Abstract
The interaction between adenosine and soluble epoxide hydrolase (sEH) in vascular response is not known. Therefore, we hypothesized that lack of sEH in mice enhances adenosine-induced relaxation through A2A adenosine receptors (AR) via CYP-epoxygenases and peroxisome proliferator-activated receptor γ (PPARγ). sEH−/− showed an increase in A2A AR, CYP2J, and PPARγ by 31%, 65%, and 36%, respectively, and a decrease in A1AR and PPARα (30% and 27%, respectively) vs. sEH+/+. 5′-N-ethylcarboxamidoadenosine (NECA, an adenosine receptor agonist), CGS 21680 (A2A AR-agonist), and GW 7647 (PPARα-agonist)-induced responses were tested with nitro-l-arginine methyl ester (l-NAME) (NO-inhibitor; 10−4 M), ZM-241385, SCH-58261 (A2A AR-antagonists; 10−6 M), 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE, an epoxyeicosatrienoic acid-antagonist; 10−5 M), 12-(3-adamantan-1-yl-ureido) dodecanoic acid (AUDA; 10 μM) or trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB, sEH-inhibitors; 10−5 M), and T0070907 (PPARγ-antagonist; 10−7 M). In sEH−/− mice, ACh response was not different from sEH+/+ (P > 0.05), and l-NAME blocked ACh-responses in both sEH−/− and sEH+/+ mice (P < 0.05). NECA (10−6 M)-induced relaxation was higher in sEH−/− (+12.94 ± 3.2%) vs. sEH+/+ mice (−5.35 ± 5.2%); however, it was blocked by ZM-241385 (−22.42 ± 1.9%) and SCH-58261(−30.04 ± 4.2%). CGS-21680 (10−6 M)-induced relaxation was higher in sEH−/− (+37.4 ± 5.4%) vs. sEH+/+ (+2.14 ± 2.8%). l-NAME (sEH−/−, +30.28 ± 4.8%, P > 0.05) did not block CGS-21680-induced response, whereas 14,15-EEZE (−7.1 ± 3.7%, P < 0.05) did. Also, AUDA and t-AUCB did not change CGS-21680-induced response in sEH−/− (P > 0.05), but reversed in sEH+/+ (from +2.14 ± 2.8% to +45.33 ± 4.1%, and +63.37 ± 7.2, respectively). PPARα-agonist did not relax as CGS 21680 (−2.48 ± 1.1 vs. +37.4 ± 5.4%) in sEH−/−, and PPARγ-antagonist blocked (from +37.4 ± 5.4% to +9.40 ± 3.1) CGS 21680-induced relaxation in sEH−/−. Our data suggest that adenosine-induced relaxation in sEH−/− may depend on the upregulation of A2A AR, CYP2J, and PPARγ, and the downregulation of A1 AR and PPARα.
Keywords: soluble epoxide hydrolase, adenosine A2a receptor, adenosine A1 receptor, CYP2J5-epoxgenase, ω-hydroxylase, contraction, relaxation
the physiological effects of adenosine have been observed in nearly every tissue and organ (1, 19, 32–35, 53). Adenosine plays an important role in cardiac protection (15–17), as well as the maintenance of vascular tone via activation of four receptor subtypes (A1, A2A, A2B, and A3). In blood vessels, vasodilation is primarily caused by the activation of A2A AR (36–39). Moreover, A2A AR-mediated relaxation was found to be dependent on the presence of a functional endothelium (1, 25, 36, 42).
Several cytochrome P-450 (CYP)-epoxygenases, including members of the CYP2C (CYP2C29) and CYP2J (CYP2J2, CYP2J5) subfamilies, have been identified in vascular endothelial cells (13, 41, 63, 64). Yang et al. (59) showed that overexpression of CYP2J2 protects endothelial cells against hypoxia-reoxygenation injury (59). Ma et al. (28) found abundant CYP2J5 in both the kidney and liver. The CYP-epoxygenases add an epoxide group across one of the four double bonds of arachidonic acid, forming four EET regioisomers, 5,6-, 8,9-, 11,12-, and 14,15-EET. Endothelial cells express CYP2C and CYP2J, which are the main source of EETs generation in the vascular system (13, 41, 46). The most potent biological effects of EETs occur in small-resistance vessels, as well as in the aorta (9, 36). For example, 14,15-EET has been observed to produce relaxation of isolated coronary microvessels at concentrations as low as 10 pM (9). This process occurs through hyperpolarization and suggests that the EETs function as an endothelium-derived hyperpolarizing factor (EDHF) in a number of vascular beds, including the coronary artery and aorta (3, 4, 13, 36, 37). The EDHF response in bovine coronary arteries and mouse aorta is inhibited by the EET antagonist, 14,15-epoxyeicosa-5(Z)-enoic acid (14, 36). The peroxisome proliferator-activated receptor (PPAR) transcription factors are members of the nuclear receptor superfamily that are activated by fatty acids and fatty acid derivatives (11). Both PPARα and PPARγ are expressed in blood vessels (5, 6), indicating that they have a role in vascular function. PPARγ is activated when bovine aortic endothelial cells are exposed to laminar flow through a process that is dependent on CYP-epoxygenases (26), and this leads to increases in the formation of 8,9-, 11,12-, and 14,15-EET in the endothelial cells within 15 min.
The soluble epoxide hydrolase (sEH) enzyme is detected in a variety of mammalian tissues, including the liver, kidney, intestine, and blood vessels (40, 56, 60). Within these tissues, sEH metabolizes epoxide-containing compounds to their corresponding diols (31, 40). Conversion of arachidonic acid epoxides to diols by sEH diminishes the beneficial cardiovascular properties of these epoxyeicosanoids. Inhibition of sEH causes EETs to accumulate and be retained for longer periods after they are formed (9). Some reports provide further evidence that sEH inhibition may be an effective approach for the treatment of hypertension and diseases associated with vascular inflammation (7, 8, 18, 47, 61). Also, the targeted disruption of the sEH gene in male mice lowers systolic blood pressure (51). Recently, we found that there is a possible link between the upregulation of A2A AR, CYP-epoxygenases, and downregulation of sEH with adenosine-induced relaxation in mouse aorta (38). Also, we found that there is a possible link between the downregulation of A2A AR, CYP-epoxygenases, and upregulation of sEH with 5′-N-ethylcarboxamidoadenosine (NECA)-induced contraction in mouse aorta (38, 39). Therefore, we hypothesized that the lack of sEH enhances adenosine-induced vascular relaxation through A2A AR via CYP-epoxygenases and PPARγ.
MATERIALS AND METHODS
The generation of sEH−/− mice was described by Sinal et al. (51). sEH−/− and sEH+/+mice were provided by Dr. Zeldin, National Institute of Environmental Health Sciences/National Institutes of Health (NIH). All animal care and experimentation protocols were approved and carried out in accordance with the West Virginia University Institutional Animal Care and Use Committee and were in accordance with the principles and guidelines of the NIH's Guide for the Care and Use of Laboratory Animals. Both male and female mice (14–18 wk old) in equal ratio were used in our study.
Protein extraction, gel electrophoresis, and Western blot analysis.
Mice were killed with pentobarbital sodium (100 mg/kg ip). According to our previously described protocol (36–39), after thoracotomy, the aorta was gently removed and cleaned of fat and connective tissue. In brief, aortas from both sEH−/− and sEH+/+mice were treated with 1 ml of lysis buffer for protein extraction. Gel electrophoresis and Western blot analysis were done according to the protocol described by us (36–39). Following blocking with nonfat dry milk, the nitrocellulose membranes were incubated with polyclonal primary antibodies for CYP2J5 (Dr. Zeldin, NIEHS/NIH), A1 AR (Sigma Chemicals), A2A AR (Alpha Diagnostic), CYP4A, PPARα, PPARγ, and β-actin (Santa Cruz Biotechnology). The secondary antibody, horseradish peroxidase-conjugated anti-rabbit IgG, was used. The membranes were developed using enhanced chemiluminescence (Amersham Biosciences) and exposed to X-ray film for appropriate time.
sEH−/− and sEH+/+ aortic rings.
