Abstract
The peptide hormone arginine vasopressin (AVP) plays a critical role in regulating salt and water transport in the mammalian kidney. Recent studies have also demonstrated that AVP can promote cell survival in neuronal cells through V1 receptors. The current study addresses whether AVP can inhibit apoptosis in kidney collecting duct cells via V2 receptors and also explores the downstream signaling pathways regulating this phenomenon. Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling analysis and caspase cleavage assays demonstrated that 1-desamino-8-d-arginine vasopressin (dDAVP) inhibited apoptosis induced by various agents (staurosporine, actinomycin D, and cycloheximide) in cultured mouse cortical collecting duct cells (mpkCCD). Incubation with dDAVP also inhibited apoptosis induced by the phosphatidylinositol 3-kinase (PI3K) pathway inhibitor LY294002, suggesting that the antiapoptotic effects of dDAVP are largely independent of PI3K signaling. The V2 receptor antagonist SR121463 completely abolished the antiapoptotic effects of dDAVP. In addition, incubation with 8-cpt-cAMP, a cell-permeable analog of cAMP, reproduced the antiapoptotic effects of dDAVP. Both dDAVP and 8-cpt-cAMP increased phosphorylation of proapoptotic Bcl-2 family members Bad and Bok. Bad phosphorylation at Ser-112 and Ser-155 is known to inhibit its proapoptotic activity. Preincubation with H89 blocked dDAVP-induced phosphorylation of both Bad and Bok, suggesting dependence on protein kinase A (PKA). This study provides evidence that AVP can inhibit apoptosis through the V2 receptor and downstream cAMP-mediated pathways in mammalian kidney. The antiapoptotic action of AVP may be relevant to a number of physiological and pathophysiological conditions including osmotic tolerance in the inner medulla, escape from AVP-induced antidiuresis, and polycystic kidney disease.
Keywords: apoptosis, caspase, collecting duct, phosphorylation, vasopressin
arginine vasopressin (AVP) is a nine-amino acid neurohypophyseal peptide hormone which regulates a diverse array of physiological processes, including regulation of peripheral vascular resistance, a diverse array of neurological functions, and the urine concentration process. One of the main targets of AVP in the mammalian kidney is the renal collecting duct principal cell, which expresses the vasopressin V2 receptor and is involved in regulating water and solute transport. In these cells, AVP signals through the heterotrimeric G protein Gs and subsequent activation of adenylyl cyclases, which mediate a rise in intracellular cAMP, activation of protein kinase A (PKA), and phosphorylation of the water channel aquaporin-2 (11, 18) as well as the urea channel UT-A1 (2). A recent large-scale phosphoproteomics study by our group revealed that AVP may regulate additional signaling pathways in the kidney collecting duct, including various MAP kinase pathways, Wnt/β-catenin signaling, and apoptosis (17). In that study, we provided preliminary evidence that AVP may attenuate apoptosis in isolated rat inner medullary collecting duct (IMCD) cells, a process that may influence cell survival in a variety of physiological [e.g., adaptive osmotic tolerance (4)] and pathophysiological [e.g., autosomal dominant polycystic kidney disease; ADPKD (37)] conditions. In the current study, we set out to both confirm and further expand on these findings by addressing the potential signaling pathways regulating this phenomenon.
Prior studies in neurons (5, 6) and glomerular mesangial cells (15) have demonstrated that AVP inhibits apoptosis and promotes cell survival through V1 receptors coupled to G protein Gq which can activate phospholipase C, stimulating various PKC isoforms as well as the phosphatidylinositol 3-kinase (PI3K)-Akt pathway. Activation of the ERK MAP kinase pathway via transactivation of EGFR has also been implicated in the AVP effect on cell survival and proliferation (6, 12). In the kidney collecting duct, however, V2 receptors, rather than V1 receptors, predominate in principal cells (38, 43), and it is well established that AVP binding to the V2 receptor does not signal through PKC (7). In addition, incubation with AVP has been shown to inhibit ERK MAP kinase signaling in the collecting duct (28). Taking these results into consideration, we propose that fundamentally different signaling mechanisms must be responsible for modulating the effect of AVP on apoptosis in collecting duct cells. In this study, we address the potential signaling pathways regulating this phenomenon in a mouse cortical collecting duct (mpkCCD) cell line.
EXPERIMENTAL PROCEDURES
Cell culture.
All experiments were performed in mpkCCD11 cells (passages 8–12) as previously described (43); mpkCCD11 cells are a subclone of the mpkCCD14 cell line derived from microdissected cortical collecting ducts from SV-PK/Tag transgenic mice (1). Briefly, cells were expanded to ∼80% confluence on 25-cm2 plastic flasks (Corning), trypsinized (0.05% trypsin, 1.5 mM EDTA) and resuspended in 10 ml DMEM/F12, and then seeded on permeable supports (24-mm2 diameter, 0.4-μm pore size, Corning) at a ratio of 1:10 and grown in 1:1 DMEM/F12 (Invitrogen) containing 2% fetal bovine serum, insulin, dexamethasone, triiodothyronine, epidermal growth factor, selenium, and transferrin as previously described (1). Once cells reached confluence and demonstrated a transepithelial resistance (RTE) of ≥5 KΩ·cm2, they were washed in 1× PBS containing MgCl2 and CaCl2 and grown overnight in serum- and hormone-free 1:1 DMEM/F12. mpkCCD cells were selected for these studies since they express the V2 receptor, are responsive to AVP, and possesses a phenotype consistent with principal cells (30, 43).
Reagents.
[Desamino-Cys1,d-Arg8]vasopressin (dDAVP) was purchased from Bachem (Torrance, CA). 8-(4-Chlorophenylthio)adenosine 3′,5′-cyclic monophosphate (8-cpt-cAMP) and 8-(4-chlorophenylthio)-2′-O-methyladenosine 3′,5′-cyclic monophosphate (Me-cAMP) were from Sigma (St. Louis, MO). H89 and LY294002 were from EMD Millipore (Billerica, MA). Staurosporine was from Cell Signaling Technology (Danvers, MA). Actinomycin D, camptothecin, cycloheximide, dexamethasone, and etoposide were from EMD Millipore. The V2 receptor antagonist SR121463, also known as satavaptan, was a kind gift from Dr. Claudine Serradeil-Le Gal (Sanofi-Synthélabo Recherche, Nancy, France) (32).
Immunoblotting.
