Abstract
The incretin hormone glucagon-like peptide-1 (GLP-1) is released from the gut in response to fat or carbohydrate and contributes to negative feedback control of blood glucose by stimulating insulin secretion, inhibiting glucagon, and slowing gastric emptying. GLP-1 receptors (GLP-1R) are also expressed in the proximal tubule, and possibly elsewhere in the kidney. Presently, we examined the effect of a GLP-1R agonist on single-nephron glomerular filtration rate (GFR; SNGFR), proximal reabsorption (Jprox), tubuloglomerular feedback (TGF) responses, and urine flow rate in hydropenic male Wistar and Wistar-Froemter rats. Micropuncture and whole-kidney data were obtained before and during infusion of the GLP-1 agonist exenatide (1 nmol/h iv). SNGFR and Jprox were measured by late proximal collection at both extremes of TGF activation, which was achieved by perfusing Henle's loop at 0 or 50 nl/min. Primary changes in Jprox were revealed by analysis of covariance for Jprox with SNGFR as a covariate. Effects on TGF activation were determined in a separate set of experiments by comparing early distal and late proximal collections. Exenatide increased SNGFR by 33–50%, suppressed proximal tubular reabsorption by 20–40%, doubled early distal flow rate, and increased urine flow rate sixfold without altering the efficiency of glomerulotubular balance, TGF responsiveness, or the tonic influence of TGF. This implies that exenatide is both a proximal diuretic and a renal vasodilator. Since the natural agonist for the GLP-1R is regulated by intake of fat and carbohydrate, but not by salt or fluid, the control of salt excretion by the GLP-1R system departs from the usual negative-feedback paradigm for regulating salt balance.
Keywords: exenatide, glomerular filtration, glucagon-like peptide 1, tubular reabsorption, tubuloglomerular feedback
incretins are hormones produced by endocrine cells of the gut upon exposure to fat or carbohydrate (7, 8). These hormones have several glucose-lowering effects that have been harnessed in the treatment of type 2 diabetes mellitus. Therapeutic manipulation of the incretin known as glucagon-like peptide-1 (GLP-1) has been a principal focus of clinical and basic diabetes research in recent years with >5,000 PubMed citations on the subject and 4 drugs approved by the FDA since 2005. Two of these agents (sitagliptin and saxigliptin) are inhibitors of the enzyme dipeptidyl peptidase IV (DPP-4), which degrades GLP-1. The other two agents (exenatide and liraglutide) are peptide agonists of the GLP-1 receptor (GLP-1R) and are resistant to degradation by DPP-4 (33).
While most of the incretin literature focuses on glucose metabolism, a handful of publications suggest that activating GLP-1R might affect renal hemodynamics or salt handling. GLP-1R is expressed in cultured monolayers of proximal tubule cells, where activation by GLP-1 inhibits sodium transport (24). GLP-1R is expressed in human renal arteries (17). GLP-1R mRNA is found in rat glomeruli and proximal tubules (5), and recombinant GLP-1 infusion vasodilates the rat kidney (5), more so with intact renal nerves (21). DPP-4, an enzyme that rapidly degrades GLP-1, is expressed in proximal tubule brush border, where it associates with Na/H exchanger 3 (NHE3) (13) and where its expression (both mRNA and protein) is augmented by a high-fat diet (34). Recombinant GLP-1 is also natriuretic in the rat (4), and continuous infusion in Dahl salt-sensitive rats accelerates the return to salt balance following an increase in sodium intake (36). Sustained exposure to the GLP-1R agonist exenatide lowers blood pressure in rats with metabolic syndrome induced by corticosterone (19). Exenatide also lowers blood pressure in salt-sensitive obese db/db mice and ANG II-infused C57BLK6/J mice (15). In obese men with glomerular hyperfiltration, intravenous infusions of GLP-1 increased sodium excretion and reduced the glomerular filtration rate (GFR) toward normal, suggesting a diuretic action at the proximal renal tubule leading to activation of tubuloglomerular feedback (TGF) (14).
In the present study, we examined the acute effects of the DPP-4-resistant GLP-1R agonist exenatide on glomerular filtration, proximal reabsorption, and TGF responses using micropuncture in rats. We report that exenatide is both a renal vasodilator and a proximal diuretic and that it does not suppress the responsiveness of TGF.
METHODS
All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals with an Institutional Animal Care and Use Committee-registered protocol.
Surgical Preparation for Micropuncture
Adult male Wistar and Wistar-Froemter (MWF) rats were surgically prepared for micropuncture according to previously established protocols (29). Briefly, animals were anesthetized with Inactin (100 mg/kg ip, Research Biochemicals, Natick MA), and body temperature was maintained on a servo-controlled heating table. The airway was maintained with a tracheostomy. Catheters were placed in the jugular vein, femoral artery, and urinary bladder. The left kidney was exposed through a flank incision, immobilized in a lucite cup, and bathed with warm Ringer saline. The left ureter was cannulated for separate urine collection. Ringer saline containing [3H]inulin (80 μCi/ml) was infused at 2 ml/h for maintenance fluid and as a marker of GFR. Blood pressure was monitored throughout by an intra-arterial catheter. Each experiment consisted of two micropuncture periods. One hour was allowed for equilibration between the end of surgery and the start of the first period. Control data were obtained during the first micropuncture period, after which intravenous infusion of exenatide (1 nmol/h, Sigma) was begun. The second period of micropuncture was begun 30 min after the start of the exenatide infusion. This protocol for delivering exenatide yields a stable plasma exenatide concentration of 2 nM in the rat (23). At the beginning and end of each micropuncture period, a blood sample was obtained to measure hematocrit and radioactivity. Urine collected during micropuncture was used to measure urine flow rate and whole-kidney GFR.
