Abstract
Chlorine is a reactive gas that is considered a chemical threat agent. Humans who develop acute lung injury from chlorine inhalation typically recover normal lung function; however, a subset can experience chronic airway disease. To examine pathological changes following chlorine-induced lung injury, mice were exposed to a single high dose of chlorine, and repair of the lung was analyzed at multiple times after exposure. In FVB/NJ mice, chlorine inhalation caused pronounced fibrosis of larger airways that developed by day 7 after exposure and was associated with airway hyperreactivity. In contrast, A/J mice had little or no airway fibrosis and had normal lung function at day 7. Unexposed FVB/NJ mice had less keratin 5 staining (basal cell marker) than A/J mice in large intrapulmonary airways where epithelial repair was poor and fibrosis developed after chlorine exposure. FVB/NJ mice had large areas devoid of epithelium on day 1 after exposure leading to fibroproliferative lesions on days 4 and 7. A/J mice had airways covered by squamous keratin 5-stained cells on day 1 that transitioned to a highly proliferative reparative epithelium by day 4 followed by the reappearance of ciliated and Clara cells by day 7. The data suggest that lack of basal cells in the large intrapulmonary airways and failure to effect epithelial repair at these sites are factors contributing to the development of airway fibrosis in FVB/NJ mice. The observed differences in susceptibility to chlorine-induced airway disease provide a model in which mechanisms and treatment of airway fibrosis can be investigated.
Keywords: acute lung injury, airway hyperreactivity, basal cells
chlorine is a widely used industrial chemical and is one the top ten chemicals produced by gross weight (9). Chlorine gas is considered a chemical threat agent and is a highly toxic respiratory irritant that when inhaled causes cellular injury, alveolar-capillary barrier disruption, inflammation, pulmonary edema, and, at very high levels of exposure, death (9, 21, 39, 44). Human exposure to chlorine has occurred from both intentional and accidental releases of chlorine (9, 17, 33, 41, 43). Humans exposed to high levels of chlorine develop acute lung injury, but most individuals recover from these acute effects (17, 43). However, additional follow-up studies suggest the existence of a subset of exposed individuals who experience chronic impairment of pulmonary function, which may include airway hyperreactivity, airway obstruction, or decreased residual volumes (1, 18, 23, 33). Long-term effects of chlorine inhalation have been studied in the context of acute irritant-induced asthma (2, 23). Patients who developed irritant-induced asthma as a result of chemical exposure (most commonly chlorine) had evidence of airway obstruction, fibrosis, and hyperreactivity (1, 4, 11, 37).
Because of the sporadic nature of accidental exposures, mechanistic information on airway disease caused by acute chlorine exposure in humans is difficult to obtain. Animal models in which exposure conditions and analysis of lung pathology are carefully controlled can be a useful adjunct in this regard. Previous studies have shown that chlorine inhalation in mice results in lung injury characterized by epithelial cell death, pulmonary edema, neutrophilic inflammation, and impaired lung function during the acute phase (24, 35, 39). Pathological changes during subacute and chronic phases of chlorine-induced lung injury have not been fully characterized. In the present study, mice from inbred strains exhibiting differential responses to chlorine-induced lung injury were exposed to a single high dose of chlorine. Airway injury and subsequent repair and remodeling were investigated by analyzing pulmonary function, histology, and markers for epithelial cell types at multiple times after exposure.
MATERIALS AND METHODS
Mice.
Eight-week-old male FVB/NJ and A/J mice were obtained from the Jackson Laboratory. Mice were housed for 1–2 wk and were then randomly assigned to chlorine-exposed or unexposed groups. All experiments involving animals were approved by the University of Louisville Institutional Animal Care and Use Committee and were performed in accordance with the Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals (16).
Chlorine exposure.
Mice (9–10 wk of age) were exposed to a target dose of 240 ppm-h chlorine (240 ppm for 1 h) followed by a 10-min period of airflow to purge the chamber before opening as described in our previous publications (13, 14, 39). The mean deviation between the target dose and actual values was 3.6%. This concentration of chlorine gas was chosen since our previous study showed that it resulted in acute lung injury with extensive damage to airway epithelium in FVB/NJ mice but with low acute mortality (39).
Pulmonary function and airway reactivity to methacholine.
Pulmonary function and airway reactivity to methacholine were measured by forced oscillation by using a FlexiVent system (SCIREQ, Montreal, Quebec, Canada) as described (5, 13).
Lung tissue preparation.
Lung tissues were collected at multiple times after chlorine exposure. Following euthanasia the chest was opened and lungs were fixed with 10% neutral buffered formalin at a constant pressure of 25 cm water for 10 min at room temperature. Then the lungs were removed and fixed in 10% neutral buffered formalin overnight at 4°C. The left lung and the superior, middle, and inferior lobes of the right lung were excised and embedded in paraffin. Initial studies revealed that the large intrapulmonary airways were the sites of inefficient epithelial repair and the development of airway fibrosis in FVB/NJ mice; therefore, in subsequent analysis, lobes were oriented during embedding to produce cross sections of the main lobar bronchi.
Reticulin staining.
Reticulin staining was used to assess the integrity of the epithelial basement membrane after chlorine exposure according to Gordon and Sweet's staining protocol (silver impregnation technique) with minor modifications. Tissues were deparaffinized with xylene and taken through alcohol to water followed by oxidation in acidified 0.5% potassium permanganate for 3 min. After decolorization with 2% oxalic acid for 1 min and mordanting in 4% iron alum for 10 min, tissues were impregnated in ammoniacal silver solution for 11 s, rinsed quickly in distilled water, then immediately reduced with 10% aqueous formalin for 2 min followed by washing in running tap water for 2 min. Sections were incubated with 0.2% gold chloride for 2 min, fixed with 2% aqueous sodium thiosulfate for 2 min, and counterstained with 0.1% toluidine blue for 3 min. After dehydrating quickly through 95% ethanol and two changes of 100% ethanol, slides were cleared in xylene and mounted.
Trichrome staining.
Accustain Trichrome stain (Masson) kit (Sigma-Aldrich, St. Louis, MO) was used according to the manufacturer's instructions with minor modifications. Lung tissues were deparaffinized and mordanted in Bouin's solution (Sigma-Aldrich) at room temperature overnight to intensify the final coloration. After being washed in running tap water to remove the yellow color from sections, tissues were stained in working Weigert's iron hematoxylin solution (Sigma-Aldrich) for 2 min. After the washing, tissues were stained in Biebrich scarlet-acid fuchsin for 2 min. After rinsing with deionized water, slides were treated with working phosphotungstic/phosphomolybdic acid solution for 10 min, followed by staining in aniline blue solution for 10 min. After briefly being rinsed in 1% acetic acid and water, slides were dehydrated through alcohol, cleared in xylene, and mounted.
Hydroxyproline assay.
To estimate the changes of total amount of collagen in the lungs after chlorine exposure, hydroxyproline content in mouse left lungs was measured based on published methods (22, 45). Tissues were hydrolyzed in 1 ml of 6 N HCl at 100°C overnight. After cooling to room temperature, 200 μl of hydrolysate was transferred to a 1.5-ml test tube and neutralized with 6 N NaOH to approximately pH 6. Water was added to a total volume of 600 μl. Aliquots of the samples (25 μl) were mixed with 275 μl of 0.001 N HCl. Then 375 μl of 3% chloramine T (Sigma-Aldrich, St. Louis, MO) and 50% isopropanol in 0.5 M sodium acetate (pH 6.0) were added and incubated at room temperature for 20 min, followed by the addition of 75 μl of perchloric acid (Sigma) and further incubation at room temperature for 5 min. Next 250 μl of Ehrlich's reagent (5% 4-dimethylaminobenzaldehyde in methanol) was added and allowed to incubate at 60°C for 20 min. Absorbance was measured at 560 nm, and the amount of hydroxyproline was determined against a standard curve generated by using known concentrations of hydroxyproline (Sigma-Aldrich).
