Abstract
Overactive bladder (OAB) is often associated with increased involuntary detrusor smooth muscle (DSM) contractions during the bladder-filling phase. To develop novel therapies for OAB, it is critical to better understand the mechanisms that control DSM excitability and contractility. Recent studies showed that small-conductance Ca2+-activated K+ (SK) channels, SK3 channels, in particular, regulate human DSM function. However, the concept that SK channel-selective pharmacological activation can decrease the excitability and contractility directly in human DSM needs further exploration. Here, we studied the effect of the novel and potent SK channel activator, SKA-31 (or naphtho [1,2-d]thiazol-2-ylamine), on human DSM excitability and contractility at the cellular and tissue level. We used isometric tension recordings on human DSM-isolated strips and the perforated patch-clamp technique on freshly isolated native human DSM cells. SKA-31 significantly decreased spontaneous phasic contractions of DSM-isolated strips. In the presence of the SK channel blocker, apamin, the inhibitory effects of SKA-31 on the DSM spontaneous phasic contractions were significantly reduced. SKA-31 decreased the carbachol- and KCl-induced contractions in human DSM strips. Electrical field stimulation-induced contractions were significantly attenuated in the presence of SKA-31 at all stimulation frequencies (0.5–50 Hz). SKA-31 hyperpolarized the resting membrane potential of human DSM cells. Apamin abolished the hyperpolarizing effect of SKA-31, indicating the involvement of SK channel activation. These results support the concept that pharmacological activation of SK channels with selective openers may represent an attractive new pharmacological approach for decreasing DSM excitability and contractility, thus controlling OAB.
Keywords: SK channels, patch-clamp, detrusor
overactive bladder (oab) is characterized by symptoms that include urinary urgency, frequency, nocturia, with or without incontinence (1, 33). OAB is associated with significant costs and medical morbidity (32). Detrusor overactivity (DO) caused by myogenic or neurogenic factors has often been implicated as an underlying cause of OAB (7, 8). Antimuscarinics, currently the most widely used pharmacotherapy for OAB, have limited efficacy and poor tolerability due to substantial side effects, such as dry mouth, blurred vision, dizziness, and cognitive dysfunction, all of which can limit their use (3, 33). To develop a novel and more effective therapy for OAB, we need a better understanding of detrusor smooth muscle (DSM) physiology and pharmacology, and particularly the function of ion channels that determine DSM excitability and contractility. Among the ion channels, Ca2+-activated K+ (KCa) channels are arguably the most important regulator of DSM action potential, resting membrane potential, Ca2+ homeostasis, and contractility (25). Activation of KCa channels leads to cell membrane hyperpolarization, which reduces L-type voltage-gated Ca2+ channel activity and, thus global Ca2+, causing DSM relaxation (4, 6, 12, 15, 17, 24, 25, 31). Hence, KCa channel openers can serve as promising drug therapies aimed at reducing DO.
On the basis of their biophysical properties and single-channel conductance, KCa channels are divided into three major groups: large-conductance Ca2+-activated K+ (BK) channels, small-conductance Ca2+-activated K+ (SK) channels, and intermediate-conductance Ca2+-activated K+ (IK) channels (25). Whereas BK channels have been extensively studied in DSM (6, 12, 15, 17, 26), the role of SK and IK channels in human DSM is less clear. Recently, we demonstrated the expression and function of SK channels in freshly isolated guinea pig DSM cells (24). Genetic modifications of SK channels in mice have confirmed the critical roles of these channels in DSM function (13, 31). The above reports suggest that SK channels modulate DSM excitability and contractility in experimental animals; however, there is limited information about the functional role of SK/IK channels in human DSM (2, 9, 21, 22). Because considerable differences exist between species, the results obtained in animal models cannot unconditionally be extrapolated to humans.
Recent studies showed that SK channels are involved in the regulation of phasic contractile activity in DSM of patients without OAB symptoms (2) and in patients exhibiting neurogenic DO (22), suggesting the critical role of the SK channel in human DSM function. Our recent in vitro studies using the selective SK channel inhibitor apamin, as well as the selective IK channel inhibitor TRAM-34, showed that SK channels, but not IK channels have an important role in human DSM contractility (2). Further, we found that SK3 channels are the most predominant subtype in human DSM (2). Another study reported an inhibitory effect of SK/IK channel opener NS4591 on carbachol-evoked contractions in DSM-isolated strips from various species (21). However, the effects of pharmacological activation of SK channels with highly selective openers and electrophysiological approaches in freshly isolated human DSM cells have never been explored.
In this study, we investigated the mechanism of pharmacological activation of SK channels in human DSM using a multimethodological experimental approach, including perforated patch-clamp technique, isometric tension recordings, and a novel, potent, and selective SK/IK channel opener, naphtho [1,2-d]thiazol-2-ylamine (SKA-31). SKA-31 has been demonstrated to have a significantly improved selectivity for SK/IK channels (EC50: 250 nM for IK channel and EC50: 2 to 3 μM for all three SK channel isoforms) over other ion channels (28). This compound increases the Ca2+ sensitivity of SK/IK channels resulting in an apparent leftward shift of their Ca2+ activation curves and thus the channel open probability (28). We have recently shown that SKA-31 decreased DSM electrical and mechanical activity in animal models and that this effect was mediated by activation of the SK channels (24). However, the pharmacological effects of SK channel activation by the novel compound SKA-31 have never been examined in human DSM. Because humans are the target species of interest for therapeutic intervention, the present study on human DSM was critical to validate earlier studies on animal models. Our data showed that SKA-31 significantly decreased human DSM excitability and contractility, and this response was due to the selective potentiation of the SK channel activity.
MATERIALS AND METHODS
Tissue collection.
The human studies were reviewed and approved by the Medical University of South Carolina Institutional Review Board (protocol HR 16918). All human DSM specimens were obtained from patients who did not have a preoperative history of OAB and had an American Urological Association symptom score of less than 8. We only used DSM tissues not affected by the disease obtained from the bladder dome from a total of 21 patients who had radical cystectomy for bladder cancer (16 men and 5 women; 16 Caucasians, and 5 African-Americans). The average patient age was 62.7 ± 2.6 yr (from 25 to 76 yr of age). Mucosa-free DSM strips (2–3 mm wide and 5–7 mm long) were cut for DSM single-cell isolation and isometric DSM tension recordings.
