Abstract
Investigations in the rat model of pregnancy indicate an important role for the corpus luteal (CL) hormone relaxin in the maternal circulatory and osmoregulatory changes in pregnancy, which are epitomized by profound vasodilation and modest hypoosmolality, respectively. In a pilot study of infertile women who became pregnant through donor eggs, in vitro fertilization, and embryo transfer, the gestational rise in glomerular filtration and fall in plasma osmolality were markedly subdued. Because these women were infertile, they lacked a CL and circulating relaxin (and possibly other vasoactive CL hormones). Based on these findings in pregnant rats and women, we hypothesize that infertile women conceiving through donor eggs will have overall subdued circulatory changes (e.g., attenuated reduction in systemic vascular resistance and subdued increase in cardiac output) particularly during early pregnancy when CL hormones predominate before the full development and maturation of the placenta. In contrast, infertile women conceiving by autologous eggs retrieved after ovarian stimulation and fresh embryo transfer may have a relatively hyperdynamic circulation due to the presence of many CL (up to 20 or more) and higher circulating levels of vasodilatory ovarian hormones such as relaxin. Emerging evidence suggests that women undergoing Assisted Reproductive Technologies (ART) have increased risk for adverse pregnancy outcomes such as preeclampsia and small for gestational-age babies. This increased risk may be partly caused by the maternal milieu, which is not physiological in ART pregnancies due to the abnormal status of the CL.
Keywords: cardiovascular, renal, preeclampsia, intrauterine growth restriction, relaxin, in vitro fertilization
assisted reproductive technology (ART) began in 1978 with the birth of Louise Brown, the first baby to be conceived by in vitro fertilization. Since 1978, there have been 3–4 million live births conceived by ART worldwide, and the use of ART has doubled over the past decade (2a). In 2010, there were 154,417 ART cycles performed yielding over 47,000 live births in the United States (2a). In pregnancies achieved through ART, the number of corpora lutea (CL) vary, while during a natural pregnancy, there is typically one CL. In general, there are two types of ART pregnancies: those achieved by using either autologous or donor eggs. Briefly, in the case of autologous eggs, after in vitro fertilization (IVF) and fresh embryo transfer (ET), there are multiple CL secondary to the ovarian stimulation used to enhance follicle numbers. Frozen embryos can be transferred either during a natural or medicated cycle. During a natural cycle, there is typically one CL, while in a medicated cycle involving pituitary suppression, there is no CL. Donor-egg recipients may have ovarian failure or be medicated, and therefore, in either case do not have a CL. To summarize, ART can occur in the setting of nil, one, or multiple CL. Because the CL serves as a major source of reproductive hormones during early pregnancy before establishment of the placenta, we hypothesize that the altered CL status of ART conceptions could impact maternal physiology and pregnancy outcomes.
Indeed, emerging epidemiological evidence suggests that pregnancies conceived by ART may be at increased risk for abnormal pregnancy outcomes including pregnancy-induced hypertension and compromised fetal growth (9, 11, 14, 17, 23, 27, 31). Furthermore, women and their children who suffer from these obstetrical complications may be at higher risk for future adverse cardiovascular events (8, 21, 25). Of perhaps even greater concern is that many of the women who conceive by ART are typically of advanced maternal age, which alone may compromise cardiovascular adaptation during pregnancy. Potential explanations for adverse obstetrical outcomes following ART are 1) multiple gestations, although increased risk is observed with singleton pregnancies, 2) increased maternal age, although increased risk is observed after matching for maternal age, 3) underlying infertility, 4) increased immunological challenge in the case of donor gametes, and 5) iatrogenic reasons related to embryo culture conditions. However, another possible explanation is that other naturally occurring or iatrogenic causes may contribute to adverse pregnancy outcomes, i.e., absence of a CL in donor-egg recipients or excessive number of CL after ovarian stimulation in women receiving autologous eggs.