After thoracotomy the aorta was gently removed, cleaned of fat and connective tissue, and cut transversely into rings of 3–4 mm in length. Care was taken not to damage the endothelium. The rings were hung vertically between two wire hooks. Two rings were suspended in organ baths containing 10 ml of modified Krebs-Henseleit buffer (36–39). After the equilibration period (60 min), tissues were contracted with KCl (50 mM) to assess the viability of the tissue. Rings were then constricted with phenylephrine (PE; 10−7 M), and tension was monitored continuously with a fixed range precision force transducer (125C; BIOPAC Systems) connected to amplifiers (Data Acquisition system 100B; BIOPAC Systems). Data were recorded using MP100 WSW, BIOPAC digital acquisition system and analyzed using Acknowledge 3.5.7 software (BIOPAC Systems). The functionality of endothelium was tested with ACh (10−7 M) on precontracted (PE) rings, as previously described by our laboratory (36–39).The aortic rings were washed several times with Krebs-Henseleit solution and allowed to equilibrate for 30 min before the experimental protocol began. Results are expressed as % downward or upward on PE-induced precontraction.
Effect of nitric oxide inhibitor (l-NAME) on ACh-induced response in sEH−/− and sEH+/+ mice.
Concentration-response curves (CRCs) were obtained by cumulative addition of drugs in 1-log increments as described by us (36–39). A single CRC was constructed for each ring in parallel in pairs of rings from either sEH−/− or sEH+/+ in the same organ bath. l-NAME (100 μM) was added 30 min before the PE contraction and was present throughout the ACh CRC.
Effect of A2A AR antagonists (ZM 241385 or SCH 58261) on NECA-induced CRC in sEH−/− and sEH+/+ mice.
Adenosine analog NECA-induced CRC was obtained as described above. ZM 241385 or SCH 58261 (1 μM) was added 30 min before PE contraction and was present throughout the experiment as described above.
Effects of nitric oxide (l-NAME)/epoxyeicosatrienoic acids (EETs) receptor antagonist 14,15-EEZE on CGS 21680-induced CRC in sEH−/− and sEH+/+ mice.
CGS 21680 (A2A AR agonist)-CRC was obtained with and without l-NAME (100 μM) or 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE; 10 μM), as described above.
Effects of sEH inhibitors on CGS 21680-induced CRC in sEH−/− and sEH+/+ mice.
CGS 21680 CRC was obtained with and without 12-(3-adamantan-1-yl-ureido) dodecanoic acid (AUDA, a sEH inhibitor; 10 μM) or trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB, a sEH inhibitor; 10 μM) as described above.
CRC for CGS 21680 and PPARα-agonist (GW 7647) and the effect of PPARγ-antagonist (T0070907) in sEH−/− and sEH+/+ mice.
CGS 21680-induced CRC was compared with GW 7647-induced CRC in sEH−/− and sEH+/+mice. Also, CGS 21680-induced CRC was obtained with and without T0070907 (PPARγ-antagonist; 0.1 μM), as described above.
Chemicals, drugs, and antibodies.
Phenylephrine and ACh (Sigma Chemicals, St. Louis, MO) were dissolved in distilled water. NECA and CGS 21680 (Sigma Chemicals, St. Louis, MO) were dissolved in 100% DMSO as 10 mM stock solutions, which were followed by serial dilutions in distilled water. T0070907, GW 7647 (Cayman Chemicals),14,15-EEZE (Dr. Falck), and AUDA and t-AUCB (Dr. Morisseau) were dissolved in DMSO. CYP2J5 (Dr. Zeldin, NIEHS/NIH), A1 AR (Sigma Chemicals), CYP4A, PPARα, and PPARγ (Santa Cruz Biotechnology), and A2A AR (Alpha Diagnostic) antibodies were obtained and used for Western blot experiments.
Statistical analysis.
Statistical data were reported as means ± SE. One-way ANOVA was used to compare difference among groups, and two-way ANOVA was used for repeated measures, followed by Tukey post hoc test to compare the NECA, CGS 21680, and GW 7647-induced vascular responses to antagonist (l-NAME, 14,15-EEZE, T0070907, ZM 241385, SCH 58261, AUDA, and t-AUCB). Differences were considered significant when P < 0.05. Further, densitometry of Western blot analysis (CYP2J5, CYP4A, PPARα, PPARγ, A2A AR, and A1AR) was expressed as means ± SE in arbitrary units. All the statistical analyses were performed using GraphPad Prism statistical package.
RESULTS
Expression of A2A AR, A1 AR, CYP2J5, CYP4A, PPARα, and PPARγ proteins in aortas from sEH−/− and sEH+/+ mice.
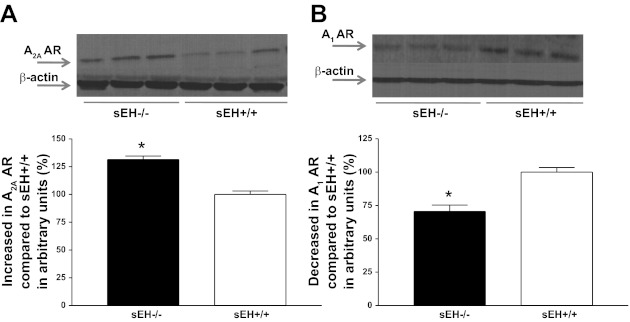
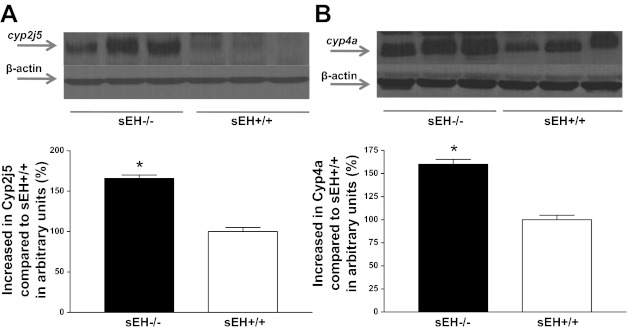
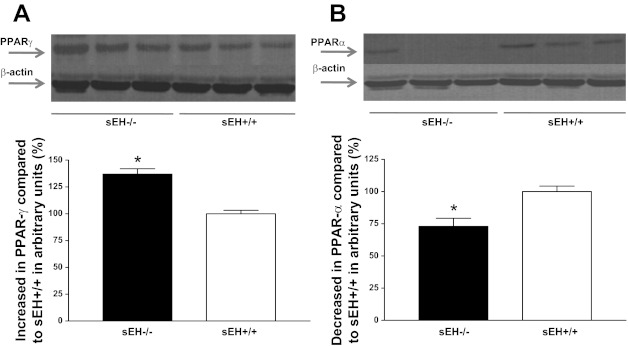
Western blot analysis for A2A AR (∼45 kDa) protein showed 31% more in sEH−/− than sEH+/+ mouse aorta (P < 0.05, Fig. 1A) whereas, A1 AR (∼37 kDa) protein showed 30% less in sEH−/− than sEH+/+ mice (P < 0.05, Fig. 1B) . The amount of CYP2J5 (∼58 kDa) protein in sEH−/− was increased by 65% compared with sEH+/+ mouse aorta (P < 0.05, Fig. 2A). Further, the level of CYP4A (∼50 kDa) protein in sEH−/− was increased by 60% compared with sEH+/+ mouse aorta (P < 0.05, Fig. 2B). Western blot analysis for PPARγ (∼58 kDa) protein showed 36% more in sEH−/− than sEH+/+ mouse aorta (P < 0.05, Fig. 3A), whereas PPARα (∼52 kDa) protein showed 27% less in sEH−/− than sEH+/+ mice (P < 0.05, Fig. 3B).
Fig. 1.
Representative Western blots and densitometric analysis for A2A AR (∼45 kDa; A) and A1AR (∼37 kDa; B) proteins in aortas of sEH−/− and sEH+/+ mice. Values are expressed as means ± SE. *P < 0.05, sEH+/+ compared with sEH−/− aortas; n = 6.
Fig. 2.
