Abstract
Maternal separation (MatSep) is a model of behavioral stress during early life. We reported that MatSep exacerbates ANG II-induced hypertension in adult male rats. The aims of this study were to determine whether exposure to MatSep in female rats sensitizes blood pressure to ANG II infusion similar to male MatSep rats and to elucidate renal mechanisms involved in the response in MatSep rats. Wistar Kyoto (WKY) pups were exposed to MatSep 3 h/day from days 2 to 14, while control rats remained with their mothers. ANG II-induced mean arterial pressure (MAP; telemetry) was enhanced in female MatSep rats compared with control female rats but delayed compared with male MatSep rats. Creatinine clearance (Ccr) was reduced in male MatSep rats compared with control rats at baseline and after ANG II infusion. ANG II infusion significantly increased T cells in the renal cortex and greater histological damage in the interstitial arteries of male MatSep rats compared with control male rats. Plasma testosterone was greater and estradiol was lower in male MatSep rats compared with control rats with ANG II infusion. ANG II infusion failed to increase blood pressure in orchidectomized male MatSep and control rats. Female MatSep and control rats had similar Ccr, histological renal analysis, and sex hormones at baseline and after ANG II infusion. These data indicate that during ANG II-induced hypertension, MatSep sensitizes the renal phenotype in male but not female rats.
Keywords: maternal separation, sex hormones, renal damage, orchidectomy
maternal separation (MatSep) is an established model of chronic early life stress in rodents that induces permanent phenotypic changes, especially in response to secondary stressors in adulthood (21–23, 33). Numerous studies show a sexual dimorphism in adult MatSep rats related to behavioral and/or neurological outcomes. For instance, environmental stress models, such as early handling and prenatal stress, have revealed that male rats are more susceptible to develop anxiety and exacerbated acute stress responses than female rats (8, 36, 55). Yet, several studies have reported that female rats exposed to MatSep distinctively display greater emotional reactivity to novel stressors (41), higher levels of corticosterone, and ACTH (46), greater hypercapnic respiratory response (7), and depressive-like behavior (32), compared with male MatSep rats. Thus, MatSep may influence distinct mechanistic pathways in a sex-specific manner. Conversely, discrepancies in the literature exist regarding outcomes of MatSep, which may be due to the characteristics of the stressor used, strain of animal, and/or duration of the MatSep protocol.
Men are at greater risk for cardiovascular and renal disease than age-matched premenopausal women (17, 56). Furthermore, sex-related differences have been reported in the current treatment of cardiovascular disease, in which women display worse outcomes than men (49). In animal models, ANG II-infused male rats show exacerbated hypertension that correlates with greater vascular and renal damage (20, 30, 38, 59). Sex steroids modulate the expression and activity of the various components of the renin-angiotensin-aldosterone sytem (RAAS), especially in the kidney (12, 18, 34, 48), thus playing an important role in the mechanism underlying hypertension in males. Androgens, specifically testosterone, contribute to the progression of hypertension and renal injury in Wistar-Kyoto (WKY) rats, spontaneously hypertensive rat (SHR) and Dahl salt-sensitive rat strains (14, 15, 51, 58, 59). A large body of evidence suggests that androgens influence the development of hypertension via direct actions within the kidney. Ganten et al. (10) have shown that surgical or chemical castration of SHR resulted in a marked reduction in hypertension development. Similarly, it has been shown that testosterone is essential for the full development of hypertension in SHR (40). Coffman's laboratory (2–4), as well as others (12, 37), demonstrated the critical role of the kidney in ANG II-induced hypertension, especially related to the role of inflammation. It is well accepted that a predisposition to renal dysfunction contributes to the pathogenesis of hypertension (3, 12, 13).
Accordingly, we designed experiments to test whether MatSep enhances ANG II-induced hypertension in female rats similar to that in male rats. We found that MatSep in female rats also sensitizes these rats to ANG II-induced hypertension. Thus, renal functional and structural outcomes in the MatSep model were determined to address whether there is a sex difference in the renal mechanisms of blood pressure control developed by the exposure to early life stress. We also determined the status of plasma levels of testosterone and estradiol in male and female, control, and MatSep rats at baseline and in response to ANG II. MatSep in male rats revealed a distinct profile of sex hormones at baseline and in response to ANG II; therefore, we also performed experiments in orchidectomized rats to study the role of sex hormones as a potential mechanism contributing to blood pressure sensitivity in male rats exposed to MatSep.
METHODS
Early life stress: maternal separation protocol.
Maternal separation (MatSep) was performed as previously described using offspring from WKY breeders (24). All experiments were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals, and were approved and monitored by the Georgia Health Sciences University Institutional Animal Care and Use Committee. Approximately half of the male and female pups from each litter were removed from their mother's cage, and their tails were snipped and cauterized with silver nitrate for identification as “MatSep”. From postnatal days 2 to 14 of life, MatSep pups were separated from their mothers and littermates, at the same time of day by transferring the pups to a clean cage in an incubator (30 ± 1°C) for 3 h. Groups exposed to MatSep comprised rats from at least three different litters. The control group animals were the nonhandled counterparts that remained with their mother. Weaning was performed at postnatal day 28, and experiments were conducted with adult rats (12–14 wk old).
Osmotic minipump and telemetry transmitter implantation.
Rats were implanted with telemetry transmitters at 10 wk old (Data Sciences, St. Paul, MN), as previously described (22). Mean arterial pressure (MAP) and heart rate (HR) were continuously recorded throughout the study using the Dataquest ART Acquisition program (Data Sciences International). After recovery (10 days) and a baseline period (7 days), rats were anesthetized with isoflurane (2% in O2 at 1 l/min) and shaved in the interscapular region. Osmotic minipumps with ANG II (65 ng/min, model 2002 for 14-day infusion; Alzet, Palo Alto, CA) were implanted subcutaneously under sterile conditions, according to the manufacturer's instructions.