Micropuncture Protocols
Two micropuncture protocols were employed. The first protocol was applied in six adult male Wistar rats to determine the effects of exenatide on single-nephron GFR (SNGFR) at both extremes of TGF activation and on proximal reabsorption (Jprox). The second protocol was applied to three of these same Wistar rats and in nine additional Wistar-Froemter rats for the purpose of measuring the effects of exenatide on SNGFR and distal fluid delivery at the natural TGF operating point and on the tonic influence exerted by TGF over SNGFR. The second protocol required tubular fluid collections from early distal nephrons. Wistar-Froemter rats are better suited for this due to the presence of surface glomeruli and more prevalent early distal tubules on the kidney surface.
Protocol 1: effects of exenatide on TGF reactivity and proximal reabsorption.
During each experimental period, SNGFR and Jprox were measured at both extremes of TGF activation in each of several nephrons. This was done by timed collections from the late proximal tubule while the TGF signal was manipulated by orthograde perfusion of Henle's loop. These microperfusions were done with a Hample nanoliter pump (University of Tuebingen) filled with artificial tubular fluid and positioned just downstream of a wax block in the last proximal segment while tubular fluid was collected from upstream of the wax block. Paired collections were made in each nephron with perfusion at 0 and 50 nl/min to establish values for SNGFR and Jprox under conditions of zero, and maximal, TGF activation. The TGF response is saturable and commonly represented by a hyperbolic tangent. The upper and lower limits of the TGF curve are referred to as at the “shoulder” and “elbow” of the TGF curve, respectively. Composition of artificial tubular fluid was (mM) 130 NaCl, 10 NaHCO3, 4 KCl, and 2 CaCl2 as well as 45 mg/100 ml urea and 0.1% FD&C, pH 7.4. The point of measuring Jprox at both extremes of TGF activation was to parse flow-dependent from flow-independent changes in Jprox. Flow affects Jprox through so-called glomerulotubular balance (GTB) whereas flow-independent changes in Jprox require some change in behavior of the tubule and are referred to as “primary” changes in Jprox (29, 31). Primary effects of exenatide on Jprox were sought by using TGF as a tool to gather data on Jprox as a function of SNGFR, then looking for primary effects on Jprox by applying analysis of covariance to the data with SNGFR as covariate.
Protocol 2: effects of exenatide on ambient SNGFR, early distal fluid delivery, and tonic influence of TGF.
Three micropuncture collections were made from each of multiple nephrons during each experimental period. Nephrons with accessible early distal tubules were identified by injecting a small bolus of dye-stained artificial tubular fluid into Bowman's space of a surface glomerulus or an early proximal tubule and watching for the dye to reappear in a nearby distal segment. Next, a timed collection of tubular fluid was made from that early distal segment. During early distal collection, TGF is left to operate normally such that SNGFR measured by early distal collection (SNGFRd) reflects the value of SNGFR at the natural TGF operating point. Next, the distal collection was repeated while late proximal flow was augmented in the same nephron by 15 nl/min with artificial tubular fluid delivered by the Hample pump. It was assumed that augmenting late proximal flow by this amount would saturate the TGF response and that inulin clearance in these collections provides SNGFR at the elbow of the TGF curve (SNGFRe). Finally, a third collection was made from the late proximal tubule of the same nephron to give SNGFR at the shoulder of the TGF curve (SNGFRp). In this protocol, the range of the TGF response is the difference, SNGFRp − SNGFRe. We use two indices for the tone exerted by TGF under natural operating conditions. First, is the familiar proximal-distal difference or PDD, SNGFRp − SNGFRd. Second is the difference between SNGFRd and SNGFRe, which we coin the distal-elbow difference, DED. As the tonic influence of TGF becomes greater, PDD increases and DED decreases. PDD+DED gives the range of the TGF response.
Statistical Analysis
Results are expressed as means ± SE. Initial statistical testing was by ANOVA, analysis of covariance (ANCOVA), or repeated-measures ANOVA where a degree of freedom was assigned to each tubular fluid collection, which is typical of the micropuncture literature. Analyses were repeated using a more rigorous nested design to parse the variance of a given dependent variable between effects of exenatide, rat strain, and date of the experiment as recently described (31). All tests were done using proprietary software (Systat, Evanston, IL).
RESULTS
Whole-Kidney Data
Exenatide had no immediate effect on blood pressure but caused GFR to increase by 25% (P = 0.03) and urine flow rate to increase by sixfold (P < 0.0005). These effects were not different between the two rat strains (see Table 1).
Table 1.
Whole kidney data
| Strain of Rat | Body Wt, g | MAP, mmHg | GFR, ml/min | Urine Flow Rate, μl/min | FE, % | |
|---|---|---|---|---|---|---|
| Wistar (n = 6) | 386 ± 21 | Control | 127 ± 3 | 3.6 ± 0.2 | 18 ± 6 | 0.53 |
| Exenatide | 120 ± 4 | 4.6 ± 0.5 | 89 ± 15 | 1.96 | ||
| Absolute difference or (fold-effect) | −7 ± 2 | 1.0 ± 0.5 | 71 ± 11 | (6.0 ± 0.6) | ||
| Wistar-Froemter (n = 9) | 325 ± 9 | Control | 127 ± 2 | 3.8 ± 0.4 | 9 ± 1 | 0.24 |
| Exenatide | 130 ± 2 | 4.7 ± 0.7 | 72 ± 11 | 1.83 | ||
| Difference or (ratio) | 3 ± 2 | 0.9 ± 0.6 | 63 ± 10 | (7.1 ± 1.1) | ||
| Combined (n = 15) | 349 ± 12 | Control | 127 ± 2 | 3.7 ± 0.3 | 13 ± 3 | 0.37 |
| Exenatide | 126 ± 2 | 4.6 ± 0.4 | 80 ± 9 | 1.88 | ||
| Difference or (ratio) | −1 ± 2 | 0.9 ± 0.4 | 67 ± 7 | (6.6 ± 0.9) | ||
| P value for exenatide vs. control | 0.719 | 0.030 | <0.0005 | <0.0005 |
Values are means ± SE. MAP, mean arterial pressure; GFR, glomerular filtration rate; UOP, urine output; FE, fractional excretion of fluid.
