Abstract
Exposure to chronic hypoxia during gestation predisposes infants to neonatal pulmonary hypertension, but the underlying mechanisms remain unclear. Here, we test the hypothesis that moderate continuous hypoxia during gestation causes changes in the rho-kinase pathway that persist in the newborn period, altering vessel tone and responsiveness. Lambs kept at 3,801 m above sea level during gestation and the first 2 wk of life were compared with those with gestation at low altitude. In vitro studies of isolated pulmonary arterial rings found a more forceful contraction in response to KCl and 5-HT in high-altitude compared with low-altitude lambs. There was no difference between the effects of blockers of various pathways of extracellular Ca2+ entry in low- and high-altitude arteries. In contrast, inhibition of rho-kinase resulted in significantly greater attenuation of 5-HT constriction in high-altitude compared with low-altitude arteries. High-altitude lambs had higher baseline pulmonary artery pressures and greater elevations in pulmonary artery pressure during 15 min of acute hypoxia compared with low-altitude lambs. Despite evidence for an increased role for rho-kinase in high-altitude arteries, in vivo studies found no significant difference between the effects of rho-kinase inhibition on hypoxic pulmonary vasoconstriction in intact high-altitude and low-altitude lambs. We conclude that chronic hypoxia in utero results in increased vasopressor response to both acute hypoxia and serotonin, but that rho-kinase is involved only in the increased response to serotonin.
Keywords: rho-kinase, hypoxic pulmonary vasoconstriction
persistent pulmonary hypertension of the newborn occurs in 1 to 7 out of 1,000 live births and is associated with a mortality rate of 10 to 20% (65). Although the cause of this disease is poorly understood, one risk factor appears to be chronic hypoxia in utero, as infants born at high altitude have as much as 100-fold increased risk of pulmonary hypertension (43–45).
Vascular smooth muscle contraction is known to be proportional to the cytosolic Ca2+ concentration. This is dependent on the entry of extracellular calcium through various voltage-dependent and voltage-independent Ca2+ pathways, which are critical to initiating the cascade of events that lead to myosin light-chain phosphorylation and contraction. The force of contraction achieved at a given cytosolic Ca2+ concentration is also proportional to the activity of two different rho-kinase isoforms (rho-kinase I and II), which maintain phosphorylation of myosin light chain by inhibition of myosin light chain phosphatase (61). Through rho-kinase, contractile tone can be modulated independent of changes in cytosolic Ca2+ concentrations, which leads to the idea that there is strong coordination between the elevation in Ca2+ and the overall sensitivity to cytosolic Ca2+. These pathways are often simultaneously activated, whether this is due to activation by G protein receptors, such as serotonin (12, 31, 49) or by acute alveolar hypoxia that causes hypoxic pulmonary vasoconstriction (26, 53, 55).
Rho-kinase is one of several factors that contribute to the normally elevated pulmonary vascular resistance (PVR) of the fetus (50). Exposure of the fetal sheep to chronic hypoxia in utero results in increased expression and activity of rho-kinase II in both pulmonary arteries and veins (9, 10). Likewise, exposure of the newborn rat to chronic hypoxia results in elevated PVR associated with increased expression and activity of both rho-kinase I and II (20). In adult animals, rho-kinase inhibition reverses pulmonary hypertension caused by acute and chronic hypoxia in the mouse (5) and rat (37, 55). Thus, there is significant evidence that rho-kinase plays a role in pulmonary vascular contraction during the perinatal period and that its role may be accentuated in response to chronic hypoxia.
The tendency of chronic hypoxia, during either gestation or the first few days of life, to increase pulmonary pressures and cause pulmonary hypertension has been replicated in numerous newborn experimental animals, including the rat pup (24), piglet (6, 16), rabbit (76), calf (62), and newborn lamb (18). However, the mechanisms by which chronic hypoxia leads to pulmonary hypertension are complex and poorly understood. The present experiments were designed to test the hypothesis that antenatal chronic hypoxia results in increased contributions of extracellular calcium entry and rho-kinase activity to pulmonary artery contraction. To test this hypothesis, we examined the contractile response of isolated pulmonary artery rings to 5-HT, an agonist known to stimulate increases in both intracellular calcium and rho-kinase activity (1, 11, 12, 19, 49, 72), in the presence of antagonists of both rho-kinase and various modes of calcium entry. The role of rho-kinase was further evaluated by studying the effects of rho-kinase inhibition on acute hypoxic pulmonary vasoconstriction in intact 2-wk-old lambs exposed to high-altitude hypoxia throughout the final two-thirds of gestation.
METHODS
Chronic hypoxia.
All surgical and experimental procedures were performed within the regulations of the Animal Welfare Act, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Guiding Principles in the Care and Use of Animals approved by the Council of the American Physiological Society, and the Loma Linda University Institutional Animal Care and Use Committee. Two groups of pregnant ewes were obtained from Nebeker Ranch (Lancaster, CA). Six ewes were maintained at low altitude (335 m, 1,100 ft) throughout gestation, while six different ewes were transported at 30 days of gestation to high altitude (3,801 m, 12,470 ft, White Mountain Research Station, Bishop, CA) and maintained there for the remainder of pregnancy. Ewes in both groups were allowed to give birth naturally, and when the lambs were 7 to 10 days of age, they were transported to Loma Linda University Animal Care Facility. After arrival and until study 2 to 5 days later, the high-altitude group was kept in a chamber supplied with 14 to 16% oxygen. Both groups of lambs were studied between 10 and 20 days of life.
Pharmacological considerations.
All in vitro studies were performed by measuring dose-response curves for 5-HT in the presence of various blockers of rho-kinase and pathways of extracellular Ca2+ entry. 5-HT was chosen as it is known to increase both cytosolic Ca2+ concentrations and rho-kinase activity. The effects of 5-HT on contraction via increases in cytosolic Ca2+ and activation of Ca2+ sensitization pathways, including rho-kinase, occur primarily via activation of G protein-coupled receptor pathways in pulmonary arteries of fetal and adult sheep (12, 49); however, its modulation of rho-kinase activity may also occur by uptake of 5-HT into the cell via 5-HT transporters, followed by covalent binding to and activation of rhoA, which leads to increased rho-kinase activity (15). Fasudil and Y27632 were selected for rho-kinase inhibition, since these two drugs have been used widely at 10 μM (26, 32, 57, 68). Nifedipine is commonly used to block L-type Ca2+ channels (CaL) at a concentration of 10 μM, which is at least 2 orders of magnitude above its inhibitory EC50 for CaL (12, 30, 49). A saturating concentration of the nonselective cation (NSC) and transient receptor potential (TRP) channel antagonist SKF 96365 (50 μM) (12, 36, 42, 49) was used in the presence of 10 μM nifedipine to assess the role of NSC/TRP channels during 5-HT-induced contraction. The antagonists were applied simultaneously, as SKF 96365 blocks CaL in the same concentration range in which it inhibits TRP channels (36). SKF 96365 inhibits a variety of TRP channels, including TRPC1, TRPC3, TRPC4, TRPC5, TRPC6, and TRPC7 (4, 40, 52). Because SKF 96365 blocks numerous channels, these findings were compared with experiments performed using flufenamic acid, which has an unrelated structure and also inhibits a number of TRP channels. Flufenamic acid is commonly used at 100 μM, a concentration that blocks TRPC3, TRPC5, TRPC7, TRPM2, TRPM4, and TRPM5, but may activate TRPC6 (4, 12, 49, 52).
Experiments also were performed to test for an interaction between rho-kinase and reverse-mode sodium-calcium exchange (NCX). The rationale for these studies is that activation of G protein-coupled receptors depolarizes the plasma membrane, which can accentuate Na+ influx through a number of TRP channels. Na+ entry and membrane depolarization reduce the electrochemical gradient for Na+ movement across the plasma membrane and can reverse Na+/Ca2+ exchanger transport activity. This switch of the NCX into “reverse mode” augments the increase in intracellular Ca2+ concentrations when the myocytes are stimulated and potentiates arterial contraction (12, 27, 49, 51, 74, 75).
To examine the role of Ca2+ entry through reverse-mode Na+/Ca2+ exchange, experiments were performed in the presence of SN-6. This inhibitor was used at 30 μM, as this is well above the EC50 for its inhibition of NCX1, NCX2, and NCX3 isoforms (25, 46, 47). SN-6 blocks NCX1, NCX2, and NCX3 at 30 μM without any known effect on TRPC channels or ryanodine receptors (3), and therefore, SN-6 can be used to discriminate NCX-related and unrelated effects on vascular contraction (47). Of note, the pharmacologic profile of these compounds is still not defined completely, and it is possible that there may be overlap, nonspecific effects, or unknown targets that could cause erroneous findings.
The intravenous dose of fasudil used in the in vivo studies (2.5 mg/kg) was based on studies in rats (2), pigs (60), dogs (58), and humans (28, 59). Given the nearly 20-h combined elimination half-life of fasudil and its active metabolite hydroxyfasudil, and its volume of distribution, we estimate the plasma concentration was greater than 10 μM throughout the experiment.