DSM single-cell isolation.
DSM single cells were freshly isolated from human DSM, as described previously (2, 15, 16, 18). In brief, 1–2 human DSM strips were incubated in prewarmed 2-ml dissection solution supplemented with 1 mg/ml BSA, 1 mg/ml papain (Worthington, Lakewood, NJ), and 1 mg/ml dl-dithiothreitol for 12–18 min at 37°C. DSM tissues were transferred to a prewarmed 2 ml of dissection solution supplemented with 1 mg/ml BSA, 0.5 mg/ml collagenase type II (Sigma-Aldrich), 0.5 mg/ml trypsin inhibitor, and 100 μM CaCl2 for 12–15 min at 37°C. The digested DSM tissue was washed 2 or 3 times with 1 mg/ml BSA containing dissection solution and then was gently triturated with a fire-blunted Pasteur pipette to dissociate single DSM cells. Freshly isolated DSM cells were used for patch-clamp recordings within 6–12 h following isolation.
Electrophysiological recordings.
The amphotericin-B perforated whole cell configuration of the patch-clamp technique was used to conduct the electrophysiological recordings from freshly isolated human DSM cells (14, 15, 18). A 0.2–0.5-ml suspension of isolated DSM cells was placed into a recording chamber and allowed to adhere to the glass bottom for about 20 min. Cell debris and poorly adhered cells were washed out from the recording chamber by perfusing the cells with bath solution. Freshly isolated elongated, distinct, bright, and reflective DSM cells, when viewed illuminated under Axiovert 40CFL microscope, were selected for patch-clamp recordings. The DSM cell's resting membrane potential was recorded in current-clamp mode (I=0) of the whole cell patch-clamp configuration using an Axopatch 200B amplifier, Digidata 1440A, and pCLAMP version 10.2 software (Molecular Devices, Union City, CA). All patch-clamp experiments were conducted at room temperature (22–23°C).
Isometric DSM tension recordings.
Isometric DSM tension recordings were performed as previously described (2, 14, 15, 18). Briefly, DSM-isolated strips (2–3 mm wide and 5–7 mm long) were mounted in 10-ml tissue baths containing physiological salt solution (PSS), which was maintained at 37°C and continuously gassed with 95% O2-5% CO2 (pH: 7.4). One end of each strip was secured to the bottom of the tissue bath, and the other end was attached to a force displacement transducer. For isometric DSM tension recordings, an initial tension (∼1 g) was applied to the DSM strips. The strips were allowed to equilibrate for at least 60 min, while being washed every 15 min with fresh PSS. Three different sets of experimental series were set up. In the first set of experimental series, the DSM strips exhibiting spontaneous phasic contractions were allowed to stabilize for at least 20 min followed by the application of SKA-31. In a separate experimental series, apamin was applied first, and the apamin-induced DSM contractions were allowed to reach a stable level before application of SKA-31 in the continued presence of apamin. In the second set of experimental series, the strips were either exposed to the ACh analog, carbachol (0.1 μM), or KCl (20 mM and 60 mM), and allowed to equilibrate until stable responses were obtained. Following stabilization of the pharmacologically induced contractions, SKA-31 was applied. To eliminate any potential effects caused by neurotransmitter release, the experiments with myogenic contractions were performed in the presence of TTX (1 μM), a selective blocker of neuronal voltage-gated Na+ channels.
In the third set of experimental series, nerve-evoked contractions were induced by electrical field stimulation (EFS). Two platinum electrodes were set in parallel to each other on both sides of the DSM strips with a sufficient gap between them to allow for effective EFS. EFS pulses were generated by a PHM-152I stimulator (Med Associates, St. Albans, VT), and the EFS pulse parameters were as follows: 0.75-ms pulse width, 20-V pulse amplitude, 3-s stimulus duration, and polarity was reversed for alternating pulses. EFS frequency applied was either 20 Hz at 1-min intervals or ranged from 0.5 to 50 Hz at 3-min intervals. At the end of each experiment, the DSM strips were washed out with PSS, and the ability of the DSM strips to contract was reevaluated using KCl (60 mM). The DSM contractions were recorded using a Myomed myograph system (Med Associates).
Solution and drugs.
Dissection solution used for preparation of DSM strips and enzymatic isolation of fresh DSM single cells had the following composition (in mM): 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 HEPES, 2 MgCl2, and the pH was adjusted to 7.3 with NaOH. The external bath solution used for the patch-clamp recordings contained (in mM): 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, 10 HEPES, with pH adjusted to 7.4 with NaOH. The patch-pipette solution was composed of (in mM) 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, 0.05 EGTA, with pH adjusted to 7.2 with NaOH. Pipette solution was filtered using a 0.2-μm filter and stored at −20°C. Freshly dissolved amphotericin-B was added to the pipette solution (200 μg/ml) just before the experiment, and the solution was replaced every 1–2 h. For DSM contraction studies, freshly prepared PSS had the following composition (in mM): 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, and 11 glucose, aerated with 95% O2-5% CO2 (pH = 7.4). SKA-31 was a kind gift from Dr. Heike Wulff (University of California at Davis). SKA-31 was dissolved daily in DMSO. TTX, carbachol, and apamin were purchased from Sigma-Aldrich (St. Louis, MO) and were dissolved in double-distilled water. Paxilline was purchased from Sigma-Aldrich and was dissolved in DMSO. The final concentration of DMSO in the bath solutions did not exceed 0.1%.
Data analysis and statistics.
MiniAnalysis software (Synaptosoft, Fort Lee, NJ) was used to analyze the DSM phasic contraction parameters. Because of the phasic pattern of the spontaneous contractile responses, data were analyzed as average changes in phasic contraction amplitude, muscle integral force (the area under the curve of the phasic contractions), duration (defined as the time period of the phasic contraction measured at the level of 50% of the peak of the contraction amplitude), frequency (contractions per minute), and tone (phasic contraction baseline curve). Data were normalized to the spontaneous contractions (taken to be 100%) and were expressed as percentages. For the EFS-induced contractions (0.5–50 Hz), the contraction amplitude at EFS frequency of 50 Hz under control conditions was taken to be 100%, and the data were normalized. For the patch-clamp studies, using Clampfit software (10.2 version; Molecular Devices, Union City, CA), at least 6–10 min of stable recordings in the absence of test compounds (control) and another 6–10 min of recordings in the presence of SKA-31 were averaged. Statistical analysis was performed with GraphPad Prism 4.03 software (GraphPad Software, San Diego, CA), and the data are expressed as means ± SE for the n (the number of DSM strips or cells), isolated from N (number of patients). In all experiments, each DSM strip or cell acted as its own control. Results obtained before and after compound treatment were compared with either paired Student's t-test or two-way ANOVA followed by Bonferroni's post hoc test. Data were considered statistically significant when P < 0.05. Corel Draw Graphic Suite X3 software (Corel, Mountain View, CA) was used to illustrate the data.