Metabolic cages.
Rats were placed in metabolic cages for determination of water and food intake and urine excretion. After 2 days of acclimation, 24-h collections were taken at baseline and day 14 of ANG II infusion. Blood sampling (500 μl) was obtained from tail snipping for creatinine determination following urine collection.
Creatinine clearance and proteinuria.
Creatinine concentration in plasma and urine was measured by the kinetic Jaffé method using a 10% picric acid solution in NaOH and reading after 15 min in a microplate set for dual wavelengths at 490 nm (read) and 620 nm (reference). Creatinine clearance (Ccr) was calculated using the following formula: Ccr (ml/min) = (urine creatinine/plasma creatinine) × urine flow rate.
Urinary protein excretion was determined by a colorimetric assay using the Quick Start Bradford Dye Reagent 1× (Bio-Rad, Hercules, CA). After 5 min of incubation, sample reading was performed in a microplate at 595 nm. The measurements were related to the 24-h urine volume and expressed as mg protein/day.
Renal histopathology.
Separate groups of rats were used for baseline and after 14 days of ANG II infusion for renal histological analysis. Kidney sections were stained with periodic acid-Schiff, as well as hematoxylin and eosin for scoring of renal damage (43). To assess the renal damage score, we assigned a semiquantitative grade of severity (0, 1, 2, or 3, indicating that the feature was absent, mild, moderate, or severe, respectively) to each of the following morphological attributes: thickening, necrosis, thrombosis, and hyalinosis of interstitial arteries; tuft necrosis, hyalinosis, and thrombosis of glomerular capillaries; and necrosis and thrombosis of glomerular arterioles and tubulointerstitial fibrosis. Thirty randomly selected nonoverlapping fields of renal cortical interstitium and glomeruli were examined at ×200 and ×400 magnification, respectively, and the means of the values were calculated for each sample. In a subset of male and female ANG II-infused rats, kidneys were perfusion-fixed and prepared, as described previously to assess CD68-positive macrophage infiltration (ED-1; Serotec, Kidlington, Oxford, UK) and CD3 for T-cell infiltration (43, 51). To quantify the interstitial infiltration of T cells and macrophages, the appropriate software (DPController; Olympus Optical, Tokyo, Japan) was used to convert the image, and a 500-μm × 500-μm grid was superimposed onto the image at ×40 magnification. Twenty grids from each slide were viewed, and positive cells were counted. Stained sections were viewed with an Olympus BX40 microscope (Olympus America) on a bright-field setting fitted with a digital camera (Olympus DP70, Olympus America). All of the measurements were made in a blinded fashion.
Sex hormones in plasma.
Separate groups of rats were used for baseline and after 14 days of ANG II infusion for plasma analysis. Testosterone (EIA, Cayman, Ann Arbor, MI) and estradiol (RIA, Beckman Coutler, Brea, CA) were measured in plasma stored at −80°C from male and females during baseline and after ANG II infusion.
Orchidectomy.
At 9 wk of age, male rats were castrated under anesthesia (isofluorane 2% by inhalation) using aseptic techniques as previously described (50). Orchidectomized rats recovered for up to 3 wk, and chronic ANG II infusion was performed, as described above. In a separate group of male rats, surgical castration was conducted following telemetry device implantation. A group of intact male rats were used as controls.
Statistical analysis.
Telemetry data are presented as means ± SE value of 24 h. For statistical analysis, 72 time points for every 24 h (10-s average value every 10 min) for each rat were computed. For blood pressure, comparisons between male and females were made by repeated-measures two-way ANOVA followed by a Bonferroni post hoc test. For single time point measurements, comparisons between male and females were made by two-way ANOVA followed by a Bonferroni post hoc test. Comparisons between same-sex control and MatSep measurements were by unpaired Student's t-test. A value of P < 0.05 was considered statistically significant. All statistical analyses were conducted using Prism 5.01 (GraphPad Software, 2007).
RESULTS
Metabolic parameters.
Food and water intake, urinary flow rate, body weight, and kidney weight were similar within male and female groups at baseline (Table 1). Food intake was unchanged by ANG II infusion in male and female control and MatSep rats (Table 2). However, water intake and excretion were similarly increased in male and female, control, and MatSep rats in response to ANG II (P < 0.05) compared with baseline conditions (Table 2).
Table 1.
Body weight, kidney weight, and metabolic parameters at baseline in male and female adult rats
| Baseline |
||||
|---|---|---|---|---|
| Male |
Female |
|||
| Control | MatSep | Control | MatSep | |
| Body weight, g | 284 ± 7 | 289 ± 9 | 214 ± 5¥ | 226 ± 5¥ |
| Kidney weight, g | 2.03 ± 0.05 | 1.96 ± 0.07 | 1.46 ± 0.05¥ | 1.45 ± 0.06¥ |
| Food intake, g | 18.4 ± 0.5 | 17.5 ± 1.0 | 16.8 ± 0.6 | 16.9 ± 0.5 |
| Water intake, ml | 34.2 ± 3.0 | 36.3 ± 3.7 | 25.1 ± 1.3¥ | 26.1 ± 1.6¥ |
| Urine flow rate, ml/day | 14.4 ± 1.6 | 12.9 ± 0.6 | 12.1 ± 0.4 | 13.8 ± 1.7 |
| Protein excretion, mg/day | 13.6 ± 0.7 | 18.2 ± 0.4* | 4.3 ± 0.3¥ | 3.7 ± 0.5¥ |
Values are expressed as means ± SE; n =6–8/group, 12–14 wk old. BW, body weight; KW, kidney weight; UV, urinary volume.