Contractility studies.
After lambs were humanely euthanized with an overdose of barbiturate (Euthasol, Virbac, Fort Worth, TX), the lungs were removed for contractility experiments. Pulmonary arteries were dissected free of parenchyma at the 5th or 6th generation distal to the pulmonary artery and cut into 5-mm-long rings in ice-cold, phosphate-free balanced salt solution of the following composition (in mM): 126 NaCl, 5 KCl, 10 HEPES, 1 MgCl2, 2 CaCl2, and 10 glucose, with pH adjusted to 7.4 with NaOH. Rings were rotated on a mounting wire to avoid endothelium-mediated effects (11, 49) and suspended in organ baths (Radnoti Glass Instruments, Monrovia, CA) containing modified Krebs-Henseleit solution. The baths contained in mM: 120 NaCl, 4.8 KCl, 1.2 K2HPO4, 25 NaHCO3, 1.2 MgCl2, 2.5 CaCl2, and 10 glucose and were kept at 37°C. The bath fluid was aerated with 95% O2-5% CO2. Rings were suspended between tungsten wires to measure isometric force, and responses were recorded and stored for later analysis, as described in detail (11, 49). At the beginning of each experiment, the vessel rings were equilibrated thermally without tension for >30 min and then progressively stretched. There was no difference between baseline tensions developed in 304 arteries from 11 low-altitude lambs (437 ± 70 dynes) and 244 arteries from 12 high-altitude lambs (418 ± 75 dynes), and these values were similar to our previous studies (12, 48, 49, 72). Tensions were compared with maximum responses obtained by exposure to 125 mM KCl (%TKmax). For evaluating dose-response characteristics, arteries were stimulated by cumulatively applying 10−9 to 10−4 M 5-HT in log increments without washing between step increases of 5-HT concentration. The wet weights of the arteries were determined by pooling the weights for the various segments used for each study. Measurements of the lumen diameter were based on arterial width with observations taken using a stage micrometer and stereo microscope (Nikon SMZ-1, Melville, NY). The internal lumen diameter was 707 ± 137 μm in 225 vessels from 11 low-altitude and 629 ± 131 μm in 195 vessels from 12 high-altitude newborns (P > 0.05).
Surgical instrumentation.
Anesthesia was induced with thiopental (10 mg/kg iv) and maintained with 2% isoflurane after intubation and mechanical ventilation. Catheters were inserted into the brachial artery for measurement of blood pressure, in the right femoral vein for administration of medications, and the right femoral artery for blood gas sampling. A Swan-Ganz catheter was passed from the left femoral vein to the pulmonary artery to measure pulmonary arterial blood pressure. Following thoracotomy, a transonic flow probe (Transonics, Ithaca, NY) was placed around the pulmonary artery and a second probe placed around the left femoral artery to measure pulmonary and hind limb blood flows.
Following surgery, lambs were transitioned to intravenous ketamine at 0.1 mg·kg−1·h−1 and vecuronium at 0.1 mg·kg−1·h−1. Positive pressure ventilation was provided using a Bird VIP Gold ventilator (CareFusion, San Diego, CA) in pressure-limited, time-cycled mode with positive end-expiratory pressure of 5 cmH2O and fractional inspired oxygen (FiO2) of 0.5 to overcome any ventilation/perfusion mismatch and, thus, ensure baseline arterial oxyhemoglobin saturations were approaching 100% in all lambs. Peak inspiratory pressure, ventilation rate, and the inhalation and exhalation ratios were adjusted to maintain normal levels of arterial Pco2.
Study protocol.
Following a 30-min baseline period, lambs were exposed to a 15-min period of acute hypoxia induced by decreasing the FiO2 between 0.08 and 0.10, resulting in arterial oxygen tensions of 20 to 30 Torr. After the hypoxic challenge, FiO2 was returned to 0.5 for a 30-min recovery period. Then the lambs were given an intravenous bolus of the rho-kinase inhibitor fasudil (2.5 mg/kg), followed by 30 min of observation, another 15-min period of acute hypoxia, and a final 30 min of recovery. This dose of fasudil is similar to parenteral doses known to result in rho-kinase inhibition in dogs (58), humans (7, 8, 22, 29), fetal lambs (50), and rats (2). This protocol enabled cardiovascular responses to be compared at equivalent levels of hypoxia with and without blockade of the rho-kinase pathway.
Hemodynamic measurements.
Systemic and pulmonary arterial pressures were measured continuously using pressure transducers (Cobe, Lakewood, CO). Pulmonary artery flow, also taken as cardiac output, and femoral artery flow were measured continuously by Transonics TS420 modules (Transonic Systems, Ithaca, NY). Pressure and flow signals were sampled at 200 Hz by an analog-to-digital converter (Powerlab 16; ADInstruments, Colorado Springs, CO) and recorded by computer (Chart v5.2 for Macintosh, ADInstruments). Heart rate was calculated from the arterial blood pressure waveform.
Arterial blood gases (0.4 ml each) (ABL-5; Radiometer, Copenhagen, Denmark) and hemoglobin concentration and oxyhemoglobin saturation (OSM3, Radiometer) were measured at baseline, prior to each hypoxic episode, 5 min after initiating hypoxia, and just prior to the end of each hypoxic episode. Pulmonary capillary wedge pressure was measured at each blood sampling time point by inflation of the Swan-Ganz catheter balloon for three to four breaths.
Data analysis.
Dose-response curves were fitted in Prism 5.0 using a Hill equation (12, 49, 72). Computations of pressures, flows, and heart rate were made in 5-min averages following completion of the experiment. Significance of changes with time was measured by one-way ANOVA with repeated measures. Significant differences between the low- and high-altitude groups were detected using two-way ANOVA with repeated measures. Significance relationships found with ANOVA were followed by Bonferroni's or Newman Keul's multiple-comparison post hoc analyses to detect significant differences at specific time points (GraphPad Prism, v5.0 for Macintosh). For all analyses, statistical significance was assumed for P < 0.05, unless otherwise noted.
RESULTS
Vessel ring contractility studies.
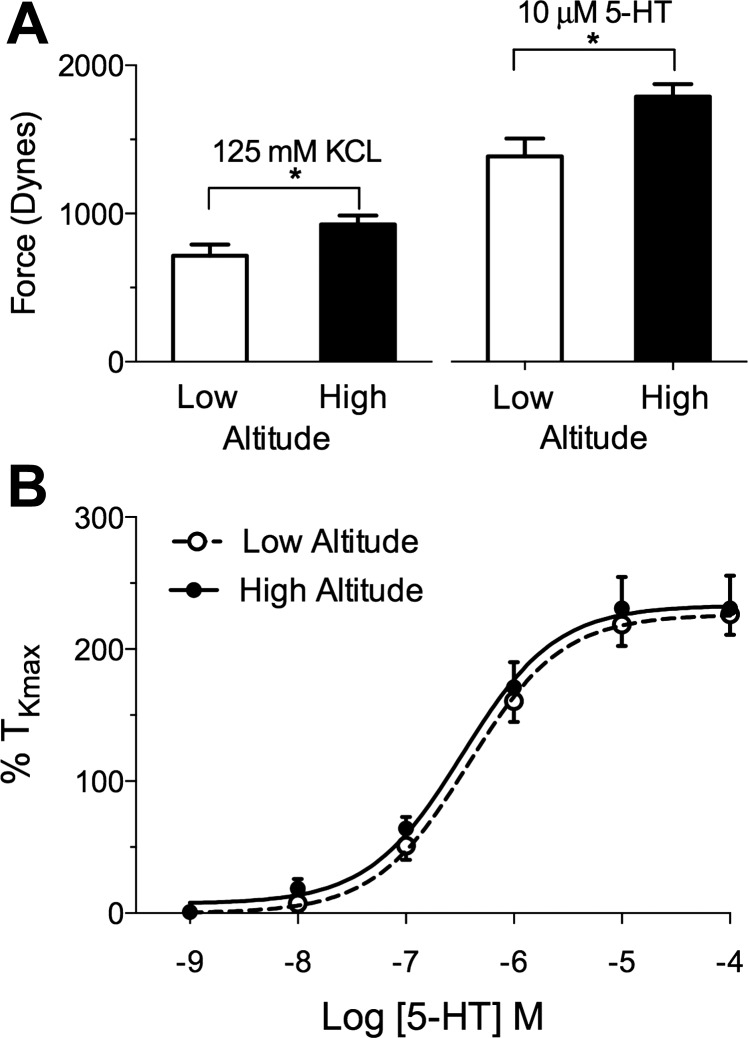
Figure 1 shows the maximal contractile response of isolated pulmonary artery rings to high concentrations of KCl (125 mM) and 5-HT (10 μM). Both KCl and 5-HT stimulated a greater contractile response in the high-altitude arteries than in controls. High-altitude hypoxia increased the KCl-induced tension by ∼30% when mean responses of low-altitude lambs (714 ± 76 dynes; 304 vessels from 11 lambs) were compared with high-altitude lambs (927 ± 57 dynes; 244 vessels from 12 lambs) (P < 0.05, unpaired Students t-test). The contraction due to 10 μM 5-HT also was increased by roughly 30% in high-altitude lambs (1,787 ± 84 dynes; 46 vessels from 8 lambs), compared with low-altitude lambs (1,385 ± 120 dynes; 86 vessels from 11 lambs) (P < 0.05, unpaired Student's t-test). Although the force of contraction was greater in high-altitude vessels, chronic hypoxia did not alter the log EC50 (−6.5 ± 0.1 M vs. −6.4 ± 0.1 M) or Emax (226 ± 9 vs. 233 ± 12% TKmax) values for 5-HT contraction.
