Abstract
The expression of intestinal Niemann-Pick C1-like 1 (NPC1L1) cholesterol transporter has been shown to be elevated in patients with diseases associated with hypercholesterolemia such as diabetes mellitus. High levels of glucose were shown to directly increase the expression of NPC1L1 in intestinal epithelial cells, but the underlying mechanisms are not fully defined. The present studies were, therefore, undertaken to examine the transcriptional regulation of NPC1L1 expression in human intestinal Caco2 cells in response to glucose. Removal of glucose from the culture medium of Caco2 cells for 24 h significantly decreased the NPC1L1 mRNA, protein expression, as well as the promoter activity. Glucose replenishment significantly increased the promoter activity of NPC1L1 in a dose-dependent manner compared with control cells. Exposure of Caco2 cells to nonmetabolizable form of glucose, 3-O-methyl-d-glucopyranose (OMG) had no effect on NPC1L1 promoter activity, indicating that the observed effects are dependent on glucose metabolism. Furthermore, glucose-mediated increase in promoter activity was abrogated in the presence of okadaic acid, suggesting the involvement of protein phosphatases. Glucose effects on several deletion constructs of NPC1L1 promoter demonstrated that cis elements mediating the effects of glucose are located in the region between −291 and +56 of NPC1L1 promoter. Consistent with the effects of glucose removal on NPC1L1 expression in Caco2 cells, 24-h fasting resulted in a significant decrease in the relative expression of NPC1L1 in mouse jejunum. In conclusion, glucose appears to directly modulate NPC1L1 expression via transcriptional mechanisms and the involvement of phosphatase-dependent pathways.
Keywords: Niemann-Pick C1-like 1 promoter, carbohydrates, glucose metabolism, intestinal cholesterol transport
the increase in intestinal cholesterol absorption has been suggested to contribute to high levels of cholesterol associated with diseases such as diabetes mellitus (12, 25). Studies have also demonstrated that the inhibition of cholesterol absorption leads to a significant reduction in plasma cholesterol (27), providing an attractive therapeutic approach for the treatment of hypercholesterolemia. Information about the molecular mechanisms involved in intestinal cholesterol absorption and its dysregulation in diseases is slowly emerging.
Recent studies have shown that NPC1L1 protein plays a pivotal role in intestinal cholesterol absorption (15). NPC1L1 is predominantly expressed in the liver and the small intestine with its expression localized to brush-border membranes of intestinal epithelial cells (15). The knockout of NPC1L1 expression in mice resulted in marked decrease in cholesterol absorption (3) and was protective against diet-induced hypercholesterolemia (8). NPC1L1 was also shown to be the molecular target of ezetamibe, the inhibitor of cholesterol absorption used in the treatment of hypercholesterolemia (11). Recent studies have shown an increase in intestinal NPC1L1 expression in metabolic disorders such as diabetes mellitus (17, 18). These findings suggest that modulation of NPC1L1 expression could occur in diseases and may play important roles in the pathophysiology of associated hypercholesterolemia.
The major pathological features of diabetes mellitus are insulin resistance and/or insulin deficiency along with hyperglycemia (19). Interestingly, glucose has been shown to directly alter insulin gene expression (4). Furthermore, previous studies have shown that glucose activates signaling molecules via several pathways, leading to stimulation of transcription factors that modulate gene transcription of enzymes involved in the regulation of lipogenesis (9). d-Glucose was recently shown to directly induce NPC1L1 mRNA (24). Whether these changes occur via transcriptional mechanisms is not known. In this regard, the expression of intestinal NPC1L1 has been shown to be modified at the transcriptional level via a number of transcription factors. Recent studies from our laboratory have demonstrated the involvement of the sterol response element-binding protein 2 (SREBP2) in mediating the modulation of NPC1L1 expression by cholesterol (1). Furthermore, the role of liver X receptor, peroxisome proliferator-activated receptor-α, and peroxisome proliferator-activated receptor-δ in the modulation of NPC1L1 expression was also indicated (10, 14, 26). However, it is not known whether glucose and the molecular signaling induced by glucose could directly alter the promoter activity of the NPC1L1 gene.
Therefore, present studies were undertaken to examine the transcriptional regulation of NPC1L1 by glucose in human intestinal Caco2 cells and to delineate the molecular pathways involved. Our data demonstrated that NPC1L1 expression and promoter activity are increased by glucose and decreased in response to glucose withdrawal. The effects of glucose were dependent on its metabolism and involved cellular protein phosphatases. These findings provide novel insights about the regulation of NPC1L1 by carbohydrates and unravel potential therapeutic targets for the treatment of hypercholesterolemia associated with diabetes mellitus.
MATERIALS AND METHODS
Materials.
Inhibitors of signaling pathways were purchased from Biomol (Farmingdale, NY). Glucose, mannitol, OMG, and okadaic acid were obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals were of reagent grade and obtained from commercial sources. Rabbit polyclonal anti-human NPC1L1 antibodies were purchased from Novus Biologicals (Littleton, CO), and anti-human α-tubulin antibodies were purchased from Sigma-Aldrich.
Cell culture.
The human colorectal adenocarcinoma cell line (Caco2) was obtained from ATCC, and cells were grown routinely in T-150-cm2 plastic flasks at 37°C in a 5% CO2-95% air environment. The cells were cultured in MEM minimum essential medium (Eagle) containing 2 mM l-glutamine and Earle's BSS adjusted to contain 1.5 g/l sodium bicarbonate, 0.1 mM nonessential amino acids, and 1.0 mM sodium pyruvate and supplemented with 20% fetal bovine serum, 100 u/ml penicillin, and 100 mg/ml gentamicin (Invitrogen, Grand Island, NY). At the time of the experiments, Dulbecco's Modified Eagle Medium (DMEM) was used when cells were incubated with no glucose or glucose replenishment conditions.
