Abstract
Precise determination of the effect of muscle temperature (Tm) on mitochondrial oxygen consumption kinetics has proven difficult in humans, in part due to the complexities in controlling for Tm-related variations in blood flow, fiber recruitment, muscle metabolism, and contractile properties. To address this issue, intracellular Po2 (PiO2) was measured continuously by phosphorescence quenching following the onset of contractions in single Xenopus myofibers (n = 24) while controlling extracellular temperature. Fibers were subjected to two identical contraction bouts, in random order, at 15°C (cold, C) and 20°C (normal, N; n = 12), or at N and 25°C (hot, H; n = 12). Contractile properties were determined for every contraction. The time delay of the PiO2 response was significantly greater in C (59 ± 35 s) compared with N (35 ± 26 s, P = 0.01) and H (27 ± 14 s, P = 0.01). The time constant for the decline in PiO2 was significantly greater in C (89 ± 34 s) compared with N (52 ± 15 s; P < 0.01) and H (37 ± 10 s; P < 0.01). There was a linear relationship between the rate constant for PiO2 kinetics and Tm (r = 0.322, P = 0.03). Estimated ATP turnover was significantly greater in H than in C (P < 0.01), but this increased energy requirement alone with increased Tm could not account for the differences observed in PiO2 kinetics among conditions. These results demonstrate that PiO2 kinetics in single contracting myofibers are dependent on Tm, likely caused by temperature-induced differences in metabolic demand and by temperature-dependent processes underlying mitochondrial activation at the start of muscle contractions.
Keywords: muscle temperature, mitochondrial respiration, oxygen consumption kinetics
the coupling of skeletal muscle mitochondrial ATP production to oxygen consumption by oxidative phosphorylation is quantitatively the most important bioenergetic pathway used to sustain muscular exercise. Although increases in muscle work can be nearly instantaneous at exercise onset, there is a significant delay before achieving the appropriate increased rate of mitochondrial respiration (see Refs. 19 and 48). This results in a temporary reliance on anaerobic metabolism, which has a finite capacity. Thus the onset kinetics of mitochondrial respiration will have implications in the development of muscle fatigue and exercise tolerance. The mechanisms controlling oxygen consumption (V̇o2) in skeletal muscle during exercise are, despite considerable efforts, still not well understood.
It is clear that altering muscle temperature (Tm) exerts widespread effects on, for example, myofibrillar ATPase activity, maximal tension, cross-bridge cycling rate, shortening velocity, and sarcoplasmic reticulum calcium sensitivity (5, 37, 39, 47), and can alter force- and power-velocity relationships and contractile efficiency (8, 10, 11, 38). In addition to direct effects on contractile properties, temperature also affects mitochondrial efficiency (49) and the rates of total ATP turnover (15), substrate-level phosphorylation (16), and V̇o2 (11, 25). However, the role of altered Tm on mitochondrial V̇o2 kinetics remains unclear (12, 13, 16).
Collectively, human studies suggest that varying Tm induces nonlinear behavior in the time constant of V̇o2 (τV̇o2) during exercise. Raising Tm before exercise onset does not have an appreciable effect on V̇o2 kinetics (11, 15, 16, 28), whereas reducing Tm during moderate exercise resulted in a significant slowing of the V̇o2 response kinetics (42). Whereas this finding is consistent with the concept that the P/O ratio decreases nonlinearly with increased Tm in isolated mitochondria (6), it remains unclear whether cellular V̇o2 kinetics are altered by Tm. For instance, the nonlinearity in temperature-induced changes in V̇o2 kinetics observed in human studies may be due, in part, to uncertainties inherent in pulmonary or whole muscle V̇o2 measurements, such as temperature-related alterations in muscle recruitment and blood flow, hemoglobin- and myoglobin-oxygen binding, as well as intramuscular temperature gradients (29). Moreover, inherent fluctuations in Tm during exercise make it difficult to perform carefully controlled assessments of the effect of Tm on V̇o2 kinetics. Therefore, significant uncertainty remains regarding the effect of Tm on V̇o2 kinetics.
The purpose of the present study was to determine intramuscular Po2 (PiO2) kinetics in isolated single Xenopus skeletal muscle fibers across a range of constant temperatures. Isolated myofibers allow the chemical environment, oxygen tension, and temperature of the myofiber, as well as the rate of contraction, to be precisely controlled (19, 20). Through the use of intracellular phosphorescence imaging, PiO2 can be continuously monitored, providing a measure for the rate of mitochondrial respiration (19). We tested the hypothesis that increased temperature would speed the fall of PiO2 in single fibers, while decreasing fiber temperature would slow PiO2 kinetics.
METHODS
Female adult Xenopus laevis were used in this study. All procedures were approved by the University of California-San Diego animal care and use committee and conform to National Institutes of Health Standards.
Single skeletal muscle fiber preparation.
Single muscle fibers (n = 24) were isolated and prepared as described previously (20). Briefly, frogs were doubly pithed and the lumbrical muscles (II-IV) were removed from the hind feet. Single muscle fibers were dissected with their tendons intact in a chamber of Ringer solution consisting of (in mM) 112 NaCl, 1.87 KCl, 0.82 CaCl2, 2.38 NaHCO3, 0.07 NaH2PO4, 0.1 EGTA, with pH set at 7.0. Fibers were injected via micropipette pressure injection (PV830 pneumatic picopump, World Precision Instruments, Sarasota, FL) with 0.5 mM Pd-meso-tetra (4-carboxyphenyl) porphyrin bound to bovine serum albumin for phosphorescence quenching measurements of PiO2 (20). After microinjection, myofibers were given >30 min recovery. Note that these fibers do not contain myoglobin. A mix of muscle fiber types was used, and no attempt was made to discriminate cells by fiber type, although skeletal muscle oxidative capacity can influence PiO2 kinetics.
Experimental protocol.
