Abstract
To determine the role of the transient receptor potential vanilloid type 1 (TRPV1) channel in the regulation of renal inflammation, lipopolysaccharide (LPS, 3 mg/kg) was intraperitoneally injected into wild-type (WT) and TRPV1-null mutant (TRPV1−/−) mice. The kidney and serum were collected 6 or 24 h after LPS injection for morphological analysis and proinflammatory cytokine assay. LPS injection led to a similar degree of transient hypotension and bradycardia in WT and TRPV1−/− mice determined by a telemetry system. LPS administration caused parenchymal red blood cell congestion and fading of intact glomerular structure in TRPV1−/− compared with WT mice. Serum creatinine levels were higher 24 h after LPS injection in TRPV1−/− than in WT mice. Neutrophil and macrophage infiltration in the kidneys was greater 6 h for the former and 24 h for both after LPS injection in TRPV1−/− than in WT mice. Serum cytokine levels including tumor necrosis factor (TNF)-α, IL-1β, and IL-6 were higher 6 h after LPS injection in TRPV1−/− compared with WT mice. Likewise, renal chemokine levels including keratinocyte-derived chemokines and macrophage inflammatory protein were higher 6 h after LPS injection in TRPV1−/− than in WT mice. Renal VCAM-1 and ICAM-1 expression was further elevated 6 h for the former and 24 h for the latter after LPS injection in TRPV1−/− than in WT mice. Renal nuclear factor-κB (NF-κB) activity was further increased 6 h after LPS injection in TRPV1−/− compared with WT mice. Pharmacological blockade TRPV1 in WT mice showed aggravated renal and serum inflammatory responses resembling that of TRPV1−/− mice. Thus TRPV1 gene ablation exacerbates LPS-induced renal tissue and function injury, including aggravated renal neutrophil and macrophage infiltration, chemokine and adhesion molecule levels, and glomerular hypercellularity accompanying with further increased serum creatinine and cytokine levels. These results indicate that TRPV1 is activated during LPS challenge, which may constitute a protect mechanism against LPS-induced renal injury via reducing renal inflammatory responses.
Keywords: endotoxin, renal inflammation, transient receptor potential vanilloid type 1 channel, mice
the transient receptor potential vanilloid type 1 (TRPV1; also known as the vanilloid receptor) channels are ligand-gated cation channels primarily expressed in sensory nerves of unmyelinated C-fibers and thinly myelinated Aδ-fibers innervating the cardiovascular tissues and kidneys (5, 18, 34). TRPV1 may function as a molecular integrator of multiple chemical and physical stimuli including capsaicin, proton, and noxious heat (18). In addition, TRPV1 may be activated by a variety of inflammatory mediators including lipoxygenase products, bradykinin, and prostaglandins (9, 18, 35). Previous studies indicated that TRPV1 activation may contribute to neurogenic inflammation (17). In contrast, recent studies showed that TRPV1 plays a protective role against the onset of sepsis and acute lung inflammatory injury induced by intratracheal administration of lipopolysaccharide (LPS) (6, 15).
Septic shock is commonly caused by gram-negative bacteria and their characteristic cell wall component, LPS, and exemplified with life-threatening cardiovascular depression with a high mortality rate (27). While the high mortality rate may result from multiorgan failure and multisystem malfunction, acute renal failure occurred during sepsis independently increases mortality (22). Regardless of the importance of the kidney in the survival during sepsis, pathogenesis of acute renal failure induced by endotoxin is poorly understood.
TRPV1-positive sensory nerves are widely distributed in the kidney (34) and play a significant functional role in mediating renal function. It has been shown that sensory nerve degeneration following neonatal capsaicin treatment or surgical denervation of sensory nerves innervating the kidney impairs sodium excretory function of the kidney in response to sodium loading (21, 31), suggesting that intact function of TRPV1-positive sensory nerves is required for the kidney to operate normally in the face of salt loading. It is unknown, however, whether TRPV1 is activated and plays a role in mediating renal inflammatory responses to LPS and LPS-induced renal damage. In the present study, TRPV1-null mutant (TRPV1−/−) or wild-type (WT) mice were used to test the hypothesis that TRPV1 serves as a protective molecule against LPS-induced renal injury possibly via alleviating the inflammatory responses of the kidney.
MATERIALS AND METHODS
Animals.
All of the experiments were approved by the Institutional Animals Care and Use Committee. TRPV1−/− mice backcrossed six generations on a C57/BL6 strain were generously provided by Dr. David Julius (University of California, San Francisco, CA). These TRPV1−/− mice have been well characterized with impaired nociception and pain sensation in the absence of other apparent phenotypes (5). Ten-week-old male TRPV1−/− mice or C57/BL6 as WT control mice (weighing ∼26 to 28 g) were used in this study. Mice were maintained on a standard rodent chow and had free access to water throughout the experiment.
Experimental protocols.
WT and TRPV1−/− mice were intraperitoneally injected with 3 mg/kg of LPS derived from Escherichia coli (Sigma Chemical, St. Louis, MO). This dose was chosen given because our preliminary data showed that this dose caused observable nephrotoxicity without apparent changes in blood pressure determined by radiotelemetry. Mice were euthanized 6 or 24 h after LPS injection for collection of blood for serum, and the samples were stored at −80°C for cytokine and creatinine assays. One kidney was frozen in liquid nitrogen for chemokine assays and Western blot analysis of adhesion molecules, and the other kidney was fixed in 4% paraformaldehyde solution for histological analysis.
To further determine the effect of TRPV1 on LPS-induced renal inflammatory responses, LPS (3 mg/kg ip) was bolus injected into conscious WT and TRPV1−/− mice with or without the selective TRPV1 antagonist capsazepine (3 mg/kg ip, CAPZ, Calbiochem, San Diego, CA) that was given 20 min before LPS injection. The effectiveness of the dose of CAPZ was verified by blockade of the hypotensive response to capsaicin (30 μg/kg), a selective TRPV1 agonist (32). CAPZ was dissolved in dimethyl sulfoxide (10%, vol/vol), Tween-80 (10%, vol/vol), and normal saline. Control WT and TRPV1−/− mice received vehicle injection. The mice were euthanized 6 h after LPS injection for collection of blood for serum that was stored at −80°C for cytokine assay and for kidneys that were fixed in 4% paraformaldehyde solution for immunohistostaining.
