Abstract
Recent studies demonstrate that mechanisms underlying gut barrier failure include systemic processes and less studied luminal processes. We thus tested the hypothesis that mucus layer oxidation is a component of trauma/hemorrhagic shock-induced gut injury and dysfunction. Male Sprague-Dawley rats underwent trauma/hemorrhagic shock. Controls underwent trauma only. Mucus from the terminal 30 cm of the ileum was collected, processed, and analyzed for reactive nitrogen intermediates (RNI)-mediated damage, reactive oxygen species (ROS)-induced damage, and total antioxidant capacity. The distal ileum was stained to quantify the mucus layer; gut permeability was assessed physiologically. A time course study was conducted to determine the temporal sequence of mucus layer damage. The role of free radical-mediated damage to the gut barrier was investigated by the effect of the free radical scavenger dimethyl sulfoxide on trauma/hemorrhagic shock-induced changes on the mucus and on gut permeability. Trauma/hemorrhagic shock increased intestinal permeability, which was associated with evidence of loss of the unstirred mucus layer. These changes correlated with increased ROS- and RNI-mediated mucus damage and loss of mucus total antioxidant capacity. Based on the time course study, ROS-mediated mucus damage and loss of total antioxidant capacity were present immediately following shock, whereas RNI-mediated damage was delayed for 3 h. Dimethyl sulfoxide ameliorated gut barrier loss, ROS-mediated changes to the mucus layer, and loss of total antioxidant capacity. There was no change in RNI-induced changes to the mucus layer. These results support the hypothesis that trauma/hemorrhagic shock leads to mucus damage and gut dysfunction through the generation of free radical species.
Keywords: intestinal mucus layer, gut-mediated sepsis
in certain clinical scenarios, including major trauma and hemorrhage, gut injury and the subsequent loss of the gut barrier function have been implicated in the development of the acute respiratory distress syndrome, systemic inflammatory response syndrome, and multiple organ dysfunction syndrome (8). Thus prevention or amelioration of gut injury in conditions associated with intestinal ischemia would be a key therapeutic strategy. Several such strategies, including early enteral nutrition, have been identified and instituted into clinical practice (1, 20). However, the development of successful new clinical therapies is based on a complete knowledge of the mechanisms of the injuries they are being developed to treat. Consequently, a considerable amount of investigative attention has been committed to elucidating the pathogenesis of stress- and trauma/shock-induced gut injury and gut barrier failure. These studies have largely focused on the splanchnic circulation and systemic host factors because it is well recognized that shock or major trauma leads to a decrease in mesenteric perfusion, resulting in an ischemia-reperfusion injury as well as an ischemia-reperfusion-induced gut inflammatory response (1). This work has led to the recognition of the important roles of inflammatory cells, proinflammatory molecules, intraluminal proteases, reactive oxygen species (ROS), and reactive nitrogen intermediates (RNI) in the pathogenesis of ischemia-reperfusion-related gut injury and dysfunction (1, 7, 8, 13, 15, 22). However, very little attention has been paid to the role of the unstirred mucus layer as a critical component of the gut barrier or the consequences of damage to this mucus layer. However, in other areas of the gastrointestinal track, mucus damage or loss has been implicated in the pathogenesis of peptic ulcer disease and celiac sprue as well as inflammatory bowel disease (11, 29). Furthermore, basic physiological transport studies of normal intestinal barrier function have documented that the unstirred mucus layer is more important in preventing the absorption of ingested molecules than the tight junctions between enterocytes (2, 20). Consequently, we began testing the hypothesis that loss of the mucus layer significantly contributes to loss of gut barrier function in conditions associated with splanchnic hypoperfusion and ischemia-reperfusion-mediated gut injuries, such as what occurs after trauma-hemorrhagic shock (T/HS) or occlusion of the superior mesenteric artery. These studies documented that the loss of the mucus layer was associated with increased gut permeability and that the anatomic sites of mucus disruption directly correlated with areas of morphological gut injury (24, 26, 19). Thus this work supports the concept that loss of the protective effects of the unstirred mucus gel layer is a critical factor in the pathogenesis of gut injury. To better understand the interactions between T/HS-induced intestinal ischemia-reperfusion injury and loss of the mucus layer, in this study, we tested the hypothesis that trauma hemorrhagic shock is associated with an oxidant-mediated damage to the mucus gel, which in turn results in the mucus gel being extruded into the gut lumen, thereby contributing to loss of gut barrier function.
MATERIALS AND METHODS
Animals.
Male Sprague Dawley rats weighing 320–400 g were housed under barrier-sustained conditions, at a temperature of 25°C, with 12-h:12-h light/dark cycles and acclimated for at least 5 days before experimentation. The rats had free access to water and chow (Teklan 22/5 Rodent Diet W-8640; Harlan Teklad, Madison, WI). All rats were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals. All animal protocols were approved by the New Jersey Medical School Animal Care Committee.