Lavage fluid cell counts.
Lung lavage and counting of cells in lavage fluid were performed as described previously (39).
EdU and immunofluorescent staining.
Mice were treated with 5-ethynyl-2′-deoxyuridine (EdU) (32) at 10 mg/kg ip 17 h before euthanasia. Dual staining for EdU and epithelial cell markers was performed. Sections were deparaffinized, rehydrated, rinsed in PBS, and treated for antigen retrieval (10 mM sodium citrate, pH 6.0, containing 0.05% Tween-20 at 95°C for 30 min) for those antibodies that required this [keratin 5 (K5) and keratin 14 (K14)]. Proliferating cells were visualized by EdU staining using Click-iT EdU Imaging Kits (Invitrogen, Eugene, OR). Tissue sections were permeabilized in 0.5% Triton X-100 for 20 min followed by incubation in Click-iT reaction cocktail for 30 min at room temperature. One milliliter of Click-iT reaction cocktail contains 860 μl of 1 × Click-iT reaction buffer, 40 μl of 100 mM CuSO4, 2.5 μl of Alexa Fluor azide 488 solution, and 100 μl of 1 × Click-iT EdU buffer additive. After EdU staining and washing in 3% (wt/vol) BSA in PBS for 5 min twice, sections were incubated in blocking solution [PBS containing 1% (wt/vol) BSA, 5% (vol/vol) normal goat or donkey serum and 0.3% Triton X-100] for 30 min at room temperature. Then tissues were incubated in primary antibody for 1 h followed by secondary antibody for an additional 1 h at room temperature. The following combinations were used: Clara cell secretory protein (CCSP) antibody (1:1,000, kind gift of Dr. Gurmukh Singh, VA Medical Center, Pittsburgh, PA) with Alexa Fluor 594 donkey anti-goat IgG; acetylated tubulin (AcTub) antibody (1:20,000, cat. no. T7451, Sigma-Aldrich, St. Louis, MO) with Alexa Fluor 594 goat anti-mouse IgG; K14 antibody (1:1,000, cat. no. MS-115-P1, Thermo Fisher, Fremont, CA) with Alexa Fluor 594 goat anti-mouse IgG; and K5 antibody (1:1,000, cat. no. PRB-160P, Covance, Princeton, NJ) with Alexa Fluor 594 donkey anti-rabbit IgG. All secondary antibodies were purchased from Invitrogen (Eugene, OR) and used at a dilution of 1:500. After vigorous washing in PBS, slides were mounted with Prolong Gold Antifade Reagent with DAPI (Invitrogen) and observed by epifluorescence.
Morphometric analysis.
The extent of chlorine-induced fibroproliferative lesions, the expression of CCSP, AcTub, K5, and K14, and the volume of EdU-stained nuclei in the epithelium of lobar bronchi of FVB/NJ and A/J mice were quantified by morphometric analysis by point/intercept counting methods (15). Images containing the entire circumference of cross sections of lobar bronchi from the left lung and the inferior lobe of the right lung were captured and analyzed with ImageJ software (http://rsbweb.nih.gov/ij/). Data from the two airways was combined to generate a single value for each parameter per mouse. The volume of specific structures of interest (i) relative to airway lumen (al) surface area was calculated by using the equation VS(i,al) = (Pi)(k)/(2)(Ial), where Pi is the number of points overlying the structure of interest, k is the line length per test point, and Ial is the number of intersections of test lines with the airway lumen (15, 42). To determine the volume of subepithelial tissue in airways, images captured from trichrome-stained sections were used. To determine the volume of EdU-stained nuclei, the numbers of points that fell on EdU-labeled nuclei within the epithelium were counted. To evaluate the expression of CCSP and AcTub in the epithelium, the number of points overlying the immunofluorescent-positive staining was counted, whereas for K5 and K14 the number of points overlying both positive staining and the surrounded unstained nuclei was counted. Therefore, for CCSP and AcTub, only the volume of staining was measured, whereas for K5 and K14, the volume of expressing cells was measured. For EdU-labeled and immunostained slides, staining of interest was assessed in viable bronchial epithelium; staining in subepithelial tissue and in detached epithelial cells was not included in the analysis.
Data analysis.
Data were expressed as means ± SE. Methacholine dose-response curves were analyzed by repeated-measures ANOVA. Data from other experiments were analyzed by ANOVA with Bonferroni correction for multiple comparisons (GraphPad Prism, GraphPad Software, La Jolla, CA). If necessary, transformation (logarithmic or square root) of data was used to achieve normally distributed data. The criterion for statistical significance was set at P < 0.05.
RESULTS
Effects of chlorine inhalation on lung function and survival.
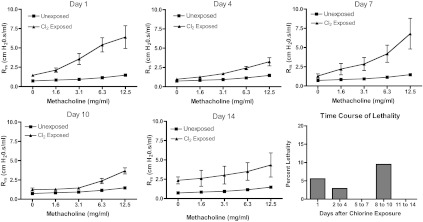
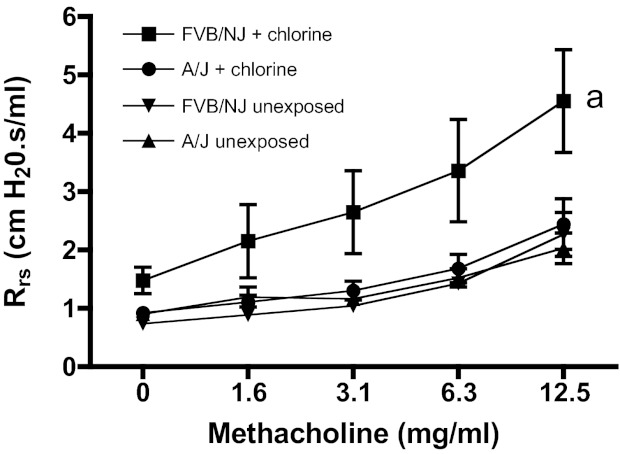
Initially lung function was measured in FVB/NJ mice at days 1, 4, 7, 10, and 14 after exposure to 240 ppm-h chlorine. The results showed that chlorine inhalation caused significantly increased baseline respiratory system resistance and airway hyperreactivity to methacholine in FVB/NJ mice one day after exposure (Fig. 1). Lung function improved by day 4 after exposure but was worse at day 7 and later times. Deaths of some chlorine-exposed animals were observed during an acute period after exposure and then during a second, delayed period 8–10 days after exposure. The elimination of these presumably more severely affected animals from the analysis possibly explains the apparent decrease in airway reactivity between days 7 and 10. No animals died between the measurements on days 4 and 7, indicating that the graphs accurately represent the increase in airway reactivity in the cohort during this time. Because previously published results indicated a different time course of airway hyperreactivity in chlorine-exposed A/J mice (40), we performed simultaneous exposures of FVB/NJ and A/J mice to determine whether they exhibited differential responses to chlorine inhalation. Chlorine exposure resulted in airway hyperreactivity in FVB/NJ mice, but not A/J mice, at day 7 after exposure (Fig. 2).
Fig. 1.
Lung function and survival in chlorine-exposed FVB/NJ mice. Mice were exposed to chlorine, and respiratory system resistance (Rrs) at baseline and in response to inhaled methacholine was analyzed at multiple times after exposure. One group of unexposed mice was measured; the same data were plotted in each graph for ease of comparison with exposed mice. Values are means ± SE (n = 3–6 mice per group); methacholine dose-response curves were significantly different (P < 0.05) between chlorine-exposed and unexposed mice at each time point. Lethality data are shown as the percentage of remaining animals that died during each time period.
Fig. 2.
