Abstract
Although abnormal soluble fms-like tyrosine kinase-1 (sFlt-1) production is thought to be an important factor in the pathogenesis of preeclampsia (PE), the mechanisms that regulate the production of sFlt-1 during PE are unclear. While our laboratory has shown tumor necrosis factor-α (TNF-α) and sFlt-1 to be elevated in pregnant rats in response to placental ischemia, the importance of TNF-α in the regulation of sFlt-1 production is unknown. Therefore, the purpose of this study was to determine the role of TNF-α in mediating the increase in sFlt-1 in response to placental ischemia or hypoxia. Reductions in uterine perfusion pressure in pregnant rats significantly increased plasma levels of sFlt-1 and tended to increase TNF-α, an effect markedly attenuated by pretreatment with a TNF-α inhibitor etanercept (0.4 mg/kg). To further assess chronic interactions between TNF-α and sFlt-1, we examined a chronic effect of TNF-α infusion (50 ng/day) into normal pregnant rats to increase plasma sFlt-1 levels, as well as the effects of acute hypoxia on placental sFlt-1 production in the absence and presence of TNF-α blockade. Placental explants exposed to hypoxic conditions had enhanced TNF-α levels versus normoxic conditions, as well as increased sFlt-1 production. Pretreatment of placental explants with etanercept (15 μM) significantly reduced TNF-α levels in response to hypoxia but did not attenuate sFlt-1 production. These data suggest that while TNF-α may not play an important role in stimulating sFlt-1 production in response to acute hypoxia, a more chronic hypoxia, or placental ischemia may be an important stimulus for enhanced sFlt-l production.
Keywords: preeclampsia, pregnancy, angiogenic imbalance, placenta
preeclampsia (PE), a pregnancy-specific disorder, is characterized by hypertension, proteinuria, decreased placental perfusion with a reduced oxygen delivery to the fetal-placental unit, and an imbalance in anti- and pro-angiogenic factors resulting in overexpression of the anti-angiogenic factor soluble fms-like tyrosine kinase-1 (sFlt-1) (1, 7, 23, 25, 31). sFlt-1 is a member the vascular endothelial growth factor (VEGF) family and is a splice variant of the VEGFR-1 (Flt-1) receptor, which acts as an antagonist of VEGF and placental growth factor (1). Increases in circulating levels of sFlt-1 reduce circulating levels of VEGF and promote endothelial cell dysfunction (5, 21). Although abnormal sFlt-1 production is thought to be an important factor in the pathogenesis of PE, the mechanisms that regulate the production of sFlt-1 during PE are unclear.
PE has been postulated to be an immunologically based disorder (8, 19); therefore, a role for inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), may be important in stimulating the overproduction of sFlt-1 during PE. Data from recent studies demonstrate women with PE to have a twofold increase in placental and plasma sFlt-1 (15, 21, 24, 29, 31) and TNF-α (3, 9, 19) levels compared with normal pregnant (NP) women. In addition, TNF-α (3, 4, 8) and sFlt-1 (27) production are increased in placental explants of preeclamptic patients. Moreover, hypoxia stimulated a twofold increase in TNF-α (4) and sFlt-1 (27) from NP human placental explants. More recent data from our laboratory shows plasma and placental levels of sFlt-1 (11) and TNF-α (18) to be significantly elevated in response to reduced uterine perfusion pressure (RUPP) and TNF-α-induced hypertension in pregnant rats (14). While these data suggest a link between the enhanced production of TNF-α and sFlt-1 in response to placental ischemia and/or hypoxia, the importance of TNF-α in stimulating sFlt-1 production is unknown.
Therefore, the purpose of this study was to test the hypothesis that TNF-α is an important stimulus for increased sFlt-1 production in response to placental ischemia and/or hypoxia. To test this hypothesis, we examined sFlt-1 production in response to placental ischemia in pregnant rats and in response to acute hypoxia in a model of placental villous explants in the absence and presence of TNF-α blockade. Furthermore, we examined the chronic effect of TNF-α infusion (50 ng/day for 5 days) into NP rats on plasma sFlt-1 levels and the direct dose-dependent effects of exogenous TNF-α on sFlt-1 levels from culture media from placental villous explants.
METHODS
All studies were performed in timed-pregnant Sprague-Dawley rats purchased from Harlan (Indianapolis, IN). Animals were housed in a temperature-controlled room (23°C) with a 12:12 light-dark cycle. All experimental procedures executed in this study were in accordance with National Institutes of Health guidelines for the use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi Medical Center.