Experimental design.
The first goal of this study was to test whether T/HS damaged the unstirred mucus layer and, if so, to characterize changes in mucus structure and function following injury. Because it is known that the large sugar moieties within the mucin molecule act as physiological free radical scavengers (14) and that reactive oxygen and nitrogen species can damage and degrade mucin molecules leading to a loss of mucus viscosity, hydrophobicity, and barrier function (14, 3), we measured the extent of oxidant stress to the mucus layer as well as its antioxidant capacity. Mucus oxidant stress was assayed by measuring carbonyl derivates and nitrated tyrosine residues in the mucus because these are markers of ROS and reactive nitrogen reactions (30, 25). Mucus function was evaluated by measuring the antioxidant activity of the mucus. In a second group of animals, in which the intestines were not manipulated to obtain specimens for mucus assays, evidence of loss of gut barrier function was assessed, and the percentage of the intestinal villi that had lost their mucus layer was quantified. Morphological injury was assessed by histological analysis, whereas intestinal permeability studies were performed to evaluate intestinal barrier function. In all groups of animals, these studies were carried out at 3 h after the end of the T/HS or trauma sham shock (T/SS) period because gut injury is well established at this point (26).
Subsequently, a second set of studies were performed to determine the time course of the changes in mucus oxidant-mediated damage and loss of antioxidant capacity. In this experiment, rats were killed and samples collected at the end of the 90-min shock period immediately after volume resuscitation (0 h) as well as at 1 h, 2 h, or 3 h after the end of the shock and volume resuscitation period.The second goal of this experiment was to investigate whether T/HS-induced ROS and RNI free radicals specifically mediated structural and functional changes to the unstirred mucus layer as well as loss of gut barrier function. To test this hypothesis, in a third set of animals, the free radical scavenger dimethyl sulfoxide (DMSO) was intravenously injected before both hemorrhagic shock and reperfusion. In the first group of animals, gut barrier function was assessed. In a second, separate, group of animals, mucus oxidant stress and function were measured.
T/HS model.
Rats were subjected to either T/HS or T/SS. Both groups were weighed, anesthetized with intraperitoneal pentobarbital sodium (dose, 50 mg/kg), and placed on a heating pad to maintain euthermia. With the use of aseptic techniques, the internal jugular vein and femoral artery were isolated and cannulated with 50-gauge silicone catheter tubing or polyethylene (PE-50) tubing containing 0.1 ml heparinized saline solution (concentration, 10 U/ml), respectively. Both catheters (internal jugular vein and femoral artery) remained in situ for the duration of the experiment. Next, a 3-cm midline laparotomy (trauma) was performed, followed by evisceration of the small and large bowel for 15 min. The small and large intestines were then returned to the abdominal cavity, and the abdomen was closed with a running 3–0 silk suture. In the rats subjected to T/HS, after the closure of the abdomen, the femoral artery catheter was attached in-line to a blood pressure monitor (BP-2 digital blood pressure monitor; Columbus Instruments; Columbus, OH) for continuous blood pressure monitoring. Blood was then withdrawn from the internal jugular vein at a rate of 1 ml/min until the mean arterial pressure reached 30 to 35 mmHg. The mean arterial pressure was maintained at 30 to 35 mmHg for 90 min by withdrawing or reinfusing the shed blood. At the end of the shock period, the animals were resuscitated by reinfusion of all the shed blood at a rate of 1 ml/min as previously described (26). The mean arterial pressure returned to the normal level within a few minutes after the animals were resuscitated and remained at that level for the duration of the experiment. In the T/SS group, animals underwent cannulation of vessels and laparotomy but were not subjected to T/HS.
DMSO (Sigma-Aldrich, St. Louis, MO) was administered to two groups of T/SS and T/HS rats. One group of T/SS and T/HS rats were assayed for gut permeability, whereas the second underwent mucus collection. DMSO (20 mg/kg) was diluted in 1 cc of sterile normal saline. Half of the volume was slowly injected via the internal jugular vein 10 min before the induction of hemorrhagic shock or sham-shock. The remainder was administered 90 min later in the sham-shock group or immediately before resuscitation in the hemorrhagic shock group as previously described (9).
Collection of mucus sample.
The distal 30-cm segment of the terminal ileum was identified and transected from the intestinal tract and the underlying mesentery. The external portion of the segment was washed with PBS to remove any debris. The lumen of this segment was then flushed with PBS to remove fecal matter. The intestinal mucus was collected by gently compressing the exterior of the segment as described by Vesterlund et al. (32). Pilot studies were done to develop a specific method of mucus collection that does not cause villous injury. In these studies, the unstirred mucus layer was removed, and the intestinal segment was subsequently stained to evaluate for villous injury. There was no evidence of damage to the intestinal villi (data not presented). The mucus samples were then homogenized in 500 μl of PBS over ice and then centrifuged (Denville 260D; Denville Scientific, Metuchen, NJ) for 75 min at 14.0 rcf. The supernatant was removed and stored at −80°C until further use.