Lung function in FVB/NJ and A/J mice 7 days after chlorine exposure. Mice were exposed to chlorine, and airway reactivity to inhaled methacholine was measured 7 days after exposure. Values are means ± SE (n = 6–10 mice per group); amethacholine dose-response curve for chlorine-exposed FVB/NJ mice significantly different (P < 0.05) from FVB/NJ unexposed.
Histopathological changes in the bronchi of chlorine-exposed mice.
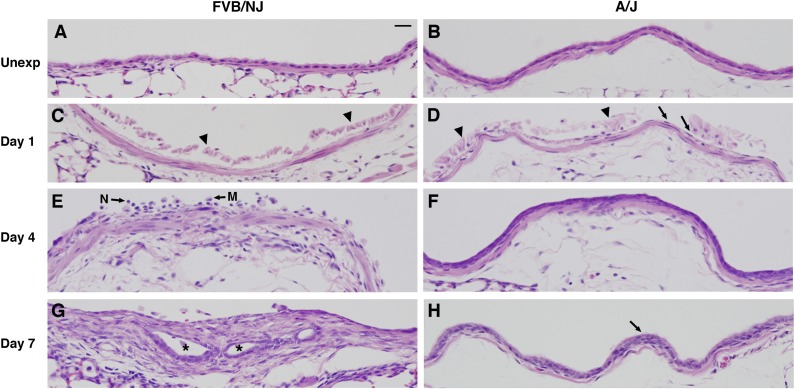
Initial histological analysis of FVB/NJ mice 7, 10, and 14 days after exposure revealed airway fibrosis localized to the distal trachea, main stem bronchi, lobar bronchi, and larger segmental airways (not shown). In some cases the development of fibrosis produced occlusion of the airways that was sufficient to prevent one or more lobes from inflating with fixative. In subsequent experiments comparing FVB/NJ and A/J mice, histological analysis was targeted to the lobar bronchi because fibrotic lesions were most pronounced and developed most reproducibly at these sites (Fig. 3). Bronchial epithelium appeared similar in unexposed FVB/NJ and A/J mice (Fig. 3, A and B). After chlorine exposure, severe injury to the airway with massive sloughing of the epithelium was observed 1 day after exposure in both FVB/NJ and A/J mice (Fig. 3, C and D). In most bronchi of FVB/NJ mice, nearly complete loss of the epithelium was observed (Fig. 3C). The bronchi of A/J mice also showed detachment of most epithelial cells, but in these mice a thin layer of flattened epithelial cells could be observed still attached to the basement membrane (Fig. 3D). In FVB/NJ mice 4 days after exposure, epithelial debris and inflammatory cells such as neutrophils and macrophages were observed in the airway lumen (Fig. 3E). At day 4 in A/J mice, a transitional regenerating epithelium was present, consisting primarily of cuboidal cells that were typically two layers thick (Fig. 3F). At day 7 after chlorine exposure, FVB/NJ mice showed widespread, pronounced fibrosis in the bronchi; in some poorly repaired areas, aberrant epithelial tubes formed within the fibroproliferative lesions (Fig. 3G). In contrast, A/J mice had little or no airway fibrosis, and at day 7 the bronchi were evenly lined primarily with a pseudostratified epithelium, although abnormal pluristratified epithelium was observed in some areas (Fig. 3H).
Fig. 3.
Histological changes in lobar bronchi of chlorine-exposed FVB/NJ and A/J mice. Mice were exposed to chlorine, and lung sections collected from mice 1, 4, and 7 days after exposure were analyzed by hematoxylin and eosin (H&E) staining. A and B are from unexposed mice (Unexp), showing normal structure of bronchi. C and D are from mice at day 1 after exposure, showing massive sloughing of bronchial epithelium in both FVB/NJ and A/J mice (arrowheads) and a thin and flattened layer of epithelial cells remaining in bronchi of A/J mice (arrows in D). E and F are from mice at day 4 after exposure, showing subepithelial thickening with inflammatory cells in the bronchial lumen of FVB/NJ mice (E) and a pluristratified, reparative epithelium in A/J mice (F). N, neutrophil; M, macrophage. G and H are from mice at day 7 after exposure, showing the development of fibroproliferative tissue with epithelial tube formation (asterisks) in FVB/NJ mice (G) and the repair of an injured bronchus by hyperplastic epithelium (arrow) in A/J mice (H). Bar in A represents 20 μm for all panels.
Reticulin staining was used to visualize the effects of chlorine exposure on the basement membrane in lobar bronchi (Fig. 4). Reticulin staining in unexposed mice revealed a dense, compact basement membrane with an additional thin layer of staining beneath the smooth muscle (Fig. 4, A and B). In chlorine-exposed FVB/NJ mice, denuded areas 1 day after exposure exhibited abnormal reticulin staining characterized by loose, disorganized basement membrane (Fig. 4C). Basement membrane structure was also disrupted by chlorine in A/J mice, but it was more compact and organized than in FVB/NJ mice (Fig. 4D). In FVB/NJ mice 4 days after exposure, fibroproliferative lesions and disorganization of the basement membrane were evident in areas of poor epithelial repair (Fig. 4E). In A/J mice, the reparative epithelium observed 4 days after exposure was associated with a basement membrane that had regained its organized structure (Fig. 4F). Fibrotic lesions in FVB/NJ mice 7 days after exposure contained a network of diffuse reticulin-stained fibers but lacked the normal densely stained basement membrane structure (Fig. 4G). In some areas, abnormal epithelial structures appeared to be growing into the airway lumen and these were associated with a densely stained basement membrane (Fig. 4H). In A/J mice 7 days after exposure, the basement membrane was repaired and appeared similar to that in the unexposed A/J mice (Fig. 4I).
Fig. 4.
Effects of chlorine inhalation on epithelial basement membrane in mouse bronchi. FVB/NJ and A/J mice were exposed to chlorine, and lung sections collected from mice 1, 4, and 7 days after exposure were analyzed by reticulin staining. A and B are from unexposed mice, showing normal structure of dense, compact basement membrane underlying the bronchial epithelium (arrowheads) and a thin layer of reticulin fibers beneath smooth muscle (arrows). C and D are from mice at day 1 after exposure, showing a loose and disorganized basement membrane and massive detachment of bronchial epithelium (arrows in C) in FVB/NJ mice (C). In A/J mice, although the basement membrane is disrupted, it is more compact and organized than in FVB/NJ mice (arrows in D) (D). E and F are from mice at day 4 after exposure, showing disorganized basement membrane in FVB/NJ mice (E) and repaired dense basement membrane in A/J mice (F). G and H are from FVB/NJ mice at day 7 after exposure, showing disorganized basement membrane, increased amount of reticulin fibers in the subepithelial area (G), and abnormal epithelial structures growing into the airway lumen (H). I is from an A/J mouse at day 7 after exposure showing repaired smooth and dense basement membrane. Bar in H represents 20 μm for all panels.
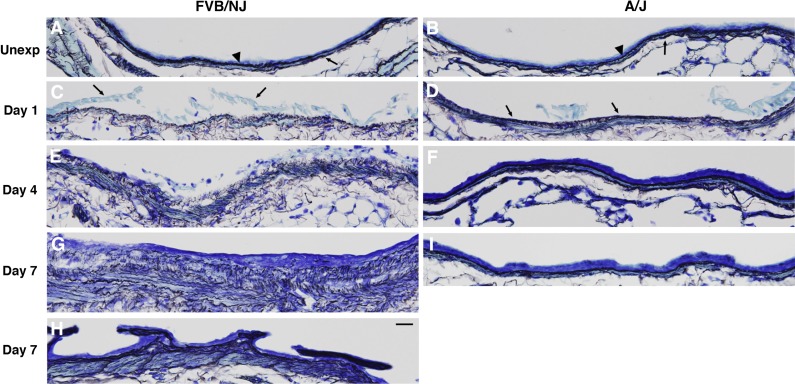
Airway collagen deposition.
