Abstract
Localized exogenous R-tetrahydrobiopterin (R-BH4) corrects the deficit in local heat-induced vasodilation (VD) in hypercholesterolemic (HC) human skin through one of two plausible mechanisms: by serving as an essential cofactor to stabilizing endothelial nitric oxide (NO) synthase (eNOS) or through generalized antioxidant effects. We used the stereoisomer S-BH4, which has the same antioxidant properties but does not function as an essential NOS cofactor, to elucidate the mechanism by which R-BH4 restores cutaneous VD in HC humans. Intradermal microdialysis fibers were placed in 20 normocholesterolemic (NC), 13 midrange cholesterolemic (MC), and 18 HC (LDL: 94 ± 3, 124 ± 3 and 179 ± 6 mg/dl, respectively) men and women to perfuse Ringer (control site) and R-BH4. In 10 NC, 13 MC, and 9 HC subjects (LDL: 94 ± 3, 124 ± 3, 180 ± 10 mg/dl), S-BH4 was perfused at a third microdialysis site. Skin blood flow was measured during a standardized local heating protocol to elicit eNOS-dependent VD. After cutaneous vascular conductance (CVC = LDF/MAP) plateaued, NO-dependent VD was quantified by perfusing NG-nitro-l-arginine methyl ester (l-NAME). Data were normalized as %CVCmax. Fully expressed VD (NC: 97.9 ± 2.3 vs. MC: 85.4 ± 5.4, HC: 79.9 ± 4.2%CVCmax) and the NO-dependent portion (NC: 62.1 ± 3 vs. MC: 45.8 ± 3.9, HC: 35.7 ± 2.8%CVCmax) were reduced in HC (both P < 0.01 vs. NC), but only the fully expressed VD was reduced in MC (P < 0.01 vs. NC). R-BH4 increased the fully expressed (93.9 ± 3.4%CVCmax; P < 0.01) and NO-dependent VD (52.1 ± 5.1%CVCmax; P < 0.01) in HC but not in NC or MC. S-BH4 increased full-expressed VD in HC (P < 0.01) but did not affect NO-dependent VD in HC or MC. In contrast S-BH4 attenuated NO-dependent VD in NC (control: 62.1 ± 3 vs. S-BH4: 41.6 ± 7%CVCmax; P < 0.001). Exogenous R-BH4 restores NO-dependent VD in HC human skin predominantly through NOS coupling mechanisms but increases full expression of the local heating response through generalized antioxidant properties.
Keywords: BH4, cholesterol, NOS coupling, skin blood flow, vascular dysfunction
hypercholesterolemia, specifically elevated low density lipoproteins (LDLs), is a major risk factor for the development of atherosclerotic vascular disease (25, 26). One early indicator of functionally manifested hypercholesterolemia-induced vascular disease is a loss of the vasoprotective molecule nitric oxide (NO) in the microcirculation. Microvascular dysfunction is detectable before the onset of atherosclerotic plaque formation in the conduit arteries and may contribute to the development of conduit artery endothelial dysfunction by inducing retrograde blood flow patterns that could cause damaging shear stress (5). The human cutaneous circulation is an accessible regional circulation to assess microvascular dysfunction and mechanisms contributing to vascular disease with hypercholesterolemia (13–15). Impairments in cutaneous microvascular function are related to end organ damage and indices of vascular function in the coronary and renal vascular beds (5, 19).
We previously showed that cutaneous NO-dependent vasodilation in response to an endothelial NO synthase (eNOS)-specific local skin heating stimulus is attenuated in human subjects with clinically defined hypercholesterolemia (LDL > 160 mg/dl) (13–15). The reduction in NO-dependent vasodilation is mediated by: 1) upregulated arginase activity that limits substrate availability through eNOS (15), 2) an increase in ascorbate-sensitive oxidant stress mechanisms (12), and 3) possibly a reduction in the essential eNOS cofactor R-tetrahydrobiopterin (R-BH4) (11). Ascorbate improves NO bioavailability through several different mechanisms including scavenging radicals that oxidize NO and through stabilizing and resynthesizing R-BH4 from BH2 (21). Furthermore, R-BH4 has both eNOS cofactor properties and generalized antioxidant properties primarily through quenching superoxide (20). It is unclear whether the restoration in cutaneous NO-dependent vasodilation to local heating with localized R-BH4 in hypercholesterolemic humans is due eNOS recoupling mechanisms or generalized antioxidant effects preventing the oxidation of NO.