RNA extraction and real-time PCR analysis.
Total RNA was prepared from Caco2 cells utilizing RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Equal amounts of RNA from both treated and control samples were reverse transcribed and amplified in one-step reaction utilizing Brilliant SYBR Green QRT-PCR Master Mix Kit (Stratagene, Clara, CA). Real-time PCR was performed utilizing Mx3000 (Stratagene). Human NPC1L1 was amplified with gene-specific primers sense primer: 5′-TATCTTCCCTGGTTCCTGAACGAC-3′, anti-sense primer: 5′-CCGCAGAGCTTCTGTGTAATCC-3′. β-Actin was amplified as an internal control utilizing gene-specific primers: sense primer: 5′ CATGTTTGAGACCTTCAACAC 3′; anti-sense primer: 5′ CCAGGAAGGAAGGCTGGAA 3′. Because the amplification efficiencies for both NPC1L1 and β-actin were approximately equal, the quantitation was expressed as a ratio of 2ΔCt−NPC1L1/2ΔCt−β−actin, where ΔCt-NPC1L1 and ΔCt-β-actin represent the difference between the threshold cycle of amplification of treated and control RNA for NPC1L1 and β-actin, respectively. All real-time qPCR reactions were performed in triplicates.
Transient transfections and luciferase assay.
For transfection studies, Caco2 cells were seeded at a density of 1.5 × 105 cells/well on 24-well plates and cotransfected while still in suspension with one of the NPC1L1 promoter-luciferase constructs and p-cytomegalovirus (CMV)-β, β-galactosidase mammalian expression vector (BD Biosciences Clontech, Palo Alto, CA), utilizing Fugene6 reagent (Roche, Indianapolis, IN). The latter plasmid served as an internal control for transfection efficiency. A total of 1.5 μg DNA/well, at a ratio of 5:1 for experimental vs. pCMV-β, was used for each transfection. After 24 h of transfection, cells were incubated with different media containing different levels of glucose. After 48 h of transfection, cells were washed with PBS and lysed using a passive lysis buffer from Promega (Madison, WI). The activities of both firefly luciferase and β-galactosidase were measured by luciferase reporter assay from Promega and β-galactosidase luminescent detection system from Clontech (Mountain View, CA) according to the manufacturer's instructions in a luminometer (Promega). The promoter activity was expressed as a ratio of luciferase to β-galactosidase activity in each sample.
Western blotting.
Whole cell lysates were obtained by first scraping the cells with a cell scraper and collecting them by centrifugation. Total protein was extracted by suspending the cell pellet in a cell lysis buffer containing 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, supplemented with protease inhibitor cocktail (Roche). The cell suspension was sonicated three times for 10 s each and then centrifuged at 13,000 rpm for 10 min. The resulting supernatant corresponds to whole protein extract. Bradford assay (Bio-Rad, Hercules, CA) was used to determine the protein concentration (6). One hundred micrograms of protein both from control and treated samples were subjected to 6% SDS PAGE. The resolved proteins in the gel were transferred to a nitrocellulose membrane electrophoretically. The membrane was incubated for 2 h with primary antibodies (1:250) and then for 1 h with goat anti-rabbit secondary antibody (1:2,000) conjugated with horseradish peroxidase followed by enhanced chemiluminescence detection (Bio-Rad).
[3H] cholesterol uptake.
[3H]cholesterol micelle solution was prepared as previously described by us with minor modifications (16). Appropriate volumes of each component from ethanol stock solutions were added to a glass tube, evaporated under nitrogen, and then dissolved in MEM media supplemented with 5% of lipoprotein-deficient calf serum (Sigma) by vigorous stirring until the solution is clear. The final concentrations of the micelle solution are as follows: 5 mM taurocholic acid, 0.3 mM oleic acid, 10 μM of l-phosphatidylcholine, 5 μM cholesterol, and 1 μci/ml of [3H]cholesterol. Briefly, cells were incubated with no-glucose media or DMEM control media with 0.2% BSA for 24 h at 37°C, and the micelles were added for 15 and 30 min. The uptake was stopped by washing the cells with ice-cold 1× PBS containing 10 mM of methyl-β-cyclodextrin. Finally, cells were solubilized with 0.5 N NaOH for at least 2–3 h. The protein concentration was measured by the method of Bradford (6). The radioactivity was counted by a Tri-CARB 1600-TR liquid scintillation counter (Packard Instruments, Downers Grove, IL). Aliquot was used to measure the total radioactivity and normalized to the amount of protein in the sample to represent total cholesterol uptake.
Animal studies.
Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the Jesse Brown VA Medical Center. Eight-week-old C57BL6 mice were obtained from Jackson Laboratories (Bar Harbor, ME) and were acclimatized for 5 days with free access to food and water and 12-h:12-h light/dark cycles. Mice were then divided into two groups (6 animals/group): control and fasted groups. Food was withheld from the fasted animals for 24 h, whereas control animals were fed ad libitum. Animals were then killed, and the intestines were harvested. Intestinal mucosa from jejunum was scraped and stored frozen at −80°C. Total RNA extraction was later prepared from these tissues and utilized for real-time PCR to amplify mouse NPC1L1, hepatocyte nuclear factor (HNF)1α, SREBP2, and GAPDH. The sequences for the primers are as follows: NPC1L1 sense primer: 5′-TGGACTGGAAGGACCATTTCC-3′, anti-sense primer: 5′-GACAGGTGCCCCGTAGTCA-3′. HNF1α sense: 5′-CGTCGAACATCCAGCACCT-3′, anti-sense: 5′-TTGGAGTCGGAACTCTGATACAAC-3′. SREBP2 sense: 5′-GACCGCTCTCGAATCCTCTTATGTG-3′, anti-sense: 5′-GTTTGTAGGTTGGCAGCAGCA-3′. GAPDH was amplified as an internal control utilizing gene-specific primers sense primer: 5′-TGTGTCCGTCGTGGATCTGA-3′, anti-sense primer: 5′-CCTGCTTCACCACCTTCTTGAT-3′.