Mean blood pressure and heart rate assay.
Mean arterial pressure (MAP) and heart rate (HR) were determined using the telemetry system (Data Sciences International, St. Paul, MN) following the manufacturer's instruction. Briefly, the mice were anesthetized with ketamine and xylazine (80 and 4 mg/kg sc, respectively). The catheter linked to the transmitter was inserted into the left carotid artery and the transmitter body placed subcutaneously in the lower right side of the abdomen. The skin incisions were then closed. The mice were returned to their individual cages and allowed recovery for 6 days before the start of the radiotelemetric recording. On day 7, MAP and HR were recorded continuously 4 h before and 24 h after LPS administration.
Histological analysis.
Paraformaldehyde-fixed and paraffin-embedded kidneys were sectioned at 4 μm and stained with hematoxylin and eosin using the standard method. These sections were viewed with a light microscopy at ×200 magnification to determine general tissue morphological changes in control and LPS-treated TRPV1−/− and WT mice.
Immunohistochemistry.
Renal neutrophil or macrophage infiltration was analyzed in 3-μm-thick paraffin-embedded sections obtained 6 or 24 h after LPS treatment. Sections were mounted on glass slides, deparaffinized, and rehydrated. The sections were then treated with 3% hydrogen peroxide for 5 min and blocked with 3% normal horse serum in phosphate buffer solution for 1 h. Rat anti-mouse monoclonal antibody to neutrophil (1: 200, Oxford, UK) or macrophage (1: 400, Oxford, UK) was used for an overnight incubation at 4°C. After the sections were washed, horse anti-rat IgG-horseradish peroxidase (Vector Laboratories, Burlingame, CA), diluted 1:400, was applied and incubated for 1 h at room temperature, and visualized by incubating the sections with the substrate vector fast red or 3,3′-diaminobenzidine (Vector Laboratories). The sections were counterstained with hematoxylin. Negative control experiments were performed by omitting the primary antibody during incubation.
Neutrophil or macrophage infiltration was quantitatively assessed on immunohistochemical-stained section in a blind fashion under ×400 magnification. At least 10 fields were counted in the cortex for each slide, and the number of positive cells was expressed as cells per square millimeter.
Creatinine assay.
Serum creatinine concentration was assayed by the use of an improved Jaffe creatinine assay kit (BioAssay Systems, Hayward, CA).
Cytokine-chemokine assay.
Levels of tumor necrosis factor (TNF)-α, IL-1β, and IL-6 in serum and protein levels of keratinocyte-derived chemokines (KC) and macrophage inflammatory protein (MIP-2) in kidneys were determined using the corresponding ELISA kit (R&D Systems, Minneapolis, MN) following the manufacturer's instruction. Fifty or less microliters of serum were used for the TNF-α, IL-1β, and IL-6 assay. For determination of KC and MIP-2 contents, whole kidneys were homogenized in ice-cold phosphate buffer containing protease inhibitor cocktail (Sigma Chemical, St. Louis, MO), and the total protein was extracted using NE-PER Cytoplasmic Extraction Reagents (Pierce, Rockford, IL). The total protein concentration in tissue extract was determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA) and used for normalizing chemokine levels in renal tissues.
Western blot analysis.
Whole kidneys were homogenized in ice-cold lysis buffer (20 mM HEPES, 75 mM NaCl, 2.5 mM MgCl2, 0.1 mM EDTA, 0.1% Triton X-100, and 1/10 volume of a protease inhibitor solution consisting of 25 μg/ml antipain, 1 μg/ml aprotinin, 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin, and 200 μM phenylmethylsulfonyl fluoride). The homogenate was centrifuged at 15,000 g at 4°C for 20 min. Protein contents of the supernatant were determined using a Bio-Rad protein assay kit (Bio-Rad Laboratories). Samples (30 μg of protein) were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. The membranes were blocked 1 h at room temperature in 5% milk solution (50 mM Tris·HCl, 100 mM NaCl, and 0.1% Tween-20 at pH 7.5). Subsequently, the membranes were incubated with goat anti-mouse ICAM-1 polyclonal antibody (1: 500; Santa Cruz Biotechnology, Santa Cruz, CA) or goat anti-human VCAM-1 polyclonal antibody (1: 500; Santa Cruz Biotechnology) in blocking solution overnight at 4°C. After being washed, membranes were incubated with bovine anti-goat horseradish peroxidase-conjugated antibody (1:3,000; Santa Cruz Biotechnology) in blocking solution at room temperature for 1 h. Membranes were developed using the enhanced chemiluminescence ECL kit (GE Healthcare, Buckinghamshire, UK) and exposed to film (Hyperfilm-ECL; GE Healthcare). Films were scanned and analyzed with the use of the Image Quantity Program (Scion) to obtained integrated densitometric values. The intensity of band was expressed as fold changes compared with control WT (100%).
Nuclear factor-κB transcription factor assay.
Nuclear protein was extracted from kidneys with a nuclear extract kit (Active Motif, Carlsbad, CA) according to the manufacturer's instruction. Protein concentrations of nuclear extract were determined with the use of a protein assay kit (Bio-Rad Laboratories). Transcription factor nuclear factor-κB (NF-κB) activity in kidneys was determined with the use of the TransAM NF-κB p65 assay kit (Active Motif, Carlsbad, CA) following the manufacturer's protocol. In brief, nuclear proteins were added into each well coated with an unlabeled oligonucleotide containing the consensus binding site for NF-κB (5′-GGGACTTTCC-3′) and incubated for 1 h. After washing was completed, a primary antibody directed against the NF-κB p65 subunit was added and incubated for 1 h. Subsequently, a secondary antibody conjugated to horseradish peroxidase was applied for 1 h. A colorimetric reaction was developed with addition of a developing solution and terminated by a stop solution. The plate was read at 450 nm with an absorbance microplate reader (Molecular Devices, Downingtown, PA).
Statistical analysis.