Model of chronic placental ischemia in pregnant rats (RUPP).
The RUPP model is a well-established model of placental ischemia in pregnant rats and has been described in detail previously (12, 28). Control NP rats underwent a sham operation. Briefly describing the sham operation, on day 14 of gestation a midline incision was made, and the abdominal aorta and ovarian arteries were isolated, followed by closure of the abdominal incision. On day 18 of gestation, RUPP and NP sham animals were treated with a soluble TNF-α receptor etanercept (0.4 mg/kg sc) as previously described by our laboratory (16).
Chronic infusion of TNF-α into normal pregnant rats.
To further assess the chronic interactions between of TNF-α and sFlt-1, we also examined the chronic effect of TNF-α infusion into NP rats on plasma sFlt-1 levels. Recombinant, purified rat TNF-α (BioSource International, Morrisville, NC) was infused via miniosmotic pump into NP rats at a rate of 50 ng/day for 5 days beginning on day 14 of gestation, as previously described by our laboratory (14, 26). This rate of infusion is comparable to the increase in serum TNF-α in our RUPP model of placental ischemia (18).
Measurement of arterial pressure in chronically instrumented, conscious rats.
Arterial pressure was determined in all groups of rats on day 19 of gestation. Pregnant rats were catheterized on day 18 of gestation under a short-acting anesthetic (2% isoflurane) delivered by an anesthesia apparatus. A catheter of V-3 tubing (SCI) was inserted into the carotid artery, tunneled to the back of the neck, and exteriorized for direct monitoring of blood pressure. On day 19 of gestation, pregnant rats were placed in individual restraining cages and blood pressure measurements were recorded continuously for two 20-min periods after 30 min of stabilization using a pressure transducer (Cobe II Transducer CDX Sema). Rats were then anesthetized using isoflurane delivered by an anesthesia apparatus for blood and tissue collection.
Villous explant culture.
Placental villous explants were collected using a protocol previously described by Caniggia et al. (6) that has been modified for rodents. Briefly, on day 19 of gestation, NP rats were euthanized and two placentas per animal, one for control and one for experimental group (i.e., hypoxia or exogenous TNF-α), were collected and washed in ice-cold PBS. The outer membrane was then cut away, the myometrium was isolated from the decidua, and the vascular bundles were excised. The vascular bundle was then plated on a culture plate insert with a pore size of 0.4 μm (Millicell-CM, Millipore, Bedford, MA) precoated with 500 μl Matrigel matrix (BD Biosciences). The explants were cultured in serum-free DMEM/F12 media supplemented with 100 μg/ml streptomyocin, 100 U/ml penicillin, and 0.25 μg/ml ascorbic acid, pH 7.4. Explants were maintained in standard condition at 6% O2-5% CO2-89% N2, or 1% O2-5% CO2-94% N2 within a hypoxia chamber. Culture media was collected after 3, 22, and 30 h and was replaced with 1 ml of fresh media. Tissues were collected after 30 h of culture and stored at −80°C. Placental sFlt-1 and TNF-α measurements were taken at the 22-h time point because significant changes in placental TNF-α and sFlt-1 were noted with no further increase beyond this time point. To determine the role of TNF-α in stimulating sFlt-1 production in response to acute hypoxia, placental explants were cultured in the presence and absence of a soluble TNF-α blocker etanercept (15 μM) in the culture media from the beginning of culture. To determine a direct effect of TNF-α on placental sFlt-1 production, placental explants were cultured in the presence of exogenous TNF-α (0.01, 0.1, 1, and 10 ng/ml).
Determination of serum TNF-α levels and TNF-α in the culture media of placental explants.
TNF-α concentrations were measured using a commercially available ELISA kit. Circulating TNF-α was measured using rat parameter TNF-α ELISA kit from R&D Systems (Quantikine) according the manufacturer's instructions. Because of possible interference from factors released from the matrigel or contained in the culture media, another ELISA kit (cat. no. ER3TNFA, Thermo Scientific) was applied in which the standard curve was developed using culture media.
Determination of plasma sFlt-1 levels and sFlt-1 in the culture media of placental explants.
Circulating sFlt-1 concentrations were measured using a commercial mouse sVEGF R1 (sFlt-1) ELISA kit available from R&D Systems (Quantikine) according to the manufacturer's directions. Placental sFlt-1 concentrations were determined after dilution of samples 1:2 with calibrator diluent.
