Abstract
The bile salt export pump, encoded by ABCB11, is the predominant canalicular transport protein for biliary bile acid secretion. The level of ABCB11 expression in humans is widely variable yet the impact of this variability on human disease is not well defined. We aim to determine the effect of hepatic Abcb11 overexpression on the enterohepatic circulation (EHC) in mice. We used a stable isotope dilution technique in transgenic mice overexpressing hepatic Abcb11 (TTR-Abcb11) to determine the pool size, fractional turnover rate (FTR), and synthesis rate of the primary bile acid, cholic acid (CA). The gallbladder was cannulated to determine bile flow, bile acid composition, and the biliary secretion rates of CA, total bile acids, phospholipid, and cholesterol. The combined data allowed for estimation of the CA cycling time and the fraction of CA lost per cycle. Hepatic and intestinal gene and protein expression were determined by qPCR and Western blot. Abcb11 overexpression strongly decreased FTR and synthesis rate of CA. Abcb11 overexpression decreased the fraction of CA that was lost per cycle of the EHC. Hepatic expression of Cyp7a1 was suppressed by nearly 50% and ileal expression of FGF15 was increased more than eightfold in TTR-Abcb11 mice. Despite the increased intestinal reabsorption of bile acids, ileal Asbt expression was suppressed. Hepatic Abcb11 overexpression in mice increases the conservation of bile acids within the enterohepatic circulation. These data provide strong evidence for the existence of feed-forward communication between hepatic expression of a bile acid transport protein and the intestine.
Keywords: bile salt export pump, cholic acid, stable isotope dilution
bile acids perform numerous critical functions within the human body. These amphipathic molecules function as detergents in the small intestine, which solubilize dietary fats, cholesterol, and fat-soluble vitamins. Bile acids are essential for generating bile flow and stimulating the biliary excretion of cholesterol. Additionally, bile acids serve as regulatory molecules within the enterohepatic circulation and beyond (14, 18, 22). When present in excess, however, bile acids produce toxicity as exemplified by the development of cholestatic liver disease. As such, the synthesis and excretion of bile acids are tightly regulated.
The enterohepatic circulation of bile acids is an essential process by which the human body conserves bile acids. Bile acids are synthesized in the liver from cholesterol and are subsequently secreted across the hepatic canalicular membrane by the ATP binding cassette transporter, ABCB11 (formerly known as the bile salt export pump or BSEP) (3, 7). Bile acids are transferred in bile to the lumen of the small intestine, where they solubilize dietary lipids into mixed micelles. Upon reaching the ileum, bile acids are taken up by the apical sodium-dependent bile acid transporter (ASBT) in ileocytes (16). The majority of bile acids are reabsorbed in the ileum and only a small fraction is lost by fecal excretion (13). Within the ileocyte, bile acids are transported to the basolateral membrane where they are secreted to enter the portal circulation by the heteromeric organic solute transporter α/β (5). Bile acids are then returned to the liver via the portal circulation.
Although the enterohepatic circulation of bile acids is highly efficient, under steady-state conditions de novo synthesis of bile acids must occur in the liver to replenish the small fraction of bile acids that is lost by fecal excretion. The rate-limiting step of the major bile acid synthetic pathway is catalyzed by the cytochrome P-450 enzyme cholesterol 7α-hydroxylase (CYP7A1) (3, 4). It is now well established that bile acids regulate their own synthesis via feedback inhibition of hepatic CYP7A1 (3, 17). Within the ileocyte, bile acids bind to the farnesoid X receptor (FXR), stimulating transcription of ileal fibroblast growth factor (FGF) 15/19 (11). FGF 15/19 travels to the liver, where it binds its receptor, FGFR4, leading to suppression of hepatic CYP7A1 via a yet undiscovered mechanism.
Transport across the canalicular membrane by ABCB11 is the rate-limiting step in the hepatocellular transport of bile acids (21). Mutations resulting in complete loss of function of ABCB11 have been identified in humans. This genetic defect causes disruption of the EHC leading to progressive familial intrahepatic cholestasis type 2, which results in cirrhosis and death at a young age if liver transplantation is not performed (23). ABCB11 has been shown to be highly polymorphic in humans, and its level of expression is widely variable (15). Yet the impact of this wide variability in expression on human disease is not well defined. Moreover, the effect of overexpression of Abcb11 on the enterohepatic circulation of bile acids is incompletely understood (6).