IMCD protein samples were diluted in Laemmli buffer (10 mM Tris, pH 6.8, 1.5% SDS, 6% glycerol, 0.05% bromophenol blue, 40 mM DTT) and subjected to SDS-PAGE. Immunoblot analysis using nitrocellulose membranes was performed as described previously (28). Both blocking buffer and IR dye-coupled secondary antibodies were obtained from LI-COR (Lincoln, NE). Fluorescence signals from discrete bands were read out using the LI-COR Odyssey system. Antibodies to full-length caspase-3 (no. 9662), cleaved caspase-3 (no. 9664), full-length caspase-7 (no. 9492), cleaved caspase-7 (no. 9491), and poly(ADP-ribose)polymerase (PARP; no. 9542) were purchased from Cell Signaling Technology and used at the manufacturer's recommended dilutions. An affinity-purified rabbit polyclonal antibody (anti-pS8-BOK) was generated against a proprietary sequence from the N terminus of rat BOK that included phosphorylation at serine-8 (PhosphoSolutions, Aurora, CA). The specificity of this antibody was tested by dotblot and immunoblot analysis (Appendix Fig. A1). Total BOK antibody (H-151) was from Santa Cruz Biotechnology (Santa Cruz, CA). Band densities for phosphorylated proteins were normalized to the corresponding total form of each protein. Changes in band density on protein immunoblots are expressed as means ± SE (n ≥3 samples) for each group. Paired t-tests or ANOVA followed by the appropriate posttest were performed for each data set.
Immunofluorescence microscopy.
Immunofluorescence labeling was done as described in Yu et al. (44) except that the blocking agent was 0.2% gelatin. Confocal fluorescence micrographs were obtained using a Zeiss LSM 510 microscope (Carl Zeiss; NHLBI Light Microscopy Core Facility).
Caspase cleavage assay.
MpkCCD cells grown to confluence on six-well permeable supports were preincubated for 15 min with a variety of inhibitors or corresponding vehicle control followed by incubation with 0.1 nM dDAVP for the indicated times. After 15-min incubation with dDAVP, the indicated proapoptotic agent was added to the cells to induce apoptosis. Following the remainder of the dDAVP incubation, cells were lysed and scraped in 2% SDS and processed for immunoblotting to detect levels of the cleaved forms of caspase-3, caspase-7, and PARP. Each experiment was repeated at least three times to produce at least three biological replicates for immunoblot analysis.
Detection of apoptotic cell death.
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) was performed using a Click-iT TUNEL Alexa Fluor Imaging Assay (Life Technologies, Grand Island, NY) by following the manufacturer's protocol except that propidium iodide replaced Hoechst 33342 as the nuclear DNA marker. Cells undergoing apoptosis were detected based on the incorporation of a modified dUTP (EdUTP) by the enzyme terminal deoxynucleotide transferase (TdT) at the 3′-OH ends of fragmented DNA (5-h incubation). The numbers of apoptotic cells (i.e., EdUTP-positive) from 15 different fields (5 fields each from 3 biological replicates) were counted to get an average number of cells per field.
RESULTS
AVP attenuates apoptosis in mpkCCD cells.
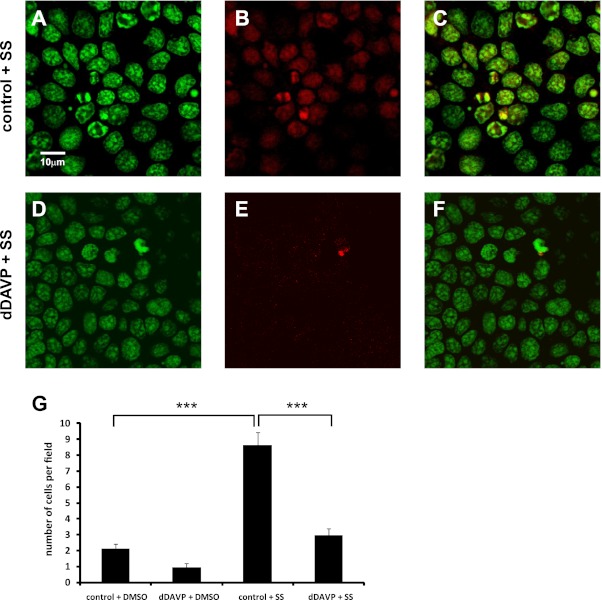
Cultured mpkCCD cells were incubated for 3 h in the presence or absence of 0.1 nM dDAVP, a V2 receptor-selective analog of AVP. Staurosporine (1 μM) was added 30 min after the start of dDAVP treatment to induce apoptosis. Staurosporine is a broad-spectrum kinase inhibitor that is known to induce cellular apoptosis at higher concentrations (0.25–1 μM) (45). Cells were then fixed and assayed by TUNEL, which detects the level of cellular apoptosis based on end-labeling of DNA breaks with a modified nucleotide, in this case EdUTP (Fig. 1). Staurosporine significantly increased the number of TUNEL-positive cells per field compared with cells incubated without staurosporine (Fig. 1G, control+DMSO vs. control+SS). In cells treated with staurosporine, the average number of TUNEL-positive cells per field was significantly decreased in the presence of dDAVP (Fig. 1, E and G, dDAVP+SS) compared with cells without dDAVP (Fig. 1, B and G, control+SS), while the level of nuclear DNA stained with propidium iodide did not change between control and dDAVP-treated cells (Fig. 1, A and D). This result demonstrates that dDAVP attenuates staurosporine-induced apoptosis in mpkCCD cells.
Fig. 1.
dDAVP significantly reduces the level of bromodeoxyuridine (BrdU)-labeled DNA breaks in staurosporine-treated cells. Mouse cortical collecting duct cells (mpkCCD) were incubated in the absence (control; A–C) or presence (dDAVP; D–F) of 0.1 nM dDAVP for 3 h in the presence of 1 μM staurosporine (SS). Cells were then fixed and assayed by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL). A and D: propidium iodide staining of total cellular DNA. B and E: EdUTP-labeled DNA breaks; C and F: merged images. G: the average number of EdUTP-labeled cells per field (i.e., cells undergoing apoptosis) were measured for each condition. Values for cells incubated in the absence of staurosporine (DMSO vehicle) are also shown to indicate baseline levels of apoptosis in the presence and absence of dDAVP. Fields = 15 (5 fields each from 3 biological replicates); ×63 objective; scale bar = 10 μm. ***P < 0.001.
AVP inhibits cleavage of multiple apoptotic proteins in mpkCCD cells.