Fig. 1.
Contractile responses of pulmonary arterial rings from newborn lambs with gestation at high altitude and low altitude. A: maximal tension in response to 125 mM KCl and 10 μM 5-HT is higher for high-altitude vessels than for those at low altitude (*P < 0.05). B: dose-response curves to cumulative additions of 5-HT show no difference in tension when expressed as a fraction of response to KCl.
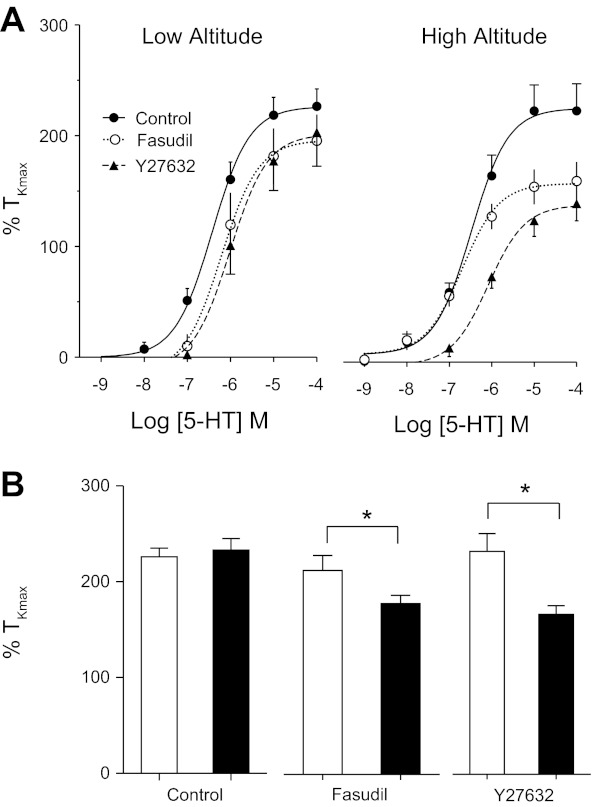
The stimulation of arteries with 5-HT results in contraction by both increases in intracellular Ca2+, as well as activation of rho-kinase. To assess the contribution of rho-kinase to the 5-HT-elicited contraction, cumulative dose-response curves were performed in the presence and absence of 10 μM fasudil or 10 μM Y27632, two commonly used rho-kinase inhibitors (23, 38, 50, 57, 68, 70). As shown in Fig. 2, arterial tension in control arteries was not significantly reduced by the presence of fasudil (18 vessels in six lambs). In contrast, in high-altitude arteries, fasudil reduced the Emax from 233% to 164% of TKmax (P < 0.01, 14 vessels from five lambs), and Y27632 reduced Emax to 144% of TKmax (P < 0.01, 13 vessels from four lambs).
Fig. 2.
Responses of pulmonary arterial rings to cumulative additions of 5-HT. A: 5-HT dose-response curves of low-altitude (●, solid line) lambs were not significantly altered by the presence of either 10 μM fasudil (○, dotted line) or 10 μM Y27632 (▲, dashed line). Lines show resultant fits with a Hill equation to the dose-response relationships and markers show means ± SE. In contrast, these rho-kinase antagonists significantly depressed responses to 5-HT in vessels from high-altitude lambs. B: bars represent means ± SE maximum tension values from the dose-response curves in arteries from low- (white bars) and high- (black bars) altitude lambs. *Significant difference between low- and high-altitude groups, P < 0.001.
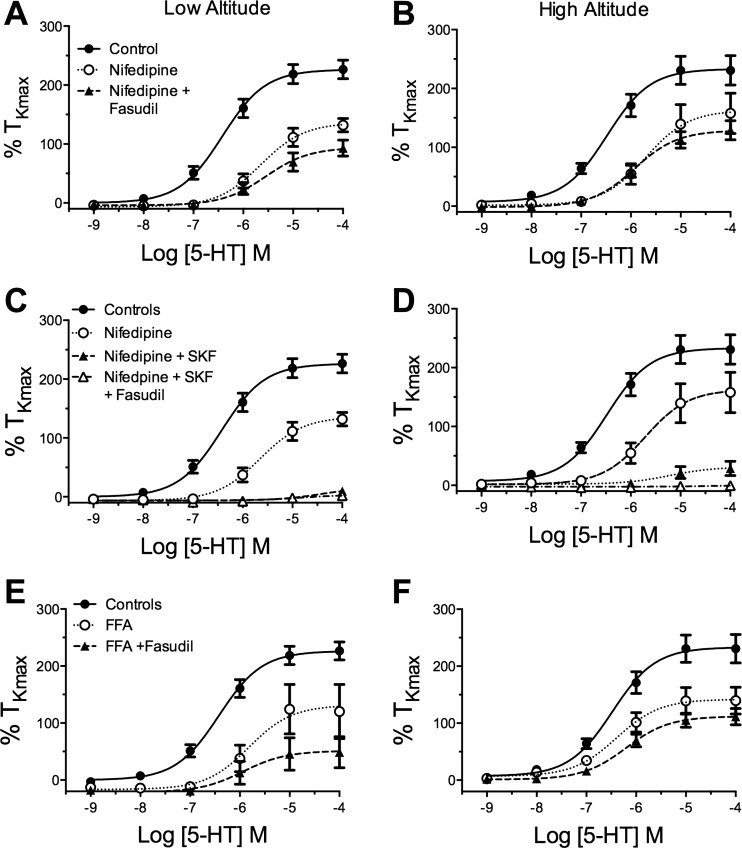
A primary mechanism by which rho-kinase increases contraction is enhancing the sensitivity of the contractile second messenger systems to increased cytosolic Ca2+. Previous studies have shown that extracellular Ca2+ entry is critical to the Ca2+-dependent contraction caused by stimulation with 5-HT (12, 14, 49, 56, 72). There are several pathways for the influx of extracellular Ca2+ into the cytosol, including CaL, nonselective cation channels (NSCC), and reverse-mode sodium/calcium exchangers (NCX). We, therefore, decided to test the possible synergy between the roles of rho-kinase and these routes of extracellular Ca2+ entry by measuring contractile responses to 5-HT in the presence of selective antagonists of these pathways with and without rho-kinase inhibition. We first examined the role of L-type Ca2+ channels in 5-HT-elicited contraction. As shown in Fig. 3, A and B, the Emax was significantly attenuated by nifedipine to a similar extent in both high-altitude (15 arteries from 5 lambs) and low-altitude (18 arteries from 6 lambs) arteries. The combination of nifedipine and fasudil attenuated the Emax to a greater extent than nifedipine alone, independent of altitude. Neither nifedipine alone, nor the combination of nifedipine and fasudil, had a significant effect on the EC50.
Fig. 3.
The role of extracellular Ca2+ entry to 5-HT-elicited contraction is unchanged in high-altitude newborns. Dose-response relationships for cumulative 5-HT-induced isometric tension values normalized to %TKmax in arteries from low- and high-altitude lambs for controls (●, solid line) and vessels in the presence of various selective Ca2+ uptake blockers (○, dotted line) or the combination of uptake blockers and 10 μM fasudil (▲, dashed line). Nifedipine (A and B), nifedipine and SKF 96365 (SKF; C and D), and flufenamic acid (FFA; E and F) all resulted in a significant attenuation of contraction that was not altered by fasudil in either low- or high-altitude vessels. Lines show resultant fits with a Hill equation to the dose-response relationships and markers show mean ± SE.
Recent evidence indicates that 5-HT stimulation activates one or more NSCCs, which contributes to increases in intracellular Ca2+ concentrations (12, 14, 49, 56), and elicits contraction in fetal and adult sheep pulmonary arteries (12). We, therefore, generated dose-response curves in the presence of a commonly used NSCC blocker, SKF 96365 (12, 14, 39, 41, 49, 66, 67, 71). To mitigate potential reactivity with L-type Ca2+ channels, the experiments were performed in the presence of 10 μM nifedipine, which does not affect NSCC activity (52). As shown in Fig. 3, C and D, the combination of SKF 96365 and nifedipine inhibited over 90% of 5-HT-elicited contraction in both low-altitude (15 vessels from 5 sheep) and high-altitude (10 vessels from 3 sheep) arteries. The addition of fasudil did not reduce contraction further than the combination of SKF 96365 and nifedipine.
