Abstract
Bile formation by the liver is initiated by canalicular transport at the hepatocyte membrane, leading to an increase in ductular bile flow. Thus, bile duct epithelial cells (cholangiocytes), which contribute to the volume and dilution of bile through regulated Cl− transport, are exposed to changes in flow and shear force at the apical membrane. The aim of the present study was to determine if fluid flow, or shear stress, is a signal regulating cholangiocyte transport. The results demonstrate that, in human and mouse biliary cells, fluid flow, or shear, increases Cl− currents and identify TMEM16A, a Ca2+-activated Cl− channel, as the operative channel. Furthermore, activation of TMEM16A by flow is dependent on PKCα through a process involving extracellular ATP, binding purinergic P2 receptors, and increases in intracellular Ca2+ concentration. These studies represent the initial characterization of mechanosensitive Cl− currents mediated by TMEM16A. Identification of this novel mechanosensitive secretory pathway provides new insight into bile formation and suggests new therapeutic targets to enhance bile formation in the treatment of cholestatic liver disorders.
Keywords: liver, cholangiocyte, purinergic signaling, ATP release, chloride channel, anoctamin-1
cholestatic liver diseases, associated with poor bile flow, comprise a significant proportion of liver disorders in children and adults. While diverse in etiology, these disorders result in a significant decrease in ductular bile flow. It has been proposed that a decrease in bile flow may directly affect bile production through mechanical effects on the bile duct epithelium, including abnormal cilia function and abnormal Ca2+ signaling (23). However, potential direct effects of fluid flow, or shear, on membrane transport and secretion are unknown.
Intrahepatic bile duct epithelial cells, or cholangiocytes, represent an important component of the bile secretory unit. While bile formation is initiated at the hepatocyte canalicular membrane, cholangiocytes subsequently modify the volume and composition of bile through regulated ion and water secretion. Cl− channels in the apical membrane of cholangiocytes provide the driving force for ductular secretion (17, 18), and two complementary Cl− channels have been identified on a molecular basis: CFTR, a cAMP-activated Cl− channel (16, 19), and TMEM16A, a Ca2+-activated Cl− channel (9). CFTR is found on the apical membrane of cholangiocytes forming the medium- and large-sized, but not small-sized, bile ducts and is regulated via binding of the hormone secretin to basolateral receptors, increases in intracellular cAMP concentration, and PKA-dependent channel phosphorylation (18). In contrast, TMEM16A is found on the small-, medium-, and large-sized bile ducts and is activated by increases in intracellular Ca2+ concentration ([Ca2+]i) (9), although the physiological events mediating channel regulation have not been defined.
TMEM16A, a 114-kDa membrane protein with eight putative transmembrane domains, has been established as a Ca2+-activated Cl− channel in other epithelia, although its regulation is far from understood. Additionally, TMEM16A has been shown to regulate tracheal cartilage development (39), appears to play a role in cancer progression (12), and may contribute to the regulation of blood flow (4, 7). The diverse clinical significance of this protein is just beginning to be appreciated. While TMEM16A is found in biliary epithelium, its regulation and overall contribution to biliary secretion and bile formation are unknown.
Mechanical fluid flow (shear) activates membrane transport in epithelial and endothelial cells (2, 31, 33, 48). In vascular endothelial cells, shear stress has been shown to activate simultaneously an inward-rectifying K+ channel and an outward-rectifying Cl− channel (22). Similarly, in kidney, flow stimulates K+ secretion in mammalian cortical collecting ducts (32). Biliary epithelial cells are also exposed to plasma membrane-directed forces, including flow/shear forces at the apical membrane due to changes in bile flow. The hormone secretin, for example, increases bile flow from 0.67 to 1.54 ml/min in humans (30), representing a potential increase in flow-induced force at the apical cholangiocyte membrane. In isolated rat bile duct segments and confluent biliary epithelial monolayers, the increase in flow results in significant increases in [Ca2+]i (34, 48). The molecular identification of the protein(s) contributing to mechanosensitive cholangiocyte transport has not been defined.
Our present studies, in human and mouse biliary epithelial models, demonstrate for the first time that the force of flow at the surface of the plasma membrane is a significant and physiological stimulus for TMEM16A-mediated Cl− transport through a process dependent on ATP release, binding of purinergic P2 receptors, and increases in [Ca2+]i. We therefore propose that the mechanical force generated by flow may directly regulate cholangiocyte secretory events and bile formation.
METHODS
Cell models.
Studies were performed in human and mouse models, including human intrahepatic biliary epithelial H69 cells, human Mz-ChA-1 cells, and mouse small and large cholangiocytes (MSC and MLC, respectively). The H69 cholangiocyte cell line is a SV-40-transformed human intrahepatic bile duct epithelial cell line originally derived from normal liver harvested for liver transplantation (25). These cells retain phenotypic markers of primary human cholangiocytes, including functional membrane channels and transporters (24, 38). Human Mz-ChA-1 cells also exhibit phenotypic features of differentiated biliary epithelium (3, 29) and have been utilized as models for biliary purinergic signaling and Ca2+-stimulated secretion (14, 15, 41, 42, 47). Immortalized MSC and MLC demonstrate properties identical to those of freshly isolated MSC and MLC (21). Importantly, MSC do not express secretin receptors, CFTR, or the HCO3−/Cl− exchanger and do not exhibit a secretory response to secretin, while MLC do (21). Cells were maintained in culture, as described elsewhere (21, 46).
Perfusion system.
Shear was applied to cells in a parallel-plate chamber (24 × 13 × 4.1 mm; model RC-25F, Warner Instruments, Hamden, CT) for Ca2+ imaging and to cells in an open-top chamber for patch-clamp recording. In each case, flow was applied by a dual-syringe pump (model 33, Harvard Apparatus, Holliston, MA). The equation relating shear stress to volumetric flow rate through the chambers is as follows: τw = 6μQ̇/a2b, where μ is viscosity of the solution (poise), Q̇ is flow rate (ml/s), a is chamber height (cm), and b is chamber width (cm).
Ca2+ imaging.
Cells were cultured for 48 h on 15-mm glass coverslips and then loaded with 2.5 μg/ml fura 2-AM (TEF Labs, Austin, TX) in isotonic extracellular buffer containing (in mM) 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 1 KH2PO4, 5 glucose, and 10 HEPES (pH 7.4) supplemented with 0.01% Pluronic F-127 for 30 min at 22°C. In selected studies, EGTA (2 mM) was used to remove Ca2+ from the bath and perfusing solutions. The coverslip was placed in the perfusion chamber on the stage of an inverted fluorescence microscope (Nikon TE2000), and the inflow and outflow ports were attached to the syringe pump. Changes in [Ca2+]i were measured at excitation wavelength of 340 nm for Ca2+-bound fura 2-AM and 380 nm for Ca2+-free fura 2-AM at emission wavelength of 510 nm. After subtraction of background fluorescence, [Ca2+]i was calculated according to the Grynkiewicz equation (44): [Ca2+]i (nM) = Kd × [(R − Rmin)/(Rmax − R)] × Sfb, where Kd is the dissociation constant (145 nM at 22°C) and Sfb is the ratio of baseline fluorescence (380 nm) under the Ca2+-free condition to fluorescence under the Ca2+-bound condition. Experiments were performed at room temperature and at 37°C.