Measurement of the protein concentration of mucus samples.
The concentration of protein in the mucus samples were measured by the Thermo Scientific Pierce BCA (bicinchoninic acid) Assay (Thermo Fisher Scientific, Rockford, IL) according the manufacturer's instructions. Protein concentrations are expressed as micrograms per milliliter.
Measurement of nitrated tyrosine residues.
Nitration of tyrosine residues, a marker for RNI-mediated injury, was measured with a competitive ELISA (nitrotyrosine ELISA; Millipore, Billerica, MA and 3-nitrotyrosine ELISA; Abcam, Cambridge, MA). Mucus samples were analyzed based on equal protein loading (10 μg) according to the manufacturer's instructions. The extent of RNI-mediated damage was expressed by comparing sample values to the provided nitrated BSA standards. Nitrated tyrosine residues are expressed as nitro-BSA equivalents (18).
Measurement of carbonyl derivatives.
Carbonyl derivatives, a marker of ROS-mediated damage, were quantified by ELISA (OxiSelect Protein Carbonyl ELISA; Cell Biolabs, San Diego, CA). Mucus samples were analyzed based on equal protein loading (10 μg/ml) of the samples according to the manufacturer's instructions. ROS-mediated damage was expressed as protein carbonyl nmol/mg.
Measurement of total antioxidant capacity.
One measure of mucus function is its ability to function as an antioxidant (6). Thus mucus sample antioxidant capacity was measured via ELISA (Total Antioxidant Capacity Assay; Abcam) as recommended by the manufacturer. Mucus samples were analyzed based on equal protein loading (10 μg/ml). Total antioxidant capacity is expressed as trolox equivalents (per nmol). Trolox equivalents refer to the antioxidant capacity of 1 nmol of trolox, which is 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid. Trolox equivalents are used as a standard measurement for antioxidant capacity (23).
Mucus coverage.
After death, a 5 cm segment of the terminal ileum was excised, opened along the mesenteric border, and pinned on cardboard with the mucosal side up. To better visualize the mucus layer, the mucosal surface was then sprayed with 3% Alcian blue followed by fixation in Carnoy's solution (19). After the processing, semithin (thickness, 2–4 μm) sections were cut and stained with hematoxylin and eosin. The total lengths of villi in 4 random ×40 visual fields were measured in a blinded fashion, and the lengths of these segments covered with mucus were also measured. Ileal mucus coverage is expressed as a percentage of the villi that were covered with mucus.
In vivo gut barrier permeability.
Ileal permeability was measured in vivo using the 4-kDa dextran permeability probe FD-4 (Sigma) as follows: after the 3-h reperfusion period was complete, a repeat laparotomy was performed through the previous incision. A loop of ileum was ligated 5 cm from the ileocecal valve, following which 10 cm of ileum was measured in a retrograde manner. At this point, the ileum was also ligated. Just distal to the proximal suture, an enterotomy was performed, and the intestinal loop was flushed with 10 ml of isotonic sodium chloride solution, after which the enterotomy incision was closed. One milliliter of FD-4 (concentration, 25 mg/ml in 0.1 M PBS solution) was injected in a retrograde fashion into the lumen of the isolated bowel segment. After 30 min, a systemic blood sample was collected. The blood sample was then centrifuged at an acceleration of 3,000 g at a temperature of 4°C for 10 min (Denville 260D; Denville Scientific). The blood sample, along with FD-4 standards, was analyzed in a BioTek Instruments FLx800 microplate fluorescence reader through an excitation filter of 485/20 and an emission filter of 528/20. Gut permeability was expressed as the amount of FD-4 found in systemic plasma in micrograms per milliliter (26).
Statistical analysis.
The results are expressed as means ± SE. Statistical analysis was conducted using Prism-4 software (GraphPad, San Diego, CA). Unpaired Student's t-tests and ANOVA were used for single and group comparisons, respectively. For data sets compared using ANOVA, the post hoc Newman-Keuls method was used to determine significance between groups. Statistical significance was considered achieved at P < 0.05.
