Abstract
Preterm infants may be at risk of necrotizing enterocolitis (NEC) due to deficiency of transforming growth factor-β 2 (TGF-β2) in the developing intestine. We hypothesized that low epithelial TGF-β2 expression in preterm intestine and during NEC results from diminished autocrine induction of TGF-β2 in these cells. Premature baboons delivered at 67% gestation were treated per current norms for human preterm infants. NEC was diagnosed by clinical and radiological findings. Inflammatory cytokines, TGF-β2, Smad7, Ski, and strawberry notch N (SnoN)/Ski-like oncoprotein (SKIL) was measured using quantitative reverse transcriptase-polymerase chain reaction, immunoblots, and immunohistochemistry. Smad7 effects were examined in transfected IEC6 intestinal epithelial cells in vitro. Findings were validated in archived human tissue samples of NEC. NEC was recorded in seven premature baboons. Consistent with existing human data, premature baboon intestine expressed less TGF-β2 than term intestine. TGF-β2 expression was regulated in epithelial cells in an autocrine fashion, which was interrupted in the premature intestine and during NEC due to increased expression of Smad7. LPS increased Smad7 binding to the TGF-β2 promoter and was associated with dimethylation of the lysine H3K9, a marker of transcriptional silencing, on the nucleosome of TGF-β2. Increased Smad7 expression in preterm intestine was correlated with the deficiency of SnoN/SKIL, a repressor of the Smad7 promoter. Smad7 inhibits autocrine expression of TGF-β2 in intestinal epithelial cells in the normal premature intestine and during NEC. Increased Smad7 expression in the developing intestine may be due to a developmental deficiency of the SnoN/SKIL oncoprotein.
Keywords: necrotizing enterocolitis, nonhuman primate, SnoN, Ski-like oncoprotein
necrotizing enterocolitis (NEC) is an idiopathic, inflammatory bowel necrosis of premature infants, affecting 5–15% of all infants born before 32 wk of gestation (20, 24, 30). Histopathologically, the disease is characterized by coagulative necrosis, bacterial overgrowth, pneumatosis (gaseous products of bacterial fermentation entrapped within the bowel wall), and a severe inflammatory response (20). Based on current evidence, NEC is believed to occur when mucosal injury in the preterm intestine permits translocation of luminal bacteria across the epithelial barrier, thereby triggering an exaggerated local inflammatory response (24, 38).
The pathogenesis of NEC is intriguing because intestinal injury and bacterial translocation occur frequently in critically ill patients of all ages, but unlike in preterm infants, these invading bacteria do not cause NEC in the mature intestine. We showed recently that the preterm intestine may be at risk of NEC because of a developmental deficiency of transforming growth factor-β 2 (TGF-β2), which is further accentuated during NEC (31). In this study, we sought to elucidate signaling mechanism(s) that suppress TGF-β2 expression in the developing intestine. TGF-β has three isoforms in humans, and one of these, TGF-β1, is induced in epithelial cells in an autocrine feed-forward loop (2, 47). Because TGF-β isoforms share several downstream signaling pathways (32), we hypothesized that TGF-β2 expression is also regulated in an autocrine fashion and that decreased TGF-β2 expression in the preterm intestine is due to impaired autocrine induction. To investigate this hypothesis, we used intestinal epithelial cell (IEC) lines and tissue samples from premature baboons that spontaneously developed NEC during the neonatal period. Delivered at 125 days (67% gestation, term = 185 days), the premature baboon has a clinical course similar to human infants delivered at 25–27 wk of gestation and is an excellent model for prematurity and its complications (5, 10, 48).
MATERIALS AND METHODS
Baboons.
Animal studies were conducted at the Texas Biomedical Research Institute and University of Texas at San Antonio, San Antonio, TX after approval by the local animal care and use committee. Olive/yellow baboons (Papio cynocephalus anubis/P. cynocephalus cynocephalus) were delivered prematurely via cesarean section at 125 days gestation (67% gestation; term = 185 days; equivalent to 27-wk gestation in humans). These animals were treated in a primate intensive care nursery per current norms for premature human infants (5, 40). Animals were intubated at birth and provided assisted ventilation for 14, 21, or 28 days as planned a priori. Intravenous fluids were initiated at birth through a central line and parenteral nutrition was started at 24 h. Enteral feeds were initiated after day 5 using a primate (Bio-Serv, Frenchtown, NJ) or human infant formula (Similac, Abbott Pharmaceuticals, Columbus, OH). In the first 24 h, arterial blood gases were checked every 1 h, hematocrit every 2 h, and electrolytes at 12 and 20 h. A chest/abdomen radiograph was obtained at birth, 4 h, and then every 8 h. After 24 h, blood gases were monitored every 2 h, hematocrit every 4 h, electrolytes twice daily, and radiographs daily. Blood, urine, or cerebrospinal fluid were cultured at birth and as needed. Sepsis was defined by at least one positive body fluid culture. All animals received antibiotics (ampicillin 50 mg/kg at 6 h and then every 12 h, gentamicin 2.5 mg/kg at 6 and 30 h) until the bacterial cultures were negative for 48 h. Other antibiotics such as vancomycin, piperacillin/tazobactam, amikacin, and fluconazole were used per discretion of the treating team when a clinical diagnosis of sepsis was entertained after the first 5–7 days.
NEC was diagnosed based on clinical/radiological signs characteristic of human NEC or at autopsy. For each NEC animal, another preterm baboon without signs of NEC was selected randomly as control for tissue analysis. In addition, three preterm baboons born within 1 day prior/subsequent to the birth of each NEC animal were selected and the following data were recorded: birth weight, vasopressor use, postnatal steroid use, days with an umbilical arterial catheter, persistent patency of the ductus arteriosus, ibuprofen/indomethacin use to achieve ductal closure, day of initiation of feedings, volume and type of enteral feeds, days needed to reach full enteral feeds, day of onset of radiological signs of NEC, and day of death.
Term baboons were delivered vaginally at the Texas Biomedical Research Institute or at the baboon facility at University of Oklahoma, Oklahoma City, OK. These animals were nursed by their mothers for the initial few days and were subsequently fed infant formula (Similac) by the veterinary staff. All animals were autopsied following death or euthanasia at the end of the study. Tissues were stored at −80°C or fixed in formalin for further study. Animal studies were performed using tissue samples from seven preterm and three term animals without NEC, and seven preterm animals with NEC.
Human NEC and controls.
Human intestinal tissue samples were collected after approval by the Institutional Review Board at the University of Illinois, Chicago, IL. Using immunohistochemistry, deidentified tissue samples of NEC were compared with healthy margins of tissue resected for other indications (intestinal obstruction, spontaneous intestinal perforation). Deidentified frozen tissue samples were available for RNA isolation from 11 patients with NEC and eight controls.
Immunohistochemistry.
Human and baboon tissues were stained (44) for TGF-β2, Smad7, and strawberry notch N (SnoN)/Ski-like oncoprotein (SKIL) using our previously described protocol (31). Briefly, tissue sections were deparaffinized and antigen retrieval was achieved using the EZ-AR solution (Biogenex, San Remon, CA) per manufacturer's protocol. Slides were then treated with Proteinase K (20 μg/ml) (Promega, Madison, WI) for 10 min at room temperature, rinsed in PBS (5 min), blocked using SuperBlock T20 blocking buffer (Thermo Scientific, Rockford, IL) for 30 min at room temperature, and then incubated overnight at 4°C with an appropriate primary antibody: mouse monoclonal anti-human TGF-β2 (R&D Systems, Minneapolis, MN), goat anti-human Smad7 polyclonal IgG, or goat anti-human SnoN/SKIL (both from Santa Cruz Biotechnology, Santa Cruz, CA). Secondary staining was performed at room temperature for 30 min with Alexa-Fluor 488-conjugated rabbit anti-mouse IgG or rabbit anti-goat IgG antibody (Invitrogen, San Diego, CA). Controls included slides with no primary antibody, appropriate isotype control, and with competing recombinant cytokine. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Calbiochem, San Diego, CA). Quantitative fluorescence imaging was performed using a Zeiss LSM 710 confocal microscope. Laser intensity was lowered to a level where the fluorescence staining was barely visible in the negative control, and the same settings were then used to image normal preterm and NEC tissues. Fluorescence intensity was measured using the Zeiss ZEN Lite software package (Carl Zeiss, Thornwood, NY).
IECs and reagents.
IEC6 cells (ATCC, Manassas, VA) were cultured at cell passages 17–21 in DMEM with 4 mM l-glutamine adjusted to contain 1.5 g/l sodium bicarbonate and 4.5 g/l glucose and supplemented with 10% (vol/vol) FBS, 50 U/ml penicillin and 50 μg/ml streptomycin at 37°C and 5% (vol/vol) CO2 (33). Media were changed at 24 h postplating and every 72 h thereafter.
IEC6 cells were treated with 0.5–5 μg/ml Escherichia coli 0111:B4 lipopolysaccharide (Sigma, St. Louis, MO) and/or recombinant human TGF-β2 (rTGF-β2; R&D Systems) for up to 24 h. Listeria monocytogenes were grown on brain-heart infusion agar/broth as previously described (19). Bacteria were heat killed by incubating for 1 h to 60°C in PBS (7) and added to cell cultures at a concentration of 107–108 killed bacteria per milliliter of media for up to 24 h.