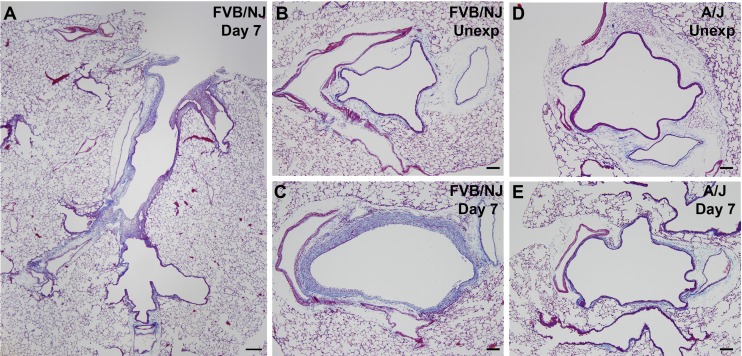
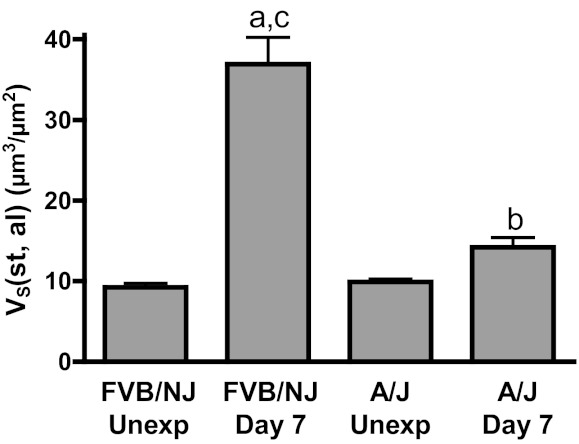
Trichrome staining of longitudinal airway sections showed dramatic fibrosis in the larger intrapulmonary airways of FVB/NJ mice and the absence of fibrosis in smaller airways (Fig. 5A). Lobar bronchi in FVB/NJ mice were consistently affected with fibrosis, and this anatomical site was targeted for subsequent analysis of airway repair and development of fibrosis. Trichrome staining of cross sections of lobar bronchi revealed extensive collagen deposition in areas of poor epithelial repair in FVB/NJ mice at day 7 following exposure compared with unexposed mice (Fig. 5, B and C). Chlorine inhalation resulted in little or no airway fibrosis in A/J mice (Fig. 5, D and E). The extent of lung pathology was quantified by morphometric analysis, which showed a modest increase in subepithelial tissue in the bronchi of A/J mice at day 7 after exposure compared with the unexposed group (Fig. 6). In FVB/NJ mice, a much larger increase in subepithelial tissue was observed, which was significantly different from unexposed mice and from chlorine-exposed A/J mice. Fibrogenic lesions in FVB/NJ mice also showed staining for α-smooth muscle actin (not shown), indicating the presence of myofibroblasts within the fibrotic tissue. To further document chlorine-induced airway fibrosis in inbred mouse strains, hydroxyproline content of left lungs was analyzed. FVB/NJ mice exhibited increased hydroxyproline content 7 days after chlorine exposure [81.0 ± 3.2 μg hydroxyproline/left lung in chlorine exposed vs. 69.2 ± 2.6 in unexposed (means ± SE, n = 5–6 mice/group, P < 0.05)], but A/J mice did not [67.6 ± 2.3 μg hydroxyproline/left lung in chlorine exposed vs. 66.6 ± 1.2 in unexposed (means ± SE, n = 5–8 mice/group, not significant)].
Fig. 5.
Trichrome staining in chlorine-exposed FVB/NJ and A/J mice. Mice were exposed to chlorine, and lung sections collected from mice 7 days after exposure were analyzed by trichrome staining to reveal collagen deposition (blue staining). Bar in A represents 200 μm and bars in B–E represent 100 μm.
Fig. 6.
Morphometric analysis of airway fibrosis. Lung sections collected from mice 7 days after chlorine exposure or from unexposed mice were evaluated by trichrome staining. The volume of subepithelial tissue normalized to airway lumen surface area [VS(st,al)] in the lobar bronchi of left lung and the inferior lobe of right lung was measured as described in materials and methods. Values are means ± SE (n = 5–8 mice per group); aP < 0.001 vs. FVB/NJ unexposed; bP < 0.05 vs. A/J unexposed; cP < 0.001 vs. A/J day 7.
Lavage fluid inflammatory cells.
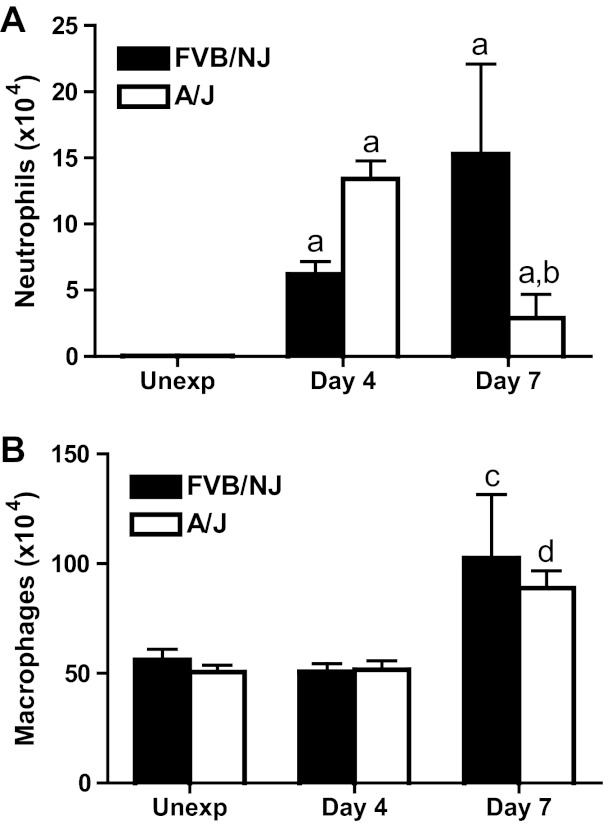
To investigate potential differences in inflammation between FVB/NJ and A/J mice, total and differential cell counts in lavage fluid 4 and 7 days after chlorine inhalation were analyzed. The number of neutrophils in lung lavage fluid from chlorine-exposed FVB/NJ and A/J mice was significantly higher than that in unexposed groups, and this neutrophil response peaked earlier in A/J mice (Fig. 7A). Chlorine exposure resulted in increased numbers of lavage fluid macrophages in both strains at 7 days after exposure, but there was no significant difference between the strains (Fig. 7B).
Fig. 7.
Neutrophils and macrophages in lung lavage fluid. Mice were exposed to chlorine, and neutrophils (A) and macrophages (B) in lung lavage fluid collected 4 and 7 days after exposure were counted. Values are means ± SE (n = 6–12 mice per group); aP < 0.001 vs. corresponding unexposed; bP < 0.05 vs. FVB/NJ day 7; cP < 0.01 vs. corresponding unexposed; dP < 0.05 vs. corresponding unexposed.
Bronchial epithelial repair in chlorine-exposed mice.