Statistical analysis.
Results are expressed as means ± SE. Student's t-test was utilized for statistical analysis. Comparisons of multiple treatment conditions with controls were analyzed by one-way ANOVA with the Tukey's test for postanalysis. A P value of 0.05 or less was considered statistically significant.
RESULTS
Glucose alters NPC1L1 expression.
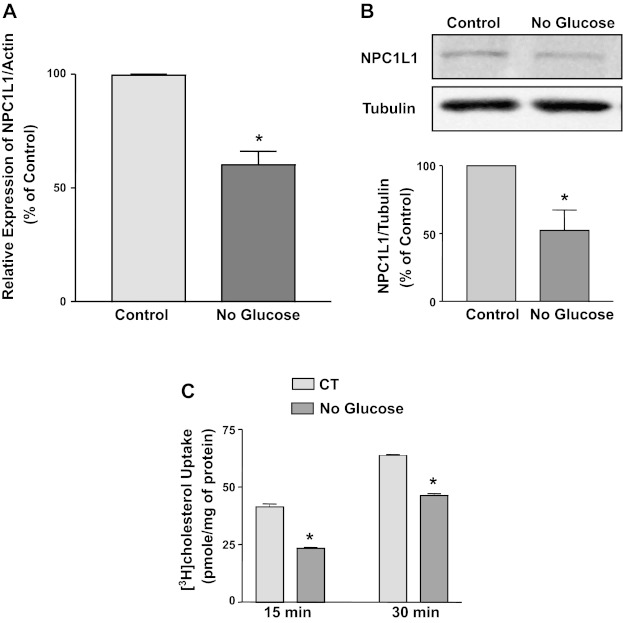
Several biological processes are altered by increasing glucose levels or by removing glucose (5). NPC1L1 expression has been recently shown to be increased in intestinal epithelial cells by high levels of glucose (24). To examine whether glucose removal has a direct effect on NPC1L1 expression, we incubated Caco2 cells for 24 h with media containing no glucose. Glucose removal caused a significant decrease in the relative expression of NPC1L1 mRNA expression compared with normal DMEM media with glucose (Fig. 1A). Concomitant with decrease in mRNA levels, NPC1L1 protein expression was also decreased in response to 24-h incubation with no glucose media (Fig. 1B). The decrease in NPC1L1 protein and mRNA expression was also associated with a significant reduction in the uptake of micellular [3H]cholesterol in Caco2 cells in response to glucose removal as shown in Fig. 1C. These findings suggest that the removal of glucose leads to a decrease in NPC1L1 expression and function in intestinal epithelial cells.
Fig. 1.
Glucose withdrawal decreases Niemann-Pick C1-like 1 (NPC1L1) expression. Caco2 cells were incubated with regular DMEM culture medium (control) or with no-glucose medium for 24 h. Cells were then harvested, and total RNA and protein were extracted. The expression of NPC1L1 mRNA relative to actin mRNA was assessed by real-time PCR (A). Data are presented as percentages of control and represent means ± SE from 3 independent determinations. *P < 0.05 compared with control. B: NPC1L1 protein expression was evaluated by Western blotting using Tubulin as a loading control. Results of the densitometric analysis representing the density of NPC1L1 relative to tubulin are shown. Data are expressed as percentages of control and represent the means ± SE from 3 independent experiments. *P < 0.05. C: micellar [3H]cholesterol uptake was measured as described in materials and methods. Cholesterol uptake is expressed as pmol/mg protein from 3 independent values. *P < 0.05 compared with control (CT).
NPC1L1 promoter activity is modulated by glucose.
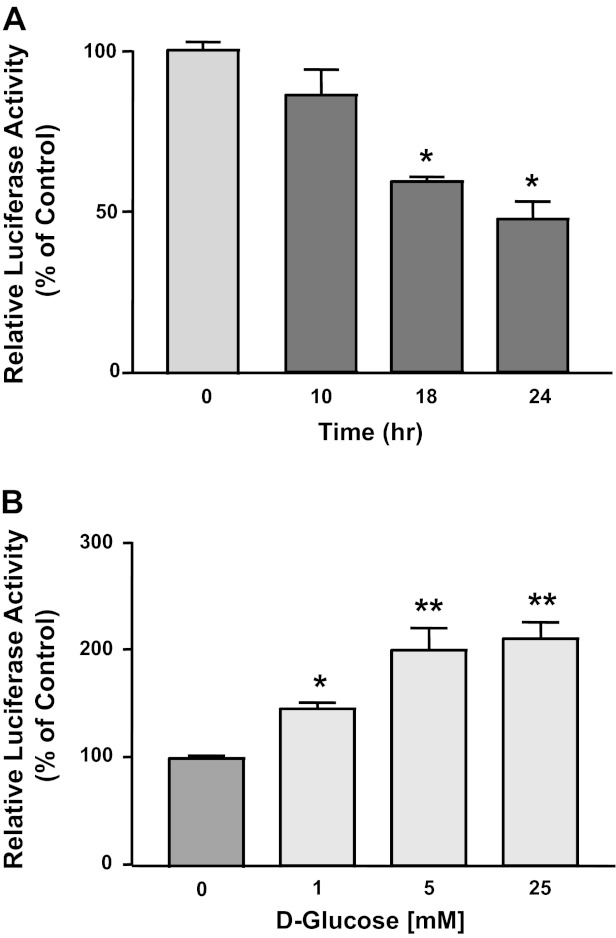
The observed changes in NPC1L1 expression by glucose may occur at the transcriptional level. To test this, we investigated the effect of glucose removal or glucose addition on NPC1L1 promoter activity. We have previously cloned and characterized the activity of human NPC1L1 promoter fragment (−1,741/+56, +1 represents transcription initiation site) in Caco2 cells. Caco2 cells were transiently transfected with NPC1L1 promoter, and the cells were incubated with no glucose media or with regular DMEM media containing glucose. As shown in Fig. 2A, NPC1L1 promoter activity was significantly decreased by the removal of glucose in a time-dependent fashion with maximum reduction occurring after 18 h from the start of glucose removal. On the other hand, the NPC1L1 promoter activity was significantly increased when glucose was added back to the media in a concentration-dependent manner, with maximum induction at glucose concentration of 5 mM (Fig. 2B).