Cholic acid is a primary bile acid in humans and rodents. Cholic acid pool size, fractional turnover rate (FTR), and synthesis rate are the kinetic parameters that allow description of its production and conservation in the body and estimation of relevant parameters of enterohepatic cycling, such as cycling time and efficiency of intestinal reabsorption. Isotope dilution techniques, applying radioactive or stable isotopes, are the preferred method to study bile acid kinetics in vivo and have contributed significantly to the present knowledge of bile acid physiology in humans. We have developed a microscale stable isotope dilution procedure for cholic acid that allows isotope enrichment measurements in small amounts of plasma. Bile salt pool size, FTR, and synthesis rate can be determined simultaneously in vivo in small animals, without interruption of the enterohepatic circulation (9).
We have previously generated transgenic mice overexpressing Abcb11 on a liver-specific transthyretin promoter (TTR-Abcb11) (6). We now aim to determine the effect of Abcb11 overexpression on the enterohepatic circulation of bile acids using microscale stable isotope dilution.
METHODS
Animals.
Male TTR-Abcb11 mice (C57BL/6 background) and wild-type C57BL/6J control mice (Jackson Laboratories, Bar Harbor, ME) age 8–10 wk were housed in colony cages with a 14:10-h light-dark cycle and were given free access to standard rodent chow (Harlan-Teklad, Madison, WI) and water. All animal protocols were approved by the Northwestern University Animal Care and Use Committee.
Experimental procedure.
The stable isotope dilution technique was performed in TTR-Abcb11 mice and control mice (each n = 10) under steady-state conditions without interruption of the EHC as previously described and validated (9, 10, 12, 24). Briefly, the isotope enrichment of administered 2,2,4,4-2H4-cholate was measured in plasma over time, from which the pool size, FTR, and synthesis of cholic acid was calculated. The atom percent excess was determined on plasma total cholic acid, i.e., combining the conjugated (glycine, taurine) and unconjugated cholate, as previously described (9). After the mice were anesthetized with Hypnorm-diazepam mixture, the gallbladder was cannulated during 30 min and bile flow was determined gravimetrically (1 g/ml). During the cannulation, body temperature was maintained by placing the mice in a humidified incubator (37°C). At the end of the experimental protocol, mice were euthanized by heart puncture to obtain a large blood sample, followed by cervical dislocation (bile cannulation experiment) or by CO2 inhalation (hepatic and intestinal mRNA analysis). The livers were rapidly excised, sectioned, and snap frozen in liquid nitrogen. The small intestine was removed, flushed with ice-cold saline, sectioned into 5-cm segments, and snap frozen in liquid nitrogen. Ileal tissue was defined as the distal 5 cm of the small intestine. The livers and small intestine were stored at −80°C until analysis.
Calculations.
The isotope dilution technique calculations, enterohepatic cycling time, and intestinal reabsorption of cholate have been described in detail by Kok et al. (12). The cholate cycling time was calculated by dividing the cholate pool size (mmol/100 g) by the biliary secretion rate of cholate (mmol·100 g−1·h−1). The cholate biliary secretion rate was calculated by multiplying the bile flow (ml·100 g−1·h−1) by the cholate concentration (mM) in a single 30-min fraction, obtained between 5 and 35 min after cannulation of the gallbladder. The amount of reabsorbed cholate per day was calculated by multiplying cholate pool size by cycling frequency and subsequent subtraction of the daily cholate synthesis rate.
Analysis of gene expression by quantitative PCR.
Total RNA from frozen liver and intestinal samples was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA). Two micrograms of total RNA was used for reverse transcription PCR with a qScript cDNA Synthesis Kit (Quanta BioSciences, Gaithersburg, MD). Real-time quantitative PCR was performed as previously described (8) by using Quantitect SYBR Green PCR Mastermix (Qiagen, Valencia, CA) along with primers specific for the gene of interest. GAPDH was employed as a housekeeping gene. Amplification was performed on an ABI 7300 sequence detector (Applied Biosystems, Foster City, CA).
Analysis of bile and of protein expression.
