Abstract
Maternal undernutrition during pregnancy and placental insufficiency are characterized by impaired development of fetal pancreatic β-cells. Prolonged reduced glucose supply to the fetus is a feature of both. It is unknown if reduced glucose supply, independent of other complications of maternal undernutrition and placental insufficiency, would cause similar β-cell defects. Therefore, we measured fetal insulin secretion and β-cell mass following prolonged reduced fetal glucose supply in sheep. We also tested whether restoring physiological insulin concentrations would correct any β-cell defects. Pregnant sheep received either a direct saline infusion (CON = control, n = 5) or an insulin infusion (HG = hypoglycemic, n = 5) for 8 wk in late gestation (75 to 134 days) to decrease maternal glucose concentrations and reduce fetal glucose supply. A separate group of HG fetuses also received a direct fetal insulin infusion for the final week of the study with a dextrose infusion to prevent a further fall in glucose concentration [hypoglycemic + insulin (HG+I), n = 4]. Maximum glucose-stimulated insulin concentrations were 45% lower in HG fetuses compared with CON fetuses. β-Cell, pancreatic, and fetal mass were 50%, 37%, and 40% lower in HG compared with CON fetuses, respectively (P < 0.05). Insulin secretion and β-cell mass did not improve in the HG+I fetuses. These results indicate that chronically reduced fetal glucose supply is sufficient to reduce pancreatic insulin secretion in response to glucose, primarily due to reduced pancreatic and β-cell mass, and is not correctable with insulin.
Keywords: pancreatic islet, glucose, insulin, fetus, hypoglycemia
reduced fetal glucose supply is a feature of two important complications during pregnancy: maternal undernutrition and placental insufficiency (11, 44). While these are two distinct entities, they share many features. Both are found in developed and developing countries, are associated with intrauterine growth restriction (IUGR), and have consequences for the long-term health of an individual (1, 4, 5, 17, 27, 55). Both also result in decreased insulin secretion and β-cell mass in the offspring (22, 24, 45, 49, 67). This may underlie the increased risk for developing Type 2 diabetes in these offspring (4, 27, 54).
The effects of reduced glucose supply on the fetal pancreatic β-cell independent of other features of maternal undernutrition and placental insufficiency are important to investigate, as restoring the fetal glucose supply has been considered as a therapeutic strategy for treating IUGR (8, 34, 56, 64). Prior studies have used a continuous maternal insulin infusion in pregnant sheep to show that reducing fetal glucose supply for relatively short, 1- to 2-wk periods during the final 10% of gestation decreases fetal β-cell function without significantly decreasing β-cell mass (43, 57, 59). Many cases of maternal undernutrition and placental insufficiency, however, are characterized by much longer periods of reduced glucose supply (17, 49), often over the entire second half of gestation when fetal growth and development are particularly vulnerable to restricted nutrient supply. Previous studies that have measured fetal effects of reduced fetal glucose supply over the second half of gestation using maternal insulin infusions are limited, but they have documented normal fetal oxygen and amino acid supply, normal umbilical and uterine blood flow rates, and normal fetal glucagon concentrations (10, 48). Fetal glucose and insulin concentrations in such conditions were significantly lower than control fetuses (10, 48). However, fetal insulin-like growth factor-1 (IGF-1), cortisol, and norepinephrine were not measured nor were fetal insulin secretion or pancreatic morphology including β-cell mass. Therefore, the main objective of the current study was to test the hypothesis that reducing the fetal glucose supply over the second half of gestation by chronic insulin infusion into pregnant ewes would decrease fetal insulin secretion primarily due to reduced β-cell mass and also to measure secondary effects that might influence β-cell function such as changes in IGF-1, cortisol, and norepinephrine concentrations.
Because plasma insulin concentrations are important for β-cell function, a second objective of the current study was to test the hypothesis that restoring fetal insulin concentrations to physiological levels for 1 wk following prolonged reduced fetal glucose supply would correct observed defects in fetal insulin secretion and/or β-cell mass. There is strong rationale for this hypothesis. Most evidence indicates that insulin is an important growth and survival factor for the β-cell. Insulin signals through the Raf-1/mitogen-activated protein kinase (MAPK) pathway to enhance β-cell proliferation and through PDX-1 to inhibit β-cell apoptosis (3, 32, 33). Both of these signaling pathways act to increase β-cell mass. Furthermore, in adults short-term hyperinsulinemia without an associated decrease in glucose concentrations enhances glucose-stimulated insulin secretion (GSIS), a finding consistent with in vitro experiments (2, 7). We prospectively designed into the current experiment, therefore, a group of animals that received a direct fetal insulin infusion for 1 wk at the end of the period of maternal insulin infusion to restore physiological fetal insulin concentrations and test whether GSIS and β-cell mass can be enhanced following chronic restriction of the fetal glucose supply.
MATERIALS AND METHODS
Animal preparation.
Experiments were conducted in Columbia-Rambouillet sheep with singleton pregnancies. An initial surgery was performed at 70.0 ± 0.8 days gestational age (dGA; term = 148 dGA) to place maternal femoral venous and arterial catheters through a left groin incision. The animal was allowed to recover at least 5 days before the initiation of experimental infusions described below. At 119.4 ± 0.5 dGA, a second surgery was performed to place fetal catheters into the fetal inferior vena cava via hindlimb pedal veins and into the fetal abdominal aorta via hindlimb pedal arteries (65). Maternal infusions were continued while each ewe was allowed to recover from the second surgery for at least 5 days before initiation of fetal infusions. Postoperatively, the sheep were kept in individual carts and given an ad libitum diet of alfalfa pellets, water, and mineral supplements. At least two sheep were always housed together. All animal procedures were in compliance with guidelines of the United States Department of Agriculture, the National Institutes of Health, and the American Association for the Accreditation of Laboratory Animal Care. The animal care and use protocols were approved by the University of Colorado Denver Institutional Animal Care and Use Committee.
Experimental design.
After the first surgery, sheep were placed into one of two randomly assigned groups. One group (n = 9) received a continuous maternal infusion of intravenous insulin prepared in saline (0.9% NaCl) with bovine serum albumin (BSA, 0.5% wt/vol) for 8 wk. Maternal arterial plasma glucose was measured at least twice daily, and the insulin infusion was adjusted to achieve a 40% reduction in maternal glucose concentrations and to restrict fetal glucose supply by ∼40% (10, 48, 65). The other group (CON; n = 5) received a maternal saline infusion at rates matched to the insulin infusion rates.
After the fetal surgery, sheep receiving an insulin infusion were further divided into two randomly assigned groups. Fetuses in one of these groups received a direct fetal insulin infusion (Humulin R; Eli Lilly, Indianapolis, IN) for the final week of the study (HG+I; n = 4) at a constant rate chosen to achieve insulin concentrations that were twice that of CON fetuses (100 mU/h; using necropsy weights = 38.9 ± 228 mU·kg−1·h−1). This ran concurrently with a 33% dextrose (wt/vol in saline) infusion to prevent a further fall in fetal glucose concentrations and achieve a 1-wk hyperinsulinemic-isoglycemic clamp. Fetal arterial plasma glucose concentrations were measured at least twice daily, and the dextrose infusion was adjusted accordingly. The other group received a direct fetal saline infusion matched at equal infusion rates to the combined fetal insulin and dextrose infusion (HG; n = 5). Finally, fetuses in the CON group (n = 5) also received a direct fetal saline infusion at equal rates (65).
