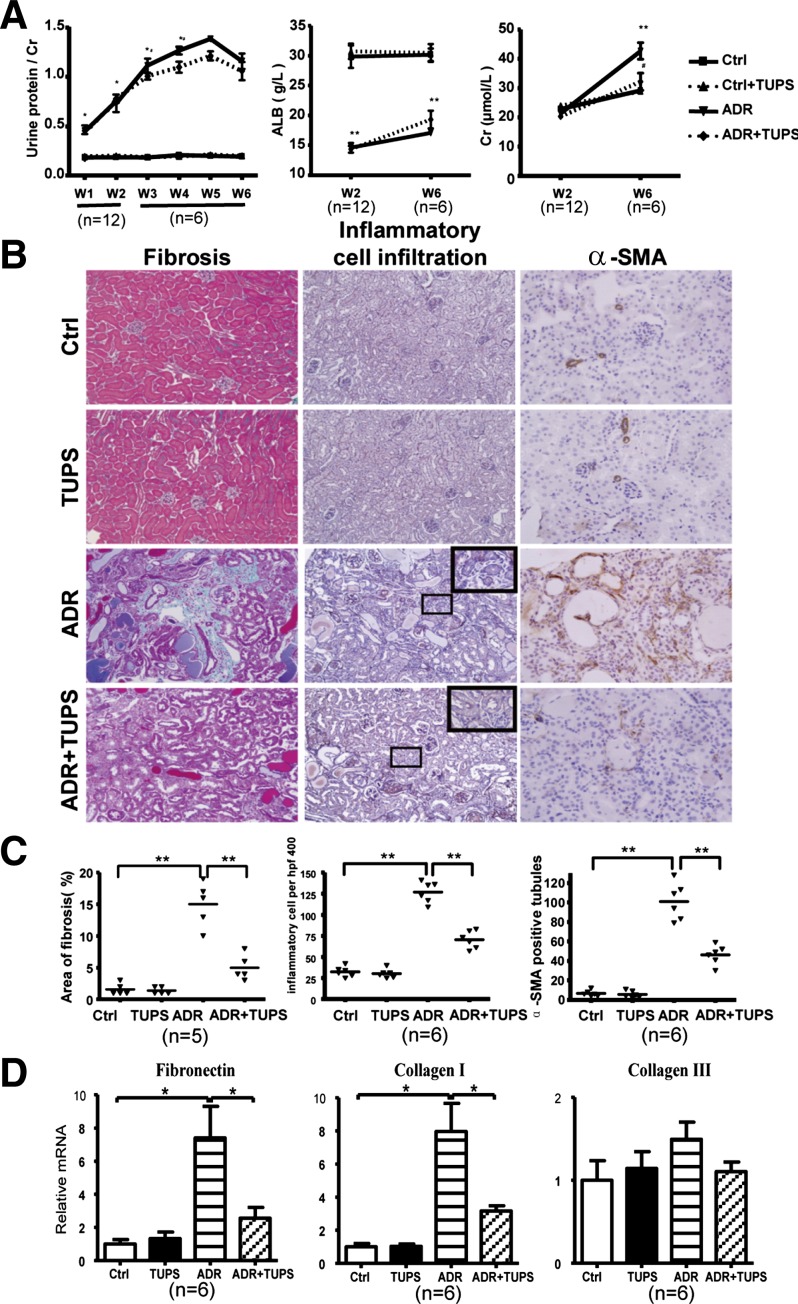
To explore whether sEH affects the development of proteinuria-mediated renal damage in vivo, we used the ADR-induced nephropathy mouse model. The ratio of protein to creatinine in urine was increased significantly starting from 1 wk after ADR injection and peaked at 5 wk (ADR group). Serum albumin in the disease model was significantly decreased at 2 wk after ADR injection up to 6 wk. Administration of TUPS had no effect on ratio of protein to creatinine in urine and levels of serum albumin in controls, and a moderate improvement in ADR group but did not reach significance (Fig. 5A). Serum creatinine levels in diseased mice increased at 6 wk in the ADR group. Administration of TUPS significantly decreased the levels of serum creatinine at 6 wk after ADR injection (Fig. 5A, right).