P < 0.05 vs. control,
P < 0.05 vs. male rats of same group.
Table 2.
Body weight, kidney weight, and metabolic parameters in ANG II-infused male and female adult rats
| ANG II |
||||
|---|---|---|---|---|
| Male |
Female |
|||
| Control | MatSep | Control | MatSep | |
| Body weight, g | 300 ± 7 | 286 ± 6 | 210 ± 6¥ | 222 ± 4¥ |
| Kidney weight, g | 1.93 ± 0.08 | 2.04 ± 0.06 | 1.41 ± 0.10¥ | 1.43 ± 0.1¥ |
| Food intake, g | 17.8 ± 0.9 | 18.4 ± 1.4 | 17.4 ± 1.4 | 15.9 ± 0.9 |
| Water intake, ml | 63.0 ± 7.8 | 75.0 ± 6.4 | 101.0 ± 3.0¥ | 89.5 ± 3.7¥ |
| Urine flow rate, ml/day | 40.4 ± 3.2 | 44.2 ± 3.3 | 54.3 ± 1.7¥ | 52.1 ± 2.7¥ |
| Protein excretion, mg/day | 41.2 ± 4.4 | 48.7 ± 5.1 | 60.3 ± 2.3¥ | 70.0 ± 10.1¥ |
Values are expressed as means ± SE; n =6–8/group, 12–14 wk old. BW, body weight; KW, kidney weight; UV, urinary volume.
P < 0.05 vs. male rats of same group.
Cardiovascular function.
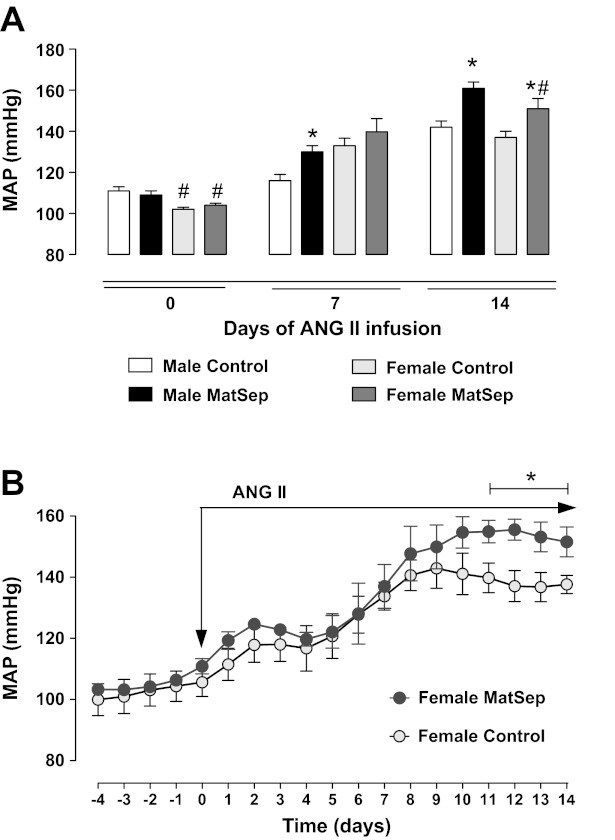
As we have reported previously (22), MatSep does not influence baseline blood pressure in male rats, yet MatSep significantly enhances ANG II-induced hypertension compared with male control rats (Fig. 1A). Female rats exposed to MatSep also have similar baseline MAP to control rats (Fig. 1B), and when infused chronically with ANG II, they displayed an exaggerated hypertension compared with control female rats given ANG II, specifically in days 11 through 14 (Fig. 1B). The increase in MAP to ANG II infusion in either the control rats or the MatSep rats was similar between the males and females, and the absolute MAP from day 0 to day 14 remained lower in the females (Fig. 1, A and B). Although, ANG II-induced hypertension in the female MatSep rats was temporally delayed compared with male MatSep rats (Fig. 1, A and B).
Fig. 1.
Effect of chronic infusion of ANG II in MAP of control and MatSep female rats. A: ANG II-induced hypertension is delayed and attenuated in female MatSep rats compared with control rats. B: changes in MAP in response to ANG II infusion is greater in female MatSep rats (n = 7) than control female rats (n = 6) in the last 4 days of the infusion period (65 ng/day sc). *P < 0.05 vs. corresponding sex control group, #P < 0.05 vs. corresponding male group.
At baseline, HR was similar in female control and MatSep rats (371 ± 7 and 376 ± 10 bpm, respectively) and male control and MatSep rats (364 ± 8 and 372 ± 7 bpm, respectively). ANG II infusion increased HR similarly in female control and MatSep rats (day 14: 412 ± 17 and 418 ± 16 bpm, P < 0.05). Conversely, ANG II infusion induced differences between male control and MatSep (day 14: 309 ± 16 and 415 ± 11 bpm, P < 0.05), as previously reported (24). Left ventricle/heart weight ratio was similar after ANG II infusion (day 14) in male control and MatSep (0.83 ± 0.01 and 0.81 ± 0.01, respectively) and female control and MatSep rats (0.78 ± 0.01 and 0.79 ± 0.01, respectively).
Renal function.