Plasmids and transfection studies.
IEC6 cells were transfected with pCMV-Smad7 (gift of Dr. Randy Rosier, University of Rochester, Rochester, NY) (16) or with the Smad7 shRNA plasmid sc-36508-SH (Santa Cruz Biotechnology) using the Fugene-6 reagent per manufacturer's instructions (Roche, Branchburg, NJ). Cells were used at 24 h posttransfection (predetermined optimum).
Real-time RT-PCR.
Primers were designed using the Beacon Design software (Bio-Rad, Hercules, CA) and are shown in Table 1. We used our previously described SYBR green protocol to measure mRNA expression; data were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and group-wise comparisons were made by the 2−ΔΔCT method (3, 41). GAPDH was used as housekeeping gene because it shows minimal regional variation at the mRNA level in the gastrointestinal tract, does not change significantly with gender, and provides excellent consistency between donor replicates (1).
Table 1.
Primer sequences used for real-time RT-PCR
| mRNA | Forward Primer, 5′-/-3′ | Reverse Primer, 5′-/-3′ |
|---|---|---|
| IL-8/CXCL8 | CATAAGGCACAAACTTTCAGAG | ATGTTTACACACAGTGAGATGG |
| CXCL12 | CACATCTAACCTCATCTTC | GACTTACTCTTCACATAGC |
| CCL2 | TGTGCCTGCTGCTCATAG | CTTGCTGCTGGTGATTCTTC |
| CCL3 | GGGTCCAGAAATATGTCAG | AGTCCATAGAAGAGGTAGC |
| CCL4 | TGACCTGGAACTGAACTGAG | AATGACACCTAATACAATAACACG |
| CCL5 | GCTACTCGGGAGGCTAAG | TTGTTGTTGTTGTTGTGACG |
| CX3CL1 | ACTGTGCTGAGTCTGAAGG | GGTGCTCTGCTGGTAAGG |
| TNF | GTGAGGAGGACGAACATC | GAGCCAGAAGAGGTTGAG |
| IL-1A | GCAGCCAACCTAAGCAAG | TCACCTGACACATTCAAGTTC |
| IL-1B | GACCAACCTCCTCTTCTTC | TGAGACTCCAGACCTACG |
| IL-6 | CTCTCACCTCTCCTACTCAC | GACACTGCTACTTCTTGCC |
| IL-17A | CTGGCACTTGGAGACTTG | CAACATATACAGCATTCATTCG |
| NF-κB1 | TCCGAGACAGTGACAGTG | GTTGAGAGTTAGCAGTGAGG |
| NF-κB2 | GCTTCTCTGCCTTCCTTAG | TGCTGTCTTGTCCATTCG |
| RELA | CGCATCCAGACCAACAACAAC | AGCCGCACAGCATTCAGG |
| FOS | GTCTTCCTTCGTCTTCACCTAC | GGCTCATTGCTGCTGCTG |
| JUN | CTGTAGATTGCTTCTGTAG | AAGAGTTCATCTGTAGGC |
| TGF-β2 | GCTTCTCCTTGCTGCGTGTC | TCATCGTTGTCGTCGTCATCATC |
| SMAD1 | CTGCTTACCTGCCTCCTG | AACCGCCTGAACATCTCC |
| SMAD2 | CTACAGCGATTAAGATGAGAACAG | ACAGCCCTCCGATTACAAAG |
| SMAD3 | GTGCTGGTGACTGGATAG | AAGGTGCTGAAGACAAGG |
| SMAD4 | GTGTGAATGCTTATAGATGATG | CTCTTCTCTGGCTTCTGG |
| SMAD5 | GGAGAGGTGTATGCGGAATG | TGACAGATTGAGCCAGAAGC |
| SMAD6 | CCTACTCTCGGCTGTCTCCTC | CAGTGGCTCGGCTTGGTG |
| SMAD7 | AGGCTGTGTTGCTGTGAATC | AGAGTCGGCTAAGGTGATGG |
| SKIL | TCGTTGAGTCTTGGAATCTTC | CAGGAGAATGGCTTGAACC |
| SKI | GCCAAGTTCAAGTGAGTAAGC | ATGTTCCAGCAAGCAGAGG |
| Baboon TGF-β2 promoter primer pair 1 | CTTGGGAGGCTGTGACTGAG | GGAGGAAGGAGGTGGAATGTG |
| Baboon TGF-β2 promoter primer pair 2 | AAGATTGGAAGGTATGTC | TCCCTCCTGTATTATGTG |
| Baboon TGF-β2 promoter primer pair 3 | CCACAGCGGTCCTCATTTC | CAGTCAGAAGTCCTCTCGTTAC |
| Rat TGF-β2 | AGGATACAATGCTAACTTCTG | GTAGAGGATGGTCAGTGG |
| Rat SMAD6 | CCTATTCTCGGCTGTCTCCTC | TGGCTTGGTGGCATCTGG |
| Rat SMAD7 | GGCTGTGTTGCTGTGAATC | CATCGGGTATCTGGAGTAAGG |
| Rat TGF-β2 promoter primer pair 1 | CTCGGTCTTTGAACATCTC | GTGGCTGATCTGAACTCC |
| Rat TGF-β2 promoter primer pair 2 | CGGACCTTCTCGTCTCTTC | ATGGAGTTCAGTGTGTCAGG |
| Rat TGF-β2 promoter primer pair 3 | TTACCCTAAGCGAGAAAGTG | TCAGCCACCTCCTTAACC |
REL, v-rel reticuloendotheliosis viral oncogene homolog; FOS, FBJ murine osteosarcoma viral oncogene homolog; SKIL, Ski-like oncoprotein; TGF-β2, transforming growth factor β2.
Western blot analysis.
TGF-β2, Smad7, and SnoN/SKIL in intestinal tissues and IEC6 cells, and histone H3 dimethyl lysine 9 (H3K9me2), H3K27me, and H4K20me (Cell Signaling Technology, Boston, MA) in IEC6 cells were measured as described previously (41). We used β-actin as the housekeeping control because, consistent with existing information (18), it provided better interassay consistency at the protein level in our hands than GAPDH.
ELIZA.
TGF-β2 concentrations were measured in cell culture supernatants by using a commercially available ELISA (R&D Systems). Optical densities and standard concentrations were log transformed, and a linear equation was obtained (accepted if r2 ≥ 0.95). TGF-β2 concentrations in test samples were calculated by regression. The linear range of measurement of the assay was 31.2–1,000 pg/ml.
Chromatin immunoprecipitation.
Chromatin was harvested from IEC6 cells or tissue samples using the magnify chromatin immunoprecipitation (ChIP) system (Invitrogen, Carlsbad, CA) per manufacturer's instructions. Briefly, cells were cross-linked for 10 min with formaldehyde at 1% final concentration, and the reaction was then stopped by adding glycine to final concentration 1.25 M. Chromatin was sheared by sonication (8 times, 20-s pulses), separated from debris by centrifugation, and then used for immunoprecipitation with protein A/G dynabeads coupled with mouse anti-human Smad7 (R&D Systems), antibodies against H3K9me2 (Cell Signaling Technology), or the isotype control from a different species (rabbit) included in the kit. Precipitated DNA was reverse cross-linked using provided buffers and then amplified by real-time PCR using primers specific for TGF-β2 promoter (Table 1). Data were expressed as percent input after normalization for background levels and chromatin input going into the ChIP (8).
Statistical methods.
Parametric and nonparametric tests were applied using the Sigma Stat 3.1.1 software (Systat, Point Richmond, CA) or SPSS for Windows (version 17.0, SPSS, Chicago, IL). For PCR data, crossing-threshold (ΔΔCT) values were compared for genes with a ≥ 2-fold increase by Mann-Whitney U-test. Number of samples and statistical analyses are indicated in each figure legend. Each sample was tested in duplicate. A P value of <0.05 was considered significant.
RESULTS
Baboon NEC is similar to NEC in human premature infants.
We identified seven baboons with clinical and radiological evidence of NEC. These animals were similar to controls in birth weight, gender, exposure to corticosteroids and vasopressors, and feeding experience (Table 2). The clinical course of these seven animals with NEC is summarized in Table 3.
Table 2.
Demographic and clinical characteristics from preterm baboons by group
| Number of Animals | Birth Weight, g* | Intention to Treat | Length of Stay, days* | Males | Postnatal Steroids | Vasopressor | Maximum Feeding Volume, ml · kg−1 · day−1* | |
|---|---|---|---|---|---|---|---|---|
| Controls utilized for tissue analysis | 6 | 361 ± 37 | 14 animals for 14 days | 14 | 3 (50%) | 1 (16%) | 2 (33%) | 99 ± 38 |
| All matched controls | 18 | 386 ± 44 | 6 animals for 14 days 12 animals for 21 days | 16 ± 3 | 12 (66%) | 7 (38%) | 12 (66%) | 69 ± 45 |
| NEC animals | 7 | 353 ± 43 | 2 animals for 14 days 4 animals for 21 days 1 animal for 28 days | 16 ± 6 | 5 (71%) | 1 (14%) | 4 (57%) | 60 ± 62 |
Results expressed as means ± SD. No significant differences were detected between these groups. NEC, necrotizing enterocolitis.