RESULTS
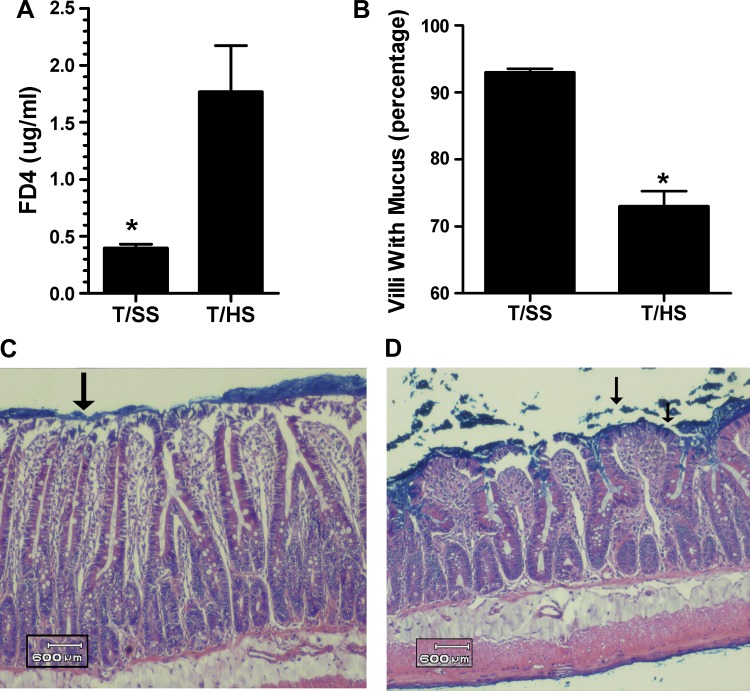
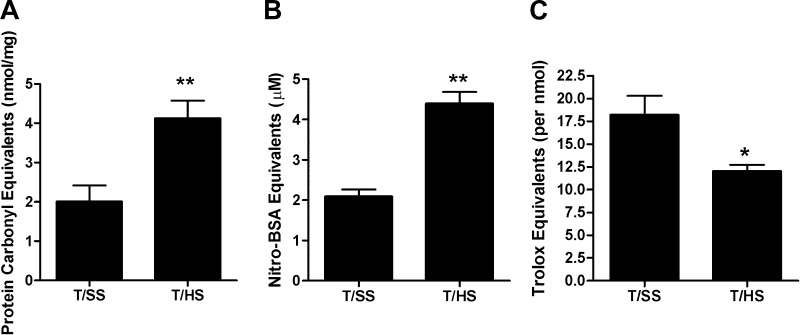
As previously reported (19, 26), T/HS-induced gut injury and loss of gut barrier function was observed at 3 h after volume resuscitation from shock as reflected by an approximate 4.5-fold increase in gut permeability (Fig. 1A). These T/HS-induced changes in gut morphology and function were associated with a significant loss of the mucus layer (Fig. 1, B–D). We next assessed whether the T/HS insult resulted in oxidant-mediated damage of the mucus layer. The observation that the protein carbonyl levels were twofold higher in the mucus of the rats subjected to T/HS than the T/SS controls (Fig. 2A) indicated that the mucus layer had been chemically modified by exposure to ROS. Likewise, the twofold increase in nitrated tyrosine residues in the T/HS compared with the T/SS mucus samples (Fig. 2B) indicated that the mucus had been exposed to RNI. To determine whether these oxidant-mediated structural changes to the mucus layer had a functional correlate, we measured the antioxidant neutralizing capacity of the mucus samples and found that it was significantly decreased in the T/HS compared with the T/SS rats (Fig. 2C).
Fig. 1.
A: trauma-hemorrhagic shock (T/HS) causes loss of gut barrier function as reflected by increased permeability to FD-4. B: T/HS-induced gut barrier failure is also reflected as a decrease in the percentage of villi covered with mucus. [Data are expressed as means ± SE with 6 animals per group. *P < 0.01 vs. trauma sham shock (T/SS)]. Representative photomicrographs of intestinal segments following T/SS (C) and T/HS (D). The T/SS photomicrograph demonstrates an intact unstirred mucus layer (arrow), whereas the T/HS photomicrograph demonstrates disruption of the mucus layer (arrows). A 600-μm reference point is added for scale.
Fig. 2.
A: carbonyl equivalents, a degenerative protein modification caused by reactive oxygen species (ROS), were increased following T/HS. B: damage mediated by reactive nitrogen intermediates (RNI), expressed as a nitrated bovine serum albumin standard, was also increased following T/HS. C: ability of the collected mucus to neutralize oxidants, reflected as total antioxidant capacity, was decreased following T/HS. This is a functional marker of mucus damage. (Data are expressed as means ± SE with 6 animals per group. *P < 0.01 vs. T/SS, **P < 0.05 vs. T/SS.)
