Abstract
The GTP-binding protein Rac regulates diverse cellular functions including activation of NADPH oxidase, a major source of superoxide production (O2·−). Rac1-mediated NADPH oxidase activation is increased after myocardial infarction (MI) and heart failure both in animals and humans; however, the impact of increased myocardial Rac on impending ischemia-reperfusion (I/R) is unknown. A novel transgenic mouse model with cardiac-specific overexpression of constitutively active mutant form of Zea maize Rac D (ZmRacD) gene has been reported with increased myocardial Rac-GTPase activity and O2·− generation. The goal of the present study was to determine signaling pathways related to increased myocardial ZmRacD and to what extent hearts with increased ZmRacD proteins are susceptible to I/R injury. The effect of myocardial I/R was examined in young adult wild-type (WT) and ZmRacD transgenic (TG) mice. In vitro reversible myocardial I/R for postischemic cardiac function and in vivo regional myocardial I/R for MI were performed. Following 20-min global ischemia and 45-min reperfusion, postischemic cardiac contractile function and heart rate were significantly reduced in TG hearts compared with WT hearts. Importantly, acute regional myocardial I/R (30-min ischemia and 24-h reperfusion) caused significantly larger MI in TG mice compared with WT mice. Western blot analysis of cardiac homogenates revealed that increased myocardial ZmRacD gene expression is associated with concomitant increased levels of NADPH oxidase subunit gp91phox, O2·−, and P21-activated kinase. Thus these findings provide direct evidence that increased levels of active myocardial Rac renders the heart susceptible to increased postischemic contractile dysfunction and MI following acute I/R.
Keywords: ZmRacD expression, cardiac function, ischemia-reperfusion, infarction
the small GTP-binding protein Rac is a member of the Rho subfamily and acts as a molecular switch for multiple signaling pathways (9, 23, 26). There is now growing evidence that Rac is activated in response to diverse extracellular stimuli such as growth factors (15), heavy metals (40), reoxygenation and reperfusion (31, 41), and cytokines (48); and regulates the production of O2·− by an NADPH oxidase in phagocytic as well as in nonphagocytic cells (1, 29, 35). Rac1 has been implicated in different cardiovascular disorders including vascular dysfunction (55), hypertension, and cardiac hypertrophy (12, 27, 29, 35, 55). We have reported that constitutive overexpression of human Rac1 in smooth muscle cells results in increased vascular O2·−, hypertension, and mild cardiac hypertrophy in transgenic mice (27).
Recently, we have demonstrated that cardiac-specific transgenic overexpression of a constitutively active mutant form of Zea maize Rac D (ZmRacD) gene resulted in increased myocardial Rac-GTPase activity and reactive oxygen species (ROS) generation in the transgenic mice (TG) of all ages (19). Aging caused markedly higher levels of myocardial Rac expression and Rac-GTPase activity in ZmRacD transgenic mice (19). Importantly, while normal at young age, with aging these TG mice develop a distinct cardiac phenotype manifested by cardiac hypertrophy, relative ventricular chamber dilation, and moderate systolic dysfunction (19). Consistent with these findings, it has been reported that increased endogenous myocardial Rac/NADPH oxidase activity in aging rats causes cardiomyocyte hypertrophy and cardiac remodeling (57). Interestingly, the activation of myocardial ZmRacD along with endogenous Rac expression with thyroxine treatment led to cardiac dilation and severe systolic dysfunction in young adult transgenic mice (19, 20). Thus a potential molecular link exists between increased Rac activity and myocardial susceptibility to cardiac pathologies.
Myocardial ischemia-reperfusion (I/R) injury is associated with increased ROS, intracellular Ca2+ overload, endothelial dysfunction, and altered myocardial metabolism (3, 10, 17, 42, 53, 58, 59). Xanthine oxidase and the mitochondrial electron transport chain are important sources of ROS during reperfusion (4, 54, 60); there is also evidence that NADPH oxidase and the Rac1 pathway mediates ROS production during hepatic I/R (25, 41). Increased expression of Rac1 and/or NADPH oxidases has been reported in animals and humans with myocardial infarction (MI), heart failure (HF), diabetes, oxidant stress, or inflammation (2, 18, 22, 33, 37–39). Using different experimental models, it has been shown that adenoviral-mediated inactivation of Rac1 GTPase by transient expression of dominant negative Rac1 (N17Rac1) can protect a wide variety of cells including endothelial cells, vascular smooth muscle cells, fibroblast and ventricular myocytes from ROS-induced I/R injury (31, 41). Recently, it has been reported that disruption of Rac1 signaling can reduce prolonged myocardial ischemia-induced Rac1 activity and I/R injury in mice (44); however, the impact of increased Rac proteins on impending or acute myocardial I/R injury is unknown partly due to the lack of an adequate animal model. Thus the question remains does expression of activated Rac in cardiac myocytes predispose the heart to enhanced I/R injury?