Micropuncture Data
Micropuncture data included 243 tubular fluid collections from late proximal or early distal tubules in 15 rats (6 Wistars and 9 MWF). Of these 243 tubular fluid collections, 72 were done according to protocol 1 and 171 were done according to protocol 2.
SNGFR
Initial experiments were done in six Wistar rats to test for effects of exenatide on SNGFR at both the shoulder and elbow of the TGF curve (see Table 2). Samples were obtained from each nephron at both extremes of TGF activation according to protocol 1 (see above). Data from 72 tubular fluid collections were analyzed by repeated-measures ANOVA, where the between-subjects effect gives the effect of exenatide on SNGFR overall and the within-subjects effect gives the effect of exenatide on the TGF responsiveness. Exenatide caused SNGFR to increase by ∼50% at both the extremes of TGF activation (P = 0.0002 for the between-subjects effect). The vasodilatory effect of exenatide did not compromise the TGF responsiveness. In fact, the range of the TGF response appeared to increase with exenatide (18.5 ± 3.6 vs. 8.1 ± 4.1 nl/min, P = 0.06 for effect of exenatide on the within-subjects effect), although the effect disappeared when the TGF response was expressed as a fraction of the SNGFR at the TGF midpoint (0.38 ± 0.10 vs. 0.31 ± 0.11, P = 0.64 by 1-way ANOVA).
Table 2.
Analysis of 72 tubular fluid collections from 2-period micropuncture studies according to protocol 1
| Group | SNGFR0, nl/min | SNGFR50, nl/min | TGFrange, nl/min | TGFrange/SNGFR | Jprox0, nl/min | Jprox50, nl/min | FRP0 | FRP50† | GTBslope | Jprox*, nl/min |
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 16) | ||||||||||
| Raw data | 43.7 ± 3.5 | 31.0 ± 3.9 | 12.7 ± 2.7 | 0.42 ± 0.11 | 23.5 ± 1.6 | 18.7 ± 1.9 | 0.55 ± 0.02 | 0.66 ± 0.04 | 0.42 ± 0.07 | 25.8 ± 0.9 |
| LS ANOVA | 44.3 ± 3.7 | 31.4 ± 5.6 | 12.9 ± 4.4 | 0.41 ± 0.13 | 22.9 ± 1.6 | 18.8 ± 2.4 | 0.53 ± 0.02 | 0.65 ± 0.03 | na | 24.7 ± 0.9 |
| Exenatide (n = 20) | ||||||||||
| ANOVA | 67.6 ± 3.8 | 48.4 ± 4.7 | 19.2 ± 3.7 | 0.39 ± 0.09 | 21.9 ± 2.5 | 17.3 ± 2.0 | 0.31 ± 0.02 | 0.35 ± 0.02 | 0.38 ± 0.05 | 15.8 ± 0.8 |
| Nested | 64.4 ± 3.4 | 46.9 ± 5.0 | 17.5 ± 4.0 | 0.35 ± 0.11 | 19.3 ± 1.5 | 15.6 ± 2.2 | 0.29 ± 0.02 | 0.32 ± 0.03 | na | 15.0 ± 0.8 |
| P value for exenatide | ||||||||||
| ANOVA | <0.0005 | 0.001 | 0.786 | 0.453 | 0.343 | 0.397 | <0.0005 | <0.0005 | 0.959 | <0.0005 |
| Nested | 0.001 | 0.050 | 0.450 | 0.749 | 0.114 | 0.339 | <0.0005 | <0.0005 | na | <0.0005 |
Values are means ± SE for raw data (top row in each cell) and for least-squares (LS) ANOVA adjusted for nesting of values within rats. (bottom row in each cell). SNGFR, single-nephron GFR; TGF, tubuloglomerular feedback; GTB, glomerulotubular balance; FRP, fractional reabsorption by proximal tubule. Subscripts 0 and 50 refer to rate of Henle's loop perfusion with artificial tubular fluid (in nl/min). GTB slope is obtained by linear regression; Jprox, a + b
SNGFR; Jprox*, proximal reabsorption adjusted for SNGFR by ANCOVA.
FRP50>FRP0 (P < 0.0005), affirming that fractional reabsorption declines as SNGFR increases within a given nephron.
Protocol 2 was applied in three Wistar and nine MWF rats to test for effects of exenatide on ambient nephron filtration rate, SNGFRd, and tonic influence exerted by TGF (see Table 3). These experiments also yielded information on the range of the TGF response by a less conventional protocol in which SNGFR at the elbow of the TGF curve, SNGFRe, was obtained by collecting from the early distal nephron while late proximal flow was augmented by 15 nl/min in the free-flowing nephron to saturate the TGF response. Exenatide increased SNGFRd by 30% (P < 0.01), similar to its effect on whole-kidney GFR (see above). Similarly, exenatide increased SNGFRp (P < 0.03) and tended to increase SNGFRe (P = not significant). On average, SNGFRd operated about one-third of the way from shoulder to elbow of the TGF curve, as previously observed for hydropenic rats by other methods (27). There was no apparent effect of exenatide on the range of the TGF response or on the orientation of SNGFd relative to the shoulder or elbow of the TGF curve. Results in Table 3 are shown for both the standard least-squares ANOVA and for the nested statistical design, which adjusts for effects of rat strain and date of individual experiments. The same overall conclusions are supported by both analyses.
Table 3.