Bile salt concentration in bile was determined by an enzymatic fluorometric assay (19). Bile salt composition of bile samples was determined by capillary gas chromatography (12). Western blot analysis was performed as previously described (8). Protein detection was performed with polyclonal rabbit antibodies to ASBT (Santa Cruz Biotechnology, Santa Cruz, CA) and monoclonal mouse antibodies to β-actin (Sigma-Aldrich). Bound antibody was detected by use of donkey anti-goat or goat anti-mouse polyclonal HRP antibody (Santa Cruz Biotechnology) and developed with ECL Western Blotting Substrate (Thermo Scientific). A representative Western blot of pooled samples is shown.
Statistics.
Statistical analyses was performed by Mann-Whitney U-test using the SPSS version 12.0.2 software (Chicago, IL) or by Student's t-test. Differences were considered significant at the level of P < 0.05.
RESULTS
Effect of Abcb11 overexpression on kinetic parameters of cholate metabolism.
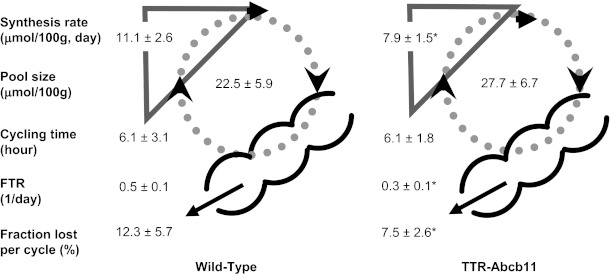
To evaluate the physiological consequences of Abcb11 overexpression, kinetic parameters of the enterohepatic circulation of cholate were determined by stable isotope dilution (Fig. 1). The percentage of cholic acid in biliary bile was used to calculate pool size, cycling frequency, cholic acid cycling time, daily cholic acid absorption, and the percentage of cholic acid absorption. Analysis of plasma cholate enrichment over time demonstrated that the cholate pool size was similar in TTR-Abcb11 and wild-type mice. The FTR was lower in TTR-Abcb11 mice compared with wild-type controls. The calculated cholate synthesis rate was also significantly decreased in TTR-Abcb11 mice. The calculated cholate cycling frequency was unaffected by Abcb11 overexpression. The calculated absolute amount of cholic acid reabsorbed in the intestine tended to be higher in TTR-Abcb11 mice compared with control mice but did not reach statistical significance (P = 0.06). The fraction of cholic acid lost by fecal excretion was significantly decreased in TTR-Abcb11 mice compared with wild-type controls.
Fig. 1.
Effect of Abcb11 overexpression on parameters of cholic acid metabolism. Cholic acid synthesis rate, pool size, cycling time, fractional turnover rate (FTR), and fraction lost per cycle were determined by using a microscale stable isotope dilution technique in mice overexpressing hepatic Abcb11 (TTR-Abcb11). *P < 0.05 vs. wild-type controls.
Effect of Abcb11 overexpression on bile formation.
Biliary bile flow was not significantly different in TTR-Abcb11 mice compared with wild-type controls (Table 1). Biliary bile acid secretion in TTR-Abcb11 mice also did not differ statistically from wild-type mice. Biliary cholic acid secretion in TTR-Abcb11 mice showed a trend toward increase that was not statistically different from wild-type mice. Biliary cholesterol and phospholipid secretion was unchanged between TTR-Abcb11 and wild-type mice.
Table 1.
Biliary bile flow and secretion rates in TTR-Abcb11 mice
| Wild-type | TTR-Abcb11 | |
|---|---|---|
| Bile flow, μl · min−1 · 100 g−1 | 4.6 ± 1.1 | 4.2 ± 0.5 |
| Biliary bile acid secretion, nmol · min−1 · 100 g−1 | 101.1 ± 24.5 | 112.4 ± 42.6 |
| Biliary cholic acid secretion, nmol · min−1 · 100 g−1 | 69.3 ± 20.4 | 83.1 ± 33.2 |
| Biliary cholesterol secretion, nmol · min−1 · 100 g−1 | 1.2 ± 0.4 | 1.3 ± 0.4 |
| Biliary phospholipid secretion, nmol · min−1 · 100 g−1 | 21.4 ± 3.8 | 21.4 ± 5.4 |
Effect of Abcb11 overexpression on bile acid pool composition.
Analysis of biliary bile acid composition is shown in Table 2. Cholic acid was the major biliary bile acid in both TTR-Abcb11 mice and in wild-type mice, and this fraction was significantly higher in TTR-Abcb11 mice compared with wild-type controls. Biliary deoxycholic acid was increased significantly in TTR-Abcb11 mice compared with wild-type mice. β-Muricholic acid and chenodeoxycholic acid were decreased in bile of TTR-Abcb11 mice compared with controls.