RESULTS
Pharmacological activation of SK channels with SKA-31 caused inhibition of spontaneous phasic contractions in human DSM-isolated strips.
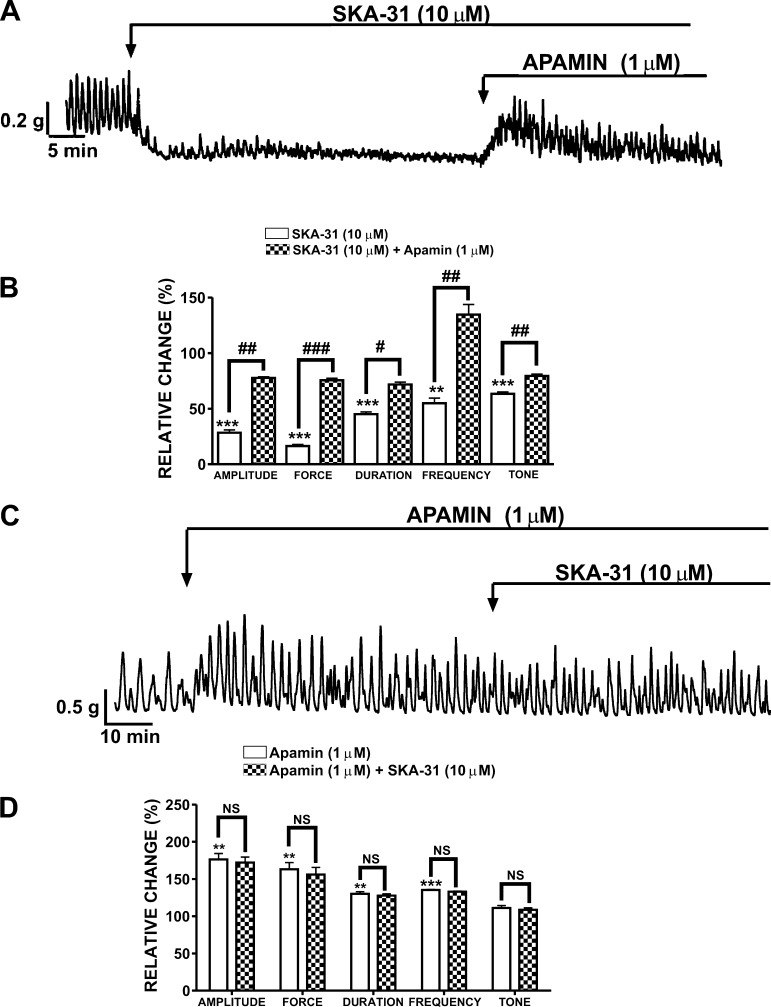
Human DSM-isolated strips exhibited spontaneous phasic contractions with an irregular amplitude, duration, and frequency. The spontaneous contractions were myogenic, as they were not blocked by inhibiting nerve activity with 1 μM TTX. Our recent study reported EC50 values in submicromolar to lower micromolar range for the inhibitory effects of SKA-31 on guinea pig DSM contractility (24). Hence, in the present study, we used 10 μM SKA-31, which falls into this concentration range. Pharmacological activation of SK channels with SKA-31 (10 μM) decreased the spontaneous phasic contraction amplitude by 71.6 ± 3.2%, muscle integral force by 83.6 ± 3.4%, contraction duration by 54.9 ± 1.9%, contraction frequency by 45.1 ± 4.5%, and muscle tone by 36.6 ± 1.7% (n = 4, N = 3; P < 0.05; Fig. 1, A and B). Using apamin, a selective SK channel blocker, we evaluated whether SKA-31 inhibitory effects were mediated by the SK channel activity. The inhibition of DSM spontaneous contractile activity by 10 μM SKA-31 was restored by 1 μM apamin, suggesting an involvement of the SK channels (n = 4, N = 3; P < 0.05; Fig. 1, A and B). In a separate set of experiments, DSM strips were pretreated with apamin (1 μM) alone for at least 20 min. Apamin (1 μM) significantly increased the contraction amplitude to 176.3 ± 7.9%, muscle integral force to 163.0 ± 8.9%, contraction duration to 130.8 ± 2.8%, and contraction frequency to 135.1 ± 0.9% (n = 4, N = 4; P < 0.05; Fig. 1, C and D). These data are consistent with our recent report demonstrating the effects of apamin on human DSM contractility (2). Preincubation of human DSM strips with apamin (1 μM) antagonized the inhibitory effects of SKA-31 on spontaneous phasic contractions (Fig. 1, C and D). These results suggest that SKA-31-mediated relaxation in human DSM is due to activation of the SK channels.
Fig. 1.
Inhibitory effects of naphtho [1,2-d]thiazol-2-ylamine (SKA-31) on the spontaneous phasic contractions of human detrusor smooth muscle (DSM) isolated strips. A: representative recording from a spontaneously contracting human DSM strip. SKA-31 (10 μM) robustly decreased the spontaneous contractile activity in human DSM-isolated strips. In the continuous presence of SKA-31, the SK channel blocker apamin (1 μM) counteracted the relaxant activity of SKA-31 and restored the phasic contractions. B: summary data and statistical analysis showing the inhibitory effects of SKA-31 (10 μM) and subsequent apamin (1 μM) effects on the spontaneous phasic contraction amplitude, muscle integral force, contraction duration, contraction frequency, and muscle tone (n = 4, N = 3; ***P < 0.005; **P < 0.01 vs. control, ###P < 0.005; ##P < 0.01; #P < 0.05 vs. apamin effect). C: representative recording illustrating that the relaxant effects of SKA-31 were suppressed in the presence of 1 μM apamin. D: summary data and statistical analysis showing the effects of SKA-31 on the spontaneous phasic contraction amplitude, muscle integral force, duration, frequency, and muscle tone in the presence of 1 μM apamin (n = 4, N = 4; ***P < 0.005; **P < 0.01 vs. control. NS, not significant). All experiments were conducted in the presence of 1 μM TTX.