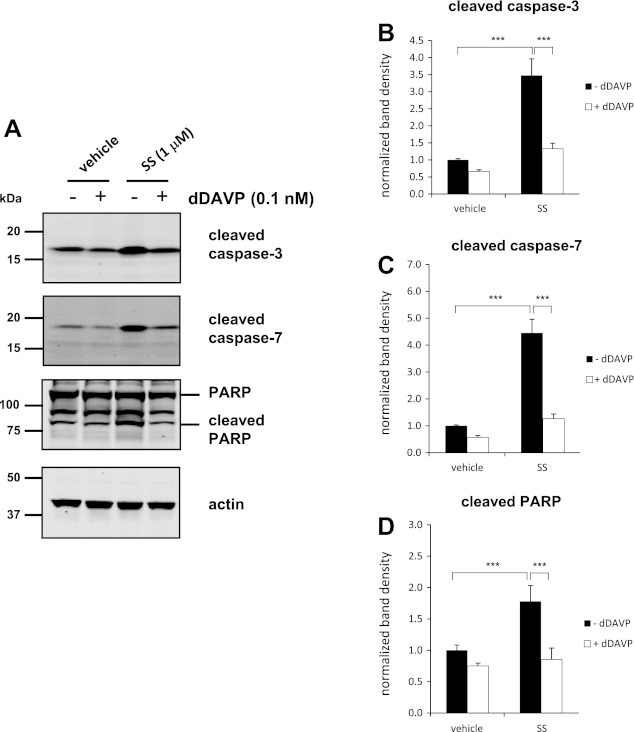
Previously, we demonstrated that AVP reduced the levels of cleaved caspases in isolated native rat IMCD cells treated with dDAVP (17). Caspase cleavage is considered one of the hallmarks of apoptosis (8, 10, 27, 35). To determine whether AVP inhibits caspase cleavage in mpkCCD cells, we incubated cells with or without dDAVP for 2 h in the presence or absence of staurosporine and quantified the levels of the cleaved forms of caspase-3, caspase-7, and PARP (one of the main cleavage targets of caspase-3 in vivo) (27, 34) (Fig. 2). Incubating cells with 1 μM staurosporine for 2 h in the absence of dDAVP dramatically increased the levels of the cleaved forms of all apoptotic proteins compared with cells without staurosporine. Incubation with dDAVP significantly reduced the levels of these cleaved proteins in staurosporine-treated cells.
Fig. 2.
dDAVP significantly reduces levels of the cleaved forms of caspase-3, caspase-7, and poly(ADP-ribose)polymerase (PARP) in cells treated with staurosporine. mpkCCD cells were incubated in the absence (−) or presence (+) of 0.1 nM dDAVP for 2 h. SS or DMSO vehicle was added 15 min after the start of dDAVP treatment. A: representative immunoblots show the levels of the cleaved forms of caspase-3 (17 kDa), caspase-7 (20 kDa), and PARP (89 kDa), as well as full-length PARP (166 kDa), and actin (45 kDa) as a loading control. Also shown is quantification of band densities from replicate immunoblots of the cleaved forms of caspase-3 (B), caspase-7 (C), and PARP (bottom band; D). P values were calculated using paired ANOVA and a Bonferroni posttest. ***P < 0.001.
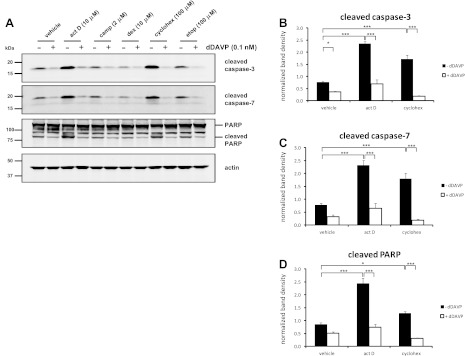
To determine whether dDAVP can inhibit the cleavage of apoptotic proteins induced by other proapoptotic agents, cells were treated for 4 h with 10 μM actinomycin D, 2 μM camptothecin, 10 μM dexamethasone, 100 μM cycloheximide, or 100 μM etoposide. Only actinomycin D and cycloheximide significantly increased the levels of cleaved apoptotic proteins compared with cells treated with vehicle (Fig. 3). Incubation with dDAVP significantly reduced the levels of these cleaved proteins in both actinomycin D-treated and cycloheximide-treated cells. At 4 h, dDAVP also significantly reduced the level of cleaved caspase-3 in cells treated only with vehicle (Fig. 3B).
Fig. 3.
dDAVP significantly reduces levels of the cleaved forms of caspase-3, caspase-7, and PARP in cells treated with a variety of proapoptotic agents. MpkCCD cells were incubated in the absence (−) or presence (+) of 0.1 nM dDAVP for 4 h. Actinomycin D (10 μM), camptothecin (2 μM), dexamethasone (10 μM), cycloheximide (100 μM), etoposide (100 μM), or DMSO vehicle was added 15 min after the start of dDAVP treatment. A: representative immunoblots show the levels of the cleaved forms of caspase-3 (17 kDa), caspase-7 (20 kDa), and PARP (89 kDa), as well as full-length PARP (166 kDa), and actin (45 kDa) as a loading control. Also shown is quantification of band densities from replicate immunoblots of the cleaved forms of caspase-3 (B), caspase-7 (C), and PARP (bottom band; D) from cells treated with actinomycin D, cycloheximide, or DMSO vehicle. P values were calculated using paired ANOVA and a Bonferroni posttest. ***P < 0.001. *P < 0.05.
AVP signals through the V2 receptor to inhibit apoptosis.
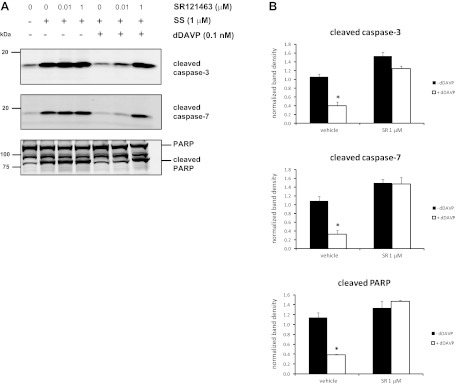
To determine whether the antiapoptotic effects of dDAVP are mediated through the V2 receptor, mpkCCD cells were preincubated with varying doses of the V2 receptor antagonist SR121463 (0.01 or 1 μM) followed by treatment with dDAVP and staurosporine. Again, the levels of the cleaved forms of caspase-3, caspase-7, and PARP were significantly elevated in staurosporine-treated cells compared to cells without staurosporine, and the addition of dDAVP significantly reduced the levels of these cleavage products (Fig. 4). This effect was completely blocked in cells preincubated with 1 μM SR121463.
Fig. 4.
V2 receptor antagonist SR121463 blocks the reduction in cleaved apoptotic proteins in response to dDAVP. MpkCCD cells were preincubated for 15 min with SR121463 (0.01 or 1 μM) or vehicle control, followed by incubation in the absence (−) or presence (+) of 0.1 nM dDAVP for 2 h. SS or DMSO control was added 15 min after the start of dDAVP treatment. A: representative immunoblots show the levels of the cleaved forms of caspase-3, caspase-7, and PARP (bottom band). B: quantification of band densities from replicate immunoblots of the cleaved forms of caspase-3, caspase-7, and PARP. P values were calculated using paired t-tests. *P < 0.05.