Therefore, the present study was designed to determine the susceptibility of mice with or without cardiomyocyte-specific overexpression of active Rac on both in vitro and in vivo acute myocardial I/R injury, and its impact on postischemic cardiac function and MI.
MATERIALS AND METHODS
All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee, and the investigation conforms with the American Physiological Society (APS) Guiding Principles for the Care and Use of Vertebrate Animals in Research and Training (revision approved by the APS Council on July 16, 2010).
Mice.
Details regarding the generation and characterization of ZmRacD transgenic (TG) mice have been described previously (19). Briefly, the αMHC-RacD transgene was constructed using the cDNA of a constitutively active mutant of ZmRacD gene (28) and α-MHC promoter was used to induce selective overexpression of ZmRacD in myocytes of FVB/N wild-type (WT) mice. Each mouse was genotyped when it was weaned and only respective littermates served as control (WT). Experiments were performed in age-matched young adult mice (5–8 mo), and tail-clips were kept to reconfirm the genotypes. Most of the mice used in this study were males except six female mice.
Echocardiographic evaluation of left ventricular function.
In vivo cardiac dimension and contractile function was evaluated by transthoracic M-mode and two-dimensional (2-D) echocardiography under light isoflurane (0.5–1%) anesthesia as described previously (51). Briefly, with a GE Vivid7 echocardiography system and intraoperative epicardial probe (model i13L; FREQ 14 MHz), the 2-D left parasternal short-axis view was used as a guide and LV M-mode tracings were obtained close to the papillary muscle. LV internal diameters at diastole and systole (LVIDd and LVIDs, respectively) were measured, and LV fractional shortening (FS) was calculated as FS (%) = (LVIDd − LVIDs)/LVIDd × 100.
Measurement of superoxide (O2·−) in the heart.
In situ production of myocardial O2·− was assessed by oxidative fluorescent dye, dihydroethidium (DHE), as described previously (19). The cell-permeable DHE is oxidized to fluorescent hydroxyethidine (HE) by O2·− and then intercalated into DNA. Briefly, hearts from WT and TG mice were placed immediately in ice-cold PBS, washed, and then embedded in OCT for cryosectioning. Frozen sections (6-μm) from the hearts were incubated with DHE (10 μM; Sigma-Aldrich) for 30 min at 37°C in a dark chamber. After PBS wash, sections were fixed with aqueous mounting medium (Gel Mount, Sigma) and images were obtained using a fluorescence microscope (Nikon TE 300, Tokyo). The red fluorescence intensity was determined using the MetaMorph image analysis software 7.1.2.0 (Molecular Devices).
In vitro myocardial ischemia-reperfusion.
Hearts were isolated from age-matched mice of both strains as described previously (51, 52). Briefly, mice were anesthetized with pentobarbital (50 mg/kg ip), and hearts were excised, aorta cannulated, and perfused in a Langendorff mode at a constant pressure of 80 mmHg with a modified Krebs-Henseleit buffer (KHB) equilibrated with 95% O2-5% CO2 at 37°C. The constituents of KHB were (in mmol/l) 120 NaCl, 5.9 KCl, 25 NaHCO3, 1.2 MgCl2, 2.5 CaCl2, 0.5 EDTA, and 16.7 glucose. A fluid-filled balloon was inserted into the left ventricle (LV) across the mitral valve and connected to a pressure transducer permitting continuous measurement of LV pressure (LVP). Hearts were immersed in a water-jacketed bath maintained at 37°C, and the LV balloon was filled with water to yield a left ventricular diastolic pressure of 3–6 mmHg. Coronary flow was continuously monitored via a Doppler flow probe (T206, Transonic Systems, Ithaca, NY) placed in the aortic perfusion line. Aortic and LV developed pressures were recorded on a PowerLab/400 multichannel data-acquisition system (ADInstruments; Castle Hill, Australia). The LVP signal was digitally processed (using PowerLab Chart software version 4.2, ADInstruments) to yield diastolic and systolic pressures as well as heart rate. LV developed pressure (LVDP) was calculated as the difference between systolic and diastolic pressures. Following 30 min equilibration, hearts underwent 20 min of global ischemia, followed by 45 min of reperfusion.
In vivo myocardial ischemia-reperfusion.
In vivo myocardial I/R was performed as described previously (52). Briefly, mice were anesthetized with a mixture of intraperitoneal ketamine (55 mg/kg) and xylazine (15 mg/kg). After adequate anesthesia and aseptic preparations, the mouse was intubated and ventilated with room air by a MiniVent (Type 845, Harvard Apparatus). After opening the chest and visualizing the heart, the left anterior descending (LAD) coronary artery was ligated 2 mm below the tip of the left auricle by a 7–0 silk ligature. The rectal temperature of the mouse was maintained at 37°C by a thermo-heating pad. After 30-min LAD artery occlusion, the knot was released to start reperfusion, and reperfusion was confirmed by return of the pink-red color and motion of the anterior wall of the LV, and the chest was closed in layers. When mice resumed a normal breathing pattern and started to wake up, the ventilator was taken off and mice were transferred to a clean cage with free access to food and water.