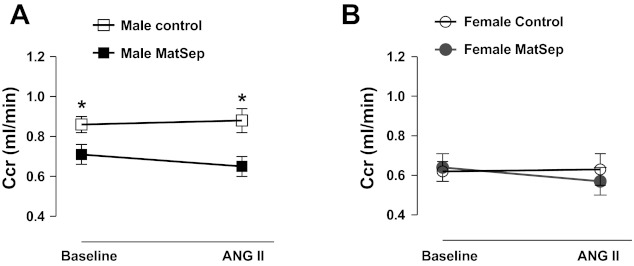
Measurement of 24-h Ccr showed a significant reduction in male MatSep rats at baseline conditions compared with male control rats. This reduction in Ccr was not significantly worsened in male ANG II-infused rats (Fig. 2A). However, female control and MatSep rats displayed similar Ccr either at baseline or after ANG II infusion (Fig. 2B).
Fig. 2.
Changes in creatinine clearance (CCr) as a parameter of renal function at baseline and after 2 wk of ANG II infusion. A: CCr in male MatSep rats was reduced at baseline. ANG II infusion did not aggravate CCr in MatSep rats significantly. B: female MatSep rats displayed similar CCr than control rats. *P < 0.05 vs. male control group.
At baseline, urinary protein excretion was increased in male MatSep rats compared with male control rats (P < 0.05). In female control and MatSep rats, protein excretion was similar although significantly lower compared with male rats. In response to ANG II, urinary protein excretion was similarly increased in male and female rats from both control and MatSep groups (Table 2).
Renal histopathology analysis.
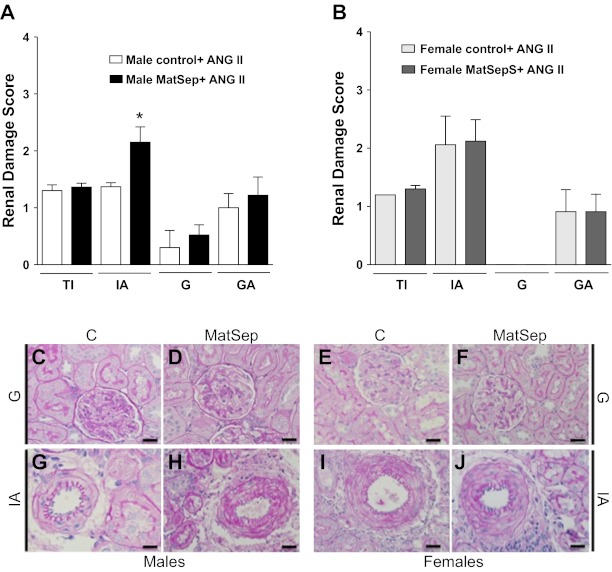
No significant renal damage was observed under baseline conditions in either male or female, control, and MatSep rats (data not shown). In response to chronic ANG II infusion, male MatSep rats displayed a renal damage score in the mild to moderate range, specifically in renal interstitial arteries (Fig. 3A). Mild to absent renal damage was observed in tubules and glomeruli in male control and MatSep rats infused with ANG II. Female control and MatSep rats given ANG II showed a moderate renal damage score in interstitial arteries (Fig. 3B). Renal damage score in tubulointerstitial and glomerular arteries and glomeruli were mild to absent in all groups (Fig. 3, A and B).
Fig. 3.
Renal damage scores in ANG II-infused rats. A: damage in interstitial arteries was exacerbated in male MatSep rats compared with control rats in response to ANG II infusion. B: renal damage in response to ANG II was similar in control and female MatSep rats. Lower panel pictures show representative pictures of glomerulus in male control (C) and MatSep (D) and female control (E), and MatSep (F) rats; interstitial artery in male control (G) and MatSep (H) and female control (I) and MatSep (J) rats. *P < 0.05. TI, tubulointerstitium; IA, interstitial arteries; G, glomerulus; GA, glomerular arteriolae. Score: 0, nil; 1, mild; 2 moderate; 3 severe. Scale bar = 25 μM.
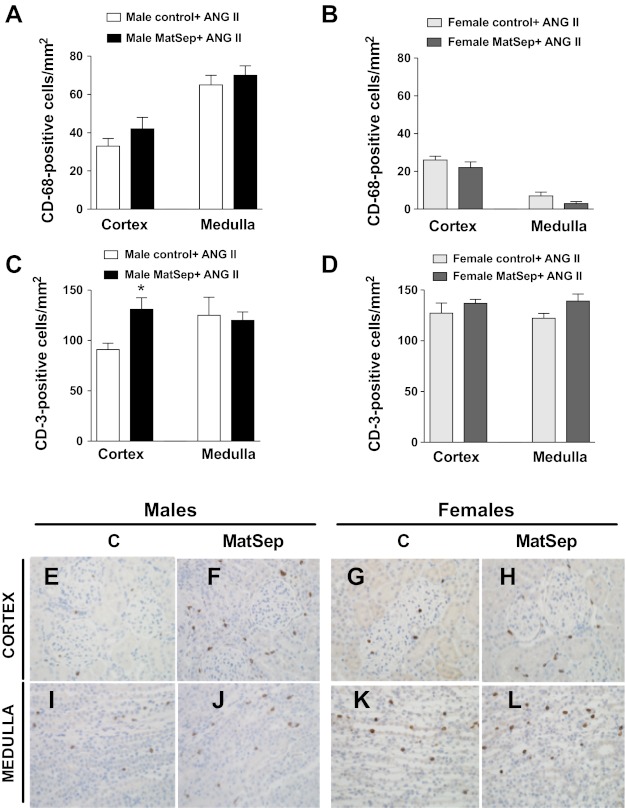
The number of renal inflammatory cells was determined in male and female, MatSep, and control rats infused with ANG II. The number of monocytes/macrophages (CD68-positive cells) was similar in male control and MatSep rats in the renal medulla and cortex (Fig. 4A). Female control and MatSep rats showed similar CD68-positive cells in renal cortex and medulla; however, they were significantly reduced compared with male rats (Fig. 4B). Male MatSep rats displayed a greater number of CD-positive cells (general marker of T cells) in the renal cortex compared with male control rats, while CD3-positive cells were unchanged in renal medulla (Fig. 4C). Female control and MatSep rats showed similar numbers of CD3-positive cells in renal cortex and medulla (Fig. 4D).