Platinum clips were attached to the tendons of each myofiber to facilitate positioning within the Ringer solution-filled chamber. One tendon was fixed and the contralateral end was attached to an adjustable force transducer (model 400A, Aurora Scientific, Aurora, Ontario, Canada), allowing the muscle fiber to be set at optimum length (i.e., the length at which maximal tetanic tension was produced). The analog signal from the force transducer was recorded via a data acquisition system (AcqKnowledge, Biopac Systems, Santa Barbara, CA) for subsequent analysis.
Fibers were perfused throughout the experiment with Ringer solution equilibrated with 5% CO2 and ∼5% O2 in N2 balance. Constant perfusion was maintained throughout the protocol to maintain the extracellular Po2 at ∼45 Torr and to minimize the occurrence of an unstirred layer around the myofiber. The initial metabolic response to contractions in this model is independent of extracellular Po2 above at least 20 Torr (27, 46). Tetanic contractions were elicited using direct (8–10 V) end-to-end stimulation of the myofiber (model S48, Grass Instruments, Warwick, RI). The stimulation protocol consisted of 250-ms trains of 70 Hz of 1 ms duration. Myofibers were subjected to a stimulation frequency of 0.167–0.5 Hz for 100–200 s, depending on contractile properties and appearance of the fiber. Particular care was taken that tension did not drop considerably.
Two experimental groups were used for this study: a control condition [termed normal (N), 20°C] vs. hot (H, 25°C; n = 12) and N vs. cold (C, 15°C; n = 12). The temperature in the chamber was continually monitored with a Thermalert TH-5 thermistor (Physitemp, Clifton, NJ) to ensure the desired temperature was maintained during each contractile bout. The temperature in each group was randomized, such that 7 of 12 fibers were first exposed to N in the each group. The experimental protocol was repeated at the other temperature after confirmation of full recovery of peak tension during a rest period of >15 min to ensure recovery of tension and resting PiO2.
Assessment of PiO2.
Myofibers were visualized with a Nikon ×40 fluor objective (0.70 numerical aperture). The phosphorescence quenching of the porphyrin compound within the fiber was measured via a system consisting of a flash lamp (Oxygen Enterprises, Philadelphia, PA), a 425-nm band-pass excitation filter, a 630-nm cut-on emission filter, and a photomultiplier tube for collection of the phosphorescence signal. To calculate phosphorescence lifetimes from the intracellular O2 probe, the phosphorescent decay curves from a series of 10 flashes (15 Hz) were averaged, and a monoexponential function was fit to the subsequent best-fit decay curve (analysis software from Medical Systems, Greenvale, NY). The O2 dependence of phosphorescence quenching is described by the Stern-Volmer equation where:
| (1) |
thus,
| (2) |
where τo and τ are the phosphorescence lifetimes at anoxia and a given PiO2, respectively, and kq, the quenching constant (in Torr/s), is a second-order rate constant related to the frequency of collisions between O2 and the excited triplet state of the porphyrin and the probability of energy transfer when collisions occur. The constants kq and τo were set at 100 Torr−1·s−1 and 690 μs (at 20°C) for Pd-meso-tetra (4-carboxyphenyl) porphyrin bound to albumin in solution for this preparation as established previously (20). Although temperature-dependent effects of these phosphorescence characteristics were ≤15% (33), this effect has no effect on the τ and time delay of PiO2 fall over the range of temperatures used. However, the absolute values of PiO2 may differ in the three temperature conditions by ≤15%. Phosphorescent decay curves for measurement of PiO2 were recorded every 4 s from each cell throughout the experimental period.
Data analysis.
Contractile properties were calculated for each contraction during the stimulation protocol and normalized to total duration of the stimulation protocol (start/middle/end). All values are reported relative to the first contraction in N (20°C). Peak tension was measured as the highest value during the contraction. Maximal rate of contraction (MRC, in s−1) and relaxation (MRR, in s−1) were calculated as respectively the highest and lowest value of the differentiated tension signal, divided by peak tension. The tension-time integral (TTI) was measured by integration of the tension-time curve for each contraction to obtain a measure of mechanical output of the fiber. MRR is an indication of the rate of ATP-dependent cross-bridge detachment during tetanic contractions, allowing ATP turnover to be estimated from MRR × peak tension (the latter term accounting for the number of cross-bridges attached); the assumptions required in this estimation are explained in more detail by Jones et al. (26). Similarly, an estimate for contractile economy of each fiber was made from the quotient of TTI (mechanical output) and MRR × peak tension [estimated ATP turnover (26)]. Values at the middle and end of contractions were determined from the mean of two contractions.
PiO2 kinetics were estimated using nonlinear least squares regression (OriginPro 8.0, Microcal Software). Because PiO2 does not begin to fall immediately after the onset of stimulation, the exponential portion of the response was isolated using an iterative method by moving the fitting window forward until the modeled parameters stabilized (41). The time course of PiO2 was fitted to:
| (3) |
where PiO2,bl is baseline PiO2 (determined from the average of ∼30 s before the onset of stimulated contractions), and ΔPiO2 is the amplitude between projected PiO2 and PiO2,bl. The time delay (TD) is the back-projected time delay where the exponential phase meets PiO2,bl and τ the time constant of the response. It is important to note that isolation of the exponential phase of the PiO2 response dynamics by this method is effective to determine whether the dynamics deviate from an expected first-order behavior (where TD is zero). However, a positive TD estimated by this model does not necessarily reflect that PiO2(t) remains at PiO2bl until TD is achieved. PiO2 may begin to fall during TD, but typically at a slower rate than the subsequent exponential region. The goodness-of-fit was assessed by the flatness of the residuals, confidence interval of τ, and R2. The mean response time (MRT) of PiO2 fall was also estimated from the sum of TD and τ.
Statistical analysis.
The effect of temperature on contractile properties was tested using a 3 × 2 repeated measures ANOVA with time (start/middle/end) and temperature as within-factors (SPSS 18.0, SPSS, Chicago, IL). Where significant main effects were observed, an LSD-corrected test was performed post hoc to identify the differences between conditions. The effects of temperature on PiO2 dynamics were tested via a paired t-test within each fiber group (C vs. N, or N vs. H). Statistical significance was accepted at P < 0.05. Data are presented as means ± SD, unless otherwise stated.