At baseline and throughout the fetal infusion period, fetal arterial blood gases and acid-base balance and plasma glucose, lactate, and insulin concentrations were measured. Immediately before measurement of fetal insulin secretion, fetal arterial plasma concentrations of amino acids, IGF-1, glucagon, cortisol, and norepinephrine were measured.
In vivo fetal insulin and glucagon secretion.
On the seventh day of the direct fetal infusions (132.5 ± 2.4 dGA), a square-wave hyperglycemic clamp followed by a 4-min arginine infusion during sustained hyperglycemia was used to determine fetal insulin and glucagon secretion as previously described (43). All sample times are relative to the start of the fetal hyperglycemic clamp.
Biochemical analysis.
Whole blood was collected in EDTA-coated syringes and immediately centrifuged (14,000 g) for 3 min at 4°C. Plasma was removed, and the glucose and lactate concentrations were immediately determined using the YSI model 2700 select biochemistry analyzer (Yellow Springs Instruments, Yellow Springs, OH) (65). The remainder of the plasma was stored at −70°C for hormone and amino acid measurements. The arterial amino acid concentrations were measured using a Dionex 300 model 4500 amino acid analyzer (Dionex, Sunnyvale, CA) (57).
Plasma insulin concentrations were measured by an ovine insulin ELISA (Alpco Diagnostics, Salem, NH; interassay and intra-assay CV's: 4.7 and 5.4%) (65). Cortisol levels were measured using a Cortisol ELISA (Alpco Diagnostics, Salem, NH; interassay and intra-assay coefficient of variations (CVs) 5.7 and 4.4%) (65). All cortisol samples were measured in duplicate with a single ELISA assay with repeat measurements made for a CV greater than 15%. Glucagon (Millipore, Billerica, MA; interassay and intra-assay CV's: 11.7 and 6.1%) and IGF-1 (Diagnostics Systems Laboratories, Webster, TX; interassay and intra-assay CV's 4.5 and 2.6%) were measured by RIA (65). Blood gases, pH, and hematocrit were determined using an ABL 520 analyzer (Radiometer, Copenhagen, Denmark). Oxygen content of the blood was calculated by the ABL 800 analyzer (65). Norepinephrine concentrations were measured by HPLC as previously described (65). Samples were measured in a single assay with repeat determination for a CV > 15%.
Fetal tissue collection.
After the in vivo insulin secretion measurements, animals were maintained for one more day on their respective chronic infusions. To obtain fetal tissues under conditions closely approximating in vivo study conditions, the ewes and fetuses were anesthetized with ketamine (1,000 mg) and diazepam (10 mg) given to the mother. The fetus was removed, blotted dry, and weighed. The ewe and the fetus were then killed with intravenous concentrated pentobarbital sodium (4,680 mg and 940 mg, respectively). The fetal pancreas was dissected free, weighed, and divided. The hepatic portion was snap-frozen in liquid nitrogen and stored at −80°C. The splenic portion was fixed in 4% (wt/vol) paraformaldehyde in PBS overnight and then embedded in paraffin.
Pancreatic insulin and glucagon mRNA and protein concentrations.
Frozen fetal pancreas tissue from the hepatic portion was pulverized in liquid nitrogen. Pancreatic insulin and glucagon were measured as previously described (59).
Total RNA was extracted from pulverized pancreas (100 mg) and reverse transcribed into cDNA as previously described (58). Insulin (GenBank Accession no. U00659), glucagon (GenBank Accession no. AF529185), somatostatin (GenBank Accession no. AF031488), pancreatic polypeptide (GenBank Accession no. AY427976), and β-actin (Accession no. U39357) were previously cloned and sequenced (13, 42). Quantitative real-time PCR for insulin and β-actin were performed as previously described (13). Other assays were performed with the following primers: glucagon forward 5′-TCACTCTCTCTTCACCTGCTCTGT-3′ and reverse 5′-GACACACTTACTTCCTGTCAG-3′, somatostatin forward 5′-TCTCCATCGTCCTGGCTCTTG-3′ and reverse 5′-CTCCAGCCTCATTTCATCCTG-3′, and pancreatic polypeptide forward 5′-TGCTCCTTCTGTCCACGTG-3′ and reverse 5′-ACCTGGGGACTGCTGAG-3′. Specificity of the primers for all genes was confirmed with agarose gel electrophoresis, melting curve analysis, and sequencing of PCR products. cDNA samples were analyzed in triplicate, and the standard curve method of relative quantification was used to compare results (68). β-Actin was used as a housekeeping gene and was not different between treatment groups, and results are normalized to the CON group.
Histology of fetal pancreas.
Tissue sections from the splenic portion of the pancreas were cut from paraffin-embedded pancreases at 70-μm minimum intervals for histological and morphometric evaluation. Pancreatic sections were processed and insulin, glucagon, somatostatin, and pancreatic polypeptide were identified as previously described (42).
Morphometric analysis was performed as previously described (59). Insulin+ and glucagon+ areas were determined for 20 fields of view (FOV) on four pancreatic sections per animal and expressed as a percentage of total pancreas area evaluated. The percent area for all FOV obtained from one section were averaged to provide the mean percent area for each section. Then the percent area for all sections from each animal were averaged to provide the mean percent area for each animal. This average was used for summary and comparative statistics. β-Cell and α-cell mass were calculated as the product of the relative β-cell and α-cell area, respectively, and the weight of the pancreas. Islet size was determined by measuring all islets in three randomly selected FOV per section in four sections per animal for a total of 46.3 ± 3.9 islets measured per animal. Islets were defined as endocrine cell clusters of at least 500 μm2 as previously described (42).
Statistical analysis.
Statistical analysis was performed using SAS v.9.1 (SAS Institute). A mixed models analysis of variance (ANOVA) was performed to determine effects of treatment group (CON, HG, or HG+I), time, and treatment group by time interactions. A term was included to account for repeated measures within a single animal, and posttest comparisons were made using Fishers least squares difference if the overall ANOVA had a P < 0.05. Results are expressed as means ± SE. P values <0.05 were considered significant.
RESULTS
Nutrients, hormones, and blood gasses.