Fig. 4.
Immune cell infiltration in renal medulla and cortex of ANG II-infused rats. A: numbers of CD-68- (macrophages) positive cells were similar in medulla and cortex from control and male MatSep rats. C: numbers of CD-3- (lymphocytes) positive cells were similar in medulla from control and male MatSep rats. CD-3 cells were increased in renal cortex from male MatSep rats compared with control. B and D: cortical and medullary CD-68- and CD-3 positive cells in medulla and cortex from control and female MatSep rats. Representative kidney sections of males (E, F, I, and J) and females (G, H, K, and L). *P < 0.05 vs. male control group.
Plasma levels of sex hormones.
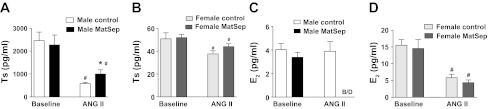
At baseline conditions, MatSep did not influence testosterone or estradiol levels in either male or female adult rats although, as expected, the levels were significantly different between the sexes (Fig. 5, A and B). Chronic ANG II infusion significantly reduced sex hormone levels in male and female rats (P < 0.05). ANG II-infused male MatSep rats displayed reduced testosterone levels compared with control rats (Fig. 5A). Female control and MatSep rats showed a similar reduction in testosterone levels (Fig. 5B). ANG II-infused male MatSep rats displayed a dramatic decrease (to levels below detection) in the estradiol levels (Fig. 5C). ANG II reduced sex hormone levels in female control and MatSep rats similarly (Fig. 5, B and D).
Fig. 5.
Plasma sex hormone levels in ANG II-infused rats. ANG II infusion significantly reduces Ts levels compared with baseline; however, male MatSep rats presented significantly elevated Ts levels compared with control MatSep rats (A). E2 levels were below detection in male MatSep rats compared with control rats after ANG II infusion (C). Although ANG II reduced significantly testosterone and estrogen levels in female rats, these levels were similar MatSep and female control rats (B and D). *P < 0.05 vs. ANG II male control group. #P < 0.05 vs. baseline corresponding sex group.
Effects of castration on renal function and blood pressure.
As indicated in Table 3, orchidectomy (ORX) in male rats did not have an effect on body weight, water and food intake, and urine volume in control or MatSep rats. Ccr was similar in control and MatSep ORX rats at baseline conditions. Proteinuria was significantly attenuated in control and MatSep ORX rats compared with intact rats. Chronic ANG II infusion reduced Ccr similarly in ORX control rats (from 1.46 ± 0.07 to 0.71 ± 0.05 ml/min, P < 0.05) and MatSep rats (from 1.28 ± 0.14 to 0.68 ± 0.08 ml/min, P < 0.05). Proteinuria was similar after ANG II infusion in male control and MatSep ORX rats.
Table 3.
Body weight and metabolic parameters in male ORX rats
| Baseline |
ANG II |
|||
|---|---|---|---|---|
| Control ORX | MatSep ORX | Control ORX | MatSep ORX | |
| Body weight, g | 278 ± 8 | 269 ± 6 | 270 ± 8 | 251 ± 7* |
| Food intake, g | 20.7 ± 0.3 | 20.2 ± 0.9 | 92.5 ± 1.6* | 95.6 ± 1.3* |
| Water intake, ml | 38.1 ± 3.1 | 31.6 ± 2.0 | 177.8 ± 13.1* | 169.6 ± 10.1* |
| Urine flow rate, ml/day | 27.1 ± 2.8 | 31.6 ± 2.3 | 64.8 ± 10.6* | 63.6 ± 2.4* |
| Protein excretion, mg/day | 6.8 ± 2.3 | 6.0 ± 1.1 | 33.2 ± 5.8* | 28.3 ± 2.8* |
Values are expressed as means ± SE; n =4 each group.
P < 0.05 vs. baseline control. ORx, orchidectomy.
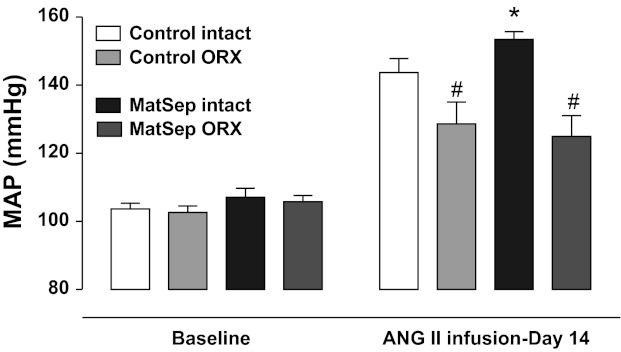
ORX had no effect on baseline MAP in control or MatSep rats (102 ± 2 and 105 ± 1 mmHg, respectively). ORX significantly attenuated the MAP response to ANG II in control and MatSep groups compared with intact rats (Fig. 6). There was no difference in blood pressure response between control and MatSep rats following ANG II infusion; at day 14, ANG II infusion increased blood pressure to a similar level in male control and MatSep ORX rats (137 ± 4 and 132 ± 3 mmHg, respectively) in contrast to the enhanced ANG II-induced hypertension observed in control and MatSep intact rats (145 ± 3 and 159 ± 4 mmHg, respectively).
Fig. 6.
Effect of orchidectomy (ORX) on ANG II-induced hypertension. ORX significantly reduced the response to ANG II in control and MatSep rats compared with intact rats. *P < 0.05 vs. intact ANG II control. #P < 0.05 vs. MatSep intact group.