RESULTS
Muscle temperature during contractions in the two experimental groups was 20.5 ± 0.6°C (N) vs. 25.9 ± 0.8°C (H) and 15.4 ± 0.2°C (C) vs. 20.7 ± 0.5°C (N). There was no statistical difference in contractile properties and kinetic parameters between the two N groups. The results for the two N groups were therefore pooled.
Effect of temperature on intracellular PiO2 kinetics.
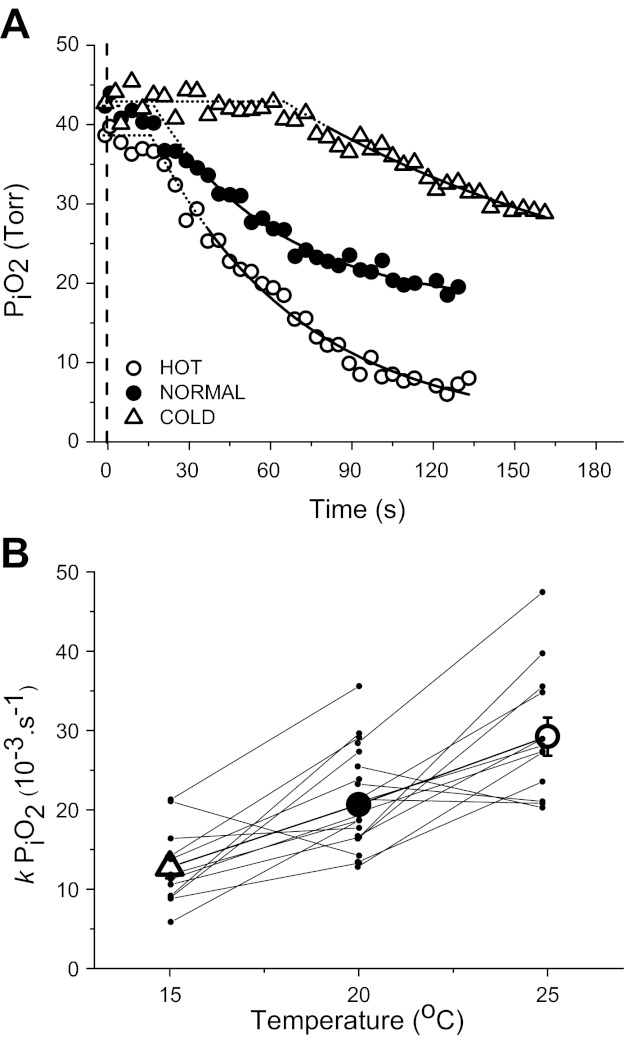
Typical examples of the PiO2 kinetic responses and fitted exponential curves in C, N, and H are displayed in Fig. 1A. Baseline PiO2 was not significantly different among all three groups and averaged 43.7 ± 1.4 Torr.
Fig. 1.
A: representative example of intracellular Po2 (PiO2) responses to stimulated contractions of single Xenopus skeletal muscle fibers at different muscle temperatures: cold (C, 15°C), normal (N, 20°C), and hot (H, 25°C). The fitted response is displayed as a solid line, while extrapolated fit is represented by the dotted line. The dashed vertical line shows the start of contractions. B: linear relationship between the rate constant (k = 1/τ) for PiO2 and muscle temperature for all data points (r = 0.322, P = 0.03).
Temperature significantly influenced the kinetics of PiO2 during stimulated contractions (Table 1). The calculated time delay (TD) for the onset of the extrapolated exponential response phase was similar in N and H but was significantly greater in C (P = 0.01). The subsequent τPiO2 was significantly greater in C (P < 0.01) and less in H (P < 0.01) compared with N. Therefore, MRT was significantly greater in C (P < 0.01) and less in H (P = 0.04) compared with N. There was a hyperbolic relationship between PiO2 kinetics (TD and τPiO2) and temperature, with a greater kinetic speeding between C and N compared with N and H (Table 1). This hyperbolic relationship between PiO2 kinetics and temperature was revealed by expressing τ as a rate constant (kPiO2 = 1/τPiO2; r = 0.322 P = 0.03, Fig. 1B).
Table 1.
Mean values of the kinetic parameters for the decline in PiO2 at the start of contractions of single Xenopus laevis skeletal muscle fibers at different temperatures
| PiO2,bl, Torr | ΔPiO2, Torr | TD, s | τPiO2, s | MRT, s | |
|---|---|---|---|---|---|
| 15°C (C) | 41.3 ± 5.3 | 17.2 ± 7.3* | 59 ± 35* | 89 ± 34* | 148 ± 55* |
| 20°C (N) | 44.2 ± 5.4 | 24.8 ± 8.1 | 35 ± 26 | 52 ± 15 | 85 ± 36 |
| 25°C (H) | 45.3 ± 7.7 | 36.3 ± 8.4* | 27 ± 14 | 37 ± 10* | 61 ± 15* |
Values are means ± SD. C, cold; N, normal; H, hot; PiO2bl, baseline resting intracellular Po2 (PiO2) value; ΔPiO2, projected amplitude of the exponential above baseline; TD, time delay of the exponential phase; τPiO2, time constant of the exponential phase; MRT, mean response time (calculated from TD + τPiO2).
P < 0.05 compared with N.
The projected amplitude of the exponential response (ΔPiO2) was temperature dependent: ΔPiO2 was significantly less in C (P < 0.001) but greater in H (P < 0.001) compared with N (Table 1).
Effect of temperature on muscle contraction.