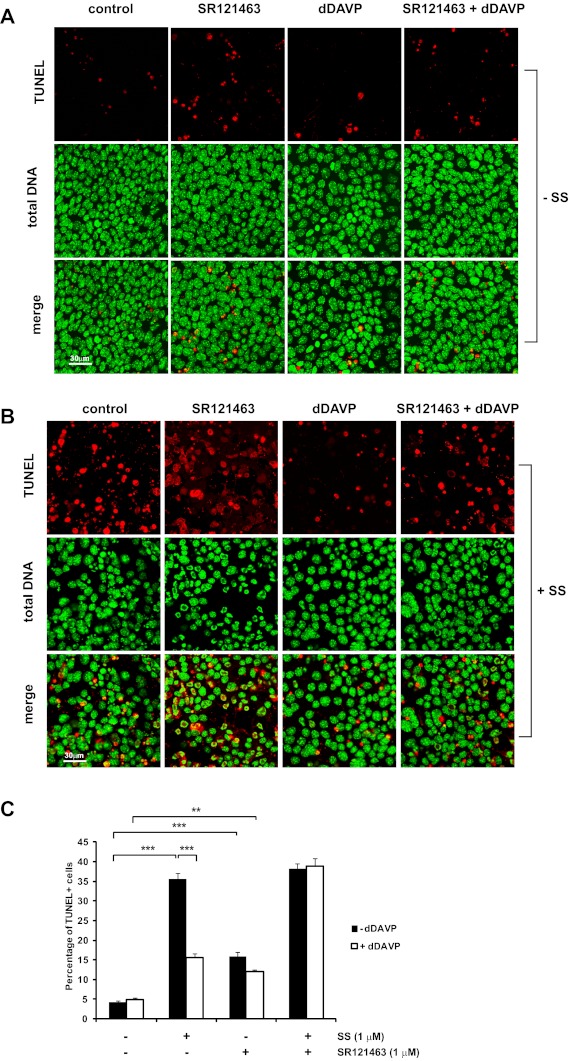
The effects of SR121463 were also analyzed by TUNEL to determine whether V2 receptor signaling is involved in reducing the number of apoptotic cells in response to dDAVP (Fig. 5). MpkCCD cells were preincubated for 15 min with or without 1 μM SR121463 followed by incubation with or without 0.1 nM dDAVP for 3 h. After 15 min of dDAVP treatment, cells were treated with either vehicle (Fig. 5A) or 1 μM staurosporine (Fig. 5B) for the remainder of the experiment. As in Fig. 1, staurosporine significantly increased the number of TUNEL-positive cells per field compared with cells incubated without staurosporine. Also, the presence of dDAVP significantly reduced the percentage of staurosporine-treated cells undergoing apoptosis. However, dDAVP did not significantly reduce the number of TUNEL-positive cells pretreated with SR121463, suggesting that V2 receptor signaling is required for the antiapoptotic effects of dDAVP. Interestingly, the presence of 1 μM SR121463 alone slightly increased the percentage of TUNEL-positive cells compared with cells without the drug, indicating that this drug may possess properties beyond its competitive effects on the V2 receptor.
Fig. 5.
SR121463 blocks the antiapoptotic effects of dDAVP. MpkCCD cells were treated as indicated, fixed, and analyzed by TUNEL. Total cellular DNA was stained with propidium iodide. The percentage of TUNEL-positive cells per field (i.e., cells undergoing apoptosis) was measured for each condition (C). Fields = 15 (5 fields each from 3 biological replicates); ×40 objective; scale bar = 30 μm. ***P < 0.001. **P < 0.01.
Analogs of cAMP regulate the levels of cleaved apoptotic proteins in mpkCCD cells.
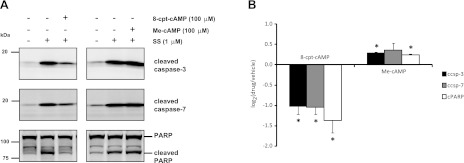
One of the main secondary messengers elevated by AVP in collecting duct cells is cAMP (13). In mpkCCD cells, cAMP levels increase within 2 min of stimulation with dDAVP and remain elevated for as long as 4 days with sustained exposure (23, 24). Downstream targets of cAMP include the regulatory subunit of protein kinase A (PKA) and the Rap1-GEF family member exchange protein directly activated by cAMP (EPAC). To determine whether cAMP can regulate the level of apoptosis in mpkCCD cells, staurosporine-treated cells were incubated with either a general analog of cAMP (8-cpt-cAMP, 100 μM), an EPAC-selective analog of cAMP (Me-cAMP, 100 μM), or vehicle control for 2 h followed by quantification of cleaved apoptotic proteins by immunoblotting (Fig. 6A). The general cAMP analog 8-cpt-cAMP was chosen over a PKA-specific analog to determine the predominant effects of a general elevation in intracellular cAMP on the levels of cleaved apoptotic proteins. Incubation of cells with 8-cpt-cAMP significantly decreased levels of the cleaved forms of caspase-3, caspase-7, and PARP, while incubation of cells with Me-cAMP produced a small but significant increase in the levels of cleaved caspase-3 and cleaved PARP (Fig. 6B). These results suggest that a general elevation of intracellular cAMP can mimic the antiapoptotic effects of AVP, while selective activation of EPAC may increase apoptosis.
Fig. 6.
Incubation of cells with cAMP analogs affects SS-induced apoptosis. MpkCCD cells were incubated with 8-cpt-cAMP (100 μM), the EPAC-specific agonist Me-cAMP (100 μM), or vehicle control for 2 h with or without SS. A: representative immunoblots show the levels of the cleaved forms of caspase-3, caspase-7, and PARP (bottom band). B: quantification of log2 ratio of cleaved caspase-3 (ccsp-3), cleaved caspase-7 (ccsp-7), and cleaved PARP (cPARP) from cAMP analog-treated vs. vehicle-treated samples from replicate immunoblots. P values were calculated using paired t-tests. *P < 0.05.
Inhibition of the PI3K-Akt pathway induces apoptosis in mpkCCD cells, and this effect is blocked by AVP.