DISCUSSION
This study reveals that MatSep, a model of early life stress, sensitizes the chronic blood pressure response to ANG II in male and female rats. The enhanced hypertension due to MatSep in female rats was temporally delayed compared with male MatSep rats. Male rats exposed to MatSep display reduced Ccr at baseline, and ANG II infusion promotes greater renal vascular damage in the interstitial arteries, as well as T-cell infiltration in the renal cortex correlating to increased blood pressure sensitivity. ANG II infusion also induced a higher testosterone/estradiol ratio in male MatSep rats. Furthermore, castration attenuated the ANG II-induced hypertension in male MatSep rats; thus, gonadal steroids most likely contribute to a more profound hypertensive response in males. We did not observe any differences in renal functional or structural measures or sex hormone profiles in the female control and MatSep rats, either at baseline or with ANG II infusion. Taken together, these results led us to conclude that androgens play a key role in the mechanism by which early life stress increases ANG II sensitivity to the development of hypertension in male rats. Early life stress in female rats also induces a phenotype with enhanced blood pressure sensitivity to ANG II, while the mechanism is apparently independent of renal tissue damage, although further studies are required.
The kidney plays a critical role in chronic blood pressure control (3, 13, 26). This study focused on several aspects of renal functional and structural outcomes in male and female rats exposed to MatSep. Numerous studies have now shown that the immune system mediates the development of hypertension. Immune cells have the capability to secrete proinflammatory cytokines implicated in immune cell recruitment and tissue damage. Rodriguez-Iturbe et al. (42) have shown that immune cells in the kidney play a role in mediating the development of hypertension. In our studies, male MatSep rats showed greater T-cell, but not macrophage, infiltration in the renal cortex, without a difference in the renal medulla in response to ANG II infusion. Female MatSep rats showed no differences compared with control MatSep rats regarding macrophage or T-cell infiltration. However, the numbers of macrophages were significantly less in the renal cortex and medulla of female rats compared with male rats. These data agree with the literature showing that the immune response, including cytokines and proinflammatory factors, may sensitize blood pressure responses in male more than female rats (1, 31, 54). Another potential explanation is related to the type and/or quantity of T cells. Recent studies in rats and mice have reported the role of several T-cell subpopulations regulating the inflammatory response associated to hypertension (25, 28). Naïve CD4+ T cells can differentiate into T helper 17 cells (Th 17) or regulatory T cells (Tregs), which can either promote the development of hypertension or suppress the innate and adaptive immune response, respectively (53). Also, it has been suggested that female ANG II-infused SHR rats may have more Tregs, and male rats may have more effector T cells or Th17 cells (52). In this study, a higher number of Tregs in females may contribute to the delayed ANG II-dependent increase in MAP in female vs. male MatSep rats. However, whether exacerbated MAP in MatSep rats is dependent on an imbalance in Tregs/Th17 ratio in males and/or females will provide a rationale for further investigations. At this point, it is unclear whether increased infiltration and/or activation of renal T cells mediate MatSep-dependent sensitivity to ANG II infusion. Future studies are planned that will focus on this hypothesis.
Testosterone has been shown to mediate interstitial fibrosis and glomerular damage in male rats (39,59). On the other hand, estradiol has potent and direct antiproliferative effects on the glomerular mesangium and inhibits mesangial extracellular matrix accumulation, both of which are key events in the development of fibrosis and glomerular sclerosis (11,19, 57). However, our data show that ANG II-induced hypertension is correlated to greater renal vascular damage of interstitial arteries in male MatSep rats compared with control rats, without significant damage in tubular structures, glomeruli, or interstitium. Previously, we demonstrated that ANG II infusion increased aortic wall thickness and wall/lumen ratio similarly in control and MatSep rats (24). We also did not find differences in control and MatSep rats in ANG II-mediated ventricular hypertrophic response. Thus, the study of the renal vasculature may reveal whether the kidney is a specific target of early behavorial stress in male rats.
The presence of higher urinary protein excretion at baseline associated with a lower Ccr in MatSep male rats suggests impaired renal function as a possible mechanism by which MatSep impairs the control of blood pressure. Androgens may play a role in the dysregulation of renal hemodynamics induced by early life stress. This potential mechanism is supported by several studies showing that androgens have increased vascular reactivity in many vascular beds, including the kidney (47). For instance, it has been shown that androgens mediate ANG II-induced renal artery vasoconstriction (47). Chronic exposure to physiological concentrations of testosterone increases vascular resistance (35). In addition, testosterone increased vascular reactivity in coronary artery (16) and aorta (29) in animals and in men (27). Therefore, male MatSep rats may undergo exacerbated ANG II-induced responses, considering the known effects of androgens in the progress of renal damage and the role of testosterone mediating vascular reactivity (39, 58, 59).
