Abstract
Pulmonary artery endothelial cells (PAEC) isolated from fetal lambs with in utero pulmonary hypertension (IPH) have phenotypical changes that lead to increased reactive oxygen species (ROS) formation and impaired angiogenesis. AMP-activated protein kinase (AMPK) is known to be activated by ROS, which is expected to help angiogenesis in IPH-PAEC. The objectives of this study were to investigate AMPK responses in IPH and its role in angiogenesis. We observed that, compared with control PAEC, IPH-PAEC have decreased phosphorylation of AMPKα catalytic subunit and AMPK downstream enzymes, indicating a decrease in AMPK activity. In addition, the expression of AMPK kinases is decreased, and protein phosphatase 2 is increased in IPH-PAEC, potentially contributing to the decreased AMPK activation. Metformin, an AMPK activator, improved IPH-PAEC angiogenesis while increasing endothelial NO synthase (eNOS) serine1179 phosphorylation and decreasing the eNOS-caveolin-1 association. Metformin also increased MnSOD activity and the expression of both eNOS and MnSOD. The increase in angiogenesis by Metformin is abolished by pretreatment with AMPK inhibitor, Compound C. Expression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor β (PDGFβ) are decreased in IPH-PAEC compared with control PAEC and were not altered by Metformin. These data indicate that Metformin improves angiogenesis through mechanisms independent of these angiogenic factors. In conclusion, activation of AMPK restores angiogenesis and increases the bioavailability of nitric oxide in IPH. Whether Metformin is beneficial in the management of pulmonary hypertension requires further investigation.
Keywords: Metformin, endothelial nitric oxide synthase, persistent pulmonary hypertension of the newborn, MnSOD, vascular endothelial growth factor
amp-activated protein kinase (AMPK) is a serine/threonine protein kinase, and, as a sensor of cellular energy status, AMPK maintains energy homeostasis and regulates a wide range of cell functions (14, 20, 32). In mammals, AMPK is a heterotrimeric protein with three subunits (α, β, and γ) that can occur in seven different isoforms (α1, α2, β1, β2, γ1, γ2, and γ3) (19). The α1/α2-subunit is catalytically active, and its phosphorylation at threonine 172 (Thr172) is the key to activation of AMPK (21). Upon ATP depletion, AMPK is phosphorylated and activated by the upstream kinases (AMPKKs) such as liver kinase B-1 (LKB1) (40) and calcium/calmodulin-dependent protein kinase-β (CAMKII) (22, 56). LKB1 is constitutively expressed and activated in most cell types, whereas CAMKII, which is activated by intracellular calcium, is expressed only in certain cell types, including endothelial cells (42). The third AMPK phosphorylating kinase is transforming growth factor-β-activated kinase 1 (TAK1). However, the normal physiological conditions under which TAK1 phosphorylates AMPK are presently unclear. AMPK can also be activated by other calcium-dependent signaling pathways (3); activation of AMPK in response to Ca2+ fluxes provides a mechanism for cells to anticipate the increased demand for ATP. Additional mechanisms for activation of AMPK include circulating factors, such as vascular endothelial growth factor (VEGF) and sphingosine-1-phosphate (S1P) (30). Dephosphorylation by protein phosphatase 2 (PP2) inactivates AMPK (33) upon the accumulation of intracellular ATP (43).
1,1-Dimethylbiguanide (Metformin) and 5-amino-imidazole carboxamide riboside (AICAR) are two most commonly used AMPK activators. AICAR is converted intracellularly into ZMP, an analog of AMP, which directly activates AMPK (8). Metformin, on the other hand, affects multiple signaling pathways and activates AMPK via either inhibition of complex I of mitochondrial electron transfer chain (50) or inhibition of AMP deaminase (36). Although not a specific activator, Metformin is a drug that can be used in humans to activate AMPK (50). 6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-3-pyridin-4-yl-pyrrazolo(1,5-α)-pyrimidine (Compound C or Dorsomorphine), together with either AICAR or Metformin, is commonly used as an inhibitor to study the role of AMPK in multiple biological systems. However, one recent study in zebrafish demonstrated that Compound C also inhibits bone morphogenetic protein-mediated angiogenesis (57).
Pulmonary artery endothelial cells (PAEC) from fetal sheep with persistent pulmonary hypertension created by in utero ductal constriction (IPH) demonstrate a phenotype of increased reactive oxygen species (ROS) formation (13, 46). Increased NADPH-oxidase activity (46) and endothelial nitric oxide synthase (eNOS) uncoupling (28, 29) contribute to the increased ROS formation and impaired angiogenesis in IPH-PAEC. The association of increased ROS formation with impaired angiogenesis in IPH-PAEC (44, 46) is similar to the findings reported in diabetic endothelial cells (17). A recent report demonstrated that AMPK activation ameliorated the decreased MnSOD expression/activity and impaired angiogenesis in endothelial progenitor cells from type I diabetic mice (53).
Decreasing ROS formation has been shown to restore eNOS expression and function in both PAEC (45) and resistance pulmonary arteries from newborn sheep with IPH (13). Increased ROS formation has been reported to mediate AMPK activation (52, 53). AMPK signaling in endothelial cells is essential for angiogenesis under hypoxic stress (35), which is mediated via Akt activation (30). However, the effect of increased ROS on AMPK activity in IPH is unknown. Our previous reports have shown that ROS have different and distinct effects on IPH-PAEC, systemic EC, or normal PAEC (12, 44). It is possible that the relationship between ROS and AMPK activation in IPH-PAEC is also different from that in systemic vascular EC. For the present studies, we hypothesize that 1) ROS fail to increase AMPK activity due to the downregulation of upstream kinases in IPH or upregulation of the downstream phosphatases and 2) pharmacological activation of AMPK by Metformin improves eNOS function and angiogenesis in IPH-PAEC.
In this report, we demonstrate decreased expression of upstream AMPKKs and increased expression of downstream PP2s in IPH-PAEC, which may contribute to the decreased AMPK-α phosphorylation. AMPK activation by Metformin and inhibition by Compound C have reciprocal effects on the angiogenesis function of IPH-PAEC. These findings provide new insights into the mechanistic role of AMPK in PAEC angiogenesis after the establishment of IPH.
MATERIALS AND METHODS
Animals.
All animal studies were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee (IACUC) and conformed to the current guidelines of NIH for care and use of laboratory animals. IPH was induced by fetal ductus arteriosus constriction at 128 ± 2 days gestation (term gestation ≈ 145 days). Control fetal lambs received sham operation. After 8 days of ductal constriction, the ewe was euthanized and fetal lungs were removed en bloc. PAEC were isolated from pulmonary arteries with the use of 0.25% collagenase type A, and cells were grown in endothelial growth media (29). Identity of the cells was verified by staining for factor VIII antigen (23) and by acetylated-LDL uptake (51). PAEC from at least four fetal sheep each with IPH or control were used for experiments between passages 4 and 6. Passage numbers of the control and persistent pulmonary hypertension of the newborn (PPHN)-PAEC were the same for all experiments whenever indicated for comparisons. Segments of pulmonary artery obtained from each fetal sheep right after delivery were snap frozen in liquid nitrogen for later use.
Antibodies and chemicals.