Analysis of 171 tubular fluid collections from 2-period micropuncture studies in 9 male Wistar-Froemter+3 Wistar rats according to protocol 2
| SNGFRd | SNGFRp | SNGFRe | PDD | DED | TGFrange | Vdist | FDdist | Jdist* | Jprox* | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 26) | ||||||||||
| ANOVA | 31.2 ± 2.3 | 35.0 ± 2.7 | 22.7 ± 3.1 | 3.8 ± 1.7 | 8.0 ± 2.5 | 12.2 ± 2.8 | 5.8 ± 0.9 | 0.19 ± 0.02 | 28.9 ± 0.7 | 15.0 ± 0.9 |
| Nested | 31.9 ± 2.2 | 36.3 ± 2.8 | 23.9 ± 3.3 | 4.5 ± 1.9 | 7.0 ± 2.3 | 12.1 ± 2.7 | 5.9 ± 0.9 | 0.18 ± 0.02 | 29.0 ± 0.8 | 15.1 ± 0.8 |
| Exenatide (n = 31) | ||||||||||
| ANOVA | 40.0 ± 2.1 | 43.4 ± 2.4 | 29.0 ± 2.8 | 3.4 ± 1.6 | 11.1 ± 2.4 | 15.5 ± 2.5 | 11.8 ± 0.8 | 0.30 ± 0.02 | 25.3 ± 0.6 | 12.4 ± 0.8 |
| Nested | 39.1 ± 2.1 | 43.4 ± 2.6 | 27.9 ± 3.0 | 4.3 ± 1.7 | 11.9 ± 2.1 | 16.5 ± 2.5 | 11.8 ± 0.9 | 0.30 ± 0.02 | 25.0 ± 0.7 | 13.0 ± 0.8 |
| P value for exenatide | ||||||||||
| ANOVA | 0.007 | 0.024 | 0.139 | 0.874 | 0.343 | 0.387 | <0.0005 | <0.0005 | <0.0005 | 0.027 |
| Nested | 0.023 | 0.072 | 0.379 | 0.963 | 0.128 | 0.229 | <0.0005 | <0.0005 | 0.001 | 0.079 |
Values are means ± SE. The first row in each cell is result of basic least-squares ANOVA, and the second row in each cell was generated by a nested design, which corrects for influence of experimental date and rat strain. Vdist, early distal flow rate; FDdist, fractional fluid delivery to early distal nephron; Jdist*, absolute reabsorption up to early distal nephron corrected for SNGFRd by ANCOVA; Jprox*, absolute reabsorption up to late proximal tubule corrected for SNGFRp by ANCOVA.
Proximal Tubular Reabsorption
Protocol 1 was designed to test for primary effects of exenatide on Jprox. Applying ANCOVA to 72 late proximal collections obtained by this protocol revealed exenatide to be a potent proximal diuretic that does not suppress GTB (see Table 2 and Fig. 1). Details are as follows. To discriminate direct effects of GLP-1R activation on Jprox from changes in Jprox due to GTB, we applied ANCOVA with state of GLP-1R activation as an independent categorical variable and SNGFR as a covariate. ANCOVA estimates the impact of SNGFR on Jprox by linear regression and computes a variance for Jprox as the average distance from the regression line. Then, it assigns means ± SE to the variate (Jprox*), which represents Jprox at the mean value for SNGFR from the pooled data. Doing ANOVA on Jprox* is the equivalent of doing ANCOVA on Jprox. This approach was previously described (25, 31). We were interested to know how exenatide affects Jprox* at the normal operating point of the nephron. However, the operating point of a nephron is rendered indeterminate by the act of late proximal collection. So, to determine Jprox* in the neighborhood of the operating point, we made use of the fact that each nephron operates somewhere between the shoulder and elbow of its own TGF curve and manipulated TGF activity to obtain values for SNGFR and Jprox that bracket the operating point. Linear regression for Jprox as a function of SNGFR for the pooled data (Jprox = a + b*SNGFR) gave nearly identical values of the GTBslope, b, for control and exenatide (0.43 ± 0.05 vs. 0.39 ± 0.04, P = 0.6). In other words, the GTBslope was not appreciably altered by exenatide. ANCOVA for the effect of exenatide on Jprox assumes that the treatment does not affect the regression slope, so the finding also validates ANCOVA as an appropriate test for the direct effect of exenatide on Jprox. The analysis revealed exenatide to be a potent primary inhibitor of proximal reabsorption (Jprox* =15.6 ± 0.8 vs. 25.5 ± 0.9 nl/min, P < 0.0005). Data from protocol 2 provided a less rigorous test of Jprox because all proximal collections were at the shoulder of the TGF curve. Using ANCOVA to adjust for effects of SNGFRp, exenatide was also shown to be a proximal diuretic in these experiments (P = 0.03).
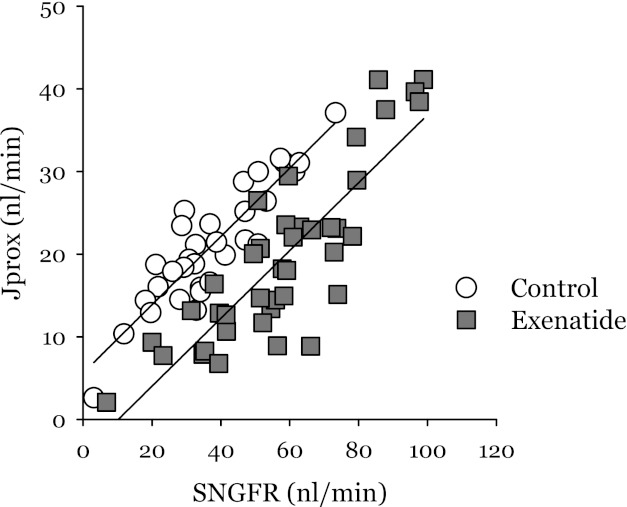
Fig. 1.
Proximal reabsorption (Jprox) as a function of single-nephron glomerular filtration rate (SNGFR) for data obtained according to protocol 1. Each point is an individual micropuncture collection. Each nephron was sampled at the shoulder and elbow of its tubuloglomerular feedback (TGF) curve. Separate regression lines are drawn for control and exenatide and are shown to have similar slopes. The primary effect of exenatide on Jprox is represented by a 10-nl/min downward displacement of the regression line, which implies that exenatide is a potent proximal diuretic (P < 0.0005 by ANCOVA).