Fig. 5.
sEH inhibition improved parameters in adriamycin (ADR)-induced nephropathy in mice. The ADR-induced nephropathy mouse model was established with tail vein injection of 10 mg/kg of ADR. The animals were divided into 4 groups (at least 6 mice in each group): control (Ctrl), ADR, TUPS, and ADR+TUPS. PBS treatment was a control. For TUPS treatment, sEH inhibitor TUPS (1.0 mg·kg−1·day−1) was given by oral gavage for 2 or 6 wk. A: 24-h urine protein level was determined every week; serum albumin and creatinine levels were measured 2 or 6 wk after ADR administration. B: cross sections of mouse kidneys at 6 wk after ADR injection histochemically stained with Masson (fibrosis), pulmonary artery smooth muscle (inflammation), and α-SMA (×400). C: area of fibrosis, number of inflammatory cells and number of α-SMA positive tubules in the cross-sections were measured. D: real-time RT-PCR quantification of mRNA levels of collagen I, collagen III, and fibronectin, Data are means ± SD of the relative mRNA normalized to that of GAPDH from at least 6 mice in each group (*P < 0.05; **P < 0.01).
Kidney cross sections were prepared for light microscopy. Sections were stained with Masson to evaluate the degree of renal histological injury and fibrosis. Interstitial fibrosis, inflammatory cell infiltration, and α-SMA staining of tubular cells were greater in kidney sections with ADR treatment, but significantly attenuated by TUPS (Fig. 5, B and C). For fibrosis markers, we found that the mRNA levels of collagen I and fibronectin, but not collagen III, were higher in the ADR group and reduced by TUPS treatment (Fig. 5D). Thus sEH inhibition significantly ameliorated inflammation, EMT, and fibrosis in ADR-induced nephropathy in mice.
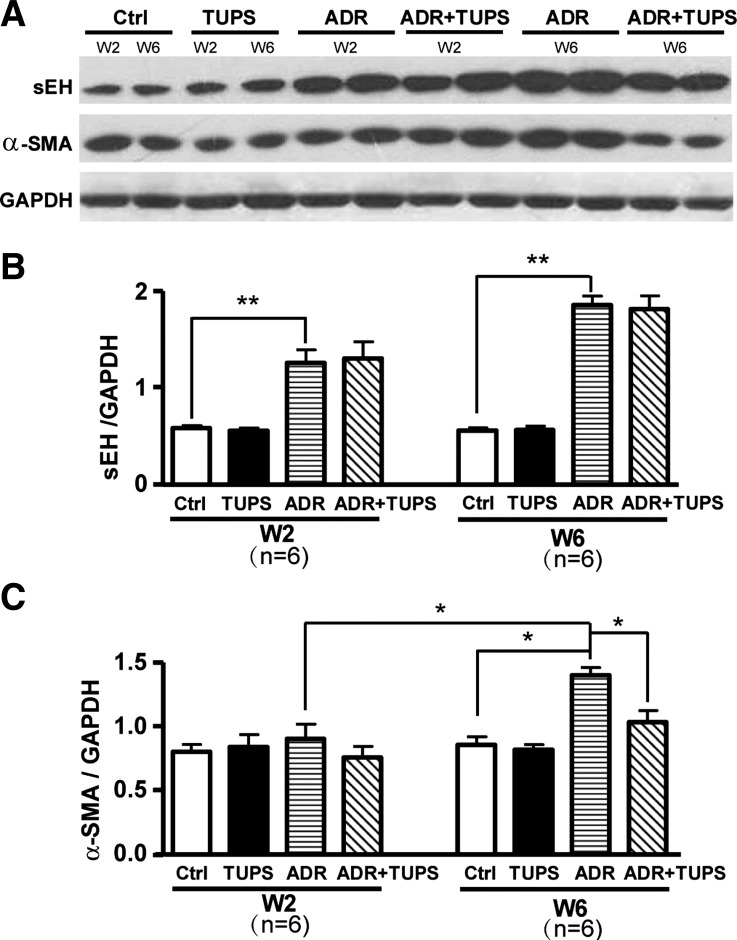
Western blot analysis of the renal cortex revealed both sEH and α-SMA were significantly upregulated at 2 and 6 wk after ADR injection; TUPS had no effect on the expression of α-SMA for a short time (2 wk), but, consistent with the improvement in renal function and histology, TUPS could reduce α-SMA expression after long-term treatment (6 wk) (Fig. 6).