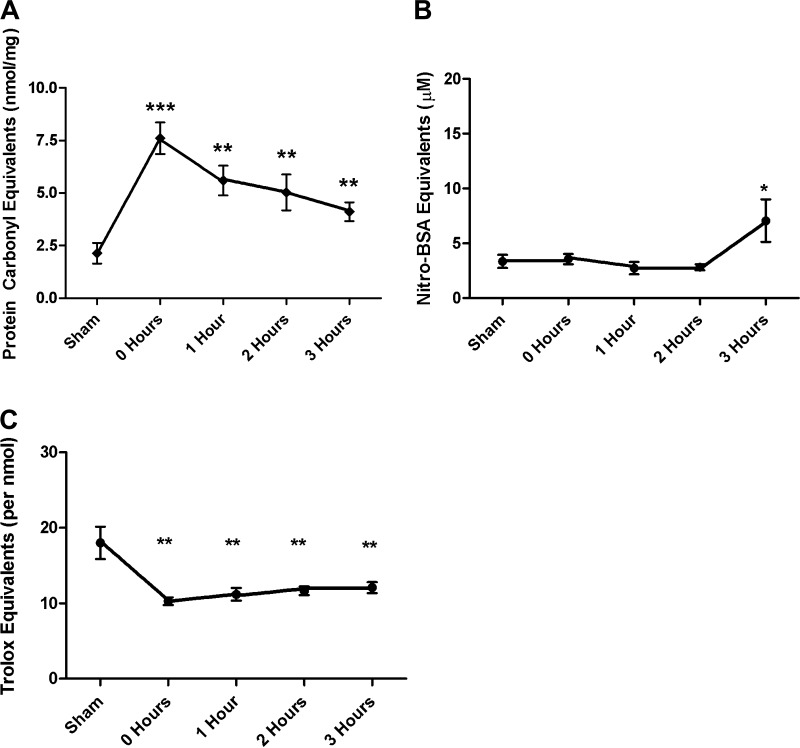
Because the studies carried out at 3 h after the end of shock period indicated that the mucus layer had been damaged by ROS and RNI, we next carried out a time course study to investigate the timing of these T/HS-induced changes in mucus structure and function. Evidence of ROS-mediated damage to the mucus layer was apparent and maximal at the end of the shock period with carbonyl protein levels decreasing during the remaining 3-h post-shock period (Fig. 3A). The temporal appearance of RNI-mediated damage of the mucus occurred at a later time point and appeared to be present only at 3 h after the end of the shock period (Fig. 3B). Consistent with the early increase in ROS-induced mucus damage, the antioxidant-neutralizing capacity of the mucus was reduced at the end of the shock period and remained at this reduced level during the rest of the study period (Fig. 3C).
Fig. 3.
A: time course data demonstrate that ROS-mediated injury occurs immediately following shock and remains elevated throughout the resuscitation period. B: RNI-mediated damage was present only at the end of the resuscitation period. C: total antioxidant capacity was lost immediately following shock and remained decreased throughout the entire resuscitation period. (Data are expressed as means ± SE with 6 animals per group. *P < 0.01 vs. T/SS, **P < 0.05 vs. T/SS, ***P < 0.05 vs. all other points.)
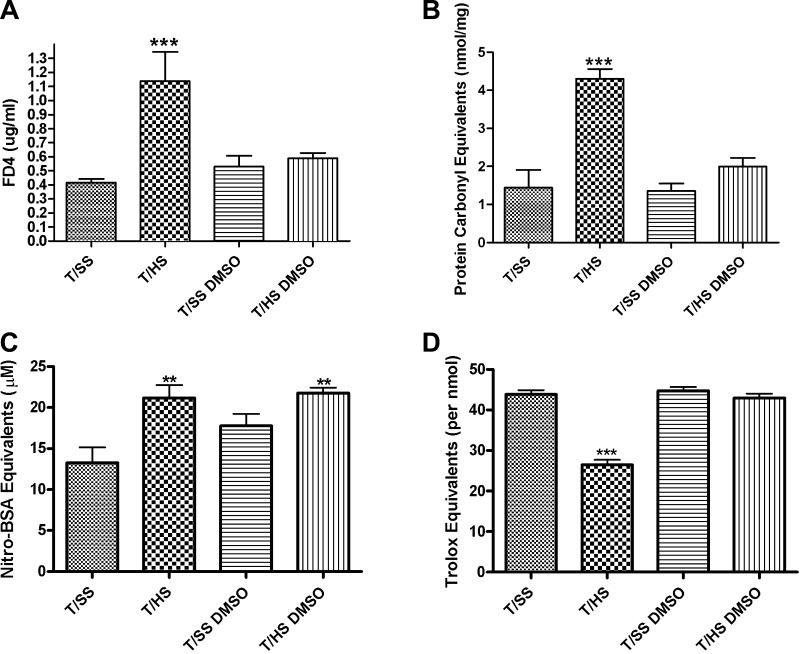
Treatment with the free radical scavenger DMSO abrogated the increase in gut barrier permeability seen following T/HS (Fig. 4A). The normalization of gut barrier permeability was associated with an absence of ROS-mediated changes to the mucus layer (Fig. 4B) as well as an increased mucus antioxidant capacity (Fig. 4D). However, there was no significant reduction in RNI-mediated mucus damage (Fig. 4C).
Fig. 4.