An alternate method for looking at the efficiency of GTB is to examine the impact on TF/P inulin in a given nephron when SNGFR is manipulated through the TGF system (see Fig. 2). GTB efficiency for a tubular segment is obtained by imposing a change in flow and measuring the resultant change in reabsorption. This is quantified with the GTB efficiency index
where J is reabsorption and Q is the volume flow rate. Perfect GTB corresponds to a value of unity for this index. Applying this to the proximal tubule, there is perfect GTB efficiency when late proximal TF/P inulin is insensitive to changes in SNGFR. Applying this to the present data, TF/P inulin appears to be less sensitive to SNGFR in nephrons during exenatide, which gives the impression that exenatide improves GTB efficiency in the proximal tubule. However, it can be shown mathematically that if reabsorption is proportional to the rate of random encounters between tubular fluid elements and a fixed amount transport machinery, then GTB efficiency will appear to improve with increased SNGFR. Hence, the effect of exenatide on SNGFR likely creates an illusion that exenatide improves GTB. The data are shown in Fig. 2, a model for predicting the general appearance of the data from probability theory is shown in Fig. 3, and derivation of the model is shown in the appendix.
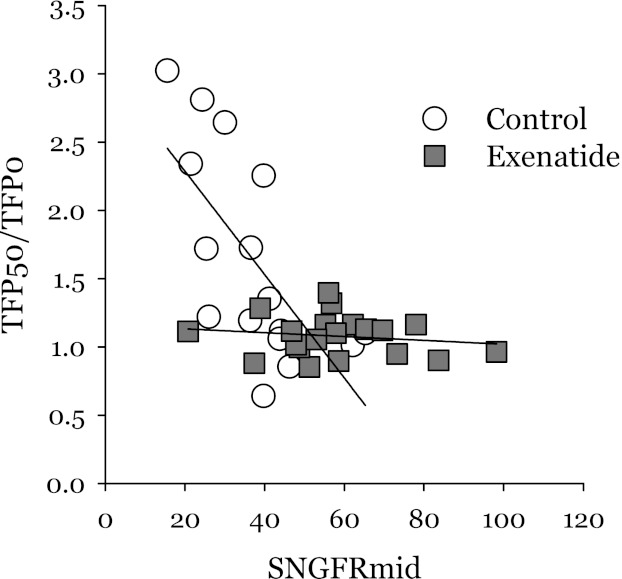
Fig. 2.
Effect of TGF activation on proximal reabsorption. Data were obtained by protocol 1. TFP50/TFP0 is the fold-change in late proximal TF/P inulin when SNGFR is reduced by activating TGF. SNGFRmid is the SNGFR midway between the elbow and shoulder of the TGF curve. A ratio of unity implies perfect glomerulotubular balance (GTB) where fractional reabsorption is independent of SNGFR. If reabsorption of a fluid element is a random event with constant probability, then late proximal TF/P inulin is expected to vary inversely with SNGFR because a fluid element has more opportunity to be reabsorbed when exposed to the epithelium for a longer period of time. This behavior is demonstrated nicely under control conditions, but not during exenatide. This gives the appearance of more efficient GTB during exenatide. But the apparent increase in GTB efficiency during exenatide could arise from the nonsaturable first-order nature of proximal reabsorption (see Fig. 3).
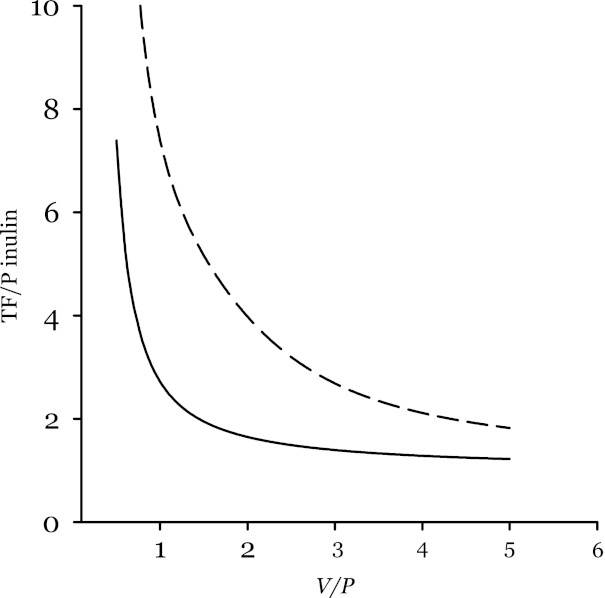
Fig. 3.
Model prediction for effect of tubular flow rate on apparent GTB efficiency. TF/P inulin at the downstream end of a tubule of unit length is shown as a function of the ratio V/P, where V represents axial flow velocity at the inlet to a segment and P is the probability per unit time that a fluid element present in the tubule will be reabsorbed. P is related to the amount of machinery available to perform reabsorption. V is related to SNGFR. If P is constant along the tubule and independent of V, then TF/P inulin decreases with increasing V, especially at low V. The solid line applies where V is constant along the tubule and the diameter declines. The dashed line applies to the case where V declines along the tubule to remain in constant proportion to the amount of remaining fluid. Perfect GTB efficiency occurs where TF/P inulin is independent of V. Lowering V reduces the apparent efficiency of GTB. This model predicts the behavior shown in Fig. 2.
Loop of Henle Reabsorption
Tubular reabsorption up to the early distal nephron was determined from early distal collections in the second set of experiments (Table 3). Exenatide increased the fractional delivery of fluid to the early distal nephron by 50% (P < 0.0005). Using ANCOVA to adjust for SNGFRd, exenatide was shown to cause a primary decrease in reabsorption by the combined proximal tubule plus loop of Henle (Jdist*) by 14% (P > 0.0005).