Statistical analysis.
All data are expressed as means ± SE. Differences between control and experimental groups were analyzed using unpaired t-tests. Data were considered statistically different at P values < 0.05. Multigroup and multifactorial analyses were performed using ANOVA with Tukey's post hoc test.
RESULTS
Effect of TNF-α blockade on plasma TNF-α and sFlt-1 levels in response to placental ischemia in pregnant rats.
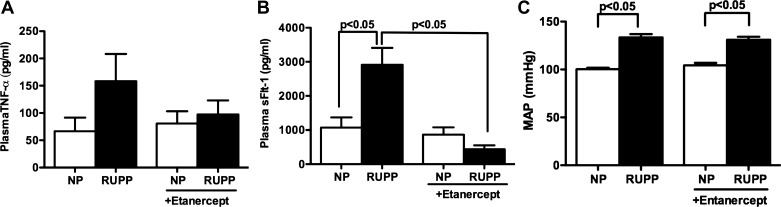
Plasma levels of TNF-α tended to increase in response to placental ischemia in pregnant rats (n = 9) compared with NP (n = 7) rats (158 ± 50 vs. 66 ± 25 pg/ml, P < 0.06) (Fig. 1A). Upon treatment with a soluble TNF-α inhibitor etanercept (0.4 mg/kg), there was a decrease in plasma levels of TNF-α in placental ischemic pregnant rats (97 ± 25 pg/ml, n = 5, not signifcant) compared with control. Figure 1B shows plasma levels of sFlt-1 to be significantly increased in response to RUPP in pregnant rats (2,910 ± 499 pg/ml, n = 7, vs. 1,071 ± 300, n = 6, P < 0.05), an effect that was blocked by TNF-α blockade (437 ± 115 pg/ml, n = 8, P < 0.05). TNF-α inhibition had no effect on circulating sFlt-1 in NP rats (864 ± 213 pg/ml, n = 7, not significant). Mean arterial pressure was significantly increased in response to RUPP in pregnant rats (133 ± 4 mmHg, n = 9, P < 0.05) compared with NP controls (100 ± 1 mmHg, n = 7) (Fig. 1C). Treatment with entancercept had no significant effect on blood pressure in either RUPP (131 ± 3 mmHg, n = 8) or NP rats (104 ± 3 mmHg, n = 5).
Fig. 1.
Effect of etanercept on plasma tumor necrosis factor-α (TNF-α) and soluble fms-like tyrosine kinase-1 (sFlt-1) levels in normal pregnant (NP) and reduced uterine perfusion pressure (RUPP) rats. Treatment with TNF-α inhibitor, etanercept, blunted circulating levels of TNF-α in response to RUPP in pregnant rats (A). Etanercept significantly reduced circulating levels of sFlt-1 in RUPP pregnant rats (B). Mean arterial pressure (MAP) was significantly increased in response to RUPP. Treatment with etanercept had no effect on MAP in either NP or RUPP rats (C). Data were analyzed by ANOVA followed by Tukey's post hoc test and are shown as means ± SE.
Effect of TNF-α blockade on litter size and pup weight in response to placental ischemia in pregnant rats.