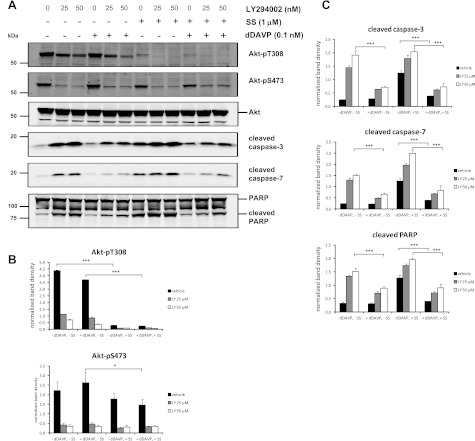
One of the major kinase pathways mediating the antiapoptotic effects of AVP in neuronal cells is the PI3K-Akt pathway (5, 6). This process is thought to be mediated through activation of the heterotrimeric G protein Gq and phospholipase C (5). To determine the involvement of the PI3K-Akt signaling pathway in regulating collecting duct cell apoptosis, quantitative immunoblotting was performed on mpkCCD cells preincubated with the PI3K inhibitor LY294002 (25 and 50 μM) followed by addition of dDAVP or vehicle control with or without staurosporine treatment (Fig. 7). Phosphorylation of Akt at its two activation sites, Thr-308 (Akt-pT308) and Ser-473 (Akt-pS473), was significantly decreased by LY294002 (Fig. 7, A and B) and resulted in elevated levels of cleaved apoptotic proteins in cells without staurosporine or dDAVP (Fig. 7A lanes 1–3). Addition of dDAVP significantly attenuated this response for the cleaved apoptotic proteins (Fig. 7, A, lanes 4–6, and C). These results demonstrate that inhibiting PI3K increases apoptosis and suggest that dDAVP can suppress this effect through a PI3K-Akt-independent pathway. Cells treated with staurosporine exhibited dramatically reduced levels of Akt-pT308, while levels of Akt-pS473 were only slightly reduced with staurosporine treatment (Fig. 7B). This result is consistent with a prior study demonstrating that the apoptotic effects of staurosporine are, at least in part, dependent on inhibition of Akt phosphorylation at Thr-308 (16). In the current study, dDAVP significantly reduced the levels of cleaved apoptotic proteins in cells treated with staurosporine alone as well as in cells treated with staurosporine in combination with LY294002 (Fig. 7C), results suggesting that a significant portion of the antiapoptotic properties of AVP in collecting duct cells are independent of PI3K-Akt signaling.
Fig. 7.
Phosphatidylinositol 3-kinase (PI3-kinase) inhibitor LY294002 inhibits Akt phosphorylation and increases apoptosis. MpkCCD cells were preincubated for 15 min with LY294002 (25 or 50 μM) or vehicle control, followed by incubation in the absence (−) or presence (+) of 0.1 nM dDAVP for 2 h. SS or DMSO control was added 15 min after the start of dDAVP treatment. A: representative immunoblots show the levels Akt phosphorylated at Thr-308 (Akt-pT308), Akt phosphorylated at Ser-473 (Akt-pS473), total Akt (Akt), cleaved caspase-3, cleaved caspase-7, cleaved PARP (bottom band), and full-length PARP (top band). B: quantification of band densities from replicate immunoblots of both forms of phosphorylated Akt. C: quantification of band densities from replicate immunoblots of the cleaved forms of caspase-3, caspase-7, and PARP. P values were calculated using paired ANOVA and a Bonferroni posttest. ***P < 0.001. *P < 0.05.
Multiple Bcl-2 family member proteins are regulated in response to short-term AVP treatment.
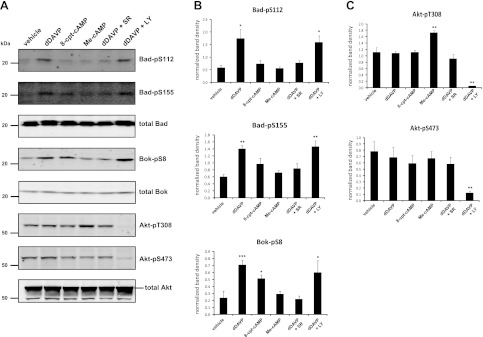
Previous studies have shown that the proapoptotic proteins Bad and Bok, both members of the Bcl-2 family, become phosphorylated with short-term dDAVP treatment (17, 30). Phosphorylation at these sites by various basophilic kinases (predominantly PKA and Akt) has either been predicted or directly demonstrated to inhibit apoptosis in other cell types (25, 39, 46). Thus, in the current study, we wanted to demonstrate these phosphorylation changes with dDAVP in mpkCCD cells and also to address whether the cAMP-PKA and/or the PI3K-Akt pathways may be involved in mediating these phosphorylation events. Quantitative immunoblotting with phospho-specific antibodies demonstrated that phosphorylation of Bad at Ser-112 and Ser-155 significantly increased within 30 min of dDAVP treatment, as was phosphorylation of Bok at Ser-8 (Fig. 8, A and B), while levels of phosphorylated Akt remained unchanged with dDAVP (Fig. 8C). Preincubation of cells with the V2 receptor antagonist SR121463 (1 μM) blocked dDAVP-mediated increases in Bad and Bok phosphorylation. However, preincubation of cells with the PI3K inhibitor LY294002 (50 μM) significantly reduced Akt phosphorylation (Fig. 8, A and C) but had no effect on levels of phosphorylated Bad or Bok (Fig. 8, A and B). These results suggest that the PI3K-Akt pathway is not involved in dDAVP-dependent phosphorylation of Bad or Bok at these sites in mpkCCD cells. To address the potential cAMP dependence of these phosphorylation events, cells were also incubated with either 100 μM 8-cpt-cAMP or the EPAC-selective analog Me-cAMP followed by quantitative immunoblotting for the phosphorylated forms of Bad and Bok (Fig. 8, A and B). Bok-pS8 was significantly increased in the presence of 8-cpt-cAMP. Levels of Bad-pS155 were elevated, although not significantly, by 8-cpt-cAMP. Bad-pS112 was completely unaffected by 8-cpt-cAMP. None of these sites was affected by Me-cAMP. Taken together, these results suggest that the increase in Bok-pS8 and Bad-pS155 with dDAVP may be at least partly dependent on PKA.
Fig. 8.
Phosphorylation of Bcl-2 family member proteins is regulated by dDAVP and cAMP, independently of PI3K. A: mpkCCD cells were incubated with either dDAVP (0.1 nM), 8-cpt-cAMP (100 μM), Me-cAMP (100 μM), or vehicle control for 30 min followed by immunoblotting for phosphorylated as well as total levels of Bad, Bok, and Akt. Cells preincubated with either SR121463 (1 μM) or LY294002 (50 μM) before dDAVP treatment were also included (dDAVP+SR and dDAVP+LY, respectively). B: quantification of band densities from replicate immunoblots probed for Bad phosphorylated at Ser-112 (Bad-pS112), Bad phosphorylated at Ser-155 (Bad-pS155), and Bok phosphorylated at Ser-8 (Bok-pS8). C: quantification of Akt phosphorylated at Thr-308 (Akt-pT308) and Ser-473 (Akt-pS473). P values were calculated using paired ANOVA followed by Dunnett's posttest. *, **, and ***: P < 0.05, <0.01, and <0.001, respectively, vs. vehicle control.