Reabsorption in Henle's loop can be estimated from the data in protocol 2 by subtracting PDD × GTBslope from Jprox to obtain a value for reabsorption up to the late proximal tubule and subtracting this value from Jdist. Assuming the proximal GTBslope of 0.4 (vide supra), this procedure gives pre- and postexenatide estimates for LOH reabsorption of 13.4 and 14.5 nl/min, respectively. Thus it appears that the diuretic effect of exenatide does not extend to Henle's loop.
Reabsorption in Distal Tubule and Collecting Duct
Applying the mean fractional fluid delivery obtained from the early distal micropuncture collections to the whole kidney GFR, one obtains a whole kidney early distal flow rate of 0.7 ml/min in control and 1.41 ml/min during exenatide. Subtracting the respective urine flow rates from these distal deliveries, the fraction of the delivered load reabsorbed by the distal tubules and collecting ducts must be less during exenatide (94.3 vs. 98.7%) while the corresponding net reabsorption must be greater during exenatide (1.33 vs. 0.71 ml/min). Thus, in transition from control to exenatide, fractional reabsorption and net reabsorption change in opposite directions, which is a qualitative attribute of load-dependent transport. The problem of detecting other effects on tubular reabsorption in this situation is to determine whether the slope of the relationship between reabsorption and incident flow is quantitatively compatible with GTB acting alone. Using the same definition of GTB efficiency described above in Proximal Tubular Reabsorption, we previously surmised that a GTB efficiency index of 0.95 for the distal nephron is required to explain the effect of dietary salt on lithium clearance in humans (26). Calculations based on published effects of low-dose (0.2 or 0.5 mg/kg iv) furosemide on sodium and lithium excretions (22) yields values of 0.97 and 0.92 for the efficiency of distal GTB in the anesthetized rat. Meanwhile, the effect on distal reabsorption that we impute for exenatide is compatible with pure GTB, assuming an efficiency index of 0.93. Hence, our findings can be explained without invoking a direct effect of exenatide on reabsorption downstream of the early distal tubule.
DISCUSSION
There is a fairly long list of secretagogs for GLP-1 and another list of its downstream effects vis a vis energy nutrient metabolism (1–3). Because it includes several variables, the network of cause-effect relationships among these factors is seemingly complex. However, the ensemble behavior is that of a simple negative-feedback system for the homeostasis of energy nutrients and the known pairwise relationships within the network are mostly predictable when this is taken into account.
The present data demonstrate potent effects of GLP-1R activation on renal hemodynamics and proximal reabsorption, which have no other direct connection to energy nutrient metabolism. In fact, exenatide proved to be as potent a renal vasodilator and as potent a proximal diuretic as any we have encountered in the hydropenic rat. The magnitude of these effects, per se, is remarkable, but it is also interesting to note that the incretins exert ballistic, rather than negative-feedback control over salt excretion (4). This latter feature makes the incretin system unique among the hormones known to exert strong effects on glomerular and tubular function.
Why the body, in its wisdom, should assign control over GFR and proximal reabsorption to GLP-1R is not obvious. A closed loop of cause-effect relationships generally exists between any hormone and each process affected by it. For renal GLP-1 to meet this standard, its activity must be governed by some constituent of the milieu intérieur, whose abundance is influenced, in turn, by glomerular filtration and proximal reabsorption. There are major and minor GLP-1 secretagogs, but the principal mechanism for GLP-1 secretion is direct contact of lipid or carbohydrate nutrients with enteroendocrine L cells of the intestinal mucosa (6, 20, 32). It is difficult to envision a role in energy nutrient, or fluid volume, homeostasis for an endocrine system that responds to dietary fat and carbohydrate by reducing the extracellular fluid volume, which is the most obvious consequence of simultaneously increasing GFR and reducing proximal reabsorption.
An alternative to consider is that the incretin system responds to macronutrients, overall, and that GLP-1 acts on the kidney as a premonitory control system to prevent urea from accumulating after a protein meal. There are at least two problems with this concept. First, although enteroendocrine L cells will secrete GLP-1 in response to peptone (9), the GLP-1 responses to intact protein or protein digests are too weak to detect in vivo (35). Second, suppressing proximal reabsorption is not an efficient means for augmenting urea clearance, which is mainly regulated in the collecting duct (16, 18).
Exenatide (synonymous with exendin-4) is a potent GLP-1 mimetic that is resistant to degradation by DPP-4 and has a long plasma half-life in humans or rodents. This 39-amino acid peptide was originally identified in saliva of the Gila monster (Heloderma suspectum) (11). A synthetic version (BYETTA) is FDA approved for treatment of type 2 diabetes. The exenatide dose for the current study was based on a detailed pharmocokinetic analysis of exenatide in the rat (23) and designed to achieve a plasma concentration of ∼2 nM for the agonist, which proved to be potent in the kidney. The plasma concentration of exenatide targeted for these studies is within the range of steady-state values achieved in long-term studies on the metabolic effects of exenatide in ZDF rats. Those metabolic effects of exenatide extended over 3 log orders, with EC50 and EC90 for lowering HbA1C being 20 pM and 1 nM, respectively (11).
The present studies address what is possible to achieve by pharmacologically activating GLP-1R but do not address the underlying tone exerted by renal GLP-1R in normal physiology. For this, acute blockade of GLP-1R would be required. A peptide antagonist of GLP-1R, exendin 9–39, is commercially available, but requires 100 nM concentration to effectively displace GLP-1, which makes it impractical for systemic administration in vivo (10). It is not possible to know the concentration of GLP-1 in the proximal tubule, since GLP-1 is subject to local degradation by DPP-4. The postprandial GLP-1 concentration in rat venous plasma is 15 pM (6). This would be the upper limit on its normal concentration in proximal tubular fluid. The concentration of exenatide we achieved in tubular fluid was certainly higher than this.