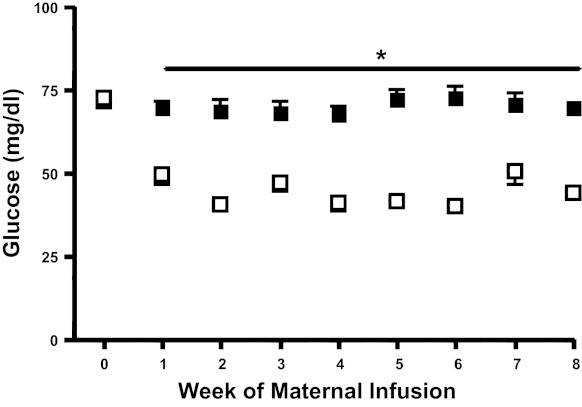
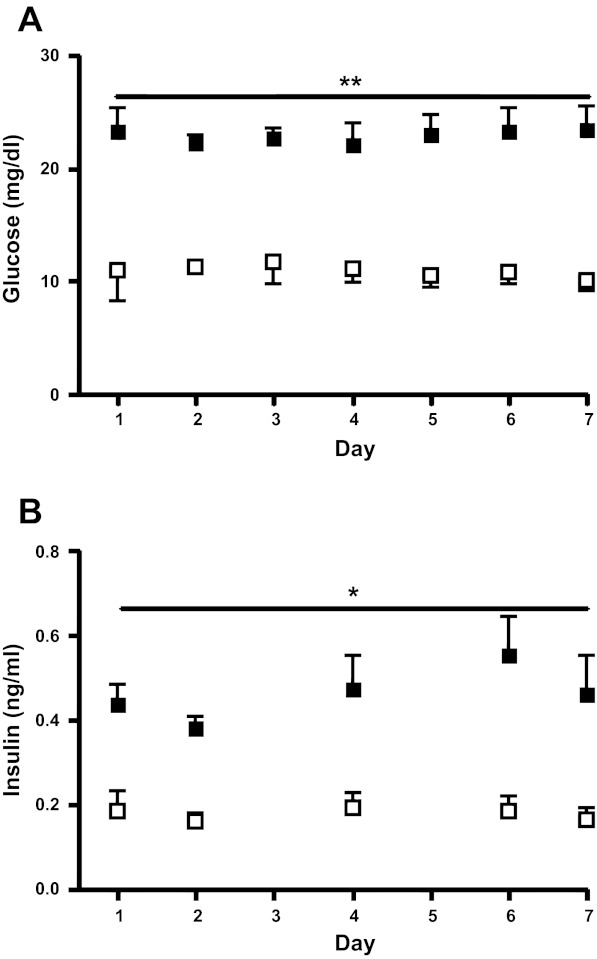
Consistent with study design, maternal arterial plasma glucose concentrations in the HG group were lower compared with those in the CON group (P < 0.0001, Fig. 1). Fetal arterial plasma glucose and insulin concentrations were only measured throughout the final week of the study when fetal vascular access was present. In HG fetuses arterial plasma glucose was 55% lower (P < 0.0001) and insulin wa 60% lower than CON fetuses (P < 0.05; Fig. 2). Chronically restricting fetal glucose supply and plasma glucose concentrations had no effect on fetal arterial pH, Pco2, lactate, Po2, hemoglobin-O2 saturation, blood O2 content, or hematocrit (Table 1).
Fig. 1.
Maternal arterial plasma glucose concentrations. Maternal arterial plasma glucose concentrations were measured throughout the 8-wk infusion period. Solid squares, control (CON); open squares, hypoglycemic (HG). *P < 0.0001 between CON and HG (mixed models ANOVA). Values are means ± SE.
Fig. 2.
Fetal glucose and insulin concentrations for the final week of study. Fetal arterial plasma glucose (A) and insulin (B) concentrations were measured throughout the final experimental week (while fetal vascular access was available). Solid squares, CON; open squares, HG. **Fetal arterial plasma glucose concentrations were significantly lower in HG fetuses compared with CON (P < 0.0001). *Fetal arterial plasma insulin concentrations were significantly lower in HG compared with CON fetuses (P < 0.05) throughout the final week. All statistics by mixed models ANOVA. Values are means ± SE.
Table 1.
Fetal arterial biochemistry
| Control | Hypoglycemic | HG + I | |
|---|---|---|---|
| Lactate, mmol/l | 1.6 ± 0.11 | 1.3 ± 0.18 | 1.5 ± 0.42 |
| Amino acids, μmol/l | |||
| Alanine | 230.2 ± 18.0 | 233.9 ± 14.9 | 197.1 ± 63.1 |
| Arginine | 136.2 ± 12.8 | 90.6 ± 14.3 | 71.9 ± 23.6a |
| Asparagine | 40.0 ± 3.4 | 56.1 ± 5.2 | 37.7 ± 10.4 |
| Aspartate | 21.1 ± 3.0 | 20.2 ± 1.5 | 21.6 ± 3.3 |
| Cysteine | 13.6 ± 1.7 | 24.7 ± 2.2b | 11.8 ± 5.0d |
| Glutamate | 30.1 ± 4.1 | 24.4 ± 0.8 | 23.3 ± 2.4 |
| Glutamine | 410.4 ± 40.6 | 465.1 ± 21.9 | 340.3 ± 71.9 |
| Glycine | 254.4 ± 31.0 | 315.5 ± 29.9 | 442.0 ± 53.1b |
| Histidine | 60.3 ± 5.3 | 64.8 ± 4.1 | 60.3 ± 10.8 |
| Isoleucine | 96.1 ± 9.9 | 95.2 ± 9.5 | 47.0 ± 12.7a, d |
| Leucine | 152.9 ± 10.7 | 175.7 ± 12.9 | 89.6 ± 27.7a, e |
| Lysine | 91.8 ± 5.3 | 133.5 ± 17.2 | 78.3 ± 34.3 |
| Methionine | 85.1 ± 13.9 | 90.8 ± 6.0 | 86.8 ± 16.7 |
| Ornithine | 69.2 ± 5.0 | 71.8 ± 10.0 | 62.7 ± 28.4 |
| Phenylalanine | 104.7 ± 4.8 | 144.9 ± 13.0 | 94.2 ± 29.3 |
| Proline | 134.8 ± 3.7 | 149.1 ± 11.3 | 115.0 ± 36.4 |
| Serine | 374.0 ± 60.4 | 394.2 ± 60.1 | 217.9 ± 32.5 |
| Taurine | 69.0 ± 19.9 | 239.6 ± 41.9c | 59.2 ± 18.6f |
| Threonine | 282.1 ± 28.2 | 274.8 ± 23.1 | 192.8 ± 61.9 |
| Tryptophan | 39.2 ± 5.0 | 35.1 ± 4.6 | 28.5 ± 2.1 |
| Tyrosine | 124.2 ± 13.4 | 165.9 ± 39.5 | 86.6 ± 21.8 |
| Valine | 423.5 ± 32.2 | 443.7 ± 42.0 | 231.4 ± 72.7a, b |
| Hormones | |||
| IGF-1, ng/ml | 30.4 ± 6.8 | 13.2 ± 1.8a | 16.6 ± 3.1 |
| Cortisol, ng/ml | 4.0 ± 1.1 | 8.2 ± 3.3 | 7.9 ± 0.8 |
| Norepinephrine, pg/ml | 464 ± 19 | 549 ± 149 | 843 ± 177 |
| Glucagon, pg/ml | 34.4 ± 2.8 | 55.2 ± 9.8 | 50.2 ± 13.2 |
| Arterial blood pH, gasses, and hematocrit | |||
| pH | 7.35 ± 0.01 | 7.34 ± 0.03 | 7.33 ± 0.03 |
| Pco2, mmHg | 50.9 ± 1.6 | 49.3 ± 1.5 | 53.8 ± 2.1 |
| Po2, mmHg | 20.4 ± 0.7 | 21.5 ± 1.1 | 16.9 ± 2.3 |
| Hct, % | 34.7 ± 1.6 | 30.7 ± 0.9 | 25.1 ± 3.0c, d |
| Hgb-O2 saturation, % | 52.4 ± 2.6 | 54.0 ± 4.2 | 34.0 ± 7.9a, e |
| O2 content, mmol/l | 3.4 ± 0.13 | 3.5 ± 0.25 | 1.8 ± 0.6c, f |
Concentrations were determined at the end of the study. All values are means ± SE.