Table 3.
Clinical course of animals with NEC
| Subject No. | Gender | Birth Weight, g | Day of Death | Pressors | Duration of Umbilical Arterial Catheter Use, days | Closure of Ductus Arteriosus, days | Use of Ibuprofen or Indomethacin to Close the Ductus Arteriosus | Initial Feed, days | Initial Feed, ml · kg−1 · day−1 | Max Feeds Achieved, days | Max Feeds Achieved, ml · kg−1 · day−1 | First Day Residuals or Emesis Reported | First Day of Abnormal X-ray |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 316 | 21 | Yes | 21 | No | No | 10 | 12 | 14 | 19 | 10 | 16 |
| 2 | Male | 373 | 14 | No | 10 | 6 | No | 8 | 8 | 8 | 8 | None | 9 |
| 3 | Female | 332 | 17 | Yes | 17 | 6 | No | 7 | 6 | 14 | 72 | 9 | 16 |
| 4 | Male | 291 | 13 | Yes | 13 | 8 | No | 7 | 7 | 11 | 28 | 7 | None |
| 5 | Male | 386 | 11 | No | 11 | 11 | No | 3 | 10 | 9 | 75 | None | 10 |
| 6 | Female | 364 | 12 | Yes | 12 | No | No | 5 | 5 | 8 | 33 | None | 11 |
| 7 | Male | 415 | 28 | No | 7 | 6 | Ibuprofen on Days 1–5 | 5 | 5 | 21 | 188 | 18 | 17 |
To estimate the incidence of NEC in premature baboons, we reviewed animal records at the Texas Biomedical Research Institute from the last 10 yr. Most premature baboons were euthanized on day 14 and therefore, may not have lived long enough to have developed NEC. In a 2-yr period in which a majority of premature baboons were kept alive for 21 days, NEC was recorded in three of fifty-two (5%) animals.
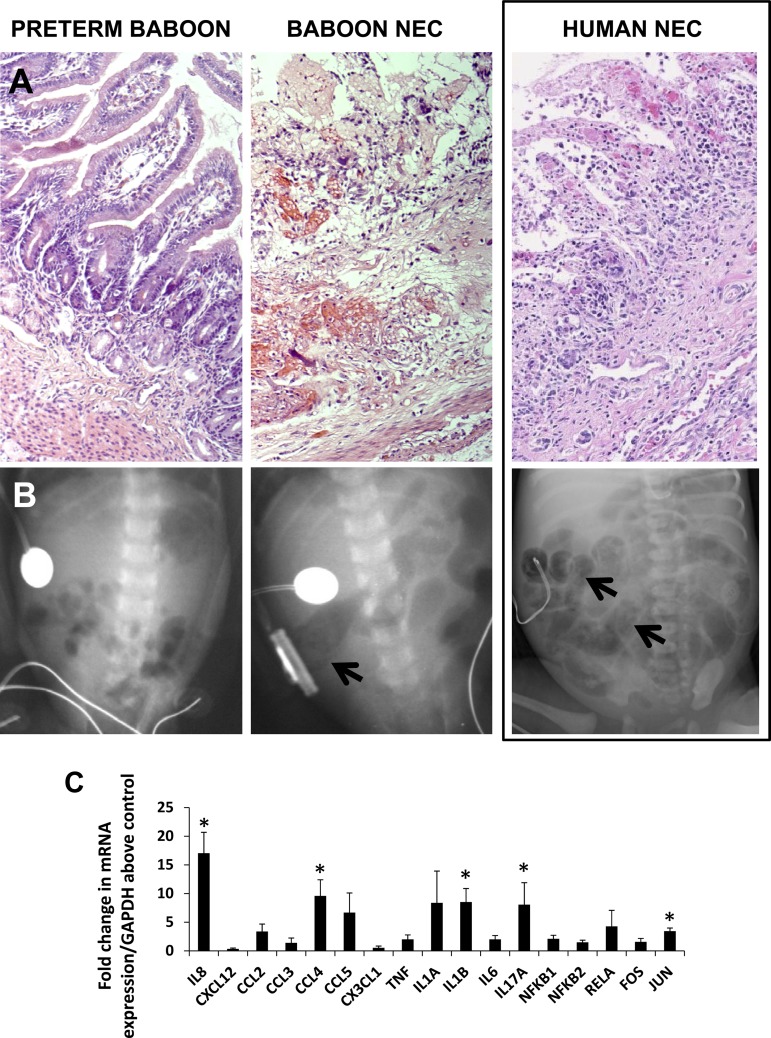
Baboon NEC showed a strong clinical and radiological resemblance to human NEC. Clinical signs of NEC (prefeed residuals, abdominal distension) were first noted at median 9.5 (range, 7 to 18) days. Baboon NEC also showed histopathological changes similar to human NEC, including coagulative necrosis, hemorrhages, inflammation, and vascular congestion (Fig. 1A). Radiological changes, such as the presence of dilated bowel loops and pneumatosis (Fig. 1B) were noted in six of seven NEC animals, starting on median, 13.5 (range, 9 to 17) days.
Fig. 1.
Baboon necrotizing enterocolitis (NEC) is similar to NEC in human premature infants. A: histopathological changes of NEC. Left, normal premature intestine (ileum) showing normal cellularity and histoarchitecture (hematoxylin and eosin; magnification, ×100); middle, photomicrograph of baboon NEC showing necrosis, focal hemorrhages, and inflammation; right, photomicrograph of human NEC, showing marked similarity between baboon and human NEC in histopathological changes. B: radiological changes of NEC. Left, abdominal radiograph of a normal premature baboon; middle, radiograph from a premature baboon with NEC showing dilated bowel loops and pneumatosis (arrow, cystic radiolucencies); right, abdominal radiograph from a human infant with NEC for comparison shows dilated bowel loops and pneumatosis (arrows). C: increased tissue expression of inflammatory cytokines and transcriptional regulators in baboon NEC. Bar diagram (means ± SE) shows fold change in mRNA expression in NEC over premature baboon ileum. N = 5 animals per group. *P < 0.05.
To characterize the inflammatory response in baboon NEC, we used real-time RT-PCR to measure key cytokines and transcriptional regulators. Similar to human NEC, baboon NEC was associated with increased IL-8/CXC-motif ligand (CXCL)8, CCL4/macrophage inflammatory protein-1β, IL-1β, IL-17A, and c-jun (Fig. 1C).
Decreased TGF-β2 expression in preterm baboon intestine and during NEC.
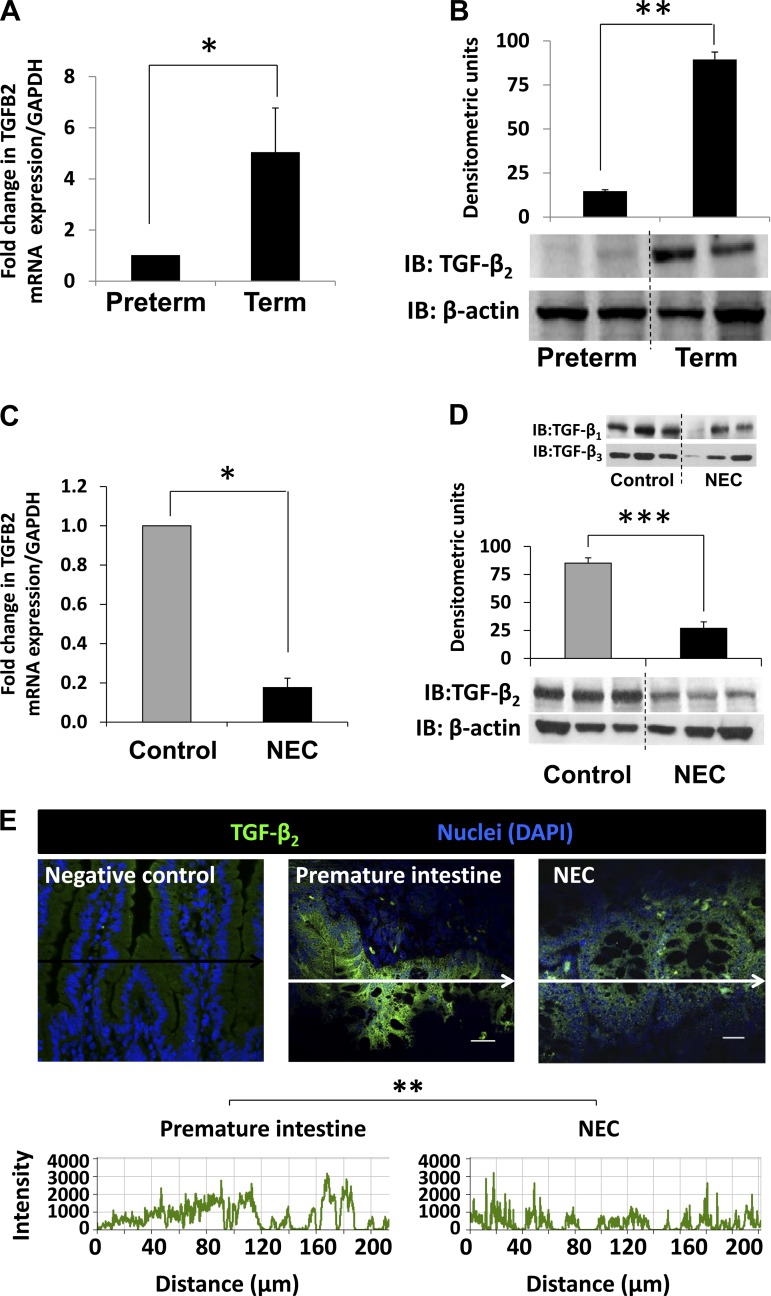
Because baboon NEC closely resembles human NEC, we asked whether the premature baboon intestine is also deficient in TGF-β2, similar to the preterm human intestine (31). Indeed, TGF-β2 mRNA and protein expression in the preterm baboon intestine was less than the term intestine (Fig. 2 A–B) and decreased further in tissue samples of NEC (Fig. 2, C–E). No significant changes were detected in TGF-β1 and TGF-β3 expression (Fig. 2D, inset).