Rabbit phospho-AMPK-α (1: 1,000), phospho-AMPK-β (1: 1,000), phospho-acetyl-CoA carboxylase (pACC, 1: 1,000), phospho-P70S6K (1: 1,000), phospho-S6 ribosomal protein (1: 1,000), phospho-eukaryotic initiation factor 4E-binding protein (4E-BP1, 1;1,000), phospho-eukaryotic initiation factors-4E (eIF4E, 1: 1,000), phospho-Akt, AMPK-α (1: 1,000), AMPK-β (1: 1,000), and ACC (1: 1,000) antibodies were from Cell Signaling Technology (Beverly, MA). Rabbit phospho-eNOSSer1179 (1: 1,000), eNOS (1: 1,000), HSP90 (1: 1,000), LKB1 (1: 500), CAMKII (1: 500), PP2A-Cα/β (1: 500), PP2-Cα/β (1: 500), peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC-1, 1: 500) antibodies, and mouse VEGF antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit platelet-derived growth factor-β (PDGF-β) antibody and mouse caveolin-1 antibody were from Novus Biologicals (Littleton, CO). MitoSOX, dihydroethidium (DHE), and 4-amino-5-methylamino-2′, 7′-difluorofluoresceine diacetate (DAF-FM-DA) were from Invitrogen (Carlsbad, CA). Growth-factor-reduced Matrigel was from BD Biosciences (Belford, MA). Metformin and AICAR were from Sigma (St. Louis, MO). Compound C was from Chemdea (Ridgewood, NJ).
Drug treatment, immunoprecipitation, and immunoblotting.
PAEC were cultured in high-glucose DMEM supplemented with L-glutamine, 20% FCS, and 1% antibiotic/antimitotic in a humidified incubator at 37°C, 5% CO2 and 95% room air. Metformin (5 × 10−6-5 × 10−4 M) was added to the culture medium for either 14 h or 48 h when the cells were at 70% confluence. Metformin and culture medium were replaced every 12 h. Cells were scraped into MOPS buffer (20 mM 3-N-morpholino-propanesulfonic acid, 2 mM EGTA, 5 mM EDTA, 30 mM NaF, 10 mM β-glycerophosphate, 10 mM Na pyrophosphate, 2 mM Na orthovanadate, 1 mM PMSF, 0.5% NP-40, 1% protease inhibitor cocktail, and 1% phosphatase inhibitor cocktails 2 and 3, pH 7.0) to obtain cell lysates. Cell lysates (250 μg) were immunoprecipitated with rabbit eNOS antibody (1 μg) or nonspecific rabbit IgG (1 μg) as negative control. Segments of pulmonary artery, from four IPH and four control sheep each, were homogenized in MOPS buffer with protease and phosphatase inhibitors and then sonicated (550 Sonic Dismembrator; Fisher Scientific, Waltham, MA) for 30 s with 15% output, twice on ice to obtain tissue homogenate. For immunoblots analyses, 30 μg of cellular protein was resolved by SDS-PAGE, transferred to nitrocellulose membranes, and probed with appropriate antibodies for overnight in 4°C. Signals were generated after incubation with horseradish peroxidase-conjugated goat anti-rabbit (1: 10,000), or anti-mouse (1;10,000), IgG using SuperSignal West Pico (Pierce, Rockford, IL), and recorded on CL-Xposure films (Pierce). The integrated optical density was processed by Image J, and β-actin was used as loading control.
In vitro angiogenic activities.
In vitro angiogenesis was studied as we previously reported (46, 47).
Tube formation assay.
PAEC (4 × 104/well) suspended in 200 μl of culture medium (2% FCS) were plated onto individual wells containing Matrigel with/without Metformin or AICAR. Compound C (10−5 M) was added 30 min before the addition of AMPK activator to study the role of AMPK in tube formation. Capillary-like structures were measured 6 h after plating by one of the coauthors blinded to the treatment under ×10 (objective) magnification.
Scratch recovery assay.
PAEC were grown to confluence in 12-well plates. Scratch lines were created by 1-ml pipette tip, and then the wells were gently rinsed with Hanks's Balanced Salt Solution to remove the detached cells. The cells were serum starved in DMEM with 2% FCS for 60 min, and then the medium was changed back to DMEM with 20% FCS and different concentrations of Metformin for 24 h. The distance of the gap between the frontlines of recovery was measured for comparisons.
Compound C was used to study the mechanism by which Metformin affects the scratch assay. After the scratch was created, Compound C (10−5 M) was added during serum starvation for 60 min. The medium was then changed back to DMEM with 20% FCS, with/without Compound C (10−5 M) and/or Metformin (5 × 10−4 M). Due to the toxic effect of Compound C on IPH-PAEC, we decided to observe the gaps after 6 h.
Measurement of MnSOD activity.
PAEC were grown in six-well plates to 90% confluence and then were treated with Metformin for 14 h. Cells were trypsinized and pelleted by centrifugation at 3,500 revolution/min for 10 min at 4°C. Cell pellets were homogenized or sonicated in cold 20 mM HEPES buffer (1 mM EGTA, 210 mM mannitol, and 70 mM sucrose, pH 7.2) with 15% power output for 10 s, and cell debris was removed by centrifugation at 13,000 revolution/min, 4°C, for 10 min. MnSOD-specific activity was measured after adding 1 mM potassium cyanide (KCN) to the lysates to block both cytosolic Cu,Zn-SOD and extracellular SOD by a colorimetric assay kit (Cayman Chemical, Ann Arbor, MI) that detects the conversion of tetrazolium salt to formazan dye by superoxide (O2−) generated by xanthine and xanthine oxidase. SOD activity was measured by the ability of each sample to scavenge O2− and prevent the formation of formazan dye. Absorbance was read at 440–460 nm using a microplate reader (FLUOstar Omega; BMG LABTECH, Durham, NC). One unit (U) SOD activity was defined as the amount of enzyme needed to exhibit 50% dismutation of the O2·−. The protein concentration in each well was measured by BCA method to calculate the MnSOD activity per milligram of protein (U/mg protein).
Evaluation of NO production in PAEC.
NO production was quantified using DAF-FM-DA (Molecular Probes, Eugene, OR) fluorescence as we previously described (45). PAEC were grown to ∼60% confluence and were then treated with/without Metformin for 14 h. The medium was then replaced with HBSS containing L-arginine (25 μM) and DAF-FM-DA (5 μM), and PAEC were incubated at 37°C for 30 min. The DAF fluorescence was imaged under a fluorescence microscope (Ex 495/Em 515 nm) after fixation with 4% formalin. Epifluorescence was quantified using MetaView software and expressed as relative light units.
Evaluation of ROS production in PAEC.
Mitochondrial O2− formation was studied using fluorescence for mitochondrial-targeted dihydroethidine (MitoSOX Red, Molecular Probes) (36), whereas total cell O2− formation was studied using DHE fluorescence. PAECs were incubated with/without Metformin for 14 h at ∼60% confluence. MitoSOX Red (5 μM) was added for 15 min at 37°C, and the cells were washed twice with HBSS followed by 4% formalin fixation. Fluorescence was imaged with excitation and emission at 510 and 590 nm, respectively. Epifluorescence was quantified using MetaView software and expressed as relative light units .
AMPK-α knockdown.
SiRNAs against bovine AMPK-α1 (sc-270395, Santa Cruz Biotechnology) and ovine AMPKα2 (sc-270396, Santa Cruz Biotechnology) for transfection were used according to the Lipofectamine RNAiMAX Transfection Reagent (Invitrogen) protocol. SiRNA (30 pM), or scramble siRNA, was mixed with 500 μl Opti-MEM-I reduced serum medium in six-well plates, and then Lipofectamine RNAiMAX (5 μl) was added and incubated for 20 min at room temperature. IPH-PAEC (1.5 × 105) suspended in antibiotic-free culture medium was added into the siRNA mixture for 72 h at 37°C in a humidified CO2 incubator. AMPK-α expression was assessed by immunoblotting, and angiogenesis was studied by tube formation assay and monolayer scratch recovery assay.
Statistical analysis.
Data are shown as means ± SE. Student's t-test or Mann-Whitney U-test were used for comparing two groups wherever appropriate. One-way ANOVA followed by Student-Newman-Keuls post hoc test was used for comparisons among more than two groups. A P value <0.05 was considered statistically significant.
RESULTS
AMPK activation is decreased in IPH-PAEC.