P < 0.05;
P < 0.01;
P < 0.005 Control vs. Hypoglycemic or HG+I.
P < 0.05;
P < 0.01;
P < 0.005, Hypoglycemic vs. HG+I. HG+I, hypoglycemic + insulin. lactate, hormones, and O2 content have been previously published (78).
Fetal arterial plasma amino acid concentrations were not different between CON and HG fetuses, with the exception of taurine and cysteine, which were higher in HG fetuses (P < 0.01, Table 1). Fetal arterial plasma IGF-1 concentrations were lower in the HG group (P < 0.05, Table 1). Fetal arterial plasma cortisol, norepinephrine, and glucagon concentrations were not different between CON and HG fetuses (Table 1).
Insulin and glucagon secretion.
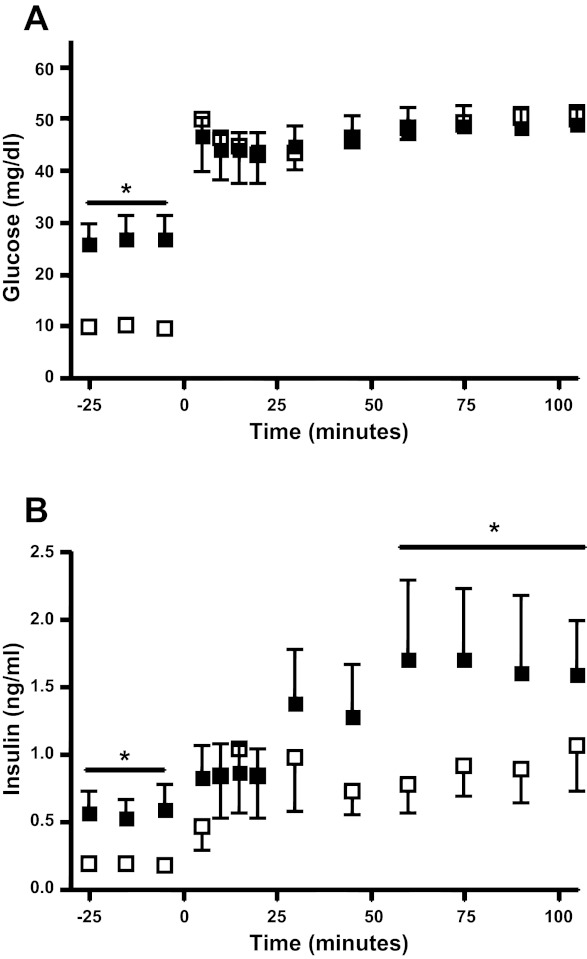
During the fetal square-wave hyperglycemic clamp at the end of the chronic study period, maximal steady-state hyperglycemic insulin concentrations in the HG group were 45% lower compared with the CON group (P < 0.05) despite achieving similar glucose concentrations (Fig. 3). The glucose infusion rates required to achieve steady-state glucose concentrations during the hyperglycemic clamp were 11.0 ± 1.8 mg·kg−1·min−1 in CON fetuses and 20.9 ± 1.4 mg·kg−1·min−1 in HG fetuses (P < 0.005). Maximum glucose-potentiated arginine-stimulated insulin concentrations were measured 5 min after the arginine bolus and were not different between the HG and CON groups (3.11 ± 0.58 ng/ml CON, 3.42 ± 0.34 ng/ml HG). Maximum glucagon concentrations also were measured 5 min after the arginine infusion in both groups but were not different from each other (127.4 ± 20.7 pg/ml CON, 130.3 ± 14.9 pg/ml HG).
Fig. 3.
Fetal glucose and insulin concentrations during glucose-stimulated insulin secretion (GSIS). Fetal arterial plasma glucose (A) and insulin (B) concentrations were measured during the square wave hyperglycemic clamp, which began at time 0. Solid squares, CON; open squares, HG. *P < 0.05 between CON and HG. Values are means ± SE.
Fetal whole body and organ weights and pancreatic morphology.
Fetal characteristics at necropsy are presented in Table 2. Gestational age was not different between CON and HG. There were more male CON fetuses (80%) compared with the HG group (20%, P = 0.06). HG fetuses weighed 40% less (P < 0.0001) and had lower extremity lengths that were 15% shorter compared with CON (P < 0.05). Pancreatic weights were 35% lower in HG compared with CON fetuses (P < 0.05). Most other organ weights were lower in HG fetuses (Table 2), but relative to body weight these organ weights, including the pancreas, were not different between groups (data now shown).
Table 2.
Fetal measurements and organ weights
| Control | Hypoglycemic | HG+I | |
|---|---|---|---|
| Gestational age, day | 133.2 ± 1.1 | 133.0 ± 1.3 | 134.5 ± 0.9 |
| Fetal weight, kg | 3.66 ± 0.12 | 2.20 ± 0.14‡ | 2.61 ± 0.2† |
| Organ weights, g | |||
| Liver | 137.8 ± 13.2 | 76.3 ± 5.5† | 104.2 ± 13.7 |
| Heart | 31.1 ± 0.4 | 18.6 ± 1.8† | 22.4 ± 2.8* |
| Lungs | 120.5 ± 8.7 | 82.6 ± 6.6* | 87.4 ± 8.8* |
| Kidneys | 24.2 ± 1.0 | 16.9 ± 1.0† | 17.3 ± 1.4* |
| Spleen | 14.3 ± 2.0 | 6.6 ± 0.8† | 7.0 ± 1.0* |
| Adrenals | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 |
| Brain | 46.1 ± 2.7 | 44.1 ± 2.2 | 40.7 ± 1.6 |
| Pancreas | 4.3 ± 0.8 | 2.8 ± 0.2* | 2.5 ± 0.4* |
| Placenta | 379.5 ± 25.7 | 294.2 ± 37.7 | 247.6 ± 22.6* |
| Lower limb length, cm | 35.3 ± 1.2 | 30.1 ± 1.4* | 29.9 ± 1.3* |
All values are means ± SE.
P < 0.05;
P < 0.005;
P < 0.0001. Control vs. Hypoglycemic or HG+I. HG+I, hypoglycemic + insulin. Gestational age refers to age at organ isolation. Glucose-stimulated insulin secretion was measured 1 day earlier. Fetal age, weight, and liver weight have been previously published (78).
Pancreatic characteristics are shown in Table 3. Islet size did not differ between groups, but the number of islets per FOV was 23% lower in HG compared with CON fetuses (P < 0.05). The average β-cell area also was 20% lower in HG fetuses, but this difference did not reach statistical significance. β-Cell mass, however, was 50% lower in HG fetuses (P < 0.05). α-Cell mass also was lower (P < 0.005) in the HG versus CON fetuses. Pancreatic insulin content was 54% lower in the HG group compared with the CON group (P < 0.005) and remained lower even when normalized to β-cell area (P < 0.05). Pancreatic glucagon content was not different between HG and CON groups. Pancreatic insulin and glucagon mRNA values were not statistically lower in HG compared with CON. There was no difference in pancreatic polypeptide or somatostatin mRNA between HG and CON groups.
Table 3.