Representative Western blots and densitometric analysis for CYP2J5 (∼58 kDa; A) and CYP4A (∼50 kDa; B) proteins in aortas of sEH−/− and sEH+/+ mice. Values are expressed as means ± SE. *P < 0.05, sEH+/+ compared with sEH−/− aortas; n = 6.
Fig. 3.
Representative Western blots and densitometric analysis for PPARγ (∼58 kDa; A) and PPARα (∼52 kDa; B) proteins in aortas of sEH−/− and sEH+/+ mice. Values are expressed as means ± SE. *P < 0.05, sEH+/+ compared with sEH−/− aortas; n = 6.
CRC for ACh and the effect of nitric oxide inhibitor in sEH−/− and sEH+/+ mice.
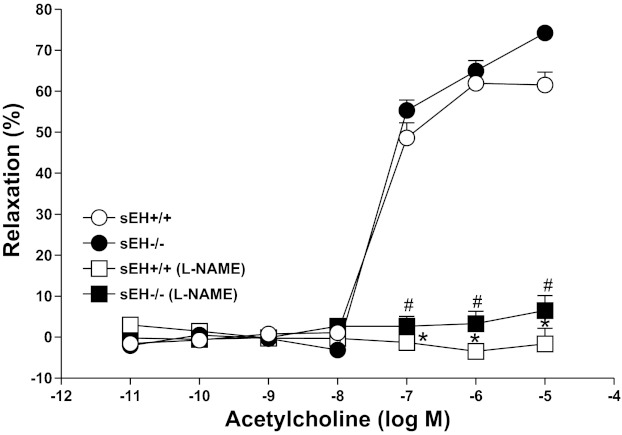
ACh caused a concentration (10−7–10−5 M)-dependent relaxation in both sEH−/− and sEH+/+, but the response was not significantly different (P > 0.05) between aortas from sEH−/− and sEH+/+ (Fig. 4). Also, l-NAME (100 μM) had altered vascular response significantly (P < 0.05) in both sEH−/− (+3.32 ± 6.0% at 10−6 ACh) and sEH+/+ (−3.4 ± 2.9% at 10−6 M ACh) compared with untreated sEH−/− and sEH+/+mouse aortas (P < 0.05, Fig. 4). But, no significant difference was observed in concentration response curves between sEH−/− and sEH+/+ (P > 0.05, Fig. 4).
Fig. 4.
Effect of l-NAME (100 μM) on ACh-induced vascular response in aortic rings of sEH+/+ and sEH−/− mice. Values are expressed as means ± SE. *P < 0.05, sEH+/+ controls vs. sEH+/+ mice treated with l-NAME. #P < 0.05, sEH−/− controls vs. #sEH−/− mice treated with l-NAME; n = 8.
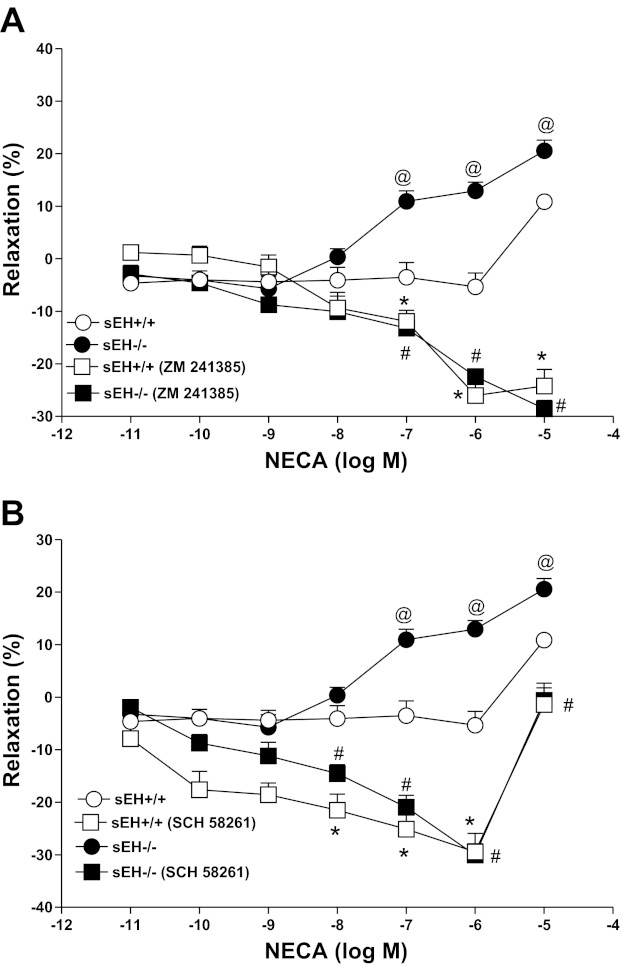
CRC for NECA with and without ZM 241385 or SCH 58261 in sEH−/− and sEH+/+ mice.
NECA produced a concentration-dependent relaxation in sEH−/− as opposed to contraction in sEH+/+ (Fig. 5, A and B). For example, the response to 10−6 M NECA in sEH−/− aorta was +12.94 ± 3.2% relaxation, while in sEH+/+ had −5.35 ± 5.2% contraction (P < 0.05, Fig. 5, A and B). CRC in sEH−/− vs. sEH+/+ for NECA (10−7–10−5 M, P < 0.05) were significantly different. ZM 241385(1 μM), an A2A AR antagonist produced a change from NECA-induced relaxation to contraction in sEH−/− (from +12.94 ± 3.2% to −22.42 ± 1.9 at 10−6 NECA, P < 0.05, Fig. 5A). No significant difference was found with ZM 241385 treatment between sEH−/− and sEH+/+ mice (P > 0.05, Fig. 5A). Another A2A AR antagonist, SCH 58261 produced a similar change from NECA-induced relaxation to contraction in sEH−/− (from +12.94 ± 3.2% to −30.04 ± 4.2 at 10−6 NECA, P < 0.05, Fig. 5B). No significant difference was found between SCH 58261 treated sEH−/− and SCH 58261 treated sEH+/+ mice (P > 0.05, Fig. 5B).
Fig. 5.
Effect of ZM 241385 (1 μM; A) and SCH 58261 (1 μM; B) on NECA-induced vascular response in aortic rings of sEH+/+ and sEH−/− mice. @P < 0.05 between sEH+/+ vs. sEH−/− mice. #P < 0.05, sEH−/− vs. sEH−/− mice treated with ZM 241385. *P < 0.05, sEH+/+ vs. sEH+/+ mice treated with ZM 241385, n = 8 (A). @P < 0.05, between sEH+/+ vs. sEH−/− mice. *P < 0.05, sEH+/+ vs. sEH+/+ mice with SCH 58261. #P < 0.05, sEH−/− vs. sEH−/− mice with SCH 58261; n = 8 (B). Values are expressed as means ± SE.
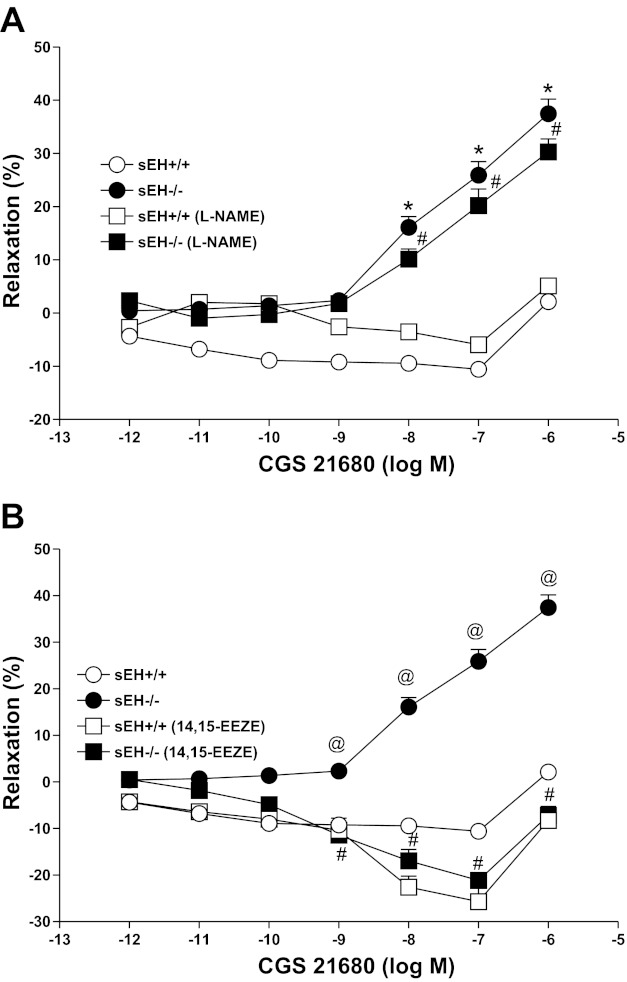
CRC for CGS 21680 and the effects of nitric oxide/epoxyeicosatrienoic acid receptor-antagonist in sEH−/− and sEH+/+ mice.