Fig. 2.
Decreased transforming growth factor-β2 (TGF-β2) expression in preterm baboon intestine and during NEC. A and B: preterm baboon intestine expresses less TGF-β2 than the term baboon intestine. Bar diagram (means ± SE) shows mRNA expression in preterm vs. term animals (A). Representative immunoblots (IB) show lower TGF-β2 expression in preterm than in term intestine. Bar diagrams (means ± SE) show densitometric measurements (B). C, D, and E: NEC is associated with decreased TGF-β2 expression. Bar diagram (means ± SE) showing fold change in TGF-β2 mRNA in preterm baboon ileum vs. baboon NEC (C). IB confirmed decreased TGF-β2 in NEC. Bar diagram (means ± SE) shows densitometric data. Inset: TGF-β1 and TGF-β3 expression in same samples (D). Immunofluorescence photomicrographs of negative control, normal preterm intestine, and NEC highlight decreased TGF-β2 staining (green) in NEC (E). Laser intensity was lowered until fluorescence staining was barely visible in negative control and the same settings were then used to image control and NEC tissues. Nuclear staining was obtained with 4,6-diamidino-2-phenylindole (DAPI). Frequency histograms below the photomicrographs show the fluorescence intensity for TGF-β2 (488 nm; measured in arbitrary units along arrows). *P < 0.05, **P < 0.01, ***P < 0.001.
Smad7 inhibits autocrine expression of TGF-β2 in IECs.
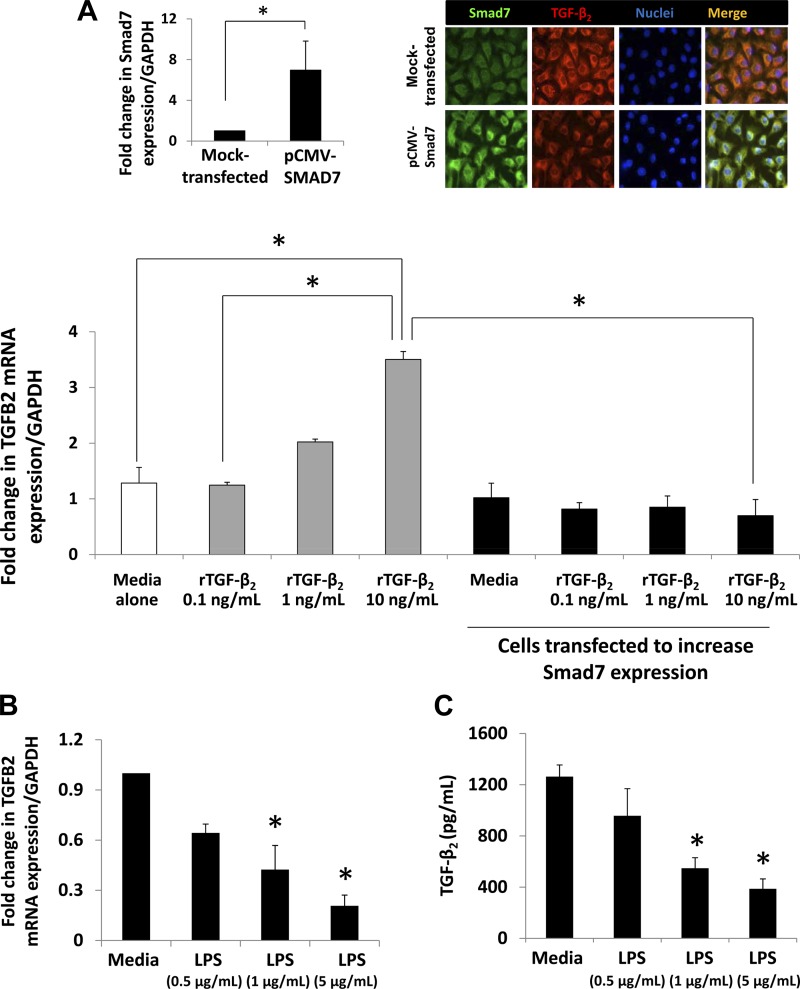
To investigate whether TGF-β2 induces its own expression in IECs, we treated IEC6 cells, a nontransformed IEC line, with rTGF-β2 in vitro. Consistent with our hypothesis, rTGF-β2 increased TGF-β2 mRNA expression in a dose-dependent fashion (Fig. 3A).
Fig. 3.
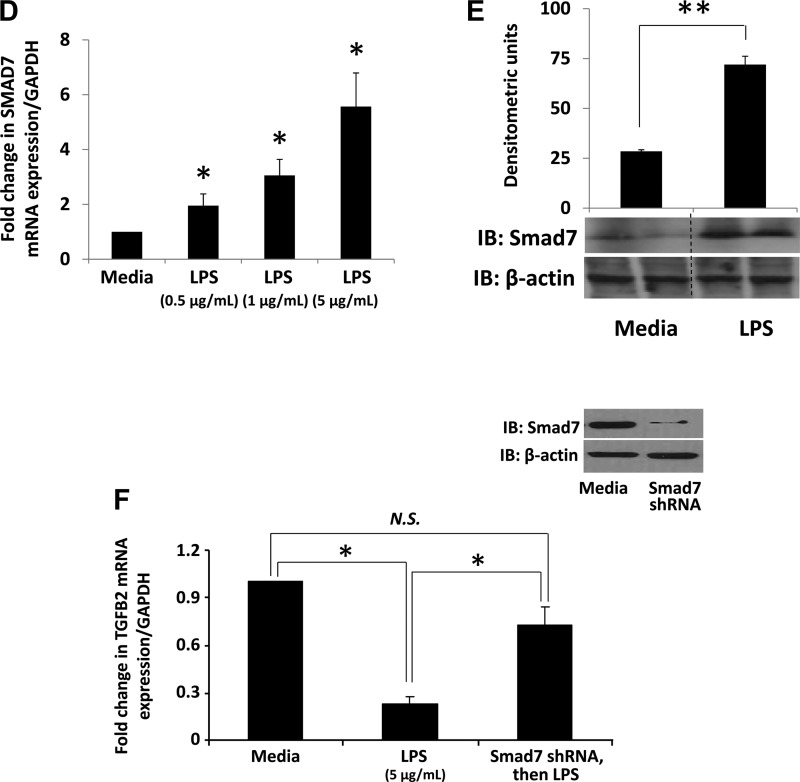
Smad7 inhibits autocrine expression of TGF-β2 in intestinal epithelial cells (IECs). A: Smad7 inhibits the autocrine feed-forward activation of TGF-β2 expression in IEC6 cells. Native and pCMV-Smad7-transfected IEC6 cells were treated with recombinant TGF-β2 (rTGF-β2). Bar diagram (means ± SE) shows fold change in TGF-β2 mRNA. rTGF-β2 increased TGF-β2 mRNA in native IEC6 cells in a dose-dependent fashion, but this autocrine feed-forward activation of TGF-β2 expression was abrogated in cells transfected with pCMV-Smad7. N = 4 per group; Left, inset: bar diagram (mean fold change ± SE) shows increased Smad7 expression in IEC6 cells transfected with pCMV-Smad7; right, inset: Fluorescence photomicrographs show that Smad7 (green) over-expression in IEC6 cells decreased TGF-β2 (red) expression. B and C: LPS inhibits TGF-β2 expression in IEC6 cells. Bar diagram (means ± SE) shows fold change in TGF-β2 mRNA following LPS treatment for 18 h. N = 5 per group (B). LPS suppresses TGF-β2 protein expression. Bar diagram (means ± SE) shows TGF-β2 concentrations in culture supernatants measured by ELISA. N = 3 per group (C). D and E: LPS increases Smad7 expression in IEC6 cells. Bar diagram (means ± SE) shows fold change in Smad7 mRNA following LPS treatment for 18 h (D). IB shows increased Smad7 protein in following LPS treatment (1 μg/ml). Bar diagrams (means ± SE) show densitometric data (E). N = 3 per group. F: Smad7 knockdown prevents LPS-mediated suppression of TGF-β2 expression in IEC6 cells. Bar diagram (means ± SE) shows fold change in TGF-β2 mRNA in IEC6 cells maintained in media alone, after LPS treatment (5 μg/ml), or in IEC6 cells expressing Smad7 shRNA and treated with LPS. Cells were harvested 18 h after LPS treatment. Inset: IB showing inhibition of Smad7 expression in cells expressing Smad7 shRNA. N.S., not significant; *P < 0.05, **P < 0.01, ***P < 0.001.