All values are expressed as means ± SE. The significance of differences among groups was evaluated using one-way analysis of variance following by a Bonferroni's adjustment for multiple comparisons. Differences were considered statistically significant at P < 0.05.
RESULTS
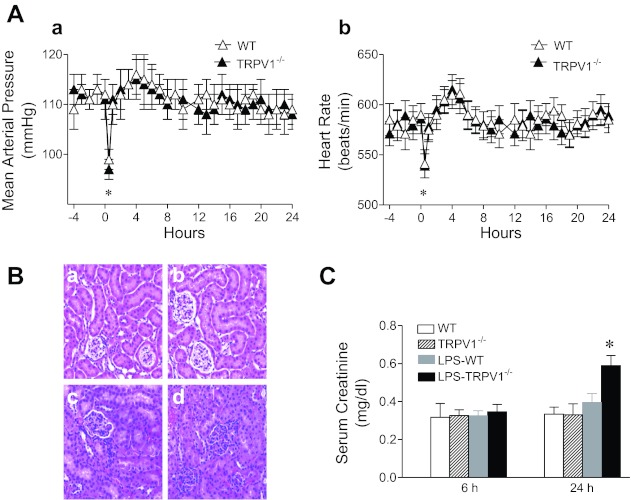
As shown in Fig. 1, administration of LPS caused a transient fall of MAP and bradycardia in both groups of mice, and the changes in MAP and HR were similar in WT and TRPV1−/− mice (111 ± 2 to 99 ± 2 mmHg in WT mice, 112 ± 3 to 97 ± 2 mmHg in TRPV1−/− mice; 579 ± 15 to 541 ± 14 beats/min in WT mice, 581 ± 14 to 539 ± 12 beats/min in TRPV1−/− mice, respetively). MAP and HR returned to the baseline by 1 h after LPS injection in both strains.
Fig. 1.
A: time-course responses of mean arterial pressure (a) and heart rate (b) to bolus injection of lipopolysaccharide (LPS, 3 mg/kg ip) in wild-type (WT) and TRPV1-null mutant (TRPV1−/−) mice. B: renal hematoxylin and eosin-stained sections from WT and TRPV1−/− mice (a and b) and LPS-treated WT and TRPV1−/− mice (c and d). Tissue samples were harvested at 24 h after LPS (3 mg/kg ip) treatment. Magnification, ×200. C: time-course responses of serum creatinine to bolus injection of LPS (3 mg/kg ip) in WT and TRPV1−/− mice. Serum samples were collected at 6 or 24 h after LPS treatment. Values are means ± SE (n = 7–8). *P < 0.05 compared with baseline, control WT, or TRPV1−/− mice.
General morphological view of kidneys (Fig. 1) was not different between TRPV1−/− and WT mice before LPS treatment, with typical glomeruli occasionally containing a few numbers of red blood cells. Twenty four hours after LPS treatment, WT mice displayed slight renal injury with generalized hypercellularity but well-preserved glomerular morphology. In contrast, renal injury induced by LPS treatment in TRPV1−/− mice included parenchymal red blood cell congestion especially in glomeruli, resulting in the fading of intact glomerular structure.
As shown in Fig. 1, serum creatinine levels in WT mice were not elevated 6 or 24 h after LPS treatment. In contrast, serum creatinine levels were not altered 6 h but markedly elevated 24 h after LPS treatment in TRPV1−/− compared with WT mice (0.59 ± 0.05 vs. 0.40 ± 0.05 mg/dl, P < 0.05).
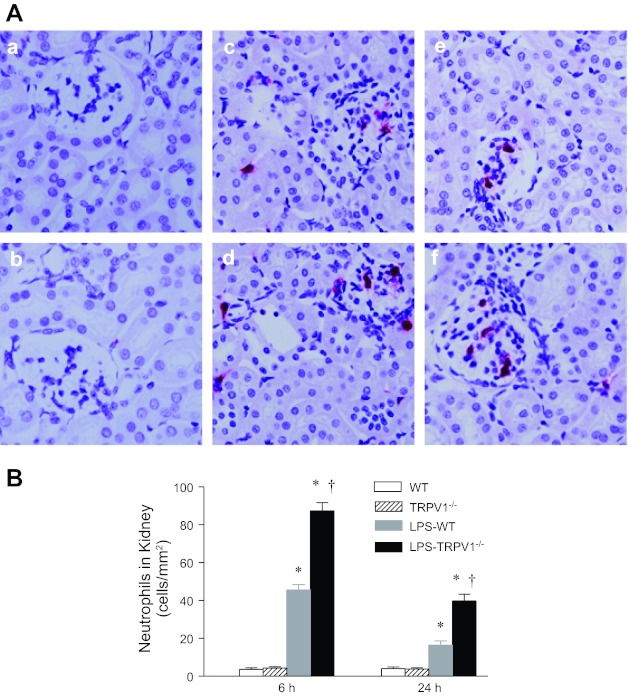
Neutrophil recruitment is a feature of endotoxin-induced inflammation. As demonstrated in Fig. 2, neutrophil infiltration in the kidneys of WT mice was markedly increased 6 h after LPS administration, with gradually diminishing infiltration 24 h after LPS injection. There was an intensified neutrophil infiltration in the kidneys of TRPV1−/− compared with WT mice either 6 or 24 h after LPS treatment (6 h, 87 ± 4 vs. 46 ± 3 neutrophils/mm2; 24 h, 40 ± 4 vs. 16 ± 2 neutrophils/mm2, P < 0.05).
Fig. 2.
A: representative immunohistochemical staining of neutrophil in kidney sections from WT and TRPV1−/− mice (a and b) and WT and TRPV1−/− mice treated with LPS (3 mg/kg ip) for 6 h (c and d) or 24 h (e and f). Magnification, ×400. B: bar graph shows the number of renal cortical neutrophil expressed as cells per square millimeter. Values are means ± SE (n = 7–8). *P < 0.05 compared with control WT or TRPV1−/− mice; †P < 0.05 compared with LPS-treated WT mice.