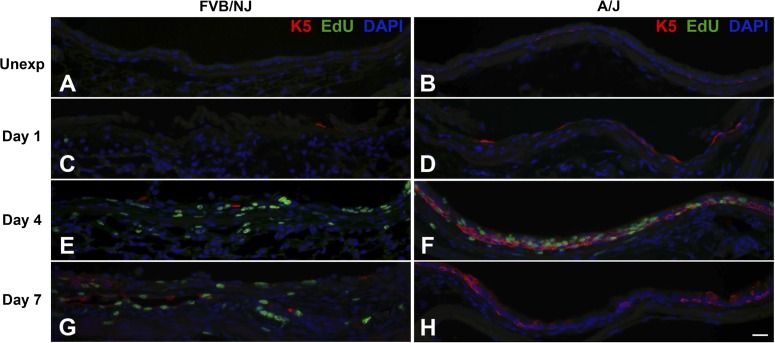
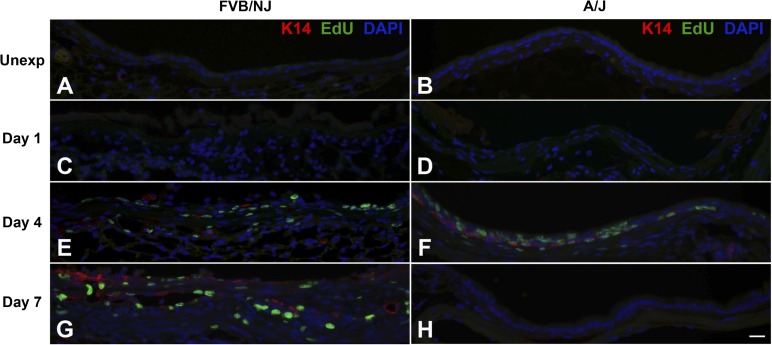
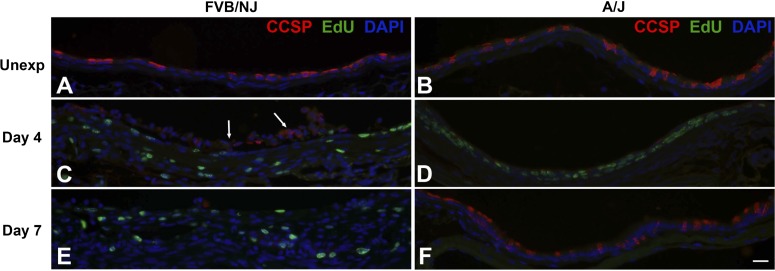
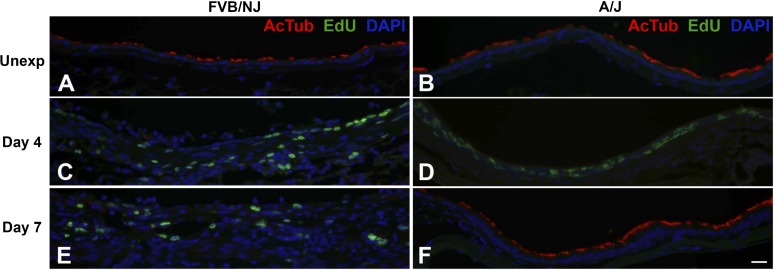
Bronchial epithelial repair after chlorine exposure was investigated by dual staining to detect EdU-labeled proliferating cells in conjunction with epithelial cell markers [K5 (Fig. 8) and K14 (Fig. 9) for basal cells, CCSP (Fig. 10) for Clara cells, and AcTub (Fig. 11) for ciliated cells]. In unexposed FVB/NJ mice, the lobar bronchi contained only a few scattered basal cells as revealed by K5 immunostaining (Fig. 8A). In contrast, unexposed A/J mice contained more abundant basal cells in lobar bronchi (Fig. 8B), although at lower density than in the trachea (not shown). Morphometric analysis confirmed a significantly higher volume of K5-expressing cells in A/J mice than in FVB/NJ mice in these airways (Fig. 12A). In unexposed FVB/NJ and A/J mice, no K14-positive cells were found in the lobar bronchi in either strain (Fig. 9, A and B). Clara (Fig. 10, A and B) and ciliated (Fig. 11, A and B) cells were numerous and distributed throughout the epithelium. There was no significant difference in the amount of staining for these markers between unexposed FVB/NJ and A/J mice (Fig. 12, C and D). In the bronchial epithelium and subepithelial area, few or no proliferating cells were observed, as indicated by staining for EdU (Figs. 8–11, A and B).
Fig. 8.
Dual fluorescence staining for 5-ethynyl-2′-deoxyuridine (EdU) and basal cell marker keratin 5 (K5). FVB/NJ and A/J mice were exposed to chlorine, and lung sections collected from mice 1, 4, and 7 days after exposure were analyzed by dual staining. Nuclei in proliferating cells are stained green (EdU staining) and K5 is stained red. Tissues were counterstained with DAPI (blue). Bar in H represents 20 μm for all panels.
Fig. 9.
Dual fluorescence staining for EdU and basal cell marker keratin 14 (K14). FVB/NJ and A/J mice were exposed to chlorine, and lung sections collected from mice 1, 4, and 7 days after exposure were analyzed by dual staining. Nuclei in proliferating cells are stained green (EdU staining) and K14 is stained red. Tissues were counterstained with DAPI (blue). Bar in H represents 20 μm for all panels.
Fig. 10.
Dual fluorescence staining for EdU and Clara cell marker Clara cell secretory protein (CCSP). FVB/NJ and A/J mice were exposed to chlorine, and lung sections collected from mice 4 and 7 days after exposure were analyzed by dual staining. Nuclei in proliferating cells are stained green (EdU staining) and CCSP is stained red. Tissues were counterstained with DAPI (blue). Arrows in C show detached epithelial cells, some of which retain CCSP immunoreactivity. Bar in F represents 20 μm for all panels.
Fig. 11.
Dual fluorescence staining for EdU and ciliated cell marker acetylated tubulin (AcTub). FVB/NJ and A/J mice were exposed to chlorine, and lung sections collected from mice 4 and 7 days after exposure were analyzed by dual staining. Nuclei in proliferating cells are stained green (EdU staining) and AcTub is stained red. Tissues were counterstained with DAPI (blue). Bar in F represents 20 μm for all panels.
Fig. 12.
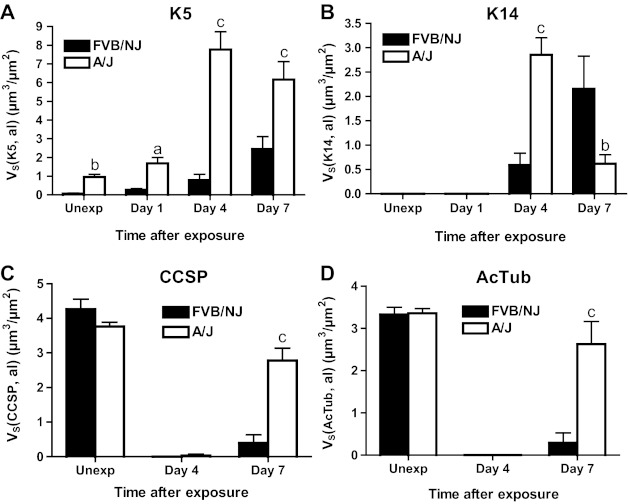
Morphometric analysis of K5, K14, CCSP, and AcTub staining in bronchial epithelium. The volume of K5- (A) and K14-expressing (B) cells and of CCSP (C) and AcTub (D) staining normalized to airway lumen (al) surface area (VS) in the bronchial epithelium of the left lung and the inferior lobe of the right lung was measured as described in materials and methods. Values are means ± SE (n = 5–8 mice per group); aP < 0.05 vs. FVB/NJ; bP < 0.01 vs. FVB/NJ; cP < 0.001 vs. FVB/NJ.
Chlorine exposure resulted in widespread death and sloughing of the epithelium observed 1 day after exposure; remaining epithelial cells, which were sparse in FVB/NJ and more abundant in A/J mice, expressed K5 (Fig. 8, C and D). The volume of K5-positive basal cells was significantly higher in A/J than FVB/NJ mice at this time (Fig. 12A). At day 1 after exposure, K14-positive cells were still not observed (Fig. 9, C and D), and proliferating cells were rare. CCSP and AcTub staining were also not observed in surviving epithelial cells at this time (not shown).