Fig. 6.
Expression of sEH and α-SMA in renal cortex of ADR-induced mouse nephropathy. Proteins were extracted from the renal cortex of animals in Fig. 5. Western blot analysis of sEH, α-SMA, and GAPDH proteins. A: representative results from each group of animals and the mean ± SD of the ratio of sEH to GAPDH (B) or α-SMA to GAPDH (C) from mice 2 and 6 wk (W2 and W6, respectively) after injection (*P < 0.05; **P < 0.01).
DISCUSSION
To explore the role of sEH in glomerulonephritis in humans, we found that 1) sEH expression was mainly located in proximal tubules and upregulated in patients; Western blot analysis confirmed the elevated levels of sEH in patients with MN, MCD, and IgAN; 2) the level of sEH was positively correlated with severity of proteinuria and incidence of edema and negatively with serum albumin level; 3) the protein level of sEH was elevated along with increased α-SMA protein and MCP-1 mRNA and decreased E-cadherin in RPTECs in vitro, whose effects were attenuated by an sEH inhibitor and mimicked with adenovirus-mediated sEH overexpression; and 4) in ADR-induced nephropathy mice, the sEH inhibitor did not improve proteinuria or serum albumin level but reduced long-term elevated serum creatinine level, interstitial inflammation, fibrosis, and α-SMA expression. Thus upregulation of sEH in proximal tubular cells in chronic proteinuric kidney diseases may mediate the proteinuria-induced renal damage.
A comprehensive knowledge of tissue- or cell type-specific patterns of expression of sEH is essential for evaluating its functional significance, especially with the growing interest in its role in many biological activities. The enzyme activity of sEH in human tissue homogenates showed the kidney with the highest levels of enzyme activity (27). Yu and colleagues (37) reported that the expression of sEH localized largely in the renal vasculature, with relatively low levels in the surrounding tubules and glomeruli in human kidneys. The expression of sEH in renal arteries was localized mostly in smooth muscle layers of the arterial wall. The authors did not observe differences in sEH expression between normal and diseased human kidney tissue in the 15 samples examined (37). Using immunohistochemistry, Enayetallah et al. (7, 8) demonstrated abundant expression of sEH protein and high expression in proximal tubules of human kidney, in which sEH is both cytosolic and peroxisomal. They found that CYP2C9 and sEH were very similar in their tissue-specific patterns of expression, but the other two major EET-related CYPs, 2C8 and 2J2, were less frequently associated with sEH (8). We examined 153 human samples of glomerulonephritis and 10 normal tissue samples and found sEH expression mainly located in proximal tubules and upregulated in diseased specimens. Agreeing with the finding by Enayetallah and colleagues (8), we found that CYP2C9 expression was highly colocalized with that of sEH in proximal tubule and increased in samples of patients parallel with the expression of sEH (data not shown). The levels of sEH in different patients vary and were correlated with severity of proteinuria, which would explain the difference with the previous report. Nevertheless, sEH has been proposed to play a role in epithelial cell proliferation (4), which is supported by the distribution in proximal tubule epithelial cells.
sEH was previously found upregulated in hypertensive renal damage, diabetic nephropathy, and Cisplatin-induced nephrotoxicity (28, 39), and sEH was the main effector in AngII-induced hypertension (17). We previously reported that sEH expression was upregulated by AngII and homocysteine in vascular endothelial cells via activation of AP-1 and ATF6 and DNA demethylation (1, 2, 38). Renal AngII, the intrarenal renin-angiotensin system enhanced in chronic glomerulonephritis, could elevate intraglomerular pressure, increase glomerular cell hypertrophy, and augment extracellular matrix accumulation (35). Furthermore, we also found that hepatic sEH upregulation could be induced by systemic inflammation on high-fat diet mice (24). In the present study, we found that sEH expression upregulated in patients with glomerulonephritis. The possible mechanism would be involved in elevated the intrarenal renin-angiotensin system and inflammation.