Table 2.
Biliary bile acid composition in TTR-Abcb11 mice
| Wild-type | TTR-Abcb11 | |
|---|---|---|
| α-Muricholic acid, % | 7.3 ± 1.3 | 6.3 ± 0.9 |
| β-Muricholic acid, % | 12.4 ± 4.9 | 6.6 ± 2.1* |
| ω-Muricholic acid, % | 6.2 ± 2.0 | 6.0 ± 3.0 |
| Cholic acid, % | 68.2 ± 5.4 | 73.2 ± 4.6* |
| Chenodeoxycholic acid, % | 3.5 ± 0.5 | 2.7 ± 0.6* |
| Deoxycholic acid, % | 2.4 ± 1.0 | 5.1 ± 1.9* |
P < 0.05 versus wild-type.
Effect of Abcb11 overexpression on gene expression of transporters within the enterohepatic circulation.
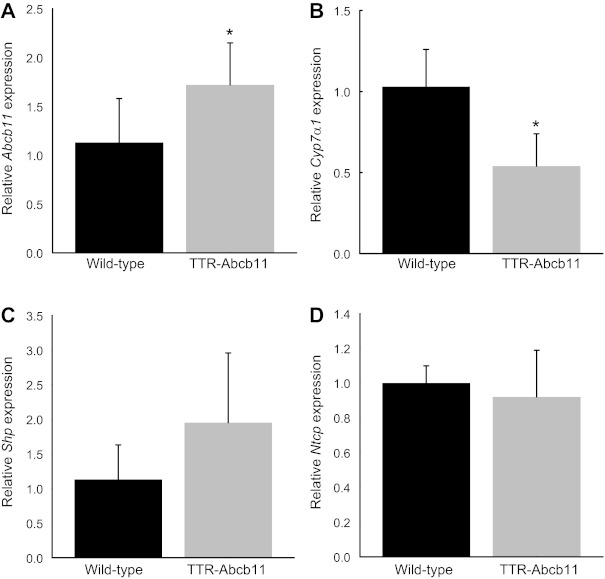
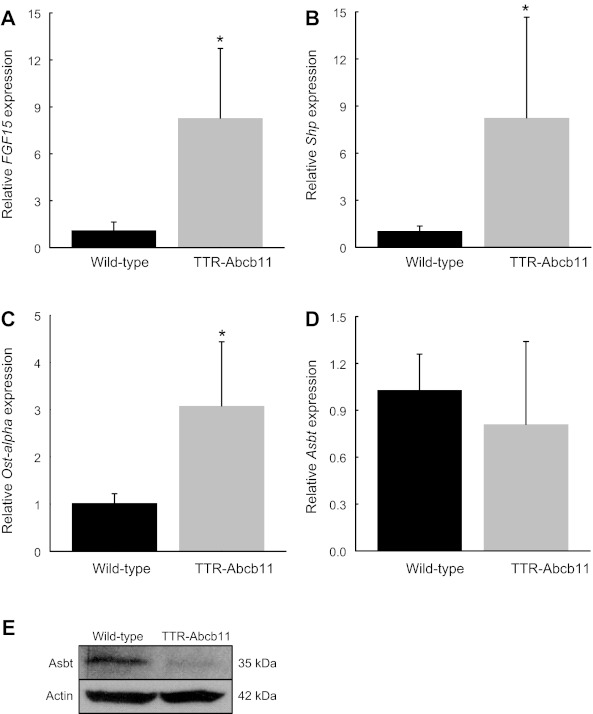
Following the observed changes in cholate synthesis rate and fecal excretion, we measured the hepatic and intestinal gene expression of key enzymes and transporters within the enterohepatic circulation. Hepatic expression of Abcb11 was increased by 66% in TTR-Abcb11 mice compared with WT controls (Fig. 2A). Hepatic expression of Cyp7a1, the rate-limiting enzyme for bile acid synthesis, was suppressed by nearly 50% in TTR-Abcb11 mice (Fig. 2B). Hepatic expression of the short heterodimer partner (Shp) and the sodium taurocholate cotransporting polypeptide (Ntcp) were not significantly different between TTR-Abcb11 mice and wild-type controls (Fig. 2, C and D). Ileal expression of FGF15 was significantly increased in TTR-Abcb11 mice (Fig. 3A). Ileal expression of Shp and Ostα was also increased in TTR-Abcb11 mice compared with controls (Fig. 3, B and C). TTR-Abcb11 mice showed a trend toward decreased expression of ileal Asbt at the transcriptional level (Fig. 3D). Given that Asbt is also subject to posttranscriptional regulation (20), we measured ileal Asbt protein expression. There was a significant decrease in ileal Asbt protein in TTR-Abcb11 mice (Fig. 3E).