Characteristics of the fetal pancreas
| Control | Hypoglycemic | HG+I | |
|---|---|---|---|
| Islet size, μm2 | 3,002 ± 137 | 3,392 ± 481 | 3,009 ± 197 |
| Islet density, number/FOV | 4.85 ± 0.32 | 3.75 ± 0.33* | 3.29 ± 0.35* |
| β-Cell area, % | 5.74 ± 0.73 | 4.44 ± 0.89 | 3.06 ± 0.58* |
| β-Cell mass, mg | 240 ± 50 | 120 ± 30* | 70 ± 10† |
| Pancreatic insulin content, mg/g | 52.0 ± 6.2 | 24.0 ± 5.5† | 19.9 ± 4.4† |
| Pancreatic insulin content per β-cell area (ratio) | 9.61 ± 1.27 | 5.50 ± 0.49* | 6.51 ± 0.86 |
| Pancreatic insulin mRNA (ratio to actin) | 1.00 ± 0.35 | 0.49 ± 0.13 | 0.15 ± 0.05* |
| α-Cell area, % | 3.18 ± 0.21 | 1.99 ± 0.21 | 1.88 ± 0.69* |
| α-Cell mass, mg | 130 ± 20 | 50 ± 10† | 50 ± 20† |
| Pancreatic glucagon content, mg/g | 4.05 ± 0.66 | 3.45 ± 0.71 | 1.80 ± 0.65* |
| Pancreatic glucagon content per α-cell area (ratio) | 1.26 ± 0.17 | 1.71 ± 0.24 | 0.66 ± 0.25‡ |
| Pancreatic glucagon mRNA (ratio to actin) | 1.00 ± 0.24 | 0.58 ± 0.19 | 0.37 ± 0.18 |
| Pancreatic polypeptide mRNA (ratio to actin) | 1.00 ± 0.48 | 1.27 ± 0.24 | 0.72 ± 0.27 |
| Pancreatic somatostatin mRNA (ratio to actin) | 1.00 ± 0.22 | 0.82 ± 0.25 | 0.52 ± 0.16 |
All values are means ± SE.
P < 0.05;
P < 0.005 Control vs. Hypoglycemic or HG+I;
P < 0.05 Hypoglycemic vs. HG+I. FOV, field of view.
Hypoglycemic + insulin group results.
This group was used to test the hypothesis that restoring fetal insulin concentrations to physiological levels for 1 wk following prolonged reduced fetal glucose supply (and associated fetal hypoinsulinemia) would correct observed defects in insulin secretion and/or β-cell mass. Maternal arterial plasma glucose concentrations were not different in the HG+I group compared with the HG group. Fetal arterial plasma insulin in the HG+I group was similar to HG fetuses on day one (0.13 ± 0.01 ng/ml) but higher throughout the fetal insulin infusion (days 2–7, mean arterial plasma insulin concentration 0.89 ± 0.12 ng/ml, P < 0.01). By study design, glucose concentrations did not change throughout the infusion and were similar to HG fetuses (mean arterial plasma glucose concentrations 9.0 ± 0.8 mg/dl). The dextrose infusion required to prevent a further fall in glucose concentrations in the HG+I group progressively increased from a starting rate of 7.0 ± 1.7 to 17.1 ± 2.7 mg/min on the final day of insulin infusion. With the use of necropsy weights, the final infusion rate of dextrose was 6.6 ± 1.1 mg·kg−1·min−1.
Neither β-cell mass nor GSIS were corrected in the HG+I fetuses. GSIS was suppressed in the HG+I fetuses (baseline insulin 0.61 ± 0.17 vs. maximal glucose stimulated insulin 0.83 ± 0.05, ng/ml), and there were no differences between HG+I and HG fetuses for any characteristics of the pancreas except for a lower pancreatic glucagon content when normalized to α-cell area (P < 0.05) (Table 3). Maximum glucose-potentiated arginine-stimulated insulin concentrations were measured at 5 min after the arginine infusion and were lower compared with HG fetuses (1.64 ± 0.81 ng/ml, P < 0.05). Maximum glucagon concentrations also were measured at 5 min after the arginine infusion and were lower compared with HG fetuses (100.7 ± 24.6 pg/ml, P < 0.05).
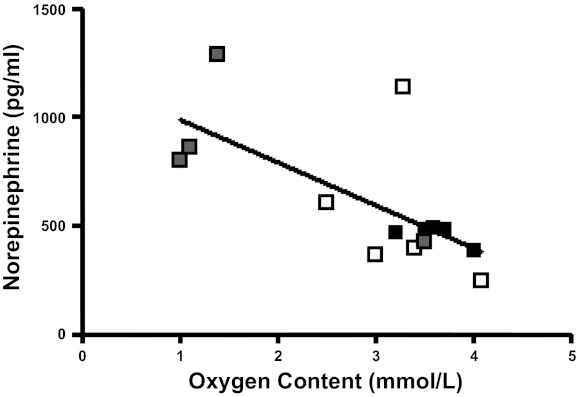
Hemoglobin-O2 saturation (P < 0.01) and blood O2 content (P < 0.005) decreased in HG+I fetuses during the insulin infusion without associated hypercapnea or metabolic acidosis (Table 1). The hematocrit in HG+I also decreased during the insulin infusion (Table 1, P < 0.05). Branched chain amino acids (valine, leucine, isoleucine) and taurine were all lower in HG+I compared with HG (P < 0.05, Table 1). Although no significant difference was seen in norepinephrine levels between HG and HG+I (Table 1), there was an inverse correlation between norepinephrine and oxygen content in all groups (Fig. 4).
Fig. 4.
Fetal arterial norepinephrine concentrations. Fetal arterial plasma norepinephrine concentrations plotted as a function of fetal arterial O2 content. Soild squares, CON; open squares, HG, shaded squares, HG+I. r2 = 0.46, P < 0.01, for all fetuses.
DISCUSSION
Reduced glucose supply to the fetus is a complication of placental insufficiency and maternal undernutrition, both of which may result in IUGR (11, 44). Increasing the fetal glucose supply has been considered as a therapeutic strategy for IUGR (8, 34, 56, 64), but the specific role of reduced glucose supply in the pathogenesis of pregnancies complicated by maternal undernutrition or placental insufficiency is not completely understood. Decreased fetal glucose and insulin concentrations are critical for β-cell function and are likely to be involved in the pathogenesis of decreased insulin secretion and decreased β-cell mass found in such conditions (2, 18, 24, 28, 50). However, whether experimentally reducing the fetal glucose supply independent of other complications of maternal undernutrition and placental insufficiency, such as decreased umbilical and uterine blood flows, decreased fetal oxygen supply and oxygen concentrations, and decreased fetal amino acid supply, causes decreased fetal insulin secretion and β-cell mass has not been directly tested. Therefore, the study reported herein quantified defects in fetal insulin secretion and β-cell mass following prolonged reduction of glucose supply to the fetus throughout the latter half of pregnancy. Furthermore, having previously identified low fetal insulin concentrations in this model of reduced fetal glucose supply (48), we prospectively designed into this study a group of animals in which we tested the ability of restored physiological fetal insulin concentrations for the final week of reduced fetal glucose supply to correct any observed defects in fetal insulin secretion or β-cell mass. The novel and important findings of this study are that following prolonged reduced glucose supply, fetuses are characterized by lower GSIS, pancreatic islet density, pancreatic β-cell mass, pancreatic insulin content, and circulating IGF-1 concentrations. Increasing fetal insulin concentrations following prolonged reduced glucose supply with a direct fetal insulin infusion does not correct any of these defects.