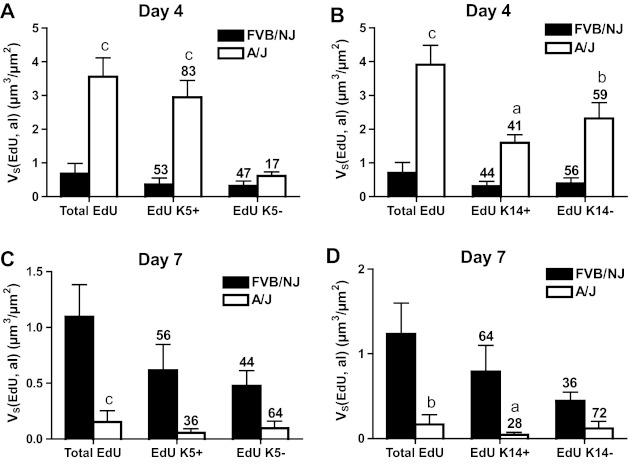
At day 4 after chlorine exposure, the abundance of K5 and K14 staining increased in A/J mice (Figs. 8F and 9F) and was higher than in FVB/NJ mice (Figs. 8E, 9E, 12A, and 12B). Moreover, significantly more EdU staining in epithelial cells was observed in A/J than FVB/NJ mice (Fig. 13A), indicating more active epithelial proliferation and regeneration in A/J mice. In FVB/NJ mice, abundant proliferating cells were observed in the subepithelial area, consistent with the fibroproliferative lesions observed by hematoxylin and eosin staining. In repairing A/J airways, proliferating cells were strongly associated with K5 staining, since 83% of EdU-stained nuclei were in K5-stained cells (Fig. 13A). In FVB/NJ airways, only 53% of EdU-stained nuclei were in K5-stained cells. Similar to day 1 after chlorine exposure, Clara (Fig. 10, C and D) and ciliated cells (Fig. 11, C and D) were scant in both FVB/NJ and A/J mice at day 4 after exposure (Fig. 12, C and D).
Fig. 13.
Morphometric analysis of EdU staining in K5- and K14-expressing cells in bronchial epithelium. The volume of EdU staining normalized to airway lumen surface area (VS) in the bronchial epithelium of the left lung and the inferior lobe of right lung was measured as described in materials and methods. Values are means ± SE (n = 5–8 mice per group). The numbers above the bars indicate the percentage of total EdU staining that was in K5+ and K5− cells (A and C) or in K14+ and K14- cells (B and D). aP < 0.05 vs. FVB/NJ; bP < 0.01 vs. FVB/NJ; cP < 0.001 vs. FVB/NJ.
At day 7 after chlorine exposure, K5 and K14 staining decreased in A/J mice compared with day 4 (Figs. 8H and 9H). This correlated with increases in CCSP (Fig. 10F) and AcTub (Fig. 11F) staining that were lower than normal but returning toward normal values (Fig. 12, C and D). In FVB/NJ mice, although few or no K5- or K14-stained cells were observed in the areas of poorly repaired airways, staining for these basal cell markers, especially K14, increased in other less severely injured areas compared with day 4 (Figs. 8G and 9G). K5- and K14-stained cells were also obvious within aberrant epithelial tubes that formed within fibrotic airways. Moreover, very little CCSP (Fig. 10E) and AcTub (Fig. 11E) staining was observed in FVB/NJ mice, and this was significantly less than in A/J mice (Fig. 12, C and D). At day 7 after exposure, epithelial cell proliferation was low in A/J mice and was higher in FVB/NJ mice (Fig. 13, C and D). In the subepithelial area in FVB/NJ mice, proliferating cells were still observed (Figs. 8G, 9G, 10E, and 11E), consistent with the continued growth of fibrotic lesions.
DISCUSSION
Chlorine is considered a chemical threat agent, and human exposures leading to lung injury have occurred as a result of industrial accidents, train derailments, and intentional releases (9, 17, 33, 41, 43). Animal models, in which lung injury and repair following chlorine exposure can be systematically examined, are useful for investigating repair mechanisms and treatments for injury (44). A hallmark of chlorine exposure that has been demonstrated in mice and rats is damage to the airway epithelium (7, 10, 21, 24, 35, 39, 40). Previous studies have shown that epithelial repair following chlorine exposure occurs over the time frame of 1–7 days (7, 10, 25, 39, 40). Chlorine exposure produces changes in lung function, including altered airway resistance, elastance, and reactivity to methacholine (7, 10, 13, 24, 26, 35, 40). These changes are complex and appear to depend on the dose of chlorine as well as the species and strain of the animal. In the present investigation, we developed a model in which the repair of injured airways was investigated in inbred mouse strains that exhibited differential responses to chlorine exposure. The study of the efficient repair process in A/J mice in contrast with the inefficient repair in FVB/NJ mice provides insights into factors that govern the restoration of lung structure and function after injury.
Chlorine inhalation in both A/J and FVB/NJ mice resulted in severe damage to the tracheobronchial epithelium, as evidenced by the detachment and sloughing of epithelium and the absence of Clara and ciliated cells. However, the subsequent response to this airway injury differed substantially between the two strains. On the day after chlorine exposure in A/J mice, damaged airways were lined with a thin layer of K5-expressing cells, indicating that surviving basal cells had spread to cover the lumen of the injured airways. By day 4 after exposure, A/J mice showed a proliferating, pluristratified epithelium that contained cells expressing basal cell markers but was still devoid of Clara and ciliated cells. A pseudostratified epithelium containing basal, Clara, and ciliated cells was observed on day 7, and proliferation had largely ceased by this time. In FVB/NJ mice, chlorine inhalation also caused severe epithelial damage, and on the day following exposure, the lumens of large airways had extensive areas devoid of epithelium. As K5 immunostaining of unexposed animals revealed significantly fewer basal cells in FVB/NJ than in A/J mice, the data suggest that the number of bronchial basal cells that survive chlorine exposure in FVB/NJ mice is insufficient to quickly recover the injured airway surface with epithelium. On days 4 and 7 after exposure, bronchial airways contained widespread patches still devoid of epithelium, and these were associated with the development of fibrotic lesions containing proliferating mesenchymal cells and collagen deposition. The fact that a period of delayed death was observed in FVB/NJ animals 7–10 days after exposure suggests that these lesions, at least in some animals, did not resolve at later times but continued to progress.
Multiple lines of evidence supported a role for basal cells in epithelial repair following chlorine inhalation, as has been shown for other agents that injure the bronchial epithelium (6, 31). First, in A/J mice 1 day after exposure, injured airways were lined with squamous epithelium expressing the basal cell marker K5. This observation suggested that basal cells were more resistant to the toxic effects of chlorine than Clara and ciliated cells, so that some basal cells survived and spread to cover the damaged airways. The location of basal cells adjacent to the basement membrane may afford some protection from inhaled irritants, which would react initially with secretory and ciliated cells that extend farther into the airway lumen. Moreover, basal cells are anchored to the basement membrane through hemidesmosome attachments, thus preventing these cells from detaching easily (8). Second, high rates of proliferation were observed in cells expressing the basal cell markers K5 and K14 in the reparative epithelium 4 days after exposure. These cells differed from normal basal cells in that they were larger, were generally cuboidal rather than pyramidal, and were not always in contact with the basement membrane. Although not formally demonstrated by lineage tracing, it is highly likely that these reparative cells are generated from basal cells because basal cells were the only epithelial cells detected at early times after exposure, the reparative cells expressed basal cell markers, and by analogy to other injury models (6, 31). Third, the most pronounced difference in airway structure between unexposed inbred strains was the higher abundance of basal cells in the bronchial airways of A/J compared with FVB/NJ mice, which suggested a mechanistic basis for the increased susceptibility of FVB/NJ mice to chlorine-induced airway fibrosis.