Fig. 2.
Effects of glucose on NPC1L1 promoter activity. Caco2 cells were transiently transfected with NPC1L1 promoter construct along with mammalian expression vector for β-galactosidase to control for transfection efficiency. 24 h after transfection, glucose was removed (A) from the culture medium and replaced with an equal concentration of mannitol. Cells were then harvested after different periods of time, and the promoter activity was expressed as a ratio of the firefly luciferase to the β-galactosidase. B: different doses of d-glucose were added to the no-glucose culture medium for 24 h; cells were then harvested, and NPC1L1 promoter activity was assessed. Data in the figures are percentages of control and represent means ± SE of 3 independent determinations. *P < 0.05 compared with control. **P < 0.05 compared with 1 mM glucose.
Stimulation of NPC1L1 promoter activity by glucose is dependent on its metabolism.
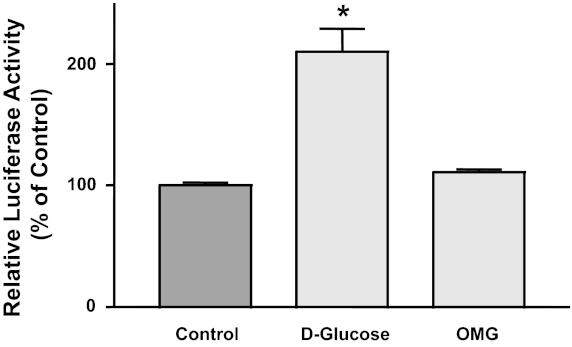
Effect of glucose on biological processes may depend on its entry to the cells and subsequent metabolism (20). To investigate whether glucose metabolism is required to elicit its effect on NPC1L1 promoter activity, we tested NPC1L1 promoter activity in the presence of the nonmetabolizable analog of glucose, OMG. As shown in Fig. 3, d-glucose increased NPC1L1 promoter activity when added to the no-glucose media, whereas the presence of equal concentration of OMG failed to induce the promoter activity, suggesting that the effects of glucose on NPC1L1 promoter are dependent on its transport into the cells and subsequent metabolism.
Fig. 3.
Effects of glucose are dependent on its metabolism. NPC1L1 promoter was transiently transfected in Caco2 cells for 24 h and then incubated with no-glucose culture medium. Cells were then exposed either to 5 mM d-glucose or to 5 mM of the nonmetabolizable glucose 3-o-methyl-d-glucopyranose (OMG) for 24 h. NPC1L1 promoter activity was then assessed. Data are the means ± SE of 3 independent determinations and presented as percentages of control. *P < 0.05 compared with control.
Protein phosphatases are involved in glucose-mediated induction of NPC1L1.
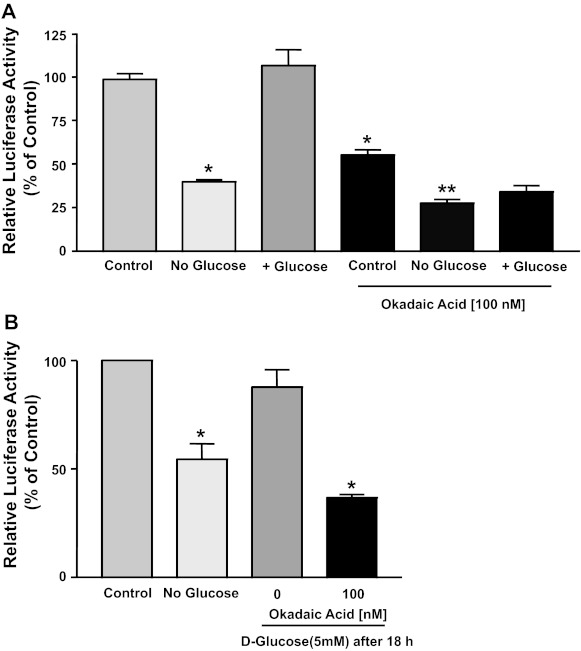
To further decipher the mechanisms mediating the induction of NPC1L1 by glucose, we utilized inhibitors of potential signaling pathways. Inhibitors of PKC-, phosphatidylinositol 3-kinase-, and AKT-dependent pathways failed to block the increase in NPC1L1 (data not shown). On the other hand, incubation of Caco2 cells with the protein phosphatases inhibitor okadaic acid (100 nM) blocked the increase in NPC1L1 promoter activity when glucose was added to glucose-free media as shown in Fig. 4A. Also, there was a significant difference in the promoter activity of NPC1L1 in the control cells incubated with the regular DMEM medium with and without okadaic acid, indicating that inhibition of phosphatases decreases basal promoter activity. Furthermore, Fig. 4B shows that the initial removal of glucose for 18 h followed by the replenishment of glucose for additional 24 h reverted NPC1L1 promoter activity back to control levels, the effect that was also blocked by the presence of okadaic acid. Taken together, the activation of protein phosphatases appears to be mediating the effects of glucose on NPC1L1 promoter activity.