CGS 21680 produced a concentration-dependent relaxation (P < 0.05) in sEH−/− compared with the contraction in sEH+/+ mice (P < 0.05; Fig. 6). For example, at 10−6 M CGS 21680, the relaxation response was +37.4 ± 5.4% in sEH−/− compared with +2.1 ± 2.8% in sEH+/+ mice (P < 0.05; Fig. 6A). l-NAME (100 μM) did not significantly alter vascular responses in both the treated sEH−/− (+30.28 ± 4.8% at 10−6 CGS 21680) and the control sEH−/− (+37.4 ± 5.4%, P > 0.05, Fig. 6A) tissues. Also, no significant difference was observed in CRC between treated (l-NAME) and control sEH+/+ aorta (+5.1 ± 2.4 vs. +2.1 ± 2.8% at 10−6 CGS 21680, P > 0.05, Fig. 6A). Whereas, a significant blockade was found in the CRC in CGS 21680-induced relaxation with 14,15-EEZE (10 μM, a EETs receptor antagonist) compared with control in sEH−/− aortas. At 10−6 M CGS 21680, 14,15-EEZE had changed the relaxation response into contraction in sEH−/− (−7.1 ± 3.7%, Fig. 6B) aortas compared with controls (+37.46 ± 5.4%, P < 0.05; Fig. 6B). The CGS 21680-induced CRC unchanged among 14,15-EEZE-treated sEH+/+, 14,15-EEZE-treated sEH−/−, and control sEH+/+ tissues (P > 0.05, Fig. 6B).
Fig. 6.
Effect of l-NAME (100 μM; A) and 14,15-EEZE (10 μM; B) on CGS 21680-induced vascular response in aortic rings of sEH+/+ and sEH−/− mice. *P < 0.05, sEH+/+ vs. sEH−/− mice. #P < 0.05, sEH+/+ vs. sEH−/− treated with l-NAME, and sEH+/+ treated with l-NAME vs. sEH−/− treated with l-NAME; n = 8 (A). @P < 0.05, between sEH+/+vs. sEH−/− mice. @P < 0.05, sEH+/+ mice treated with 14,15-EEZE vs. sEH−/−; n = 8 (B). #P < 0.05, sEH−/− vs. sEH−/− mice treated with 14,15-EEZE. Values are expressed as means ± SE.
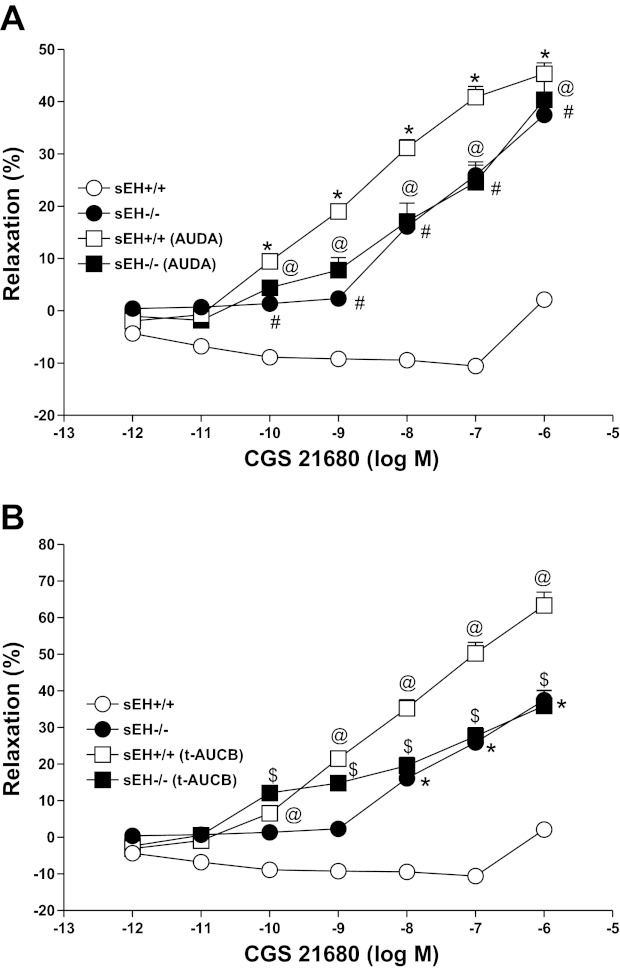
Effects of sEH inhibitors on CGS 21680 CRC in sEH−/− and sEH+/+ mice.
AUDA (10 μM), a soluble epoxide hydrolase (sEH) inhibitor, reversed and produced a significantly higher relaxation (+45.33 ± 4.1%, P < 0.05, Fig. 7A) with CGS 21680 in sEH+/+ as opposed to its control (+2.1 ± 2.8%; Fig. 7A). In contrast, AUDA had no significant effect on CGS 21680 CRC in treated and untreated sEH−/− tissues (P > 0.05, Fig. 7A). Also, no significant differences were observed in CGS 21680 CRC among sEH+/+ and sEH−/− tissues treated with AUDA and untreated EH−/− (P > 0.05, Fig. 7A). t-AUCB (trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid; 10 μM), another soluble epoxide hydrolase inhibitor, once again reversed and produced a significantly higher relaxation (+63.37 ± 7.2%, P < 0.05, Fig. 7B) at 10−6 M CGS 21680 in sEH+/+ as opposed to its control (+2.1 ± 2.8%; Fig. 7B). In contrast, t-AUCB had no significant effect on CGS 21680 CRC in sEH−/− (P > 0.05, Fig. 7B).
Fig. 7.
Effect of AUDA (10 μM) on CGS 21680 induced vascular response in aortic rings of sEH+/+ and sEH−/− mice (A). The control curve is the same as in Fig. 6. #P < 0.05, sEH+/+vs. sEH−/− mice. *P < 0.05, sEH+/+ vs. sEH+/+ mice treated with AUDA. @P < 0.05, sEH+/+ vs. sEH−/− treated with AUDA; n = 8 (A). Effect of t-AUCB (10 μM) on CGS 21680-induced vascular response in aortic rings of sEH+/+ and sEH−/− mice (B). The control curve is the same as in Fig. 6. *P < 0.05, sEH+/+ vs. sEH−/− mice. @P < 0.05, sEH+/+ vs. sEH+/+ mice treated with t-AUCB. $P < 0.05, sEH+/+ vs. sEH−/− mice treated with t-AUCB; n = 8 (B). Values are expressed as means ± SE.