To elucidate the mechanism(s) that suppress TGF-β2 expression in the preterm intestine and in NEC, we investigated the role of the inhibitory Smads, Smad6 and Smad7 (32). Because bacterial products, which play a key role in NEC (30), can induce Smad7 (but not Smad6) in macrophages (27), subsequent experiments in this study were focused on Smad7. Indeed, Smad7 overexpression blocked the autocrine induction of TGF-β2 in IEC6 cells (Fig. 3A). LPS treatment of IEC6 cells suppressed TGF-β2 mRNA and protein expression (Fig. 3, B and C). Heat-killed Listeria monocytogenes had a similar effect [fold change 0.63 ± 0.15 and 0.58 ± 0.19 at multiplicity of infection 50 and 100, respectively (data not depicted)]. LPS treatment also increased Smad7 expression in a dose-dependent fashion (Fig. 3, D and E). Finally, Smad7 knockdown blocked LPS-mediated suppression of TGF-β2 expression (Fig. 3F). Taken together, these data emphasized Smad7 as a mediator of LPS effects on epithelial TGF-β2 expression.
Increased Smad7 expression in preterm baboon intestine and during NEC.
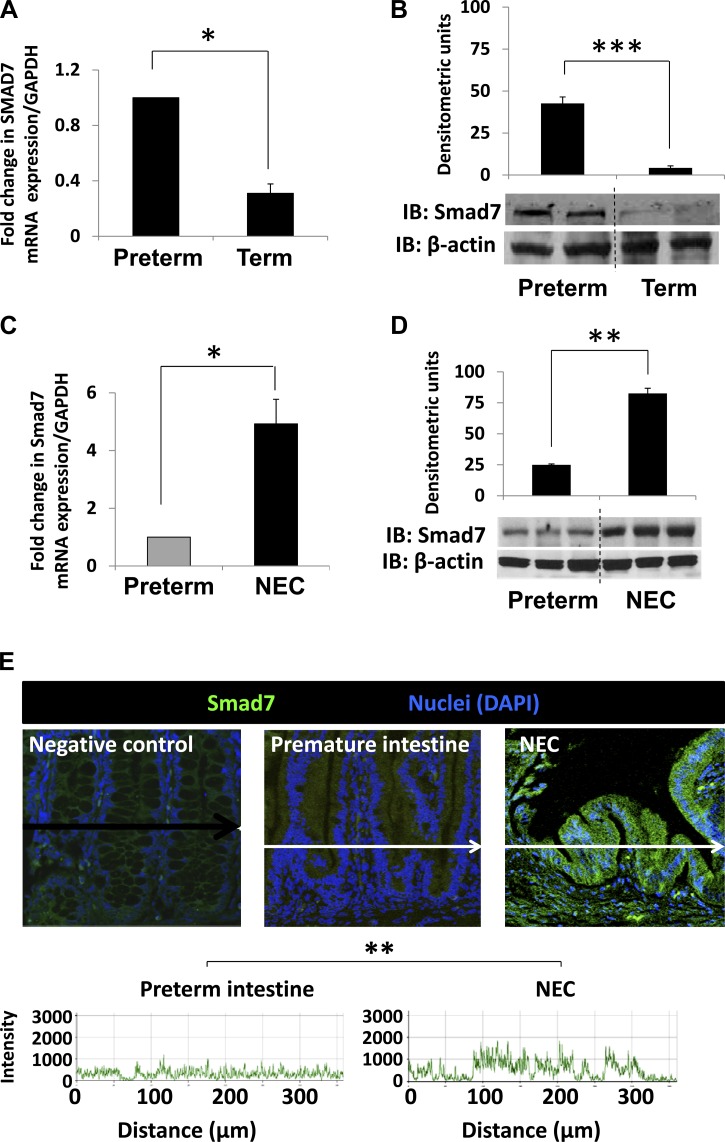
We hypothesized that TGF-β2 deficiency in the preterm intestine and during NEC is due to increased Smad7 expression. This postulation was confirmed upon direct measurements of Smad7 mRNA and protein in preterm and term baboon ileum (Fig. 4, A and B). In tissue samples of NEC, Smad7 expression was even higher than in the normal preterm baboon intestine (Fig. 4, C–E).
Fig. 4.
Increased Smad7 expression in preterm baboon intestine and during NEC. A and B: preterm baboon intestine expresses more Smad7 than the term baboon intestine. Bar diagram (means ± SE) shows fold change in Smad7 mRNA in preterm vs. term baboon ileum (A). IB shows higher Smad7 protein in preterm than in term intestine. Bar diagrams (means ± SE) show densitometric data (B). C, D, and E: Smad7 expression is increased during NEC. Bar diagram (means ± SE) shows fold change in Smad7 mRNA expression in preterm baboon ileum vs. baboon NEC (C). IB shows increased Smad7 protein expression in NEC. Bar diagrams (means ± SE) show densitometric data (D). Immunofluorescence photomicrographs of negative control, normal preterm intestine, and NEC tissues show increased Smad7 immunoreactivity (green) in NEC. Nuclei were stained with DAPI (blue) (E). Fluorescence intensity (488 nm) is depicted in frequency histograms below the photomicrographs. F: bar diagram (mean fold change ± SE) shows mRNA expression for Smad1–7 in human preterm intestine vs. NEC. N = 11 patients with NEC and 8 controls. G: immunofluorescence photomicrographs of negative control, normal preterm human intestine, and human NEC tissues show increased Smad7 immunoreactivity (green) in NEC similar to baboon NEC. Nuclei were stained with DAPI (blue). Fluorescence intensity (488 nm) is depicted in frequency histograms below the photomicrographs. *P < 0.05, **P < 0.01, ***P < 0.001.
Increased Smad7 expression in surgically resected human tissue samples of NEC.
Using PCR and quantitative immunofluorescence, we next measured the mRNA expression of all seven Smad genes in human preterm intestine and NEC. As depicted in Fig. 4F, NEC was associated with a specific increase in Smad7 expression. Smad7 protein was also readily immunolocalized in tissue samples of NEC but not in the normal human premature intestine (Fig. 4G).
LPS promotes Smad7 binding to TGF-β2 promoter and is associated with repressive histone modifications on the nucleosome of TGF-β2.
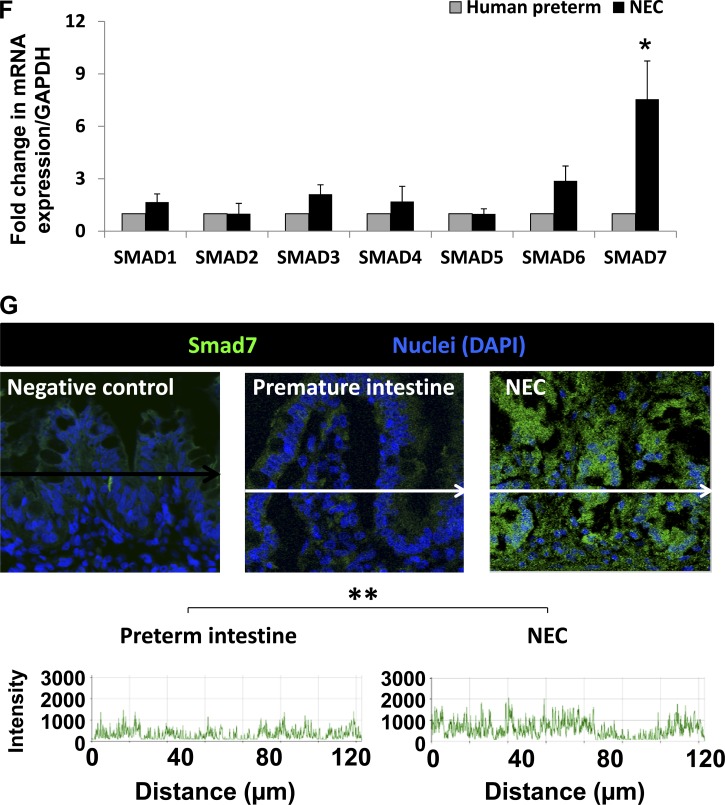
We next investigated the mechanisms by which Smad7 suppresses TGF-β2 expression in IEC6 cells. Using ChIP, we demonstrated that LPS increases Smad7 binding to the TGF-β2 promoter in a dose-dependent fashion (Fig. 5A).
Fig. 5.
LPS promotes Smad7 binding to TGF-β2 promoter and is associated with repressive histone modifications on the nucleosome of TGF-β2. A: LPS treatment increases Smad7 binding to the TGF-β2 promoter in a dose-dependent fashion. Bar diagram (means ± SE) shows data from chromatin immunoprecipitation (ChIP) assay of Smad7 binding to TGF-β2 promoter region. Quantification of Smad7 binding was performed using real-time PCR and is shown as %input. To calculate %input, ChIP results for the specific antibody were determined using a standard curve of input DNA from the same cells. Control IgG ChIP results are subtracted from specific antibody ChIP results. Data are representative of 3 experiments. B: LPS promotes the development of repressive histone modifications in IEC6 cells. IB shows increased H3K9me2 and H4K20me. Bar diagrams (means ± SE) show densitometric data. N = 3 per group. C: LPS promotes the development of repressive histone modifications specifically on the nucleosome of TGF-β2. Bar diagram (means ± SE) show data from ChIP assay of H3K9me2 on TGF-β2 promoter region. Quantification of H3K9me2 was performed using real-time PCR and is shown as %input; D: increased Smad7 binding to the TGF-β2 promoter in baboon NEC. Bar diagram (means ± SE) show data from ChIP assay of Smad7 binding to TGF-β2 promoter region (%input). *P < 0.05, **P < 0.01, ***P < 0.001.