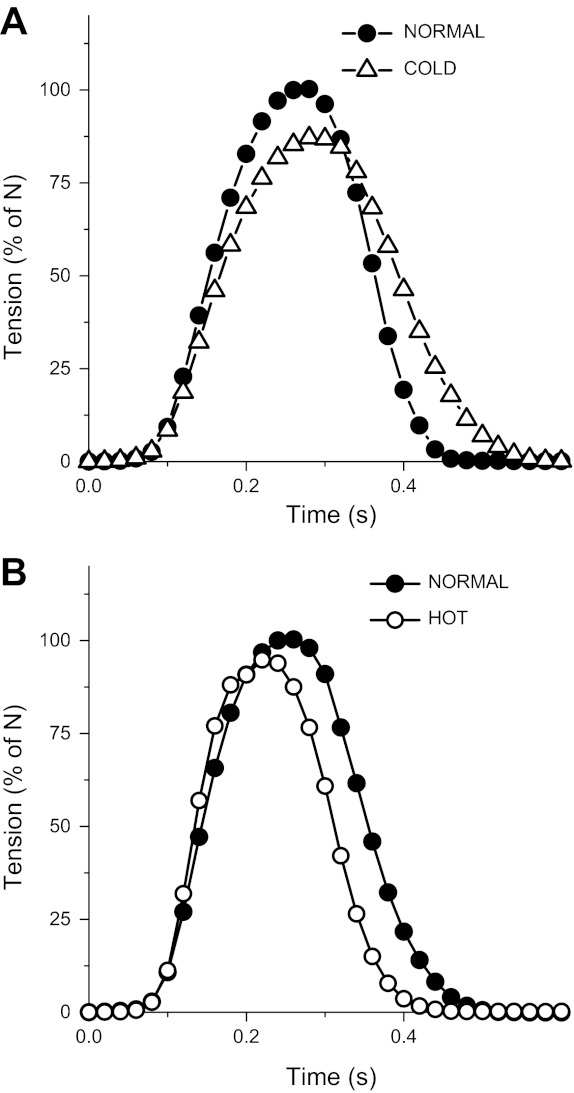
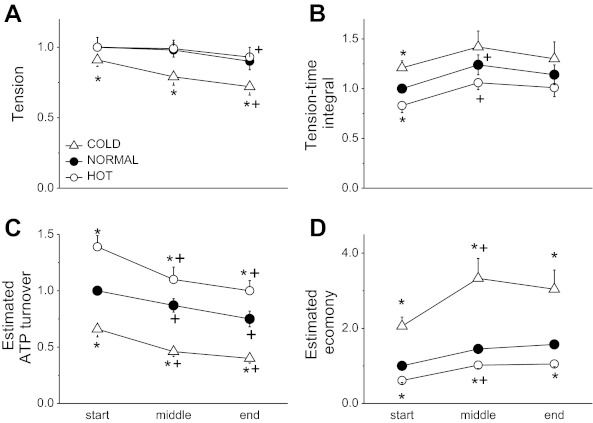
The group mean tension-time response for the first contraction in each condition is displayed in Fig. 2. At rest, peak tension (Fig. 3A) was lower in C relative to N (C was 0.89 ± 0.15 of N; P = 0.04) but was not different between N and H (0.99 ± 0.24). MRC was significantly reduced in C (0.92 ± 0.14; P = 0.04) and increased in H (1.26 ± 0.26; P = 0.02) compared with N. Similarly, MRR was significantly reduced in C (0.72 ± 0.16; P < 0.01) and increased in H (1.40 ± 0.23; P < 0.01) compared with N. Tension-time integral (TTI, Fig. 3B) was greater in C (1.21 ± 0.24; P = 0.02) compared with N but lower in H (0.83 ± 0.25; P = 0.04). The MRR × tension (an estimate of ATP turnover; Fig. 3C) was significantly reduced in C (0.66 ± 0.24; P < 0.01) and increased in H (1.39 ± 0.36; P < 0.001) compared with N. The result of these two changes was that the estimate of economy (TTI/ATP turnover) was significantly higher in C (2.06 ± 0.8; P = 0.001) and lower in H (0.61 ± 0.18; P < 0.001) compared with N.
Fig. 2.
Mean tension response of the first stimulated contraction in cold (C, 15°C) (A) and the corresponding normal condition (N, 20°C, normalized) and hot (H, 25°C) (B) and the corresponding N (20°C, normalized). Error bars are omitted for clarity.
Fig. 3.
Group mean changes in contractile properties in single myofibers during the stimulation protocol expressed in cold (C, 15°C) and hot (H, 25°C). All values are expressed relative to the starting normal time point (N, 20°C): relative tension (A), tension-time integral (TTI) (B), estimated ATP turnover (MRR × peak tension) (C), and estimated relative economy (estimated as TTI/(MRR × peak tension); reflecting the ratio of mechanical output to total energy consumption) (D). *P < 0.05 compared with N. +P < 0.05 compared the previous time point. Values are means ± SE.
During the contraction protocol, the peak tension tended to fall at the end of the contraction period in all conditions (Fig. 3A) but remained lower for the entire contraction protocol in C compared with N and H (P < 0.01). The TTI increased after the start of contractions, but this small increase only reached statistical significance in N and H, and the temperature dependency observed at the start and middle of the contractile bout disappeared by the end of the contractile period (Fig. 3B). MRR declined significantly and to a similar degree over time at all temperatures. As such, estimated ATP turnover (MRR × peak tension) declined over time in all conditions (P < 0.001; Fig. 3C). The difference in estimated ATP turnover between C and H at the onset of contractions remained during the stimulation protocol (P < 0.01). The combination of an increased TTI and a decreased estimated ATP turnover resulted in a significantly higher estimated relative economy between the first contraction and the middle of the contraction period (P < 0.001 in all conditions) but remained constant thereafter (Fig. 3D). The estimated relative economy in C was significantly greater throughout the stimulation protocol compared with N (P < 0.01), which in turn was greater than H (P < 0.001).
DISCUSSION
This is the first study to directly measure the dynamics of intracellular oxygen pressure during contractions in single skeletal muscle fibers at different constant muscle temperatures. In accordance with our hypothesis and consistent with observations in isolated mitochondria, PiO2 kinetics during stimulated contractions were temperature dependent, with higher Tm (across a physiological range of temperatures for this muscle) associated with a more rapid fall in PiO2. The time delay and time constant were greatest in the coolest condition (C) and became progressively lower as temperature was increased (N and H). In addition, the relative increase in ΔPiO2 from C to N and from N to H was reflected by a similar magnitude change in the estimated ATP turnover, suggesting that PiO2 kinetics remained a good proxy for V̇o2 kinetics in this model, even at different temperatures. These data indicate, therefore, that the processes underlying the activation of mitochondrial oxidative phosphorylation at the onset of contractions are dependent on Tm.