Pharmacological activation of SK channels with SKA-31 caused inhibition of carbachol-induced contractions in human DSM-isolated strips.
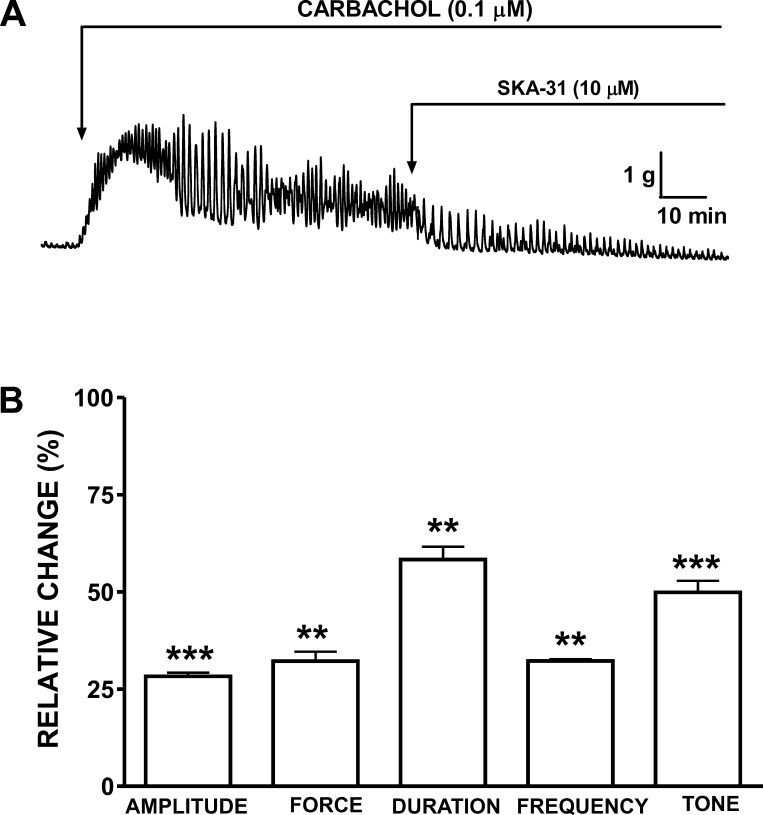
Human DSM-isolated strips were precontracted with carbachol (0.1 μM). Carbachol provided the DSM preparations with a high degree of well-maintained phasic and tonic contractions (Fig. 2A). Exposure of DSM strips to 10 μM SKA-31 significantly reduced the amplitude of carbachol-induced contractions by 71.7 ± 0.9%, muscle integral force by 67.8 ± 2.4%, contraction duration by 41.7 ± 3.3%, contraction frequency by 67.8 ± 0.5%, and muscle tone by 50.2 ± 3.0% (n = 5, N = 5; P < 0.05; Fig. 2B).
Fig. 2.
Inhibitory effects of SKA-31 on carbachol-induced contractions in human DSM-isolated strips. A: representative recording from a DSM-isolated strip demonstrating the inhibitory effects of SKA-31 (10 μM) on 0.1 μM carbachol-induced contractions. B: summary data and statistical analyses showing the inhibitory effects of SKA-31 on the carbachol-induced phasic contraction amplitude, muscle integral force, contraction duration, contraction frequency, and muscle tone in human DSM-isolated strips (n = 5, N = 5; ***P < 0.005; **P < 0.01 vs. control). All experiments were performed in the presence of 1 μM TTX.
Differential effects of SKA-31 on 20 mM and 60 mM KCl-induced contractions in human DSM-isolated strips.
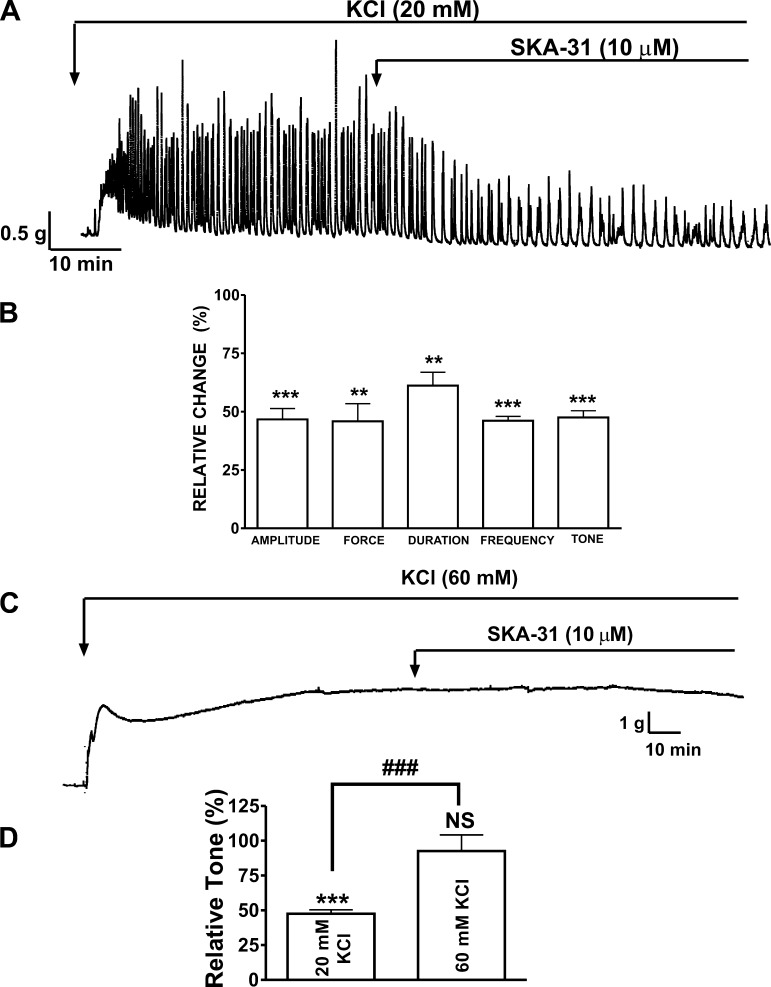
Next, we examined the ability of SKA-31 to relax human DSM strips precontracted by different concentrations of the depolarizing agent KCl. Maximal stable phasic contractions were produced within 20–30 min of exposure to KCl, after which time, the DSM strips were exposed to SKA-31 (10 μM). Figure 3A shows that 20 mM KCl increased DSM phasic and tonic contractions, and subsequent addition of SKA-31 significantly reduced these contractions. SKA-31 (10 μM) reduced the 20 mM KCl-induced phasic contraction amplitude by 53.3 ± 4.6%, muscle integral force by 54.2 ± 7.6%, contraction duration by 38.8 ± 5.7%, contraction frequency by 53.9 ± 1.8%, and muscle tone by 52.5 ± 2.9% (n = 5, N = 5; P < 0.05; Fig. 3B). In a separate set of experiments, the effects of SKA-31 were also examined in human DSM strips, which were precontracted with 60 mM KCl. The relaxant effect of SKA-31 was significantly reduced in the solution containing high K+ concentration (60 mM) (Fig. 3, C and D). In the presence of 20 mM KCl, the muscle tone was reduced by 52.5 ± 7.5%; however, in the presence of 60 mM KCl, the tone was reduced by only 7.5 ± 4.8% (n = 6, N = 5; P > 0.05; Fig. 3D). These results suggest that the inhibitory effect of SKA-31 depends on the K+ gradient, and this is consistent with a mechanism of SKA-31 activating the K+ channels.