Addition of the free radical scavenger DMSO. A: DMSO abrogated the effects of T/HS-induced gut barrier failure. B: DMSO abrogated the effects of T/HS-induced ROS-mediated damage to the mucus layer. C: DMSO decreased some of the effects of T/HS-induced RNI-mediated damage to the mucus layer. D: DMSO abrogated the effects of T/HS-induced loss of mucus function by preserving the total antioxidant capacity. (Data are expressed as means ± SE with 6 animals per group. ***P < 0.001 vs. all groups, **P < 0.01 vs. T/SS.)
DISCUSSION
The physiological importance of the mucus layer as a crucial component of the intestinal barrier has been well documented in pharmacological transport and absorption studies of the gastrointestinal tract (2, 20, 29). However, with the exception of studies investigating peptic and stress-induced gastric and duodenal ulcers and erosions (12), little attention has been focused on mucus components of the rest of the gut. However, one major role of the mucus layer is to limit the ability of bacteria, bacterial products, and other potentially tissue-injurious factors contained within the intestinal lumen from coming into direct contact with the underlying enterocytes. In fact, although the epithelium contains tight junctions that limit the passage of bacteria and other luminal factors across the mucosal barrier, it is a less effective barrier than the unstirred mucus layer (27, 31). Recently, we have reported that disruption of the small intestinal mucus layer is associated with T/HS-induced loss of gut barrier function (24, 26, 19) and that removal of the mucus layer from the normal small intestine results in gut injury and loss of barrier function (28). Because the mechanism by which T/HS leads to loss of the mucus layer is unknown and gut ischemia-reperfusion is associated with a severe oxidant stress state, the goal of this study was to test the hypothesis that T/HS leads to ischemia-reperfusion-mediated alterations in the intestinal mucus layer that involves increased oxidation and nitration. To our knowledge, the results of this study show for the first time that the intestinal mucus layer sustains oxidant-mediated damage and that the mucus layer as well as the intestinal mucosa itself is susceptible to an ischemia-reperfusion injury during shock. A second potentially important observation from this study is that T/HS leads to a decrease in the antioxidant-neutralizing capacity of the mucus. The antioxidant properties of mucus appear to be due to the large sugar moieties within the mucin molecule, which act as physiological free radical scavengers (14). This oxidant-scavenging capacity of mucus is important because oxidant-mediated damage to these mucin molecules leads to a loss of mucus viscosity, hydrophobicity, and barrier function (14, 3). In fact, this oxidant-scavenging capacity of intestinal mucus helps explain the paradox of why enterocytes tested in vitro are very sensitive to ROS-mediated damage, yet oxidant-generating systems put into the gut cause little to no enterocyte damage (3). Thus, during normal physiological conditions, intestinal mucus is a physiologically important antioxidant and free radical scavenger for oxidants produced within the gut lumen. However, under conditions of gut ischemia-reperfusion, the presence of increased levels of ROS and RNI could result in mucin molecules losing their viscoelastic properties, thereby compromising the barrier function of the mucus layer. Based on the time course results of this study, it appears that ROS are more important than RNI in the loss of the antioxidant-neutralizing capacity of the mucus after T/HS. This conclusion is based on two major observations. First, increased ROS damage to mucus and loss of mucus antioxidant capacity occur shortly after the end of the shock period, whereas evidence of RNI damage to mucus only occurs several hours later. Second, treatment with the ROS scavenger DMSO prevents T/HS-induced increased gut permeability and oxidation, but not nitration, of the mucus layer.