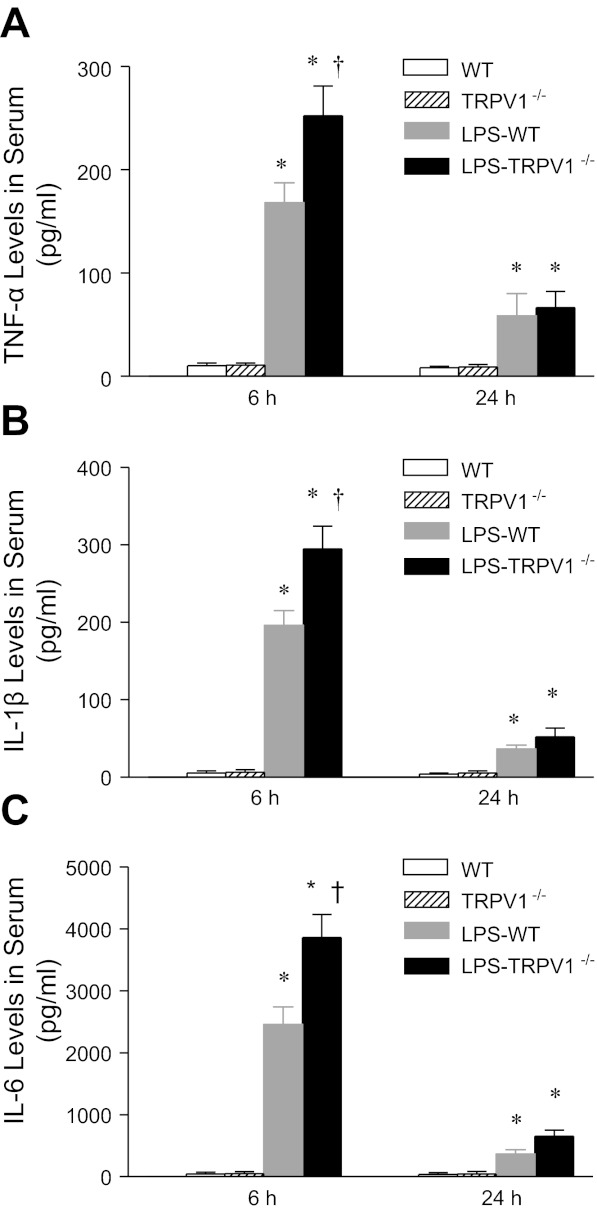
Given that TNF-α is known to contribute to endotoxin-induced tissue damage, TNF-α levels in blood were examined using ELISA. As shown in Fig. 3, serum TNF-α levels markedly increased in WT mice 6 h after LPS injection, with substantial levels remaining at 24 h. Serum TNF-α levels after LPS were greater in TRPV1−/− compared with WT mice at 6 h (252 ± 29 vs. 168 ± 19 pg/ml, P < 0.05) but not 24 h after LPS injection.
Fig. 3.
Time-course responses of serum tumor necrosis factor (TNF)-α (A), IL-1β (B), and IL-6 (C) to bolus injection of LPS (3 mg/kg ip) in WT and TRPV1−/− mice. Serum samples were collected at 6 or 24 h after LPS treatment. Values are means ± SE (n = 7–8). *P < 0.05 compared with control WT or TRPV1−/− mice. †P < 0.05 compared with LPS-treated WT mice.
TNF-α controls expression of a number of cytokines, which were quantitatively determined as shown in Fig. 3. Serum IL-1β and IL-6 increased after LPS challenge in WT and TRPV1−/− mice, and the patterns of changes were similar to that of TNF-α. Enhanced elevation of serum IL-1β and IL-6 was observed in TRPV1−/− compared with WT mice 6 h but not 24 h after LPS injection (IL-1β, 295 ± 29 vs. 196 ± 19 pg/ml; IL-6, 3,860 ± 372 vs. 2,457 ± 281 pg/ml, P < 0.05).
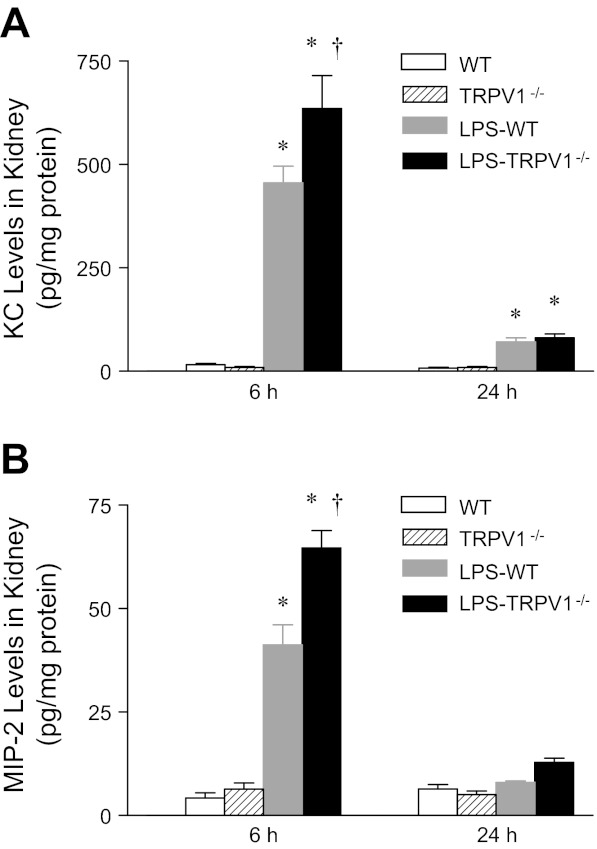
Levels of chemotactic molecules in the kidney were determined 6 or 24 h after LPS challenge as shown in Fig. 4. Renal levels of neutrophil chemoattractants, KC and MIP-2, markedly increased in WT and TRPV1−/− mice 6 h after LPS treatment, with a greater degree in the latter group (KC, 456 ± 40 pg/mg protein vs. 636 ± 79 pg/mg protein; MIP-2, 41 ± 5 pg/mg protein vs. 65 ± 4 pg/mg protein, P < 0.05). KC but not MIP-2 remained slightly elevated 24 h after LPS treatment with no difference between WT and TRPV1−/− mice.
Fig. 4.