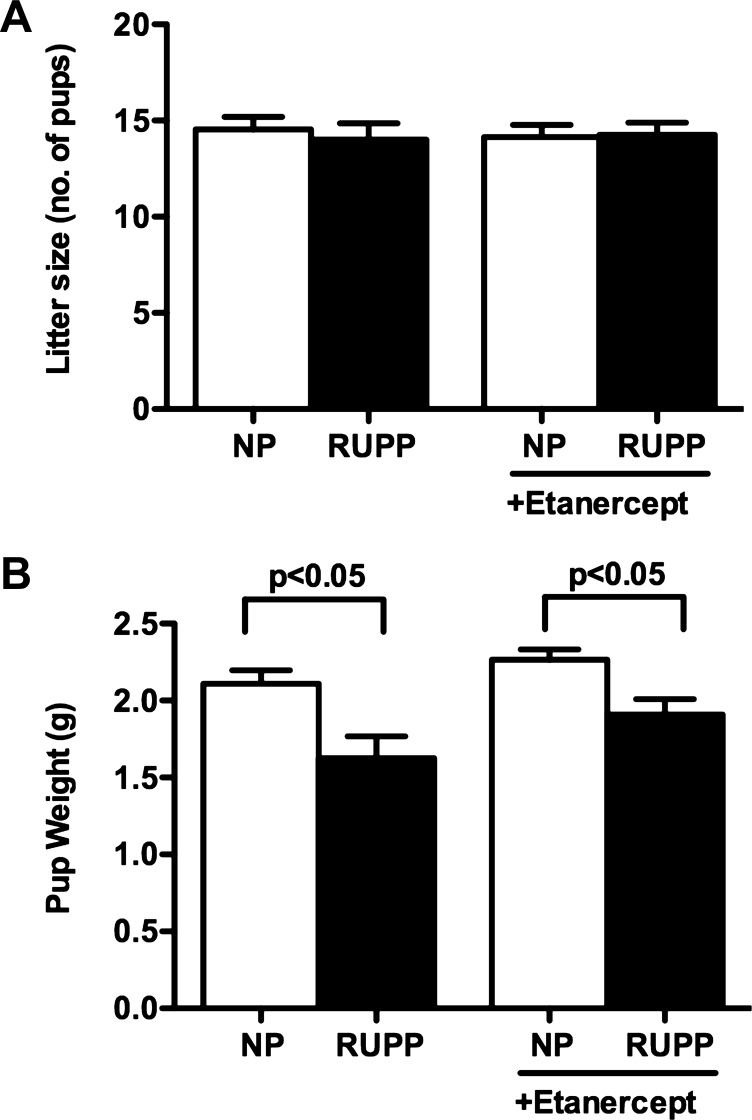
Litter size remained unaffected in response to RUPP (14 ± 1) versus NP (15 ± 1) (Fig. 2A). Treatment with etanercept did not change litter size in either group (14 ± 1 vs. 14 ± 1). However, pup size was significantly reduced in response to RUPP compared with NP rats in both control (1.6 ± 0.1 vs. 2.1 ± 0.08 g, P < 0.05) and entanercept-treated groups (1.9 ± 0.1 vs. 2.3 ± 0.6 g) (Fig. 2B).
Fig. 2.
Effect of etanercept on litter size and pup weights in NP and RUPP pregnant rats. Pup weights (B) were significantly reduced in response to RUPP, whereas litter size (A) remained unaffected. Treatment with etanercept had no effect on litter size or pup weight. Data were analyzed by ANOVA followed by Tukey's post hoc test and are shown as means ± SE.
Plasma sFlt-1 levels and arterial pressure responses in control and TNF-α-treated pregnant rats.
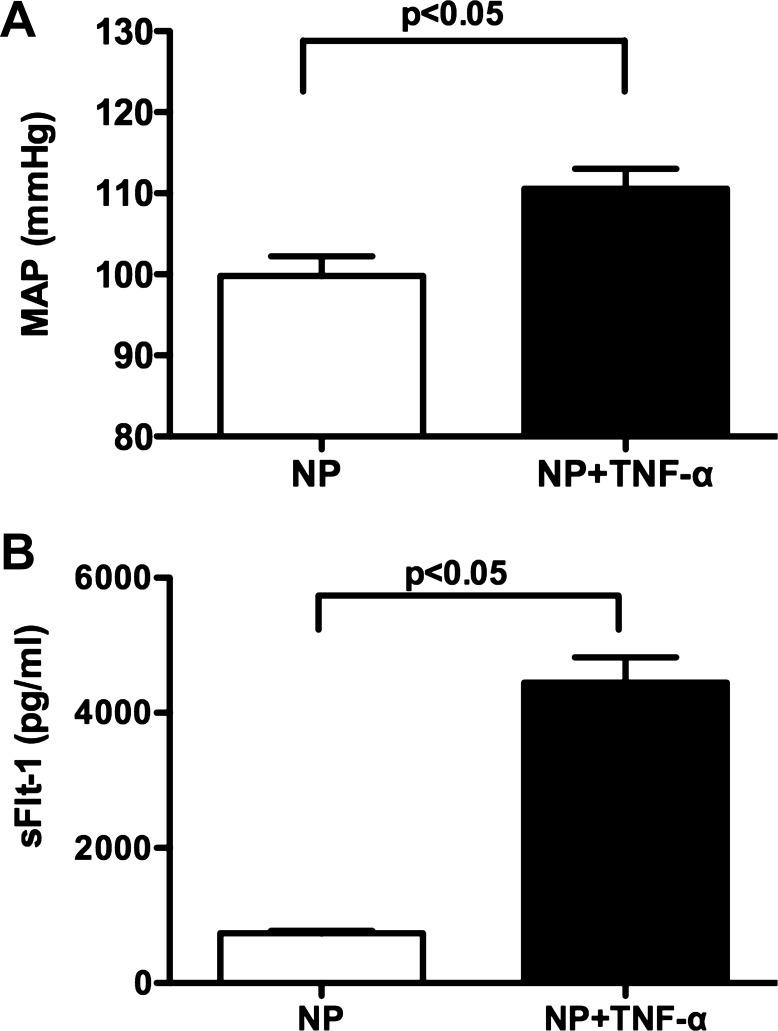
Chronic infusion of TNF-α at a rate of 50 ng/day for 5 days into NP rats significantly increased mean arterial pressure (111 ± 3 mmHg, n = 11, P < 0.05) compared with untreated control rats (99 ± 2 mmHg, n = 5) (Fig. 3A). Associated with the rise in blood pressure, TNF-α infusion increased circulating levels of sFlt-1 from 736 ± 34 (n = 5) to 4,447 ± 37 pg/ml (n = 11, P < 0.05) (Fig. 3B).