Measurement of flow-stimulated currents.
Membrane currents were measured using whole cell patch-clamp techniques. Cells on a coverslip were mounted in the chamber, and whole cell currents were measured during basal and perfused conditions with a standard extracellular solution containing (in mM) 140 NaCl, 4 KCl, 1 CaCl2, 2 MgCl2, 1 KH2PO4, 10 glucose, and 10 HEPES/NaOH (pH ∼7.40). The standard intracellular (pipette) solution for whole cell recordings contained (in mM) 130 KCl, 10 NaCl, 2 MgCl2, 10 HEPES/KOH, 0.5 CaCl2, and 1 EGTA (pH 7.3), corresponding to a free Ca2+ concentration of ∼100 nM (6, 13). Patch pipettes, which were pulled from Corning 7052 glass, had a resistance of 3–10 MΩ. Recordings were made with an Axopatch ID amplifier (Axon Instruments, Foster City, CA), digitized (2 kHz), and analyzed using pCLAMP version 10 (Axon Instruments, Burlingame, CA), as previously described (13, 20). Two voltage protocols were utilized: 1) holding potential of −40 mV with 200-ms steps to 0 and −80 mV at 10-s intervals and 2) holding potential of −40 mV with 400-ms steps from −100 to +100 mV in 20-mV increments. Current-voltage relations were generated from the “step” protocol. Pipette voltages are referred to the bath. Results are compared with control studies measured on the same day to minimize effects of day-to-day variability. While mean cell capacitance was 18.4 ± 0.7 pF (n = 101), results are reported as current density (pA/pF) to normalize for differences in cell size (13).
TMEM16A and CFTR silencing.
TMEM16A was suppressed by specific anti-TMEM16A small interfering RNA (siRNA; TMEM16A-HSS123904), as described in our previous studies (9). Briefly, 25-nucleotide siRNAs were designed and synthesized by Invitrogen [AAG UUA GUG AGG UAG GCU GGG AAC C (antisense) and GGU UCC CAG CCU ACC UCA CUA ACU U (sense)] and transfected using FuGENE (5 μg/100 μl). Noncoding Stealth RNAi (medium guanine-cytosine duplex, Invitrogen) was utilized in control (mock) transfections. Similarly, CFTR was suppressed by specific anti-CFTR siRNA (catalog no. 4392421, Life Technologies). BLOCK-iT Fluorescent Oligo (catalog no. 2013, Invitrogen) was used to optimize transfection conditions and to select transfected cells for whole cell patch-clamp recording. Whole cell patch-clamp experiments were performed 24–48 h after transfection. Transfection efficiency and the degree of TMEM16A and CFTR silencing were measured at the message level by real-time PCR and at the protein level by Western blot analysis (9).
Reagents.
The CFTR inhibitors CFTR(inh)-172 and malic hydrazide (MalH) were kind gifts from Drs. Nitin Sonawane and Alan Verkman (University of California, San Francisco, CA). Anti-CFTR (clone M3A7) monoclonal antibody (catalog no. 05-583) was purchased from Millipore. All other reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Statistics.
Values are means ± SE, with n representing the number of culture plates or repetitions for each assay. Statistical analysis included Fisher's paired and unpaired t-test and ANOVA for multiple comparisons to assess statistical significance. P < 0.05 was considered to be statistically significant.
RESULTS
Flow (shear) activates membrane Cl− currents.
To characterize the biophysical and pharmacological properties of membrane Cl− currents in response to shear, whole cell patch-clamp studies were performed in single Mz-ChA-1 and H69 cells and MSC and MLC in the presence or absence of defined shear. Representative traces of a Mz-ChA-1 cell and a H669 cell are shown in Fig. 1. Under basal conditions with standard intra- and extracellular buffers, Cl− current was small (−1.9 ± 0.5 pA/pF). Exposure to flow (shear = 0.24 dyn/cm2) resulted in activation of currents within 95 ± 17 s, increasing current density to −18.0 ± 4.0 pA/pF at −80 mV (P < 0.001, n = 13 for Mz-ChA-1 cells; P < 0.05, n = 4 for H69 cells). The currents were sustained for the duration of flow exposure and were fully reversible within 5 min of flow cessation. Interestingly, currents demonstrated two distinct patterns. In the majority (∼85%) of studies, the currents exhibited reversal near 0 mV [Cl− reversal (equilibrium) potential], outward rectification, and time-dependent activation at depolarizing potentials above +60 mV (Fig. 1), characteristics associated with Ca2+-activated Cl− currents previously described in these cells (9, 16). However, in a minority (∼15%) of studies, currents demonstrated time-dependent inactivation at positive depolarizing potentials above +60 mV (Fig. 2). In some studies, currents with both types of biophysical properties were observed in the same cell (5 of 35). Inclusion of MgATP2− in the patch pipette increased (from 15% to 38%) the relative percentage of the currents displaying time-dependent inactivation. Therefore, to minimize the currents demonstrating time-dependent inactivation, the majority of studies were performed without additional MgATP−2 in the pipette.
Fig. 1.
Characterization of flow-stimulated currents in human biliary epithelial cells. Whole cell currents were measured during basal conditions and during exposure to flow of isotonic extracellular buffer. A and B: representative whole cell recordings of an Mz-ChA-1 cell and an H69 cell. Currents were measured at −80 mV (○), representing Cl− current, and at 0 mV (●), representing K+ current. Flow exposure (shear = 0. 24 dyn/cm2) is indicated by horizontal bar. Currents were activated within 2 min of the onset of flow and were partially reversible when flow was stopped. A voltage-step protocol (inset in A), from a holding potential of −40 mV with 100-ms steps from +100 to −100 mV in 20-mV increments, was obtained at ☆a and ☆b, representing basal and maximal inward currents, respectively. Currents demonstrated time-dependent activation at membrane potentials >60 mV. Zero-current level is indicated by dotted lines. Current-voltage (I-V) plot was generated from these protocols during basal (●) and flow-stimulated (○) conditions. C: representative whole cell current recordings of Mz-ChA-1 cells in the presence or absence of the Ca2+-activated Cl− channel inhibitor niflumic acid (n. acid, 100 μM, top) and in the presence of the CFTR inhibitor CFTR(inh)-172 (5 μM, bottom). Flow is indicated by horizontal bar. D: cumulative data demonstrating percentage of maximum flow-stimulated currents in the presence or absence of the Cl− channel inhibitors 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB, 100 μM), niflumic acid (100 μM), DIDS (100 μM), CFTR(inh)-172 (5 μM), and malonic hydrazide (MalH, 5 μM). Values represent percentage of current density measured at −80 mV (n = 4–5 each). *Significantly different from control (P < 0.05).