Fig. 2.
Hepatic gene expression in TTR-Abcb11 mice. Hepatic mRNA levels of Abcb11 (A), Cyp7a1 (B), Shp (C), and Ntcp (D) in TTR-Abcb11 mice relative to wild-type controls. Means (n = 5) ± SD. *P < 0.05.
Fig. 3.
Intestinal gene and protein expression in TTR-Abcb11 mice. Ileal mRNA levels of FGF15 (A), Shp (B), Ostα (C), and Asbt (D) in TTR-Abcb11 mice relative to wild-type controls. Means (n = 5) ± SD. *P < 0.05. E: representative Western blot of ileal Asbt protein expression in wild-type and TTR-Abcb11 mice. Samples are pooled (n = 4). *P < 0.05 vs. wild-type.
DISCUSSION
Bile acids perform essential physiological functions including facilitating intestinal lipid absorption, generating bile flow, promoting hepatic excretion of cholesterol into bile, and activating FXR and MAPK signaling pathways (1, 14, 18, 22). Tight regulation of the enterohepatic circulation of bile acids is crucial to maintain these processes without producing toxicity.
Abcb11 controls the rate-limiting step in the hepatocellular transport of bile acids, yet the effect of Abcb11 overexpression on the kinetics of the enterohepatic circulation of bile acids was previously unknown. Mice bearing a genetic deletion of Abcb11 have a more hydrophilic bile acid pool and therefore do not spontaneously develop severe cholestasis despite having impaired bile acid secretion (25). Furthermore, heterozygosity of Abcb11 in mice has minimal phenotypic effects suggesting that decreasing the gene dose by 50% does not affect the system, at least not under physiological circumstances. In the present study we used a microscale stable isotope dilution technique to measure the kinetics of cholate metabolism in mice overexpressing hepatic Abcb11. We have determined that Abcb11 overexpression in mice decreases bile acid synthesis and decreases fecal bile acid loss. These data indicate that Abcb11 overexpression promotes the conservation of bile acids within the enterohepatic circulation.
The mechanism by which Abcb11 overexpression decreases fecal bile acid excretion is not clear. Asbt is the ileal transporter responsible for the active reabsorption of intestinal bile acids (16). TTR-Abcb11 mice did not demonstrate enhanced expression of Asbt to account for the decreased fecal excretion of bile acids. In fact, Asbt expression was suppressed at the protein level consistent with FXR-mediated negative feedback regulation of Asbt by bile acids (2). These results are in contrast to data in lactating rats, in which increased hepatic expression of Abcb11 is associated with increased ileal taurocholate absorption and increased ileal expression of Asbt protein (20). The reduced fecal bile acid excretion in the setting of decreased Asbt expression may indicate an Asbt-independent mechanism of enhanced intestinal uptake of bile acids. Similarly, Kok et al. (12) have shown that fxr−/− mice demonstrate a twofold increase in intestinal bile acid reabsorption yet exhibit no increase in ileal Asbt protein expression. In our model the Asbt-independent bile acid uptake may be evoked by overexpression of Abcb11. Alternatively, it must be considered that despite reduced Asbt expression in our model, the remaining expression may suffice for uptake and thus does not exert a rate-limiting influence on bile acid reuptake, similar to reported data in fxr−/− mice (12).
Hepatic Abcb11 overexpression increased the expression of numerous bile acid-responsive genes without expanding the cholic acid pool size. It was previously postulated that this seemingly paradoxical finding could be due to enhanced cycling of bile acids in mice overexpressing Abcb11 (6). We have now directly tested this hypothesis and have determined that hepatic Abcb11 overexpression does not decrease the estimated cycling time of bile acids. Instead we hypothesize that the stimulation of bile acid-responsive genes in this model is due to the shift in bile acid pool composition. Hydrophobic bile acids, such as deoxycholic acid, have a stronger affinity for FXR than hydrophilic bile acids. The increased content of deoxycholic acid in the bile acid pool of TTR-Abcb11 mice may account, at least partly, for the increased expression of bile acid-responsive genes including FGF15. Stimulation of FGF15, and the resultant inhibition of Cyp7a1, causes the observed suppression of bile acid synthesis in TTR-Abcb11 mice.