We next evaluated the role of reverse-mode NCX in 5-HT-elicited contraction by performing experiments in the presence of SN-6, a selective reverse mode NCX inhibitor (25). Exposure to 30 μM SN-6 did not depress the Emax in 12 vessels from 4 control lambs or in 9 vessels from 3 high-altitude lambs, nor did it influence the EC50 of 5-HT (data not shown), indicating no role for the NCX in 5-HT-stimulated contraction in both high-altitude and low-altitude arteries.
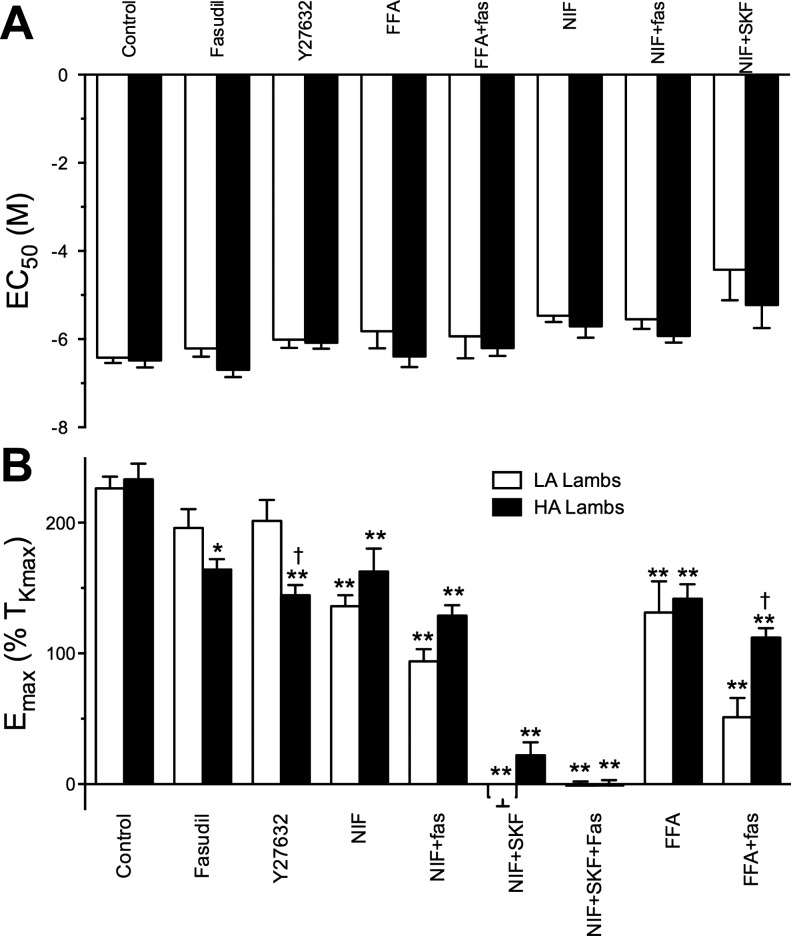
Figure 4 displays the calculated EC50 and Emax values for the dose-response experiments. Overall, no combination of Ca2+ entry or rho-kinase blockers resulted in a significant change in the EC50 for 5-HT. In contrast, Emax was highly sensitive to the various blockers, and many of these sensitivities were affected by high-altitude hypoxia. In general, rho-kinase inhibition was more effective at attenuating constriction in vessels from hypoxic lambs compared with normoxic controls. Nifedipine significantly reduced the Emax to similar extents in vessels from control and high-altitude lambs, and the addition of fasudil reduced contraction further in vessels from both study groups. In both control and high-altitude vessels, contraction was abolished by combined blockade of L-type Ca2+ channels and NSCCs, while reverse-mode NCX blockade had no significant effect in either study group.
Fig. 4.
Effect of selective inhibition of Ca2+ entry and rho-kinase inhibitors on EC50 and Emax. Half-maximal effective concentration for 5-HT (EC50) (A) and the maximal contraction (Emax) (B). Bars show means ± SE. The data were analyzed by two-way ANOVA with a Bonferroni post hoc test analysis. Statistical significance is noted relative to the control group (*P < 0.05, **P < 0.01) or between low- and high-altitude groups (†P < 0.05). Emax values for low-altitude data in the presence of NIF plus SKF were estimated on the basis of the contraction response recorded at 10 μM 5-HT because the curve fit analysis failed to fit the data properly. The number of vessel segments and animals studied is provided in the results section. NIF, nifedipine; Fas, fasudil; LA, low altitude; HA, high altitude.
In vivo protocol.
To further investigate the above evidence of an increased role for rho-kinase in artery contractility in high-altitude lambs, we conducted an in vivo study of the effects of fasudil on pulmonary artery pressure in response to acute hypoxia. Twelve lambs were studied, six in the low-altitude group (3 males and 3 females) and six in the high-altitude group (3 males and 3 females). There was no significant difference between the ages of the low-altitude and high-altitude lambs at the time of study (15.0 ± 0.7 vs. 15.2 ± 0 0.2 days, respectively). Lambs in the low-altitude group weighed less than those in the high-altitude group (5.7 ± 0.6 vs. 7.8 ± 0.7 kg, respectively, P < 0.05).
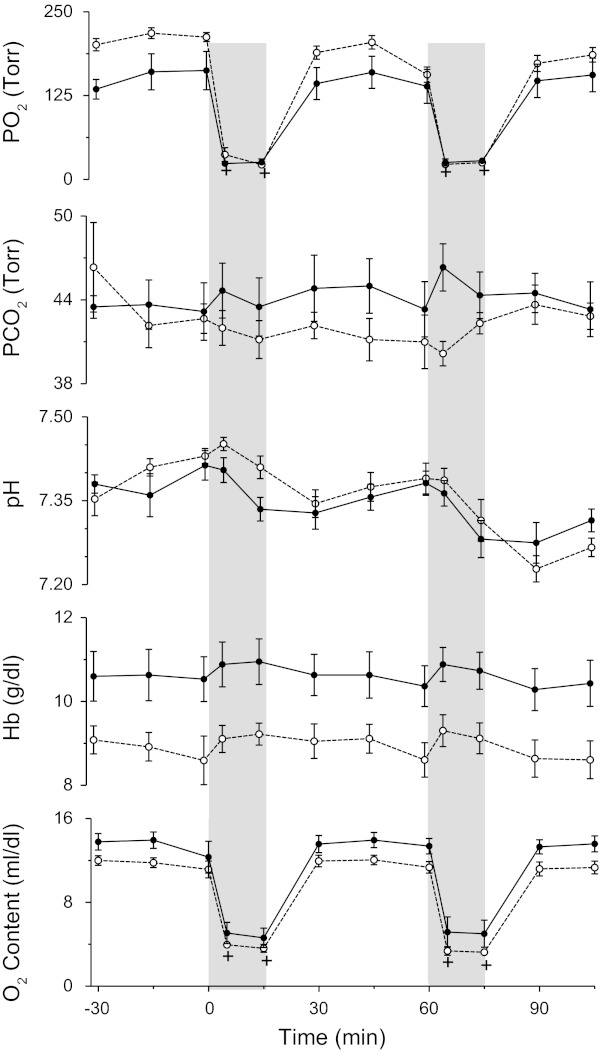
There was no significant difference between the two study groups with respect to baseline arterial pH, Pco2, or arterial oxygen content (Fig. 5). Hemoglobin concentrations were significantly higher in the high-altitude group than in the low-altitude group (10.7 ± 0.4 vs. 9.1 ± 0.2 g/dl, respectively, P < 0.01), resulting in higher baseline arterial oxygen contents (13.8 ± 0.6 vs. 12.0 ± 0.3 vol%, respectively, P = 0.01). Both groups received comparable acute hypoxic challenges with arterial oxygen contents falling to 4.7 ± 0.7 and 4.0 ± 0.3 ml/dl during the first hypoxic challenge and 5.2 ± 1.0 and 4.2 ± 0.3 ml/dl during the second hypoxic challenge in the high-altitude and low-altitude groups, respectively. Arterial pH decreased comparably from baseline during the hypoxic challenge in both low-altitude and high-altitude groups (P < 0.01).
Fig. 5.
Arterial blood gases, hemoglobin concentration, and oxygen concentration responses in lambs with gestation at high elevation (●) with those at low altitude (○). Vertical shading indicates 15-min intervals of hypoxia, first without and then with inhibition of the rho-kinase pathway with fasudil (2.5 mg/kg iv). +Significant difference from baseline (one-way ANOVA with repeated measures).
Pulmonary hemodynamics.