Fig. 3.
Differential effects of SKA-31 on 20 mM and 60 mM KCl-induced contractions in human DSM-isolated strips. A: representative recording illustrating the inhibitory effects of SKA-31 on contractions induced by 20 mM KCl in human DSM strip. 20 mM KCl increased the phasic contractions in DSM preparations, and SKA-31 significantly reduced the 20 mM KCl-induced contractions. B: summary data and statistical analyses showing the inhibitory effects of SKA-31 (10 μM) on 20 mM KCl-induced phasic contraction amplitude, muscle integral force, contraction duration, contraction frequency, and tone in human DSM (n = 5, N = 5; ***P < 0.005; **P < 0.01). C: A representative recording from a DSM-isolated strip showing a lack of SKA-31 (10 μM) effect on 60 mM KCl-induced tonic contraction. D: summary data illustrating a lack of significant effect of SKA-31 (10 μM) on 60 mM KCl-induced tonic contraction (n = 6, N = 5; ***P < 0.005 vs. control, ###P < 0.005; 60 mM KCl vs. 20 mM KCl. NS, not significant vs. control). All experiments were performed in the presence of 1 μM TTX.
Pharmacological activation of SK channels with SKA-31 caused inhibition of EFS-induced contractions of human DSM-isolated strips.
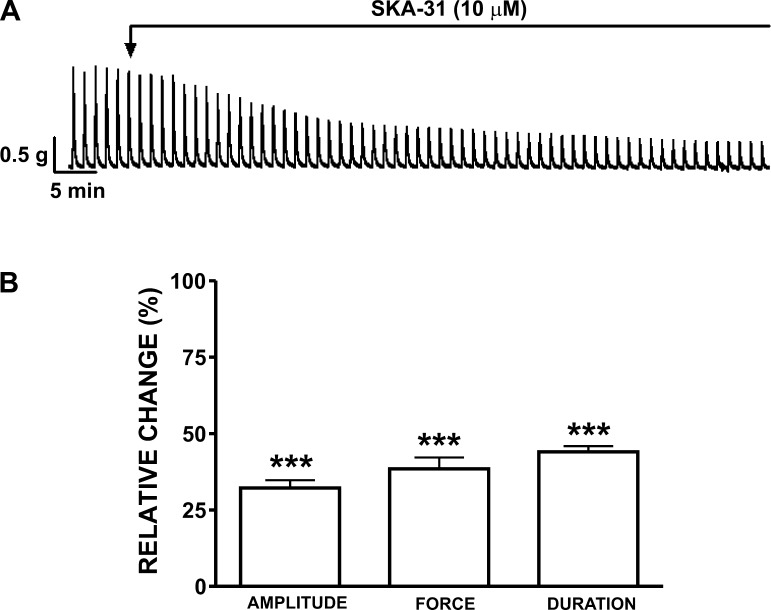
EFS stimulates neuronal release of the excitatory neurotransmitters-ACh and ATP. EFS at 20-Hz frequency produced stable and highly reproducible contractile responses (Fig. 4A). SKA-31 (10 μM) decreased the 20-Hz EFS-induced contraction amplitude by 67.8 ± 2.6%, muscle integral force by 61.5 ± 3.7%, and contraction duration by 56.0 ± 1.9%, compared with the control responses evoked by EFS in the absence of SKA-31 (n = 6, N = 6; P < 0.05; Fig. 4B).
Fig. 4.
Inhibitory effects of SKA-31 on 20 Hz EFS-induced contractions in human DSM-isolated strips. A: representative recording of 20 Hz EFS-induced contractions in a human DSM-isolated strip and the inhibitory effects of 10 μM SKA-31. B: summary data demonstrating a significant decrease in 20 Hz EFS-induced contraction amplitude, muscle integral force, and duration in the presence of SKA-31 (10 μM) (n = 6, N = 6; ***P < 0.005).