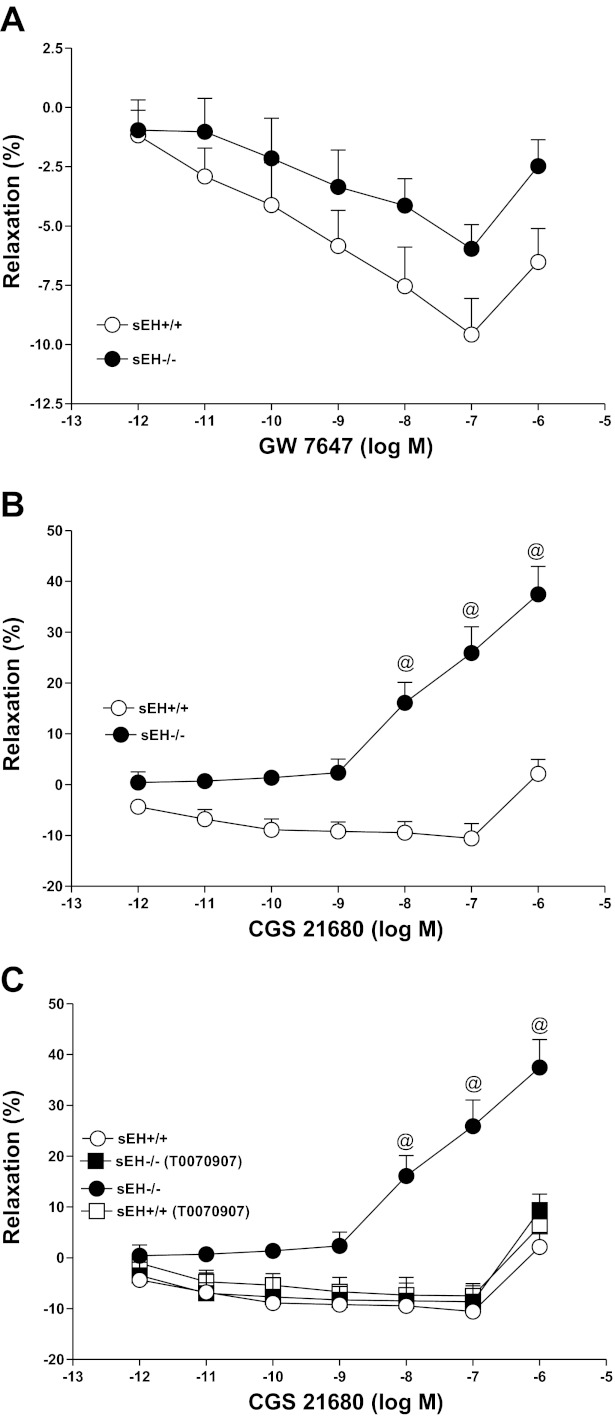
CRCs for PPARα-agonist (GW 7647) and CGS 21680 and the effect of PPARγ-antagonist (T0070907) in sEH−/− and sEH+/+ mice.
GW 7647 produced a concentration-dependent contraction in both sEH−/− and sEH+/+ mice (P > 0.05; Fig. 8A). For example, at 10−6 M GW 7647, the contraction response was −2.48 ± 1.1% in sEH−/− compared with −6.52 ± 1.4% in sEH+/+ mice (P > 0.05; Fig. 8A), while CGS 21680 produced a concentration-dependent enhanced relaxation in sEH−/− compared with less relaxation in sEH+/+ mice (P < 0.05; Fig. 8B). For example, at 10−6 M CGS 21680, the relaxation response was +37.4 ± 5.4% in sEH−/− compared with +2.1 ± 2.8% in sEH+/+ mice (P < 0.05; Fig. 8B). In comparison between CGS 21680 and GW 7647, the CGS 21680 (10−6 M)-induced relaxation response was +37.4 ± 5.4% in sEH−/− mice, whereas the GW 7647 (10−6 M)-induced contraction response was −2.48 ± 1.1% in sEH−/− mice (P < 0.05; Fig. 8, A and B). Also, a significant blockade was found in the CRC of CGS 21680-induced relaxation with T0070907 (PPARγ-antagonist; 0.1 μM) compared with control in sEH−/− aortas. At 10−6 M CGS 21680, T0070907 had changed the vascular response in sEH−/− (+9.40 ± 3.1%; Fig. 8C) aortas compared with controls (+37.46 ± 5.4%, P < 0.05; Fig. 8C).
Fig. 8.
GW 7647 (PPARα-agonist)-induced vascular response in aortic rings of sEH+/+ and sEH−/− mice. P > 0.05 for GW 7647-sEH+/+ vs. GW 7647-sEH−/−; n = 8 (A). CGS 21680-induced vascular response in aortic rings of sEH+/+ and sEH−/− mice. @P < 0.05, CGS 21680-sEH+/+ vs. CGS 21680-sEH−/− mice; n = 8 (B). Effect of PPARγ-antagonist (0.1 μM; B) on CGS 21680-induced vascular response in aortic rings of sEH+/+ and sEH−/− mice. @P < 0.05, sEH+/+vs. sEH−/− mice. @P < 0.05, sEH+/+ treated with PPARγ-antagonist vs. sEH−/− mice alone. @P < 0.05, sEH−/− treated with PPARγ-antagonist vs. sEH−/− mice alone; n = 8 (C). Values are expressed as means ± SE.
DISCUSSION
The sEH−/− mice show an increase in aortic A2A AR, CYP2J, and PPARγ protein expressions, and a decrease in A1 AR, PPARα proteins compared with sEH+/+ mice (Figs. 1–3). The relationship between sEH and adenosine-induced vascular responses in mice is not known. Therefore, this study was designed to investigate the role of A2A, A1 ARs, CYP-epoxygenases, PPARα, and PPARγ in sEH+/+ and sEH−/− mice. Our data demonstrate that 1) ACh-induced vascular relaxation was not different between sEH−/− and sEH+/+mice, and l-NAME was able to block ACh-induced vascular relaxation equally in both sEH−/− and sEH+/+ mice; 2) A2A AR modulates nitric oxide (NO)-independent vascular relaxation in sEH−/− mice compared with sEH+/+; 3) A2A AR-mediated relaxation was blocked by EET receptor antagonist, but not with NO inhibitor; 4) sEH inhibitors (AUDA and t-AUCB) reversed the CGS 21680-mediated vascular response to enhanced relaxation in sEH+/+, but ineffective in sEH−/−mice; 5) CGS 21680 enhanced dose-dependent relaxation in sEH−/− mice, whereas GW 7647 (PPARα-agonist) reduced relaxation significantly, and finally 6) T0070907 (PPARγ-antagonist) significantly inhibited the CGS 21680-enhanced vascular relaxation in sEH−/− mice.
Genetic polymorphisms in CYP-epoxygenases have been observed in different populations, which affect cardiovascular function, including hypertension (10, 12, 20, 23, 54, 55, 57, 66). Genetic variation in sEH and CYP4A was also found in human population with risk of coronary heart disease, ischemic stroke, restenosis, diabetes heart, heart failure, ischemic stroke in white Europeans, Chinese populations, and in the African American population with hypertension (2, 10, 21, 24, 30, 66). As the earlier studies showed the differential renal sEH gene expression in prehypertensive, hypertensive, and spontaneously hypertensive rats (49), and mice lacking A2A AR have hypertensive characteristics (22). Therefore, there is a need to identify the possible targets and develop novel pharmacological agents to treat vascular deregulation in patients in the long run, who have allelic variants that may possibly act similar to our gene-manipulated mice (A2A AR−/−, sEH−/−), which may be involved in the regulation of blood pressure and vascular tone.
ACh data between sEH+/+ and sEH−/− suggest that there is no relationship between ACh and the presence or absence of sEH (Fig. 4). Also, l-NAME, a nitric oxide synthase inhibitor completely blocked ACh-induced vascular response equally in both sEH+/+ and sEH−/− mice (Fig. 4).These data also suggest that the presence or absence of sEH does not matter in ACh-induced vascular response in both sEH+/+ and sEH−/− mice (Fig. 4). Similarly, Zhang et al. (65) reported that the inhibition of sEH ameliorates endothelial dysfunction and effects in the db/db mice, which is independent of NO, but dependent on CYP-epoxygenase-derived metabolites. Other studies have also shown that nitric oxide synthase inhibition does not affect the increase of blood flow in forearm or leg during exercise in humans, rats, and rabbits (43, 44, 50, 58). But, during exercise, adenosine originates from skeletal muscle fibers and acts on A2A AR to evoke vasodilatation independent of NO (45). Our own studies have shown that adenosine-induced mouse aortic relaxation through A2A AR is independent of NO and COX (36–38).