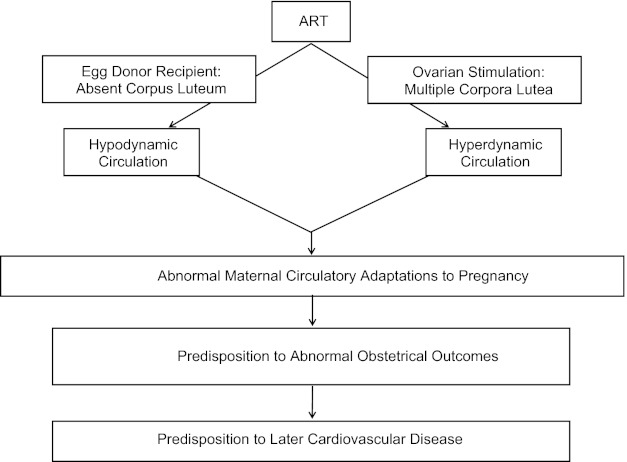
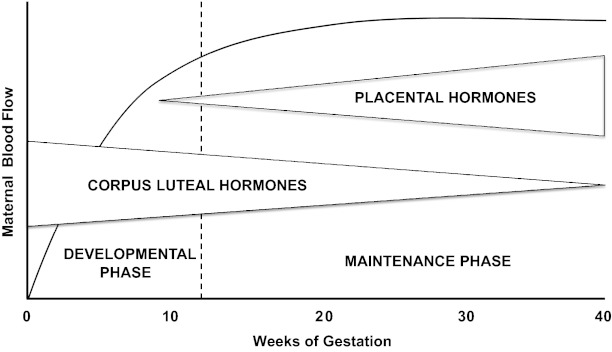
More specifically, we hypothesize that women conceiving by ART manifest abnormal cardiovascular, renal, and plasma volume regulation particularly during early pregnancy that, in turn, predispose to pathological outcomes such as preeclampsia (PE). That is, ART pregnancies begin in a maternal milieu, which is not physiological due to the abnormal status of the CL and its production of hormones including relaxin and sex steroids. Although relaxin is a likely candidate for vasodilatory and angiogenic effects, other CL products besides relaxin may circulate and have similar actions. Absent or excessive circulating levels of these CL factors in the case of donor-egg recipients and ovarian stimulation, respectively, may perturb maternal circulatory adaptations (and decidualization) in pregnancy, thereby precluding optimal placental development and fetal growth. In pregnancies conceived by ART, there is a CL “dose response,” the number of CL ranging from 0 to >20, with CL products such as relaxin being either undetectable or supraphysiological in the blood (24). (Note that progesterone is administered for luteal support in ART cycles including those with absent CLs.) We propose that the absence or excess of CL and CL hormones will result in a relatively “hypodynamic” or “hyperdynamic” maternal circulation, respectively, during early gestation (Fig. 1). As part of an endocrinological continuum beginning in the luteal phase, CL hormones are likely to be major regulators of maternal adaptations in early pregnancy. Thus the concept of the luteal-placental shift pertains not only to the organ source of circulating sex steroids but should be expanded to include maternal cardiovascular, renal, and plasma volume regulation, of which the development and maintenance phases will be mainly dictated by hormones derived from the CL and placenta, respectively, but with overlap during midgestation (Fig. 2).
Fig. 1.
Hypothetical impact of Assisted Reproductive Technologies (ART) on maternal circulatory changes in pregnancy. Both mother and offspring who suffer obstetrical complications such as preeclampsia and fetal growth restriction are predisposed to future cardiovascular disease (see Text).
Fig. 2.
The concept of the luteal-placental shift applied to maternal blood flow in pregnancy (e.g., cardiac output, and renal blood flow among other organ circulations). The vertical dotted line demarcates the end of the 1st trimester.