The results of the present study are in general agreement with previous studies investigating the role of basal cells in epithelial repair after exposure to naphthalene or sulfur dioxide (6, 31). Naphthalene is toxic to Clara cells and therefore injures airway epithelium from the trachea to the terminal bronchioles. In the trachea, concurrent loss of ciliated cells can also occur, which is postulated to be caused by loss of contacts with depleted Clara cells (6). Repair of the tracheobronchial epithelium following naphthalene injury has been shown to be carried out by basal cells, which act as facultative progenitor cells that proliferate to restore the epithelium and have the capacity to produce basal, Clara, and ciliated cells (6). Chlorine inhalation differs from naphthalene exposure in that chlorine is toxic to all cells as opposed to the restricted toxicity of naphthalene. However, both types of exposures can result in injured airways that contain predominantly basal cells (caused by resistance of basal cells to chlorine injury or by specific Clara cell injury by naphthalene coupled with consequent ciliated cell loss). For this reason, repair processes for regenerating the pseudostratified epithelium from residual basal cells in the two models may be similar. Repair of the tracheobronchial epithelium following naphthalene treatment should be more efficient, since the injured airways have a full complement of basal cells, whereas chlorine exposure may kill a proportion of these cells. Sulfur dioxide inhalation is similar to chlorine exposure in that it can damage all types of airway epithelial cells. Sulfur dioxide exposure resulted in damage and sloughing of tracheal epithelium, and lineage tracing was used to show that basal cells repaired the epithelium by the production of Clara and ciliated cells (31).
The inbred mouse strains with differential responses to chlorine inhalation represent a model in which mechanistic connections between epithelial loss, the development of fibrosis, and airway hyperreactivity can be investigated. Epithelial damage, particularly chronic injury that results in loss of basement membrane integrity, has been implicated as a trigger for the development of fibrosis (36). Loss of type II alveolar epithelial cells has been postulated as an initiating event in idiopathic pulmonary fibrosis (34). Chronic injury to bronchiolar epithelium, for example in transplanted lungs, is thought to trigger bronchiolitis obliterans (38). More definitive evidence for this concept comes from experiments in which Clara cells were ablated in adult mice by inducible expression of a diphtheria toxin transgene. Transgene induction for 10 days led to extensive Clara cell loss, and airway fibrosis was observed when lungs were examined 19 wk later (29). A similar phenomenon was observed in chlorine-exposed FVB/NJ mice, in which airways that had a poorly repaired epithelium developed fibrosis. The fibrogenic response in chlorine-exposed mice was rapid, with pronounced growth of fibrotic lesions surrounding and within the airways by 7 days after exposure. The mechanisms by which epithelial cell loss triggers fibrogenesis are unclear. Epithelial cells, either through interaction with the extracellular matrix or through secreted factors, typically repress the growth of fibroblasts (3, 27, 36). Loss of these repressive signals following epithelial damage may then allow the growth of fibroblasts to occur unchecked. Inflammatory cells, such as macrophages and neutrophils observed in airways of chlorine-exposed FVB/NJ mice, also have the capacity to secrete profibrotic cytokines and growth factors (12).
The structural changes in the airways of mice susceptible to chlorine-induced airway fibrosis were associated with alterations in lung function. FVB/NJ mice that developed airway fibrosis 7 days after chlorine exposure showed a small increase in lung resistance at baseline and a large increase in reactivity to inhaled methacholine. The timing of the delayed airway hyperreactivity coincided with the development of airway fibrosis. Theoretical models have shown that thickening of the inner airway wall or the peribronchial layer (both of which were observed in chlorine-exposed FVB/NJ mice) can lead to pronounced airway hyperreactivity, even with remodeling that produces only a modest increase in baseline resistance (28). Thus the data in chlorine-exposed FVB/NJ mice fit well with theoretical considerations regarding predicted physiological effects of the histological observations. Data from human studies suggest that related abnormalities may occur in individuals with irritant-induced asthma caused by an acute exposure to high levels of an irritant gas. A study of patients with acute irritant-induced asthma (over half of whom had disease that was attributed to chlorine exposure) found that respiratory symptoms such as wheezing and dyspnea continued to be present when assessed at a mean duration of 12 years after diagnosis (23). These patients had impaired lung function evidenced by reduced FEV1, which did not improve over the course of the follow-up period. Pathophysiological studies have suggested that chlorine exposure can result in airway fibrosis and that patients with acute irritant-induced asthma caused by chlorine exposure may have a greater extent of airway fibrosis than nonasthmatic or other types of asthmatic subjects. Subepithelial thickening indicative of airway fibrosis has been reported in multiple studies in bronchial biopsies from patients who developed irritant-induced asthma after chlorine exposure (1, 4, 11, 37). Patients with irritant-induced asthma appear to have more pronounced subepithelial thickening of the airways and less reversibility of airway obstruction with bronchodilator treatment compared with patients with other forms of occupational asthma (1, 11, 37). Subepithelial fibrosis was found to be associated with airway hyperreactivity to inhaled methacholine (1, 37). These studies are in accord with our observations that mice develop airway fibrosis and hyperreactivity following chlorine inhalation and epithelial injury.
Most victims of chlorine poisoning, even those who are exposed to high levels of the gas, recover normal lung function (17, 43). However, there appears to be a subset of exposed individuals who experience chronic impairment of pulmonary function, which may include airway hyperreactivity, airway obstruction, or decreased residual volumes (1, 18, 23, 33). These observations suggest that there are interindividual differences in responses to irritant gas exposure that are under genetic control. This concept is supported by work identifying in mice genetic differences that affect susceptibility to acute lung injury caused by inhalation of toxic industrial chemicals (19, 20, 30). Likewise, we identified differences between inbred mouse strains in their susceptibility to chlorine-induced airway fibrosis. A clear phenotypic difference between the strains was the greater abundance of basal cells in the bronchi of A/J mice, which correlated with more efficient epithelial repair, compared with FVB/NJ mice. Studies investigating the genetic basis for differential susceptibility to chemical-induced acute lung injury in inbred mouse strains have typically revealed that this phenomenon is under the control of multiple genes (19, 20, 30). Thus other genes, such as those controlling antioxidant defense, inflammatory responses, intracellular signaling, and cellular proliferation, are likely to differ between A/J and FVB/NJ mice and contribute to susceptibility to chlorine-induced lung injury. The studies presented here identify a model for investigating fibrogenesis resulting from epithelial injury and identify a novel phenotypic marker that may potentially affect recovery from chlorine-induced lung injury.
GRANTS
This work was supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD) and the National Institute of Environmental Health Sciences (NIEHS), grant numbers R21 ES020123 and U01 ES022564.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.M. and G.W.H. conceived and designed the experiments; Y.M., J.C., C.F.S., and G.W.H. performed the experiments; Y.M., J.C., C.F.S., and G.W.H. analyzed data; Y.M. and G. W. H. interpreted results of experiments; Y.M., C.F.S., and G.W.H. prepared figures; Y.M. and G.W.H. drafted the manuscript; Y.M., J.C., C.F.S., and G.W.H. edited and revised the manuscript; Y.M., J.C., C.F.S., and G.W.H. approved the final version of the manuscript.