Fig. 3.
Effect of TNF-α administration on MAP and plasma sFlt-1 levels in NP rats. MAP increased in response to TNF-α infusion (50 ng/day for 5 days) into NP rats (A). Plasma levels of sFlt-1 increased significantly in TNF-α-induced hypertensive pregnant rats compared with NP rats (P < 0.05) (B). Data were analyzed by an unpaired t-test and are shown as means ± SE.
Effect of TNF-α blockade on sFlt-1 production in normal pregnant explants in response to acute hypoxia.
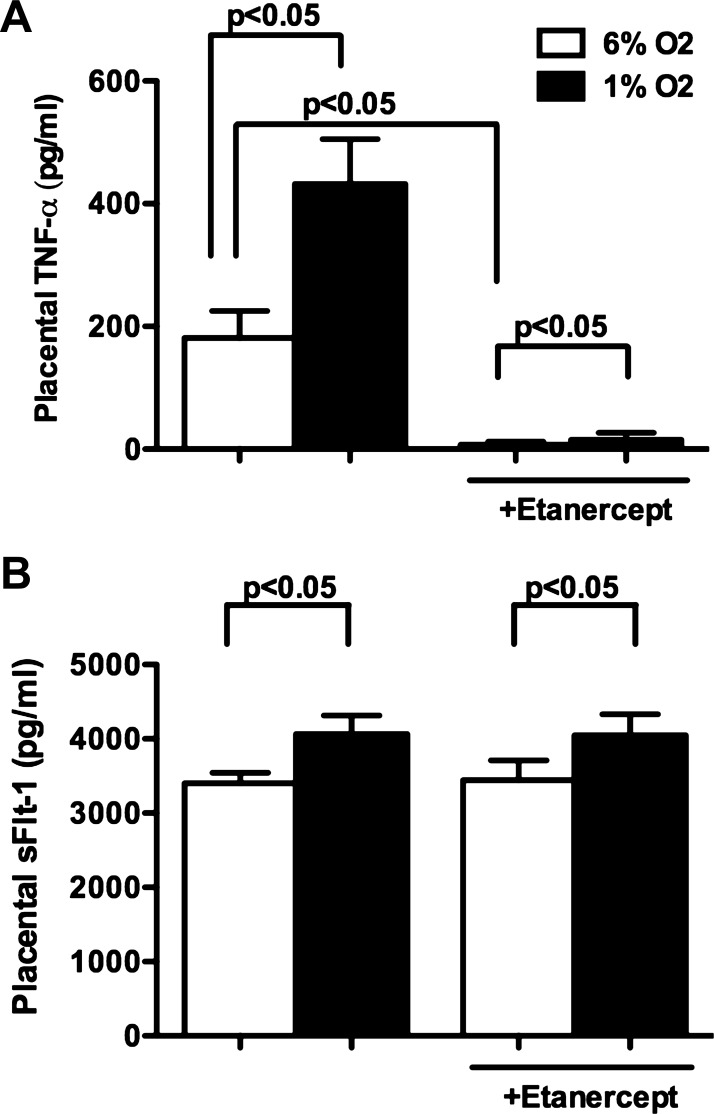
Placental explants from NP rats cultured under hypoxic conditions (1% O2) showed enhanced production of TNF-α (432 ± 73 pg/ml, n = 7) versus normoxic conditions (6% O2) (181 ± 44 pg/ml, n = 6) (Fig. 4A). Associated with the increased TNF-α production, placental sFlt-1 production was significantly increased in response to acute hypoxia (4,065 ± 248, n = 8 vs. 3,402 ± 142 pg/ml, n = 10, P < 0.05). Inhibition of TNF-α with etanercept (15 μM) significantly reduced bioavailable levels of TNF-α in the culture media of NP placental explants in both normoxic (7 ± 5 pg/ml, n = 6, P < 0.05) and acute hypoxic (15 ± 29 pg/ml, n = 7, P < 0.05) conditions compared with untreated controls (Fig. 4A). TNF-α blockade had no effect on sFlt-1 production from NP placental explants under normoxic conditions (3,445 ± 263 pg/ml, n = 9 vs. untreated controls) or in response to acute hypoxia (4,046 ± 289 pg/ml, n = 8 vs. untreated controls) (Fig. 4B).