The purpose of this study was to differentiate how R-BH4 augments NO-dependent vasodilation during local heating of hypercholesterolemic human skin. We used the stereoisomer S-BH4, which has the same antioxidant properties but does not function as an eNOS cofactor (17, 22), to elucidate the mechanism by which R-BH4 restores cutaneous vasodilation in this population. We hypothesized that local administration of R-BH4 restores cutaneous NO-dependent vasodilation during local heating in the skin of hypercholesterolemics via stabilization of eNOS and not through generalized antioxidant mechanisms.
METHODS
Subjects.
All experimental procedures were approved by the Institutional Review Board at The Pennsylvania State University, which follows the guidelines set forth by the Declaration of Helsinki. Twenty healthy normocholesterolemic, thirteen midrange cholesterolemic, and eighteen hypercholesterolemic men and women participated in the study after giving written and oral consent (Table 1). The age of the subjects ranged from 40 to 69 yr old, and the groups (normocholesterolemic, midrange cholesterolemic, and hypercholesterolemic) were age-matched to account for any age-related differences in local heating response. The subjects were normally active and not currently taking statins or other medications including low-dose aspirin, vitamins, antioxidants, or hormone replacement.
Table 1.
Subject characteristics
| Normocholesterolemic | Midrange Cholesterolemic | Hypercholesterolemic | |
|---|---|---|---|
| Number of subjects | 20 | 13 | 18 |
| Age, yr | 53 ± 1.7 | 49 ± 1.6 | 54 ± 1.8 |
| Total cholesterol, mg/dl | 166 ± 4.4 | 194 ± 4.6* | 262 ± 6.6*† |
| HDL, mg/dl | 55 ± 3.0 | 53 ± 3.8 | 53 ± 4.5 |
| LDL, mg/dl | 94 ± 2.5 | 124 ± 3.0* | 179 ± 5.6*† |
| oxLDL, U/l) | 43 ± 2.1 | 59 ± 2.1* | 88 ± 4.2*† |
| Trig, mg/dl | 89 ± 7.6 | 87 ± 11.0 | 154 ± 14.9*† |
| MAP, mmHg | 90 ± 2 | 90 ± 2 | 89 ± 2 |
| Heart rate, beats/min | 62 ± 5 | 67 ± 4 | 67 ± 3 |
Values are means ± SE. HDL, high-density lipoprotein; LDL, low-density lipoprotein; oxLDL, oxidized LDL; Trig, triglyceride; MAP, mean arterial pressure.
P < 0.001 vs. normocholesterolemic subject group;
P < 0.001 vs. midrange cholesterolemic subject group.
During the protocol, subjects were in a thermoneutral laboratory in a semisupine position with their arm positioned at the level of the heart. Four intradermal microdialysis probes were inserted into the dermal layer of the ventral left forearm for local delivery of pharmacological agents (16). Microdialysis sites were perfused with: 1) 20 mM NG-nitro-l-arginine methyl ester (l-NAME) to inhibit NO production via a nonspecific NO synthase inhibition during local heating; 2) 10 mM R-BH4 to locally supplement the essential cofactor for eNOS (Sigma-Aldrich, St. Louis, MO) (14); 3) 10 mM S-BH4, to have the same antioxidant properties of R-BH4 but without the cofactor properties for eNOS (22); and 4) lactated Ringer to serve as control. The S-BH4 site was utilized in 11 of the normocholesterolemic, 13 midrange cholesterolemic, and 9 of the hypercholesterolemic subjects. Pharmacological solutions were mixed in lactated Ringer solution immediately before use and sterilized using syringe microfilters (Acrodisc, Pall, Ann Arbor, MI). Solutions were wrapped immediately in foil to prevent photodegradation.