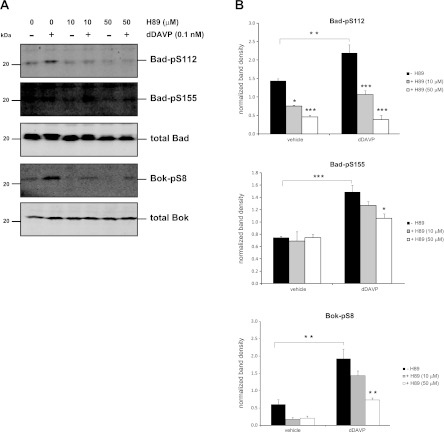
To further explore potential involvement of PKA in mediating these phosphorylation events, quantitative immunoblotting was performed on cells preincubated with various concentrations of the PKA inhibitor H89 (10 and 50 μM) for 15 min followed by incubation for 30 min with either 0.1 nM dDAVP or vehicle control (Fig. 9). Incubation of cells with dDAVP significantly increased levels of Bad-pS112, Bad-pS155, and Bok-pS8, while preincubation with H89 produced a dose-dependent reduction in this response for all three phosphorylation sites. The sensitivity of Bad-pS155 and Bok-pS8 to the H89 inhibitor, in combination with our finding that both of these sites are increased by 8-cpt-cAMP, suggests that these sites are either directly or indirectly regulated by PKA in response to dDAVP in mpkCCD cells. While Bad-pS112 was not elevated by 8-cpt-cAMP, the sensitivity of this site to H89 suggests that it may be the target of other kinases that are known to be inhibited by H89, including members of the ribosomal S6 kinase (RSK) family (26, 31, 36). Indeed, multiple isoforms of p90RSK (Rsk1, Rsk2, and Rsk3) have been shown to phosphorylate Bad at Ser-112 (3, 33).
Fig. 9.
Dose-dependent inhibition of dDAVP-induced phosphorylation of Bcl-2 family member proteins by H89. Cells were preincubated in the absence or presence of the PKA inhibitor H89 at 2 concentrations (10 or 50 μM) before addition of 0.1 nM dDAVP for 30 min. A: separate immunoblots were performed using antibodies that recognized phosphorylated as well as total levels of Bad and Bok. B: quantification of band densities from replicate immunoblots of phosphorylated Bad (Bad-pS112, Bad-pS155) and phosphorylated Bok (Bok-pS8). P values without brackets indicate significance vs. group vehicle control without H89 (−H89). *, **, and ***: P values <0.05, <0.01, and <0.001, respectively (paired ANOVA with Bonferroni posttest).
DISCUSSION
The current study establishes that the V2 receptor-selective AVP analog dDAVP exerts strong antiapoptotic activity in collecting duct cells, indicating a previously unrecognized role for AVP in kidney physiology. Incubating cells with dDAVP significantly inhibited the amount of nick end-labeled DNA by TUNEL analysis and significantly reduced the amount of the cleaved forms of caspase-3, caspase-7, and PARP in cells treated with the proapoptotic agent staurosporine. dDAVP also significantly reduced the levels of cleaved apoptotic proteins in cells treated with two other proapoptotic reagents, actinomycin D (an inhibitor of RNA synthesis) and cycloheximide (an inhibitor of protein synthesis), indicating that the antiapoptotic properties of dDAVP are likely a general phenomenon. Another agent found to promote apoptosis in mpkCCD cells was the PI3K inhibitor LY294002. This was not surprising as the proliferative effects of PI3K-mediated signaling are well documented in the current literature. Specifically, PI3K signaling has been shown to stimulate proliferation of pancreatic β-cells (42), and in renal proximal tubular cells, overexpression of a constitutively active form of Akt was able to block hyperglycemia-induced apoptosis associated with diabetic nephropathy (29). In the current study, incubation of cells with the PI3K inhibitor LY294002 dramatically increased the levels of cleaved apoptotic proteins compared with cells without the inhibitor, suggesting that the PI3K pathway actively suppresses apoptosis under basal conditions. However, dDAVP significantly reduced the levels of cleaved apoptotic proteins in cells treated with LY294002 both alone and in combination with staurosporine, a result suggesting that the majority of the antiapoptotic effects of AVP are independent of PI3K-Akt signaling. This indicates a major difference from studies implicating the PI3K-Akt pathway in V1 receptor-mediated inhibition of apoptosis (5, 6).
This study provides initial evidence that the antiapoptotic effects of AVP in the collecting duct occur through the V2 receptor and likely involve cAMP-mediated signaling. The V2 receptor antagonist SR121463 completely blocked the antiapoptotic effects of dDAVP as determined by TUNEL analysis and also abrogated the reduction in cleaved apoptotic proteins produced by dDAVP, demonstrating that dDAVP binding to the V2 receptor is critical for this response. In addition, incubation of staurosporine-treated cells with a general analog of cAMP, 8-cpt-cAMP, dramatically reduced levels of cleaved apoptotic proteins, suggesting that elevation of intracellular cAMP can mimic the antiapoptotic effects of dDAVP in the collecting duct. Incubation of cells with the EPAC-selective cAMP analog Me-cAMP slightly increased levels of cleaved apoptotic proteins, indicating that cAMP may increase apoptosis when signaling through EPAC. The observation that cAMP can exhibit both antiapoptotic and proapoptotic properties within the same cell type is well established in the literature (reviewed in Ref. 21). However, in most of these cases, one response usually predominates. In the case of mpkCCD cells, our results suggest that cAMP produces a predominantly antiapoptotic response and that elevation of intracellular cAMP, at least in part, regulates the antiapoptotic effects of AVP in collecting duct cells.
Our results also implicate the Bcl-2 family member proteins Bad and Bok as downstream targets of AVP in the regulation of apoptosis. The Bcl-2 protein family includes both proapoptotic (Bax, Bak, Bad, and Bok) and antiapoptotic members (Bcl-2, Bcl-xL, and Bcl-w) that can dimerize with each other and ultimately regulate the release of cytochrome C from mitochondria, a crucial step in initiating caspase cleavage, which leads to cellular apoptosis (reviewed in Ref. 20). In the current study, dDAVP increased Bad phosphorylation at Ser-112 and Ser-155. Ser-112 is located within a potential Akt/PKB consensus motif (…ETRSRHSS*YPA…) and can be phosphorylated by Akt (9), members of the p90RSK family (3, 33), as well as mitochondrial-anchored PKA (14). Ser-155 falls within a PKA consensus motif (…ELRRMS*DE…) and is predominantly phosphorylated by PKA (25, 39). Phosphorylation of Bad at these two sites inhibits its proapoptotic activity by blocking its dimerization with the antiapoptotic protein Bcl-xL as well as promoting its interaction with 14-3-3 proteins in the cytoplasm (22, 25, 39). In our study, neither of these sites was sensitive to concentrations of LY294002 that blocked Akt phosphorylation, indicating that the PI3K-Akt pathway is not involved in phosphorylating these sites in response to AVP. Both sites were sensitive to the PKA inhibitor H89; however, Ser-155 was only moderately increased by cAMP and Ser-112 was completely unaffected by cAMP. As H89 is known to inhibit kinases other than PKA (e.g., S6K1, MSK1, ROCK-II, PKBα, and Rsk2) (26), this result indicates that additional kinases likely contribute to phosphorylation of these residues in response to AVP.