Fig. 4.
Effect of etanercept on placental production of TNF-α and sFlt-1 from NP placental explants cultured in normoxic (6% O2-5% CO2-89% N2) or hypoxic (1% O2-5% CO2-94% N2) conditions. Placental production of TNF-α (A) and sFlt-1 (B) was significantly increased in NP placental explants in response to acute hypoxia. TNF-α levels were significantly reduced in the culture media of NP placental explants cultured in both normoxic and hypoxic conditions when in the presence of TNF-α blockade. sFlt-1 production was unaltered in the presence of etanercept with in the culture media. Placental sFlt-1 and TNF-α measurements were taken at the 22-h time point because significant changes in placental TNF-α and sFlt-1 were noted with no further increase beyond this time point. Data are analyzed using ANOVA followed by Tukey's post hoc test, and data are expressed as means ± SE.
Effect of exogenous TNF-α on sFlt-1 production in normal pregnant explants.
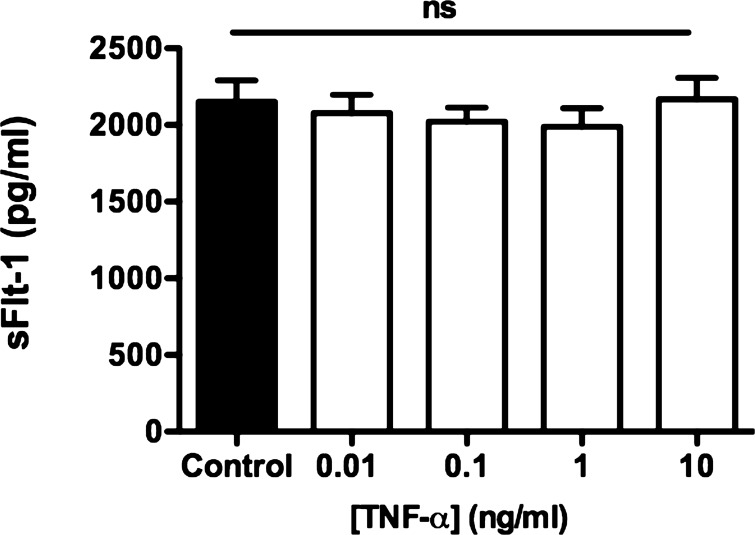
Exogenous administration of TNF-α (0.01, n = 6; 0.1, n = 7; 1, n = 6; 10 ng/ml, n = 10) to the culture media of NP placental explants had no effect on sFlt-1 production after 22 h of culture compared with untreated (n = 7) NP placental explants (2,077 ± 119, 2,020 ± 93, 1,989 ± 119, 2,167 ± 140 vs. 2,151 ± 139 pg/ml) (Fig. 5).
Fig. 5.
Effect of exogenous TNF-α on placental sFlt-1 production. NP placental explants were cultured in the presence of exogenous TNF-α (0.01, 0.1, 1, and 10 ng/ml). sFlt-1 production was unaltered in the presence of exogenous TNF-α under normoxic conditions compared with untreated NP control explants. Data are analyzed using ANOVA followed by Tukey's post hoc test, and data are expressed as means ± SE.