Fig. 4.
Okadaic acid inhibits the effects of glucose on NPC1L1 promoter activity. A: Caco2 cells were transiently transfected with NPC1L1 promoter and then incubated with regular culture medium (control), no-glucose culture medium containing 5 mM mannitol, or medium replenished with 5 mM d-glucose for 24 h in the presence or the absence of 100 nM okadaic acid. NPC1L1 promoter activity was then assessed. B: cells transfected with NPC1L1 promoter were incubated with no-glucose culture medium containing 5 mM mannitol for 18 h and were either left with this medium for an additional 24 h or exposed to culture medium containing 5 mM d-glucose. NPC1L1 promoter activity was then measured. Data are means ± SE of 3 independent determinations and are expressed as percentages of control. *P < 0.05 compared with control without okadaic acid. **P < 0.05 compared with control with okadaic acid.
Effects of glucose on deletion constructs of NPC1L1 promoter.
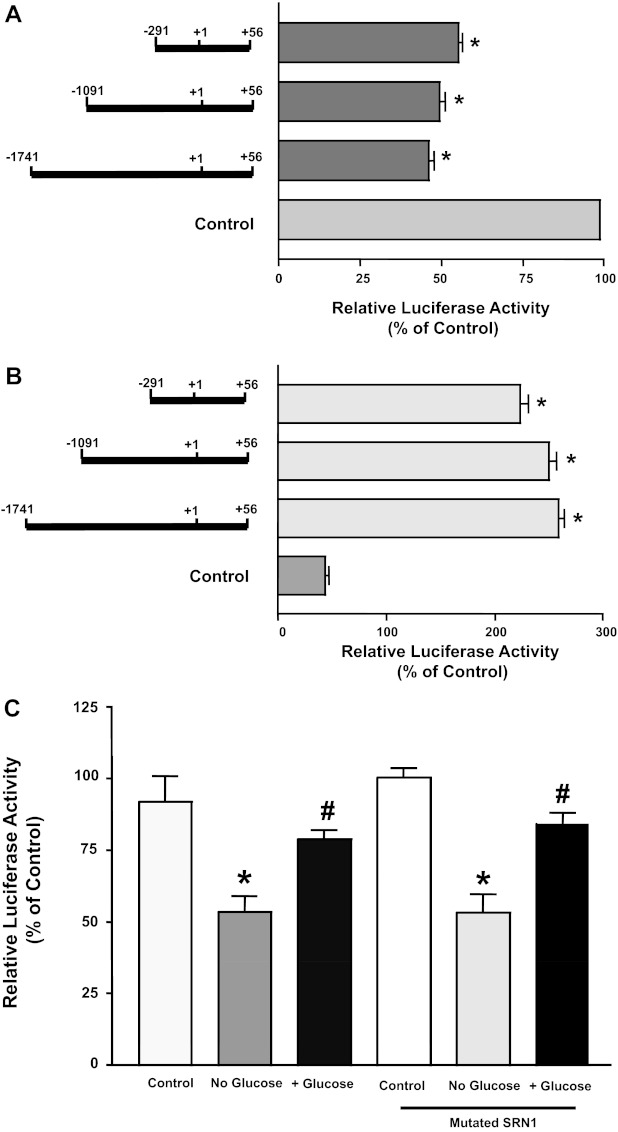
We next examined the influence of glucose on progressive 5′ deletions in the NPC1L1 promoter to identify the region in the promoter fragment responsible for mediating the observed effects of glucose. Caco2 cells were transiently transfected with different promoter constructs representing three fragments flanking the regions, −1,741/+56, −1,091/+56, and −291/+56, of the NPC1L1 gene and then incubated without glucose or with 5 mM glucose added to the no-glucose media. As shown in Fig. 5A, the removal of glucose inhibited the promoter activity of the three fragments. Data shown in Fig. 5B also show that the addition of glucose to the no-glucose media was able to increase the promoter activity of the three constructs. These data clearly indicate that the region flanking the area between −291/+56 of the NPC1L1 gene harbors the glucose-response elements.
Fig. 5.
Glucose effects on different deletion constructs of NPC1L1 promoter. Caco2 cells were transfected with different deletion constructs of NPC1L1 promoter. A: cells were incubated with no-glucose culture medium containing 5 mM mannitol for 24 h and then NPC1L1 promoter activity was measured. B: cells were incubated with no-glucose culture medium containing 5 mM mannitol or exposed to medium with 5 mM glucose for 24 h, and NPC1L1 promoter activity was assessed. C: Caco2 cells were transfected with NPC1L1 promoter or with a promoter construct harboring mutated sterol response element and then incubated with no-glucose culture medium or with medium containing 5 mM d-glucose for 24 h; NPC1L1 promoter activity was then evaluated. Results are shown as percentages of control and represent means ± SE of 3 independent determinations. *P < 0.05 compared with respective control. #P < 0.05 compared with no-glucose media.
We have previously shown that the region −291/+56 in the NPC1L1 gene harbors SRE for the binding of SREBP2 transcription factor (1). SREBP2 was previously shown as a potential mediator of glucose effects on gene transcription. We next examined whether SREBP2 is involved in the regulation of NPC1L1 promoter activity by glucose. Figure 5C shows that mutation in the SRE on the NPC1L1 failed to block glucose-mediated alterations in NPC1L1 promoter activity, indicating that SREBP2 is not involved.