Skin blood flow was measured using integrated laser-Doppler flowmeter. Local temperature was controlled using a local heater placed directly above each microdialysis membrane (MoorLAB, Temperature Monitor SH02, Moor Instruments, Devon, UK). The laser Doppler probe was secured in the local heater and monitored blood flow over each microdialysis fiber. An automated brachial cuff (Cardiocap) measured arterial blood pressure every 5 min on the right arm. Cutaneous vascular conductance (CVC) was calculated as laser-Doppler flux divided by mean arterial pressure (MAP). Data were normalized as percentage maximal (%CVCmax).
Local heating protocol.
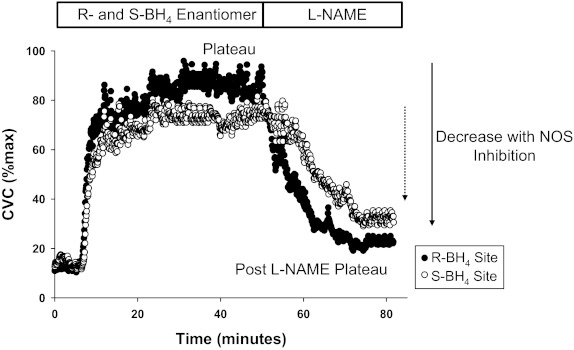
The insertion of the microdialysis fibers results in a transient period of hyperemia. Sixty to ninety minutes were allowed for hyperemia to cease before a standard local heating protocol to induce eNOS-dependent vasodilation (1, 24). Local heater temperature was increased from the baseline clamped temperature of 33°C to 42°C at a rate of 0.1°C every second and then clamped at 42°C for the remainder of the heating protocol. After ∼30–40 min, when skin blood flow reached an established plateau, 20 mM l-NAME was perfused to quantify NO-dependent vasodilation at all sites. The phases of the local heating response include the initial peak and nadir (small NO contribution) followed by a predominately NO-dependent plateau. Figure 1 is a representative tracing of a local heating response in a hypercholesterolemic subject at their R-BH4 and S-BH4 sites, the control site has been omitted for clarity. After infusion of l-NAME and subsequent stabilization of a post-l-NAME plateau in skin blood flow, 28 mM sodium nitroprusside (SNP) was perfused and local temperature increased to 43°C to elicit CVCmax (18).
Fig. 1.
Representative tracing. The time course of cutaneous vascular conductance (%CVCmax) throughout the local heating response in a hypercholesterolemic subject for the R-tetrahydrobiopterin (R-BH4) and S-BH4 sites. The nitric oxide (NO)-dependent plateau and the post-NG-nitro-l-arginine methyl ester (l-NAME) plateau are labeled. The difference between the plateau and the post-l-NAME plateau is the decrease with NO synthase (NOS) inhibition respresented by the arrows for each site. The control site was omited for clarity.
Data and statistical analysis.
Data were collected continuously at 40 Hz and stored for offline analysis with signal-processing software (Windaq, DATAQ Instruments). All skin blood flow data (CVC) were averaged for a stable 5 min of baseline, local heating plateau, post-l-NAME plateau, and maximal vasodilation and normalized to %CVCmax. The NO-dependent vasodilation during local heating was calculated by the difference between the local heating plateau and the post-l-NAME plateau. A three-way mixed model ANOVA was conducted to distinguish differences in %CVCmax between subject groups, local heating phase, and pharmacological treatment sites (SAS, version 9.1). A separate two-way ANOVA was conducted to examine differences between groups and localized pharmacological treatments for the vasodilation due to NO. A priori specific planned comparisons with Bonferroni correction were performed when appropriate to determine where differneces between groups and localized drug treatments existed. The level of significance was set at α = 0.05. Values are presented as means ± SE unless specified otherwise.
RESULTS
Subject characteristics are presented in Table 1. The subject groups differed in total cholesterol, LDLs, and oxidized LDL, by design, but there were no differences among groups for high-density lipoprotein. Otherwise the subjects were well matched for age, body mass index, fasting blood glucose, and MAP.