Fig. 2.
Flow-stimulated Cl− currents demonstrate a second and distinct biophysical profile, as shown in a representative whole cell recording of a Mz-ChA-1 cell. Currents were measured at −80 mV (○), representing Cl− current, and at 0 mV (●), representing K+ current. Flow exposure (shear = 0. 24 dyn/cm2) is indicated by horizontal bar. A voltage-step protocol as described in Fig. 1A legend (test potentials between −100 and +100 mV in 20-mV increments) was obtained at ☆a (basal) and ☆b (maximal current response). Currents demonstrate time-dependent inactivation at membrane potentials >60 mV. Zero-current levels are indicated by dotted lines. I-V plot was generated from these protocols during basal (●) and flow-stimulated (○) conditions.
MSC and MLC also demonstrated flow-stimulated Cl− currents with biophysical properties similar to those of the human biliary cells (Fig. 3). In a number of studies the flow-stimulated Cl− currents were preceded by small K+ currents, which occurred early and were transient. The biophysical properties of these K+ currents were consistent with the small- or intermediate-conductance Ca2+-activated K+ channels previously described in biliary cells (10, 14).
Fig. 3.
Characterization of flow-stimulated currents in mouse large and small cholangiocytes (MLC and MSC, respectively). A: representative whole cell current recordings from MSC and MLC. Currents in MLC were measured in the presence of CFTR channel inhibitor CFTR(inh)-172 (5 μM) at −80 mV (○) and 0 mV (●). Flow exposure (shear = 0. 18 dyn/cm2) is indicated by horizontal bar. Currents were activated within 2 min of the onset of flow. A voltage-step protocol (test potentials between −100 and +100 mV in 20-mV increments) was obtained at ☆a (basal) and ☆b (maximal current response). Currents demonstrate time-dependent activation at membrane potentials >60 mV. Zero-current levels are indicated by dotted lines. I-V plot was generated from these protocols during basal (●) and flow-stimulated (○) conditions. B: cumulative data demonstrating maximum current density in response to addition of the CFTR-activating cocktail (chlorophenylthio-cAMP, forskolin, and IBMX) or exposure to flow (shear = 0.18 dyn/cm2) in MLC and MSC. Flow-stimulated currents were recorded in the presence or absence of niflumic acid (100 μM) or CFTR(inh)-172 (5 μM). Values represent maximum current density measured at −80 mV (n = 4–11 each). *CFTR-activating cocktail significantly increased currents from basal in MLC (P < 0.05). **Flow-stimulated currents were significantly inhibited by niflumic acid in MLC (P < 0.05). ns, Not significant.
Flow-stimulated Cl− currents are independent of CFTR.
To determine if CFTR contributes to the flow-stimulated Cl− current, three complementary strategies were utilized: 1) pharmacological inhibition, 2) differential models of CFTR expression, and 3) molecular silencing. 1) Flow-stimulated currents were inhibited by the nonspecific Cl− channel blocker 5-nitro-2-(3-phenylpropylamino)benzoic acid and by the Ca2+-activated Cl− channel blockers niflumic acid and DIDS (Fig. 1, C and D) but were unaffected by pharmacological inhibitors of CFTR, including CFTR(inh)-172 and MalH (Fig. 1, C and D). 2) Comparison studies were performed in mouse cholangiocytes with and without CFTR expression. MSC (∼8 μm), isolated from the epithelium forming the small intrahepatic bile ducts, do not express CFTR (46), while MLC (∼14 μm), isolated from the epithelium forming the large bile ducts, do. In MSC, exposure to a CFTR-activating cocktail (10 μM forskolin, 100 μM IBMX, and 500 μM chlorophenylthio-cAMP) did not increase Cl− currents from the basal level, consistent with the absence of CFTR channel protein (Fig. 3B). However, exposure to flow (shear = 0.12 dyn/cm2) significantly increased current density (Fig. 3A). In comparison, addition of the CFTR-activating cocktail to MLC increased Cl− current density (from −2.7 ± 0.9 to −8.7 ± 2.1 pA/pF), consistent with functional CFTR expression. Interestingly, subsequent exposure to flow (shear = 0.12 dyn/cm2) in the same cell further increased the magnitude of Cl− currents (−29.2 ± 12.6 pA/pF). The CFTR inhibitor CFTR(inh)-172 completely inhibited the currents in response to the CFTR cocktail but had no effect on the flow-stimulated currents (Fig. 3, A and B). The biophysical properties of the flow-stimulated currents in both cell types were identical to those of the Ca2+-activated and ATP-stimulated Cl− currents previously reported in these cells (9), with time-dependent activation, outward rectification, and reversal at ∼0 mV. While CFTR(inh)-172 had no effect, the Ca2+-activated Cl− channel blocker niflumic acid significantly inhibited flow-stimulated Cl− currents in the MLC (Fig. 3B). Thus, flow exposure resulted in activation of Cl− currents in mouse cholangiocytes lacking CFTR expression (MSC) and in the presence of pharmacological inhibitors of CFTR in CFTR-expressing cholangiocytes (MLC). 3) Studies were performed in human Mz-ChA-1 cells with and without CFTR silencing. Transfection of cells with CFTR siRNA significantly decreased expression of CFTR protein by 54 ± 6%; however, no significant differences in the magnitude of Cl− currents in response to flow (shear = 0.18 dyn/cm2) were observed compared with cells transfected with nontargeting siRNA (Fig. 4). Together, these findings provide evidence that CFTR contributes negligibly to flow-stimulated Cl− currents and suggest the existence of alternate (non-CFTR) channels, which contribute to flow-stimulated Cl− transport.
Fig. 4.
Flow-stimulated currents are independent of CFTR channel. A: representative Western blot demonstrating CFTR protein levels in Mz-ChA-1 cells transfected with nontargeting small interfering RNA (siRNA, mock) and anti-CFTR siRNA. Actin was used as loading control. B: representative whole cell current recordings from Mz-ChA-1 cells transfected with CFTR siRNA under basal and flow-stimulated conditions. Flow exposure (shear = 0.18 dyn/cm2) is indicated by horizontal bar. Currents were measured at −80 mV (○) and 0 mV (●). Voltage-step protocols were obtained at ☆a (basal) and ☆b (maximal current response). Currents demonstrate time-dependent activation at membrane potentials >60 mV. I-V plots were generated from the step protocols during basal (●) and flow-stimulated (○) conditions. C: cumulative data demonstrating maximal current density measured at −80 mV in response to flow in cells transfected with nontargeting siRNA (mock) or cells transfected with anti-CFTR siRNA. Values are means ± SE; n = 4–5. P = not significant.