DISCUSSION
PE or hypertension during pregnancy with proteinuria has been associated with the overproduction of anti-angiogenic factors such as sFlt-1 (1, 10, 20). sFlt-1 is produced by circulating monocytes and within the trophoblast layer of the placenta (10). Trophoblasts are an invasive cell type responsible for the remodeling of the maternal vasculature during placentation. Through this remodeling process chorionic villi, the site of fetal-maternal blood exchange, are formed. In PE, there is shallow trophoblast invasion and inadequate vascular remodeling leading to a relatively hypoxic environment and increases in sFlt-1 production (13, 25). However, the stimulus for this enhanced production is unclear at the present time. It has been suggested that PE is an immunologically based disorder (8, 9, 19); therefore, inflammatory cytokines, such as TNF-α, may play an important role in stimulating the overproduction of sFlt-1. Supporting this hypothesis are recent data showing that circulating and placental levels of TNF-α (9, 18) and sFlt-1 (15, 21, 22, 24, 29, 31) are elevated in women with PE and in our model of placental ischemia in pregnant rats. However, whether TNF-α stimulates sFlt-1 production during the disorder remains unclear. Data from the current study is novel in that we wanted to determine whether endogenously formed TNF-α within the placenta plays a role in stimulating sFlt-1 in response to placental ischemia. To test this hypothesis, RUPP pregnant rats were treated with the soluble TNF-α inhibitor etanercept. Similar to what we have previously reported in the RUPP model, plasma levels of sFlt-1 were significantly increased in response to placental ischemia (11, 18). When both NP and RUPP rats were administered etanercept, a soluble TNF-α receptor, sFlt-1 levels were markedly reduced, again suggesting a link between TNF-α and increases in sFlt-1 production.
Another goal of the current study was to determine whether chronic increases in plasma levels of TNF-α could stimulate sFlt-1 production in vivo. To this end, a model of TNF-α-induced hypertension in pregnant rats was utilized, in which TNF-α was infused at a rate to increase plasma levels approximately twofold (14) comparable to what is seen in our RUPP model of placental ischemia in pregnant rats. Utilizing this model of TNF-α induced hypertension in pregnant rats, we found circulating sFlt-1 levels were significantly elevated compared with NP rats. This is consistent with a previous report from our laboratory by Parrish et al. (26), in which he examined the interaction of TNF-α and the AT1-AA on sFlt-1 and soluble endoglin (sEng) production.
However, whether TNF-α had a direct effect to stimulate sFlt-1 production from the placenta remains unclear. Therefore, we examined sFlt-1 production in response to acute hypoxia in a model of placental villous explants in the absence and presence of TNF-α blockade. In response to hypoxia (1% O2), TNF-α and sFlt-1 release into the culture media from NP placental explants was found to be significantly elevated after 22 h of culture. When etanercept was added to the culture media of these explants, TNF-α levels were significantly reduced. This result was surprising to the authors and can only be explained by the fact that the ELISA kit measures only free TNF-α and not TNF-α bound to the soluble receptor inhibitor etanercept. Also surprising was that placental explant sFlt-1 production was not altered in response to acute hypoxia after treatment with etanercept or in response to acute exogenous TNF-α administration. From these data it can be suggested that TNF-α does not directly stimulate sFlt-1 production in vitro after an acute hypoxic event. However, it cannot be discounted that TNF-α may increase sFlt-1 production through an indirect mechanism.
One such mechanism recently proposed is the renin-angiotensin system. While ANG II levels are reduced during PE, women with this disorder have an increased sensitivity to ANG II, increases in the placental production of ANG II (2, 33) and sFlt-1 (25), as well as the presence of an agonistic autoantibody directed at the angiotensin type-1 receptor (AT1-AA) has been identified (30). In addition, Zhou and colleagues (32, 33) have demonstrated that exogenous administration of ANG II and AT1-AA in the culture media of NP placental explants significantly stimulates sFlt-1 production, a response that can be blocked by pretreatment with an AT1 receptor antagonist. In a more recent report from our laboratory, we found a significant increase in AT1-AA and sFlt-1 production in response to TNF-α-induced hypertension in pregnant rats (17, 26). Thus TNF-α-stimulated sFlt-1 production may occur through an indirect mechanism, possibly mediated by the AT1-AA. Alternatively, the direct actions of TNF-α to stimulate sFlt-1 production may be more powerful under chronic conditions. Moreover, tissue sources other than the placenta, such as activated maternal leukocytes and endothelial cells, may also contribute to the increase in sFt-1 levels in response to placental ischemia. However, the quantitative importance of each of these tissues sources remains to be determined.