Figure 1 illustrates time course representative tracings at the R-BH4 and S-BH4 sites for a hypercholesterolemic subject. Full expression of the plateau of local heating-induced vasodilation and the post-l-NAME plateau are labeled. The difference between the plateau and the post-l-NAME plateau is calculated as functional NO-dependent vasodilation (13–15).
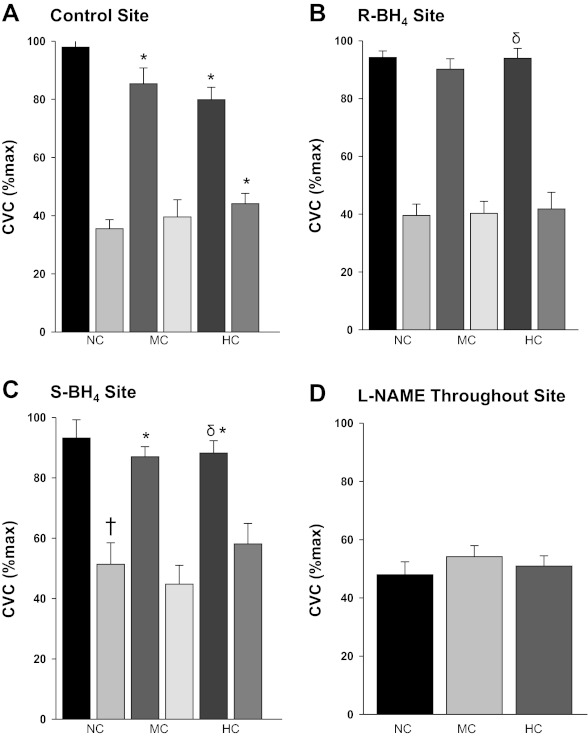
The group means %CVCmax for the local heating-induced plateau and the post-l-NAME plateau are illustrated in Fig. 2. At the control sites full expression of the local heating response was attenuated in the midrange cholesterolemic and hypercholesterolemic groups compared with the normocholesterolemic group (both P < 0.01). The post-l-NAME plateau in the hypercholesterolemic group was elevated compared with the normocholesterolemic group (P <0.05) (Fig. 2A), but there was no difference between the midrange and normocholesterolemic groups. Localized R-BH4 administration increased the local heating plateau in hypercholesterolemic subjects compared with their control site (P < 0.01) (Fig. 2B). S-BH4 augmented the full expression of the local heating plateau in the hypercholesterolemic group compared with their control site (P <0.01), but the post-l-NAME plateau remained elevated. Furthermore, S-BH4 had no effect on the full local heating response in the normocholesterolemic or midrange cholesterolemic group, but the post-l-NAME plateau was elevated compared with the control site in the normocholesterolemic group (Fig. 2C). There was no difference at the l-NAME site throughout the heating protocol among groups (Fig. 2D).
Fig. 2.
Mean skin blood flow responses for the plateau and the post-l-NAME plateau. CVC represented at percent maximum at the plateau in skin blood flow as a result of local heating and after NOS inhibition with l-NAME in normocholesterolemic (NC) control subjects, midrange cholesterolemic (MC) subjects, and hypercholesterolemic (HC) subjects. Each panel illustrates a different localized microdialysis treatment site: control site (A), R-BH4 site (B), S-BH4 site (C), and l-NAME (D) throughout local heating. *P < 0.01 different vs. NC group, †P < 0.05 vs. NC control site.δP < 0.001 vs. HC control site.
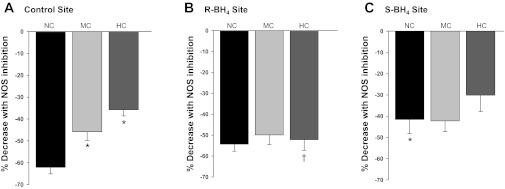
Figure 3 illustrates the decrease in skin blood flow following NOS inhibition within each site for all of the cholesterol groups. NO-dependent vasodilation was attenuated in the hypercholesterolemic and the midrange cholesterolemic group compared with the normocholesterolemic group (P < 0.01). Localized R-BH4 treatment augmented NO-dependent vasodilation in the hypercholesterolemic group (P < 0.01) with no change in the midrange cholesterolemic group. There was no difference in NO-dependent vasodilation with local S-BH4 treatment for the midrange and hypercholesterolemic groups compared with their control sites. In contrast, in the normocholesterolemic group local S-BH4 treatment reduced NO-dependent vasodilation compared with their control sites (P <0.01).