REFERENCES
- 1. Boulet LP, Laviolette M, Turcotte H, Cartier A, Dugas M, Malo JL, Boutet M. Bronchial subepithelial fibrosis correlates with airway responsiveness to methacholine. Chest 112: 45–52, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Brooks SM, Weiss MA, Bernstein IL. Reactive airways dysfunction syndrome (RADS). Persistent asthma syndrome after high level irritant exposures. Chest 88: 376–384, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Caniggia I, Tseu I, Rolland G, Edelson J, Tanswell AK, Post M. Inhibition of fibroblast growth by epithelial cells in fetal rat lung. Am J Respir Cell Mol Biol 13: 91–98, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Chang-Yeung M, Lam S, Kennedy SM, Frew AJ. Persistent asthma after repeated exposure to high concentrations of gases in pulpmills. Am J Respir Crit Care Med 149: 1676–1680, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Chang W, Chen J, Schlueter CF, Rando RJ, Pathak YV, Hoyle GW. Inhibition of chlorine-induced lung injury by the type 4 phosphodiesterase inhibitor rolipram. Toxicol Appl Pharmacol 263: 251–258, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cole BB, Smith RW, Jenkins KM, Graham BB, Reynolds PR, Reynolds SD. Tracheal basal cells: a facultative progenitor cell pool. Am J Pathol 177: 362–376, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Demnati R, Fraser R, Ghezzo H, Martin JG, Plaa G, Malo JL. Time-course of functional and pathological changes after a single high acute inhalation of chlorine in rats. Eur Respir J 11: 922–928, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Evans MJ, Moller PC. Biology of airway basal cells. Exp Lung Res 17: 513–531, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Evans RB. Chlorine: state of the art. Lung 183: 151–167, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Fanucchi MV, Bracher A, Doran SF, Squadrito GL, Fernandez S, Postlethwait EM, Bowen L, Matalon S. Post-exposure antioxidant treatment in rats decreases airway hyperplasia and hyperreactivity due to chlorine inhalation. Am J Respir Cell Mol Biol 46: 599–606, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gautrin D, Boulet LP, Boutet M, Dugas M, Bherer L, L'Archeveque J, Laviolette M, Cote J, Malo JL. Is reactive airways dysfunction syndrome a variant of occupational asthma? J Allergy Clin Immunol 93: 12–22, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Homer RJ, Elias JA, Lee CG, Herzog E. Modern concepts on the role of inflammation in pulmonary fibrosis. Arch Pathol Lab Med 135: 780–788, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Hoyle GW, Chang W, Chen J, Schlueter CF, Rando RJ. Deviations from Haber's Law for multiple measures of acute lung injury in chlorine-exposed mice. Toxicol Sci 118: 696–703, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoyle GW, Hoyle CI, Chen J, Chang W, Williams RW, Rando RJ. Identification of triptolide, a natural diterpenoid compound, as an inhibitor of lung inflammation. Am J Physiol Lung Cell Mol Physiol 298: L830–L836, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181: 394–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Institute of Laboratory Animal Resources, Commission of Life Sciences, National Research Council Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press, 1996 [Google Scholar]
- 17. Jones RN, Hughes JM, Glindmeyer H, Weill H. Lung function after acute chlorine exposure. Am Rev Respir Dis 134: 1190–1195, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Kowitz TA, Reba RC, Parker RT, Spicer WS., Jr Effects of chlorine gas upon respiratory function. Arch Environ Health 14: 545–558, 1967 [DOI] [PubMed] [Google Scholar]
- 19. Leikauf GD, Concel VJ, Liu P, Bein K, Berndt A, Ganguly K, Jang AS, Brant KA, Dietsch M, Pope-Varsalona H, Dopico RA, Jr, Di YP, Li Q, Vuga LJ, Medvedovic M, Kaminski N, You M, Prows DR. Haplotype association mapping of acute lung injury in mice implicates activin a receptor, type 1. Am J Respir Crit Care Med 183: 1499–1509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leikauf GD, Pope-Varsalona H, Concel VJ, Liu P, Bein K, Berndt A, Martin TM, Ganguly K, Jang AS, Brant KA, Dopico RA, Jr, Upadhyay S, Di YP, Li Q, Hu Z, Vuga LJ, Medvedovic M, Kaminski N, You M, Alexander DC, McDunn JE, Prows DR, Knoell DL, Fabisiak JP. Integrative assessment of chlorine-induced acute lung injury in mice. Am J Respir Cell Mol Biol 47: 234–244, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol 295: L733–L743, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li J, Poovey HG, Rodriguez JF, Brody A, Hoyle GW. Effect of platelet-derived growth factor on the development and persistence of asbestos-induced fibroproliferative lung disease. J Environ Pathol Toxicol Oncol 23: 253–266, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Malo JL, L'Archeveque J, Castellanos L, Lavoie K, Ghezzo H, Maghni K. Long-term outcomes of acute irritant-induced asthma. Am J Respir Crit Care Med 179: 923–928, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med 168: 568–574, 2003 [DOI] [PubMed] [Google Scholar]
- 25. McGovern T, Day BJ, White CW, Powell WS, Martin JG. AEOL10150: a novel therapeutic for rescue treatment after toxic gas lung injury. Free Radic Biol Med 50: 602–608, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGovern TK, Powell WS, Day BJ, White CW, Govindaraju K, Karmouty-Quintana H, Lavoie N, Tan JJ, Martin JG. Dimethylthiourea protects against chlorine induced changes in airway function in a murine model of irritant induced asthma. Respir Res 11: 138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan T, Mason RJ, Westcott JY, Shannon JM. Rat alveolar type II cells inhibit lung fibroblast proliferation in vitro. Am J Respir Cell Mol Biol 25: 353–361, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Pare PD, McParland BE, Seow CY. Structural basis for exaggerated airway narrowing. Can J Physiol Pharmacol 85: 653–658, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Perl AK, Riethmacher D, Whitsett JA. Conditional depletion of airway progenitor cells induces peribronchiolar fibrosis. Am J Respir Crit Care Med 183: 511–521, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prows DR, Leikauf GD. Quantitative trait analysis of nickel-induced acute lung injury in mice. Am J Respir Cell Mol Biol 24: 740–746, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 106: 12771–12775, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA 105: 2415–2420, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwartz DA, Smith DD, Lakshminarayan S. The pulmonary sequelae associated with accidental inhalation of chlorine gas. Chest 97: 820–825, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 134: 136–151, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Postexposure administration of a β2-agonist decreases chlorine-induced airway hyperreactivity in mice. Am J Respir Cell Mol Biol 45: 88–94, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strieter RM. What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc Am Thorac Soc 5: 305–310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takeda N, Maghni K, Daigle S, L'Archeveque J, Castellanos L, Al-Ramli W, Malo JL, Hamid Q. Long-term pathologic consequences of acute irritant-induced asthma. J Allergy Clin Immunol 124: 975–981, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Tazelaar HD, Prop J, Nieuwenhuis P, Billingham ME, Wildevuur CR. Airway pathology in the transplanted rat lung. Transplantation 45: 864–868, 1988 [DOI] [PubMed] [Google Scholar]
- 39. Tian X, Tao H, Brisolara J, Chen J, Rando RJ, Hoyle GW. Acute lung injury induced by chlorine inhalation in C57BL/6 and FVB/N mice. Inhal Toxicol 20: 783–793, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Tuck SA, Ramos-Barbon D, Campbell H, McGovern T, Karmouty-Quintana H, Martin JG. Time course of airway remodelling after an acute chlorine gas exposure in mice. Respir Res 9: 61, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, Garbe P, Moolenaar RL. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med 27: 1–7, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weibel ER. Principles and methods for the morphometric study of the lung and other organs. Lab Invest 12: 131–155, 1963 [PubMed] [Google Scholar]
- 43. Weill H, George R, Schwarz M, Ziskind M. Late evaluation of pulmonary function after acute exposure to chlorine gas. Am Rev Respir Dis 99: 374–379, 1969 [PubMed] [Google Scholar]
- 44. White CW, Martin JG. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc 7: 257–263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93: 440–447, 1961 [DOI] [PubMed] [Google Scholar]