The sEH enzyme metabolizes EET that serve as substrates for the sEH (31, 40). The conversion of EETs in the presence sEH into the corresponding dihydroxyeicosatrienoic acids (DHETs) results in loss of beneficial effects (29, 52, 62). The conversion of epoxides to diols by sEH diminishes the beneficial cardiovascular properties of these epoxyeicosanoids. Inhibition of sEH causes EETs to accumulate and be retained for longer periods after they are formed (9). Some reports provide further evidence that sEH inhibition may be an effective approach for the treatment of hypertension and diseases associated with vascular inflammation (7, 8, 18, 47, 61). Also, the targeted disruption of the sEH gene in male mice lowers systolic blood pressure (51). Recently, we found that there is a possible link between the upregulation of A2A AR, CYP-epoxygenases, and downregulation of sEH with adenosine-induced relaxation in high-salt diet-fed WT mice (C57BL/6J) and eNOS−/− (C57BL/6J background) compared with low-salt diet-fed mice (38, 39). Also, we found that there is a possible link between the downregulation of A2A AR, CYP-epoxygenases and upregulation of sEH with NECA-induced contraction in low-salt diet-fed WT mice and eNOS−/− (C57BL/6J background) compared with high-salt diet-fed mice (38, 39). The current study shows that in the absence of sEH, there is an increase in aortic A2A AR, CYP2J5, PPARγ, and a decrease in A1 AR, PPARα proteins compared with WT mice. Therefore, we hypothesize that, in the absence of sEH, adenosine induces vascular relaxation through A2A AR via CYP-epoxygenases and PPARγ, whereas in the presence of sEH, adenosine induces vascular contraction through PPARα.
NECA (nonselective adenosine agonist) induces significant relaxation in sEH−/− compared to sEH+/+ mice (Fig. 5), suggesting that sEH deletion enhances NECA-induced vascular relaxation. Our data show that NECA-induced vascular response in aortas of sEH−/− mice is similar to that from our earlier reports in which we found that high-salt diet-fed WT (C57BL/6J) mice had an enhanced NECA-induced relaxation with upregulation of A2A AR, CYP2J-epoxygenase and downregulation of sEH, whereas, NECA-induced vascular response in aortas of sEH+/+ mice is similar to that found in our earlier reports in which low-salt diet-fed WT (C57BL/6J) mice had an enhanced NECA-induced contraction with downregulation of A2A AR, CYP2J-epoxygenase and upregulation of sEH (37, 38). Therefore, in the present study, we used specific A2A AR antagonists (ZM-241385 and SCH-58261) to rule out the involvement of other adenosine receptors (A1, A2B, A3). Both ZM 241385 and SCH-58261 (A2A AR-antagonists) blocked completely the NECA-induced vascular relaxation in sEH−/− compared with untreated mice (Fig. 5, A and B). We also confirmed the involvement of A2A AR with the use of a highly selective A2A AR agonist (CGS 21680) in sEH+/+ and sEH−/− mouse aortas. A highly robust CGS 21680-induced vascular relaxation was observed in sEH−/− compared with sEH+/+ mice (Fig. 6). Also, in the present study, we found a significant upregulation of A2A AR protein in sEH−/− compared with sEH+/+ mice (Fig. 1A), whereas a significant downregulation of A1 AR protein in sEH−/− compared with sEH+/+ mice (Fig. 1B). These data suggest that A2A AR is the only adenosine receptor involved in enhancing adenosine-induced relaxation in sEH−/− compared with sEH+/+ mouse aortas.
In the present study, we also tested whether A2A AR-induced vascular relaxation is NO-dependent or not. No significant changes were found between l-NAME-treated and untreated sEH−/− and sEH+/+ mouse aorta with CGS 21680-induced vascular response (Fig. 6A). These data suggest that there is no role for NO in adenosine-induced vascular response through A2A AR in sEH−/− and sEH+/+mice. These data confirm our earlier reports from this laboratory in which l-NAME and indomethacin (COX inhibitor) were unable to block aortic relaxation in A2A AR+/+ compared with A2A AR−/− mice, and in high-salt diet-fed compared with low-salt diet-fed C57BL/6J mice (36–39). Also, Ray and Marshall (45) reported that during exercise, skeletal muscle fibers release adenosine, which is independent of NO.
In this study, NECA- and CGS 21680-induced relaxation in sEH−/− mouse aortas (Figs. 5–8) suggests that this relaxation depends on A2A AR via CYP-epoxygenases leading to EETs formation. Therefore, we were able to block CGS 21680-induced vascular relaxation with 14,15-EEZE (EET receptor antagonist) in sEH−/− mouse aortas (Fig. 6B). Similarly, the EDHF response in bovine coronary arteries was inhibited by the EET antagonist, 14,15-epoxyeicosa-5(Z)-enoic acid (14). Recently, a report from our laboratory also showed that in A2A AR+/+ mouse aorta, the adenosine-induced vascular relaxation was inhibited by the EET antagonist, 14,15-epoxyeicosa-5(Z)-enoic acid (36). In the present study, we also found an upregulation of CYP2J5 proteins in sEH−/− compared with sEH+/+ mouse aortas (Fig. 2A). Our data suggest that the endothelium-dependent NECA- and CGS-21680-induced vascular relaxation is mediated through EETs possibly by increased in CYP2J-epoxygenase in sEH−/− compared sEH+/+ mouse aortas. The current study further confirmed that the adenosine-induced vascular relaxation through A2A AR is dependent on CYP-epoxygenases, including CYP2J-epoxygenase, but not NO or COX.
We also demonstrated that the treatment with 12-(3-adamantan-1-yl-ureido) dodecanoic acid (AUDA) or trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB), inhibitors of the sEH enzyme have reversed the CGS 21680-induced weak vascular response into a strong vascular relaxation in sEH+/+ compared with controls (Fig. 7, A and B), while, no significant change was observed with AUDA or t-AUCB treated and untreated sEH−/− mice (Fig. 7, A and B). These data suggest that, in the presence of sEH, AUDA or t-AUCB effectively block the endogenous sEH enzyme activity. Therefore, we observed complete reversal of CGS 21680-induced vascular response in AUDA- or t-AUCB-treated sEH+/+ mouse aortas compared with controls. Also, it is obvious that, no significant change was observed with CGS 21680-induced vascular response between AUDA or t-AUCB-treated and untreated sEH−/− mice, because these mice do not have endogenous sEH enzyme activity. Use of sEH enzyme inhibitors or deleting the sEH gene increases endogenous EETs by decreasing the metabolic conversion of EETs into less active DHETs. If the mechanism by which sEH inhibitors (AUDA or t-AUCB) or deletion of sEH gene enhance CGS 21680-induced vascular relaxation occurs by increasing endogenous EET levels, then it would be expected that inhibiting EET synthesis would attenuate the CGS 21680-induced vascular response in sEH−/− mice. As expected, pretreatment of sEH−/− mouse aorta with the EETs antagonist 14,15-EEZE did alter significantly CGS 21680-induced vascular response from relaxation to contraction (Fig. 6B). These data suggest that in absence or blocking of sEH, enhanced CGS 21680-induced vascular relaxation is mostly likely due to an increase EET levels. Liu et al. (26) suggests that selective sEH inhibitors will potentiate the anti-inflammatory effect in the endothelial cells, presumably by increasing the retention of 11,12- and 14,15-EET so that PPARγ activation is prolonged. Acute activation of PPARγ leads to endothelium-dependent aortic relaxation in nondiabetic (+db/+m) mice (48). Therefore, we found upregulation of PPARγ and down regulation of PPARα with A2A AR enhanced relaxation in sEH−/− compared with sEH+/+ mice (Fig. 3, A and B). The PPAR expression data are also supported by the functional data, in which PPARα agonist dose-dependent vascular response in sEH−/− was not significantly different compared with sEH+/+ mice (Fig. 8A), whereas CGS 21680 dose-dependent vascular response in sEH−/− was significantly different compared with sEH+/+ mice (Fig. 8B). There was no significant difference found between CGS 21680-induced vascular response in sEH+/+ mice compared with PPARα-agonist-induced response in both sEH−/− and sEH+/+ mice (Fig. 8, A and B), suggesting that the vascular contraction in sEH+/+ mouse aorta is possible due to PPARα activity (Figs. 3B and 8A). Moreover, PPARγ antagonist was able to block significantly the CGS 21680-induced vascular relaxation (Fig. 8C), and there was no significant difference found between CGS 21680-induced vascular response in sEH+/+ mice compared with CGS 21680 + PPARγ antagonist response in both sEH−/− and sEH+/+-treated mice (Fig. 8C). These data suggest a possible role for PPARγ in CGS 21680-induced vascular relaxation in the absence of sEH compared with the presence of sEH in mice (Figs. 3A and 8C). Also, the data suggest a possible role of PPARα in vascular contraction (Figs. 3B and 8A).