Fig. 3.
Within-site NO-dependent vasodilation during local heating. The decrease in CVC with NOS inhibition in NC control subjects, MC subjects, and HC subjects. Each panel illustrates a different localized microdialysis treatment site: control site (A), R-BH4 site (B), and S-BH4 site (C). *P < 0.001 vs. NC control site, †P = 0.005 vs. HC control site.
Absolute maximal CVC (flux/mmHg) values for each site are presented in Table 2. There were no differences in absolute maximal CVC among localized microdialysis treatment sites or cholesterol groups.
Table 2.
Maximal cutaenous vascular conductance
| Normocholesterolemic | Midrange Cholesterolemic | Hypercholesterolemic | |
|---|---|---|---|
| Control | 1.7 ± 02 | 1.8 ± 0.2 | 1.9 ± 0.3 |
| R-BH4 | 1.5 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.3 |
| S-BH4 | 1.7 ± 0.2 | 2.0 ± 0.3 | 1.5 ± 0.3 |
Values are means ± SE (in AU/mmHg). BH4, tetrahydrobiopterin. Absolute cutaneous vascular conducatance in all microdialysis treatment sites for the normocholesterolemic, midrange cholesterolemic, and hypercholesterolemic groups. There was no significant difference due to localized drug treatment or between groups.
DISCUSSION
The major new finding of this study is that supplementation with the essential NOS cofactor BH4 augments NO-dependent vasodilation during local heating of the skin predominantly by improving NOS coupling mechanisms. Local S-BH4 administration modestly increased full expression of the local heating plateau in hypercholesterolemics but did not improve NO-dependent vasodilation in any of the cholesterols groups, suggesting that the generalized antioxidant effects of BH4 may increase non-NO-dependent mechanisms. These results suggest that the reduced NO bioavailability associated with hypercholesterolemic-induced microvascular dysfunction is mediated in part through a reduction in the eNOS essential cofactor BH4 leading to NOS uncoupling, and localized administration of R-BH4 likely augments NO-dependent vasodilation through recoupling eNOS. We also found that vasodilation during local heating was reduced in a cohort of subjects with moderately elevated LDL cholesterol (midrange), but there was not a detectable difference in NO-dependent vasodilation with localized R-BH4 administration in this group. Finally, a secondary unexpected finding was that S-BH4 treatment increased the post-l-NAME plateau in the normocholesterolemic group and reduced NO-dependent vasodilation, suggesting that supplementing antioxidants to healthy vasculature may decrease functional NO bioavailability.
We have previously demonstrated that the hypercholesterolemia-induced microvascular dysfunction is mediated by a variety of mechanisms contributing to eNOS uncoupling where the eNOS dimer uncouples to produce superoxide instead of functional NO (7). These mechanisms include 1) increased arginase activity, which limits l-arginine availability for eNOS (15); 2) through ascorbate-sensitive oxidant stress mechanisms (13); and 3) through a reduction in the essential eNOS cofactor BH4 (14). In these prior experiments it was not possible to determine whether localized ascorbate or BH4 supplementation improved NO-dependent mechanisms through eNOS coupling mechanisms or through acting as an antioxidant. This was in part because ascorbate is a powerful generalized antioxidant capable of quenching hydroxyl, alkoyxyl, preoxyl, thiol, and tocopheroxyl radicals (6) and stabilizing/resynthesizing BH4 from the salvage pathway (21). Furthermore, BH4 independently possesses both eNOS coupling actions through acting as an essential cofactor and weak generalized antioxidant properties. The antioxidant effects are primarily a result of the rapid reaction of superoxide radical reducing BH4 to BH3 (21), in essence quenching superoxide. At present, there has been no direct examination of the antioxidant capacity between ascorbate and BH4; however, superoxide reacts with BH4 6–10 times faster than it reacts with ascorbate (21). In the present study we utilized the S enantiomer of the functional eNOS cofactor R-BH4. We found that providing a BH4 compound with the same antioxidant properties but without functional cofactor properties did not affect NO-dependent vasodilation in our hypercholesterolemic subjects. This is in contrast to R-BH4, which significantly augmented NO-dependent vasodilation in the hypercholesterolemics. Based on these new data it is likely that localized BH4 supplementation increases functional NO-dependent vasodilation predominantly through acting as an essential cofactor to recouple eNOS.