The combined effects of exenatide on SNGFR and proximal reabsorption require at least two sites of action, one in the preglomerular vasculature and another in the proximal tubule. We have deduced the requirement for exenatide to be a proximal diuretic by applying ANCOVA to control for the effects of SNGFR on proximal reabsorption. By comparing the effect of exenatide on segmental reabsorption downstream of the late proximal tubule to what is expected for normal GTB, we also conclude that the net effect of exenatide on urinary flow rate can be explained by its effects on SNGFR and Jprox and that changes in reabsorption beyond the late proximal tubule can all be accounted for by the normal operation of flow-dependent transport working in those segments. SNGFRd increased with exenatide despite a primary decline in Jprox, which would otherwise reduce SNGFRd by activating TGF. This implies a primary increase in SNGFR, as opposed to an increase mediated through the tubule. Furthermore, exenatide increased SNGFR at both extremes of TGF activation, without interfering with the TGF response, per se. The present data are sufficient to prove vasodilation upstream of the glomerulus since the constraint of filtration equilibrium makes it physically impossible for the changes in SNGFR shown in Table 2 to occur without a decline in preglomerular resistance. The current data don't permit us to draw conclusions about postglomerular resistance although a published account of PAH and creatinine clearance suggests that exenatide does not affect the filtration fraction (5), in which case its effect on GFR could be explained by parallel decreases in pre- and postglomerular resistance with no change in glomerular capillary pressure.
Exenatide increased GFR and suppresses proximal reabsorption by independent mechanisms. Yet administering exenatide caused SNGFRd to occupy the same position along a new TGF curve as it occupied along the original curve (See Fig. 4). This is unlikely to occur without some coordination between the tubular and vascular effects of exenatide. This coordination could be mediated by temporal adaptation of the TGF curve to an initial increase in distal delivery. The tendency for TGF to reset to accommodate increased distal flow is a general property of the TGF system and not specific to GLP-1R signaling (28, 30).
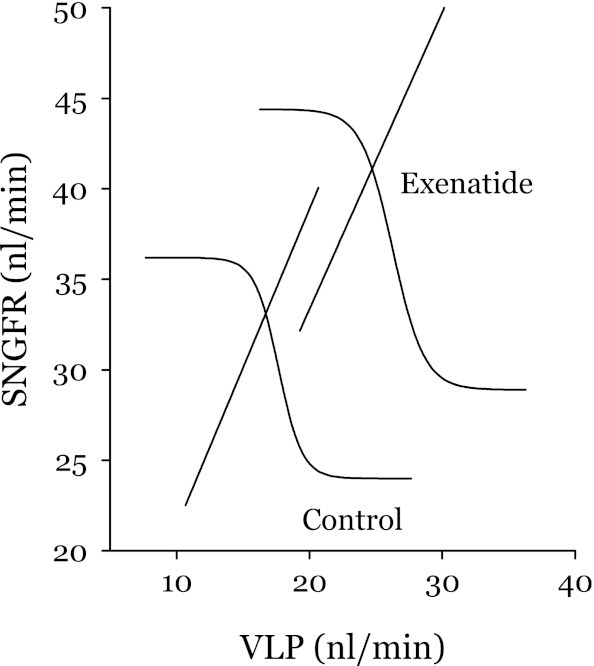
Fig. 4.
Simultaneous effects of exenatide on TGF (hyperbolic tangents) and GTB (straight lines). TGF and GTB curves intersect at the operating point. VLP, late proximal flow. Upper and lower limits of the TGF curves and SNGFR at the operating points were obtained by protocol 2. Slopes for the GTB curves were obtained by protocol 1. VLP values at the operating points were calculated from VLP at the TGF shoulder (protocol 2) and the GTBslope. Inflection point slopes for the TGF curve is the ratio of peak open loop gain (OLG) for the intact TGF-GTB system to the GTBslope (27). In the present case, we assumed a peak OLG of 2, which is typical of the hydropenic rat (27). Since exenatide did not reduce the range of the TGF response, it is reasonable to assume no major effect on peak OLG. Exenatide caused the TGF curve to shift upward (P = 0.0002) and the GTB curve to shift rightward (P = 0.0005) but did not appear to affect the range of the TGF response, the GTBslope, or the position of the operating point along the TGF curve.
We have demonstrated individual “primary” effects of systemic exenatide on glomerular and tubular function. We suppose that these effects are mediated by activating GLP-1R in the renal vasculature and proximal tubule, but have not verified this to be the case. It is possible, however, unlikely, for the primary vascular and/or tubular effects of intravenous exenatide to be mediated by some intermediary released by activating GLP-1R in a remote location. Proving that tubular GLP-1R mediates the effect on Jprox could be done by perfusing the tubule with exenatide. Proving that the primary vascular effect is owed to local GLP-1R would be more difficult.
To summarize, we have demonstrated that the GLP-1R agonist exenatide is a potent renal vasodilator and inhibitor of proximal reabsorption in the hydropenic rat. Meanwhile, we find no evidence for direct effects of exenatide on the range of the TGF response, the efficiency of proximal GTB, or transport downstream of the late proximal tubule. Whether, and by what mechanism, the tonic influence of renal GLP-1 signaling is altered under various circumstances to influence GFR, total body salt, and blood pressure is a subject for future investigation.
GRANTS
This work was supported by NIH RO1 DK56248 and the Department of Veterans Affairs Research Service. P. Singh was supported by K08-DK084305. A. Kashkouli was supported by training grant T32-HL07261. Technical support was provided by Ser Khang.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.C.T. and P.S. provided conception and design of research; S.C.T. and A.K. performed experiments; S.C.T. and P.S. analyzed data; S.C.T., A.K., and P.S. interpreted results of experiments; S.C.T. prepared figures; S.C.T. drafted manuscript; S.C.T. and P.S. edited and revised manuscript; S.C.T. and P.S. approved final version of manuscript.