Intestinal NPC1L1 mRNA expression is decreased by fasting.
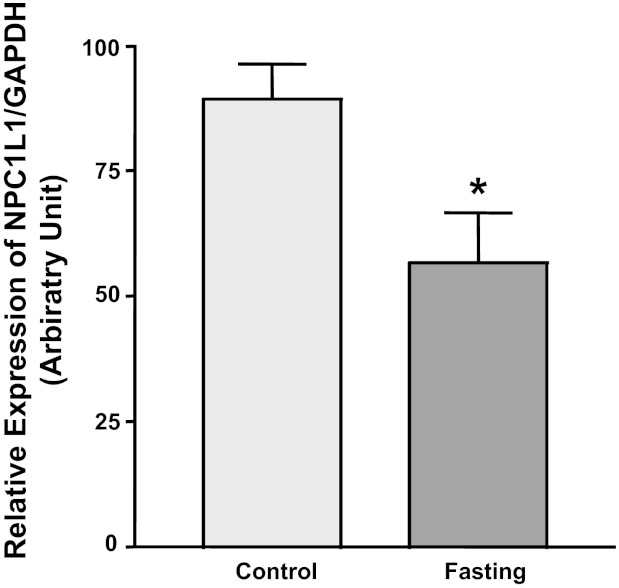
Effects of glucose withdrawal may mimic condition of nutrient depletion, such as those that occur during fasting. We next aimed to examine alterations in NPC1L1 expression under conditions of energy depletion in native mouse intestine. Figure 6 shows that the relative expression of NPC1L1 was significantly decreased in mouse jejunum after 24-h fasting, suggesting that energy depletion reduces NPC1L1 expression in mouse intestine similar to the effects of glucose depletion in Caco2 cells. Because HNF-1α and SREBP2 are important regulators of NPC1L1 (1, 22), we also investigated the expression of thee transcription factors in the jejunum of mice fasted for 24 h. No significant changes were observed in relative mRNA expression of HNF-1α [0.60 ± 0.2 arbitrary units (A.U.) in control vs. 0.57 ± 0.1 A.U. in fasted mice] and SREBP2 (1.72 ± 0.7 A.U. in control vs. 1.19 ± 0.1 A.U. in fasted mice). The observation that SREBP2 expression was unaltered in fasting further supports the conclusion derived from the experiments in Caco2 cells, demonstrating that SREBP2 may not be involved in modulating NPC1L1 expression in response to changes in the levels of glucose.
Fig. 6.
NPC1L1 expression is reduced in fasted mice. 8-wk-old C57BL6 mice were divided into 2 groups (6 mice/group). Food was withheld from the fasted group for 24 h, and the control group was left with free access to food ad libitum. Mice were then killed, and the mucosa was scraped from the jejunum. Total RNA was extracted from the mucosal scrapings, and NPC1L1 mRNA expression relative to GAPDH mRNA expression was assessed by real-time PCR. Data are means ± SE of each animal group and presented as percentages of control. *P < 0.05 compared with control group.
DISCUSSION
In the present studies, we provide novel data showing that glucose depletion causes a reduction in NPC1L1 expression in human intestinal epithelial cells. Our data also show that changes observed by adding or removing glucose were concomitant with alterations in NPC1L1 promoter activity, indicating the involvement of transcriptional regulation. The effects of glucose appear to be dependent on glucose metabolism and the activation of cellular protein phosphatases.
NPC1L1 inhibition has been proven to be beneficial in reducing cholesterol absorption and lowering plasma cholesterol (3, 11). In this regard, significant advancement has been made for the treatment of hypercholesterolemia. However, dire need still exists for developing improved effective therapeutic modalities to reduce high levels of plasma cholesterol, especially in high-risk patients such as those with chronic disorders such as metabolic syndrome and diabetes mellitus (27). Interestingly, NPC1L1 expression was shown to be increased in diabetic patients and in animal models of diabetes mellitus (17, 18). These findings suggest that high levels of NPC1L1 may be involved in the pathophysiology of the associated hypercholesterolemia and provide strong rationale to determine the molecular mechanisms involved in the observed increase in NPC1L1 expression. Little is known about the regulation of NPC1L1 expression by glucose metabolism. Ravid et al. (24) have recently demonstrated that glucose directly increases the expression of NPC1L1 in intestinal Caco2 cells. Our present studies further extend their findings showing that glucose also increases NPC1L1 promoter activity in a dose-dependent manner, indicating transcriptional regulation. Surprisingly, 25 mM concentration of glucose was previously shown to significantly increase NPC1L1 mRNA expression compared with the effects of 5 mM glucose concentration. However, our data showed maximum induction of NPC1L1 promoter activity at 5 mM concentration, the effect that was not significantly different from that of 25 mM concentration. This observation suggests that glucose promotes the basal expression of NPC1L1 and may increase NPC1L1 expression via two different mechanisms, transcriptional and posttranscriptional. It will be interesting to investigate in the future the potential role of posttranscriptional mechanisms involved in the induction of NPC1L1 mRNA expression by glucose.