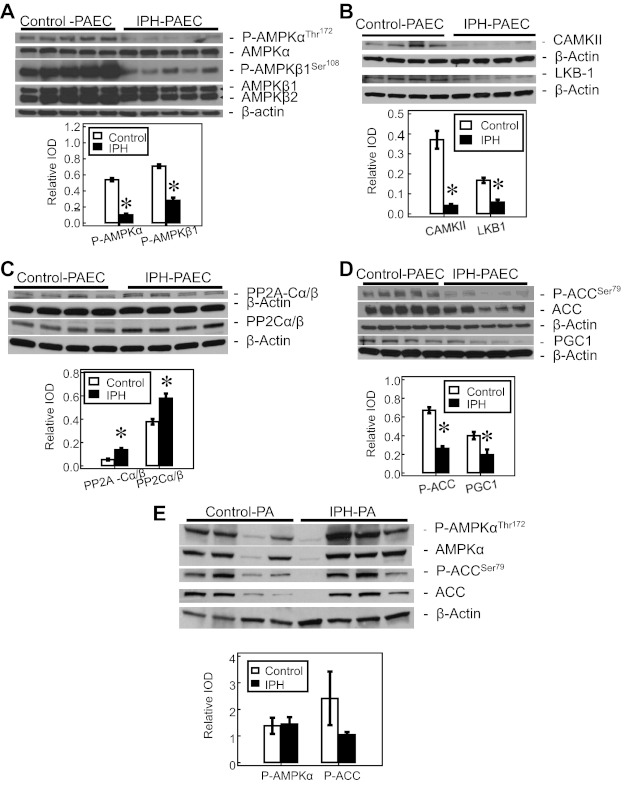
The expression of AMPK-α and AMPK-β subunits was similar between IPH-PAEC and control PAEC. However, phospho-AMPK-αThr172 and phospho-AMPK-β1Ser108 are both decreased in IPH-PAEC compared with control PAEC (Fig. 1A). Phosphorylation of Thr172 in the catalytic site of AMPK is required for AMPK activity, and dephosphorylation by PP2 leads to AMPK inactivation (33). The expression of two AMPK kinases (AMPKK), CAMKII and LKB1, is decreased in IPH-PAEC compared with control PAEC (Fig. 1B). Expression of PP2A-Cα/β and PP2-Cα/β is increased in IPH-PAEC (Fig. 1C). These findings together indicate that IPH-PAEC acquire a phenotype with decreased AMPK activation.
Fig. 1.
AMP-activated protein kinase (AMPK) phosphorylation is decreased in pulmonary artery endothelial cells (PAEC) with in utero pulmonary hypertension (IPH). A: there is no difference in the expression of total AMPK-α and AMPK-β, but the phosphorylation of both AMPK-α (n = 5, P < 0.05) and -β (n = 5, P < 0.05) subunits is decreased in IPH-PAEC compared with control PAEC. B: protein levels of AMPK kinases, liver kinase B-1 (LKB1, n = 4, P < 0.05), and calcium/calmodulin dependent protein kinase-β (CAMKII, n = 4, P < 0.05) are decreased in IPH-PAEC compared with control PAEC. C: expression of 2 types of protein phosphatase 2 (PP2), PP2A-Cα/β (n = 4, P < 0.05) and PP2-Cα/β (n = 4, P < 0.05), is increased in IPH-PAEC. D: expression of AMPK downstream enzymes, acetyl-CoA carboxylase (ACC), phospho-ACC (n = 5, P < 0.05), and peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC-1; 0.43 ± 0.05 vs. 0.20 ± 0.06, n = 4, P < 0.05) are decreased in IPH-PAEC. E: AMPK-α Thr172 phosphorylation, on the contrary, are not decreased in IPH pulmonary arteries (n = 4, P = 0.92). ACC Ser79 phosphorylation is also not significantly different in IPH-PA (n = 4, P = 0.12). *P < 0.05 compared with control PAEC. IOD, integrated optical density.
AMPK activation leads to phosphorylation of several downstream proteins including ACC, which is involved in lipid metabolism (32). AMPK phosphorylates and inactivates ACC; thus ACC phosphorylation has been commonly used as an indicator for AMPK activation (18). We observed that both ACC (1.36 ± 0.14 for IPH-PAEC vs. 1.76 ± 0.06 for control PAEC; n = 5, P < 0.05) and phospho-ACC (0.37 ± 0.07 for IPH-PAEC vs. 1.18 ± 0.08 for control PAEC; n = 5, P < 0.05) levels were decreased in IPH-PAEC. Decrease in ACC phosphorylation in IPH-PAEC (Fig. 1D) supports the concept that AMPK activation was decreased in IPH-PAEC. Another downstream enzyme for AMPK is PGC-1, which is the master regulator that controls biogenesis of mitochondria (4, 31, 38). The expression of PGC-1 was decreased in IPH-PAEC compared with control PAEC (Fig. 1D), providing further evidence that AMPK activity is decreased in IPH-PAEC.
AMPK-α expression in pulmonary artery homogenates was quite variable among different animals with no significant difference between IPH and control (0.80 ± 0.28 for IPH vs. 0.86 ± 0.28 for control; n = 4, P = 0.90). Similarly no difference was seen in AMPK-α Thr172 phosphorylation between IPH and control PA (Fig. 1E). There were also no differences in the levels of ACC and phospho-ACC between the two groups (n = 4, P = 0.64). These discrepant findings between PAEC and PA suggest that the phenotypical changes of IPH are limited to PAEC in our model.
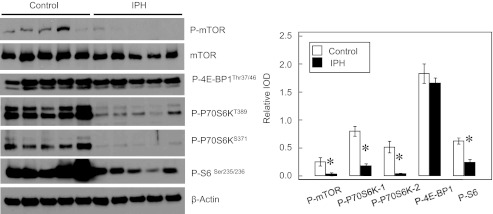
Protein synthesis factors are decreased in IPH-PAEC.
Phosphorylation of mammalian target of rapamycin (mTOR) is known to promote protein synthesis and cell growth through phosphorylation of its downstream factors including P70S6K and S6 ribosomal protein (1). EIF4E is a factor that controls the initiation of translation (27). AMPK activation is known to attenuate S6K1, 4E-BP1, and eEF2 signaling responses in stimulated skeletal muscle, resulting in decreased protein synthesis (11, 48), which may impair endothelial function. IPH-PAEC show decreased phosphorylation of mTOR, P70S6K, and S6 ribosomal protein (Fig. 2) compared with control PAEC, suggesting decreased protein synthesis activity in IPH-PAEC. However, no significant differences in eIF4E, Akt, phosphor-Akt, or phospho-4E-BP1 phosphorylation were observed between control and IPH-PAEC (data not shown). These results indicate that the observed difference in AMPK activation between control and IPH PAEC may not be mediated by mTOR/p70S6K pathway.
Fig. 2.
Protein synthesis factors are decreased in IPH-PAEC. Phosphorylation of mammalian target of rapamycin (mTOR) (n = 5, P < 0.05), P70S6KT389 (n = 5, P < 0.05), P70S6KS371 (n = 5, P < 0.05), and S6Ser235/236 (n = 5, P < 0.05) are significantly lower in IPH-PAEC compared with control PAEC, indicating that higher AMPK activity in control PAEC does not affect protein synthesis negatively. *P < 0.05 compared with control PAEC.
AMPK activation improves the in vitro angiogenesis of IPH-PAEC.