To determine whether Smad7 binding to TGF-β2 promoter in LPS-treated IEC6 cells was associated with repressive posttranslational modifications on histones (45), we measured H3K9me2, H3K27me, and H4K20me first on the entire chromatin and then specifically on the nucleosome of TGF-β2. In Western blot analysis (Fig. 5B), we detected a global increase in H3K9me2 in LPS-treated IEC6 cells. A modest increase was also observed in H4K20me. To confirm the presence of H3K9me2 marks specifically on the TGF-β2 nucleosome, we treated IEC6 cells with LPS and performed a ChIP-quantitative PCR assay. Nuclear extracts of formaldehyde-fixed cells were sonicated and then subjected to immunoprecipitation using anti- H3K9me2 or control IgG. As depicted in Fig. 5C, quantitative PCR confirmed that LPS treatment of IEC6 cells was associated with H3K9me2 marks on the nucleosome of TGF-β2.
To confirm Smad7 binding to TGF-β2 promoter in vivo, we repeated the ChIP assay on tissue samples of normal preterm baboon intestine and baboon NEC. We detected Smad7 binding to the TGF-β2 promoter in the normal preterm baboon intestine and further enrichment of Smad7 in tissue samples of NEC (Fig. 5D).
Developmental deficiency of SKIL can explain increased Smad7 expression in preterm intestine and during NEC.
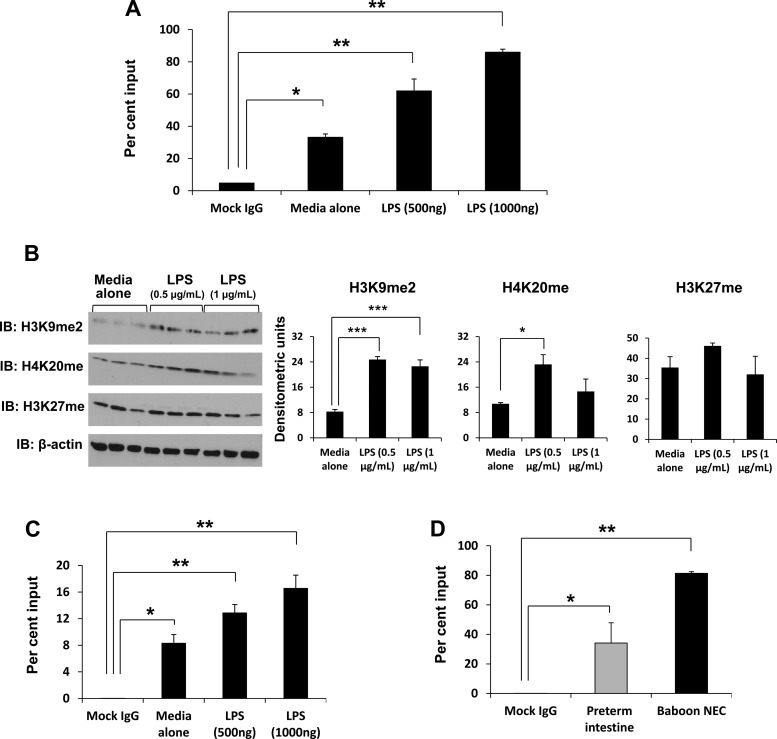
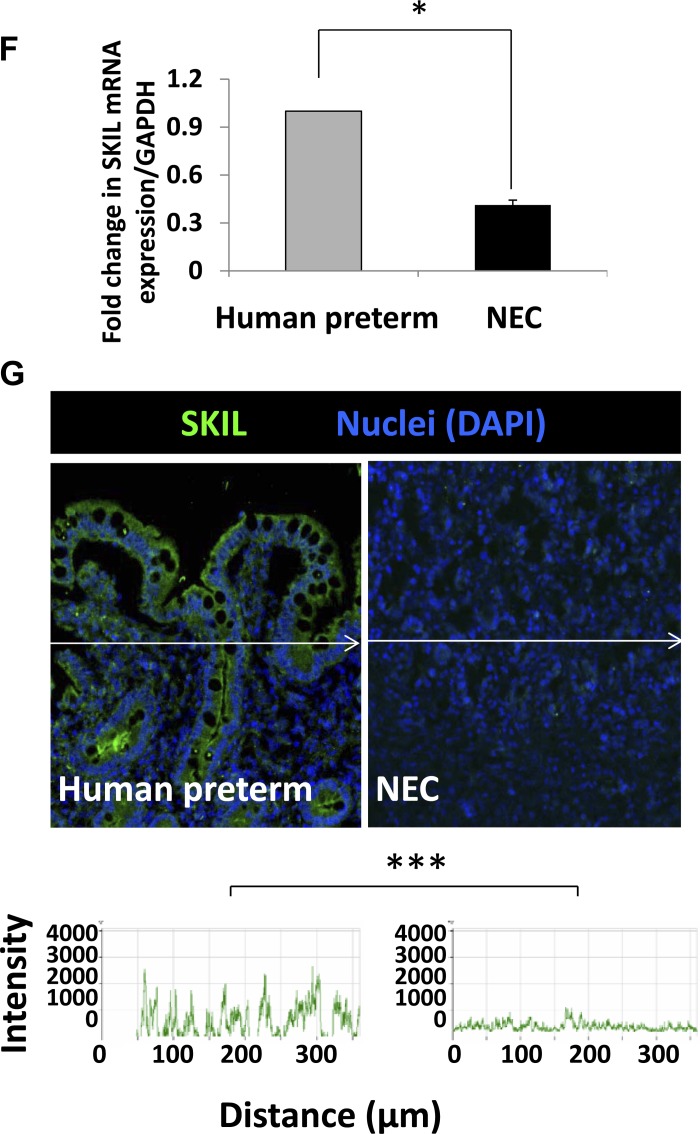
To determine whether increased Smad7 expression in preterm intestine and during NEC could be explained by the deficiency of the Smad7 corepressors SKI and SnoN/SKIL (6, 12, 46), we first measured SKI and SnoN/SKIL mRNA and protein expression in premature vs. term baboon intestine and also in premature intestine vs. baboon NEC. There was a trend toward less SKI expression in preterm intestine (79.5 ± 15.6% of term) and during NEC (66.81 ± 0.11% of premature intestine) that did not reach significance (data not depicted). In contrast, preterm baboon intestine expressed significantly less SnoN/SKIL than term intestine (Fig. 6, A and B). SnoN/SKIL expression was further decreased during baboon NEC (Fig. 6, C–E). These findings were confirmed by real-time RT-PCR and quantitative immunohistochemistry in surgically resected tissue samples of human NEC, indicating that a similar pathway was active in human NEC (Fig. 6, F and G).
Fig. 6.
Developmental deficiency of SKI-like oncoprotein (SKIL) can explain increased Smad7 expression in preterm intestine and during NEC. A and B: SKIL expression is lower in normal preterm baboon intestine (in the absence of NEC) than in the term intestine. Bar diagram (means ± SE) shows fold change in SKIL mRNA in preterm vs. term baboon ileum (A). IB shows lower SKIL in preterm than in term intestine. Bar diagrams (means ± SE) show densitometric data (B). C and D: Increased SKIL expression during baboon NEC. Bar diagram (means ± SE) showing fold change in SKIL mRNA in control preterm baboon ileum vs. baboon NEC (C). Immunofluorescence photomicrographs from baboon control and NEC show decreased SKIL (green) immunoreactivity in NEC (D). Nuclei were stained with DAPI (blue). Fluorescence intensity (488 nm) is depicted in frequency histograms below the photomicrographs. E and F: surgically resected human tissue samples of NEC show lower expression of SKIL than in preterm intestine resected for other indications. Bar diagram (means ± SE) showing fold change in SKIL mRNA in human preterm intestine vs. NEC (E). N = 11 patients with NEC and 8 controls. G: immunofluorescence photomicrographs from control and NEC tissues show decreased SKIL (green) immunoreactivity in NEC. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
We present a detailed investigation of the signaling mechanism(s) that suppress TGF-β2 expression in the developing intestine and during NEC. We demonstrate TGF-β2 expression in IECs is regulated via autocrine induction, and also show that this process is interrupted in the normal preterm intestine and during NEC due to increased expression of Smad7. Smad7 binds to TGF-β2 promoter, where it induces repressive posttranslational modifications on histones H3 and H4 that are characteristically identified with heterochromatin (45). Finally, we provide correlative evidence that increased Smad7 expression in the preterm intestine may be due to the developmental deficiency of the oncoprotein SnoN/SKIL.