Severely growth-restricted human fetuses are characterized by decreased β-cell mass and decreased in vivo GSIS (49, 67). The focus of this study, therefore, was to determine the role of prolonged reduced glucose supply on fetal insulin secretion and β-cell mass using an experimental large animal model. While HG fetal islets were the same size as CON fetal islets, we found a significant reduction in HG fetal pancreatic islet density. Combined with a 35% smaller pancreas, decreased pancreatic islet density contributed to a 50% lower β-cell mass in the HG fetuses. Normal islet size but decreased pancreatic islet density is consistent with decreased isletogenesis in the HG fetuses. In rodents, islets do not form until the final 22% of gestation (31, 62). But in sheep, like humans, isletogenesis begins in the first trimester, persists throughout gestation, and overlaps with the experimental insult tested herein (6, 15, 52). Future studies will determine which specific downstream features of reduced fetal glucose supply (i.e., low glucose, low insulin, or low IGF-1) might be responsible for decreased islet formation and the precise timing of such an insult. We also identified lower pancreatic insulin content in the HG fetuses, even when normalized to β-cell area, indicating decreased insulin per β-cell. Both decreased β-cell mass and decreased pancreatic insulin content likely limited maximum glucose-stimulated insulin concentrations in the HG fetuses.
We also found that α-cell mass was 60% lower in HG fetuses compared with controls. Despite this, there was only a 15% (and not statistically significant) reduction in pancreatic glucagon content and no difference in arginine-stimulated glucagon secretion, suggesting increased responsiveness of the α-cell. Decreased insulin concentrations and secretion in HG fetuses is a possible explanation for increased α-cell responsiveness in this group, as insulin and other β-cell secreted factors suppress glucagon release (25, 29, 46).
Because of the chronic nature of the experimental insult, we hypothesized a reduction in the β-cell mass of the HG fetuses and therefore focused on changes in pancreatic morphology to explain decreased fetal insulin concentrations and insulin secretion. This precluded isolation of the pancreatic islets for in vitro functional analysis. Therefore, we cannot exclude the possibility of additional mechanisms contributing to decreased maximal glucose-stimulated insulin concentrations. The classical pathway by which glucose stimulates insulin secretion from the β-cell involves the generation of ATP, which inhibits K+ channels on the β-cell membrane. This leads to depolarization and entry of calcium into the β-cells, which triggers insulin granule exocytosis and insulin release. This pathway is thought to regulate early phase insulin secretion (28), which was normal in the HG fetuses in the present study, indicating suppression of sustained insulin secretion rather than complete interruption of the normal pathway. We speculate that defects in the generation of secondary messengers by the HG islets may contribute to the inability of the β-cells to sustain a normal insulin response to the hyperglycemic clamp (63). These secondary messengers, which include cAMP, nicotinamide adenine dinucleotide phosphate (NADP)/NADPH, anaplerotic input to the Krebs cycle (e.g., α-ketoglutarate), and adenosine monophosphate-activated kinase (AMPK), are regulated by glucose availability and the overall energy status of the β-cell making them particularly important to test in future experiments (20, 26, 30, 51, 60). Some of these secondary messengers such as AMPK not only regulate GSIS but also insulin gene transcription and β-cell survival (16, 23, 35, 36). Normal ASIS in the HG fetuses, despite lower β-cell mass and pancreatic insulin content, also supports a defect in the generation of secondary messengers. Arginine acutely stimulates insulin secretion by directly depolarizing the β-cell leading to calcium entry and insulin granule exocytosis, therefore, bypassing the pathways stimulated by these secondary messengers (12).
We also report lower IGF-1 concentrations following chronic reduction of the fetal glucose supply from maternal insulin infusions. IGF-1 signaling in the β-cell overlaps significantly with insulin signaling and, therefore, could be hypothesized to increase insulin secretion and β-cell mass in the HG fetuses (38). Furthermore, chronic fetal IGF-1 infusions are not associated with fetal hypoxemia, as found in the HG+I fetuses in the current study (21, 37). However, at higher doses of IGF-1 circulating fetal insulin concentrations are decreased and acute IGF-1 infusions inhibit GSIS in adults (37, 53). This is the first to the identification of lower IGF-1 concentrations in this model, thus precluding prospective inclusion of a group of HG fetuses in which IGF-1 concentrations were experimentally increased.
In the HG+I group, we used direct fetal insulin infusions for 1 wk to determine whether elevated fetal insulin concentrations would correct defects in insulin secretion and β-cell mass in the HG group. Hyperinsulinemic-isolglycemic clamps in adult humans potentiate GSIS (7). This in vivo result is supported by in vitro studies that show that insulin increases insulin granule exocytosis from the β-cell by stimulating increases in intracellular calcium concentrations with minimal effects on cell membrane potential (2). Furthermore, at physiological doses insulin acts via the Raf-1/MAPK pathway to enhance β-cell proliferation and via PDX-1 to inhibit β-cell apoptosis (3, 32, 33). Decreased insulin signaling in the β-cell results in decreased β-cell mass (38, 39, 66). Taken together, these studies provided the rationale for testing the hypothesis that increased fetal insulin concentrations would act to increase β-cell function and mass. However, we found that raising fetal insulin concentrations for 1 wk suppressed GSIS, reduced ASIS, and did not change β-cell mass or pancreatic insulin content. These results may be due to progressive fetal hypoxemia and/or increases in norepinephrine concentrations, which can have direct inhibitory effects on the β-cell (19, 40, 56) and were not present in the adult studies noted above that demonstrated insulin potentiation of GSIS.
Progressive hypoxia following a direct fetal insulin infusion has been previously reported in normally growing fetuses (9, 47, 61). The decrease in fetal arterial oxygen content was postulated to be due to either increased fetal oxygen consumption, restricted placental oxygen transfer to the fetus, or decreased and/or redistributed fetal blood flow. Data are conflicting as to the exact mechanism (9, 47, 61). Experiments measuring blood flows, fetal and placental oxidative metabolism, and oxygen transfer across the placenta are underway to test these possibilities as well as the effects of prolonged hyperinsulinemia on GSIS in normal fetuses. Lower taurine or the branched chain amino acids leucine, isoleucine, and valine also could have contributed to decreased insulin secretion in the HG+I fetuses, because these amino acids enhance β-cell function (14, 41). We also observed a decrease in hematocrit in the HG+I group compared with HG, which could not be explained by differences in daily infusate volume or blood volume sampling.
Perspectives and Significance
Prolonged reduced fetal glucose supply for 8 wk in pregnant sheep made hypoglycemic by maternal insulin infusion caused a defect in fetal insulin secretion, as well as 50% lower β-cell mass and lower pancreatic insulin content even when normalized to β-cell area, indicating decreased insulin per β-cell. A direct fetal insulin infusion for 1 wk after prolonged reduced fetal glucose supply did not improve any of the β-cell defects. On the contrary, it caused a more severe impairment in insulin secretion, a novel observation that needs further investigation to determine causation, such as the concomitant fetal hypoxemia and secondarily increased concentrations of norepinephrine. Whereas these results do not rule out the possibility that other complications of placental insufficiency and maternal nutrient deprivation contribute to β-cell defects in IUGR individuals, they show that reduction of the fetal glucose supply is sufficient to cause these defects.