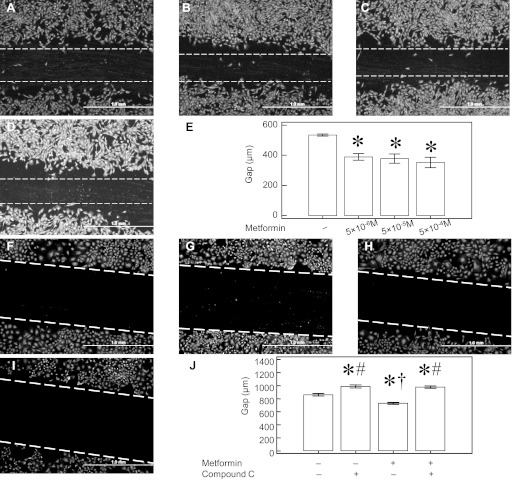
We previously reported that IPH-PAEC show impaired angiogenesis. In the present study, we used AICAR and Metformin to activate AMPK in IPH-PAEC. AICAR (10−4 M) increased tube formation by 5.7 ± 0.4-fold (P < 0.05, n = 7), whereas Metformin (10−3 M) increased tube formation by 2.0 ± 0.2-fold (P < 0.05, n = 7). We focused our studies on Metformin because it has been clinically used in diabetic patients for several decades with therapeutic serum levels between 5 × 10−6-5 × 10−5 M. Plasma levels of Metformin can be 10-fold higher (5 × 10−4 M) in some animals treated with the same doses (56). Three concentrations (5 × 10−6, 5 × 10−5, and 5 × 10−4 M) were therefore used in our tube formation assay, and all three concentrations increased IPH-PAEC tube formation (Fig. 3, A–E). We used Compound C to study the role of AMPK activation by Metformin in tube formation. Compound C (10−5 M, Fig. 3G) abolished IPH-PAEC (Fig. 3F) tube formation. Although Metformin (5 × 10−4 M) increased IPH-PAEC tube formation (Fig. 3H), pretreatment with Compound C completely blocked the stimulation of tube formation by Metformin (Fig. 3, I and J). Metformin (5 × 10−4 M) treatment did not affect tube formation (106.7 ± 4.4%) in control PAEC. Compound C decreased control PAEC tube formation (16.2 ± 3.3%, P < 0.05, n = 4), even in the presence of Metformin (43.7 ± 4.5%, P < 0.05, n = 4).
Fig. 3.
Metformin increases IPH-PAEC tube formation through AMPK activation. IPH-PAEC (A) have impaired tube formation, whereas treatment with Metformin at 5 × 10−6 M (B), 5 × 10−5 M (C), and 5 × 10−4 M (D) significantly increases the tube formation (E). 6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-3-pyridin-4-yl-pyrrazolo(1,5-a)-pyrimidine (Compound C) is an AMPK inhibitor. IPH-PAEC (F) treated with Compound C (10−5 M) form no tubes (G). Although 5 × 10−4 M of Metformin alone increases tube formation in IPH-PAEC (H), there is no tube formation when Compound C (10−5 M) is added together with Metformin (5 × 10−4 M) (I and J). *P < 0.05 compared with untreated IPH-PAEC. ‡P < 0.05 compared with IPH-PAEC treated with 5 × 10−6 M Metformin. #P < 0.05 compared with IPH-PAEC treated with 5 × 10−5 M Metformin. †P < 0.05 compared with IPH-PAEC treated with 5 × 10−4 M Metformin. ¶P < 0.05 compared with IPH-PAEC treated with 10−5 M Compound C. §P < 0.05 compared with IPH-PAEC treated with only 5 × 10−4 M Metformin.
We also used scratch recovery assay to further determine the effects of Metformin and Compound C on IPH-PAEC angiogenesis. The gaps after 24 h in IPH-PAEC without treatment were 534.5 ± 7.3 μm (Fig. 4A). The gaps decreased to 389.1 ± 22.0 (Fig. 4B), 378.2 ± 30.6 (Fig. 4C), and 352.7 ± 33.9 μm (Fig. 4D) after treatment with Metformin at 5 × 10−6, 5 × 10−5, and 5 × 10−4 M, respectively. All three concentrations improved the scratch recovery (P < 0.05, n = 5, Fig. 4E). The gaps after 6 h were 861.1 ± 17.9 μm (Fig. 3F) and increased to 988.9 ± 23.3 μm (Fig. 4G) with Compound C pretreatment. The gaps decreased to 729.6 ± 14.4 μm (Fig. 4H) with Metformin at 5 × 10−4 M but increased to 975.9 ± 21.2 μm (Fig. 4I) when Metformin was applied after Compound C pretreatment. Compound C inhibited IPH-PAEC scratch recovery with or without the presence of Metformin (P < 0.05, n = 12, Fig. 4J)
Fig. 4.
Metformin improves IPH-PAEC scratch recovery through AMPK activation. The gaps after 24 h of recovery (A) decreased in the presence of Metformin 5 × 10−6 M (B), 5 × 10−5 M (C), and 5 × 10−4 M (D). All 3 concentrations of Metformin improved the scratch recovery, but no difference was seen among the 3 treatment groups (E). The effect of Compound C on scratch recovery was obtained 6 h after the scratch. Compared with the untreated IPH-PAEC (F), the gaps were increased after Compound C treatment (G). Metformin treatment decreased the gaps (H), but this effect was abolished by Compound C (I). Compound C inhibited scratch recovery in the presence/absence of Metformin (J). *P < 0.05 compared with untreated IPH-PAEC. #P < 0.05 compared with Metformin-only-treated IPH-PAEC. †P < 0.05 compared with Compound C-treated IPH-PAEC.
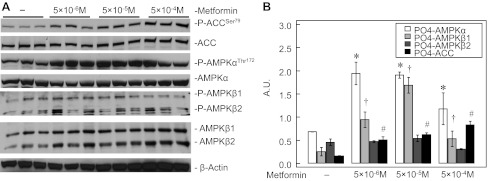
Metformin activates IPH-PAEC AMPK.
Metformin treatment for 14 h increased the phosphorylation of AMPK-α (P < 0.05, n = 3) and AMPK-β1 (P < 0.05, n = 3) in IPH PAEC at all three concentrations. Metformin also increased the phosphorylation of ACC (P < 0.05, n = 3, Fig. 5). These results support the observation that Metformin activates AMPK in IPH-PAEC.
Fig. 5.
Metformin activates AMPK in IPH-PAEC. Metformin increased AMPK-α phosphorylation at Thr172, AMPK-β1 phosphorylation at Ser108, and ACC phosphorylation at Ser79 at 14 h, and the increased phosphorylation occurred at all 3 concentrations (5 × 10−6, 5 × 10−5, and 5 × 10−4 M) of Metformin (B). *P < 0.05 compared with nontreated IPH-PAEC for AMPK-α. †P < 0.05 compared with nontreated IPH-PAEC for AMPK-β1. #P < 0.05 compared with nontreated IPH-PAEC for ACC.
AMPK activation increases IPH-PAEC eNOS activity.
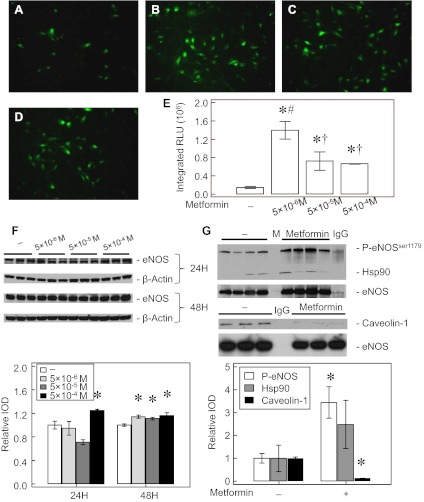
Intrauterine pulmonary hypertension is known to increase ROS formation in PAEC due to eNOS uncoupling (28). Providing antioxidants (46) or supplementing BH4 to IPH-PAEC recouples eNOS with improved angiogenesis (45). Metformin increased the DAF-FM-DA epifluorescence (probe for NO) at all three concentrations, with the highest signal seen at 5 × 10−6 M (Fig. 6, A–E). We investigated whether increased expression of eNOS contributes to the increased NO levels after Metformin treatment. After 24-h treatment, eNOS expression increased only at 5 × 10−4 M concentration of Metformin. However, all three concentrations of Metformin increased eNOS expression after 48 h by ∼15%, (Fig. 6F). We also studied the association of eNOS with two key proteins known to modulate eNOS activity, hsp90 (29) and caveolin-1 (15, 25). Metformin (at 5 × 10−4 M) treatment for 14 h tended to increase eNOS-hsp90 association, but this effect did not reach the statistical level of significance. However, treatment with the same concentration of Metformin significantly decreased eNOS-caveolin-1 association and increased Ser1179 phosphorylation (Fig. 6G). These results indicate that Metformin improves eNOS expression and coupling in IPH-PAEC, which may account for the increased NO formation observed in Metformin-treated IPH-PAEC. The small increase in eNOS expression may be less important than eNOS recoupling after Metformin treatment.