Several studies have reported sex differences in the endogenous RAAS pathway (44, 49, 58). Although the RAAS plays an important role in control of blood pressure, this pathway also seems to play a role in the sexual dimorphism of renal injury (39, 49). Classically, ANG II type 1 receptor (AT1R) activation mediates vasoconstriction, vascular hypertrophy and remodeling, inflammatory cell activation, as well as sodium and water retention (45). Sex steroids are able to modulate the intrarenal renin-angiotensin system in normotensive and hypertensive rat models. Estradiol has been shown to down-regulate AT1R expression in the kidney, adrenal vasculature, and brain in female normotensive rats (6, 9). In contrast, testosterone was previously shown to up-regulate angiotensinogen in kidneys of male SHR and mediate hypertension in several rat models (5, 40, 58, 59). Moreover, many studies support the concept that the presence of testosterone may be more relevant than the absence of estradiol in the renal and vascular damage in hypertensive models, such as SHR or ANG II (39, 40, 48). However, whether the effect of testosterone on renal injury is hemodynamically mediated in our model needs further investigation. Sullivan et al. (49, 51) have shown that the “nonclassical” RAAS (such as angiotensin type 2 receptor, angiotensin-converting enzyme 2, and angiotensin 1–7) plays an important role in the counterbalancing of blood pressure in SHR female rats, conferring a protective mechanism in the maintenance of lower blood pressure compared with male rats (49, 51). Because ANG II-induced changes in renal vascular damage and immune cell infiltration were not distinct in MatSep female rats, we speculate that attenuation of the nonclassical RAAS components may be a potential mechanism underlying exaggerated ANG II-induced hypertension in female MatSep rats. However, this hypothesis will need further investigation.
Perspectives and Significance
These data led us to hypothesize that an imbalance in sex steroids plays a pivotal role in the underlying mechanism of enhanced ANG II-induced hypertension in male MatSep rats. Sex differences in disease susceptibility have been consistently attributable to the protective effects of estrogen. However, recent evidence suggests that male hormones may also have an important role in these differences. Further research is needed to address whether an excess of androgens or lack of estradiol plays an important role in the mechanism by which male MatSep rats display heightened hypertension associated with greater renal vascular damage and inflammation.
GRANTS
This study was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (J.S. Pollock and D.M. Pollock: P01 HL69999) and from the American Heart Association (A. S. Loria Postdoctoral Fellowship: AHASE00027).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.S.L. and J.S.P. conception and design of research; A.S.L. performed experiments; A.S.L. and T.Y. analyzed data; A.S.L., D.M.P., and J.S.P. interpreted results of experiments; A.S.L. prepared figures; A.S.L. and J.S.P. drafted manuscript; A.S.L., T.Y., D.M.P., and J.S.P. edited and revised manuscript; A.S.L., T.Y., D.M.P., and J.S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the outstanding technical support from Hiram Ocasio, Janet Hobbs and Amy Dukes.
REFERENCES
- 1. Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Tumor necrosis factor-α and vascular angiotensin II in estrogen-deficient rats. Hypertension 48: 497–503, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Crowley SD, Gurley SB, Coffman TM. AT1 receptors and control of blood pressure: the kidney and more. Trends Cardiovasc Med 17: 30–34, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115: 1092–1099, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis DD, Ruiz AL, Yanes LL, Iliescu R, Yuan K, Moulana M, Racusen LC, Reckelhoff JF. Testosterone supplementation in male obese Zucker rats reduces body weight and improves insulin sensitivity but increases blood pressure. Hypertension 59: 726–731, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dean SA, Tan J, O'Brien ER, Leenen FH. 17β-estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. Am J Physiol Regul Integr Comp Physiol 288: R759–R766, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Dumont FS, Biancardi V, Kinkead R. Hypercapnic ventilatory response of anesthetized female rats subjected to neonatal maternal separation: insight into the origins of panic attacks? Respir Physiol Neurobiol 175: 288–295, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Erskine MS, Stern JM, Levine S. Effects of prepubertal handling on shock-induced fighting and ACTH in male and female rats. Physiol Behav 14: 413–420, 1975 [DOI] [PubMed] [Google Scholar]
- 9. Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension 33: 323–328, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Ganten U, Schroder G, Witt M, Zimmermann F, Ganten D, Stock G. Sexual dimorphism of blood pressure in spontaneously hypertensive rats: effects of anti-androgen treatment. J Hypertens 7: 721–726, 1989 [PubMed] [Google Scholar]
- 11. Guccione M, Silbiger S, Lei J, Neugarten J. Estradiol upregulates mesangial cell MMP-2 activity via the transcription factor AP-2. Am J Physiol Renal Physiol 282: F164–F169, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Hall JE, Brands MW, Henegar JR. Angiotensin II and long-term arterial pressure regulation: the overriding dominance of the kidney. J Am Soc Nephrol 12: S258–S265, 1999 [PubMed] [Google Scholar]
- 13. Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl 55: S35–S41, 1996 [PubMed] [Google Scholar]
- 14. Hu J, Tan S, Zhong Y. Effects of testosterone on renal function in salt-loaded rats. Am J Med Sci 342: 38–43, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Ji H, Menini S, Mok K, Zheng W, Pesce C, Kim J, Mulroney S, Sandberg K. Gonadal steroid regulation of renal injury in renal wrap hypertension. Am J Physiol Renal Physiol 288: F513–F520, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Karanian JW, Ramwell PW. Effect of gender and sex steroids on the contractile response of canine coronary and renal blood vessels. J Cardiovasc Pharmacol 27: 312–319, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Khoury S, Yarows SA, O'Brien TK, Sowers JR. Ambulatory blood pressure monitoring in a nonacademic setting. Effects of age and sex. Am J Hypertens 5: 616–623, 1992 [DOI] [PubMed] [Google Scholar]