Effect of temperature on PiO2 kinetics.
In human exercise studies, pulmonary V̇o2 kinetics are slowed at lowered Tm but unchanged at increased Tm (28, 42). Although the single fiber preparation removes a number of confounding factors that are inherent to human studies (e.g., blood flow dynamics, contraction-related Tm changes, fiber recruitment), we also observed a nonlinear effect of Tm on the parameters of PiO2 kinetics (larger decrease in τPiO2 from C to N compared with N to H; Table 1). The influence of Tm on kinetic control of PiO2, however, was revealed by expressing the dynamics in terms of the rate constant k (because the exponent in the transfer function is 1/τ; Fig. 1B). This showed that the kinetics of PiO2 fall during contractions was linearly related to Tm between 15 and 25°C (r = 0.322, Fig. 1B). In other words, the biochemical effect of temperature within fibers is linearly related to k, predicting a plateau in τ with increasing temperature. It is expected that extending the experimental variation in Tm and by using fibers of similar mitochondrial density would further strengthen this relationship. The change in oxidative energy provision to transient energy turnover that is reflected by altered PiO2 kinetics is greater between C and N than N and H (Fig. 1). While species and temperature differences between this study and previous studies in humans complicate a direct comparison, it is interesting that the temperature-related effects on τPiO2 (or τV̇o2) appear to follow similar patterns across the two species.
This speeding of PiO2 kinetics with increasing temperature may be a consequence of the temperature dependence of overall enzyme activity (the Q10 effect). In this regard, the present data are consistent with recent findings of Gray and colleagues (16) who observed a greater increase in both V̇o2 and muscle ATP turnover in humans at the start of exercise from a raised Tm. The calculated Q10 effect using the PiO2 data in these fibers was 2.02 in C and 2.07 in H (compared with N); a value very close to the typical range of values for Q10 in fish, amphibians and mammals of 2 to 2.5 at similar temperatures. Interestingly, using the contractile properties as an independent estimate of ATP turnover (Fig. 3C), similar values for Q10 were obtained, supporting the robustness of the methodology. However, whether a Q10 effect could differentially alter rates of ATP demand and oxidative ATP supply, and thereby influence τV̇o2 in vivo is not well known; that some human studies show little effect of increased Tm on τV̇o2 perhaps suggests that it does not (16, 28).
While it is possible that a temperature-dependent speeding of ADP and/or creatine fluxes to the mitochondrion contribute to the faster PiO2 kinetics (23, 24), it should be noted that adenine nucleotide diffusion was independent of temperature in fish muscle (although the diffusion coefficients of lactate, 2-deoxyglucose, and Ca2+ were altered) (43). Furthermore, the observed increases in estimated ATP turnover (16) and sensitivity of the mitochondrion to cytosolic ADP (i.e., decreased mitochondrial Km for ADP) at elevated temperatures (45) may lead to a more rapid increase in mitochondrial [ADP] and could explain the faster V̇o2 kinetics at increased Tm.
Alternatively, temperature-induced activation of regulated and rate-limiting enzymes might underlie the speeded PiO2 kinetics we observed. It is known that prior activation of pyruvate dehydrogenase (21) or prior contractions [within 5 min (19)] can speed PiO2 kinetics in the single fiber preparation that was used in this present study. This suggests that activation of mitochondrial matrix or membrane-bound proteins can contribute to τPiO2 and may therefore underlie the temperature effects observed here. A temperature-dependent activation of the voltage-dependent anion channel (which increases ADP sensitivity) and/or (mitochondrial) calcium accumulation might modulate cytosolic and mitochondrial phosphate concentrations influencing V̇o2 kinetics. It is striking that the modeled TD for PiO2 fall, which may be indicative of the “inertia” in these activation processes (4, 14, 19), was dramatically longer at 15°C than at 20 or 25°C. Therefore, the behavior of the TD was also consistent with a temperature-dependent activation of aerobic pathways at the onset of contractions.
These data add to the mounting evidence that the control of skeletal muscle mitochondrial oxidative phosphorylation, predominantly mediated through ADP feedback through the creatine kinase reaction (34), is subject to modulation by specific activation of regulated enzyme activities at the start of exercise (in this study related to Tm) (1, 30, 50). Multiple (to date unknown) mechanisms may exist by which exercise and temperature may alter the kinetics of mitochondrial respiration in contracting skeletal muscle fibers in vivo. Features consistent with higher-order control mechanisms, such as a positive TD, are difficult to elucidate in intact systems where tissue and vascular O2 capacitances separate the site of O2 utilization and V̇o2 measurement. Nonetheless, the best attempts to account for these capacitances suggest that a delay may exist between contractions onset and a rapid increase in mitochondrial O2 use (2, 17). Calculations from Behnke et al. (3) in rat spinotrapezius muscle suggests that V̇o2 increases essentially instantaneously at contractions onset, whereas more direct measurements in frog fibers suggest that intracellular V̇o2 may increase slowly over the first few seconds of contractions [8 s or more (27)]. The measurements made in the present study are a few steps removed from elucidating the transfer function for V̇o2 control, i.e., we measured PiO2 kinetics not V̇o2 (27), and the dynamics of putative control molecules such as ADP were not measured. However, the dramatic Tm-dependent changes in TD are not consistent with a first-order control system, which would be expected to respond with a monoexponential time course without delay. Therefore, the current data are strongly supportive of recent suggestions of nonlinear (higher order) control in skeletal muscle V̇o2 kinetics in vivo (14, 30, 50).
Effect of temperature on PiO2 amplitude.