Fig. 6.
Metformin improves endothelial nitric oxide synthase (eNOS) activity in IPH-PAEC. 4-Amino-5-methylamino-2′, 7′-difluorofluoresceine diacetate (DAF-DM-DA) epifluorescence was used to quantify NO levels. The basal signal for NO was very low in IPH-PAEC (A). Metformin treatments for 14 h at 5 × 10−6 M (B), 5 × 10−5 M (C), and 5 × 10−4 M (D) increased the epifluorescence, and the strongest signal observed was with 5 × 10−6 M of Metformin (E). The expression of eNOS did not change with low concentration of Metformin after 24-h treatment, except at 5 × 10−4 M, but by 48 h all 3 concentrations of Metformin increased eNOS expression (F). Ser1179 phosphorylation, hsp90 association, and caveolin-1 association of eNOS were also examined after Metformin (5 × 10−4 M) treatment for 14 h. eNOS Ser1179 phosphorylation increased, whereas eNOS-caveolin-1 association decreased, but no significant increases in eNOS-hsp90 association were observed (G). M, molecular weight marker; IgG, nonspecific IgG; RLU, relative light units. *P < 0.05 compared with nontreated IPH-PAEC. #P < 0.05 compared with high-concentration Metformin-treated IPH-PAEC. †P < 0.05 compared with low-concentration Metformin-treated IPH-PAEC.
AMPK activation increases IPH-PAEC MnSOD activity.
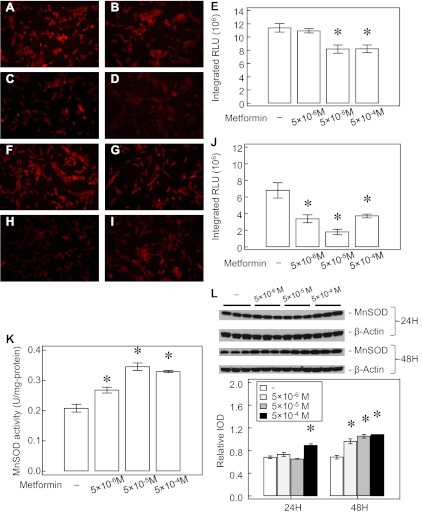
AMPK is known to regulate the antioxidant status of vascular endothelial cells including MnSOD expression (7, 53). Because MnSOD regulates mitochondrial superoxide levels, we assessed the potential effects of Metformin on this source of ROS. We used MitoSOX fluorescence to study the effect of Metformin on ROS formation in the mitochondria. Metformin at 5 × 10−5 and 5 × 10−4 M for 14 h decreased MitoSOX epifluorescence (∼30%) in IPH-PAEC (n = 10, P < 0.05, Fig. 7, A–E). We also tested the effects of Metformin on total cell ROS formation using DHE fluorescence. We observed that all three Metformin concentrations decrease the DHE signal (∼50%, n = 10, P < 0.05), especially at 5 × 10−5 M (∼75%, Fig. 7, F–J). We also measured the MnSOD-specific activity in PAEC after blocking Cu, Zn-SOD, and ECSOD activities by KCN. All three concentrations of Metformin increased MnSOD activity in IPH-PAEC by ∼20–50% (P < 0.05, n = 3, Fig. 7K). The expression of MnSOD in IPH-PAEC increased after 24 h, only at the highest concentration (5 × 10−4 M) of Metformin treatment. All three concentrations of Metformin increased MnSOD expression (Fig. 7L) after 48 h. Our results indicate that Metformin attenuates ROS formation by increasing MnSOD expression and activity in IPH-PAEC, and eNOS recoupling probably also contributes to the reduction in whole cell ROS formation (28, 45).
Fig. 7.
Metformin decreases superoxide formation in IPH-PAEC. MitoSOX fluorescence for IPH-PAEC (A) decreased as Metformin concentration increased. No change in epifluorescence was seen at 5 × 10−6 M Metformin (B) for 14 h, but the epifluorescence decreased at 5 × 10−5 M (C) and 5 × 10−4 M (D) of Metformin (P < 0.05, E). Dihydroethidium (DHE) staining for total cell superoxide levels (F) was decreased by Metformin in IPH-PAEC (P < 0.05, n = 10, G–J). MnSOD activity increased with Metformin treatment for 24 h (K). The expression of MnSOD did not change at the lower concentrations of Metformin after 24-h treatment but increased at the highest concentration of 5 × 10−4 M (P < 0.05, n = 3). By 48 h, all 3 concentrations increased MnSOD expression (P < 0.05, n = 3, L). *P < 0.05 compared with nontreated IPH-PAEC.
AMPK activation by Metformin does not involve the increased expression of angiogenic factors in IPH-PAEC.
Both VEGF and PDGF are known to facilitate endothelial growth and angiogenesis (30). We first compared the expressions of VEGF and PDGF-β in control and IPH-PAEC and found that both angiogenic factors were decreased in IPH-PAEC (Fig. 8A). Although Metformin increases angiogenesis in IPH-PAEC, we did not see any changes in VEGF (Fig. 8B) or PDGF expression (Fig. 8C) after 24- or 48-h treatment with Metformin.
Fig. 8.
Metformin does not alter the expression of angiogenic factors in IPH-PAEC. The expression of vascular endothelial growth factor (VEGF, P < 0.05, n = 4) and platelet-derived growth factor-β (PDGF-β, P < 0.05, n = 4) were decreased in IPH-PAEC compared with control PAEC (A). The expression of VEGF (B) and PDGF-β (C) were not changed after Metformin treatment for either 24 h or 48 h. *P < 0.05 compared with control PAEC.
AMPK activation by Metformin does not affect the factors that modulate protein synthesis in IPH-PAEC.
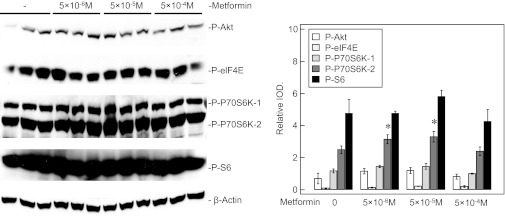
Protein synthesis is vital to endothelial cell functions and angiogenesis. VEGF enhances angiogenesis partly through Akt and enhances protein synthesis through increasing the phosphorylation of mTOR and P70S6K (41). We observed decreased phosphorylation of mTOR, P70S6K, and S6 in IPH-PAEC compared with control PAEC. We investigated the potential decrease in phosphorylation of these protein synthesis factors and impaired angiogenesis in response to AMPK activation by Metformin (11, 48). We studied IPH-PAEC treated with different concentrations of Metformin for 24 h for phosphorylation of Akt, eIF4E, P70S6K, and S6. We did not observe changes in phosphorylation in any of these proteins with the exception of an increase in P70S6K phosphorylation at low concentrations of Metformin (Fig. 9). These results indicate that Metformin does not affect protein synthesis factors in IPH-PAEC.
Fig. 9.