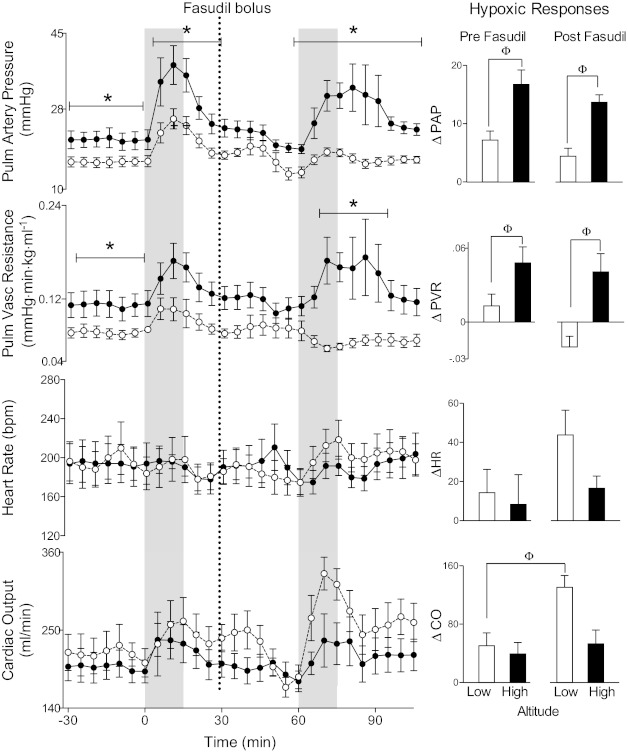
Following gestation at high-altitude, lambs had significantly higher baseline pulmonary artery pressures (21.1 ± 1.9 vs. 16.1 ± 1.1 mmHg, respectively, P < 0.05) and PVRs (0.112 ± 0.016 vs. 0.078 ± 0.006 mmHg·min·kg·ml−1 respectively, P < 0.05) than low-altitude controls, as shown in Fig. 6. Other baseline values, including pulmonary capillary wedge pressures (not shown), cardiac output, and heart rate, were comparable. In response to 15 min of acute hypoxia, pulmonary artery pressures and vascular resistances increased significantly from baseline in both high-altitude and low-altitude animals, while cardiac output and heart rate did not change significantly. Pulmonary capillary wedge pressures did not change significantly with hypoxia and did not vary significantly between the two study groups (data not shown).
Fig. 6.
Rho-kinase is not critical to the enhanced HPV response of newborn lambs exposed to chronic hypoxia. Pulmonary (Pulm) artery pressure, pulmonary vascular resistance, and cardiac output responses of lambs with gestation at high altitude (●) with those at low altitude (○). Vertical shading denotes intervals of acute hypoxia, and the vertical line shows the time of fasudil administration. Bar graphs indicate changes from baseline for pulmonary artery pressure (PAP), pulmonary vascular resistance (PVR), heart rate (HR), and cardiac output (CO) during hypoxia. The similar increases of PAP and PVR, irrespective of fasudil administration, do not support a role for rho-kinase in causing hypoxia-induced hypertension in newborn lambs. *Significant difference between study groups (two-way ANOVA), ΦSignificant difference (one-way ANOVA).
Responses to acute hypoxia, expressed as changes from averages of the 15 min preceding the start of hypoxia, are shown in Fig. 6. Both pulmonary artery pressure and PVR increased significantly more in the high-altitude animals than in the low-altitude controls during acute hypoxia, whereas cardiac output and heart rate responses were similar in the two groups of animals.
Figures 6 and 7 show responses to an intravenous bolus infusion of fasudil (see broken vertical line in figures). Fasudil significantly decreased pulmonary artery pressures of both high-altitude (from 23 ± 2 to 19 ± 1 mmHg, P < 0.05, 1-way ANOVA) and low-altitude animals (from 19 ± 2 to 14 ± 1 mmHg, P < 0.05). Fasudil administration also reduced cardiac output (from 234 ± 22 to 184 ± 17 (ml/min), P < 0.05) in the low-altitude animals, with no significant change in high-altitude animals. No significant changes were observed in PVR or heart rate following fasudil administration to either group of animals.
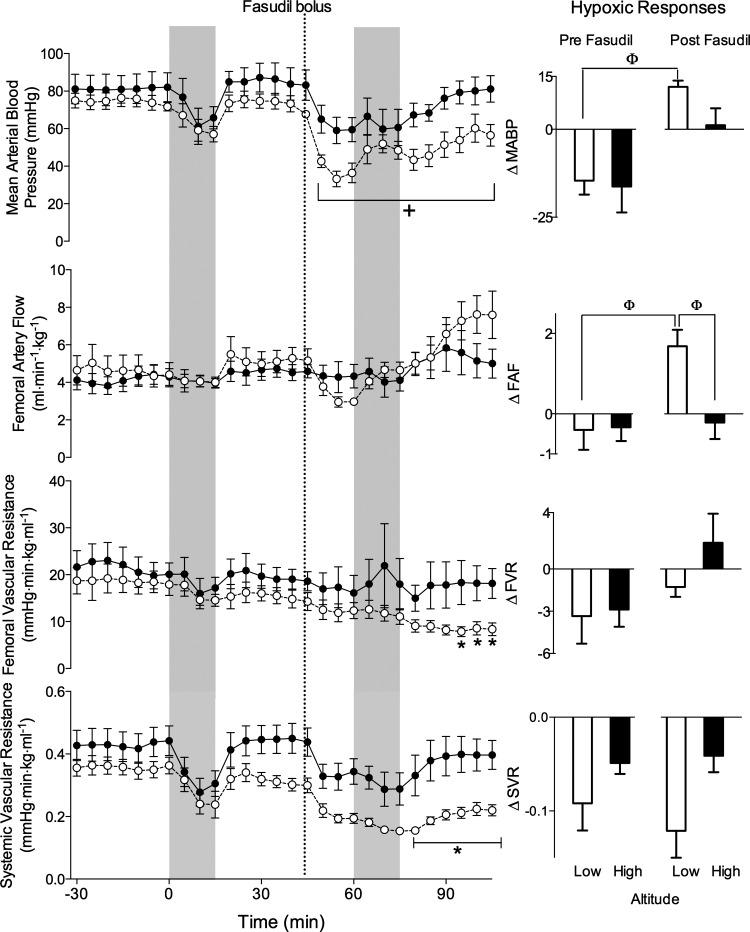
Fig. 7.
Rho-kinase inhibition results in greater systemic vasodilation in low-altitude lambs than in lambs exposed to chronic hypoxia. Systemic blood pressure, femoral artery flow, and femoral and systemic resistance to flow responses in lambs with gestation at high altitude (●) or low altitude (○). Vertical shading shows periods of acute hypoxia before and after administration of fasudil. Bar graphs indicate changes from baseline for mean arterial blood pressure (MABP), femoral artery flow (FAF), femoral vascular resistance (FVR), and systemic vascular resistance (SVR) during hypoxia. +Significant difference from mean baseline (−15 to 0 min, one-way ANOVA with repeated measures). *Significant difference between study groups (two-way ANOVA). ΦSignificant difference (one-way ANOVA).
Fasudil had no effect on pulmonary artery pressure or vascular resistance responses to acute hypoxia (Fig. 6). Following fasudil administration, the animals at low-altitude demonstrated a greater increase in cardiac output during acute hypoxia, compared with the response observed prior to fasudil administration (Fig. 6). In contrast, cardiac output responses to hypoxia were not affected by fasudil administration in the high-altitude group. As was observed prior to fasudil administration, heart rate did not change significantly during acute hypoxia in either group after fasudil.
Systemic hemodynamics.
There were no significant differences between the high-altitude and low-altitude groups for baseline mean arterial blood pressure, femoral artery blood flow, and systemic and femoral vascular resistance (Fig. 7). Acute hypoxia did not result in any significant changes in these three parameters, although systemic blood pressure and femoral vascular resistances tended to decrease in both the high-altitude and low-altitude animals.
Fasudil administration significantly reduced mean arterial blood pressure in both the low-altitude (from 73 ± 4 to 36 ± 5 mmHg, P < 0.01, one-way ANOVA) and high-altitude (from 84 ± 7 to 60 ± 6 mmHg, P < 0.01) animals. The magnitude of responses to acute hypoxia, expressed as a change from the 15-min period just prior to hypoxia, are shown to the right in Fig. 7. Following fasudil administration, acute hypoxia increased arterial blood pressure (from 36 ± 5 to 48 ± 5 mmHg, P < 0.01) and femoral artery flow of low-altitude animals relative to the postfasudil baseline (from 3.0 ml·kg−1·min−1 to 4.7 ± 0.4, P < 0.01). This contrasts with the decrease or lack of pressure and flow responses observed during hypoxia prior to fasudil administration. There was no significant difference in the response of high-altitude animals to acute hypoxia before or after fasudil administration. During recovery from the second hypoxic exposure, systemic and femoral vascular resistances of control lambs were significantly lower than those of the high-altitude lambs (P < 0.01), suggesting the vasodilating effects of fasudil on the peripheral vasculature were less in the high-altitude lambs.
DISCUSSION
Experiments in the present study in newborn lambs confirm that perinatal chronic hypoxia due to high-altitude living is detrimental to lung vascular function. The sustained moderate antenatal hypoxia of high altitude during the latter portion of gestation, and continuing in the first few days of life, increased basal pulmonary artery pressures and vascular resistance. This prolonged hypoxic exposure enhanced contraction of isolated pulmonary arteries in the presence of KCl or 5-HT and in response to acute hypoxia in vivo. The increased contractility of these arteries in the presence of 5-HT is not likely due to changes in modes of extracellular Ca2+ entry, but rather involves an increase in rho-kinase activity. In comparison, rho-kinase activity does not appear to be responsible for the increased response to acute hypoxia in vivo.