Flow-stimulated currents are dependent on [Ca2+]i.
It has previously been shown that fluid flow (shear) increases [Ca2+]i in rat bile duct epithelial cells (34, 48), isolated duct segments (34), and single Mz-ChA-1 cells (48). To confirm that shear increases [Ca2+]i in mouse cholangiocytes, studies were performed in MSC and MLC. In both cell types, exposure to flow resulted in an increase in [Ca2+]i (Fig. 5). The increase in [Ca2+]i was shear-dependent, as [Ca2+]i was increased to a significantly greater extent by exposure to shear of 0.09 than 0.02 dyn/cm2 in both cell types. However, there was no significant difference in the magnitude of the flow-stimulated [Ca2+]i between MSC and MLC at the same flow rates (Fig. 5B). In parallel whole cell patch-clamp studies, flow-stimulated Cl− currents were dependent on Ca2+, as removal of Ca2+ from the patch pipette (2 mM EGTA) and bath completely inhibited Cl− currents. To determine the relative contributions of intra- vs. extracellular Ca2+ to shear-stimulated Cl− currents, Ca2+ was individually removed from the pipette or the bath and Cl− currents were measured in response to shear. While removal of extracellular Ca2+ had little effect on the magnitude of Cl− currents, removal of intracellular Ca2+ significantly decreased shear-stimulated Cl− currents (Fig. 5, C and D), suggesting that the operative channels are primarily dependent on intracellular Ca2+ stores for activation.
Fig. 5.
Flow-stimulated currents are dependent on intracellular Ca2+ concentration ([Ca2+]i). A: representative studies demonstrating the increase in [Ca2+]i, associated with flow in MLC and MSC. Maximal and minimal [Ca2+]i was obtained by exposure to ionomycin (2 μM) and EGTA (10 mM), respectively. F340/F380, ratio of fluorescence at 340 nm to fluorescence at 380 nm. B: cumulative data demonstrating flow-stimulated increase in [Ca2+]i in MLC and MSC (n = 10 each) *Significant increase in [Ca2+]i at shear = 0.09 dyn/cm2 compared with shear = 0.02 dyn/cm2 (P < 0.05). **No difference in [Ca2+]i between MSC and MLC at either shear (P = not significant). C: representative whole cell current recordings from Mz-ChA-1 cells with removal of extracellular Ca2+ (1 mM EGTA in bath, top), removal of intracellular Ca2+ (2 mM EGTA in pipette, middle), and removal of extra- and intracellular Ca2+ (bottom). Currents were measured at −80 mV (●) and 0 mV (○). Flow exposure (shear = 0.24 dyn/cm2) is indicated by horizontal bar. D: cumulative data demonstrating maximum current density with removal of extracellular [(E)Ca2+ free], intracellular [(I)Ca2+ free], or both (Ca2+ free) compared with control. Values represent maximum current density measured at −80 mV (n = 4–8 each). Flow-stimulated currents were significantly inhibited: *P < 0.001, #P < 0.01.
Functional expression of TMEM16A in human intrahepatic cholangiocytes.
We previously demonstrated that biliary epithelial cells (including human, rat, and mouse) express the Ca2+-activated Cl− channel TMEM16A (9). We performed additional studies to determine if H69 human intrahepatic cholangiocytes also express functional TMEM16A channel proteins. 1) Expression of TMEM16A was confirmed by Western blotting (Fig. 6A). 2) Whole cell currents were measured in response to increasing [Ca2+]i. As shown in Fig. 6B, addition of 1 μM free Ca2+ in the patch pipette increased Cl− currents from −0.9 ± 0.3 to −39.5 ± 29.2 pA/pF (P < 0.01, n = 5). Ca2+-activated currents exhibited time-dependent activation at membrane potentials above +60 mV, outward rectification, and a reversal (equilibrium) potential (ECl) near 0 mV, biophysical properties consistent with TMEM16A. Thus, human intrahepatic H69 cells express TMEM16A and functional Ca2+-activated Cl− currents similar to other biliary models. To our knowledge, these findings represent the first biophysical characterization of Ca2+-activated Cl− channels in human intrahepatic biliary epithelial cells.
Fig. 6.
Functional expression of TMEM16A channels in human intrahepatic biliary epithelial (H69) cells. A: representative Western blot demonstrating TMEM16A protein in H69 and Mz-ChA-1 cells. B: representative whole cell current recordings. Currents were measured in standard extracellular (bath) and intracellular (pipette) solution buffered with 1 μM free intracellular Ca2+ in the pipette. Currents were measured at −80 mV (○), representing Cl− current, and 0 mV (●), representing K+ current. Voltage-step protocols were obtained at ☆a (initial) and ☆b (maximal current response). Currents demonstrate time-dependent activation at membrane potentials >60 mV. I-V plots were generated from step protocols during basal (●) and Ca2+-stimulated (○) conditions. C: cumulative data demonstrating maximal current density in H69 cells measured at −80 mV in response to [Ca2+]i (1 μM) or flow (shear = 0.18 dyn/cm2). Values are means ± SE; n = 4–5.
Flow-stimulated Cl− currents are mediated by TMEM16A.
To determine if TMEM16A represents the flow-stimulated Cl− channel, studies were performed in Mz-ChA-1 cells with and without TMEM16A silencing. Transfection of cells with anti-TMEM16A siRNA significantly decreased expression of TMEM16A mRNA by 66 ± 13% and protein by 64 ± 8% and significantly inhibited Cl− currents in response to flow (shear = 0.12 dyn/cm2) compared with cells transfected with nontargeting siRNA (Fig. 7). Together, these studies demonstrate that TMEM16A contributes to flow-stimulated Cl− currents in human biliary cells. While TMEM16A siRNA significantly inhibited flow-stimulated currents with biophysical properties consistent with the Ca2+-activated Cl− channel, in 14% of cells, currents with time-dependent inactivation (as shown in Fig. 2) were still observed (data not shown). The transient flow-activated K+ currents observed in some trials were not significantly affected by TMEM16A silencing (data not shown). Together, these studies demonstrate that TMEM16A contributes to flow-stimulated Cl− currents in human biliary cells.
Fig. 7.