In this study we designed our experiments to provide equamolar concentrations (10 mM) of both BH4 enantiomers. We observed a positive effect of R-BH4 on NO-dependent vasodilation in our hypercholesterolemic subjects that was not observed with S-BH4. Similar results have also been seen chronic smokers with preexisting endothelial dysfunction (10). However, other studies in humans using similar methodology and relative concentrations of these drugs have found that both enantiomers are equally effective at augmenting endothelium-dependent vasodilation after ischemia-reperfusion injury (22). Together, these results suggest in models of chronic endothelial dysfunction (i.e., smoking, hypercholesterolemia) the principle target BH4 results in increased NO-dependent vasodilation through the reversal of NOS uncoupling, whereas in acute cases of endothelial injury, where there is an increase in superoxide, BH4 may be beneficial as a generalized antioxidant.
In our previous studies examining hypercholesterolemia-induced microvascular dysfunction, we have focused on two distinct, but well-matched groups, to draw comparisons based on different cholesterol concentrations. In this study we have also tested an intermediary “midrange” cholesterol group. Using our standardized local heating protocol to induce eNOS-dependent vasodilation, the midrange cholesterol group demonstrated an attenuated plateau response (Fig. 2A) due to a reduction in NO-dependent vasodilatory mechanisms (Fig. 3A). With R-BH4 administration there was no longer a difference the in plateau of the local heating response (Fig. 2B) or vasodilation due to the production of NO (Fig. 3B); however, there was not a significant improvement in NO-dependent vasodilation within the group after the localized R-BH4 treatment. This is likely because of the small decrement in cutaneous vascular function observed in the midrange subject group. Our study was powered to detect a meaningful physiological difference of 15% CVCmax due to group and localized microdialysis treatment. While we saw group differences in the control site there was no difference within group due to localized R-BH4 treatment.
One unexpected finding in the present study was that localized supplementation of S-BH4 increased NO-independent vasodilatory mechanisms in both hypercholesterolemics and normocholesterolemics, and there was a reduction in functional NO-dependent vasodilation in the normocholesterolemic group. Vascular studies conducted in healthy (i.e., no evidence of endothelial dysfunction) models have shown that supplementing supraphysiological doses of antioxidants can alter redox potential and decrease NO bioavailability (8). Using a similar local heating protocol in young healthy subjects, Stewart et al. (23) have shown that H2O2 can increase NO-independent vasodilatory mechanisms. There is also increasing evidence that ascorbate and BH4 can potentiate endothelium-derived hyperpolarizing factors (EDHF) through H2O2. Because the NO-independent portion of the local heating response is in part mediated by EDHFs (2), it is possible that S-BH4 is increasing EDHFs through altering the redox potential within the vessel. We have observed a similar phenomenon in young healthy human subjects with the local administration of ascorbate (16). However, it remains unclear why we do not observe a similar increase in NO-independent vasodilation with R-BH4 or ascorbate in our healthy middle-aged cohort of subjects.