Phosphorylation of Bok at Ser-8 was also increased by short-term dDAVP treatment in this study. There is mounting evidence that phosphorylation of Bok is PKA dependent. This site lies within a strong PKA consensus motif (…VLRRSS*VF…). In addition, PKA is capable of phosphorylating this residue as determined by in vitro kinase assays (17). The current study reveals that Bok-pS8 is sensitive to both H89 and is significantly elevated by cAMP. Future studies will be required to address whether phosphorylation of Bok can inhibit its proapoptotic activity in a fashion similar to Bad.
In conclusion, we have demonstrated that vasopressin can inhibit apoptosis in collecting duct cells through a V2 receptor-mediated pathway that likely involves elevation of intracellular cAMP, activation of PKA, and regulated phosphorylation of Bad and Bok (summarized in Fig. 10). Although we are beginning to elucidate the signaling pathways regulating the antiapoptotic actions of AVP in the collecting duct, the physiological role of AVP in promoting kidney cell survival remains unclear. One possibility is that increased cell survival in the presence of AVP may contribute to osmotic tolerance in the kidney inner medulla, a region which is often subjected to extremely high levels of NaCl and urea during the urine concentration process. In a recent study, Cai et al. (4) found that treating Brattleboro rats with dDAVP caused a fourfold increase in expression of inner medullary Pax2, a transcription factor crucial for kidney cell differentiation. The same study also found that small interfering RNA-mediated inhibition of Pax2 expression increased both high NaCl-induced cleavage of caspase-3 as well as the number of apoptotic bodies in mouse inner medullary collecting duct cells. These results suggest that AVP may also control cell survival at the transcriptional level through regulation of genes important for normal kidney development. At the pathophysiological level, there is evidence that cellular apoptosis may be regulated during the phenomenon of escape from the antidiuretic action of vasopressin (i.e., “vasopressin escape”), a process that limits the severity of various hyponatremic disorders. Hoorn et al. (19) found increased levels of various proteins involved in apoptosis, including caspase-3 and RACK1, in inner medullary collecting duct protein samples from a rat model undergoing the later stages of vasopressin escape. There is also growing evidence that the effects of AVP on cell survival could figure prominently in renal proliferative disorders such as autosomal dominant polycystic kidney disease (ADPKD). Cystic epithelia display expansion in response to increases in cAMP and the resulting proliferative response is mediated through PKA (40). In addition, V2 receptor antagonists can inhibit cystogenesis in animal models, presumably by downregulating cAMP signaling (41). Thus the evidence that AVP can regulate cell growth and survival in both physiological and pathophysiological settings is growing and warrants further investigation of the signaling pathways regulating apoptosis in the renal collecting duct.
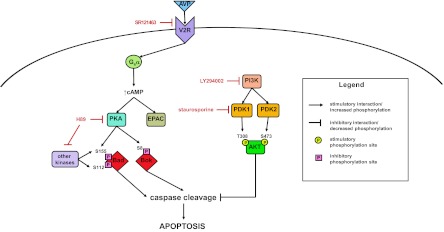
Fig. 10.
Signaling networks involved in regulation of apoptosis in collecting duct cells. Schematic shows regulation of caspase cleavage and apoptosis through the cAMP-PKA pathway downstream of the vasopressin V2 receptor as well as the PI3K-Akt signaling pathway. Sites of action for the various inhibitors used in the study are indicated by red lettering.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute intramural budget (ZO1-HL001285).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.L.M., P.C.S., and J.D.H. performed experiments; R.L.M., P.C.S., T.P., M.A.K., and J.D.H. edited and revised manuscript; R.L.M., P.C.S., T.P., M.A.K., and J.D.H. approved final version of manuscript; P.C.S. and J.D.H. prepared figures; T.P. and J.D.H. provided conception and design of research; J.D.H. analyzed data; J.D.H. interpreted results of experiments; J.D.H. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Claudine Serradeil-Le Gal for providing the V2 receptor antagonist and Jae H. Song for technical assistance.
Appendix
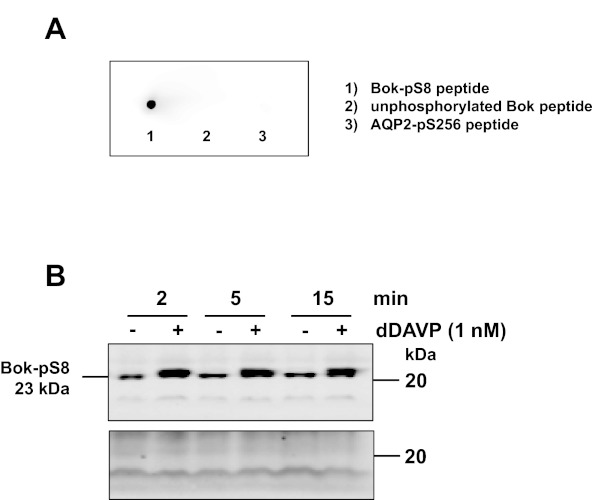
Fig. A1.
Characterization of a newly generated phospho-specific Bok antibody. A: a rabbit polyclonal antibody recognizing Bok phosphorylated at Ser-8 was tested for reactivity with the immunizing phosphopeptide (lane 1), the unphosphorylated version of the same peptide (lane 2), and a peptide corresponding to the phosphorylated C terminus of aquaporin-2 (AQP2-pS256) as a negative control (lane 3). B: rat collecting duct samples incubated in the absence (−) or presence (+) of 1 nM dDAVP were probed with the phospho-specific Bok antibody preadsorbed with either the unphosphorylated Bok antigenic peptide (top) or the phosphorylated Bok antigenic peptide (bottom).