To our knowledge, this is the first study to report spontaneous NEC in a premature nonhuman primate model. Our data illustrate the remarkable clinical, radiological, and histopathological similarity between human and baboon NEC. Baboon NEC showed inflammatory changes similar to human NEC, including increased expression of cytokines such as IL-8/CXCL8, CCL4/MIP-1β, and IL-1β, which have been previously documented in human NEC (14, 22, 42, 51). Baboons (and other nonhuman primates) have several advantages in the study of prematurity and its complications: 1) constant environmental conditions can be maintained over long periods of time, which provides an opportunity to investigate the complex interaction between perinatal risk factors for NEC, intensive care practices, and comorbidities related to prematurity; 2) invasive monitoring (and intervention) is feasible over prolonged periods of time, which is difficult in rodent pups; and 3) high degree of homology at the DNA level (nearly 96%) allows scientific interrogation of biological phenomena using the same reagents and techniques as in humans (5, 40, 50).
We detected less TGF-β2 expression in preterm than in the term baboon intestine. These findings are consistent with our previous observations in the premature human intestine (31) and could explain the occurrence of NEC in premature baboons. In the mature intestine, resident macrophages display a profound inflammatory anergy to bacterial products, an adaptive mechanism to minimize mucosal inflammation despite the close physical proximity to luminal bacteria (44). This inflammatory downregulation of macrophages occurs in the developing intestine under the influence of TGF-β2 (31). In the premature intestine, which is deficient in TGF-β2, resident macrophages are yet to undergo this noninflammatory differentiation and display robust inflammatory responses, increasing the risk of inflammatory injury and NEC (31). Consistent with our previous observations in human NEC, TGF-β2 expression was further suppressed in baboon NEC to levels lower than the normal premature intestine. This reduction in TGF-β2 expression is a unique feature of NEC and contrasts with inflammatory conditions of the gastrointestinal tract in older children/adults such as ulcerative colitis and Crohn's disease (11, 36), celiac disease (29), and shigellosis (39), which are associated with increased TGF-β expression. In a recent study, we showed that the inflammatory changes in NEC may be related not to specific etiological factors but may instead represent a generic tissue injury response of the developing gastrointestinal tract (35). We induced nonspecific intestinal injury in neonatal and adult mice by administering 2,4,6-trinitrobenzene sulfonic acid (TNBS); TNBS-enterocolitis increased TGF-β2 expression in adult mice but inhibited its expression in pups (unpublished observation, MohanKumar K, Kaza N, Jagadeeswaran R, Garzon SA, Bansal A, Kurundkar AR, Namachivayam K, Remon JI, Bandepalli R, Feng X, Weitkamp J-H, Maheshwari A.). Our findings in the present study can potentially explain developmental differences in gut mucosal injury and the occurrence of NEC almost exclusively in preterm infants.
Smad7 is an important negative regulator of TGF-β signaling in the gastrointestinal tract (32, 37). In this study, we demonstrate that Smad7 inhibited the autocrine expression of TGF-β2 in IECs. LPS treatment of IEC6 cells was associated with increased Smad7 binding to the TGF-β2 promoter and with dimethylation of the lysine H3K9 on the nucleosome of TGF-β2. H3K9 dimethylation is a repressive modification associated with facultative heterochromatin assembly and the resulting transcriptional silencing (45). In previous studies, Smad7 has been shown to inhibit TGF-β signaling via multiple mechanisms. In the nucleus, Smad7 can introduce epigenetic changes by binding histone deacetylases (HDACs) such as HDAC1 and SIRT1 (silent mating type information regulation-1) and the acetyltransferase p300 (21, 28). It can also block induction of target genes by interfering with the attachment of activating Smads to target promoters (53). In the cytosol, Smad7 can bind TGF-β receptor (TBR)-I directly or in a ternary complex with BAMBI (BMP and activin membrane-bound inhibitor) to inhibit TGF-β signaling (23, 52). It can also recruit E3 ubiquitin ligases to promote TBRI ubiquitination and degradation (13, 26). Smad7 can also engage the phosphatase GADD34 (growth-arrest and DNA-damage-inducible protein 34)-PP1c (protein phosphatase 1c) to dephosphorylate TBRI (43).
We detected increased Smad7 expression in both baboon and human NEC. High Smad7 expression has been previously reported in inflammatory bowel disease, where Smad7 inhibits TGF-β signaling in spite of increased TGF-β expression (unlike NEC, where TGF-β expression is decreased) (37). Smad7 transcription can be activated during intestinal inflammation by bacterial products (as shown in this study) or by inflammatory cytokines through activation of NF-κB or STAT1 (signal transducer and activator of transcription 1) (4, 49). Besides inhibiting TGF-β signaling, Smad7 can also directly activate inflammatory pathways. For instance, Smad7 can bind TBRII to activate extracellular signal-regulated kinases, induce apoptosis by promoting the formation of a TAK1 (TGF-β-activated kinase 1)-MKK3 (mitogen-activated protein kinase kinase)-p38 complex, activate p38 kinase, and facilitate MKK4/JNK activation (15, 34).
We considered several possible mechanism(s) to explain increased Smad7 expression in the preterm intestine and during NEC: 1) deficiency of SKI and/or SnoN/SKIL, corepressors of the Smad7 promoter (6, 12, 46); 2) deficiency of Fox-P3, which normally inhibits Smad7 expression (17); and 3) Smad7 induction by bacterial products and cytokines such as interferon-γ (4, 49). FoxP3 was less relevant because it is not expressed in IECs (9), and similarly, LPS and interferon-γ may be important during NEC but not in the normal premature intestine. Deficiency of SKI and/or SnoN/SKIL could potentially explain increased Smad7 and decreased TGF-β2 in both normal premature intestine and during NEC. In the absence of TGF-β signaling, SnoN/SKIL binds to the Smad7 promoter and maintains it in a state of repression (6, 46). Our findings of decreased SnoN/SKIL expression in the premature intestine is consistent with existing information; SnoN/SKIL is expressed at very low levels in most cell types during the fetal period except for specific developmental epochs (25). SnoN/SKIL can be induced in some tissues by prolonged stimulation with TGF-β (6), and in the developing intestine, decreased TGF-β bioactivity (31) can provide another explanation for the deficiency of SnoN/SKIL.
In conclusion, we have shown that Smad7, which interrupts the normal autocrine expression of TGF-β2 in IECs, may be a potential therapeutic target in NEC. Although the present study has important limitations in the small sample size and the lack of information on the microbiota, a major strength is the use of a nonhuman primate model that accurately mimics human disease. Further study is needed to identify strategies to manipulate Smad7 in vivo and to investigate the global import of this concept in the context of the developing intestine.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants HD-059142 (to A. Maheshwari) and HD-061607 (to J. H. Weitkamp) and research grants from the Robert Wood Johnson Foundation (67067) and the UTHSCSA CTSA UL1RR025767 (to C. L. Blanco). The study was made possible in part by the National Institutes of Health Grants for Facility Support HL-52636 (to Bronchopulmonary Dysplasia Resource Center) and NCRR-P51-RR-013986 (to the Southwest National Primate Research Center now Texas Biomedical Research Institute). C. Blanco received grant ID7-11-BS13 from the American Diabetes Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.N., C.L.B., K.M., and A.M. conception and design of research; K.N., C.L.B., K.M., R.J., M.V., L.M.-V., S.A.G., S.K.J., R.K.G., N.E.F., J.-H.W., S.R.S., and A.M. performed experiments; K.N., C.L.B., K.M., R.J., M.V., L.M.-V., S.A.G., S.K.J., R.K.G., N.E.F., J.-H.W., S.R.S., and A.M. analyzed data; K.N., C.L.B., K.M., R.J., M.V., L.M.-V., S.A.G., S.K.J., R.K.G., N.E.F., J.-H.W., S.R.S., and A.M. interpreted results of experiments; K.N., C.L.B., K.M., and A.M. drafted manuscript; K.N., C.L.B., K.M., R.J., M.V., L.M.-V., S.A.G., S.K.J., R.K.G., N.E.F., J.-H.W., S.R.S., and A.M. edited and revised manuscript; K.N., C.L.B., K.M., R.J., M.V., L.M.-V., S.A.G., S.K.J., R.K.G., N.E.F., J.-H.W., S.R.S., and A.M. approved final version of manuscript; K.M. and A.M. prepared figures.