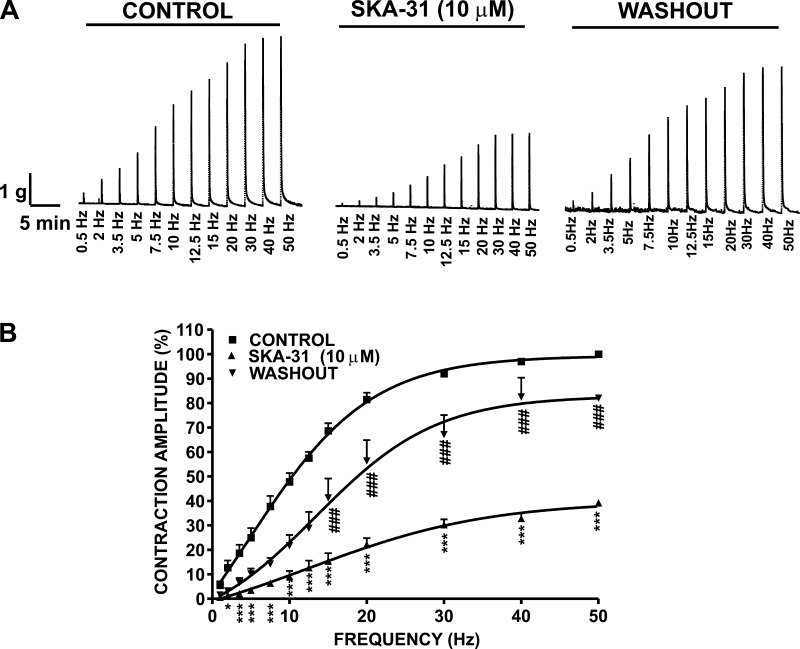
In the next experimental series, EFS at stimulation frequencies of 0.5, 2, 3.5, 5, 7.5, 10, 12.5, 15, 20, 30, 40, and 50 Hz were applied to human DSM-isolated strips. The amplitude of DSM contractile responses caused by EFS (0.5–50 Hz) increased in a frequency-dependent manner (Fig. 5). After an initial EFS frequency-response curve was generated under control conditions, DSM strips were preincubated with 10 μM SKA-31 for 30 min, and then a second EFS frequency-response curve was generated using the same EFS parameters as indicated above. As shown in Fig. 5A, SKA-31 caused an inhibition of the EFS frequency-response curve with a significant decrease above stimulation frequencies of 3.5 Hz. At 50 Hz, the contraction amplitude was reduced by 60.8 ± 1.1% (n = 6, N = 4; P < 0.05; Fig. 5B). The inhibitory effect of SKA-31 was reversed by washing the DSM strips with fresh PSS (7 washes within 5 min), and the contraction amplitude at 50 Hz after washout of SKA-31 recovered to 82.0 ± 1.4% of the control response (n = 6, N = 4; P < 0.05; Fig. 5B).
Fig. 5.
Inhibitory effect of SKA-31 on 0.5–50 Hz EFS-induced contractions in human DSM. A: representative recording illustrating EFS frequency-dependent contractile responses induced by EFS at increasing stimulation frequencies (0.5–50 Hz) in a human DSM-isolated strip in the absence (control), or presence of SKA-31 (10 μM), and following the washout of SKA-31. B: frequency-response curves on the amplitude of EFS-induced contractions for the control, 10 μM SKA-31, and following the washout of SKA-31. SKA-31 caused a significant decrease in the EFS-induced contraction amplitude above 3.5 Hz EFS. Data are expressed as a percentage of the control response at 50 Hz (n = 6, N = 4; ***P < 0.005, *P < 0.05 vs. control; ###P < 0.005 vs. SKA-31).
SKA-31 hyperpolarized the resting membrane potential in freshly isolated human DSM cells by activation of the SK channels.
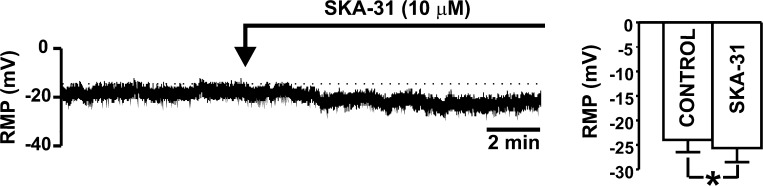
Amphotericin-B perforated whole cell patch-clamp experiments were performed in current-clamp mode to study the effect of SKA-31 (10 μM) on DSM cell resting membrane potential. The average cell capacitance of all recorded human DSM cells was 19.2 ± 1.6 pF (n = 18, N = 6). All experiments were performed in the presence of paxilline (300 nM) to eliminate the contribution of the BK channels to the resting membrane potential. In freshly isolated human DSM cells, SKA-31 (10 μM) significantly hyperpolarized the resting membrane potential (n = 12, N = 4; P < 0.05; Fig. 6). To test whether this hyperpolarizing effect was due to activation of the SK channels, we also recorded the resting membrane potential in the presence of 1 μM apamin. Pretreatment with apamin (1 μM) prevented the SKA-31-induced membrane hyperpolarization in human DSM cells. The mean value of the resting membrane potential in the presence of apamin (1 μM) was −27.1 ± 4.0 mV, and after the application of SKA-31 (10 μM), the mean value of the resting membrane potential did not change significantly and remained at −25.4 ± 3.6 mV (n = 6, N = 4; P > 0.05). These results suggest that pharmacological activation of the SK channels with SKA-31 hyperpolarizes the resting membrane potential in freshly isolated human DSM cells.
Fig. 6.
Activation of the SK channels with SKA-31 hyperpolarizes the resting membrane potential (RMP) of freshly isolated human DSM cells. A representative recording illustrating that SKA-31 (10 μM) hyperpolarizes the RMP in human DSM cells. Bar graph shows summary data for the effect of SKA-31 (10 μM) on the RMP in human DSM cells (n = 12, N = 4; *P < 0.05). All experiments were performed in the presence of 300 nM paxilline, a BK channel-selective inhibitor.
DISCUSSION
The present study reveals the cellular mechanism and functional role of SK channel pharmacological activation on the excitability and contractility of human DSM. The novel, potent, and selective SK/IK channel opener SKA-31 was tested directly on freshly isolated human DSM strips and native human DSM cells. Our experimental observations provide persuasive evidence that pharmacological activation of SK channels exerts a direct inhibitory effect on the contractility of human DSM. Furthermore, our results suggest that SKA-31 hyperpolarized human DSM cell resting membrane potential, thus inhibiting DSM contractions by its direct action on SK channels. Results from this study strongly suggest that pharmacological activation of SK channels can effectively reduce excitability and contractility in human DSM.
We studied the effect of an SK/IK channel opener on human DSM excitability using the patch-clamp technique. Our current-clamp experiments revealed that SKA-31 (10 μM) caused a small, but statistically significant, hyperpolarization of the resting membrane potential in human DSM cells (Fig. 6). As evident from our in vitro studies on DSM contractility, this reduction in membrane excitability was sufficient to inhibit human DSM spontaneous contractions (Fig. 1). This finding is consistent with previous studies on experimental animals, suggesting that modest membrane hyperpolarization, either by SKA-31 or KATP channel openers, can cause a substantial inhibition of spontaneous DSM contractions (24, 27). Furthermore, SKA-31-induced hyperpolarization in human DSM cells was blocked by pretreatment with 1 μM apamin, implicating the involvement of SK channels. Thus, our electrophysiological studies support the concept that SKA-31 hyperpolarizes the human DSM cell membrane by activation of the SK channels, consistent with our recent findings in guinea pig DSM (24) and human DSM (2).