Metformin does not affect protein synthesis factors in IPH-PAEC. IPH-PAEC was treated with Metformin (5 × 10−6, 5 × 10−5, and 5 × 10−4 M) for 24 h. The phosphorylation of Akt, eukaryotic initiation factors-4E (eIF4E), and S6 did not change after treatment. Phosphorylation of P70S6K-2 increased with Metformin at 5 × 10−6 and 5 × 10−5 M but was not associated with increased S6 phosphorylation.
AMPK-α1 knockdown impairs IPH-PAEC response to metformin in angiogenesis.
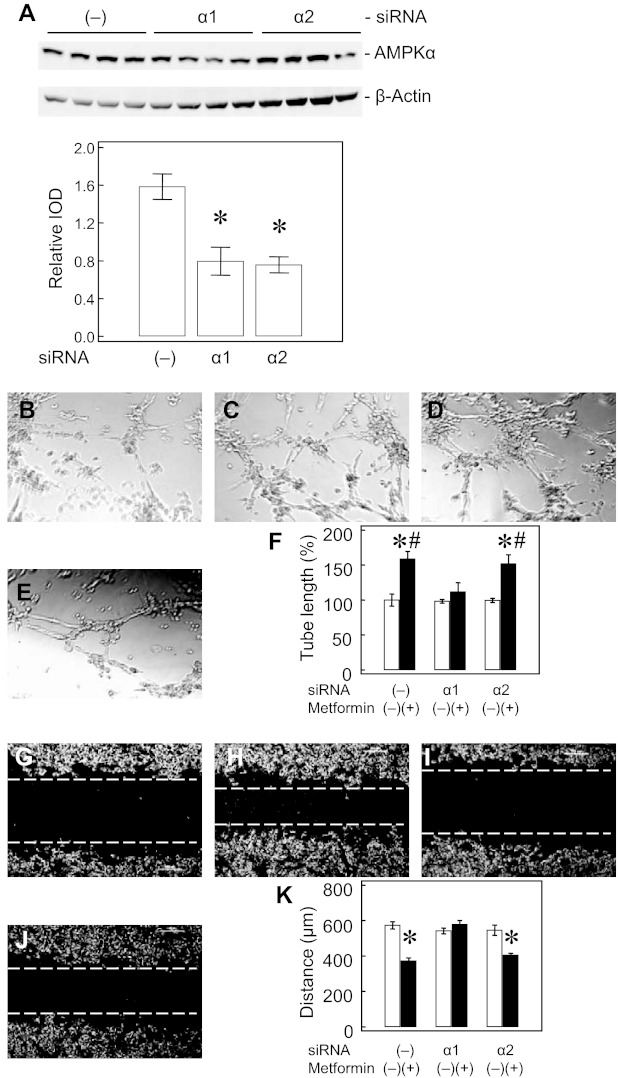
Transfection of PAEC with both siRNAs (AMPK-α1 and AMPK-α2) decreased AMPK-α expression by ∼50% (Fig. 10A). AMPK-α1 knockdown impaired IPH-PAEC response to Metformin in both tube formation (Fig. 10, B–F) and scratch recovery (Fig. 10, G–K). However, similar effects were not observed with AMPK-α2 knockdown. This difference suggests that AMPK-α1 may be the major isoform in modulating angiogenesis in IPH-PAEC.
Fig. 10.
AMPK-α1 knockdown impairs IPH-PAEC response to Metformin in angiogenesis. Knockdown of both AMPK-α1 and AMPK-α2 decreased AMPK-α expression by ∼50% (A). AMPK-α1 and -α2 knockdowns did not affect the impaired tube formation in IPH compared with scramble siRNA transfection (B). Metformin (5 × 10−5 M) increased tube length in scramble siRNA-treated IPH-PAEC (C) but not in AMPK-α1 knockdown IPH-PAEC (D). AMPK-α2 knockdown did not affect the Metformin effect on IPH-PAEC tube formation (E). Only AMPK-α1 knockdown abolished the Metformin effect in improving IPH-PAEC tube formation (F). Similarly, AMPK-α1 and -α2 knockdown did not affect scratch recovery in IPH-PAEC (G). Metformin improved scratch recovery in scramble siRNA-treated IPH-PAEC (H) but not AMPK-α1 knockdown IPH-PAEC (I). AMPK-α2 knockdown did not affect Metformin effect on IPH-PAEC scratch recovery (J). Only AMPK-α1 knockdown affected Metformin-treated scratch recovery in IPH-PAEC (K). *P < 0.05 compared with nontreated IPH-PAEC. #P < 0.05 compared with Metformin-treated AMPK-α1 knockdown IPH-PAEC. -, Scramble siRNA; α1, AMPK-α1 siRNA; α2, AMPK-α2 siRNA.
DISCUSSION
PPHN is a relatively common complication of respiratory diseases during neonatal period with an incidence of 1 in 500 live births (54). Both impaired pulmonary vasodilation and decreased blood vessel density in lungs are characteristics of PPHN (16). Inadequate NO production (10) plays a major role in the increased pulmonary vascular resistance and impaired blood vessel formation in the developing lungs, which may subsequently prolong the recovery process.
Persistent pulmonary hypertension induced by constriction of fetal ductus arteriosus has been widely used as a model to study PPHN because it reproduces the hemodynamic and structural features of this illness (34). Increased ROS formation and eNOS uncoupling contribute to pulmonary vasoconstriction (28), increased autophagy (44), apoptosis, and impaired angiogenesis (46) in this model. These endothelial cell alterations may contribute in part to the pulmonary vascular dysfunction observed in neonates with PPHN. Using PAEC from this model, we have shown that reducing ROS by antioxidants (46) or recoupling eNOS activity by BH4 analog, sepiapterin, improves angiogenesis (44, 45). Increased ROS formation has been shown to activate AMPK (52, 53) in some vascular beds, which may increase the activity of eNOS (5, 6) and antioxidant enzymes (7) in endothelial cells as part of overall adaptive changes. However, we did not observe this compensation in our study with IPH-PAEC, which raises the possibility of inadequate response of AMPK to increased ROS formation in our model.
In this report, we show that basal phosphorylation of AMPK-α (Thr172) and AMPK-β1 (Ser108) is decreased in IPH-PAEC compared with control PAEC. The AMPK downstream enzyme, ACC, phosphorylation (Ser79) is also decreased in IPH-PAEC together with decreased expression of PGC1, further supporting the concept that basal AMPK activation is decreased in IPH-PAEC (14, 20). The mechanism by which AMPK activation is decreased in IPH-PAEC may be related to the decreased expression of two major upstream AMPKKs, CAMKII (22, 56) and LKB1 (40). We also observed a modest increase in the expression of PP2A-Cα/β and PP2-Cα/β, which are known to dephosphorylate and inactivate AMPK (33). These results show that increased ROS formation does not lead to increased AMPK activation in IPH-PAEC.
AMPK activation is known to decrease protein synthesis by attenuating S6K1, 4E-BP1, and eEF2 signaling responses in skeletal muscle (11, 48). The decreased protein synthesis may in turn impair angiogenesis. Although basal AMPK activation is higher in control PAEC, we did not see a decrease in protein synthesis factors in these cells. Instead, we observed that phosphorylation of mTOR, P70S6K, and S6 was actually higher in control PAEC, which may facilitate protein synthesis (Fig. 2). These findings suggest that the higher AMPK activity in the control PAEC is adequate for rapidly proliferating PAEC from developing lungs and does not impair angiogenesis compared with IPH-PAEC. The findings are also consistent with our previous report that control PAEC have more active proliferation, less apoptosis, and better angiogenesis (46).