Perspectives and Significance
In the current study we utilized supraphysiological concentrations of both BH4 enantiomers to examine eNOS coupling mechanisms in humans with hypercholesterolemia. One criticism of these localized infusion studies is the potential for nonspecific effects of the highly concentrated drugs on the vessel wall. In this study we specifically chose a compound (S-BH4) to examine the potential nonspecific antioxidant effects delivered at equimolar concentrations. A different approach that others have used examining the mechanisms of BH4 on hypercholesterolemia-induced microvascular dysfunction is a chronic oral BH4 intervention. Similar to the results from this study with localized administration of BH4, monotherapy with chronic dosing of oral BH4 in hypercholesterolemic humans reverses endothelial dysfunction and decrease systemic markers of oxidant stress (3). While treatment of hypercholesterolemia-induced vascular dysfunction with BH4 appears to be efficacious, it is cost prohibitive and the long-term repercussions are unclear. For example, recent studies in populations with more significant atherosclerotic vascular disease indicate that high doses of oral BH4 oxidize rapidly adversely affecting the BH4-to-BH2 ratio and resulting in increased oxidant stress through inducing eNOS uncoupling (4).
We have explored the effects of an oral atorvastatin intervention on hypercholesterolemia-induced cutaneous microvascular dysfunction. In this series of studies we consistently observed an improvement in NO-dependent vasodilation after 12 wk on an oral atorvastatin intervention, with no further improvements from the local delivery of arginase inhibitors, nonspecific antioxidants (ascorbate), or BH4 (13–15). Furthermore, our biochemical studies support that oral atorvastatin at relatively low clinical doses (10 mg) decreases arginase activity and restored endothelium-dependent vasodilatory function. Because statins have many “pleiotropic” effects unrelated to their direct action of decreasing LDL, including acting as a generalized antioxidant (27) and increasing intracellular BH4 content (9), this multipronged approach to treating hypercholesterolemia-induced vasodilatory dysfunction in this preclinical model appears efficacious. However, the effects of systemic statin therapy on microvascular proconstrictor pathways and atherosclerotic vascular remodeling detectable in the cutaneous circulation remain unclear.
In summary supplementation with the essential NOS cofactor R-BH4 augments NO-dependent vasodilation, and S-BH4 modestly increased NO-independent vasodilation during local heating in subjects with hypercholesterolemia-induced cutaneous microvascular dysfunction. Local administration of the S-BH4 enantiomer, which has the same antioxidant properties as R-BH4 but does not serve as a NOS cofactor, did not improve NO-dependent vasodilation, suggesting that the reduction in NO bioavailability is predominantly mediated through NOS uncoupling mechanisms. Vasodilation to local heating was reduced in a cohort of subjects with moderately elevated LDL cholesterol (midrange), but there was not a detectable difference in NO-dependent vasodilation with localized R-BH4 administration in this group. Finally, the BH4 pathway through the use of an oral intervention or statin therapy is a viable molecular target for the treatment of hypercholesterolemia-induced microvascular dysfunction.
GRANTS
This work has been supported by National Heart, Lung, and Blood Institute Grants R01-HL-089302-05 and M01-RR-10732.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.M.A. and W.L.K. conception and design of research; L.M.A. and J.L.K. performed experiments; L.M.A. and J.L.K. analyzed data; L.M.A. and W.L.K. interpreted results of experiments; L.M.A. and J.L.K. prepared figures; L.M.A. drafted manuscript; L.M.A., J.L.K., and W.L.K. edited and revised manuscript; L.M.A. and J.L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jane Pierzga for technical assistance and help with data collection. We would also thank Caroline Smith, Rebecca Bruning, and Anna Stanhewicz for assistance with data collection.