GRANTS
J. R. Lavezzi was supported by National Institutes of Health (NIH) Training Grant T32 HD007186-32 (to W. W. Hay). This work was supported by NIH Grants R01DK-088139 and K08HD-060688, as well as American Diabetes Association Junior Faculty Award 7-08-JF-51(to P. J. Rozance). Histological services and a Pilot and Feasibility Award to P. J. Rozance were provided by the UC Denver DERC (P30DK-57516; to J. Hutton). L. D. Brown was supported as a Scholar by NIH Building Interdisciplinary Careers in Women's Health Scholar Award K12HD-057022 (to J. Regensteiner), and a Children's Hospital Colorado Research Institute Research Scholar Award (PI). S. R Thorn was supported by NIH K01DK-090199 (PI) and as a trainee on NIH training grant T32 HD007186-32 (to W. W. Hay). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or NICHD.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R.L., S.R.T., M.C.O., L.D.B., W.W.H.J., and P.J.R. conception and design of research; J.R.L., S.R.T., M.C.O., D.L., L.D.B., and P.J.R. performed experiments; J.R.L., S.R.T., M.C.O., D.L., L.D.B., W.W.H.J., and P.J.R. analyzed data; J.R.L., S.R.T., M.C.O., D.L., L.D.B., W.W.H.J., and P.J.R. interpreted results of experiments; J.R.L. and P.J.R. prepared figures; J.R.L. and P.J.R. drafted manuscript; J.R.L., S.R.T., M.C.O., D.L., L.D.B., W.W.H.J., and P.J.R. edited and revised manuscript; J.R.L., S.R.T., M.C.O., D.L., L.D.B., W.W.H.J., and P.J.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Karen Trembler, David Caprio, Alex Cheung, and Gates Roe for their technical support.
REFERENCES
- 1. Abayomi JC, Watkinson H, Boothby J, Topping J, Hackett AF. Identification of “hot spots” of obesity and being underweight in early pregnancy in Liverpool. J Hum Nutr Diet 22: 246–254, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Aspinwall CA, Lakey JR, Kennedy RT. Insulin-stimulated insulin secretion in single pancreatic beta cells. J Biol Chem 274: 6360–6365, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Beith JL, Alejandro EU, Johnson JD. Insulin stimulates primary beta-cell proliferation via Raf-1 kinase. Endocrinology 149: 2251–2260, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belkacemi L, Nelson DM, Desai M, Ross MG. Maternal undernutrition influences placental-fetal development. Biol Reprod 83: 325–331, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Bloomfield FH. How is maternal nutrition related to preterm birth? Annu Rev Nutr 31: 235–261, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Bocian-Sobkowska J, Zabel M, Wozniak W, Surdyk-Zasada J. Polyhormonal aspect of the endocrine cells of the human fetal pancreas. Histochem Cell Biol 112: 147–153, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Bouche C, Lopez X, Fleischman A, Cypess AM, O'Shea S, Stefanovski D, Bergman RN, Rogatsky E, Stein DT, Kahn CR, Kulkarni RN, Goldfine AB. Insulin enhances glucose-stimulated insulin secretion in healthy humans. Proc Natl Acad Sci USA 107: 4770–4775, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buchmiller TL, Kim CS, Chopourian HL, Fonkalsrud EW. Transamniotic fetal feeding: enhancement of growth in a rabbit model of intrauterine growth retardation. Surgery 116: 36–41, 1994 [PubMed] [Google Scholar]
- 9. Carson BS, Philipps AF, Simmons MA, Battaglia FC, Meschia G. Effects of a sustained insulin infusion upon glucose uptake and oxygenation of the ovine fetus. Pediatr Res 14: 147–152, 1980 [DOI] [PubMed] [Google Scholar]
- 10. Carver TD, Hay WW., Jr Uteroplacental carbon substrate metabolism and O2 consumption after long-term hypoglycemia in pregnant sheep. Am J Physiol Endocrinol Metab 269: E299–E308, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Chandler KD, Leury BJ, Bird AR, Bell AW. Effects of undernutrition and exercise during late pregnancy on uterine, fetal and uteroplacental metabolism in the ewe. Br J Nutr 53: 625–635, 1985 [DOI] [PubMed] [Google Scholar]
- 12. Charles S, Tamagawa T, Henquin JC. A single mechanism for the stimulation of insulin release and 86Rb+ efflux from rat islets by cationic amino acids. Biochem J 208: 301–308, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen X, Rozance PJ, Hay WW, Jr, Limesand SW. Insulin-like growth factor and fibroblast growth factor expression profiles in growth-restricted fetal sheep pancreas. Exp Biol Med (Maywood) 237: 524–529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cherif H, Reusens B, Ahn MT, Hoet JJ, Remacle C. Effects of taurine on the insulin secretion of rat fetal islets from dams fed a low-protein diet. J Endocrinol 159: 341–348, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Cole L, Anderson M, Antin PB, Limes SW. One process for pancreatic beta-cell coalescence into islets involves an epithelial-mesenchymal transition. J Endocrinol 203: 19–31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. da S, X, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J 371: 761–774, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delisle HF. Poverty: the double burden of malnutrition in mothers and the intergenerational impact. Ann NY Acad Sci 1136: 172–184, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Dickson LM, Lingohr MK, McCuaig J, Hugl SR, Snow L, Kahn BB, Myers MG, Jr, Rhodes CJ. Differential activation of protein kinase B and p70(S6)K by glucose and insulin-like growth factor 1 in pancreatic beta-cells (INS-1). J Biol Chem 276: 21110–21120, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 42: 12–21, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Dyachok O, Idevall-Hagren O, Sagetorp J, Tian G, Wuttke A, Arrieumerlou C, Akusjarvi G, Gylfe E, Tengholm A. Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab 8: 26–37, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Eremia SC, DEBOOHA, Bloomfield FH, Oliver MH, HARDINGJE. Fetal and amniotic insulin-like growth factor-I supplements improve growth rate in intrauterine growth restriction fetal sheep. Endocrinology 148: 2963–2972, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Garofano A, Czernichow P, Breant B. In utero undernutrition impairs rat beta-cell development. Diabetologia 40: 1231–1234, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem 282: 10341–10351, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Green AS, Rozance PJ, Limesand SW. Consequences of a compromised intrauterine environment on islet function. J Endocrinol 205: 211–224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greenbaum CJ, Havel PJ, Taborsky GJ, Jr, Klaff LJ. Intra-islet insulin permits glucose to directly suppress pancreatic A cell function. J Clin Invest 88: 767–773, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gunawardana SC, Liu YJ, MacDonald MJ, Straub SG, Sharp GW. Anaplerotic input is sufficient to induce time-dependent potentiation of insulin release in rat pancreatic islets. Am J Physiol Endocrinol Metab 287: E828–E833, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull 60: 5–20, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49: 1751–1760, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol 5: 330–335, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Ivarsson R, Quintens R, Dejonghe S, Tsukamoto K, in' V, Renstrom E, Schuit FC. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 54: 2132–2142, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes 49: 163–176, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Johnson JD, Alejandro EU. Control of pancreatic beta-cell fate by insulin signaling: The sweet spot hypothesis. Cell Cycle 7: 1343–1347, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Johnson JD, Bernal-Mizrachi E, Alejandro EU, Han Z, Kalynyak TB, Li H, Beith JL, Gross J, Warnock GL, Townsend RR, Permutt MA, Polonsky KS. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc Natl Acad Sci USA 103: 19575–19580, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karamatsu JT, Boyd AT, Cooke J, Vinall PS, McMahon MJ. Intravenous nutrition during a twin pregnancy. JPEN J Parenter Enteral Nutr 11: 499–501, 1987 [DOI] [PubMed] [Google Scholar]