TMEM16A contributes to flow-stimulated currents. Representative whole cell current recordings from Mz-ChA-1 cells transfected with nontargeting siRNA [control-mock (A)] or TMEM16A siRNA (B) under basal and flow-stimulated conditions. Flow exposure (shear = 0.12 dyn/cm2) is indicated by horizontal bar. Currents were measured at −80 mV (○) and at 0 mV (●). Voltage-step protocols were obtained at ☆a (basal) and ☆b (maximal current response). Currents demonstrate time-dependent activation at membrane potentials >60 mV. I-V plots were generated from step protocols during basal (●) and flow-stimulated (○) conditions. C: cumulative data demonstrating maximal current density measured at −80 mV in response to flow in cells transfected with nontargeting siRNA (mock) or TMEM16A siRNA. Values are means ± SE; n = 5. *P < 0.05 vs. control. D: representative Western blot and cumulative data demonstrating TMEM16A protein levels in control cells, cells transfected with nontargeting siRNA (mock), and cells transfected with TMEM16A siRNA. *P < 0.05 vs. control or mock. β-Actin was used as loading control.
Flow-stimulated Cl− currents are dependent on shear rate, but not viscosity.
As shown in Fig. 8, exposure to a very low shear (<0.01 dyn/cm2) did not activate currents in most of the whole cell current recordings. Currents were not observed until a shear of 0.02 dyn/cm2 was applied (Fig. 8). Whole cell Cl− currents (measured at −80 mV) reached maximal values at a shear rate of 0.12 dyn/cm2. Higher flow rates did not result in a further increase in the magnitude of currents. The calculated shear rate at half-maximal current (k½max) was 0.05 dyn/cm2 (Fig. 8B). As shear is determined not only by flow rate, but also by viscosity, the effects of increasing viscosity on the magnitude of currents were determined. These studies were designed to determine if the activation of Cl− channels by flow was due to a direct mechanical effect on the channel or an indirect effect of flow on the delivery of a soluble factor to the apical membrane. Increasing viscosity by inclusion of 5% dextran to the buffer solution (increasing viscosity from 0.95 to 2.8 cP) did not increase the magnitude of currents at a low (0.5 ml/min) or a high (2 ml/min) flow rate (Fig. 8C). Thus the currents are dependent on the flow rate, but not viscosity. This is consistent with the delivery of a soluble substance in the perfusate to the site of channel activation.
Fig. 8.
Flow-stimulated currents are dependent on flow rate. A: representative whole cell recording. Currents were measured at −80 mV (○) and 0 mV (●). Flow exposure (shear = 0. 03 or 0.06 dyn/cm2) is indicated by horizontal bar. B: flow (shear) rate-response curve for Cl− currents. Data are plotted from maximum current density measured at −80 mV in response to different flow rates. Values are means ± SE (n = 4–15) fit to data for shear activation of whole cell currents as follows: y = Vmax × xn/(Kn + xn), where y is the current density, x is shear, Vmax is maximum current density at −80 mV, K is half-maximum shear, and n is the Hill coefficient. C: cumulative data demonstrating normalized Cl− currents relative to basal values in response to low flow (0.5 ml/min) and high flow (2 ml/min) with and without 5% dextran (n = 5–10). Addition of dextran did not increase magnitude of flow-stimulated currents (P = not significant).
Flow-stimulated Cl− currents are mediated by extracellular ATP.
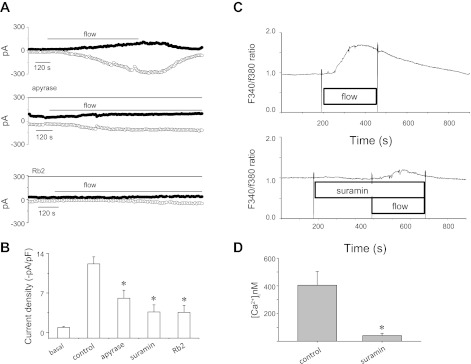
We previously showed that flow-stimulated increases in [Ca2+]i are mediated in part by release of ATP, binding of ATP to P2Y receptors, and release of Ca2+ from intracellular stores (48). To determine if flow-stimulated TMEM16A currents are dependent on activation of membrane P2Y receptors, studies were performed in the presence or absence of P2Y receptor antagonists. In the presence of the nonspecific P2 receptor inhibitor suramin or the structurally unrelated P2Y inhibitor reactive blue 2, flow-stimulated increases in [Ca2+]i and Cl− currents were inhibited (Fig. 9). Similarly, in the presence of apyrase in the bath and perfusate to hydrolyze ATP, flow-stimulated increases in [Ca2+]i and Cl− currents were inhibited. Together, these studies demonstrate that the TMEM16A-mediated Cl− currents stimulated by flow are dependent on extracellular ATP and P2Y receptor stimulation.
Fig. 9.
Flow-stimulated Cl− currents are dependent on extracellular ATP-mediated P2 receptor stimulation. A: representative whole cell patch-clamp recordings in response to flow (shear = 0.18 dyn/cm2, indicated by horizontal bar) in control conditions (top), in the presence of the ATP-hydrolyzing enzyme apyrase (5 U/ml, middle), and in the presence of the P2 receptor antagonist reactive blue 2 (Rb2, 25 μM, bottom). B: cumulative data representing maximum current density measured at −80 mV. Values are means ± SE (n = 4–11 each). *Apyrase, suramin, and Rb2 significantly inhibited flow-stimulated currents (P < 0.05). C: flow-stimulated increases in fura 2 fluorescence in control conditions (top) and in the presence of the P2Y receptor antagonist suramin (100 μM, bottom). D: cumulative data representing maximum increase in [Ca2+]i. Values are means ± SE (n = 6). *Significantly different from control [Ca2+]i (P < 0.05).
Flow-stimulated Cl− currents are regulated by PKCα.
PKC has been shown to be involved in signal transduction in response to mechanical stimuli in epithelial and endothelial cells (9, 27, 40, 48). Swelling in Mz-ChA-1 cells results in rapid translocation of PKCα to the plasma membrane, and inhibition of PKCα blocks volume-stimulated ATP release and volume-stimulated Cl− currents (40). In contrast, flow-stimulated ATP release is regulated by PKCζ (45). Thus, distinct PKC isoforms appear to be involved in transducing different mechanosensitive stimuli to intracellular signals involved in purinergic signaling. To evaluate a potential role of these PKC isoforms in flow-stimulated Cl− channel regulation, studies were performed in the presence or absence of specific antagonists. 1) Whole cell patch-clamp studies were performed utilizing intracellular dialysis to deliver a specific inhibitor of PKCζ, PKCζ pseudosubstrate (45), directly to the cell interior. Intracellular dialysis with the PKCζ pseudosubstrate or a scrambled PKCζ peptide as control did not affect the flow-stimulated Cl− currents (Fig. 10). 2) Flow-stimulated currents were measured in the presence or absence of the PKCα inhibitor Gö 6976 (10 μM). In contrast to PKCζ inhibition, inhibition of PKCα completely and reversibly inhibited flow-stimulated Cl− currents (Fig. 10). Thus, while flow-stimulated ATP release is, in part, dependent on PKCζ (48), flow-stimulated Cl− channels are regulated by the conventional isoform PKCα.