Optimal maternal cardiovascular, renal, and volume adaptations in early pregnancy are critical to successful pregnancy outcomes (1, 5, 7, 22, 30). Deficits or excesses in these maternal pregnancy adaptations in ART patients are likely to ultimately impinge upon uterine blood flow and consequently fetal and placental growth. During the clinical phase of PE, a picture opposite to normal pregnancy is observed, i.e., hypertension secondary to profound vasoconstriction of maternal organs such as the kidneys. Despite a reciprocal decrease in cardiac output consequent to raised ventricular afterload, arterial pressure is still markedly elevated in PE. Thus a low cardiac output-high arterial resistance state prevails, and plasma volume is contracted during clinical disease (a relatively “hypodynamic” circulation for pregnancy). Interestingly, recent evidence suggests that a low cardiac output-high arterial resistance state during the first trimester precedes the clinical onset of normotensive intrauterine growth restriction (IUGR) or severe early onset PE with IUGR (5, 22, 30). Thus failure or deficiency of maternal vasodilation in early pregnancy is associated with these adverse obstetrical and perinatal outcomes and may predispose or contribute to them. In contrast, the prodromal period of late-onset PE and/or gestational hypertension is characterized by an excessively high cardiac output-low arterial resistance state during the first trimester (a “hyperdynamic circulation”), which then “crosses over” to a vasoconstrictive pattern during the clinical phase (2, 5, 7). Taken together, abnormal maternal circulatory dynamics characterized either by a “hypodynamic” or “hyperdynamic” circulation predates clinical symptoms of major obstetrical disorders, and as such, may play a causal or predisposing role. Insofar as pregnancies achieved by ART may manifest similarly perturbed maternal circulatory dynamics (vide supra), they may be likewise prone to obstetrical pathologies.
Supporting Evidence for an Important Role of the Corpus Luteum in Maternal Adaptations to Pregnancy
Administration of relaxin, a CL hormone, mimics maternal vasodilation of pregnancy.
Gravid rats and women undergo similar maternal circulatory adaptations to pregnancy typified by massive vasodilation, which is mimicked by relaxin administration to nonpregnant rats and women (reviewed in Ref. 4). In addition, many maternal adaptations to pregnancy are anticipated in the luteal phase of the menstrual cycle, albeit of lesser magnitude [(3) and reviewed in Ref. 12]. The pattern of change in circulating relaxin (20, 28) corresponds with the circulatory and osmoregulatory changes in the luteal phase and early pregnancy suggesting the contribution of relaxin to these maternal adaptations.
Relaxin neutralization/elimination from the circulation inhibits maternal vasodilation.
Not only does relaxin administration to nonpregnant rats mimic maternal circulatory and osmoregulatory responses to pregnancy (vide supra), but immunoneutralization/elimination of relaxin inhibits maternal vasodilation, the increase in global arterial compliance (AC) and osmoregulatory changes in midterm pregnant rats [(6, 19), and reviewed in Ref. 4]. Interestingly, in late-pregnant rats, relaxin immunoneutralization only partly inhibits maternal systemic vasodilation and increases in global AC (Debrah DO, Shroff SE, K. P. Conrad; unpublished information), suggesting a vasodilatory role for placental-derived hormone(s) such as PlGF [(18), and citations therein].
Absence of the CL in women impairs renal function changes during pregnancy.
In women conceiving by egg donation, IVF, and ET, we observed a markedly subdued gestational increase in glomerular filtration rate (GFR) (and blunted decline in plasma osmolality) during the first trimester; later time points were not studied (26). One explanation is that these women lack a CL and circulating CL factors like relaxin. Do these deficiencies represent just the “tip of the iceberg?” In fact we do not know, because to our knowledge, there are no reports of other maternal cardiovascular adaptations such as systemic vascular resistance, cardiac output, and global AC in ART pregnancies. Since the women who received donor eggs lack a CL, CL factors such as relaxin are implicated in mediating these physiological adaptations in the first trimester; however, unlike the animal studies in which specific neutralizing antibodies were employed, it was not possible to prove a specific intermediary role for relaxin in this study (26). In fact, residual changes were observed despite the lack of circulating relaxin, suggesting the contribution of other hormones during early pregnancy in women, e.g., human chorionic gonadotropin (hCG) (10), estrogen, and progesterone, or their metabolites. While estrogen has little or no effect on plasma osmolality (15) nor on the renal circulation, progesterone may exert modest renal vasodilation (reviewed in Ref. 12). Estrogen may contribute to the vasodilation of other maternal circulations during early pregnancy (16) if systemic vasodilation is discovered to be partly, but not completely, attenuated in donor-egg recipients.