Time-course responses of renal chemokine levels to bolus injection of LPS (3 mg/kg ip) in WT and TRPV1−/− mice. Tissue samples were collected at 6 or 24 h after LPS treatment. Values are means ± SE (n = 7–8). *P < 0.05 compared with control WT or TRPV1−/− mice. †P < 0.05 compared with LPS-treated WT mice.
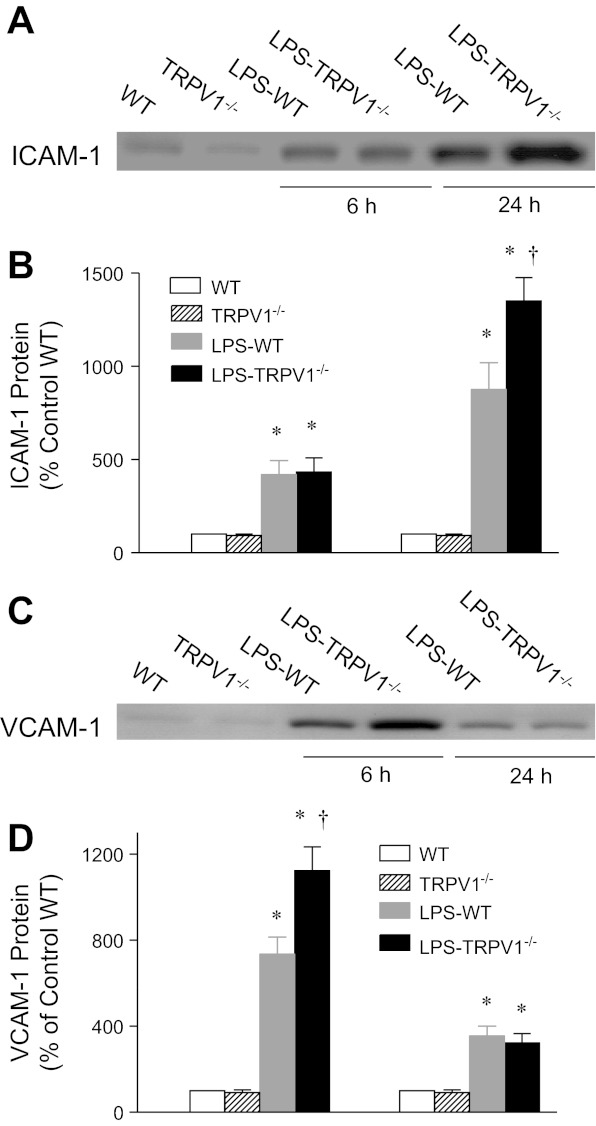
Western blot analysis showed that renal expression of an adhesion molecule, VCAM-1, was markedly elevated in WT mice 6 h after LPS challenge, with substantial levels remaining at 24 h (Fig. 5). Enhanced upregulation of VCAM-1 was seen in TRPV1−/− compared with WT mice 6 h but not 24 h after LPS treatment (1,123 ± 111% of control WT vs. 735 ± 79% of control WT, P < 0.05). In contrast, ICAM-1 expression increased in WT and TRPV1−/− mice 6 h after LPS treatment, which further increased at 24 h with a bigger magnitude in TRPV1−/− compared with WT mice (1,350 ± 126% of control WT vs. 875 ± 144% of control WT, P < 0.05).
Fig. 5.
Representative Western blot of ICAM-1 (A) and VCAM-1 (C) in kidneys from WT and TRPV1−/− mice and WT and TRPV1−/− mice treated with LPS (3 mg/kg ip) for 6 or 24 h. Bar graphs show the relative optical density values for ICAM-1 (B) and VCAM-1 (D) in WT and TRPV1−/− mice with and without LPS. Tissue samples were collected at 6 or 24 h after LPS treatment. Protein expression levels are normalized with the control WT protein expression. Values are means ± SE (n = 4–5). *P < 0.05 compared with control WT or TRPV1−/− mice. †P < 0.05 compared with LPS-treated WT mice.
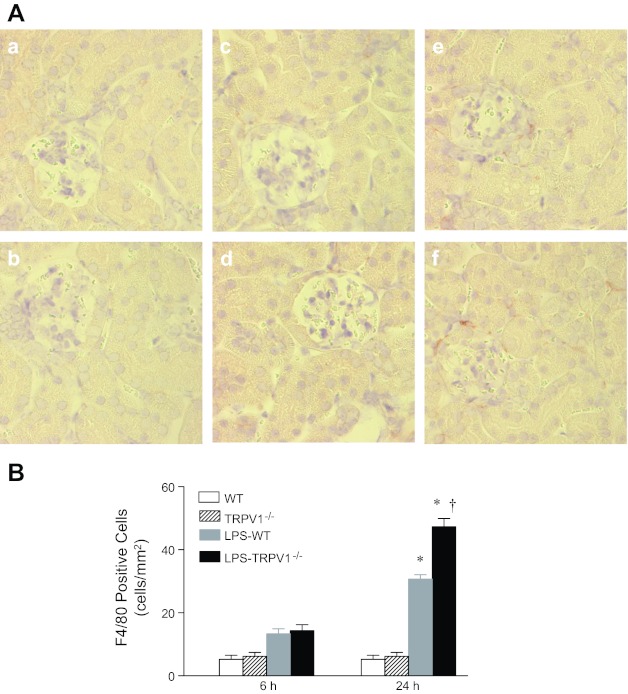
As shown in Fig. 6, analysis of a marker of monocyte/macrophages (F4/80) showed significantly increased staining of F4/80 in the kidney of WT and TRPV1−/− mice 24 h but not 6 h after LPS treatment, with a greater level in the latter group (31 ± 3 F4/80 positive cells/mm2 vs. 47 ± 6 F4/80 positive cells/mm2, P < 0.05).
Fig. 6.
A: representative immunohistochemical staining of F4/80-positive cell in kidney sections from WT and TRPV1−/− mice (a and b) and WT and TRPV1−/− mice treated with LPS (3 mg/kg ip) for 6 (c and d) or 24 h (e and f). Magnification, ×400. B: bar graph shows the number of renal cortical F4/80-positive cell expressed as cells per square millimeter. Values are means ± SE (n = 7 to 8). *P < 0.05 compared with control WT or TRPV1−/− mice; †P < 0.05 compared with LPS-treated WT mice.