- 35. Kefas BA, Heimberg H, Vaulont S, Meisse D, Hue L, Pipeleers D, Van de CM. AICA-riboside induces apoptosis of pancreatic beta cells through stimulation of AMP-activated protein kinase. Diabetologia 46: 250–254, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Kim JW, Cho JH, Ko SH, Park HS, Ha J, Song KH, Son HY, Kim SS, Yoon KH, Suh-Kim H. Transcriptional mechanism of suppression of insulin gene expression by AMP-activated protein kinase activator 5-amino-4-imidazolecarboxamide riboside (AICAR) in beta-cells. Biochem Biophys Res Commun 365: 614–620, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Kind KL, Owens JA, Lok F, Robinson JS, Quinn KJ, Mundy L, Gilmour RS, Owens PC. Intravenous infusion of insulin-like growth factor I in fetal sheep reduces hepatic IGF-I and IGF-II mRNAs. Am J Physiol Regul Integr Comp Physiol 271: R1632–R1637, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Kulkarni RN. New insights into the roles of insulin/IGF-I in the development and maintenance of beta-cell mass. Rev Endocr Metab Disord 6: 199–210, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Kulkarni RN, Bruning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96: 329–339, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Leos RA, Anderson MJ, Chen X, Pugmire J, Anderson KA, Limesand SW. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 298: E770–E778, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li C, Najafi H, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Matschinsky FM, Stanley CA. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J Biol Chem 278: 2853–2858, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 288: R1297–R1305, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Limesand SW, Hay WW., Jr Adaptation of ovine fetal pancreatic insulin secretion to chronic hypoglycaemia and euglycaemic correction. J Physiol 547: 95–105, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Limesand SW, Rozance PJ, Smith D, Hay WW., Jr Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 293: E1716–E1725, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147: 1488–1497, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 74: 2296–2299, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Milley JR, Rosenberg AA, Philipps AF, Molteni RA, Jones MD, Jr, Simmons MA. The effect of insulin on ovine fetal oxygen extraction. Am J Obstet Gynecol 149: 673–678, 1984 [DOI] [PubMed] [Google Scholar]
- 48. Narkewicz MR, Carver TD, Hay WW., Jr Induction of cytosolic phosphoenolpyruvate carboxykinase in the ovine fetal liver by chronic fetal hypoglycemia and hypoinsulinemia. Pediatr Res 33: 493–496, 1993 [DOI] [PubMed] [Google Scholar]
- 49. Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res 22: 426–430, 1990 [DOI] [PubMed] [Google Scholar]
- 50. Ohsugi M, Cras-Meneur C, Zhou Y, Bernal-Mizrachi E, Johnson JD, Luciani DS, Polonsky KS, Permutt MA. Reduced expression of the insulin receptor in mouse insulinoma (MIN6) cells reveals multiple roles of insulin signaling in gene expression, proliferation, insulin content, and secretion. J Biol Chem 280: 4992–5003, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Panten U, Rustenbeck I. Fuel-induced amplification of insulin secretion in mouse pancreatic islets exposed to a high sulfonylurea concentration: role of the NADPH/NADP+ ratio. Diabetologia 51: 101–109, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI, Hanley NA. Beta cell differentiation during early human pancreas development. J Endocrinol 181: 11–23, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Rennert NJ, Caprio S, Sherwin RS. Insulin-like growth factor I inhibits glucose-stimulated insulin secretion but does not impair glucose metabolism in normal humans. J Clin Endocrinol Metab 76: 804–806, 1993 [DOI] [PubMed] [Google Scholar]
- 54. Roseboom TJ, Painter RC, van Abeelen AF, Veenendaal MV, de R., Sr Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas 70: 141–145, 2011 [DOI] [PubMed] [Google Scholar]
- 55. Rosenberg A. The IUGR newborn. Semin Perinatol 32: 219–224, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Rozance PJ, Limesand SW, Barry JS, Brown LD, Hay WW., Jr Glucose replacement to euglycemia causes hypoxia, acidosis, and decreased insulin secretion in fetal sheep with intrauterine growth restriction. Pediatr Res 65: 72–78, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rozance PJ, Limesand SW, Hay WW., Jr Decreased nutrient-stimulated insulin secretion in chronically hypoglycemic late-gestation fetal sheep is due to an intrinsic islet defect. Am J Physiol Endocrinol Metab 291: E404–E411, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Rozance PJ, Limesand SW, Barry JS, Brown LD, Thorn SR, LoTurco D, Regnault TRH, Friedman JE, Hay WW., Jr Chronic late-gestation hypoglycemia upregulates hepatic PEPCK associated with increased PGC1α mRNA and phosphorylated CREB in fetal sheep. Am J Physiol Endocrinol Metab 294: E365–E370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rozance PJ, Limesand SW, Zerbe GO, Hay WW., Jr Chronic fetal hypoglycemia inhibits the later steps of stimulus-secretion coupling in pancreatic β-cells. Am J Physiol Endocrinol Metab 292: E1256–E1264, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Rutter GA, Leclerc I. The AMP-regulated kinase family: enigmatic targets for diabetes therapy. Mol Cell Endocrinol 297: 41–49, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Simmons MA, Jones MD, Jr, Battaglia FC, Meschia G. Insulin effect on fetal glucose utilization. Pediatr Res 12: 90–92, 1978 [DOI] [PubMed] [Google Scholar]
- 62. Slack JM. Developmental biology of the pancreas. Development 121: 1569–1580, 1995 [DOI] [PubMed] [Google Scholar]
- 63. Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev 18: 451–463, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Tchirikov M, Kharkevich O, Steetskamp J, Beluga M, Strohner M. Treatment of growth-restricted human fetuses with amino acids and glucose supplementation through a chronic fetal intravascular perinatal port system. Eur Surg Res 45: 45–49, 2010 [DOI] [PubMed] [Google Scholar]
- 65. Thorn SR, Sekar SM, Lavezzi JR, O'Meara MC, Brown LD, Hay WW, Jr, Rozance PJ. A physiological increase in insulin suppresses gluconeogenic gene activation in fetal sheep with sustained hypoglycemia. Am J Physiol Regul Integr Comp Physiol 303: R861–R869, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, Satin LS, Stein R, Holzenberger M, Kennedy RT, Kahn CR, Kulkarni RN. Total insulin and IGF-I resistance in pancreatic [beta] cells causes overt diabetes. Nat Genet 38: 583–588, 2006 [DOI] [PubMed] [Google Scholar]
- 67. Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol 84: 751–753, 1977 [DOI] [PubMed] [Google Scholar]
- 68. Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques 39: 75–85, 2005 [DOI] [PubMed] [Google Scholar]