REFERENCES
- 1. Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Blount MA, Mistry AC, Frohlich O, Price SR, Chen G, Sands JM, Klein JD. Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am J Physiol Renal Physiol 295: F295–F299, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286: 1358–1362, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Cai Q, Dmitrieva NI, Ferraris JD, Brooks HL, van Balkom BW, Burg M. Pax2 expression occurs in renal medullary epithelial cells in vivo and in cell culture, is osmoregulated, and promotes osmotic tolerance. Proc Natl Acad Sci USA 102: 503–508, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen J, Liu Y, Soh JW, Aguilera G. Antiapoptotic effects of vasopressin in the neuronal cell line H32 involve protein kinase Calpha and beta. J Neurochem 110: 1310–1320, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen J, Volpi S, Aguilera G. Anti-apoptotic actions of vasopressin in H32 neurons involve MAP kinase transactivation and Bad phosphorylation. Exp Neurol 211: 529–538, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chou CL, Rapko SI, Knepper MA. Phosphoinositide signaling in rat inner medullary collecting duct. Am J Physiol Renal Physiol 274: F564–F572, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Cohen GM. Caspases: the executioners of apoptosis. Biochem J 326: 1–16, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278: 687–689, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Fernandes-Alnemri T, Takahashi A, Armstrong R, Krebs J, Fritz L, Tomaselli KJ, Wang L, Yu Z, Croce CM, Salveson G. Mch3, a novel human apoptotic cysteine protease highly related to CPP32. Cancer Res 55: 6045–6052, 1995 [PubMed] [Google Scholar]
- 11. Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Ghosh PM, Bedolla R, Thomas CA, Kreisberg JI. Role of protein kinase C in arginine vasopressin-stimulated ERK and p70S6 kinase phosphorylation. J Cell Biochem 91: 1109–1129, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Grantham JJ, Burg MB. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol 211: 255–259, 1966 [DOI] [PubMed] [Google Scholar]
- 14. Harada H, Becknell B, Wilm M, Mann M, Huang LJ, Taylor SS, Scott JD, Korsmeyer SJ. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell 3: 413–422, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Higashiyama M, Ishikawa S, Saito T, Nakamura T, Kusaka I, Nagasaka S, Honda K, Saito T. Arginine vasopressin inhibits apoptosis of rat glomerular mesangial cells via V1a receptors. Life Sci 68: 1485–1493, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Hill MM, Andjelkovic M, Brazil DP, Ferrari S, Fabbro D, Hemmings BA. Insulin-stimulated protein kinase B phosphorylation on Ser-473 is independent of its activity and occurs through a staurosporine-insensitive kinase. J Biol Chem 276: 25643–25646, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Hoffert JD, Pisitkun T, Saeed F, Song JH, Chou CL, Knepper MA. Dynamics of the G protein-coupled vasopressin V2 receptor signaling network revealed by quantitative phosphoproteomics. Mol Cell Proteomics 11: M111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoorn EJ, Hoffert JD, Knepper MA. Combined proteomics and pathways analysis of collecting duct reveals a protein regulatory network activated in vasopressin escape. J Am Soc Nephrol 16: 2852–2863, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsu SY, Hsueh AJ. Tissue-specific Bcl-2 protein partners in apoptosis: an ovarian paradigm. Physiol Rev 80: 593–614, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Insel PA, Zhang L, Murray F, Yokouchi H, Zambon AC. Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol (Oxf) 204: 277–287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korsmeyer SJ, Gross A, Harada H, Zha J, Wang K, Yin XM, Wei M, Zinkel S. Death and survival signals determine active/inactive conformations of pro-apoptotic BAX, BAD, and BID molecules. Cold Spring Harb Symp Quant Biol 64: 343–350, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Kortenoeven ML, Trimpert C, van den BM, Li Y, Wetzels JF, Deen PM. In mpkCCD cells, long-term regulation of aquaporin-2 by vasopressin occurs independent of protein kinase A and CREB but may involve Epac. Am J Physiol Renal Physiol 302: F1395–F1401, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Li Y, Shaw S, Kamsteeg EJ, Vandewalle A, Deen PM. Development of lithium-induced nephrogenic diabetes insipidus is dissociated from adenylyl cyclase activity. J Am Soc Nephrol 17: 1063–1072, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Lizcano JM, Morrice N, Cohen P. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem J 349: 547–557, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev 24: 261–274, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376: 37–43, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Pisitkun T, Jacob V, Schleicher SM, Chou CL, Yu MJ, Knepper MA. Akt and ERK1/2 pathways are components of the vasopressin signaling network in rat native IMCD. Am J Physiol Renal Physiol 295: F1030–F1043, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rane MJ, Song Y, Jin S, Barati MT, Wu R, Kausar H, Tan Y, Wang Y, Zhou G, Klein JB, Li X, Cai L. Interplay between Akt and p38 MAPK pathways in the regulation of renal tubular cell apoptosis associated with diabetic nephropathy. Am J Physiol Renal Physiol 298: F49–F61, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rinschen MM, Yu MJ, Wang G, Boja ES, Hoffert JD, Pisitkun T, Knepper MA. Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci USA 107: 3882–3887, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sapkota GP, Kieloch A, Lizcano JM, Lain S, Arthur JS, Williams MR, Morrice N, Deak M, Alessi DR. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell growth. J Biol Chem 276: 19469–19482, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Serradeil-Le GC. An overview of SR121463, a selective non-peptide vasopressin V2 receptor antagonist. Cardiovasc Drug Rev 19: 201–214, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Tan Y, Ruan H, Demeter MR, Comb MJ. p90(RSK) blocks bad-mediated cell death via a protein kinase C-dependent pathway. J Biol Chem 274: 34859–34867, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 81: 801–809, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 272: 17907–17911, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Tomas-Zuber M, Mary JL, Lesslauer W. Control sites of ribosomal S6 kinase B and persistent activation through tumor necrosis factor. J Biol Chem 275: 23549–23558, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2: 40–55, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Uawithya P, Pisitkun T, Ruttenberg BE, Knepper MA. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics 32: 229–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Virdee K, Parone PA, Tolkovsky AM. Phosphorylation of the pro-apoptotic protein BAD on serine 155, a novel site, contributes to cell survival. Curr Biol 10: 1151–1154, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Wang X, Ward CJ, Harris PC, Torres VE. Cyclic nucleotide signaling in polycystic kidney disease. Kidney Int 77: 129–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 102–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yao LJ, McCormick JA, Wang J, Yang KY, Kidwai A, Colussi GL, Boini KM, Birnbaum MJ, Lang F, German MS, Pearce D. Novel role for SGK3 in glucose homeostasis revealed in SGK3/Akt2 double-null mice. Mol Endocrinol 25: 2106–2118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, Chou CL, Pisitkun T, Nelson RD, Knepper MA. Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci USA 106: 2441–2446, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu MJ, Pisitkun T, Wang G, Shen RF, Knepper MA. LC-MS/MS analysis of apical and basolateral plasma membranes of rat renal collecting duct cells. Mol Cell Proteomics 5: 2131–2145, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yue TL, Wang C, Romanic AM, Kikly K, Keller P, DeWolf WE, Jr, Hart TK, Thomas HC, Storer B, Gu JL, Wang X, Feuerstein GZ. Staurosporine-induced apoptosis in cardiomyocytes: a potential role of caspase-3. J Mol Cell Cardiol 30: 495–507, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14–3-3 not BCL-X(L). Cell 87: 619–628, 1996 [DOI] [PubMed] [Google Scholar]