Perinatal hypoxia and pulmonary hypertension.
The tendency of antenatal (17) and postnatal (6, 16, 24, 62) chronic hypoxia to result in sustained pulmonary hypertension and increased pulmonary contractility has been demonstrated in a number of species. The effects of chronic hypoxia are characterized by an increase in contractile force in response to potassium-mediated depolarization and most pulmonary vasoconstrictors, a decrease in response to endothelium-derived vasodilators, and morphometric changes, such as increased medial wall thickness [see review by Shimoda and colleagues (63)]. The current study is in agreement with many aspects of these reports, in that isolated pulmonary arteries from the high-altitude lambs had increased contractile response to KCl and 5-HT. Further, the high-altitude lambs had elevated baseline pulmonary artery pressures and vascular resistances even while breathing 21% O2 at low altitude. This observation is consistent with reports from Herrera et al. (17, 18), who observed a similar or increased pressor response to acute hypoxia in newborn lambs exposed to chronic hypoxia in utero on the Andean altiplano. Interestingly, in contrast to the lamb, exposure of adult rats to chronic hypoxia results in an attenuated hypoxic pulmonary vasoconstriction (13, 34). These differences may be due to dissimilarities between rat and sheep, adult and newborn, or whole animal, and isolated lung preparations.
Rho-kinase and 5-HT-mediated contraction.
Our in vitro studies demonstrated that 5-HT-stimulated contraction was attenuated by fasudil to a greater extent in high-altitude than low-altitude arteries, indicating rho-kinase plays a role in the increased vasopressor response of high-altitude arteries to 5-HT (Fig. 1). Vascular smooth muscle contraction requires phosphorylation of the myosin light chain. The fraction of myosin light chain that is phosphorylated is determined by the competing actions of calcium/calmodulin-dependent myosin light-chain kinase that (61) induces vasoconstriction, and myosin light-chain phosphatase (MLCP), which leads to vasodilation. Thus, changes in MLCP activity can modulate vascular tone, independent of alterations in cytosolic calcium concentrations. Rho-kinase inhibits MLCP activity by phosphorylating its regulatory subunit MYPT1, resulting in decreased myosin light-chain dephosphorylation, increased vascular tone, and, hence, increased resistance to blood flow (61).
The finding that fasudil attenuates 5-HT-stimulated contraction in high-altitude more than in low-altitude arteries is consistent with the idea that chronic hypoxia increases rho-kinase expression and activity, as previously reported in near-term fetal lambs from our chronic hypoxia model (10). There is evidence that 5-HT can stimulate contraction of pulmonary artery smooth muscle cells by at least two different pathways. The predominant pathway for 5-HT-stimulated contraction involves its binding to extracellular G protein-coupled receptors, leading to increased activation of Ca2+-dependent pathways of vasoconstriction (12, 49), including activation of L-type Ca2+ channels and various nonselective cation channels that are inhibited by various blockers. Any effect of chronic hypoxia on this pathway would likely have resulted in different responses to one or more of the selective blockers of Ca2+ entry used in our studies. Instead, we found no differences between the effects of these blockers on high-altitude and low-altitude arteries, suggesting these pathways were not changed by chronic hypoxia. In addition, 5-HT may cause contraction through uptake of 5-HT into the cell via the 5-HT transporter, where it can then be covalently linked to the monomeric GTP-ase rhoA, resulting in constitutive activation of rho A (15). Rho-kinase is activated, in turn, by rhoA, resulting in increased intracellular Ca2+ sensitivity and vasoconstriction (33). An increase in rho-kinase expression in high-altitude arteries may lead to an increase in 5-HT-stimulated contraction via this pathway and would be consistent with our finding of a greater effect of rho-kinase blockers on the high-altitude arteries.
Rho-kinase and pulmonary vasoconstriction to acute hypoxia.
Rho-kinase plays a key role in both acute (5, 54, 69) and chronic (5, 21, 35, 37, 73) hypoxic pulmonary vasoconstriction in rodents. Concordant with these findings, Raj and colleagues (10) have reported that pulmonary arteries from near-term fetal sheep in our model of antenatal chronic hypoxia have increased expression and activity of rho-kinase II. Together with our current vessel bath studies demonstrating a more prominent role for rho-kinase in 5-HT-stimulated contraction of high-altitude compared with low-altitude arteries, these findings suggest that suppression of the rho-kinase pathway would be of benefit in treatment of pulmonary hypertension and was one rationale for the present in vivo study. However, our results indicate that although rho-kinase does play a role in the hypoxic pulmonary vasoconstriction (HPV) response of newborn lambs, it is not responsible for the heightened HPV response of high-altitude lambs. This conclusion is supported by the observation that although fasudil blunted the HPV response in both high-altitude and low-altitude lambs, the vasopressor response of high-altitude lambs remained heightened compared with that of low-altitude lambs, even following treatment with fasudil. Our finding of a lack of an enhanced role for rho-kinase in HPV following chronic hypoxia is contrary to findings of the rodent studies cited above and may be due to a difference in species or a difference between responses of newborn and adult pulmonary vasculature to hypoxia. It also is possible that increases in rho-kinase activity in the pulmonary arteries of our newborn lambs were masked by a concomitant decrease in rho-kinase activity in pulmonary veins; such a decrease has been reported by Dr. Raj's group in pulmonary veins of chronically hypoxic fetal sheep (9). However, this possibility is not supported by our observation that pulmonary venous pressures did not differ between the two groups of animals and were unaltered by acute hypoxic insult.
Systemic effects of rho-kinase inhibition.
Many pulmonary vasodilators are of limited clinical use because they cause systemic hypotension. Consistent with this concern, we found that intravenous fasudil measurably depressed mean arterial blood pressure systemic and femoral vascular resistance (Fig. 7). Fasudil also resulted in a significant decrease in cardiac output with no change in heart rate, suggesting stroke volume was decreased by either a decrease in cardiac contractility or decreased preload. The effect of rho-kinase inhibition on cardiac contractility has not been studied, but in dogs, it is reported to increase contractility (64), suggesting that the decrease in cardiac output in our lambs was more likely due to decreased preload caused by the marked fall in peripheral vascular resistance. Notably, these systemic effects were less pronounced in chronically hypoxic lambs, suggesting that the systemic effects of rho-kinase inhibition may be attenuated following chronic hypoxia. This observation is in contrast to studies that show adult rats with pulmonary hypertension due to infusion of the prostaglandin analog U-46619 have a greater fall in systemic vascular resistance in response to intravenous fasudil, compared with control rats (2). When considering the potential clinical use of rho-kinase blockers in newborn infants, it is clear that the systemic effects of rho-kinase inhibition warrant further study in various models of pulmonary hypertension before any studies are performed in human infants. It is also possible that administration of rho-kinase inhibitors by an inhaled route may provide pulmonary vasodilation while minimizing systemic effects (37).
Study limitations.
The responses of the pulmonary vasculature to prolonged intrauterine hypoxia include greater contractile force and proliferation of vascular smooth muscle. The acute HPV response of isolated rat pulmonary arteries can be divided into a rapid phase of vasoconstriction—lasting about 5 min and due largely to increases in intracellular calcium—and a prolonged phase that is dependent on increased calcium sensitivity (54). Rho-kinase appears to play a greater role during the second phase of HPV, as the rho-kinase inhibitor Y-27632 causes only minor attenuation of the first phase of constriction but abolishes the second phase (55). The biphasic HPV response, if present in newborn lambs, is not detectable in vivo by measurement of pulmonary artery pressures (20). While the 15-min acute hypoxic stimulus used in the current study was obviously long enough to assess the first phase of HPV response, it may not have been long enough to fully evaluate the effect of rho-kinase inhibition on the mechanisms that maintain the second phase of HPV. A further limitation is that the effects of chronic fasudil administration may differ from those of a single dose. Prolonged administration has been found to prevent or reverse pulmonary hypertension and morphologic changes caused by chronic hypoxia in newborn rats (73), and, thus, vary from single-dose administration, as employed in the present study. Finally, we note that the arteries used for the vessel bath studies were collected from the same lambs used in the in vivo protocol, and although both study groups were subjected to the same treatments, we cannot rule out the possibility that the arteries of one group were affected in a different way by the in vivo study protocol.
Perspectives and Significance
In summary, we have shown that acclimatization of lambs to moderate hypoxia during gestation and the first few days of life results in an increased contractile response of the pulmonary arteries to both acute hypoxia and 5-HT. Rho-kinase contributes to pulmonary artery tone in both control and high-altitude lambs, but does not appear to be involved in the heightened response of lambs at high altitude to acute hypoxia. In contrast, rho-kinase does play a role in the increased response of chronically hypoxic pulmonary arteries to 5-HT. These findings imply that multiple mechanisms underlie the increased contractility of chronically hypoxic pulmonary arteries. We speculate that hypoxic upregulation of components of contraction that work in parallel with rho-kinase may be involved in increased HPV response.