Fig. 10.
Flow-stimulated Cl− currents are regulated by PKCα. A: representative whole cell patch-clamp recordings of flow-stimulated currents (shear = 0.12 dyn/cm2, indicated by horizontal bar) in the presence or absence Gö 6976 (10 μM) in bath solution (top) or with PKC-specific peptides in the patch pipette: PKCζ pseudosubstrate (20 μM, middle) or PKCζ scramble (20 μM, bottom). Currents were measured at −80 mV (○) and 0 mV (●). B: cumulative data demonstrating maximum current density in the presence or absence of Gö 6976 (10 μM, left) or PKCζ pseudosubstrate (20 μM) and control PKCζ scrambled peptide (20 μM; right). Values (means ± SE; n = 4–5 each) represent maximum current density measured at −80 mV. *Flow-stimulated currents were significantly inhibited by Gö 6976 (P < 0.05).
DISCUSSION
The present studies provide evidence that fluid flow is a stimulus for TMEM16A activation and that TMEM16A represents the downstream effector pathway mediating purinergic (P2) receptor-mediated secretion. Evidence for this includes the following. 1) Fluid flow activates Cl− currents with outward rectification, time-dependent activation at depolarizing potentials above +60 mV, and a reversal potential near 0 mV, biophysical properties consistent with TMEM16A (5, 43, 49). 2) Flow-stimulated currents are inhibited by the Ca2+-activated Cl− channel blockers niflumic acid and DIDS, but not by the CFTR inhibitors CFTR(inh)-172 and MalH. 3) MSC, which express TMEM16A, but not CFTR, exhibit robust flow-stimulated Cl− currents. 4) Fluid flow increases [Ca2+]i, and inhibition of the flow-stimulated increase in [Ca2+]i prevents current activation. 5) Flow-stimulated currents exhibit biophysical properties similar to those activated by extracellular ATP (9) and removal of ATP, or blockade of P2 receptors abolishes flow-stimulated currents. 6) CFTR siRNA has no effect on the flow-stimulated currents, while TMEM16A siRNA significantly inhibits the flow-stimulated currents. Together, the findings are consistent with TMEM16A as the operative Cl− channel responsible for the flow-stimulated membrane currents through a pathway involving ATP release, binding P2 receptors, and increases in [Ca2+]i. Thus, TMEM16A plays a critical role in mechanosensitive signaling and is, thereby, an important regulator of biliary epithelial secretion and bile formation. In light of recent studies demonstrating that the mechanical effects of fluid flow, or shear stress, at the apical membrane of biliary epithelial cells are a robust stimulus for ATP release (48), a model emerges in which mechanosensitive ATP release and Cl− secretion represent a dominant pathway regulating biliary secretion.
While CFTR has recently been shown to be mechanosensitive in other model systems (50), there is no evidence in the present studies that CFTR contributes to the flow-stimulated response. 1) The biophysical properties of the flow-stimulated Cl− currents are distinct from CFTR, which generally exhibits a linear current-voltage relation and no time dependence (28). 2) Pharmacological inhibition, or molecular silencing, of CFTR affected neither the activation nor the magnitude of flow-stimulated currents. 3) MSC, which do not express CFTR, still exhibited flow-stimulated currents. While previous studies have highlighted the importance of CFTR in driving ductular secretion, recent evidence suggests that Cl− secretion mediated by extracellular ATP is functionally of greater significance. In response to ATP, the unitary currents from single cells and the short-circuit current response from intact biliary epithelial monolayers are severalfold greater than those mediated by increases in cAMP (11). Additionally, even cAMP-mediated secretion requires extracellular ATP (37). Our present findings provide further evidence that Cl− secretion mediated by non-CFTR pathways is functionally important and, in fact, may be the predominant pathway mediating ductular bile formation.
The magnitude of flow-stimulated currents increased with increasing shear rate, with a calculated k½max of 0.05 dyn/cm2 and a steep activation curve. We did not observe an increase in the magnitude of flow-stimulated currents when shear force was increased by increasing the viscosity of the perfusate by the addition of dextran. Thus the currents are dependent on shear rate, but not viscosity. This is consistent with the delivery of a soluble substance (ATP) in the perfusate to the site of channel activation (membrane P2 receptor). Thus the currents appear to be dependent on the rate-driven delivery of ATP to the membrane (shear rate), and not on direct mechanical stimulation (shear stress) on the channel itself. In the absence of ATP (apyrase in the perfusate), flow-stimulated currents were significantly reduced. Furthermore, there is no evidence that TMEM16A is itself a mechanosensitive channel, and while TMEM16A is activated by membrane distension due to cell swelling in other models (1), this may occur via swelling-stimulated ATP release and autocrine stimulation of membrane P2 receptors (41).
If these studies, performed in human and mouse biliary epithelial cells, translate to in vivo conditions, several points, as well as uncertainties, deserve to be highlighted.
First, the actual flow rates and shear force along the intrahepatic bile ducts are unknown. The flow rates used in this study were based on observations, as well as calculations, derived from previous studies of cell and animal models. Flow-stimulated ATP release from human and rat biliary cells occurs at a shear rate of 0.16 dyn/cm2 (27, 48), similar to the shear rates used in these studies. Masyuk et al. (36) utilized three-dimensional imaging of the rat intrahepatic biliary tree to evaluate bile duct size and then used mathematical calculations to estimate the flow rates in the ducts. On the basis of these calculations, the estimated flow rate was 11.1 nl/min in small (50 μm) bile ducts and 1,064 nl/min in larger (225 μm) ducts, corresponding to a shear force of ∼0.14 dyn/cm2. These calculations utilized a constant value for viscosity and, hence, presumed a wall shear stress that is constant throughout the system. While our present studies utilized shear forces within this calculated range, direct evaluation of flow rates and shear force in the small intrahepatic bile ducts requires technical advances.
Second, while the majority of flow-stimulated Cl− currents demonstrated outward rectification and time-dependent activation at depolarizing potentials above +60 mV, in ∼15% of cells the flow-stimulated Cl− currents demonstrated slightly different biophysical properties (time-dependent inactivation) that were unaffected by TMEM16A silencing. Thus a distinct Cl− channel, of unknown molecular identity, appears also to contribute to the flow-stimulated currents. These biophysical properties are consistent with the swelling-activated Cl− currents previously described in biliary epithelial cells (42). In other cell types, currents with these biophysical properties have been attributed to Cl− channel protein 3 (ClC-3) (26); however, ClC-3 has not been definitively identified in biliary epithelium. The possibility that other TMEM16 isoforms (f, j, and k), previously identified in biliary epithelium (9), form novel Cl−-permeable heteromultimers with distinct biophysical properties cannot be excluded.