Perspectives and Significance
While preeclampsia remains the leading cause of maternal death and a major contributor to maternal and perinatal morbidity, the mechanisms underlying the pathogenesis of the disorder remain unclear. Recent data suggests an imbalance of angiogenic factors during gestation may lead to the clinical manifestations of the disorder. However, the mechanisms that lead to this angiogenic balance, weighted toward increased anti-angiogenic factor sFlt-1 and reduced VEGF and placental growth factor, still remain unknown. While previous studies show placental ischemia is associated with increased circulating and placental levels of TNF-α and sFlt-1, the possibility of TNF-α stimulating the enhanced sFlt-1 production during PE remains unclear. Data from the current study shows TNF-α to stimulate sFlt-1 production in response to placental ischemia in pregnant rats, an effect that may be abolished with TNF-α blockade. However, recent studies show a possible role for ANG II and AT1-AA in stimulating sFlt-1 in vitro (32). Therefore, future studies may be directed toward investigating a potential role of the components of the renin-angiotensin system in stimulating sFlt-1 in PE.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-51971.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.R.M. and J.P.G. conception and design of research; S.R.M., M.P., and K.C. performed experiments; S.R.M. analyzed data; S.R.M., B.B.D.L., and J.P.G. interpreted results of experiments; S.R.M. prepared figures; S.R.M. drafted manuscript; S.R.M., B.B.D.L., and J.P.G. edited and revised manuscript; S.R.M. and J.P.G. approved final version of manuscript.
REFERENCES
- 1. Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 95: 884–891, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Anton L, Merrill DC, Neves LA, Stovall K, Gallagher PE, Diz DI, Moorefield C, Gruver C, Ferrario CM, Brosnihan KB. Activation of local chorionic villi angiotensin II levels but not angiotensin (1–7) in preeclampsia. Hypertension 51: 1066–1072, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab 82: 1582–1588, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab 86: 2505–2512, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens 22: 564–568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta 21, Suppl A: S25–S30, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod 59: 1540–1508, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol 37: 240–249, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol 40: 102–11, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Genbacev O, DiFederico E, McMaster M, Fisher SJ. Invasive cytotrophoblast apoptosis in pre-eclampsia. Hum Reprod 14, Suppl 2: 59–66, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 50: 1142–1147, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med 122: 383–392, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Karumanchi SA, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology 145: 4835–4837, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Keiser SD, Veillon EW, Parrish MR, Bennett W, Cockrell K, Fournier L, Granger JP, Martin JN, Jr, Lamarca B. Effects of 17-hydroxyprogesterone on tumor necrosis factor-alpha-induced hypertension during pregnancy. Am J Hypertens 22: 1120–1125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension 46: 1077–1085, 2005 [DOI] [PubMed] [Google Scholar]
- 16. LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension 52: 1161–1167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 52: 1168–1172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 46: 1022–1025, 2005 [DOI] [PubMed] [Google Scholar]
- 19. LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep 9: 480–485, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol 48: 372–386, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Levine RJ, Vatten LJ, Horowitz GL, Qian C, Romundstad PR, Yu KF, Hollenberg AN, Hellevik AI, Asvold BO, Karumanchi SA. Pre-eclampsia, soluble fms-like tyrosine kinase 1, and the risk of reduced thyroid function: nested case-control and population based study. BMJ 339: b4336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int 71: 977–984, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, Taketani Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 145: 4838–4845, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Parrish MR, Murphy SR, Rutland S, Wallace K, Wenzel K, Wallukat G, Keiser S, Ray LF, Dechend R, Martin JN, Granger JP, LaMarca B. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens 23: 911–916, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajakumar A, Powers RW, Hubel CA, Shibata E, von Versen-Hoynck F, Plymire D, Jeyabalan A. Novel soluble Flt-1 isoforms in plasma and cultured placental explants from normotensive pregnant and preeclamptic women. Placenta 30: 25–34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol 293: H2080–H2084, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab 88: 5555–55563, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 103: 945–952, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 24: 147–158, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Zhou CC, Ahmad S, Mi T, Abbasi S, Xia L, Day MC, Ramin SM, Ahmed A, Kellems RE, Xia Y. Autoantibody from women with preeclampsia induces soluble Fms-like tyrosine kinase-1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated T-cells signaling. Hypertension 51: 1010–1019, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou CC, Ahmad S, Mi T, Xia L, Abbasi S, Hewett PW, Sun C, Ahmed A, Kellems RE, Xia Y. Angiotensin II induces soluble fms-Like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ Res 100: 88–95, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]