Low first trimester serum relaxin predisposes to preeclampsia.
Although serum relaxin levels may not be different during disease (29), spontaneously conceiving women with serum relaxin in the lowest quartile (<500 pg/ml) during the first trimester were shown to have an adjusted odds ratio of 7.5 for developing PE (P < 0.02) (13). Although provocative, the results of this pilot investigation need corroboration by a larger study.
Perspectives and Significance
In summary, we advance the concept that maternal cardiovascular, renal, osmoregulatory, and volume adaptations are abnormal during pregnancy in many women conceiving by ART. That is, in women conceiving by ART, pregnancy begins in a state that is not physiological for the mother secondary to abnormal CL status with CL numbers ranging from 0 to >20. In turn, this may be a contributing factor to the increased incidence of obstetrical complications after ART.
Clinical implications for oocyte donation and IVF with autologous oocytes.
In the case of egg donation, IVF and ET, in which circulating relaxin and other CL factors are absent, relaxin or a small molecule mimetic might be included in the hormonal regimen administered for luteal support. In the case of ovarian stimulation, IVF and ET, less ovarian stimulation might be recommended, to reduce the number of CL, and thus, circulating vasodilatory factors like relaxin, which emanate from the CL. The ultimate goal would be to determine whether these potentially corrective strategies would restore normal maternal cardiovascular and volume regulation in ART pregnancies, thereby preventing or reducing the risk of pathological pregnancy outcomes.
GRANTS
This paper was supported by National Institutes of Health Grants PO1 HD-065647, RO1 DK-063321, and RO1 HL-067937.
DISCLOSURES
K. P. Conrad holds patents related to the relaxin.
AUTHOR CONTRIBUTIONS
Author contributions: K.P.C. conception and design of research; K.P.C. prepared figures; K.P.C. drafted manuscript; K.P.C. and V.L.B. edited and revised manuscript; K.P.C. and V.L.B. approved final version of manuscript.
ACKNOWLEDGMENTS
K. P. Conrad gratefully acknowledges the productive collaborations with Lee A. Danielson, John M. Davison, Arun Jeyabalan, Jacqueline Novak, Laura J. Parry, Mark S. Segal, and Sanjeev G. Shroff.
REFERENCES
- 1. Bosio PM, McKenna PJ, Conroy R, O'Herlihy C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol 6: 978–984, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Bosio PM, McKenna PJ, Conroy R, O'Herlihy C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol 6: 978–984, 1999 [DOI] [PubMed] [Google Scholar]
- 2a. Centers for Disease Control and Prevention Assisted Reproductive Technology. 2012. http://www.cdc.gov/ART/index.htm [Google Scholar]
- 3. Chapman AB, Zamudio S, Woodmansee W, Merouani A, Osorio F, Johnson A, Moore LG, Dahms T, Coffin C, Abraham WT, Schrier RW. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol Renal Physiol 273: F777–F782, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Conrad KP. Unveiling the vasodilatory actions and mechanisms of relaxin. Hypertension 56: 2–9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Paco C, Kametas N, Rencoret G, Strobl I, Nicolaides KH. Maternal cardiac output between 11 and 13 weeks of gestation in the prediction of preeclampsia and small for gestational age. Obstet Gynecol 111: 292–300, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Debrah DO, Novak J, Matthews JE, Ramirez RJ, Shroff SG, Conrad KP. Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology 147: 5126–5131, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Easterling TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal hemodynamics in normal and preeclamptic pregnancies: a longitudinal study. Obstet Gynecol 76: 1061–1069, 1990 [PubMed] [Google Scholar]
- 8. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359: 61–73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Helmerhorst FM, Perquin DA, Donker D, Keirse MJ. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ 328: 261, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hermsteiner M, Zoltan DR, Doetsch J, Rascher W, Kuenzel W. Human chorionic gonadotropin dilates uterine and mesenteric resistance arteries in pregnant and nonpregnant rats. Pflügers Arch 439: 186–194, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol 103: 551–563, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Jeyabalan A, Conrad KP. Renal physiology and pathophysiology in pregnancy. In: Renal and Electrolyte Disorders, edited by Schrier RW. Philadelphia, PA: Lippincott Williams & Wilkins, 2010, p. 462–518 [Google Scholar]