AMPK is known to mediate basic FGF, adiponectin, hypoxia, and VEGF-induced angiogenesis (30, 55), so it is possible that decreased AMPK activation in IPH-PAEC may play a role in the impaired angiogenesis as we have previously observed (46). To explore the role of AMPK in angiogenesis in IPH, we used two commonly used AMPK activators, AICAR and Metformin. AICAR is taken up by tissues and subsequently phosphorylated into ZMP (an AMP analog) (24) to increase AMPK activity both allosterically and covalently (39, 43). Metformin, a widely used drug for type 2 diabetes, provides pleiotropic metabolic benefits via inhibition of mitochondrial respiratory chain complex I and AMPK activation (50, 58). Both AICAR and Metformin increased tube formation in IPH-PAEC but did not increase tube formation in control PAEC, indicating that basal AMPK activation is optimal in control PAEC. Compound C is considered to be a selective AMPK inhibitor (58) that prevents the phosphorylation of ACC following incubation with either AICAR or Metformin (26). In our study, Compound C inhibited tube formation and scratch recovery assays in Metformin-treated PAEC, indicating that the increased angiogenesis in IPH-PAEC is through AMPK activation. The increased phosphorylation of AMPK-α, AMPK-β1, and ACC in IPH-PAEC after Metformin treatment further supports this notion.
Another possible mechanism by which Metformin increases angiogenesis in IPH-PAEC is through increased expression of angiogenic factor(s). The expression of VEGF and PDGF-β was decreased in IPH-PAEC compared with control PAEC, further supporting this possibility. However, Metformin did not affect the expression of either VEGF or PDGF-β at any concentration or duration. These findings indicate that the mechanisms by which Metformin improves IPH-PAEC angiogenesis are not through increasing expression of angiogenic factors.
We previously reported that antioxidant treatment (46) and eNOS recoupling (45) improve IPH-PAEC angiogenesis. The increased PP2A expression may also contribute to eNOS uncoupling in IPH-PAEC (49). Metformin treatment increased DAF-FM-DA epifluorescence and eNOS Ser1179 phosphorylation and decreased eNOS-caveolin-1 association, indicating improved eNOS activity. The improved eNOS activity is expected to increase NO bioavailability and facilitate angiogenesis in IPH-PAEC. We also observed an increased expression of eNOS after Metformin treatment in IPH-PAEC. The mechanism by which Metformin increases eNOS expression deserves future investigation.
Metformin prevents endothelial cell apoptosis via mild but specific inhibition of the mitochondrial respiratory chain complex I with decreased ROS formation (2, 9). AMPK activation can increase the expression of antioxidant enzymes in vascular endothelial cells (7, 53). In this study, we observed that Metformin treatment decreased MitoSOX and DHE epifluorescence with increased MnSOD expression and activity in IPH-PAEC, consistent with previous reports. The more dramatic decrease in DHE epifluorescence suggests that Metformin decreases IPH-PAEC ROS formation by other mechanisms besides increased MnSOD activity. The explanation that recoupled eNOS activity by Metformin accounts for the reduced overall ROS formation is plausible. However, we did observe a discrepancy in dose response between MnSOD activity and MitoSOX epifluorescence, which needs to be explored in further studies.
Metformin appears to have beneficial effects on IPH-PAEC in improving angiogenesis, eNOS activity, and MnSOD activity (Fig. 11). However, the potential inhibitory effect on protein synthesis (10, 48) remains a concern, especially with the decreased basal phosphorylation of mTOR, P70S6K, and S6 in IPH-PAEC. However, we did not see any decreased phosphorylation in these protein synthesis factors after Metformin treatment at the concentrations we used, suggesting dose dependence of its side effects. However, we acknowledge the limitation that our studies tested Metformin effects in vitro in cell culture system, and these effects may not be directly extrapolated to in vivo situation.
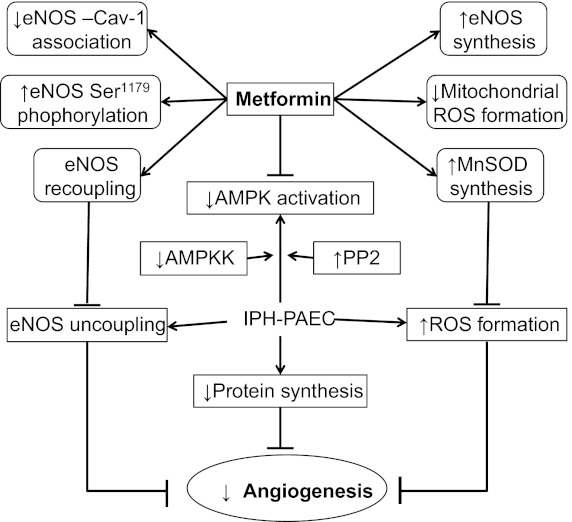
Fig. 11.
The proposed mechanism by which AMPK activation is decreased in IPH-PAEC and how Metformin improves IPH-PAEC angiogenesis. IPH-PAEC has phenotypical changes including eNOS uncoupling and increased reactive oxygen species (ROS) formation, both of which lead to impaired angiogenesis. In this study, we also observed decreased AMPK activation in IPH-PAEC with decreased upstream AMPKK expression and increased downstream PP2 expression. Metformin recouples eNOS by decreasing eNOS association with caveolin-1 and increasing ser1179 phosphorylation, which can lead to increased NO bioavailability and decreased ROS formation. Metformin also increases MnSOD expression/activity that further decreases mitochondrial ROS formation. →, indicate enhanced activity; ┤, suppressed activity.
To determine whether Metformin improves IPH-PAEC angiogenesis through AMPK activation, we performed AMPK-α1 and AMPK-α2 knockdowns. Although both siRNAs decreased AMPK-α expression in IPH-PAEC by ∼50%, only AMPK-α1 knockdown abolishes the effect of Metformin on angiogenesis. The findings suggest that AMPK-α1 may be the isoform in IPH-PAEC that contributes to the angiogenesis.
One important limitation of our study is that the phenotype changes we observed in PAEC were not reproduced in isolated pulmonary arteries. We observed decreased AMPK activation in IPH-PAEC but not in IPH-PA. It is possible that the changes in AMPK activation we observed are limited to endothelial cells and do not extend to other cell types in PA. However, it is also plausible that the changes we observed in endothelial cells are a result of alterations unique to culture conditions and do not represent in vivo changes. In the present report, we observed an increase in ROS and decrease in NO levels in IPH PAEC compared with controls. This phenotype change is consistent with the increased ROS formation, eNOS uncoupling, and impaired in vitro angiogenesis we observed in IPH PAEC in our previous studies (28, 29, 44–46). The increase in ROS in PAEC we observed was also matched by increased DHE fluorescence in the endothelial layer of freshly isolated pulmonary arteries in this model in our previous study (28). Similarly, the decrease in eNOS-hsp90 association we observed in IPH-PAEC was also present in freshly isolated pulmonary arteries (29). Our previous study, using ex vivo PA, also demonstrated impaired sprouting angiogenesis in IPH compared with control PA, similar to what we observed in IPH-PAEC (45). These data suggest that in utero pulmonary hypertension induces a consistent and reproducible phenotype alteration in PAEC that persists under culture conditions. Whether these changes include decreased AMPK activity in vivo requires further investigation.
In conclusion, basal AMPK activity is decreased in IPH-PAEC secondary to decreased AMPKK expression and increased PP2 expression. Metformin improves IPH-PAEC angiogenesis via AMPK activation, increases eNOS activity, and decreases ROS formation. The improved angiogenesis appears to be independent of the increased formation of angiogenic factors. Metformin also did not show evidence of inhibitory effects on protein synthesis. Thus metformin may be a potential therapeutic agent to restore vasodilation and angiogenesis in pulmonary circulation in PPHN.