The effects of glucose on gene transcription depend on its cellular metabolism (20). We have used d-glucose in our experiments, as it has been shown to be the preferred substrate for glucose transporters such as SGLT-1 and GLUT2 (7, 28). Apparently, the stimulatory effects of glucose on NPC1L1 promoter activity appear to be dependent on its metabolism, as the incubation of the cells with equal concentrations of the nonmetabolizable form of glucose, OMG, failed to induce NPC1L1 promoter activity. The initiation of glucose metabolism in the cells result in activation of several pathways, such as the conventional glycolytic, the hexosamine biosynthetic pathway (HBP), and the hexose monophosphate shunt (HMP shunt) (4, 13, 20). High levels of glucose were shown to induce insulin gene expression by the activation of the HBP, leading to an increase in the O-linked N-acetylglucosamine (O-GlcNAc) modification of NeuroD1 transcription factor (4). We have examined the possibility that HBP may be involved in glucose-mediated alteration in NPC1L1 promoter by using the O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate, PUGNAc, that inhibits the O-GlcNAc transferase responsible for the deglycolsylation and removal of O-GlcNAc group from regulatory proteins impacting their activities (4). The presence of PUGNAc, however, failed to block the effects of glucose alterations on NPC1L1 promoter activity (P. Malhotra and W. A. Alrefai, unpublished observations). These unpublished data, therefore, exclude the possible role of HBP in the modulation of NPC1L1 expression by glucose.
The flux through the HMP shunt was previously shown to modulate the expression of proteins critical for the lipogenesis and glycolytic pathways (13). It is well established that the protein phosphatase 2A (PP2A) is activated by xylulose-5-phosphate, the metabolite of HMP shunt, to activate transcription factors such as the carbohydrate response element-binding protein (13). Our data provided novel evidence showing that the basal level of NPC1L1 promoter activity in the presence of glucose was significantly reduced by okadaic acid, the inhibitor of protein phosphatases. This finding implies that glucose promotes NPC1L1 expression via activation of protein phosphatases. Adding glucose to no-glucose media failed to revert NPC1L1 promoter activity back to normal levels in the presence of okadaic acid. This observation was also confirmed by another protein phosphatase inhibitor, Canthradin, further suggesting the essential role of protein phosphatases in the stimulation of NPC1L1 expression by glucose. In this regard, previous studies have shown that the increase in the expression and activity of PP1A and PP2A are implicated in the cardiac dysfunction associated with diabetes mellitus (23). It is, therefore, reasonable to suggest that the increase in protein phosphatases may play a role in the pathophysiology of diabetes mellitus in various tissues, including the intestine. Future studies will need to focus on identifying the type of protein phosphatase involved in the modulation of NPC1L1 expression.
Several physiological processes are altered by changes in the levels of glucose (5). Our present data indicate that NPC1L1 expression and the promoter activity are sensitive to alterations in the levels of glucose, as the withdrawal of glucose resulted in significant decreases in NPC1L1 expression. It is unlikely that the observed decrease is secondary to cell death because the replenishment of glucose after a withdrawal for a period of time sufficient to reduce the promoter activity (18 h) was able to rescue the activity back to normal levels. The uptake of micellar cholesterol was also significantly decreased concomitant with the observed decrease in NPC1L1 expression. The fact that cholesterol uptake was significantly reduced in Caco2 cells deprived of glucose, and that such treatment did not affect cell viability, strongly suggests a reduction in a protein-mediated cholesterol uptake process parallel to the observed decrease in NPC1L1 expression.
Interestingly, the shortest fragment of the NPC1L1 promoter was responsive to both the withdrawal and the addition of glucose, indicating that cis elements mediating the two processes are located in the region between −291 and +56 of the NPC1L1 gene. It is plausible to propose that the same cis elements mediate the increase and the decrease of NPC1L1 promoter activity by glucose addition and removal, respectively. Apparently, the SRE that we have previously identified in this region of the NPC1L1 (1) is not involved in the observed modulation by glucose, as mutations in these elements did not block the response to changes in the levels of glucose. Moreover, the observation that SREBP2 expression was unaltered in fasted mice further supports the notion that SREBP2 is unlikely to be involved.
The effects of glucose withdrawal on NPC1L1 promoter activity are likely to be a result of energy depletion and cellular ATP reduction, conditions that occur during fasting. Indeed, our findings clearly indicate that the expression of intestinal NPC1L1 in mouse intestine was significantly reduced after 24-h fasting. These findings support the speculation that ATP depletion occurred by fasting led to a decrease in NPC1L1 expression in a manner similar to the effect of glucose withdrawal on NPC1L1 expression and promoter activity in Caco2 cells. Energy depletion was previously shown to negatively influence cholesterol synthesis (21). Our present findings, therefore, support a logical conclusion that cellular energy depletion reduces cholesterol entry into the cells along with decreasing cholesterol synthesis, probably as a means to save the energy for other cellular processes more essential for the survival of the cell. This conclusion, however, needs to be further investigated in future studies. It should be noted that the expression of NPC1L1 was previously shown to remain unaltered during fasting in rats (17). The discrepancy between our present data and these previous findings may be due to species differences in the regulation of intestinal NPC1L1 between rats and mice. Such divergence in the modulation of the expression on intestinal transporters between rats and mice is not unusual. Indeed, previous studies have shown that the regulation of intestinal bile acid transporters, also essential for the maintenance of cholesterol homeostasis, is different in rat intestine compared with mice and humans (2).
In conclusion, our data show that changes in the level of glucose alter the expression of intestinal NPC1L1 mainly via changes in the gene promoter activity and the involvement of cellular protein phosphatases. Our studies also demonstrate that energy depletion by glucose withdrawal or fasting decreases the expression of intestinal NPC1L1 in human Caco2 cells and mouse intestine, respectively. These findings unravel a link between glucose metabolism and cholesterol absorption and may explain the observed increase in NPC1L1 expression in diabetic patients and animals with induced diabetes mellitus.