Isolated human DSM strips often develop spontaneous phasic contractions, generally occurring upon action potential discharge, resulting in a Ca2+ influx through L-type voltage-gated Ca2+ channels and associated Ca2+ transients (10, 14, 15, 18). Simultaneous measurements of membrane potential and spontaneous contractions in guinea pig DSM revealed a clear correlation between the parameters of action potential and phasic DSM contractility (11). The analysis of DSM spontaneous contractions and electrophysiological patch-clamp recordings in the present study suggests that under physiological conditions, the activation of SK channels in human DSM decreases DSM phasic contractility by hyperpolarizing the cell membrane, moving the DSM resting membrane potential away from the threshold necessary for activation of the DSM action potential, and thus inhibiting the related spontaneous phasic contractions.
Spontaneous phasic contractions have been shown to occur more commonly and are of greater frequency and amplitude in DSM strips obtained from patients with clinically diagnosed DO compared with controls (20, 22). Therefore, these data suggest that there may be a role in heightened spontaneous activity in some OAB patients and that the modulation of this activity may reduce or prevent the symptoms of clinically diagnosed OAB. Pharmacological activation of SK channels with SKA-31 significantly inhibited all parameters of the spontaneous phasic contractions, indicating a role of SK channels in maintaining DSM contractility. Patients with DO have showed increased DSM tone due to ongoing stimulation by ACh released from nerves (20). A reduced DSM tone during the bladder-filling phase is linked to increased bladder capacity (13). In the present study, we demonstrate that pharmacological activation of SK channels with SKA-31 decreases human DSM tone, which can potentially improve functional bladder capacity. Apamin, a selective SK channel blocker, readily reversed the relaxant effect of SKA-31, suggesting that SKA-31 inhibits human DSM contractions mainly by its action on the SK channels (Fig. 1, A and B). Pretreatment of human DSM with apamin also blocked the relaxant effects of SKA-31, further supporting the concept that SKA-31 acts selectively on the SK channels to induce DSM relaxation (Fig. 1, C and D). These findings suggest that pharmacological activation of SK channels in human DSM has the potential to be an effective treatment for patients with myogenic derived OAB. Future clinical trials are needed to evaluate the value of the SK channel in developing drugs for OAB treatment and to identify any possible side effects.
IK channels have been previously identified at the molecular level by our group and others in guinea pig and murine DSM (23, 24). However, recent studies from our laboratory using RT-PCR, real-time PCR, Western blot analysis, and immunohistochemistry showed no detectable expression of IK channels in freshly isolated human DSM (2). We previously demonstrated no contribution of the IK channels to SKA-31-induced relaxation in guinea pig DSM (24). Our previous results, which suggest that IK channels do not play an important role in basal or stimulated human DSM contractility, support a conclusion that SKA-31 effects seen in the current study are due to interaction with the SK channels.
SKA-31 showed remarkable potency in inhibiting contractions induced pharmacologically by carbachol or 20 mM KCl. Muscarinic receptor-mediated Ca2+ mobilization is a key event in DSM contraction (30). SK channel activation by SKA-31 significantly reduced carbachol-induced contraction amplitude, muscle integral force, contraction duration, frequency, and muscle tone (Fig. 2), suggesting that activation of the SK channels inhibits Ca2+ influx in the DSM. Stimulation of DSM with 20 mM KCl acts by depolarizing the cell membrane, thus increasing the Ca2+ entry through L-type voltage-gated Ca2+ channels. SKA-31 caused a significant relaxation of human DSM strips precontracted with 20 mM KCl (Fig. 3, A and B), suggesting that SK channel activation counteracts pathways requiring depolarization of the DSM cell membrane. However, the relaxant effect of SKA-31 was negligible when the extracellular K+ concentration was raised to 60 mM KCl (Fig. 3, C and D). In the presence of this high concentration of extracellular KCl, the electrochemical gradient for K+ efflux is reduced, leading to a reduced effect of K+ channel openers (18, 19, 29). These results further indicate the K+ channel selectivity of SKA-31.
Neurogenic OAB involves excessive DSM contractions that occur as a result of abnormal activation of the micturition reflex (7). Nerve-evoked contractions elicited by EFS involves participation of both muscarinic and purinergic components. At lower EFS frequencies, ATP released from purinergic nerves is responsible for DSM contractions, and as the EFS frequencies increase, the neurotransmission from cholinergic nerves contributes proportionally more to DSM contractions (5, 34). In the present study, EFS experiments were designed to assess the effect of SK channel activation at various stimulation frequencies (0.5–50 Hz; Fig. 5). Our results showed that in human DSM, SK channel activation significantly reduced EFS-induced contractions (Figs. 4 and 5), thus demonstrating that the SK channels could be novel pharmacological targets for neurogenic OAB.
Perspectives and Significance
SK channels may constitute attractive new pharmacological targets for OAB that currently lack effective therapies. However, the relationship between SK channel activity and the bladder functional effects must first be clearly understood. We used a multifaceted approach, including the perforated patch-clamp technique, to study the effects of the novel SK channel opener, SKA-31, on freshly isolated native human DSM cells and correlated these findings with DSM tissue contractility. The results from our study revealed that selective pharmacological activation of SK channels with the novel compound SKA-31 effectively decreases human DSM excitability and contractility. Together with recent findings (2, 21, 22, 24), the present results point to a key role for SK channels in the control of human urinary bladder function. The use of SK channel openers could be a novel therapeutic approach for the treatment of bladder dysfunction resulting from DO.