Surprisingly, we found an upregulation of CYP4A (Fig. 2B), a vasoconstrictor enzyme in sEH−/− mouse aorta compared with sEH+/+. The reason behind the upregulation of CYP4A in the absence of sEH may be due to a compensatory response to maintain vascular tone, as well as the blood pressure in sEH knockout mouse. Similar upregulation of CYP4A in kidneys of Ephx2 gene-disrupted mice has been reported as a compensatory mechanism to maintain blood pressure regulation (27).
The present data suggest that there is a relationship between A2A AR-enhanced vascular relaxation and lack of sEH, and there is relationship between the presence of sEH and adenosine-induced vasoconstriction or less relaxation through upregulation of A1 AR. The signaling mechanism may be involved by the activation of A2A AR, functional endothelium to activate CYP2J-epoxgenases to generate more EETs than DHETs, downregulation of A1 AR, PPARα, and upregulation of A2A AR, PPARγ, leading to NECA or CGS 21680-enhanced vascular relaxation in sEH−/− mouse aorta. In contrast, the contraction or less relaxation with NECA or CGS 21680 in sEH+/+ mice provides evidence that there is a possible link among the less availability of A2A AR, less functional endothelium to activate CYP2J-epoxygenases, less generation of EETs than DHETs, and upregulation of A1 AR and PPARα. Therefore, we conclude that upregulation of CYP-epoxygenases, A2A AR, and PPARγ and downregulation of A1 AR and PPARα protein expression contribute to NECA- or CGS 21680-induced dilation in sEH−/− mouse aorta, while the upregulation of A1 AR and PPARα and downregulation of CYP-epoxygenases, A2A AR, and PPARγ protein expression contribute to NECA or CGS 21680-induced vasoconstriction or less relaxation in sEH+/+ mouse aorta.
Perspectives and Significances
These findings suggest that the relationship among A2A AR, CYP-epoxygenases, arachidonic acid-derived metabolites, and PPARγ in vasodilation, while a relationship among A1 AR, sEH, and PPARα in vasoconstriction may have clinical implication in the regulation of vascular tone and regulation of blood pressure. Any deregulation in these pathways or slight allelic variations may possibly lead to hypertension and coronary artery disease in humans. Future studies are necessary to identify the possible targets and develop novel pharmacological agents to treat vascular deregulation in patients in the long run, who have allelic variants that may possibly act similar to our gene-manipulated mice (A2A AR−/−, sEH−/−), which may play a role in the regulation of vascular tone and ultimately blood pressure.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.A.N. conception and design of research; M.A.N. and I.P. performed experiments; M.A.N. analyzed data; M.A.N. interpreted results of experiments; M.A.N. prepared figures; M.A.N. drafted manuscript; M.A.N. edited and revised manuscript; M.A.N., I.P., S.J.M., C.M., J.R.F., and D.C.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by Bridge Grant funding [West Virginia University WVU)] and startup funding (WVU) to M. A. Nayeem, National Institutes of Health Grant HL-027339 and HL-094447 to S. J. Mustafa, NIH Grant GM-31278 to J. R. Falck and National Institute of Environmental Health Sciences Grant z01 ES025034 to D. C. Zeldin.
REFERENCES
- 1. Abebe W, Makujina SR, Mustafa SJ. Adenosine receptor-mediated relaxation of porcine coronary artery in presence and absence of endothelium. Am J Physiol Heart Circ Physiol 266: H2018–H2025, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Burdon KP, Lehtinen AB, Langefeld CD, Carr JJ, Rich SS, Freedman BI, Herrington D, Bowden DW. Genetic analysis of the soluble epoxide hydrolase gene, EPHX2, in subclinical cardiovascular disease in the Diabetes Heart Study. Diab Vasc Dis Res 5: 128–134, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Campbell WB, Harder DR. Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circ Res 84: 484–488, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. Peroxisome proliferators-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J Biol Chem 274: 32048–32054, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol 169: 453–459, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Elmarakby AA, Faulkner J, Al-Shabrawey M, Wang MH, Maddipati KR, Imig JD. Deletion of soluble epoxide hydrolase gene improves renal endothelial function and reduces renal inflammation and injury in streptozotocin-induced diabetes. Am J Physiol Regul Integr Comp Physiol 301: R1307–R1317, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elmarakby AA, Ibrahim AS, Faulkner J, Mozaffari MS, Liou GI, Abdelsayed R. Tyrosine kinase inhibitor, genistein, reduces renal inflammation and injury in streptozotocin-induced diabetic mice. Vascul Pharmacol 55: 149–156, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, Thompson DA, Hammock BD, Spector AA. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem 276: 14867–14874, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Fava C, Montagnana M, Almgren P, Hedblad B, Engstrom G, Berglund G, Minuz P, Melander O. The common functional polymorphism −50G>T of the CYP2J2 gene is not associated with ischemic coronary and cerebrovascular events in an urban-based sample of Swedes. J Hypertens 28: 294–299, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res 45: 120–159, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens 21: 1288–1291, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res 90: 1028–1036, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Gross GJ, Hardman HF, Warltier DC. Adenosine on myocardial oxygen consumption. Br J Pharmacol 57: 409–412, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gross GJ, Mei DA, Sleph PG, Grover GJ. Adenosine A1 receptor blockade does not abolish the cardioprotective effects of the adenosine triphosphate-sensitive potassium channel opener bimakalim. J Pharmacol Exp Ther 280: 533–540, 1997 [PubMed] [Google Scholar]
- 17. Gross GJ, Warltier DC, Hardman HF. Effect of adenosine on myocardial oxygen balance. J Pharmacol Exp Ther 196: 445–454, 1976 [PubMed] [Google Scholar]
- 18. Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol 289: F496–F503, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Jackson EK, Zhu C, Tofovic SP. Expression of adenosine receptors in the preglomerular microcirculation. Am J Physiol Renal Physiol 283: F41–F51, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Jie Z, Hong K, Jianhong T, Biao C, Yongmei Z, Jingchuan L. Haplotype analysis of the CYP2J2 gene associated with myocardial infarction in a Chinese Han population. Cell Biochem Funct 28: 435–439 [DOI] [PubMed] [Google Scholar]
- 21. Kullmann S, Binner P, Rackebrandt K, Huge A, Haltern G, Lankisch M, Futh R, von Hodenberg E, Bestehorn HP, Scheffold T. Variation in the human soluble epoxide hydrolase gene and risk of restenosis after percutaneous coronary intervention. BMC Cardiovasc Disord 9: 48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388: 674–678, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Lee CR, North KE, Bray MS, Couper DJ, Heiss G, Zeldin DC. CYP2J2 and CYP2C8 polymorphisms and coronary heart disease risk: the Atherosclerosis Risk in Communities (ARIC) study. Pharmacogenet Genomics 17: 349–358, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee CR, North KE, Bray MS, Fornage M, Seubert JM, Newman JW, Hammock BD, Couper DJ, Heiss G, Zeldin DC. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Hum Mol Genet 15: 1640–1649, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewis CD, Hourani SM, Long CJ, Collis MG. Characterization of adenosine receptors in the rat isolated aorta. Gen Pharmacol 25: 1381–1387, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Zhu Y, Rannou F, Lee TS, Formentin K, Zeng L, Yuan X, Wang N, Chien S, Forman BM, Shyy JY. Laminar flow activates peroxisome proliferator-activated receptor-gamma in vascular endothelial cells. Circulation 110: 1128–1133, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Luria A, Weldon SM, Kabcenell AK, Ingraham RH, Matera D, Jiang H, Gill R, Morisseau C, Newman JW, Hammock BD. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J Biol Chem 282: 2891–2898, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma J, Qu W, Scarborough PE, Tomer KB, Moomaw CR, Maronpot R, Davis LS, Breyer MD, Zeldin DC. Molecular cloning, enzymatic characterization, developmental expression, and cellular localization of a mouse cytochrome P450 highly expressed in kidney. J Biol Chem 274: 17777–17788, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med 3: 562–566, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Monti J, Fischer J, Paskas S, Heinig M, Schulz H, Gosele C, Heuser A, Fischer R, Schmidt C, Schirdewan A, Gross V, Hummel O, Maatz H, Patone G, Saar K, Vingron M, Weldon SM, Lindpaintner K, Hammock BD, Rohde K, Dietz R, Cook SA, Schunck WH, Luft FC, Hubner N. Soluble epoxide hydrolase is a susceptibility factor for heart failure in a rat model of human isease. Nat Genet 40: 529–537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol 45: 311–333, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Morrison RR, Teng B, Oldenburg PJ, Katwa LC, Schnermann JB, Mustafa SJ. Effects of targeted deletion of A1 adenosine receptors on postischemic cardiac function and expression of adenosine receptor subtypes. Am J Physiol Heart Circ Physiol 291: H1875–H1882, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Nayeem MA, Matherne GP, Mustafa SJ. Ischemic and pharmacological preconditioning induces further delayed protection in transgenic mouse cardiac myocytes over-expressing adenosine A1 receptors (A1AR): role of A1AR, iNOS and K(ATP) channels. Naunyn Schmiedebergs Arch Pharmacol 367: 219–226, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Nayeem MA, Mustafa SJ. Mechanisms of delayed preconditioning with A1 adenosine receptor activation in porcine coronary smooth muscle cells. Pol J Pharmacol 54: 443–453, 2002 [PubMed] [Google Scholar]
- 35. Nayeem MA, Mustafa SJ. Protein kinase C isoforms and A1 adenosine receptors in porcine coronary smooth muscle cells. Vascul Pharmacol 39: 47–54, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS, Ansari HR, Mustafa SJ. Role of CYP epoxygenases in A2A AR-mediated relaxation using A2A AR-null and wild-type mice. Am J Physiol Heart Circ Physiol 295: H2068–H2078, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nayeem MA, Ponnoth DS, Boegehold MA, Zeldin DC, Falck JR, Mustafa SJ. High-salt diet enhances mouse aortic relaxation through adenosine A2A receptor via CYP epoxygenases. Am J Physiol Regul Integr Comp Physiol 296: R567–R574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nayeem MA, Zeldin DC, Boegehold MA, Falck JR. Salt modulates vascular response through adenosine A2A receptor in eNOS-null mice: role of CYP450 epoxygenase and soluble epoxide hydrolase. Mol Cell Biochem 350: 101–111, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nayeem MA, Zeldin DC, Boegehold MA, Morisseau C, Marowsky A, Ponnoth DS, Roush KP, Falck JR. Modulation by salt intake of the vascular response mediated through adenosine A2A receptor: role of CYP epoxygenase and soluble epoxide hydrolase. Am J Physiol Regul Integr Comp Physiol 299: R325–R333, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res 44: 1–51, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276–1279, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olanrewaju HA, Mustafa SJ. Adenosine A2A and A2B receptors mediated nitric oxide production in coronary artery endothelial cells. Gen Pharmacol 35: 171–177, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Persson MG, Gustafsson LE, Wiklund NP, Hedqvist P, Moncada S. Endogenous nitric oxide as a modulator of rabbit skeletal muscle microcirculation in vivo. Br J Pharmacol 100: 463–466, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1951–H1960, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Ray CJ, Marshall JM. Nitric oxide (NO) does not contribute to the generation or action of adenosine during exercise hyperaemia in rat hindlimb. J Physiol 587: 1579–1591, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosolowsky M, Campbell WB. Synthesis of hydroxyeicosatetraenoic (HETEs) and epoxyeicosatrienoic acids (EETs) by cultured bovine coronary artery endothelial cells. Biochim Biophys Acta 1299: 267–277, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA 102: 9772–9777, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seto SW, Lam TY, Leung GP, Au AL, Ngai SM, Chan SW, Kwan YW. Comparison of vascular relaxation, lipolysis and glucose uptake by peroxisome proliferator-activated receptor-gamma activation in +db/+m and +db/+db mice. Eur J Pharmacol 572: 40–48, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Seubert JM, Xu F, Graves JP, Collins JB, Sieber SO, Paules RS, Kroetz DL, Zeldin DC. Differential renal gene expression in prehypertensive and hypertensive spontaneously hypertensive rats. Am J Physiol Renal Physiol 289: F552–F561, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol Heart Circ Physiol 273: H2388–H2395, 1997 [DOI] [PubMed] [Google Scholar]
- 51. Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem 275: 40504–40510, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res 43: 55–90, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Talukder MA, Morrison RR, Mustafa SJ. Comparison of the vascular effects of adenosine in isolated mouse heart and aorta. Am J Physiol Heart Circ Physiol 282: H49–H57, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Theken KN, Lee CR. Genetic variation in the cytochrome P450 epoxygenase pathway and cardiovascular disease risk. Pharmacogenomics 8: 1369–1383, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Wang CP, Hung WC, Yu TH, Chiu CA, Lu LF, Chung FM, Hung CH, Shin SJ, Chen HJ, Lee YJ. Genetic variation in the G-50T polymorphism of the cytochrome P450 epoxygenase CYP2J2 gene and the risk of younger onset type 2 diabetes among Chinese population: potential interaction with body mass index and family history. Exp Clin Endocrinol Diabetes 118: 346–352, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Wang P, Meijer J, Guengerich FP. Purification of human liver cytosolic epoxide hydrolase and comparison to the microsomal enzyme. Biochemistry 21: 5769–5776, 1982 [DOI] [PubMed] [Google Scholar]
- 57. Xu Y, Ding H, Peng J, Cui G, Liu L, Cianflone K, Wang DW. Association between polymorphisms of CYP2J2 and EPHX2 genes and risk of coronary artery disease. Pharmacogenet Genomics 21: 489–494, 2011 [DOI] [PubMed] [Google Scholar]
- 58. Yamada M, Ishikawa T, Fujimori A, Goto K. Local neurogenic regulation of rat hindlimb circulation: role of calcitonin gene-related peptide in vasodilatation after skeletal muscle contraction. Br J Pharmacol 122: 703–709, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang B, Graham L, Dikalov S, Mason RP, Falck JR, Liao JK, Zeldin DC. Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol Pharmacol 60: 310–320, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Yu Z, Davis BB, Morisseau C, Hammock BD, Olson JL, Kroetz DL, Weiss RH. Vascular localization of soluble epoxide hydrolase in the human kidney. Am J Physiol Renal Physiol 286: F720–F726, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res 87: 992–998, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem 276: 36059–36062, 2001 [DOI] [PubMed] [Google Scholar]
- 63. Zeldin DC, Liao JK. Reply: cytochrome P450-derived eicosanoids and the vascular wall. Trends Pharmacol Sci 21: 127–128, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Zeldin DC, Wohlford-Lenane C, Chulada P, Bradbury JA, Scarborough PE, Roggli V, Langenbach R, Schwartz DA. Airway inflammation and responsiveness in prostaglandin H synthase-deficient mice exposed to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol 25: 457–465, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Zhang LN, Vincelette J, Chen D, Gless RD, Anandan SK, Rubanyi GM, Webb HK, MacIntyre DE, Wang YX. Inhibition of soluble epoxide hydrolase attenuates endothelial dysfunction in animal models of diabetes, obesity and hypertension. Eur J Pharmacol 654: 68–74, 2011 [DOI] [PubMed] [Google Scholar]
- 66. Zordoky BN, El-Kadi AO. Effect of cytochrome P450 polymorphism on arachidonic acid metabolism and their impact on cardiovascular diseases. Pharmacol Ther 125: 446–463, 2010 [DOI] [PubMed] [Google Scholar]