The elevated level of sEH can increase the hydrolysis of EETs to corresponding diols. EETs not only are involved in regulating vascular tone and antagonizing the vasoconstrictor actions of AngII but also directly influence tubular transport of sodium (13). In our study, the level of sEH was elevated along with increased EMT and inflammation in culture RPTECs, whose effects were mimicked with adenovirus-mediated sEH overexpression and attenuated by an sEH inhibitor in vitro and in ADR-induced nephropathy mice. Thus upregulation of sEH in chronic kidney diseases may mediate the proteinuria-induced renal damage via the decrease in the protective effect of EETs.
sEH inhibitors have been extensively studied in animal models of hypertension, with generally beneficial outcome. Interestingly, we found no correlation between sEH expression in kidney and the incidence of hypertension. Compared with patients with MCD and MN, those with IgAN had more hypertension but less sEH expression. One possible explanation is the different mechanism of hypertension and proteinuria. Many animal models used with sEH study were based on the hypertensive models, and albuminuria was the consequence of hypertension. Because hypertensive renal damage is not the index to perform a kidney biopsy, we did not collect samples with this condition. However, in patients with glomerulonephritis, proteinuria attributed to glomerular inflammation and epithelial cell damage usually being considered as initiator, hypertension would be the response of chronic injury and progression of glomerulonephritis. However, hypertensive renal damage mainly affects vasculature; the elevated sEH level in tubular cells reflects the effects of infiltrated proteins in proteinuric disease, which caused renal EMT and interstitial inflammation.
Given that proximal tubules are more a “sufferer” than an “initiator” of proteinuria, the proteins passing through renal tubules may stimulate and damage tubular epithelial cells. We investigated the effects of urinary protein on the expression of sEH in cultured RPTECs in vitro and found the expression of sEH in this epithelial cell upregulated by urine proteins from patients, along with the upregulation of α-SMA and MCP-1 and downregulation of E-cadherin, which could be largely attenuated by the sEH inhibitor. These results suggested that patients' proteinuria-induced tubular EMT, inflammatory reaction were mediated, at least in part, by upregulation of sEH. Indeed, sEH overexpression could mimic the effect of the urine proteins and deteriorate the expression of those markers of EMT and inflammation, which suggests that elevated levels of sEH expression in renal proximal tubular epithelial cells is not necessary but is sufficient to trigger or aggregate the process of EMT and renal injury.
The ADR nephropathy is a common used model of chronic proteinuric renal disease. Owing to the cytotoxic effects of ADR in podocytes, mice develop significant proteinuria and a subsequent tubule-interstitial injury that mimics many features of chronic proteinuric renal disease in humans. Thus this model was reported to be useful in unraveling the pathogenesis of chronic proteinuric renal disease (36). In our clinical data, we found the elevated levels of sEH directly associated with the proteinuria in patients. Therefore, we used this model to further test our hypothesis that sEH upregulation in proximal tubules mediated epithelial cell EMT and injury. The expression of sEH increased 2 wk after ADR injection, with the increased proteinuria and decreased serum albumin. Inhibition of sEH activity did not affect severity of proteinuria or sEH expression, which suggests that sEH inhibition with TUPS did not cause increased protein infiltration in the acute phase. However, TUPS improved the renal function at 6 wk, as evidenced by decreased serum creatinine, interstitial fibrosis, inflammatory cell infiltration, and tubular α-SMA expression. Recently, inhibition of sEH failed to improve albuminuria in mice with 5/6 nephrectomy (18). Thus sEH inhibition protects renal function by improving tubular EMT, interstitial inflammation, and fibrosis induced by proteinuria but not proteinuria. Increasing protein infiltration may have direct toxic effects on tubular epithelial cells or on promoting renal interstitial inflammation via proinflammatory factors and profibrotic mediators and stimulating EMT. Tubulointerstitial damage and progressive fibrosis are common but relatively terminal pathways that lead to renal failure (10, 33).