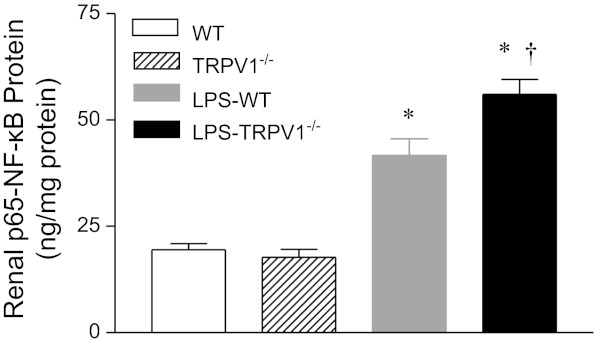
NF-κB activity in the kidney in response to LPS treatment was determined and shown in Fig. 7. Renal NF-κB activity was markedly increased in WT mice 6 h after LPS injection. LPS treatment further enhanced NF-κB activity in TRPV1−/− compared with WT mice (56.1 ± 3.5 ng/mg protein vs. 41.7 ± 3.9 ng/mg protein, P < 0.05).
Fig. 7.
Renal nuclear factor (NF)-κB responses to bolus injection of LPS (3 mg/kg ip) in WT and TRPV1−/− mice. Tissue samples were collected at 6 h after LPS treatment. Values are means ± SE (n = 7 to 8). *P < 0.05 compared with control WT or TRPV1−/− mice. †P < 0.05 compared with LPS-treated WT mice.
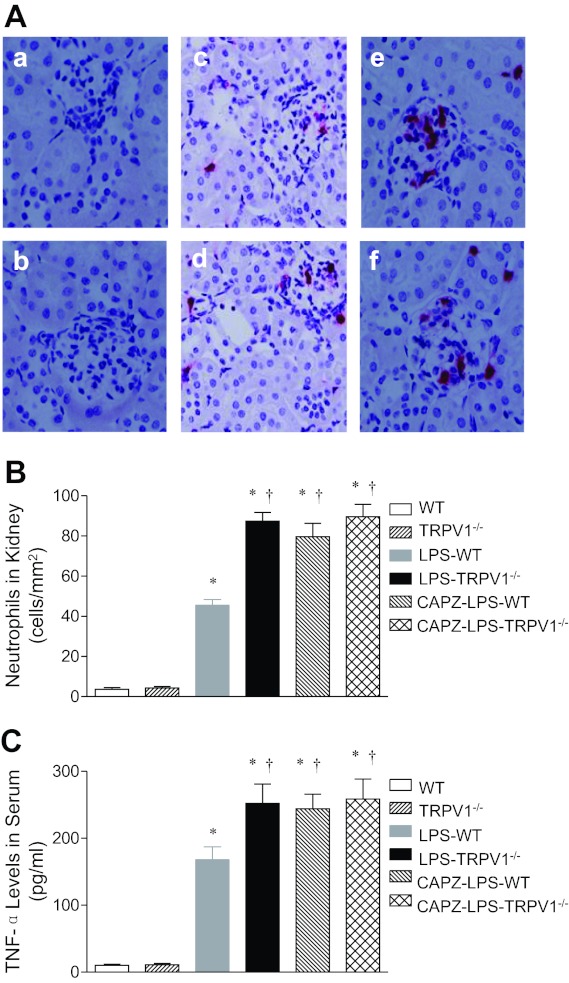
The effect of the TRPV1 antagonist CAPZ on renal neutrophil infiltration and serum TNF-α levels in WT and TRPV1−/− mice was also determined. As demonstrated in Fig. 8, blockade of TRPV1 with CAPZ further increased renal neutrophil infiltration and serum TNF-α levels 6 h after LPS treatment (45 ± 3 neutrophils/mm2 vs. 80 ± 7 neutrophils/mm2; 168 ± 20 pg/ml vs. 244 ± 22 pg/ml, P < 0.05) in WT but not TRPV1−/− mice.
Fig. 8.
A: representative immunohistochemical staining of neutrophil in kidney sections from WT and TRPV1−/− mice (a and b) and LPS (3 mg/kg ip)-treated WT and TRPV1−/− mice with (e and f) or without (c and d) capsazepine (CAPZ, 3 mg/kg ip) treatment. Tissue samples were collected at 6 h after LPS treatment. Magnification, ×400. B: bar graph shows the number of renal cortical neutrophil expressed as cells per square millimeter. C: responses of serum TNF-α to bolus injection of LPS (3 mg/kg ip) in WT and TRPV1−/− mice with or without CAPZ treatment. Serum samples were collected at 6 h after LPS treatment. Values are means ± SE (n = 7 to 8). *P < 0.05 compared with control WT or TRPV1−/− mice; †P < 0.05 compared with LPS-treated WT mice.
DISCUSSION
The major finding of this study is that targeted ablation of TRPV1 led to further impaired renal structural and functional injury after LPS injection. More severe renal damage observed in TRPV1−/− mice occurred in the absence of worsened hypotension compared with WT mice and was characterized with enhanced early neutrophilic inflammation followed by aggravated macrophage infiltration in the kidney in response to LPS injection. Furthermore, increased inflammatory responses in TRPV1−/− mice included heightened production of serum proinflammatory cytokines, renal proinflammatory chemokine, and renal adhesion molecule expression as well as NF-κB activity. These results lead to conclusive evidence showing that TRPV1 contributes to the regulation of LPS-induced renal inflammation in vivo.
The role of TRPV1 in systemic and renal inflammation induced by LPS is largely unknown, albeit Helyes et al. (15) reported that TRPV1 is protective against acute lung inflammatory injury induced by intra-tracheal administration of LPS. The results from the present study demonstrate for the first time in vivo that deletion of TRPV1 exaggerates systemic and renal inflammatory responses induced by LPS. It is known that inflammatory mediators contribute to pathophysiology of endotoxin-induced renal injury, with TNF-α playing a central role in this process (7, 8, 19, 20). TNF-α has been shown to magnify inflammatory or tissue injury responses induced by other cytokines and/or bioactive substances including IL-1β, nitric oxide, and adhesion molecules (25). In the present study, serum IL-1β and IL-6 production was also enhanced in accompanying elevated serum TNF-α levels within the same time frame in TRPV1−/− mice. Thus the increased inflammatory response to LPS after ablation of TRPV1 may rely on enhanced production of injurious proinflammatory cytokines including TNF-α, IL-1β, and IL-6.