Third, in the majority of studies, flow-activated Cl− currents were preceded by activation of K+ currents. When they were observed, the K+ currents activated rapidly in response to flow and were transient. In secretory epithelium, it is believed that activation of K+ channels leads to membrane hyperpolarization to maintain the electrical driving force for continued Cl− efflux (8). While we previously identified the Ca2+-activated K+ channels SK2 and IK1 in biliary epithelium (10, 14), the molecular identification of the flow-stimulated K+ channels is unknown.
Fourth, the mechanism by which biliary epithelium senses flow and initiates the mechanosensory signaling cascade is unknown. Cholangiocytes express a primary cilium, a mechanosensory organelle that translates flow to increases in [Ca2+]i and may play a role in mechanosensitive ATP release (48). However, Mz-ChA-1 cells do not express a primary cilium, despite exhibiting robust flow-stimulated ATP release, [Ca2+]i increases, and Cl− secretion, suggesting the existence of other mechanosensors in these cells. Furthermore, removal of the primary cilium from rat cholangiocytes (chloral hydrate) decreases, but does not eliminate, mechanosensitive ATP release (48). In other epithelia, mechanical stimulation may be transduced through microvilli, the cytoskeleton, or other membrane proteins. The identification of the mechanosensors in cholangiocytes will be an important area for future investigation of mechanosensitive signaling in the liver.
Lastly, abnormalities in mechanosensitive signaling along the bile duct may have important implications during cholestatic liver disease. A decrease in bile flow associated with cholestasis would inhibit mechanosensitive ATP release, Ca2+ signaling, and Cl− secretion, thus decreasing ductular secretion and affecting the volume and composition of bile. Conversely, targeting the elements of the mechanosensitive signaling pathway, including ATP release, P2 receptors, and/or TMEM16A, may provide new and innovative strategies to increase ductular bile formation for the treatment of cholestatic liver diseases.
GRANTS
This study was supported by a American Liver Foundation Liver Scholar Award (A. K. Dutta), the Children's Medical Center Foundation (A. K. Dutta), Cystic Fibrosis Foundation Grant FERANC08G0 (A. P. Feranchak), and National Institute of Diabetes, Digestive, and Kidney Diseases Grant DK-078587 (A. P. Feranchak).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K.D. and A.P.F. are responsible for conception and design of the research; A.K.D., K.W., A.-K.K., and C.K. performed the experiments; A.K.D., K.W., A.-K.K., C.K., and A.P.F. analyzed the data; A.K.D., K.W., A.-K.K., C.K., and A.P.F. interpreted the results of the experiments; A.K.D. and A.P.F. prepared the figures; A.K.D. and A.P.F. drafted the manuscript; A.K.D. and A.P.F. edited and revised the manuscript; A.K.D. and A.P.F. approved the final version of the manuscript.
ACKNOWLEDGMENTS
MSC and MLC were kindly provided by Dr. Gianfranco Alpini (Texas A & M Health Science Center College of Medicine, Temple, TX).
REFERENCES
- 1. Almaca J, Tian Y, Aldehni F, Ousingsawat J, Kongsuphol P, Rock JR, Harfe BD, Schreiber R, Kunzelmann K. TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem 284: 28571– 28578, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barakat AI, Leaver EV, Pappone PA, Davies PF. A flow-activated chloride-selective membrane current in vascular endothelial cells. Circ Res 85: 820– 828, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Basavappa S, Middleton JP, Mangel A, McGill J, Cohn JA, Fitz JG. Cl− and K+ transport in human biliary cell lines. Gastroenterology 104: 1796– 1805, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Bulley S, Neeb ZP, Burris SK, Bannister JP, Thomas-Gatewood CM, Jangsangthong W, Jaggar JH. TMEM16A/ANO1 channels contribute to the myogenic response in cerebral arteries. Circ Res 111: 1027– 1036, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590– 594, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Chang D, Hsieh PS, Dawson DC. Calcium: a program in BASIC for calculating the composition of solutions with specified free concentrations of calcium, magnesium and other divalent cations. Comput Biol Med 18: 351– 366, 1988 [DOI] [PubMed] [Google Scholar]
- 7. Davis AJ, Shi J, Pritchard HA, Chadha PS, Leblanc N, Vasilikostas G, Yao Z, Verkman AS, Albert AP, Greenwood IA. Potent vasorelaxant activity of the TMEM16A inhibitor T16A(inh)-A01. Br J Pharmacol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Devor DC, Singh AK, Frizzell RA, Bridges RJ. Modulation of Cl− secretion by benzimidazolones. I. Direct activation of a Ca2+-dependent K+ channel. Am J Physiol Lung Cell Mol Physiol 271: L775– L784, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Dutta AK, Khimji AK, Kresge C, Bugde A, Dougherty M, Esser V, Ueno Y, Glaser SS, Alpini G, Rockey DC, Feranchak AP. Identification and functional characterization of TMEM16A, a Ca2+-activated Cl− channel activated by extracellular nucleotides, in biliary epithelium. J Biol Chem 286: 766– 776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dutta AK, Khimji AK, Sathe M, Kresge C, Parameswara V, Esser V, Rockey DC, Feranchak AP. Identification and functional characterization of the intermediate-conductance Ca2+-activated K+ channel (IK-1) in biliary epithelium. Am J Physiol Gastrointest Liver Physiol 297: G1009– G1018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dutta AK, Woo K, Doctor RB, Fitz JG, Feranchak AP. Extracellular nucleotides stimulate Cl− currents in biliary epithelia through receptor-mediated IP3 and Ca2+ release. Am J Physiol Gastrointest Liver Physiol 295: G1004– G1015, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duvvuri U, Shiwarski DJ, Xiao D, Bertrand C, Huang X, Edinger RS, Rock JR, Harfe BD, Henson BJ, Kunzelmann K, Schreiber R, Seethala RS, Egloff AM, Chen X, Lui VW, Grandis JR, Gollin SM. TMEM16A induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res 72: 3270– 3281, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feranchak AP, Berl T, Capasso J, Wojtaszek PA, Han J, Fitz JG. p38 MAP kinase modulates liver cell volume through inhibition of membrane Na+ permeability. J Clin Invest 108: 1495– 1504, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feranchak AP, Doctor RB, Troetsch M, Brookman K, Johnson SM, Fitz JG. Calcium-dependent regulation of secretion in biliary epithelial cells: the role of apamin-sensitive SK channels. Gastroenterology 127: 903– 913, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Feranchak AP, Roman RM, Doctor RB, Salter KD, Toker A, Fitz JG. The lipid products of phosphoinositide 3-kinase contribute to regulation of cholangiocyte ATP and chloride transport. J Biol Chem 274: 30979– 30986, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Fitz JG, Basavappa S, McGill J. Ca2+- and cAMP-stimulated Cl− conductances in bile duct epithelial cells: a possible mechanism for ductular secretion (Abstract). Hepatology 14: A416, 1991 [Google Scholar]