In contrast to our prior studies (6), TTR-Abcb11 mice in the present study did not demonstrate increased secretion of biliary bile acids, cholesterol, or phospholipids. The present studies were performed using TTR-Abcb11 mice in a C57BL/6 background whereas the prior studies used TTR-Abcb11 mice in a FVB background. When comparing these two models it is important to note that the level of overexpression of Abcb11 in the C57BL/6 strain is lower than that in the FVB strain. As shown in Fig. 2, TTR-Abcb11 mice in a C57BL/6 background showed only a 66% increase in Abcb11 expression, compared with a more than twofold expression observed in the TTR-Abcb11 mice in a FVB background (6). This observation may explain the observed differences in biliary secretion as well as the differences in hepatic Shp expression between the two strains. We originally proposed that the suppression of Cyp7a1 observed in TTR-Abcb11 mice was due to increased activation of hepatic Shp (6). In TTR-Abcb11 mice exhibiting a lower level of overexpression, Cyp7a1 expression remains suppressed yet the upregulation of Shp is no longer observed. Instead we find that FGF15 expression is markedly elevated, indicating that the primary mechanism of Cyp7a1 suppression in this model is mediated by ileal FGF15 rather than hepatic Shp. At the time of our original study, the importance of ileal FGF15 in bile-acid mediated feedback regulation of Cyp7a1 was not yet appreciated and, therefore, was not measured.
We note that caution is warranted with respect to the estimation of bile acid flux rates per day derived from measurements for only 30 min as was performed in this study. These limitations apply to both wild-type and transgenic mice and hence the relative differences between groups may be more valuable than the absolute values.
The finding that intestinal reabsorption of bile salts is affected by changing the hepatic expression of Abcb11 is unexpected and suggests the existence of a “feed-forward” communication between the liver and the intestine. The nature of this communication remains to be established. Theoretically, the increased expression of Abcb11 could increase the rate of transport of bile salts within the hepatocyte, thereby decreasing their steady-state intracellular concentration and thus leading to enhanced signaling. Alternatively, the increased secretion of a presently unidentified ligand of Abcb11 could exert a signal toward the intestine, affecting the efficacy of intestinal bile salt reabsorption. Although the mechanism is not certain, the present data do indicate that increased hepatic expression of Abcb11 modulates the enterohepatic circulation of bile acids via a mechanism affecting the intestine.
The level of ABCB11 expression in humans is widely variable yet the impact of such variability on human disease remains unclear. The present data indicate that Abcb11 increases the conservation of bile acids within the enterohepatic circulation, defining a novel physiological role of Abcb11. The level of Abcb11 overexpression observed in the TTR-Abcb11 mice used in this study is within the range of the variability observed in humans. This observation highlights the potential translational relevance of our findings. Conserving bile acids within the human body is critical to maintaining the numerous physiological processes performed by bile acids such as facilitating intestinal lipid absorption and promoting hepatic excretion of cholesterol. Our work highlights the key role that ABCB11 plays in mediating these essential processes.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK080810 and F32DK076342.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.S.H., R.H., R.B., R.M.G., and H.J.V. conception and design of research; A.S.H., K.E.R.G., R.H., R.B., and H.J.V. performed experiments; A.S.H., K.E.R.G., R.B., and H.J.V. analyzed data; A.S.H., K.E.R.G., R.M.G., and H.J.V. interpreted results of experiments; A.S.H. and H.J.V. prepared figures; A.S.H. and H.J.V. drafted manuscript; A.S.H., K.E.R.G., R.M.G., and H.J.V. edited and revised manuscript; A.S.H., K.E.R.G., R.H., R.B., R.M.G., and H.J.V. approved final version of manuscript.