GRANTS
Funding for this work was supported by National Institute of Health Grants R01HL95973 (to A. B. Blood), P01 HD-31226-16 and 5R01 HD-003807-37 (LDL), and R03 HD-069746 (to S. M. Wilson). A portion of this material was also supported by the National Science Foundation under Major Research Instrumentation, Division of Biological Infrastructure Grant 0923559 (to S. M. Wilson) and the Loma Linda University School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.B.B., G.G.P., L.D.L., and S.M.W. conception and design of research; A.B.B., M.H.T., T.A.M., D.G.P., Q.B., J.M.R., and S.M.W. performed experiments; A.B.B., M.H.T., T.A.M., D.G.P., Q.B., J.M.R., and S.M.W. analyzed data; A.B.B., M.H.T., D.G.P., G.G.P., L.D.L., and S.M.W. interpreted results of experiments; A.B.B., M.H.T., D.G.P., Q.B., J.M.R., and S.M.W. prepared figures; A.B.B. drafted manuscript; A.B.B., M.H.T., J.M.R., G.G.P., L.D.L., and S.M.W. edited and revised manuscript; A.B.B., M.H.T., J.M.R., G.G.P., L.D.L., and S.M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the skillful surgical assistance of Shannon Bragg and Jeff E. Angermann for use of the CAT-12 hypoxic generator and Rachael Wilson for technical assistance with the wire myography studies. These experiments were performed in the laboratories of Sean Wilson, Gordon Power, and Arlin Blood in the Center for Perinatal Biology at Loma Linda University.
REFERENCES
- 1. Akopov SE, Zhang L, Pearce WJ. Regulation of Ca2+ sensitization by PKC and rho proteins in ovine cerebral arteries: effects of artery size and age. Am J Physiol Heart Circ Physiol 275: H930–H939, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Badejo AM, Jr, Dhaliwal JS, Casey DB, Gallen TB, Greco AJ, Kadowitz PJ. Analysis of pulmonary vasodilator responses to the Rho-kinase inhibitor fasudil in the anesthetized rat. Am J Physiol Lung Cell Mol Physiol 295: L828–L836, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Barrientos G, Bose DD, Feng W, Padilla I, Pessah IN. The Na+/Ca2+ exchange inhibitor 2-(2-(4-(4-nitrobenzyloxy)phenyl)ethyl)isothiourea methanesulfonate (KB-R7943) also blocks ryanodine receptors type 1 (RyR1) and type 2 (RyR2) channels. Mol Pharmacol 76: 560–568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clapham D, Nilius B, Owsianik G. Transient receptor potential channels. IUPHAR database (IUPHAR-DB), http://www.iuphar-db.org/DATABASE/FamilyMenuForward?familyId=78, 2010 [Google Scholar]
- 5. Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol 287: L656–L664, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Fike CD, Kaplowitz MR. Effect of chronic hypoxia on pulmonary vascular pressures in isolated lungs of newborn pigs. J Appl Physiol 77: 2853–2862, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Fujita H, Fukumoto Y, Saji K, Sugimura K, Demachi J, Nawata J, Shimokawa H. Acute vasodilator effects of inhaled fasudil, a specific Rho-kinase inhibitor, in patients with pulmonary arterial hypertension. Heart Vessels 25: 144–149, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, Abe K, Takeshita A, Shimokawa H. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart 91: 391–392, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao Y, Portugal AD, Liu J, Negash S, Zhou W, Tian J, Xiang R, Longo LD, Raj JU. Preservation of cGMP-induced relaxation of pulmonary veins of fetal lambs exposed to chronic high altitude hypoxia: role of PKG and Rho kinase. Am J Physiol Lung Cell Mol Physiol 295: L889–L896, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao Y, Portugal AD, Negash S, Zhou W, Longo LD, Usha Raj J. Role of Rho kinases in PKG-mediated relaxation of pulmonary arteries of fetal lambs exposed to chronic high altitude hypoxia. Am J Physiol Lung Cell Mol Physiol 292: L678–L684, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Goyal R, Creel KD, Chavis E, Smith GD, Longo LD, Wilson SM. Maturation of intracellular calcium homeostasis in sheep pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 295: L905–L914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goyal R, Papamatheakis DG, Loftin M, Vrancken K, Dawson AS, Osman NJ, Blood AB, Pearce WJ, Longo LD, Wilson SM. Long-term maternal hypoxia: the role of extracellular Ca2+ entry during serotonin-mediated contractility in fetal ovine pulmonary arteries. Reprod Sci 18: 948–962, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenlees KJ, Tucker A. Hypoxic pressor responses in lungs from rats acutely exposed to simulated high altitude. Respiration 45: 169–174, 1984 [DOI] [PubMed] [Google Scholar]
- 14. Guibert C, Marthan R, Savineau JP. 5-HT induces an arachidonic acid-sensitive calcium influx in rat small intrapulmonary artery. Am J Physiol Lung Cell Mol Physiol 286: L1228–L1236, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Guilluy C, Rolli-Derkinderen M, Tharaux PL, Melino G, Pacaud P, Loirand G. Transglutaminase-dependent RhoA activation and depletion by serotonin in vascular smooth muscle cells. J Biol Chem 282: 2918–2928, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Haworth SG, Hislop AA. Effect of hypoxia on adaptation of the pulmonary circulation to extra-uterine life in the pig. Cardiovasc Res 16: 293–303, 1982 [DOI] [PubMed] [Google Scholar]
- 17. Herrera EA, Pulgar VM, Riquelme RA, Sanhueza EM, Reyes RV, Ebensperger G, Parer JT, Valdez EA, Giussani DA, Blanco CE, Hanson MA, Llanos AJ. High-altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am J Physiol Regul Integr Comp Physiol 292: R2234–R2240, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Herrera EA, Riquelme RA, Ebensperger G, Reyes RV, Ulloa CE, Cabello G, Krause BJ, Parer JT, Giussani DA, Llanos AJ. Long-term exposure to high-altitude chronic hypoxia during gestation induces neonatal pulmonary hypertension at sea level. Am J Physiol Regul Integr Comp Physiol 299: R1676–R1684, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Homma N, Nagaoka T, Morio Y, Ota H, Gebb SA, Karoor V, McMurtry IF, Oka M. Endothelin-1 and serotonin are involved in activation of RhoA/Rho kinase signaling in the chronically hypoxic hypertensive rat pulmonary circulation. J Cardiovasc Pharm 50: 697–702, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nature Med 10: 1122–1127, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 97: 185–191, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, Nakano T. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J 70: 174–178, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Janssen LJ, Lu-Chao H, Netherton S. Excitation-contraction coupling in pulmonary vascular smooth muscle involves tyrosine kinase and Rho kinase. Am J Physiol Lung Cell Mol Physiol 280: L666–L674, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Kantores C, McNamara PJ, Teixeira L, Engelberts D, Murthy P, Kavanagh BP, Jankov RP. Therapeutic hypercapnia prevents chronic hypoxia-induced pulmonary hypertension in the newborn rat. Am J Physiol Lung Cell Mol Physiol 291: L912–L922, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Kita S, Iwamoto T. Inhibitory mechanism of SN-6, a novel benzyloxyphenyl Na+/Ca2+ exchange inhibitor. Ann NY Acad Sci 1099: 529–533, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Knock GA, Snetkov VA, Shaifta Y, Drndarski S, Ward JP, Aaronson PI. Role of src-family kinases in hypoxic vasoconstriction of rat pulmonary artery. Cardiovasc Res, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee CH, Poburko D, Sahota P, Sandhu J, Ruehlmann DO, van Breemen C. The mechanism of phenylephrine-mediated [Ca2+]i oscillations underlying tonic contraction in the rabbit inferior vena cava. J Physiol 534: 641–650, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li F, Xia W, Yuan S, Sun R. Acute inhibition of Rho-kinase attenuates pulmonary hypertension in patients with congenital heart disease. Pediatr Cardiol 30: 363–366, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Li F, Xia W, Yuan S, Sun R. Acute inhibition of Rho-kinase attenuates pulmonary hypertension in patients with congenital heart disease. Pediatr Cardiol 30: 363–366, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Liao P, Yu D, Li G, Yong TF, Soon JL, Chua YL, Soong TW. A smooth muscle Cav1.2 calcium channel splice variant underlies hyperpolarized window current and enhanced state-dependent inhibition by nifedipine. J Biol Chem 282: 35133–35142, 2007 [DOI] [PubMed] [Google Scholar]
- 31. MacLean MR, Sweeney G, Baird M, McCulloch KM, Houslay M, Morecroft I. 5-Hydroxytryptamine receptors mediating vasoconstriction in pulmonary arteries from control and pulmonary hypertensive rats. Br J Pharm 119: 917–930, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mair KM, MacLean MR, Morecroft I, Dempsie Y, Palmer TM. Novel interactions between the 5-HT transporter, 5-HT1B receptors and Rho kinase in vivo and in pulmonary fibroblasts. Br J Pharm 155: 606–616, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McMurtry IF, Abe K, Ota H, Fagan KA, Oka M. Rho kinase-mediated vasoconstriction in pulmonary hypertension. Adv Expt Med Biol 661: 299–308, 2010 [DOI] [PubMed] [Google Scholar]