GRANTS
The study was supported by National Institutes of Health Grants 1R03HD073274–01 (R-J. Teng), HL-080468S (Y. Shi), R03HD-65841 (G. Konduri), RO1HL-057268 (G. Konduri), CTSI grant UL1RR031973 from Advancing Healthier Wisconsin Foundation (G. Konduri), and Muma Endowed Chair in Neonatology (G. Konduri).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R-J.T., J.D., and Y.S. conception and design of research; R-J.T., J.D., A.J.A., and A.E. performed experiments; R-J.T., A.J.A., and A.E. analyzed data; R-J.T., A.E., and G.G.K. interpreted results of experiments; R-J.T. prepared figures; R-J.T. drafted manuscript; R-J.T., J.D., Y.S., and G.G.K. edited and revised manuscript; G.G.K. approved final version of manuscript.
REFERENCES
- 1. Acosta-Jaquez HA, Keller JA, Foster KG, Ekim B, Soliman GA, Feener EP, Ballif BA, Fingar DC. Site-specific mTOR phosphorylation promotes mTORC1-mediated signaling and cell growth. Mol Cell Biol 29: 4308–4324, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, Leverve XM. The ROS production induced by a reverse-electron flux at respiratory-chain complex I is hampered by metformin. J Bioenerg Biomembr 38: 33–42, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Birnbaum MJ. Activating AMP-activated protein kinase without AMP. Mol Cell 19: 289–290, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Cantó C, Auwerx J. PGC-1α, SIRT1 and AMPK, and energy sensing network that controls energy expenditure. Curr Opin Lipidol 20: 98–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JYJ. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res 104: 496–505, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen ZP, Mtichelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witter LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443: 285–289, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Colombo SL, Moncada S. AMPKa1 regulates the antioxidant status of vascular endothelial cells. Biochem J 421: 163–169, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229: 558–565, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, Leverve X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes 54: 2179–2187, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Dollberg S, Warner BW, Myatt L. Urinary nitrite and nitrate concentrations in patients with idiopathic persistent pulmonary hypertension of the newborn and effect of extracorporeal membrane oxygenation. Pediatr Res 37: 31–34, 1995 [PubMed] [Google Scholar]
- 11. Dreyer HC, Satoshi Fujita S, Cadenas JG, Chinkes DL, Elena Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576: 613–624, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Du J, Teng RJ, Guan T, Eis A, Kaul S, Konduri GG, Shi Y. The role of autophagy in angiogenesis in aortic endothelial cells. Am J Physiol Cell Physiol 302: C383–C391, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, Steinhorn RH. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L979–L987, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fisslthaler B, Flemming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res 105: 114–127, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci USA 93: 6448–6453, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geggel RL, Reid LM. The structural basis of PPHN. Clin Perinatol 11: 525–549, 1984 [PubMed] [Google Scholar]
- 17. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hardie DG, Carling D. The AMP-activated protein kinase: fuel gauge of the mammalian cell? Eur J Biochem 246: 259–273, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8: 774–785, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Hardie DG. AMP-activated protein kinase—an energy sensor that regulates all aspects of cell function. Genes Dev 25: 1895–1908, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rate liver, and identification of threonine-172 as the major site at which it phosphorylates and activates AMP-activated protein kinase. J Biol Chem 271: 27879–27887, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2: 9–19, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Hoyer LW, de los Santos RP, Hoyer JR. Antihemophilic factor antigen. Localization in endothelial cells by immunofluorescent microscopy. J Clin Invest 52: 2737–2744, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin X, Townley R, Shapiro L. Structural insight into AMPK regulation: ADP comes into play. Structure 15: 1285–1295, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem 272: 18522–18525, 1997 [DOI] [PubMed] [Google Scholar]
- 26. King TD, Song L, Jope RS. AMP-activated protein kinase (AMPK) activating agents cause dephosphorylation of Akt and glycogen synthase kinase-3. Biochem Pharmacol 71: 1637–1647, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kleijn M, Voorma HO, Thomas AAM. Phosphorylation of elF-4E and initiation of protein synthesis in P19 embryonal carcinoma cells. J Cell Biochem 59: 443–452, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Konduri GG, Bakhutashvili I, Eis A, Pritchard K., Jr Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 291: H1812–H1820, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Konduri GG, Ou J, Shi Y, Pritchard KA., Jr Decreased association of HSP90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 285: H204–H211, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Levine YC, Li GK, Michel T. Agonist-modulated regulation of AMPK-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK→Rac1→Akt →endothelial nitric-oxide synthase pathway. J Biol Chem 282: 20351–20364, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1: 361–370, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Mihaylova MM, Shaw RJ. The AMPK signaling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13: 1016–1023, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moore F, Weekes J, Hardie DG. Evidence that AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. A sensitive mechanism to protect the cell against ATP depletion. Eur J Biochem 199: 691–697, 1991 [DOI] [PubMed] [Google Scholar]
- 34. Morin FC., 3rd Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res 25: 245–250, 1989 [DOI] [PubMed] [Google Scholar]
- 35. Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem 278: 31000–31006, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Ouyang J, Parakhia RA, Ochs RS. Metformin activates AMP kinase through inhibition of AMP deaminase. J Biol Chem 286: 1–11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robinson KM, Janes MS, Beckman JS. The selective detection of mitochondrial superoxide by live cell imaging. Nat Protocols 3: 941–947, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha A, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 298: E751–E760, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 403: 139–148, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA 101: 332933–332935, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res 90: 1243–1250, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase β. Mol Cell Biol 26: 5933–5945, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem 281: 32207–32216, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Teng RJ, Du J, Welak S, Guan T, Eis A, Shi Y, Konduri GG. Cross-talk between NADPH oxidase and autophagy in pulmonary artery endothelial cells with intrauterine persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L651–L663, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Teng RJ, Du J, Xu H, Bakhutashvili I, Eis A, Shi Y, Pritchard KA, Jr, Konduri GG. Sepiapterin improves angiogenesis of pulmonary artery endothelial cells with in utero pulmonary hypertension by recoupling endothelial nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol 301: L334–L345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Teng RJ, Eis A, Bakhutashvili I, Arul N, Konduri GG. Increased superoxide production contributes to the impaired angiogenesis of fetal pulmonary arteries with in utero pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 297: L184–L195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Teng RJ, Wu TJ, Bisig CG, Eis A, Pritchard KA, Konduri GG. Nitrotyrosine impairs angiogenesis and uncouples eNOS activity of pulmonary artery endothelial cells isolated from developing sheep lungs. Pediatr Res 69: 112–117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thomson DM, Fick CA, Gordon SE. AMPK activation attenuates S6K1, 4E-BP1, and eEF2 signaling responses to high-frequency electrically stimulated skeletal muscle contractions. J Appl Physiol 104: 625–632, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Urbich C, Reissner A, Chavakis E, Dernbach E, Haendeler J, Fleming I, Zeiher AM, Kaszkin M, Dimmeler S. Dephosphorylation of endothelial nitric oxide synthase contributes to the anti-angiogenic effects of endostatin. FASEB J 16: 706–708, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci 122: 253–270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol 99: 2034–2040, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Q, Liang B, Shirwany NA, Zou MH. 2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PLoS One 6: e17234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang XR, Zhang MW, Chen DD, Zhang Y, Chen AF. AMP-activated protein kinase rescues the angiogenic functions of endothelial progenitor cells via manganese superoxide dismutase induction in type 1 diabetes. Am J Physiol Endocrinol Metab 300: E1135–E1145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, Verter J, Stoll BJ, Lemons JA, Papile L, Shankaran S, Donovan EF, Oh W, Ehrenkranz RA, Fanaroff AA. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics 105: 14–20, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Webler AC, Michaelis UR, Popp R, Barbosa-Sicard E, Murugan A, Falck JR, Fisslthaler B, Fleming I. Epoxyeicosatrienoic acids are part of the VEGF-activated signaling cascade leading to angiogenesis. Am J Physiol Cell Physiol 295: C1292–C1301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2: 21–33, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 4: 33–41, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenky-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]