In the present study, higher Tm was associated with a larger ΔPiO2. Our lab has previously demonstrated that ΔPiO2 is linearly related to submaximal contraction intensity (22). Thus the ΔPiO2 during contractions in these myofibers is considered to reflect the net rise in V̇o2 (9). It should also be noted that the different Tm conditions complicate precise determinations of V̇o2 due to temperature-related alterations in membrane-diffusing capacity and O2 solubility. Despite these complexities our results clearly suggest that the “oxygen cost” of contractions increases with Tm (40), which is in accordance with the Q10 effect for ATPase activity. The estimated ATP turnover rate in H was higher than N throughout the stimulation protocol, while the opposite was found in C (Fig. 3C). A contributing factor in the greater fall in PiO2 at higher temperatures could be the temperature-dependent effect on contractile economy (Fig. 3D). Therefore, the suggested decrease in mitochondrial efficiency [P/O, (6, 49)] might not be the sole explanation of the greater fall in PiO2 at higher temperatures. Thus it appears that the greater absolute fall in PiO2 at any point in time in H compared with other conditions is consequent to an increased ATP turnover and V̇o2 requirement (i.e., representing an increased error signal for oxidative phosphorylation), whereas the greater rate of PiO2 decline (speeded kinetics) is independent of ΔPiO2 per se.
Effect of temperature on contractile properties.
The reduced maximal isometric tension at 15°C is in accordance with previous findings in isolated frog and mammalian muscle (5, 37). That the maximal tension was unchanged at 25°C compared with 20°C confirmed that there is a plateau in the relationship between temperature and maximal isometric tension (5, 32, 37), which is near the physiological temperature for frogs. As this was not the primary aim of the study, the reader is referred to other excellent studies on the effect of temperature on contractile properties (see Refs. 5, 10, and 37).
Perspectives and Significance
The results of the present study further our current understanding of mitochondrial function, not only during exercise, but also during conditions where temperature fluctuations are commonly observed, such as in hibernation (7), hypothermic protection (35), and mechanisms of ischemia-reperfusion injury (44).
In the present study, chamber temperature was constant during the stimulation protocol, but this is not the case for voluntary exercise in humans where Tm increases (see Refs. 12 and 18). Extrapolation to mammalian body temperatures suggests that there would be very little effect of, for example, temperature changes between 35 and 40°C on τV̇o2. Indeed, when the range over which Tm changes is relatively small, such as where resting Tm is around a normal range of 35–37°C or higher or following a standard preexercise “warm-up” in humans, there is no appreciable effect on the primary exponential component of V̇o2 kinetics (28). Equally, increasing muscle temperature by 1°C did not affect steady-state V̇o2 (such as in Ref. 36). On the other hand, exercise initiated from a low resting Tm may provide the potential for a far larger change in Tm to occur, and therefore a speeding of V̇o2 kinetics may be observed (42). These data suggest that the control of oxidative phosphorylation is linearly modulated by Tm via the reciprocal of τ (k = 1/τ), therefore increasing temperature is expected to provide a diminishing return for speeding of V̇o2 kinetics at mammalian temperatures in vivo.
Moreover, temperature would reasonably be expected to affect both O2 delivery (i.e., bulk blood flow) and O2 utilization in vivo. Our methodology is isolated from physiological O2 delivery and therefore did not allow us to draw conclusions about whether Tm alters V̇o2 kinetics in humans via an O2 delivery-dependent mechanism.
In the present study estimated ATP turnover fell during all Tm conditions presumably due to the effect of muscle fatigue on energy demand. The dynamics of these changes were more closely reflected in TTI than in the fall in peak tension, consequent to slowed relaxation (Fig. 3). Importantly, the dynamic changes in estimated ATP turnover were similar in all Tm conditions and therefore should not influence the interpretation of PiO2 kinetic responses. Nevertheless, increasing Tm during exercise in vivo would be expected to lead to temporal changes in contractile economy and ATP turnover. This would mean that during repetitive contractions where Tm was allowed to increase progressively the operational relationship between maximal tension and ATP turnover would shift in time. This is represented graphically in Fig. 3C by moving from one curve to the next as exercise continues (from cold → normal → hot). Thus relative ATP turnover and economy (or efficiency) might not change as dramatically under conditions where temperature is allowed to increase compared with the present study. In theory, ATP turnover could even increase during repeated tetanic contractions if the increase in temperature is more pronounced than the development of fatigue. Muscle temperature might, therefore, be a confounding factor in the observation of a more efficient metabolism at the start of knee extension exercise in canine muscle (50) and in humans (31), particularly once additional (warmer, less efficient, and less fatigue resistant) fibers are recruited. Whether ATP turnover remains constant during constant load exercise (due to the interaction between Tm and fatigue), however, has not been empirically determined.
A striking feature of the present data was that the fall in PiO2 in C was almost completely ablated for ∼1 min from the onset of contractions (Fig. 1; Table 1), suggesting a downregulation of mitochondrial activity that was maintained even under conditions of greatly increased energy demand: an effect that would presumably be of great benefit under conditions of reduced tissue O2 delivery or oxidative stress (35, 44), such as in hypothermic protection and ischemia-reperfusion injury. Interestingly, in a recent study, Brown et al. (7) showed that skeletal muscle mitochondrial respiration declined up to 90% during hibernation. That this was accompanied by a reduced production of reactive oxygen species and free radical leak (7) will have major protective consequences for inactive, hypothermic tissue. Whether this putative evolutionary benefit of reducing mitochondrial activity and (long term) oxidative damage during cold exposure in hibernating animals comes at the expense of a very slow mitochondrial activation during activity (such as flight responses) is currently unknown.
In conclusion, these results clearly demonstrate a temperature-dependent control of PiO2 kinetics in single contracting myofibers. τPiO2 showed nonlinear behavior across a physiological range of temperatures, but the rate constant for PiO2 kinetics was linearly related to muscle temperature. The lower estimated ATP turnover at lower temperatures suggests that cold muscles might be more economical when performing mechanical work, reducing the required oxygen consumption and increasing the time before PiO2 starts to fall. These data also suggest that the dynamics of cellular V̇o2 are dramatically slowed at lower temperatures, reducing the contribution of oxidative energy provision to the total ATP turnover. Increasing muscle temperature resulted in a speeding of mitochondrial respiratory kinetics due to a more pronounced mitochondrial activation and a larger amplitude. These results can be explained by temperature-induced differences in metabolic demand and by temperature-dependent processes underlying mitochondrial activation at the start of muscle contractions.