- 13. Jeyabalan A, Steward D, McGonigal S, Powers RW, Conrad KP. Low relaxin concentrations in the first trimester are assoicated with increased risk of developing preeclampsia. Reproductive Sci 16, Suppl 3: 101A, 2009 [Google Scholar]
- 14. Keegan DA, Krey LC, Chang HC, Noyes N. Increased risk of pregnancy-induced hypertension in young recipients of donated oocytes. Fertil Steril 87: 776–781, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Lindheimer MD, Barron W, Davison J. Osmoregulation of thirst and vasopressin release in pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol 257: F159–F169, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Magness RR, Phernetton TM, Zheng J. Systemic and uterine blood flow distribution during prolonged infusion of 17β-estradiol. Am J Physiol Heart Circ Physiol 275: H731–H743, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Maman E, Lunenfeld E, Levy A, Vardi H, Potashnik G. Obstetric outcome of singleton pregnancies conceived by in vitro fertilization and ovulation induction compared with those conceived spontaneously. Fertil Steril 70: 240–245, 1998 [DOI] [PubMed] [Google Scholar]
- 18. McGuane JT, Danielson LA, Debrah JE, Rubin JP, Novak J, Conrad KP. Angiogenic growth factors are new and essential players in the sustained relaxin vasodilatory pathway in rodents and humans. Hypertension 57: 1151–1160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Novak J, Danielson LA, Kerchner LJ, Sherwood OD, Ramirez RJ, Moalli PA, Conrad KP. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J Clin Invest 107: 1469–1475, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Byrne EM, Carriere BT, Sorensen L, Segaloff A, Schwabe C, Steinetz BG. Plasma immunoreactive relaxin levels in pregnant and nonpregnant women. J Clin Endocrinol Metab 47: 1106–1110, 1978 [DOI] [PubMed] [Google Scholar]
- 21. Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension 41: 437–445, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 47: 203–208, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Salha O, Sharma V, Dada T, Nugent D, Rutherford AJ, Tomlinson AJ, Philips S, Allgar V, Walker JJ. The influence of donated gametes on the incidence of hypertensive disorders of pregnancy. Hum Reprod 14: 2268–2273, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Sherwood O. Relaxin. In: The Physiology of Reproduction, edited by Knobil ENJ, Greenwald GS, Markert CL, Pfaff DW. New York: Raven, 1994, p. 861–1009 [Google Scholar]
- 25. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 365: 785–799, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Smith MC, Murdoch AP, Danielson LA, Conrad KP, Davison JM. Relaxin has a role in establishing a renal response in pregnancy. Fertil Steril 86: 253–255, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Soderstrom-Anttila V, Tiitinen A, Foudila T, Hovatta O. Obstetric and perinatal outcome after oocyte donation: comparison with in-vitro fertilization pregnancies. Hum Reprod 13: 483–490, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Stewart DR, Celniker AC, Taylor CA, Jr, Cragun JR, Overstreet JW, Lasley BL. Relaxin in the peri-implantation period. J Clin Endocrinol Metab 70: 1771–1773, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Szlachter BN, Quagliarello J, Jewelewicz R, Osathanondh R, Spellacy WN, Weiss G. Relaxin in normal and pathogenic pregnancies. Obstet Gynecol 59: 167–170, 1982 [PubMed] [Google Scholar]
- 30. Vasapollo B, Valensise H, Novelli GP, Altomare F, Galante A, Arduini D. Abnormal maternal cardiac function precedes the clinical manifestation of fetal growth restriction. Ultrasound Obstet Gynecol 24: 23–29, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Weiss G, Goldsmith LT, Sachdev R, Von Hagen S, Lederer K. Elevated first-trimester serum relaxin concentrations in pregnant women following ovarian stimulation predict prematurity risk and preterm delivery. Obstet Gynecol 82: 821–828, 1993 [PubMed] [Google Scholar]