- 34. McMurtry IF, Petrun MD, Reeves JT. Lungs from chronically hypoxic rats have decreased pressor response to acute hypoxia. Am J Physiol Heart Circ Physiol 235: H104–H109, 1978 [DOI] [PubMed] [Google Scholar]
- 35. McNamara PJ, Murthy P, Kantores C, Teixeira L, Engelberts D, van Vliet T, Kavanagh BP, Jankov RP. Acute vasodilator effects of Rho-kinase inhibitors in neonatal rats with pulmonary hypertension unresponsive to nitric oxide. Am J Physiol Lung Cell Mol Physiol 294: L205–L213, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Merritt JE, Armstrong WP, Benham CD, Hallam TJ, Jacob R, Jaxa-Chamiec A, Leigh BK, McCarthy SA, Moores KE, Rink TJ. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J 271: 515–522, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, McMurtry IF, Oka M. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med 171: 494–499, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–L672, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Ng LC, Kyle BD, Lennox AR, Shen XM, Hatton WJ, Hume JR. Cell culture alters Ca2+ entry pathways activated by store depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 294: C313–C323, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Ng LC, McCormack MD, Airey JA, Singer CA, Keller PS, Shen XM, Hume JR. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J Physiol 587: 2429–2442, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ng LC, Wilson SM, Hume JR. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol 563: 409–419, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ng LC, Wilson SM, McAllister CE, Hume JR. Role of InsP3 and ryanodine receptors in the activation of capacitative Ca2+ entry by store depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Br J Pharm 152: 101–111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niermeyer S. Cardiopulmonary transition in the high altitude infant. High Alt Med Biol 4: 225–239, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Niermeyer S, Andrade Mollinedo P, Huicho L. Child health and living at high altitude. Arch Dis Child 94: 806–811, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Niermeyer S, Moore LG. Hypoxic responses in infants. No known mechanism links hypoxia and sudden infant death syndrome. Br Med J 317: 675–676; author reply 677–678, 1998 [PubMed] [Google Scholar]
- 46. Niu CF, Watanabe Y, Iwamoto T, Yamashita K, Satoh H, Urushida T, Hayashi H, Kimura J. Electrophysiological effects of SN-6, a novel Na+/Ca2+ exchange inhibitor on membrane currents in guinea pig ventricular myocytes. Ann N Y Acad Sci 1099: 534–539, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Niu CF, Watanabe Y, Ono K, Iwamoto T, Yamashita K, Satoh H, Urushida T, Hayashi H, Kimura J. Characterization of SN-6, a novel Na+/Ca2+ exchange inhibitor in guinea pig cardiac ventricular myocytes. Eur J Pharmacol 573: 161–169, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Papamatheakis DG, Patel JJ, Blood Q, Merritt TT, Longo LD, Wilson SM. Depolarization-dependent contraction increase after birth and preservation following long-term hypoxia in sheep pulmonary arteries. Pulm Circ 2: 41–53, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Papamatheakis DG, Vemulakonda S, Blood Q, Goyal R, Rubalcava M, Vrancken K, Bennett A, Dawson A, Osman NJ, Blood AB, Pearce WJ, Longo LD, Wilson SM. Preservation of serotonin-mediated contractility in adult sheep pulmonary arteries following long-term high-altitude hypoxia. High Alt Med Biol 12: 253–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Parker TA, Roe G, Grover TR, Abman SH. Rho kinase activation maintains high pulmonary vascular resistance in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 291: L976–L982, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Poburko D, Liao CH, Lemos VS, Lin E, Maruyama Y, Cole WC, van BC. Transient receptor potential channel 6-mediated, localized cytosolic [Na+] transients drive Na+/Ca2+ exchanger-mediated Ca2+ entry in purinergically stimulated aorta smooth muscle cells. Circ Res 101: 1030–1038, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Ann Rev Physiol 68: 619–647, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Robertson TP, Aaronson PI, Ward JP. Ca2+ sensitization during sustained hypoxic pulmonary vasoconstriction is endothelium dependent. Am J Physiol Lung Cell Mol Physiol 284: L1121–L1126, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Robertson TP, Aaronson PI, Ward JP. Hypoxic vasoconstriction and intracellular Ca2+ in pulmonary arteries: evidence for PKC-independent Ca2+ sensitization. Am J Physiol Heart Circ Physiol 268: H301–H307, 1995 [DOI] [PubMed] [Google Scholar]
- 55. Robertson TP, Dipp M, Ward JP, Aaronson PI, Evans AM. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharm 131: 5–9, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rodat L, Savineau JP, Marthan R, Guibert C. Effect of chronic hypoxia on voltage-independent calcium influx activated by 5-HT in rat intrapulmonary arteries. Pflügers Arch 454: 41–51, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res 93: 548–556, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Satoh S, Takayasu M, Kawasaki K, Ikegaki I, Hitomi A, Yano K, Shibuya M, Asano T. Antivasospastic effects of hydroxyfasudil, a Rho-kinase inhibitor, after subarachnoid hemorrhage. J Pharmacol Sci 118: 92–98, 2012 [DOI] [PubMed] [Google Scholar]
- 59. Shibuya M, Suzuki Y, Sugita K, Saito I, Sasaki T, Takakura K, Nagata I, Kikuchi H, Takemae T, Hidaka H, Nakashima M. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg 76: 571–577, 1992 [DOI] [PubMed] [Google Scholar]
- 60. Shimokawa H, Seto M, Katsumata N, Amano M, Kozai T, Yamawaki T, Kuwata K, Kandabashi T, Egashira K, Ikegaki I, Asano T, Kaibuchi K, Takeshita A. Rho-kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc Res 43: 1029–1039, 1999 [DOI] [PubMed] [Google Scholar]
- 61. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Stenmark KR, Durmowicz AG, Roby JD, Mecham RP, Parks WC. Persistence of the fetal pattern of tropoelastin gene expression in severe neonatal bovine pulmonary hypertension. J Clin Invest 93: 1234–1242, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev 92: 367–520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Takahara A, Sugiyama A, Satoh Y, Yoneyama M, Hashimoto K. Cardiovascular effects of Y-27632, a selective Rho-associated kinase inhibitor, assessed in the halothane-anesthetized canine model. Euro J Pharm 460: 51–57, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, Verter J, Stoll BJ, Lemons JA, Papile LA, Shankaran S, Donovan EF, Oh W, Ehrenkranz RA, Fanaroff AA. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics 105: 14–20, 2000 [DOI] [PubMed] [Google Scholar]
- 66. Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L848–L858, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Wang J, Shimoda LA, Weigand L, Wang W, Sun D, Sylvester JT. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 288: L1059–L1069, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Wang J, Weigand L, Foxson J, Shimoda LA, Sylvester JT. Ca2+ signaling in hypoxic pulmonary vasoconstriction: effects of myosin light chain and Rho kinase antagonists. Am J Physiol Lung Cell Mol Physiol 293: L674–L685, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Wang Z, Jin N, Ganguli S, Swartz DR, Li L, Rhoades RA. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am J Respir Cell Mol Biol 25: 628–635, 2001 [DOI] [PubMed] [Google Scholar]
- 70. Wang Z, Lanner MC, Jin N, Swartz D, Li L, Rhoades RA. Hypoxia inhibits myosin phosphatase in pulmonary arterial smooth muscle cells: role of Rho-kinase. Am J Respir Cell Mol Biol 29: 465–471, 2003 [DOI] [PubMed] [Google Scholar]
- 71. Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol 289: L5–L13, 2005 [DOI] [PubMed] [Google Scholar]
- 72. Wilson SM, Mason HS, Ng LC, Montague S, Johnston L, Nicholson N, Mansfield S, Hume JR. Role of basal extracellular Ca2+ entry during 5-HT-induced vasoconstriction of canine pulmonary arteries. Br J Pharmacol 144: 252–264, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xu EZ, Kantores C, Ivanovska J, Engelberts D, Kavanagh BP, McNamara PJ, Jankov RP. Rescue treatment with a Rho-kinase inhibitor normalizes right ventricular function and reverses remodeling in juvenile rats with chronic pulmonary hypertension. Am J Physiol Heart Circ Physiol 299: H1854–H1864, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang S, Dong H, Rubin LJ, Yuan JX. Upregulation of Na+/Ca2+ exchanger contributes to the enhanced Ca2+ entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol 292: C2297–C2305, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Zhang S, Y uan JX, Barrett KE, Dong H. Role of Na+/Ca2+ exchange in regulating cytosolic Ca2+ in cultured human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 288: C245–C252, 2005 [DOI] [PubMed] [Google Scholar]
- 76. Zhu D, Medhora M, Campbell WB, Spitzbarth N, Baker JE, Jacobs ER. Chronic hypoxia activates lung 15-lipoxygenase, which catalyzes production of 15-HETE and enhances constriction in neonatal rabbit pulmonary arteries. Circ Res 92: 992–1000, 2003 [DOI] [PubMed] [Google Scholar]