Appendix
Herein, we present the method for deriving curves in Fig. 3. Let N be the number of fluid elements transiting a short length of tubule per unit time. Let V be the flow velocity. Let P represent the probability of reabsorption per unit time for a fluid element in that short length of tubule. P is related to the frequency of chance encounters between fluid elements and available transporters. Then
where V is the fluid velocity. If P and V are constant along the tubule, then integrating along a tubule of unit length gives a fractional reabsorption
and
REFERENCES
- 1. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Brubaker PL. Update on incretin biology: focus on glucagon-like peptide-1. Endocrinology 151: 1984–1989, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Burcelin R. The incretins: a link between nutrients and well-being. Br J Nutr 93, Suppl 1: S147–S156, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Carpenter RH. Homeostasis: a plea for a unified approach. Adv Physiol Educ 28: 180–187, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, Girardi AC. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol 301: F355–F363, 2011 [DOI] [PubMed] [Google Scholar]
- 6. D'Alessio D, Lu W, Sun W, Zheng S, Yang Q, Seeley R, Woods SC, Tso P. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol 293: R2163–R2169, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Deacon CF. What do we know about the secretion and degradation of incretin hormones? Regul Pept 128: 117–124, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Drucker DJ. The biology of incretin hormones. Cell Metab 3: 153–165, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber JC. Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology 139: 3780–3786, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Fehmann HC, Jiang J, Schweinfurth J, Wheeler MG, Boyd AE, Göke G. Stable expression of the rat GLP-I receptor in CHO cells: activation and binding characteristics utilizing GLP-I(7–36)-amide, oxyntomodulin, exendin-4, and exendin(9–39). Peptides 15: 453–456, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Furman BL. The development of Byetta (exenatide) from the venom of the Gila monster as an anti-diabetic agent. Toxicon 59: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Gedulin BR, Smith P, Prickett KS, Tryon M, Barnhill S, Reynolds J, Nielsen LL, Parkes DG, Young AA. Dose-response for glycaemic and metabolic changes 28 days after single injection of long-acting release exenatide in diabetic fatty Zucker rats. Diabetologia 48: 1380–1385, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Girardi AC, Degray BC, Nagy T, Biemesderfer D, Aronson PS. Association of Na+-H+ exchanger isoform NHE3 and dipeptidyl peptidase IV in the renal proximal tubule. J Biol Chem 276: 46671–46677, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 89: 3055–3061, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Hirata K, Kume S, Araki S, Sakaguchi M, Chin-Kanasaki M, Isshiki K, Sugimoto T, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Uzu T. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun 380: 44–49, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Klein JD, Blount MA, Sands JM. Urea transport in the kidney. Compr Physiol 1: 699–729, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 48: 736–743, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Lassiter WE, Gottschalk CW, Mylle M. Micropuncture study of net transtubular movement of water and urea in nondiuretic mammalian kidney. Am J Physiol 200: 1139–1146, 1961 [DOI] [PubMed] [Google Scholar]
- 19. Laugero KD, Stonehouse AH, Guss S, Landry J, Vu C, Parkes DG. Exenatide improves hypertension in a rat model of the metabolic syndrome. Metab Syndr Relat Disord 7: 327–334, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Lim GE, Brubaker PL. Glucagon-like peptide 1 secretion by the L-cell. The view from within. Diabetes 55, Suppl 2: S70–S77, 2006 [Google Scholar]
- 21. Moreno C, Mistry M, Roman RJ. Renal effects of glucagon-like peptide in rats. Eur J Pharmacol 434: 163–167, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Nowicki S, Opezzo JA, Levin G, Gonzalez D, Elias MM. Furosemide renal excretion rate and the effects of the diuretic on different tubular sites are modified by endogenous dopamine in normohydrated rats. J Pharmacol Exp Ther 274: 1348–1354, 1995 [PubMed] [Google Scholar]
- 23. Parkes D, Jodka C, Smith P, Nayak S, Rinehart L, Gingerich R, Chen K, Young A. Pharmacokinetic actions of exendin-4 in the rat: comparison with glucagon-like peptide-1. Drug Dev Res 53: 260–267, 2001 [Google Scholar]
- 24. Schlatter P, Beglinger C, Drewe J, Gutmann H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul Pept 141: 120–128, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Singh P, Deng A, Blantz RC, Thomson SC. Unexpected effect of angiotensin AT1 receptor blockade on tubuloglomerular feedback in early subtotal nephrectomy. Am J Physiol Renal Physiol 296: F1158–F1165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomson SC, Blantz RC. Glomerulotubular balance, tubuloglomerular feedback, and salt homeostasis. J Am Soc Nephrol 19: 2272–2275, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Thomson SC, Blantz RC. Homeostatic efficiency of tubuloglomerular feedback in hydropenia, euvolemia, and acute volume expansion. Am J Physiol Renal Fluid Electrolyte Physiol 264: F930–F936, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Thomson SC, Blantz RC, Vallon V. Increased tubular flow induces resetting of tubuloglomerular feedback in euvolemic rats. Am J Physiol Renal Fluid Electrolyte Physiol 270: F461–F468, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Thomson Deng A SC, Bao D, Satriano J, Blantz RC, Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest 107: 217–224, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomson SC, Vallon V, Blantz RC. Reduced proximal reabsorption resets tubuloglomerular feedback in euvolemic rats. Am J Physiol Renal Physiol 273: F1414–F1420, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 302: R75–R83, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol 587: 27–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Viereck C, Boudes P. An analysis of the impact of FDA's guidelines for addressing cardiovascular risk of drugs for type 2 diabetes on clinical development. Contemp Clin Trials 32: 324–332, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Yang J, Campitelli J, Hu G, Lin Y, Luo J, Xue C. Increase in DPP-IV in the intestine, liver and kidney of the rat treated with high fat diet and streptozotocin. Life Sci 81: 272–279, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Yoder SM, Yang Q, Kindel TL, Tso P. Differential responses of the incretin hormones GIP and GLP-1 to increasing doses of dietary carbohydrate but not dietary protein in lean rats. Am J Physiol Gastrointest Liver Physiol 299: G476–G485, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens 21: 1125–1135, 2003 [DOI] [PubMed] [Google Scholar]