GRANTS
This work was supported, in part, by National Institutes of Health Grants NIAMSD AR-40155, HLBI, HL-17731 (to M. C. Hogan), AR-053219 (to B. Walsh), AR-48461 (to C. A. Kindig); by Biotechnology and Biological Sciences Research Council UK Grants BB/F019521/1 (to H. B. Rossiter), BB/I024798/1 (to H. B. Rossiter and S. Koga); and Grant JSPS-16500431 (to S. Koga). C. A. Kindig was a Parker B. Francis pulmonary fellow.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.K., C.A.K., and M.C.H. conception and design of research; S.K., B.W., C.A.K., and M.C.H. performed experiments; S.K., R.C.I.W., B.W., and H.B.R. analyzed data; S.K., R.C.I.W., B.W., H.B.R., and M.C.H. interpreted results of experiments; S.K., R.C.I.W., and B.W. drafted manuscript; S.K., R.C.I.W., B.W., H.B.R., and M.C.H. edited and revised manuscript; S.K., R.C.I.W., B.W., C.A.K., H.B.R., and M.C.H. approved final version of manuscript; R.C.I.W. prepared figures.
ACKNOWLEDGMENTS
The authors thank Professor David A Jones for insightful discussions.
Current address of R. C. I. Wüst: Dept. of Physiology, Institute for Cardiovascular Research, VU University Medical Center, Amsterdam, The Netherlands.
REFERENCES
- 1. Balaban RS. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol Cell Physiol 258: C377–C389, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Bangsbo J, Krustrup P, Gonzalez-Alonso J, Boushel R, Saltin B. Muscle oxygen kinetics at onset of intense dynamic exercise in humans. Am J Physiol Regul Integr Comp Physiol 279: R899–R906, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Behnke BJ, Barstow TJ, Kindig CA, McDonough P, Musch TI, Poole DC. Dynamics of oxygen uptake following exercise onset in rat skeletal muscle. Respir Physiol Neurobiol 133: 229–239, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Behnke BJ, Kindig CA, Musch TI, Sexton WL, Poole DC. Effects of prior contractions on muscle microvascular oxygen pressure at onset of subsequent contractions. J Physiol 539: 927–934, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennett AF. Thermal dependence of muscle function. Am J Physiol Regul Integr Comp Physiol 247: R217–R229, 1984 [DOI] [PubMed] [Google Scholar]
- 6. Brooks GA, Hittelman KJ, Faulkner JA, Beyer RE. Temperature, skeletal muscle mitochondrial functions, and oxygen debt. Am J Physiol 220: 1053–1059, 1971 [DOI] [PubMed] [Google Scholar]
- 7. Brown JC, Chung DJ, Belgrave KR, Staples JF. Mitochondrial metabolic suppression and reactive oxygen species production in liver and skeletal muscle of hibernating thirteen-lined ground squirrels. Am J Physiol Regul Integr Comp Physiol 302: R15–R28, 2012 [DOI] [PubMed] [Google Scholar]
- 8. De Ruiter CJ, De Haan A. Temperature effect on the force/velocity relationship of the fresh and fatigued human adductor pollicis muscle. Pflügers Arch 440: 163–170, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Elzinga G, van der Laarse WJ. Oxygen consumption of single muscle fibres of Rana temporaria and Xenopus laevis at 20 degrees C. J Physiol 399: 405–418, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Febbraio MA. Does muscle function and metabolism affect exercise performance in the heat? Exerc Sport Sci Rev 28: 171–176, 2000 [PubMed] [Google Scholar]
- 11. Ferguson RA, Ball D, Sargeant AJ. Effect of muscle temperature on rate of oxygen uptake during exercise in humans at different contraction frequencies. J Exp Biol 205: 981–987, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Ferguson RA, Krustrup P, Kjaer M, Mohr M, Ball D, Bangsbo J. Effect of temperature on skeletal muscle energy turnover during dynamic knee-extensor exercise in humans. J Appl Physiol 101: 47–52, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Fukuba Y, Shinhara Y, Houman T, Endo MY, Yamada M, Miura A, Hayashi N, Sato H, Koga S, Yoshida T. VO2 response at the onset of heavy exercise is accelerated not by diathermic warming of the thigh muscles but by prior heavy exercise. Res Sports Med 20: 13–24, 2012 [DOI] [PubMed] [Google Scholar]
- 14. Gandra PG, Nogueira L, Hogan MC. Mitochondrial activation at the onset of contractions in isolated myofibres during successive contractile periods. J Physiol 590: 3597–3609, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gray SR, De Vito G, Nimmo MA, Farina D, Ferguson RA. Skeletal muscle ATP turnover and muscle fiber conduction velocity are elevated at higher muscle temperatures during maximal power output development in humans. Am J Physiol Regul Integr Comp Physiol 290: R376–R382, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Gray SR, Soderlund K, Watson M, Ferguson RA. Skeletal muscle ATP turnover and single fibre ATP and PCr content during intense exercise at different muscle temperatures in humans. Pflügers Arch 462: 885–893, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Hernandez A, Goodwin ML, Lai N, Cabrera ME, McDonald JR, Gladden LB. Contraction-by-contraction VO2 and computer-controlled pump perfusion as novel techniques to study skeletal muscle metabolism in situ. J Appl Physiol 108: 705–712, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Hettinga FJ, De Koning JJ, de Vrijer A, Wust RC, Daanen HA, Foster C. The effect of ambient temperature on gross-efficiency in cycling. Eur J Appl Physiol 101: 465–471, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hogan MC. Fall in intracellular Po2 at the onset of contractions in Xenopus single skeletal muscle fibers. J Appl Physiol 90: 1871–1876, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Hogan MC. Phosphorescence quenching method for measurement of intracellular Po2 in isolated skeletal muscle fibers. J Appl Physiol 86: 720–724, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Howlett RA, Hogan MC. Dichloroacetate accelerates the fall in intracellular Po2 at onset of contractions in Xenopus single muscle fibers. Am J Physiol Regul Integr Comp Physiol 284: R481–R485, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Howlett RA, Hogan MC. Intracellular Po2 decreases