GRANTS
These studies were supported by grants from the Department of Veteran Affairs (W. Alrefai and P. Dudeja) and NIDDK: DK71596 (W. Alrefai), DK54016, DK81858, DK92441, P01 DK 67887 (P. Dudeja), and DK74458 (R. Gill).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.M., S.S., P.K.D., R.K.G., and W.A.A. conception and design of research; P.M., C.S.B., V.S., and W.A.A. performed experiments; P.M., C.S.B., V.S., S.S., R.K.G., and W.A.A. analyzed data; P.M., C.S.B., V.S., S.S., P.K.D., R.K.G., and W.A.A. interpreted results of experiments; P.M., P.K.D., R.K.G., and W.A.A. drafted manuscript; P.M., S.S., P.K.D., R.K.G., and W.A.A. edited and revised manuscript; P.K.D., R.K.G., and W.A.A. approved final version of manuscript; R.K.G. and W.A.A. prepared figures.
REFERENCES
- 1. Alrefai WA, Annaba F, Sarwar Z, Dwivedi A, Saksena S, Singla A, Dudeja PK, Gill RK. Modulation of human Niemann-Pick C1-like 1 gene expression by sterol: role of sterol regulatory element binding protein 2. Am J Physiol Gastrointest Liver Physiol 292: G369–G376, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res 24: 1803–1823, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 303: 1201–1204, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Andrali SS, Sampley ML, Vanderford NL, Ozcan S. Glucose regulation of insulin gene expression in pancreatic beta-cells. Biochem J 415: 1–10, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Aoh QL, Graves LM, Duncan MC. Glucose regulates clathrin adaptors at the trans-Golgi network and endosomes. Mol Biol Cell 22: 3671–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 7. Colville CA, Seatter MJ, Jess TJ, Gould GW, Thomas HM. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochem J 290: 701–706, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem 280: 12710–12720, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Denechaud PD, Bossard P, Lobaccaro JM, Millatt L, Staels B, Girard J, Postic C. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. J Clin Invest 118: 956–964, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duval C, Touche V, Tailleux A, Fruchart JC, Fievet C, Clavey V, Staels B, Lestavel S. Niemann-Pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem Biophys Res Commun 340: 1259–1263, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR, Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O'Neill K A, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetamibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci USA 102: 8132–8137, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gylling H, Tuominen JA, Koivisto VA, Miettinen TA. Cholesterol metabolism in type 1 diabetes. Diabetes 53: 2217–2222, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Iizuka K, Horikawa Y. ChREBP: a glucose-activated transcription factor involved in the development of metabolic syndrome. Endocr J 55: 617–624, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Iwayanagi Y, Takada T, Tomura F, Yamanashi Y, Terada T, Inui K, Suzuki H. Human NPC1L1 expression is positively regulated by PPARalpha. Pharm Res 28: 405–412, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Jia L, Betters JL, Yu L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu Rev Physiol 73: 239–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumar P, Malhotra P, Ma K, Singla A, Hedroug O, Saksena S, Dudeja PK, Gill RK, Alrefai WA. SREBP2 mediates the modulation of intestinal NPC1L1 expression by curcumin. Am J Physiol Gastrointest Liver Physiol 301: G148–G155, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lally S, Owens D, Tomkin GH. Genes that affect cholesterol synthesis, cholesterol absorption, and chylomicron assembly: the relationship between the liver and intestine in control and streptozotosin diabetic rats. Metabolism 56: 430–438, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Lally S, Tan CY, Owens D, Tomkin GH. Messenger RNA levels of genes involved in dysregulation of postprandial lipoproteins in type 2 diabetes: the role of Niemann-Pick C1-like 1, ATP-binding cassette, transporters G5 and G8, and of microsomal triglyceride transfer protein. Diabetologia 49: 1008–1016, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Lyons TJ, Basu A. Biomarkers in diabetes: hemoglobin A1c, vascular and tissue markers. Transl Res 159: 303–312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masson E, Lagarde M, Wiernsperger N, El Bawab S. Hyperglycemia and glucosamine-induced mesangial cell cycle arrest and hypertrophy: common or independent mechanisms? IUBMB Life 58: 381–388, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation–AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 574: 63–71, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pramfalk C, Jiang ZY, Cai Q, Hu H, Zhang SD, Han TQ, Eriksson M, Parini P. HNF1alpha and SREBP2 are important regulators of NPC1L1 in human liver. J Lipid Res 51: 1354–1362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rastogi S, Sentex E, Elimban V, Dhalla NS, Netticadan T. Elevated levels of protein phosphatase 1 and phosphatase 2A may contribute to cardiac dysfunction in diabetes. Biochim Biophys Acta 1638: 273–277, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Ravid Z, Bendayan M, Delvin E, Sane AT, Elchebly M, Lafond J, Lambert M, Mailhot G, Levy E. Modulation of intestinal cholesterol absorption by high glucose levels: impact on cholesterol transporters, regulatory enzymes, and transcription factors. Am J Physiol Gastrointest Liver Physiol 295: G873–G885, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Tomkin GH. The intestine as a regulator of cholesterol homeostasis in diabetes. Atheroscler Suppl 9: 27–32, 2008 [DOI] [PubMed] [Google Scholar]
- 26. van der Veen JN, Kruit JK, Havinga R, Baller JF, Chimini G, Lestavel S, Staels B, Groot PH, Groen AK, Kuipers F. Reduced cholesterol absorption upon PPARdelta activation coincides with decreased intestinal expression of NPC1L1. J Lipid Res 46: 526–534, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Wang DQ. Regulation of intestinal cholesterol absorption. Annu Rev Physiol 69: 221–248, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Wright EM, Loo DD, Hirayama BA, Turk E. Surprising versatility of Na+-glucose cotransporters: SLC5. Physiology (Bethesda) 19: 370–376, 2004 [DOI] [PubMed] [Google Scholar]