GRANTS
This study was supported by National Institutes of Health Grants DK-084284 and DK-083687 to Georgi V. Petkov. The study was also supported, in part, by a fellowship from the American Urological Association Foundation Research Scholars Program and the Allergan Foundation to Shankar P. Parajuli.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.P.S., S.P.P., K.L.H., and G.V.P. performed experiments; R.P.S., S.P.P., K.L.H., and G.V.P. analyzed data; R.P.S., S.P.P., K.L.H., and G.V.P. prepared figures; R.P.S., S.P.P., K.L.H., E.S.R., and G.V.P. edited and revised manuscript; R.P.S., S.P.P., K.L.H., E.S.R., and G.V.P. approved final version of manuscript; K.L.H. and G.V.P. interpreted results of experiments; G.V.P. conception and design of research; R.P.S., and G.V.P. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Heike Wulff (University of California at Davis) for kindly providing SKA-31. We thank Medical University of South Carolina (MUSC) Urology staff surgeons: Drs. Thomas Keane, Harry Clarke, Stephen Savage, Ross Rames, Jonathan Picard, and Ahmed M. El-Zawahry, as well as the MUSC Urology Residents: Drs. Avi C. Weiss, Gary W. Bong, Kelly Doyle, Matthew McIntyre, Matthew Eskridge, Jonathan N. Hamilton, Robin Bhavsar, Timothy R. Yoost, Vinh Q. Trang, Lydia Labocetta, Elizabeth Peacock, Matthew Young, Erin Burns, Vaughan Taylor, and Samuel Walker Nickles for help with human tissue collection; and Drs. John Malysz, Wenkuan Xin, Mr. Qiuping Cheng, Mr. Serge Afeli, Mr. Ning Li, and Ms. Amy Smith for the critical evaluation of the manuscript.
REFERENCES
- 1. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61: 37–49, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Afeli SA, Rovner ES, Petkov GV. SK but not IK channels regulate human detrusor smooth muscle spontaneous and nerve-evoked contractions. Am J Physiol Renal Physiol 303: F559–F568, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersson KE. Pharmacotherapy of the overactive bladder. Discov Med 8: 118–124, 2009 [PubMed] [Google Scholar]
- 4. Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol 570: 13–22, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brading AF, Williams JH. Contractile responses of smooth muscle strips from rat and guinea-pig urinary bladder to transmural stimulation: effects of atropine and alpha, beta-methylene ATP. Br J Pharmacol 99: 493–498, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. Beta-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Groat WC. A neurologic basis for the overactive bladder. Urology 50: 36–52; discussion 53–36, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Fry CH, Ikeda Y, Harvey R, Wu C, Sui GP. Control of bladder function by peripheral nerves: avenues for novel drug targets. Urology 63: 24–31, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 140: 146–158, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol 140: 159–169, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141: 183–193, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol 551: 893–903, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hristov KL, Chen M, Afeli SAY, Cheng Q, Rovner ES, Petkov GV. Expression and function of KV2-containing channels in human urinary bladder smooth muscle. Am J Physiol Cell Physiol 302: C1599–C1608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903–C912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hristov KL, Chen M, Soder RP, Parajuli SP, Cheng Q, Kellett WF, Petkov GV. KV2.1 and electrically silent KV channel subunits control excitability and contractility of guinea pig detrusor smooth muscle. Am J Physiol Cell Physiol 302: C360–C372, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of β3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hristov KL, Parajuli SP, Soder RP, Cheng Q, Rovner E, Petkov GV. Suppression of human detrusor smooth muscle excitability and contractility via pharmacological activation of large-conductance Ca2+-activated potassium channels. Am J Physiol Cell Physiol 302: C1632–C1641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malysz J, Buckner SA, Daza AV, Milicic I, Perez-Medrano A, Gopalakrishnan M. Functional characterization of large conductance calcium-activated K+ channel openers in bladder and vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol 369: 481–489, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Mills IW, Greenland JE, McMurray G, McCoy R, Ho KM, Noble JG, Brading AF. Studies of the pathophysiology of idiopathic detrusor instability: the physiological properties of the detrusor smooth muscle and its pattern of innervation. J Urol 163: 646–651, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Nielsen JS, Rode F, Rahbek M, Andersson KE, Ronn LC, Bouchelouche K, Nordling J, Bouchelouche P. Effect of the SK/IK channel modulator 4,5-dichloro-1,3-diethyl-1,3-dihydro-benzoimidazol-2-one (NS4591) on contractile force in rat, pig and human detrusor smooth muscle. BJU Int 108: 771–777, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Oger S, Behr-Roussel D, Gorny D, Bernabe J, Comperat E, Chartier-Kastler E, Denys P, Giuliano F. Effects of potassium channel modulators on myogenic spontaneous phasic contractile activity in human detrusor from neurogenic patients. BJU Int 108: 604–611, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Ohya S, Kimura S, Kitsukawa M, Muraki K, Watanabe M, Imaizumi Y. SK4 encodes intermediate conductance Ca2+-activated K+ channels in mouse urinary bladder smooth muscle cells. Jpn J Pharmacol 84: 97–100, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Parajuli SP, Soder RP, Hristov KL, Petkov GV. Pharmacological activation of small conductance calcium-activated potassium channels with naphtho[1,2-d]thiazol-2-ylamine decreases guinea pig detrusor smooth muscle excitability and contractility. J Pharmacol Exp Ther 340: 114–123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. β1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of KATP channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol 280: R1427–R1433, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, Kohler R, Wulff H. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol 75: 281–295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soder RP, Petkov GV. Large conductance Ca2+-activated K+ channel activation with NS1619 decreases myogenic and neurogenic contractions of rat detrusor smooth muscle. Eur J Pharmacol 670: 252–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takahashi R, Nishimura J, Hirano K, Seki N, Naito S, Kanaide H. Ca2+ sensitization in contraction of human bladder smooth muscle. J Urol 172: 748–752, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Thorneloe KS, Knorn AM, Doetsch PE, Lashinger ES, Liu AX, Bond CT, Adelman JP, Nelson MT. Small-conductance, Ca2+-activated K+ channel 2 is the key functional component of SK channels in mouse urinary bladder. Am J Physiol Regul Integr Comp Physiol 294: R1737–R1743, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wagner TH, Hu TW, Bentkover J, LeBlanc K, Stewart W, Corey R, Zhou Z, Hunt T. Health-related consequences of overactive bladder. Am J Manag Care 8: S598–S607, 2002 [PubMed] [Google Scholar]
- 33. Wein AJ. Diagnosis and treatment of the overactive bladder. Urology 62: 20–27, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol 292: R616–R624,2007 [DOI] [PubMed] [Google Scholar]