with increasing stimulation frequency in contracting single Xenopus muscle fibers. J Appl Physiol 91: 632–636, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Hubley MJ, Locke BR, Moerland TS. The effects of temperature, pH, and magnesium on the diffusion coefficient of ATP in solutions of physiological ionic strength. Biochim Biophys Acta 1291: 115–121, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Hubley MJ, Locke BR, Moerland TS. Reaction-diffusion analysis of the effects of temperature on high-energy phosphate dynamics in goldfish skeletal muscle. J Exp Biol 200: 975–988, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Ishii M, Ferretti G, Cerretelli P. Effects of muscle temperature on the VO2 kinetics at the onset of exercise in man. Respir Physiol 88: 343–353, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Jones DA, Turner DL, McIntyre DB, Newham DJ. Energy turnover in relation to slowing of contractile properties during fatiguing contractions of the human anterior tibialis muscle. J Physiol 587: 4329–4338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kindig CA, Howlett RA, Hogan MC. Effect of extracellular Po2 on the fall in intracellular Po2 in contracting single myocytes. J Appl Physiol 94: 1964–1970, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Koga S, Shiojiri T, Kondo N, Barstow TJ. Effect of increased muscle temperature on oxygen uptake kinetics during exercise. J Appl Physiol 83: 1333–1338, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Koga S, Shiojiri T, Kondo N, Barstow TJ. Letter to the Editor. J Appl Physiol 85: 1593–1594, 1997 [Google Scholar]
- 30. Korzeniewski B. Regulation of oxidative phosphorylation through parallel activation. Biophys Chem 129: 93–110, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Krustrup P, Ferguson RA, Kjaer M, Bangsbo J. ATP and heat production in human skeletal muscle during dynamic exercise: higher efficiency of anaerobic than aerobic ATP resynthesis. J Physiol 549: 255–269, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lannergren J, Westerblad H. The effect of temperature and stimulation scheme on fatigue and recovery in Xenopus muscle fibres. Acta Physiol Scand 133: 73–82, 1988 [DOI] [PubMed] [Google Scholar]
- 33. Lo LW, Koch CJ, Wilson DF. Calibration of oxygen-dependent quenching of the phosphorescence of Pd-meso-tetra (4-carboxyphenyl) porphine: a phosphor with general application for measuring oxygen concentration in biological systems. Anal Biochem 236: 153–160, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol Cell Physiol 254: C548–C553, 1988 [DOI] [PubMed] [Google Scholar]
- 35. Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 37: S186–S202, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Poole DC, Schaffartzik W, Knight DR, Derion T, Kennedy B, Guy HJ, Prediletto R, Wagner PD. Contribution of exercising legs to the slow component of oxygen uptake kinetics in humans. J Appl Physiol 71: 1245–1260, 1991 [DOI] [PubMed] [Google Scholar]
- 37. Rall JA, Woledge RC. Influence of temperature on mechanics and energetics of muscle contraction. Am J Physiol Regul Integr Comp Physiol 259: R197–R203, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Ranatunga KW. The force-velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J Physiol 351: 517–529, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ranatunga KW. Temperature dependence of mechanical power output in mammalian (rat) skeletal muscle. Exp Physiol 83: 371–376, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Rome LC, Kushmerick MJ. Energetics of isometric contractions as a function of muscle temperature. Am J Physiol Cell Physiol 244: C100–C109, 1983 [DOI] [PubMed] [Google Scholar]
- 41. Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ. Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J Physiol 537: 291–303, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shiojiri T, Shibasaki M, Aoki K, Kondo N, Koga S. Effects of reduced muscle temperature on the oxygen uptake kinetics at the start of exercise. Acta Physiol Scand 159: 327–333, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Sidell BD, Hazel JR. Temperature affects the diffusion of small molecules through cytosol of fish muscle. J Exp Biol 129: 191–203, 1987 [DOI] [PubMed] [Google Scholar]
- 44. Tissier R, Couvreur N, Ghaleh B, Bruneval P, Lidouren F, Morin D, Zini R, Bize A, Chenoune M, Belair MF, Mandet C, Douheret M, Dubois-Rande JL, Parker JC, Cohen MV, Downey JM, Berdeaux A. Rapid cooling preserves the ischaemic myocardium against mitochondrial damage and left ventricular dysfunction. Cardiovasc Res 83: 345–353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Toleikis A, Majiene D, Trumbeckaite S, Dagys A, Jasaitis A. The effect of collagenase and temperature on mitochondrial respiratory parameters in saponin-skinned cardiac fibers. Biosci Rep 16: 513–519, 1996 [DOI] [PubMed] [Google Scholar]
- 46. van der Laarse WJ, des Tombe AL, van Beek-Harmsen BJ, Lee-de Groot MB, Jaspers RT. Krogh's diffusion coefficient for oxygen in isolated Xenopus skeletal muscle fibers and rat myocardial trabeculae at maximum rates of oxygen consumption. J Appl Physiol 99: 2173–2180, 2005 [DOI] [PubMed] [Google Scholar]
- 47. van der Poel C, Stephenson DG. Reversible changes in Ca(2+)-activation properties of rat skeletal muscle exposed to elevated physiological temperatures. J Physiol 544: 765–776, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Whipp BJ, Rossiter HB, Ward SA. Exertional oxygen uptake kinetics: a stamen of stamina? Biochem Soc Trans 30: 237–247, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Willis WT, Jackman MR. Mitochondrial function during heavy exercise. Med Sci Sports Exerc 26: 1347–1353, 1994 [PubMed] [Google Scholar]
- 50. Wüst RCI, Grassi B, Hogan MC, Howlett RA, Gladden LB, Rossiter HB. Kinetic control of oxygen consumption during contractions in self-perfused skeletal muscle. J Physiol 589: 3995–4009, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]