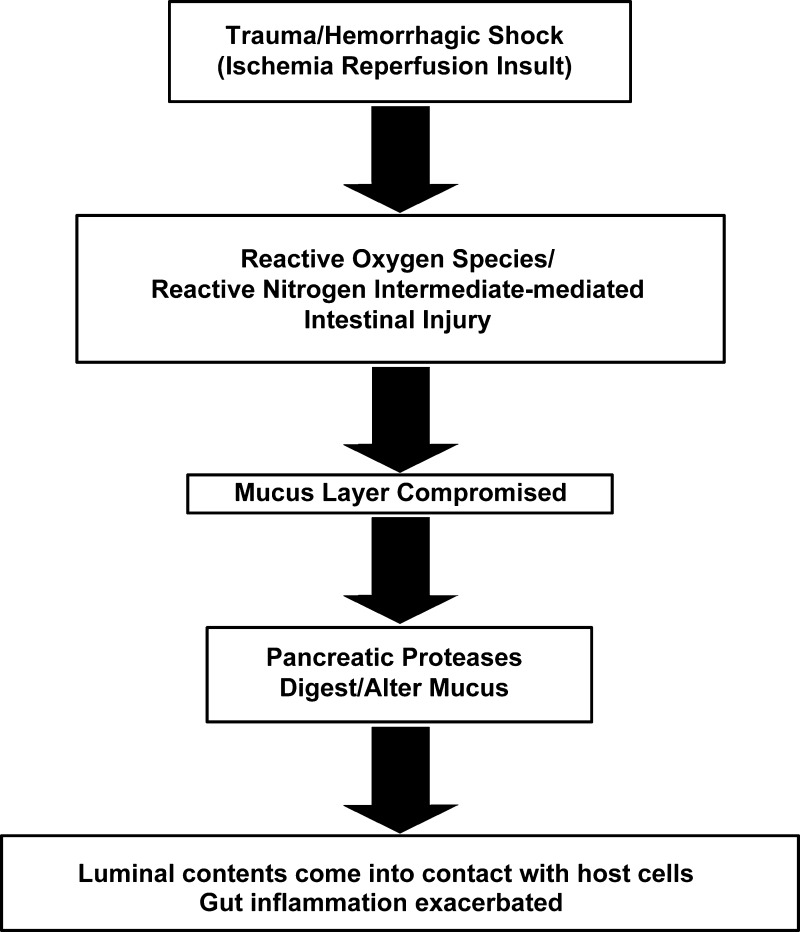
In assessing the potential importance of oxidant-mediated damage to the mucus in the pathogenesis of T/HS-induced gut injury and gut-derived systemic inflammation and organ injury, we believe it is also important to integrate these findings with recent studies documenting an important role of pancreatic proteases in ischemia-reperfusion-mediated gut injury (22, 27). Under normal physiological conditions, the mucus layer is resistant to pancreatic enzyme digestion and prevents these digestive proteases from coming into direct contact with the mucosa and thereby digesting the enterocytes and underlying tissue as they do ingested foodstuffs (10, 16). However, the ability of pancreatic proteases to cleave mucins is greatly augmented when the mucins are oxidatively modified (3, 14). Thus ischemia-reperfusion-mediated oxidative modification of the mucus may render the mucus layer susceptible to pancreatic digestive enzymes contained within the gut lumen. This notion is consistent with recent studies showing that pancreatic duct diversion of pancreatic enzymes from the intestinal lumen is associated with amelioration of T/HS-induced gut injury and preservation of both the intestinal mucus layer and gut barrier function (4). Thus, in the absence of pancreatic proteases in the gut lumen, T/HS-induced gut injury and dysfunction, as well as loss of the mucus layer, were largely abrogated. The relationship between loss of the mucus layer and pancreatic protease-induced gut injury is supported by our recent work carried out in the noninjured normal small intestine (28). In these studies, the presence of luminal pancreatic proteases only led to increased gut morphological injury and increased permeability when the mucus layer was impaired. Recent work from Schmid-Schonbein's group has also demonstrated that the intestinal mucus layer serves to limit digestive enzymes from reaching the gut epithelial layer in both normal animals and in animals subjected to a gut ischemia-reperfusion insult (5, 17). In conclusion, decreases in splanchnic blood flow resulting in a gut ischemia-reperfusion insult appear to be necessary components of gut injury and loss of barrier function after T/HS, as well as in other model systems associated with gut ischemia. However, we propose that factors on the luminal side of the gut modulate this process. Specifically, these luminal factors, especially the mucus layer and intraluminal pancreatic proteases, interact with each other as well as systemically generated mediators. This current hypothesis of integration between systemic and luminal factors in the pathogenesis of T/HS-induced gut injury and loss of gut barrier function is illustrated in Fig. 5.
Fig. 5.
Proposed integrated hypothesis illustrating interactions of systemic and luminal factors in the pathogenesis of T/HS-induced gut injury.
GRANTS
This work was supported by NIH grant R01GM05984 (E. Deitch) and NIH grant T32GM069330 (V. Alli and S. Sheth).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.E.F. and E.A.D. conception and design of research; J.E.F., G.L., V.A., S.U.S., and Q.L. performed experiments; J.E.F., G.L., V.A., S.U.S., Q.L., and E.A.D. analyzed data; J.E.F., V.A., S.U.S., and E.A.D. interpreted results of experiments; J.E.F. prepared figures; J.E.F. drafted manuscript; E.A.D. edited and revised manuscript; E.A.D. approved final version of manuscript.