Exaggerated inflammatory responses to LPS after deletion of TRPV1 gene were also evident with aggravated neutrophil infiltration in the kidney. Neutrophils, mediators of tissue injury in inflammatory diseases (4, 23), may produce an array of proinflammatory mediators including oxygen-free radicals, neutrophil-specific proteases, and products of lipid peroxidation that could be harmful to cells (11). Thus enhanced release of these products due to aggravated neutrophil infiltration in the kidneys of TRPV1−/− mice or when TRPV1 is pharmacologically blockaded may lead to irreversible renal damage. The increased neutrophil infiltration after TRPV1 ablation or blockade may underlie, at least in part, LPS-induced renal tissue and function injury observed in TRPV1−/− mice as well as in WT mice with pharmacological blockade of TRPV1.
Chemokines, a special subtype of chemotactic cytokines, play a critical role in leukocyte recruitment in the face of inflammatory conditions (2). Based on the spacing of the amino terminal cysteine residues, chemokines are classified into four subfamilies: CXC, CC, C, and CX3C (24). In general, the CXC chemokines including CXCL1/KC and CXCL2/MIP-2 are regarded as potent chemoattractants for neutrophils (24). Consistent with the observation, we found that KC and MIP2 concentrations in the kidney further increased along with enhanced neutrophil infiltration in response to LPS in TRPV1−/− mice. Moreover, although the patterns of changes in chemokines and neutrophils were similar, the effect of these chemokines appeared lasting given that changes in neutrophil infiltration lagged behind that of chemokines. These data indicate that TRPV1-induced attenuation in neutrophil infiltration could be the result of inhibition of chemokine expression.
ICAM-1 and VCAM-1 are members of adhesion molecule family known to mediate tight adherence of leukocytes to endothelial cells (1, 30). Also, ICAM-1 has been shown to play a major role in the recruitment of neutrophils to numerous tissues (10, 29). In the present study, however, renal VCAM-1, but not ICAM-1 expression, was further elevated 6 h after LPS injection in TRPV1−/− compared with WT mice, which coincided with the timeline of exacerbated renal neutrophil infiltration, i.e., 6 h after LPS injection, in TRPV1−/− mice. These results suggest and are consistent with the notion that VCAM-1 may facilitate the egress of neutrophils into the kidney in hosts with high cytokine levels (16) albeit VCAM-1 was not originally recognized as an important mediator of neutrophil infiltration.
In addition to neutrophils, monocyte/macrophage function has been shown to be modulated during sepsis. When stimulated, monocytes/macrophages differentiate into distinct phenotypes including classically activated and alternatively activated macrophages that have been shown to be involved in microbicidal and tissue repair process, respectively (13). In the present study, monocyte/macrophage infiltration was further enhanced 24 h after LPS injection in TRPV1−/− mice. The timeline of increased monocyte/macrophage infiltration in the kidney of TRPV1−/− mice overlapped with that of enhanced ICAM-1 expression, i.e., 24 h after LPS injection in these mice. These results are consistent with the recent reports showing that ICAM-1 also contributes to the regulation of monocyte/macrophage infiltration (26, 28) despite the notion that VCAM-1 is originally recognized as the important component mediating tight adherence of monocytes to endothelial cells. Further functional studies of monocytes/macrophages are required for elucidating the role of different phenotypes of macrophages in endotoxin-induced renal damage.
Many genes encoding protein products involved in inflammatory responses are regulated at the transcription level by the transcription factor NF-κB. Once activated by LPS, NF-κB leads to the release of a number of inflammatory mediators, including cytokines, chemokines, and adhesion molecules (3, 14). In the present study, we found that deletion of TRPV1 further enhanced LPS-induced renal NF-κB activity. These data suggest that inhibition of NF-κB activity may be a potential mechanism underlying TRPV1-mediated anti-inflammatory effects.
In addition to the prospect that TRPV1 per se may suppress inflammation during LPS challenge (6, 33), TRPV1-mediated release of sensory neuropeptides including calcitonin gene-related peptide and substance P may also affect the inflammatory processes (12). Further studies distinguishing the direct or downstream molecule effects of TRPV1 are required for better understanding of potential mechanisms of TRPV1 in mediating the inflammatory responses. While the present study used inbred C57/BL6 mice as WT mice compared with TRPV1−/− mice backcrossed six generations on the C57/BL6 strain, limitations exist. Genetic variability may occur with the possibility of small percentage of the genes being non-C57 genes in the knockout, thus the use of inbred C57/BL6 mice instead of littermates as controls might be less optimal albeit pharmacological blockade of TRPV1 in WT mice showed similar results as that in TRPV1−/− mice after LPS injection. Nevertheless, to obtain a more accurate picture of the effect of TRPV1 on LPS-induced inflammation, future studies are needed with the use of TRPV1−/− mice and its littermates.
In summary, the results of the study show that ablation or blockade of TRPV1 aggravates LPS-induced renal tissue and functional damage in the absence of worsened hypotension. The renal injury is accompanied with enhanced neutrophil and macrophage infiltration in the kidney and augmented induction of serum cytokines, renal chemokines, and renal adhesion molecules. These results indicate that TRPV1 is activated during LPS challenge, which may constitute a protect mechanism against LPS-induced renal injury via reducing renal inflammatory responses.
Perspectives and Significance
TRPV1-mediated protection against endotoxin-induced renal damage was demonstrated by targeted ablation of TRPV1 as well as by pharmacological blockade of TRPV1. These data support the notion that TRPV1 plays an important role in the pathophysiology of endotoxin-induced renal injury. Given the devastating outcomes of sepsis, it is intriguing to accommodate the possibility that modulation of TRPV1 function as therapeutic strategies for treating and preventing endotoxin-induced renal tissue and functional damage.