REFERENCES
- 1. Alpini G, Kanno N, Phinizy JL, Glaser S, Francis H, Taffetani S, LeSage G. Tauroursodeoxycholate inhibits human cholangiocarcinoma growth via Ca2+-, PKC-, and MAPK-dependent pathways. Am J Physiol Gastrointest Liver Physiol 286: G973–G982, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Chen F, Ma L, Dawson PA, Sinal CJ, Sehayek E, Gonzalez FJ, Breslow J, Ananthanarayanan M, Shneider BL. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem 278: 19909–19916, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 50: 1955–1966, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol 40: 539–551, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res 50: 2340–2357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Figge A, Lammert F, Paigen B, Henkel A, Matern S, Korstanje R, Shneider BL, Chen F, Stoltenberg E, Spatz K, Hoda F, Cohen DE, Green RM. Hepatic overexpression of murine Abcb11 increases hepatobiliary lipid secretion and reduces hepatic steatosis. J Biol Chem 279: 2790–2799, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem 273: 10046–10050, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Henkel AS, Anderson KA, Dewey AM, Kavesh MH, Green RM. A chronic high-cholesterol diet paradoxically suppresses hepatic CYP7A1 expression in FVB/NJ mice. J Lipid Res 52: 289–298, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hulzebos CV, Renfurm L, Bandsma RH, Verkade HJ, Boer T, Boverhof R, Tanaka H, Mierau I, Sauer PJ, Kuipers F, Stellaard F. Measurement of parameters of cholic acid kinetics in plasma using a microscale stable isotope dilution technique: application to rodents and humans. J Lipid Res 42: 1923–1929, 2001 [PubMed] [Google Scholar]
- 10. Hulzebos CV, Voshol PJ, Wolters H, Kruit JK, Ottenhof R, Groen AK, Stellaard F, Verkade HJ, Kuipers F. Bile duct proliferation associated with bile salt-induced hypercholeresis in Mdr2 P-glycoprotein-deficient mice. Liver Int 25: 604–612, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217–225, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Kok T, Hulzebos CV, Wolters H, Havinga R, Agellon LB, Stellaard F, Shan B, Schwarz M, Kuipers F. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem 278: 41930–41937, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Kramer W, Buscher HP, Gerok W, Kurz G. Bile salt binding to serum components. Taurocholate incorporation into high-density lipoprotein revealed by photoaffinity labelling. Eur J Biochem 102: 1–9, 1979 [DOI] [PubMed] [Google Scholar]
- 14. Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology 126: 322–342, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Lang T, Haberl M, Jung D, Drescher A, Schlagenhaufer R, Keil A, Mornhinweg E, Stieger B, Kullak-Ublick GA, Kerb R. Genetic variability, haplotype structures, and ethnic diversity of hepatic transporters MDR3 (ABCB4) and bile salt export pump (ABCB11). Drug Metab Dispos 34: 1582–1599, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Lazaridis KN, Pham L, Tietz P, Marinelli RA, deGroen PC, Levine S, Dawson PA, LaRusso NF. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest 100: 2714–2721, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6: 507–515, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science 284: 1362–1365, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Mashige F, Imai K, Osuga T. A simple and sensitive assay of total serum bile acids. Clin Chim Acta 70: 79–86, 1976 [DOI] [PubMed] [Google Scholar]
- 20. Mottino AD, Hoffman T, Dawson PA, Luquita MG, Monti JA, Sanchez Pozzi EJ, Catania VA, Cao J, Vore M. Increased expression of ileal apical sodium-dependent bile acid transporter in postpartum rats. Am J Physiol Gastrointest Liver Physiol 282: G41–G50, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Nishida T, Gatmaitan Z, Che M, Arias IM. Rat liver canalicular membrane vesicles contain an ATP-dependent bile acid transport system. Proc Natl Acad Sci USA 88: 6590–6594, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science 284: 1365–1368, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, Tanner MS, Kagalwalla AF, Nemeth A, Pawlowska J, Baker A, Mieli-Vergani G, Freimer NB, Gardiner RM, Thompson RJ. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet 20: 233–238, 1998 [DOI] [PubMed] [Google Scholar]
- 24. van Meer H, Boehm G, Stellaard F, Vriesema A, Knol J, Havinga R, Sauer PJ, Verkade HJ. Prebiotic oligosaccharides and the enterohepatic circulation of bile salts in rats. Am J Physiol Gastrointest Liver Physiol 294: G540–G547, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, Helgason CD, Ackerley C, Phillips MJ, Ling V. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci USA 98: 2011–2016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]