REFERENCES
- 1. Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24 h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst Rev 4: CD004080, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Bounous G, McArdle AH, Hodges DM, Hampson LG, Gurd FN. Biosynthesis of intestinal mucin in shock: relationship to tryptic hemorrhagic enteritis and permeability to curare. Ann Surg 164: 13–22, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brownlee IA, Knight J, Dettmar PW, Pearson JP. Action of reactive oxygen species on colonic mucus secretions. Free Radic Biol Med 43: 800–808, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Caputo FJ, Rupani B, Watkins AC, Barlos D, Vega D, Senthil M, Deitch EA. Pancreatic duct ligation abrogates the trauma hemorrhage-induced gut barrier failure and the subsequent production of biologically active intestinal lymph. Shock 28: 441–446, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Chang M, Alsaigh T, Kistler EB, Schmid-Schönbein GW. Breakdown of mucin as barrier to digestive enzymes in the ischemic rat small intestine. PLoS One 7: e40087, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cross CE, Halliwell B, Allen A. Antioxidant protection: a function of tracheobronchial and gastrointestinal mucus. Lancet 8390: 1328–1330, 1984 [DOI] [PubMed] [Google Scholar]
- 7. Deitch EA, Shi HP, Lu Q, Feketeova E, Xu DZ. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock 19: 452–456, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg 216: 117–134, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deitch EA, Bridges W, Berg R, Specian RD, Granger DN. Hemorrhagic shock-induced bacterial translocation: the role of neutrophils and hydroxyl radicals. J Trauma 30: 942–951, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Forstner JF. Intestinal mucins in health and disease. Digestion 17: 234–263, 1978 [DOI] [PubMed] [Google Scholar]
- 11. Gersemann M, Stange EF, Wehkamp J. From intestinal stem cells to inflammatory bowel diseases. World J Gastroenterol 17: 3198–3203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goggin PM, Northfield TC, Spychal RT. Factors affecting gastric mucosal hydrophobicity in man. Scand J Gastroenterol Suppl 181: 65–73, 1991 [PubMed] [Google Scholar]
- 13. Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 255: H1269–H1275, 1988 [DOI] [PubMed] [Google Scholar]
- 14. Grisham MB, Von Ritter C, Smith BF, Lamont JT, Granger DN. Interaction between oxygen radicals and gastric mucin. Am J Physiol Gastrointest Liver Physiol 253: G93–G96, 1987 [DOI] [PubMed] [Google Scholar]
- 15. Hierholzer C, Kalff JC, Billiar TR, Bauer AJ, Tweardy DJ, Harbrecht BG. Induced nitric oxide promotes intestinal inflammation following hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol 286: G225–G233, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Kemper AC, Specian RD. Rat small intestinal mucins: a quantitative analysis. Anat Rec 229: 219–226, 1991 [DOI] [PubMed] [Google Scholar]
- 17. Kistler EB, Alsaigh T, Chang M, Schmid-Schönbein GW. Impaired small-bowel barrier integrity in the presence of luminal pancreatic digestive enzymes leads to circulatory shock. Shock 38: 262–267, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuhn DM, Sakowski SA, Sadidi M, Geddes TJ. Nitrotyrosine as a marker for peroxynitrite-induced neurotoxicity: the beginning or the end of the end of dopamine neurons? J Neurochem 89: 529–536, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Lu Q, Xu DZ, Sharpe S, Doucet D, Pisarenko V, Lee M, Deitch EA. The anatomic sites of disruption of the mucus layer directly correlate with areas of trauma/hemorrhagic shock-induced gut injury. J Trauma 70: 630–635, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Lugea A, Salas A, Casalot J, Guarner F, Malagelada JR. Surface hydrophobicity of the rat colonic mucosa is a defensive barrier against macromolecules and toxins. Gut 46: 515–521, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med 29: 2264–2270, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Mitsuoka H, Kistler EB, Schmid-Schonbein GW. Generation of in vivo activating factors in the ischemic intestine by pancreatic enzymes. Proc Natl Acad Sci USA 97: 1772–1777, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in food and dietary supplements. J Agric Food Chem 53: 4290–302, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Qin X, Sheth SU, Sharpe SM, Dong W, Lu Q, Xu D, Deitch EA. The mucus layer is critical in protecting against ischemia-reperfusion-mediated gut injury and in the restitution of gut barrier function. Shock 35: 275–281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubbo H, Radi R. Protein and lipid nitration: role in redox signaling and injury. Biochim Biophys Acta 1780: 1318–1324, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Rupani B, Caputo FJ, Watkins AC, Vega D, Magnotti LJ, Lu Q, Xu da Z, Deitch EA. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery 141: 481–489, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Sharpe SM, Doucet DR, Qin X, Deitch EA. Role of intestinal mucus and pancreatic proteases in the pathogenesis of trauma-hemorrhagic shock-induced gut barrier function and multiple organ failure syndrome. J Organ Dysfunct 4: 168–176, 2008 [Google Scholar]
- 28. Sharpe SM, Qin X, Lu Q, Feketeova E, Palange DC, Dong W, Sheth SU, Lee MA, Reino D, Xu DZ, Deitch EA. Loss of the intestinal mucus layer in the normal rat causes gut injury but not toxic mesenteric lymph nor lung injury. Shock 34: 475–481, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Specian RD, Oliver MG. Functional biology of intestinal goblet cells. Am J Physiol Cell Physiol 260: C183–C193, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Stadtman ER, Levine RL. Protein oxidation. Ann NY Acad Sci 899: 191–208, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, Dorffel Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 56: 343–350, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vesterlund S, Karp M, Salminen S, Ouwehand AC. Staphylococcus aureus adheres to human intestinal mucus but can be displaced by certain lactic acid bacteria. Microbiology 152: 1819–1826, 2006 [DOI] [PubMed] [Google Scholar]