GRANTS
This work was supported in part by grants from the National Institutes of Health (HL-57853, HL-73287, and DK-67620) to D. H. Wang and a grant from National Natural Science Foundation of China (No. 81170243) to Y. Wang.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.W. and D.H.W. conception and design of research; Y.W. performed experiments; Y.W. analyzed data; Y.W. and D.H.W. interpreted results of experiments; Y.W. prepared figures; Y.W. drafted manuscript; Y.W. and D.H.W. edited and revised manuscript; Y.W. and D.H.W. approved final version of manuscript.
REFERENCES
- 1. Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J 8: 504–512, 1994 [PubMed] [Google Scholar]
- 2. Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol 15: 675–705, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Böhrer H, Qiu F, Zimmermann T, Zhang Y, Jllmer T, Männel D, Böttiger BW, Stern DM, Waldherr R, Saeger HD, Ziegler R, Bierhaus A, Martin E, Nawroth PP. Role of NFkappaB in the mortality of sepsis. J Clin Invest 100: 972–985, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brady HR. Leukocyte adhesion molecules: potential targets for therapeutic intervention in kidney diseases. Curr Opin Nephrol Hypertens 2: 171–182, 1993 [PubMed] [Google Scholar]
- 5. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Clark N, Keeble J, Fernandes ES, Starr A, Liang L, Sugden D, de Winter P, Brain SD. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J 21: 2747–3755, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Cunningham PN, Dyanov HM, Park P, Wang J, Newell KA, Quigg RJ. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol 168: 5817–5823, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Cuzzocrea S, Sautebin L, De Sarro G, Costantino G, Rombolà L, Mazzon E, Ialenti A, De Sarro A, Ciliberto G, Di Rosa M, Caputi AP, Thiemermann C. Role of IL-6 in the pleurisy and lung injury caused by carrageenan. J Immunol 163: 5094–5104, 1999 [PubMed] [Google Scholar]
- 9. Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid recptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405: 183–187, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Dustin ML, Springer TA. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol 107: 321–331, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fitzpatrick MM, Shah Filler G V, Dillon MJ, Barratt TM. Neutrophil activation in the hemolytic uremic syndrome: free and complexed elastase in plasma. Pediatr Nephrol 6: 50–53, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Franco-Penteado CF, De Souza IA, Camargo EA, Teixeira SA, Muscara MN, De Nucci G, Antunes E. Mechanisms involved in the enhancement of allergic airways neutrophil influx by permanent C-fiber degeneration in rats. J Pharmacol Exp Ther 313: 440–448, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Han SJ, Ko HM, Choi JH, Seo KH, Lee HS, Choi EK, Choi IW, Lee HK, Im SY. Molecular mechanisms for lipopolysaccharide-induced biphasic activation of nuclear factor-kappaB (NF-kappaB). J Biol Chem 277: 44715–44721, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Helyes Z, Elekes K, Németh J, Pozsgai G, Sándor K, Kereskai L, Börzsei R, Pintér E, Szabó Á, Szolcsányi J. Role of transient receptor potential vanilloid 1 receptors in endotoxin-induced airway inflammation in the mouse. Am J Physiol Lung Cell Mol Physiol 292: L1173–L1181, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Ibbotson GC, Doig C, Kaur J, Gill V, Ostrovsky L, Fairhead T, Kubes P. Functional alpha4-integrin: a newly identified pathway of neutrophil recruitment in critically ill septic patients. Nat Med 7: 465–470, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Jancsó N, Jancsó-Gábor A, Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother 31: 138–151, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 413: 203–210, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Karima R, Matsumoto S, Higashi H, Matsushima K. The molecular pathogenesis of endotoxic shock and organ failure. Mol Med Today 5: 123–132, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Knotek M, Rogachev B, Wang W, Ecder T, Melnikov V, Gengaro PE, Esson M, Edelstein CL, Dinarello CA, Schrier RW. Endotoxemic renal failure in mice: role of tumor necrosis factor independent of inducible nitric oxide synthase. Kidney Int 59: 2243–2249, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Kopp UC, Cicha MZ, Smith LA. Dietary sodium loading increases arterial pressure in afferent renal-denervated rats. Hypertension 42: 968–973, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA 275: 1489–1494, 1996 [PubMed] [Google Scholar]
- 23. Linas SL, Whittenburg D, Parsons PE, Repine JE. Ischemia increases neutrophil retention and worsens acute renal failure: role of oxygen metabolites and ICAM-1. Kidney Int 48: 1584–1591, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Lukacs NW, Hogaboam C, Campbell E, Kunkel SL. Chemokines: function, regulation and alteration of inflammatory responses. Chem Immunol 72: 102–120, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Morris SM, Jr, Billiar TR. New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol Endocrinol Metab 266: E829–E839, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Okada S, Shikata K, Matsuda M, Ogawa D, Usui H, Kidoy Y, Nagase R, Wada J, Shikata Y, Makino H. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes 52: 2586–2593, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med 328: 1471–1477, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Rains JL, Jain SK. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am J Physiol Endocrinol Metab 301: E298–E306, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith CW, Entman ML, Lane CL, Beaudet AL, Ty TI, Youker K, Hawkins HK, Anderson DC. Adherence of neutrophils to canine cardiac myocytes in vitro is dependent on intercellular adhesion molecule-1. J Clin Invest 88: 1216–1223, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith CW, Rothlein R, Hughes BJ, Mariscalco MM, Rudloff HE, Schmalstieg FC, Anderson DC. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest 82: 1746–1756, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Y, Chen AF, Wang DH. ETA receptor blockade prevents renal dysfunction in salt-sensitive hypertension induced by sensory denervation. Am J Physiol Heart Circ Physiol 289: H2005–H2011, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Wang Y, Kaminski NE, Wang DH. VR1-mediated depressor effects during high-salt intake: role of anandamide. Hypertension 46: 986–991, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Novotný M, Quaiserová-Mocko V, Swain GM, Wang DH. TRPV1-meidated protection against endotoxin-induced hypotension and mortality in rats. Am J Physiol Regul Integr Comp Physiol 294: R1517–R1523, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wimalawansa SJ. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev 17: 533–585, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, DiMarzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452–457, 1999 [DOI] [PubMed] [Google Scholar]