REFERENCES
- 1. Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiological genomics 21: 389–395, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Barnard JA, Beauchamp RD, Coffey RJ, Moses HL. Regulation of intestinal epithelial cell growth by transforming growth factor type-β. Proc Natl Acad Sci USA 86: 1578–1582, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benjamin JT, Smith RJ, Halloran BA, Day TJ, Kelly DR, Prince LS. FGF-10 is decreased in bronchopulmonary dysplasia and suppressed by Toll-like receptor activation. Am J Physiol Lung Cell Mol Physiol 292: L550–L558, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bitzer M, von Gersdorff G, Liang D, Dominguez-Rosales A, Beg AA, Rojkind M, Bottinger EP. A mechanism of suppression of TGF-β/SMAD signaling by NF-κB/RelA. Genes Dev 14: 187–197, 2000 [PMC free article] [PubMed] [Google Scholar]
- 5. Blanco CL, Liang H, Joya-Galeana J, DeFronzo RA, McCurnin D, Musi N. The ontogeny of insulin signaling in the preterm baboon model. Endocrinology 151: 1990–1997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Briones-Orta MA, Sosa-Garrocho M, Moreno-Alvarez P, Fonseca-Sanchez MA, Macias-Silva M. SnoN co-repressor binds and represses smad7 gene promoter. Biochem Biophys Res Commun 341: 889–894, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Brocke S, Hahn H. Heat-killed Listeria monocytogenes and L. monocytogenes soluble antigen induce clonable CD4+ T lymphocytes with protective and chemotactic activities in vivo. Infect Immun 59: 4531–4539, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carey MF, Peterson CL, Smale ST. Chromatin immunoprecipitation (ChIP). Cold Spring Harb Protoc doi:10.1101/pdb.prot5279, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Chen GY, Chen C, Wang L, Chang X, Zheng P, Liu Y. Cutting edge: broad expression of the FoxP3 locus in epithelial cells: a caution against early interpretation of fatal inflammatory diseases following in vivo depletion of FoxP3-expressing cells. J Immunol 180: 5163–5166, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med 160: 1333–1346, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Del Zotto B, Mumolo G, Pronio AM, Montesani C, Tersigni R, Boirivant M. TGF-β1 production in inflammatory bowel disease: differing production patterns in Crohn's disease and ulcerative colitis. Clin Exp Immunol 134: 120–126, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Denissova NG, Liu F. Repression of endogenous Smad7 by Ski. J Biol Chem 279: 28143–28148, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T, Miyazono K. Smurf1 interacts with transforming growth factor-β type I receptor through Smad7 and induces receptor degradation. J Biol Chem 276: 12477–12480, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics 103: 766–771, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Edlund S, Bu S, Schuster N, Aspenstrom P, Heuchel R, Heldin NE, ten Dijke P, Heldin CH, Landstrom M. Transforming growth factor-β1 (TGF-β)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-β-activated kinase 1 and mitogen-activated protein kinase kinase 3. Mol Biol Cell 14: 529–544, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eliseev RA, Schwarz EM, Zuscik MJ, O'Keefe RJ, Drissi H, Rosier RN. Smad7 mediates inhibition of Saos2 osteosarcoma cell differentiation by NF-κB. Exp Cell Res 312: 40–50, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-β induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol 172: 5149–5153, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Ferguson RE, Carroll HP, Harris A, Maher ER, Selby PJ, Banks RE. Housekeeping proteins: a preliminary study illustrating some limitations as useful references in protein expression studies. Proteomics 5: 566–571, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Freitag NE, Rong L, Portnoy DA. Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect Immun 61: 2537–2544, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frost BL, Jilling T, Caplan MS. The importance of pro-inflammatory signaling in neonatal necrotizing enterocolitis. Semin Perinatol 32: 100–106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gronroos E, Hellman U, Heldin CH, Ericsson J. Control of Smad7 stability by competition between acetylation and ubiquitination. Mol Cell 10: 483–493, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Harris MC, Costarino AT, Jr, Sullivan JS, Dulkerian S, McCawley L, Corcoran L, Butler S, Kilpatrick L. Cytokine elevations in critically ill infants with sepsis and necrotizing enterocolitis. J Pediatr 124: 105–111, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA, Jr, Wrana JL, Falb D. The MAD-related protein Smad7 associates with the TGF-β receptor and functions as an antagonist of TGF-β signaling. Cell 89: 1165–1173, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Hsueh W, Caplan MS, Tan X, MacKendrick W, Gonzalez-Crussi F. Necrotizing enterocolitis of the newborn: pathogenetic concepts in perspective. Pediatr Dev Pathol 1: 2–16, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jahchan NS, Luo K. SnoN in mammalian development, function and diseases. Curr Opin Pharmacol 10: 670–675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF-β receptor for degradation. Mol Cell 6: 1365–1375, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Kim EY, Kim BC. Lipopolysaccharide inhibits transforming growth factor-β1-stimulated Smad6 expression by inducing phosphorylation of the linker region of Smad3 through a TLR4-IRAK1-ERK1/2 pathway. FEBS Lett 585: 779–785, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Kume S, Haneda M, Kanasaki K, Sugimoto T, Araki S, Isshiki K, Isono M, Uzu T, Guarente L, Kashiwagi A, Koya D. SIRT1 inhibits transforming growth factor β-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J Biol Chem 282: 151–158, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Lahat N, Shapiro S, Karban A, Gerstein R, Kinarty A, Lerner A. Cytokine profile in coeliac disease. Scand J Immunol 49: 441–446, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Maheshwari A, Corbin LL, Schelonka RL. Neonatal necrotizing enterocolitis. Res Rep Neonatol 1: 39–53 2011 [Google Scholar]
- 31. Maheshwari A, Kelly DR, Nicola T, Ambalavanan N, Jain SK, Murphy-Ullrich J, Athar M, Shimamura M, Bhandari V, Aprahamian C, Dimmitt RA, Serra R, Ohls RK. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 140: 242–253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Massagué J. How cells read TGF-β signals. Nat Rev Mol Cell Biol 1: 169–178, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Maynard AA, Dvorak K, Khailova L, Dobrenen H, Arganbright KM, Halpern MD, Kurundkar AR, Maheshwari A, Dvorak B. Epidermal growth factor reduces autophagy in intestinal epithelium and in the rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299: G614–G622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mazars A, Lallemand F, Prunier C, Marais J, Ferrand N, Pessah M, Cherqui G, Atfi A. Evidence for a role of the JNK cascade in Smad7-mediated apoptosis. J Biol Chem 276: 36797–36803, 2001 [DOI] [PubMed] [Google Scholar]
- 35. MohanKumar K, Kaza N, Jagadeeswaran R, Garzon SA, Bansal A, Kurundkar AR, Namachivayam K, Remon JI, Bandepalli R, Feng X, Weitkamp J-H, Maheshwari A. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am J Physiol Gastrointest Liver Physiol 303: G93–G102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monteleone G, Mann J, Monteleone I, Vavassori P, Bremner R, Fantini M, Del Vecchio Blanco G, Tersigni R, Alessandroni L, Mann D, Pallone F, MacDonald TT. A failure of transforming growth factor-β1 negative regulation maintains sustained NF-κB activation in gut inflammation. J Biol Chem 279: 3925–3932, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Monteleone G, Pallone F, MacDonald TT. Smad7 in TGF-β-mediated negative regulation of gut inflammation. Trends Immunol 25: 513–517, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Neu J. Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr Clin North Am 43: 409–432, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raqib R, Lindberg AA, Wretlind B, Bardhan PK, Andersson U, Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun 63: 289–296, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seidner SR, Chen YQ, Oprysko PR, Mauray F, Tse MM, Lin E, Koch C, Clyman RI. Combined prostaglandin and nitric oxide inhibition produces anatomic remodeling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr Res 50: 365–373, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Shaik SS, Soltau TD, Chaturvedi G, Totapally B, Hagood JS, Andrews WW, Athar M, Voitenok NN, Killingsworth CR, Patel RP, Fallon MB, Maheshwari A. Low intensity shear stress increases endothelial ELR+ CXC chemokine production via a focal adhesion kinase-p38β MAPK-NF-κB pathway. J Biol Chem 284: 5945–5955, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharma R, Tepas JJ, 3rd, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, Premachandra BR. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg 42: 454–461, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Shi W, Sun C, He B, Xiong W, Shi X, Yao D, Cao X. GADD34-PP1c recruited by Smad7 dephosphorylates TGF-β type I receptor. J Cell Biol 164: 291–300, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest 115: 66–75, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Strahl BD, Allis CD. The language of covalent histone modifications. Nature 403: 41–45, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Stroschein SL, Wang W, Zhou S, Zhou Q, Luo K. Negative feedback regulation of TGF-β signaling by the SnoN oncoprotein. Science 286: 771–774, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Suemori S, Ciacci C, Podolsky DK. Regulation of transforming growth factor expression in rat intestinal epithelial cell lines. J Clin Invest 87: 2216–2221, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sutherland MR, Yoder BA, McCurnin D, Seidner S, Gubhaju L, Clyman RI, Black MJ. Effects of ibuprofen treatment on the developing preterm baboon kidney. Am J Physiol Renal Physiol 302: (10) F1286–F1292, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-β/SMAD signalling by the interferon-gamma/STAT pathway. Nature 397: 710–713, 1999 [DOI] [PubMed] [Google Scholar]
- 50. VandeBerg JL, Williams-Blangero S. Advantages and limitations of nonhuman primates as animal models in genetic research on complex diseases. J Med Primatol 26: 113–119, 1997 [DOI] [PubMed] [Google Scholar]
- 51. Viscardi RM, Lyon NH, Sun CC, Hebel JR, Hasday JD. Inflammatory cytokine mRNAs in surgical specimens of necrotizing enterocolitis and normal newborn intestine. Pediatr Pathol Lab Med 17: 547–559, 1997 [PubMed] [Google Scholar]
- 52. Yan X, Lin Z, Chen F, Zhao X, Chen H, Ning Y, Chen YG. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-β signaling. J Biol Chem 284: 30097–30104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, Han Y, Feng XH, Meng A, Chen YG. Smad7 antagonizes transforming growth factor-β signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol 27: 4488–4499, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]