- 17. Fitz JG, Basavappa S, McGill J, Melhus O, Cohn JA. Regulation of membrane chloride currents in rat bile duct epithelial cells. J Clin Invest 91: 319– 328, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fitz JG, Cohn JA. Regulation of CFTR and other chloride channels in biliary epithelial cells. In: Biliary and Pancreatic Ductal Epithelia: Pathobiology and Pathophysiology, edited by Sirica A, Longnecker D. Philadelphia: Decker, 1996. [Google Scholar]
- 19. Fitz JG, McGill J, Basavappa S, Cohn JA. Bile duct epithelial cells contain regulated chloride channels and CFTR (Abstract). Clin Res 40: 319A, 1992 [Google Scholar]
- 20. Fitz JG, Sostman A. Nucleotide receptors activate cation, potassium, and chloride currents in a liver cell line. Am J Physiol Gastrointest Liver Physiol 266: G544– G553, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Francis H, Glaser S, Demorrow S, Gaudio E, Ueno Y, Venter J, Dostal D, Onori P, Franchitto A, Marzioni M, Vaculin S, Vaculin B, Katki K, Stutes M, Savage J, Alpini G. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol 295: C499– C513, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gautam M, Shen Y, Thirkill TL, Douglas GC, Barakat AI. Flow-activated chloride channels in vascular endothelium: shear stress sensitivity, desensitization dynamics, and physiological implications. J Biol Chem 281: 36492– 36500, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, LaRusso NF. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci USA 104: 19138– 19143, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grubman SA, Fang SL, Mulberg AE, Perrone RD, Rogers LC, Lee DW, Armentano D, Murray SL, Dorkin HL, Cheng SH, Smith AE, Jefferson DM. Correction of the cystic fibrosis defect by gene complementation in human intrahepatic biliary cell lines. Gastroenterology 108: 584– 592, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Grubman SA, Perrone RD, Lee DW, Murray SL, Rogers LC, Wolkoff LI, Mulberg AE, Cherington V, Jefferson DM. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol Gastrointest Liver Physiol 266: G1060– G1070, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Hume JR, Wang GX, Yamazaki J, Ng LC, Duan D. CLC-3 chloride channels in the pulmonary vasculature. Adv Exp Med Biol 661: 237– 247, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Ishida T, Takahashi M, Corson MA, Berk BC. Fluid shear stress-mediated signal transduction: how do endothelial cells transduce mechanical force into biological responses? Ann NY Acad Sci 811: 12– 23, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev 82: 503– 568, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D, Prellwitz W, Meyer zum Buschenfelde KH. Biliary adenocarcinoma characterization of three new human tumor cell lines. J Hepatol 1: 579– 596, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Lenzen R, Elster J, Behrend C, Hampel KE, Bechstein WO, Neuhaus P. Bile acid-independent bile flow is differentially regulated by glucagon and secretin in humans after orthotopic liver transplantation. Hepatology 26: 1272– 1281, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Liu W, Morimoto T, Woda C, Kleyman TR, Satlin LM. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol Renal Physiol 293: F227– F235, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol 289: F978– F988, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998– F1012, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 131: 911– 920, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Masyuk TV, Masyuk AI, Ritman EL, LaRusso NF. Three-dimensional reconstruction of the rat intrahepatic biliary tree: physiologic implications. In: The Pathophysiology of Biliary Epithelia, edited by Alpini G, Alvaro D, Marzioni M, Lesage G, LaRusso N. Georgetown, TX: Landes Bioscience, 2004. [Google Scholar]
- 37. Minagawa N, Nagata J, Shibao K, Masyuk AI, Gomes DA, Rodrigues MA, Lesage G, Akiba Y, Kaunitz JD, Ehrlich BE, LaRusso NF, Nathanson MH. Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile. Gastroenterology 133: 1592– 1602, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ninlawan K, O'Hara SP, Splinter PL, Yongvanit P, Kaewkes S, Surapaitoon A, LaRusso NF, Sripa B. Opisthorchis viverrini excretory/secretory products induce Toll-like receptor 4 upregulation and production of interleukin 6 and 8 in cholangiocyte. Parasitol Int 59: 616– 621, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol 321: 141– 149, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Roman RM, Bodily K, Wang Y, Raymond JR, Fitz JG. Activation of protein kinase Cα couples cell volume to membrane Cl− permeability in HTC hepatoma and Mz-ChA-1 cholangiocarcinoma cells. Hepatology 28: 1073– 1080, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Roman RM, Feranchak AP, Salter KD, Wang Y, Fitz JG. Endogenous ATP regulates Cl− secretion in cultured human and rat biliary epithelial cells. Am J Physiol Gastrointest Liver Physiol 276: G1391– G1400, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Roman RM, Wang Y, Fitz JG. Regulation of cell volume in a human biliary cell line: calcium-dependent activation of K+ and Cl− currents. Am J Physiol Gastrointest Liver Physiol 271: G239– G248, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019– 1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simpson A. Fluorescent measurement of [Ca2+]. In: Methods in Molecular Biology: Calcium Signaling Protocols, edited by Lambert D. Totowa, NJ: Humana, 1999. [Google Scholar]
- 45. Sun R, Gao P, Chen L, Ma D, Wang J, Oppenheim JJ, Zhang N. Protein kinase Cξ is required for epidermal growth factor-induced chemotaxis of human breast cancer cells. Cancer Res 65: 1433– 1441, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Ueno Y, Alpini G, Yahagi K, Kanno N, Moritoki Y, Fukushima K, Glaser S, Lesage G, Shimosegawa T. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int 23: 449– 459, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Wang Y, Roman RM, Schlenker T, Hannun YA, Raymond JR, Fitz JG. Cytosolic calcium and protein kinase Cα couple cellular metabolism to membrane K+ permeability in a human biliary cell line. J Clin Invest 99: 2890– 2897, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Woo K, Dutta AK, Patel V, Kresge C, Feranchak AP. Fluid flow induces mechanosensitive ATP release, calcium signalling and Cl− transport in biliary epithelial cells through a PKCξ-dependent pathway. J Physiol 586: 2779– 2798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210– 1215, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Zhang WK, Wang D, Duan Y, Loy MM, Chan HC, Huang P. Mechanosensitive gating of CFTR. Nat Cell Biol 12: 507– 512, 2010 [DOI] [PubMed] [Google Scholar]