REFERENCES
- 1. Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol 112: 2019–2026, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 590: 3523–3534, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cosentino F, Hurlimann D, Delli Gatti C, Chenevard R, Blau N, Alp NJ, Channon KM, Eto M, Lerch P, Enseleit F, Ruschitzka F, Volpe M, Luscher TF, Noll G. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolemia. Heart 94: 487–492, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Cunnington C, Van Assche T, Shirodaria C, Kylintireas I, Lindsay AC, Lee JM, Antoniades C, Margaritis M, Lee R, Cerrato R, Crabtree MJ, Francis JM, Sayeed R, Ratnatunga C, Pillai R, Choudhury RP, Neubauer S, Channon KM. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation 125: 1356–1366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Debbabi H, Bonnin P, Ducluzeau PH, Leftheriotis G, Levy BI. Noninvasive assessment of endothelial function in the skin microcirculation. Am J Hypertens 23: 541–546, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Du J, Cullen JJ, Buettner GR. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim Biophys Acta 1826: 443–457, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Garry A, Edwards DH, Fallis IF, Jenkins RL, Griffith TM. Ascorbic acid and tetrahydrobiopterin potentiate the EDHF phenomenon by generating hydrogen peroxide. Cardiovasc Res 84: 218–226, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hattori Y, Nakanishi N, Akimoto K, Yoshida M, Kasai K. HMG-CoA reductase inhibitor increases GTP cyclohydrolase I mRNA and tetrahydrobiopterin in vascular endothelial cells. Arterioscler Thromb Vasc Biol 23: 176–182, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Munzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res 86: E36–E41, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Holowatz LA, Kenney WL. Acute localized administration of tetrahydrobiopterin and chronic systemic atorvastatin treatment restore cutaneous microvascular function in hypercholesterolaemic humans. J Physiol 589: 4787–4797, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holowatz LA, Kenney WL. Oral atorvastatin therapy increases nitric oxide-dependent cutaneous vasodilation in humans by decreasing ascorbate-sensitive oxidants. Am J Physiol Regul Integr Comp Physiol 301: R763–R768, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holowatz LA, Kenney WL. Oral atorvastatin therapy increases nitric oxide-dependent cutaneous vasodilation in humans by decreasing ascorbate-sensitive oxidants. Am J Physiol Regul Integr Comp Physiol 301: R763–R768, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holowatz LA, Kenney WL. Acute localized administration of tetrahydrobiopterin and systemic atrovastatin restores cutaneous microvascular function in hypercholesterolaemic humans. J Physiol 589: 4787–4797, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holowatz LA, Santhanam L, Webb A, Berkowitz DE, Kenney WL. Oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolaemic humans. J Physiol 589: 2093–2103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol Heart Circ Physiol 291: H2965–H2970, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Ihlemann N, Rask-Madsen C, Perner A, Dominguez H, Hermann T, Kober L., Torp-Pedersen C. Tetrahydrobiopterin restores endothelial dysfunction induced by an oral glucose challenge in healthy subjects. Am J Physiol Heart Circ Physiol 285: H875–H882, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Johnson JM, O'Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol 6: 337–346, 1986 [DOI] [PubMed] [Google Scholar]
- 19. Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser Doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 115: 295–300, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Kojima S, Ona S, Iizuka I, Arai T, Mori H, Kubota K. Antioxidative activity of 5,6,7,8-tetrahydrobiopterin and its inhibitory effect on paraquat-induced cell toxicity in cultured rat hepatocytes. Free Radic Res 23: 419–430, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Mayahi L, Heales S, Owen D, Casas JP, Harris J, MacAllister RJ, Hingorani AD. (6R)-5,6,7,8-tetrahydro-L-biopterin and its stereoisomer prevent ischemia reperfusion injury in human forearm. Arterioscler Thromb Vasc Biol 27: 1334–1339, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Medow MS, Bamji N, Clarke D, Ocon AJ, Stewart JM. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J Appl Physiol 111: 20–26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Toshima S, Hasegawa A, Kurabayashi M, Itabe H, Takano T, Sugano J, Shimamura K, Kimura J, Michishita I, Suzuki T, Nagai R. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler Thromb Vasc Biol 20: 2243–2247, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Vasankari T, Ahotupa M, Toikka J, Mikkola J, Irjala K, Pasanen P, Neuvonen K, Raitakari O, Viikari J. Oxidized LDL and thickness of carotid intima-media are associated with coronary atherosclerosis in middle-aged men: lower levels of oxidized LDL with statin therapy. Atherosclerosis 155: 403–412, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Wassmann S, Laufs U, Muller K, Konkol C, Ahlbory K, Baumer AT, Linz W, Bohm M, Nickenig G. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol 22: 300–305, 2002 [DOI] [PubMed] [Google Scholar]