- 18. Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol 293: F938–F945, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kwan G, Neugarten J, Sherman M, Ding Q, Fotadar U, Lei J, Silbiger S. Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney Int 50: 1173–1179, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Lavoz C, Rodrigues-Diez R, Benito-Martin A, Rayego-Mateos S, Rodrigues-Diez RR, Alique M, Ortiz A, Mezzano S, Egido J, Ruiz-Ortega M. Angiotensin II contributes to renal fibrosis independently of Notch pathway activation. PLos One 7: e40490, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci 25: 3091–3098, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Loria AS, D'Angelo G, Pollock DM, Pollock JS. Early life stress downregulates endothelin receptor expression and enhances acute stress-mediated blood pressure responses in adult rats. Am J Physiol Regul Integr Comp Physiol 299: R185–R191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loria AS, Kang KT, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension 58: 619–626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension 55: 494–499, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madsen K, Marcussen N, Pedersen M, Kjaersgaard G, Facemire C, Coffman TM, Jensen BL. Angiotensin II promotes development of the renal microcirculation through AT1 receptors. J Am Soc Nephrol 21: 448–459, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malkin CJ, Jones RD, Jones TH, Channer KS. Effect of testosterone on ex vivo vascular reactivity in man. Clin Sci (Lond) 111: 265–274, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Matrougui K, Abd Elmageed Z, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P, Belmadani S, Partyka M. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol 178: 434–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsuda K, Ruff A, Morinelli TA, Mathur RS, Halushka PV. Testosterone increases thromboxane A2 receptor density and responsiveness in rat aortas and platelets. Am J Physiol Heart Circ Physiol 267: H887–H893, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Matsumoto K, Morishita R, Moriguchi A, Tomita N, Yo Y, Nishii T, Nakamura T, Higaki J, Ogihara T. Prevention of renal damage by angiotensin II blockade, accompanied by increased renal hepatocyte growth factor in experimental hypertensive rats. Hypertension 34: 279–284, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Metcalfe PD, Leslie JA, Campbell MT, Meldrum DR, Hile KL, Meldrum KK. Testosterone exacerbates obstructive renal injury by stimulating TNF-α production and increasing proapoptotic and profibrotic signaling. Am J Physiol Endocrinol Metab 294: E435–E443, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Mourlon V, Baudin A, Blanc O, Lauber A, Giros B, Naudon L, Dauge V. Maternal deprivation induces depressive-like behaviours only in female rats. Behav Brain Res 213: 278–287, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Neumann ID, Wigger A, Kromer S, Frank E, Landgraf R, Bosch OJ. Differential effects of periodic maternal separation on adult stress coping in a rat model of extremes in trait anxiety. Neuroscience 132: 867–877, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Nistala R, Wei Y, Sowers JR, Whaley-Connell A. Renin-angiotensin-aldosterone system-mediated redox effects in chronic kidney disease. Transl Res 153: 102–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233–R249, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Peters SL, Gray JA, Joseph MH. Pre-weaning non-handling of rats disrupts latent inhibition in males, and results in persisting sex- and area-dependent increases in dopamine and serotonin turnover. Behav Pharmacol 2: 215–223, 1991 [PubMed] [Google Scholar]
- 37. Polichnowski AJ, Lu L, Cowley AW., Jr Renal injury in angiotensin II+l-NAME-induced hypertensive rats is independent of elevated blood pressure. Am J Physiol Renal Physiol 300: F1008–F1016, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rajapakse NW, De Miguel C, Das S, Mattson DL. Exogenous l-arginine ameliorates angiotensin II-induced hypertension and renal damage in rats. Hypertension 52: 1084–1090, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reckelhoff JF, Granger JP. Role of androgens in mediating hypertension and renal injury. Clin Exp Pharmacol Physiol 26: 127–131, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 31: 435–439, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Renard GM, Rivarola MA, Suarez MM. Sexual dimorphism in rats: effects of early maternal separation and variable chronic stress on pituitary-adrenal axis and behavior. Int J Dev Neurosci 25: 373–379, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol 282: F191–F201, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol 18: 143–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 62: 587–593, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Siragy HM, Senbonmatsu T, Ichiki T, Inagami T, Carey RM. Increased renal vasodilator prostanoids prevent hypertension in mice lacking the angiotensin subtype-2 receptor. J Clin Invest 104: 181–188, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KC. Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: gender-dependent effects. Brain Res 1097: 123–132, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Song J, Eyster KM, Kost CK, Jr, Kjellsen B, Martin DS. Involvement of protein kinase C-CPI-17 in androgen modulation of angiotensin II-renal vasoconstriction. Cardiovasc Res 85: 614–621, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Song J, Kost CK, Jr, Martin DS. Androgens potentiate renal vascular responses to angiotensin II via amplification of the Rho kinase signaling pathway. Cardiovasc Res 72: 456–463, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 294: R1220–R1226, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Sullivan JC, Sasser JM, Pollock DM, Pollock JS. Sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats. Hypertension 45: 406–411, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 293: R1573–R1579, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol 303: R359–R367, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Viel EC, Lemarie CA, Benkirane K, Paradis P, Schiffrin EL. Immune regulation and vascular inflammation in genetic hypertension. Am J Physiol Heart Circ Physiol 298: H938–H944, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Wang M, Tsai BM, Kher A, Baker LB, Wairiuko GM, Meldrum DR. Role of endogenous testosterone in myocardial proinflammatory and proapoptotic signaling after acute ischemia-reperfusion. Am J Physiol Heart Circ Physiol 288: H221–H226, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Weiner I, Feldon J, Ziv-Harris D. Early handling and latent inhibition in the conditioned suppression paradigm. Dev Psychobiol 20: 233–240, 1987 [DOI] [PubMed] [Google Scholar]
- 56. Wiinberg N, Hoegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens 8: 978–986, 1995 [DOI] [PubMed] [Google Scholar]
- 57. Xiao S, Gillespie DG, Baylis C, Jackson EK, Dubey RK. Effects of estradiol and its metabolites on glomerular endothelial nitric oxide synthesis and mesangial cell growth. Hypertension 37: 645–650, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Yanes LL, Romero DG, Iles JW, Iliescu R, Gomez-Sanchez C, Reckelhoff JF. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 291: R383–R390, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 296: F771–F779, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]