In summary, sEH may participate in proteinuria-induced renal damage by triggering or aggravating the EMT of tubular cells and interstitial inflammatory and fibrosis process. sEH could be a potential therapeutic target for preventing the progression of chronic proteinuric kidney diseases.
GRANTS
This work was supported in part by grants from the Major National Basic Research Grant of China (2010CB912504, 2012CB517504); the National Natural Science Foundation of China (30971063, 81130002, 81070213), the “111” plan of China, and the National Institute of Environmental Health Sciences, NIH Grants (RO1 HL059699; RO1 ES02710). B. D. Hammock is a George and Judy Marcus Fellow of the American Asthma Society.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Q.W., W.P., Y.W., and Y. Zhu conception and design of research; Q.W., W.P., Z.C., J.S., Y.L., B.L., and Y. Zhou performed experiments; Q.W., W.P., Y.W., and Y. Zhu analyzed data; Q.W., W.P., Y.W., and Y. Zhu prepared figures; Q.W., W.P., Y.W., and Y. Zhu drafted manuscript; Q.W., W.P., Y.W., and Y. Zhu edited and revised manuscript; Y.G., B.D.H., Y.W., and Y. Zhu interpreted results of experiments; Y.W. and Y. Zhu approved final version of manuscript.
REFERENCES
- 1. Ai D, Fu Y, Guo D, Tanaka H, Wang N, Tang C, Hammock BD, Shyy JY, Zhu Y. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci USA 104: 9018–9023, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ai D, Pang W, Li N, Xu M, Jones PD, Yang J, Zhang Y, Chiamvimonvat N, Shyy JY, Hammock BD, Zhu Y. Soluble epoxide hydrolase plays an essential role in angiotensin II-induced cardiac hypertrophy. Proc Natl Acad Sci USA 106: 564–569, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Chen JK, Falck JR, Reddy KM, Capdevila J, Harris RC. Epoxyeicosatrienoic acids and their sulfonimide derivatives stimulate tyrosine phosphorylation and induce mitogenesis in renal epithelial cells. J Biol Chem 273: 29254–29261, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol 50: 225–237, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Deng Y, Theken KN, Lee CR. Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J Mol Cell Cardiol 48: 331–341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Enayetallah AE, French RA, Barber M, Grant DF. Cell-specific subcellular localization of soluble epoxide hydrolase in human tissues. J Histochem Cytochem 54: 329–335, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Enayetallah AE, French RA, Thibodeau MS, Grant DF. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J Histochem Cytochem 52: 447–454, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Fleming I. Vascular cytochrome p450 enzymes: physiology and pathophysiology. Trends Cardiovasc Med 18: 20–25, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Hallan SI, Stevens P. Screening for chronic kidney disease: which strategy? J Nephrol 23: 147–155, 2010 [PubMed] [Google Scholar]
- 11. Huang H, Morisseau C, Wang J, Yang T, Falck JR, Hammock BD, Wang MH. Increasing or stabilizing renal epoxyeicosatrienoic acid production attenuates abnormal renal function and hypertension in obese rats. Am J Physiol Renal Physiol 293: F342–F349, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol 289: F496–F503, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Imig JD. Targeting epoxides for organ damage in hypertension. J Cardiovasc Pharmacol 56: 329–335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov 8: 794–805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension 39: 690–694, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension 46: 975–981, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jung O, Brandes RP, Kim IH, Schweda F, Schmidt R, Hammock BD, Busse R, Fleming I. Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension 45: 759–765, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Jung O, Jansen F, Mieth A, Barbosa-Sicard E, Pliquett RU, Babelova A, Morisseau C, Hwang SH, Tsai C, Hammock BD, Schaefer L, Geisslinger G, Amann K, Brandes RP. Inhibition of the soluble epoxide hydrolase promotes albuminuria in mice with progressive renal disease. PLoS One 5: e11979, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaspera R, Totah RA. Epoxyeicosatrienoic acids: formation, metabolism and potential role in tissue physiology and pathophysiology. Expert Opin Drug Metab Toxicol 5: 757–771, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Koeners MP, Wesseling S, Ulu A, Sepulveda RL, Morisseau C, Braam B, Hammock BD, Joles JA. Soluble epoxide hydrolase in the generation and maintenance of high blood pressure in spontaneously hypertensive rats. Am J Physiol Endocrinol Metab 300: E691–E698, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsen BT, Campbell WB, Gutterman DD. Beyond vasodilatation: non-vasomotor roles of epoxyeicosatrienoic acids in the cardiovascular system. Trends Pharmacol Sci 28: 32–38, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Lee CR, Pretorius M, Schuck RN, Burch LH, Bartlett J, Williams SM, Zeldin DC, Brown NJ. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with forearm vasodilator responses in humans. Hypertension 57: 116–122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee J, Dahl M, Grande P, Tybjaerg-Hansen A, Nordestgaard BG. Genetically reduced soluble epoxide hydrolase activity and risk of stroke and other cardiovascular disease. Stroke 41: 27–33, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Dang H, Li D, Pang W, Hammock BD, Zhu Y. Inhibition of soluble epoxide hydrolase attenuates high-fat-diet-induced hepatic steatosis by reduced systemic inflammatory status in mice. PLoS One 7: e39165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, Hammock BD, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol 297: F740–F748, 2009. 19553349 [Google Scholar]
- 26. Marshall T, Williams KM. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of urine: concentration of urinary proteins by precipitation with coomassie blue. Clin Chem 39: 2314–2318, 1993 [PubMed] [Google Scholar]
- 27. Pacifici GM, Temellini A, Giuliani L, Rane A, Thomas H, Oesch F. Cytosolic epoxide hydrolase in humans: development and tissue distribution. Arch Toxicol 62: 254–257, 1988 [DOI] [PubMed] [Google Scholar]
- 28. Parrish AR, Chen G, Burghardt RC, Watanabe T, Morisseau C, Hammock BD. Attenuation of cisplatin nephrotoxicity by inhibition of soluble epoxide hydrolase. Cell Biol Toxicol 25: 217–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharma M, McCarthy ET, Reddy DS, Patel PK, Savin VJ, Medhora M, Falck JR. 8,9-Epoxyeicosatrienoic acid protects the glomerular filtration barrier. Prosaglandins Other Lipid Mediat 89: 43–51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, Hammock BD. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci USA 102: 2186–2191, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res 50 Suppl: S52–S56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol 292: C996–C1012, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Taal MW, Brenner BM. Renal risk scores: progress and prospects. Kidney Int 73: 1216–1219, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Tsai HJ, Hwang SH, Morisseau C, Yang J, Jones PD, Kasagami T, Kim IH, Hammock BD. Pharmacokinetic screening of soluble epoxide hydrolase inhibitors in dogs. Eur J Pharm Sci 40: 222–238, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Urushihara M, Kinoshita Y, Kondo S, Kagami S. Involvement of the intrarenal renin-angiotensin system in experimental models of glomerulonephritis. J Biomed Biotechnol 2012: 601786, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Y, Wang YP, Tay YC, Harris DC. Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int 58: 1797–1804, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Yu Z, Davis BB, Morisseau C, Hammock BD, Olson JL, Kroetz DL, Weiss RH. Vascular localization of soluble epoxide hydrolase in the human kidney. Am J Physiol Renal Physiol 286: F720–F726, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Zhang D, Xie X, Chen Y, Hammock BD, Kong W, Zhu Y. Homocysteine upregulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Circ Res 110: 808–817, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol 15: 1244–1253, 2004 [PubMed] [Google Scholar]
- 40. Zordoky BN, El-Kadi AO. Effect of cytochrome P450 polymorphism on arachidonic acid metabolism and their impact on cardiovascular diseases. Pharmacol Ther 125: 446–463, 2010 [DOI] [PubMed] [Google Scholar]