Myocardial infarct size measurement.
In vivo myocardial infarct size was measured at 24 h postreperfusion as previously described with slight modification (52). Mice were anesthetized, intubated, ventilated, and the chest opened along the previous incision line and the left main coronary artery religated at the same location as before. The aorta was cannulated and phthalo blue dye was injected into the aorta for visualization of the nonischemic zone. The area of the myocardium not stained with Evans blue was defined as the area at risk (AAR). The heart was then rapidly excised, wrapped in polyethylene wrap, and frozen for 10 min for hardening, serially sectioned along the short axis (1-mm thick) by a heart slicer, and incubated with 1% TTC (Sigma) for 15 min at room temperature for demarcation of the viable and nonviable myocardium within the AAR. Both sides of each myocardial slice were photographed, and the area of infarction, AAR, and nonrisk area were determined by computerized planimetry as above.
Western blot analysis.
Western blots were performed to determine the relative cardiac expression of Rac1, gp91phox, and Pak1 in both WT and TG mice as described previously (19, 20). Briefly, hearts were homogenized and equal amounts (50 μg) of protein extract were separated by SDS-PAGE on polyacrylamide gel. To detect ZmRacD protein levels, we used rabbit polyclonal antibody raised against human Rac1 (1:1,000; Santa Cruz) that cross-reacts with ZmRacD. Membranes were also incubated with specific antibody for gp91phox (1:200; Santa Cruz) or PAK1 (1:1,000; Cell Signaling). Secondary antibodies (Santa Cruz) were goat anti-rabbit IgG-HRP (1:2000) for Rac1 and PAK1, and monkey anti-goat IgG-HRP (1:2,000) for gp91phox. Antibody signals were detected by an enhanced chemiluminescence kit (Pierce), and GAPDH was used as an internal control for equal protein loading.
Data analysis.
All results are expressed as means ± SE. Data were analyzed either by two-tailed Student's t-test for paired data from the same experiment and unpaired data from different experiments or by ANOVA followed by Bonferroni post hoc test. Values of P < 0.05 were considered to be significant.
RESULTS
Verification of ZmRacD expression.
Transgenic expression of ZmRacD was confirmed by tail biopsy genotyping and Western blot analysis of cardiac homogenates. Consistent with the previous data (19), PCR results demonstrated the distinct band of ZmRacD gene in the transgenic mouse, whereas no band was seen in the WT mouse (Fig. 1A). Similarly, an increased level of ZmRacD protein was clearly evident in TG hearts compared with endogenous Rac1 protein in WT hearts (Fig. 1B). To determine if ZmRacD expression resulted in increased baseline O2·− generation, in situ myocardial O2·− generation was determined. Consistent with the previous study (19), there was significantly increased levels of myocardial O2·− in TG mice compared with WT mice (Fig. 1C).
Fig. 1.
Verification of ZmRacD gene and protein expression, and myocardial O2·− generation in wild-type (WT) and ZmRacD transgenic (TG) mice. A: PCR analysis with tail sample from offspring of TG breeders. Genomic DNA with specific primer for the transgene shows distinct band of ZmRacD transgene in the TG mice only, but not in the WT mice. (+), positive control; (−), negative control. B: representative Western blots and densitometric ratios for total myocardial Rac proteins in WT and TG mice where WT was assigned as 100%. Values are means ± SE; n = 6/group. *P < 0.05 vs. WT. C: representative images of myocardial O2·− generation with DHE in frozen sections and bar graphs for mean fluorescence intensity in arbitrary units. Total intensity in each heart was normalized where WT was assigned as 100%. Values are means ± SE; n = 4–6/group. ***P < 0.001 vs. WT. Magnification, 40×.
General characteristics and baseline in vivo cardiac parameters of WT and TG mice.
Table 1 summarizes morphologic and baseline in vivo cardiac parameters in adult WT and TG mice. Consistent with previous studies (19, 20), there were no differences in the body weight (BW) between young adult (5–8 mo) WT and TG mice, and it was 30.7 ± 0.9 g and 31.3 ± 1.3 g in WT and TG mice, respectively (n = 22/group, P = NS). In vivo echocardiographic parameters between anesthetized WT and TG mice were comparable for ventricular dimensions and systolic LV fractional shortening between the two strains as reported (19, 20); however, heart rates in TG mice tended to be lower than WT mice (504 ± 16 vs. 549 ± 16 beats/min, n = 10–12/group, P = 0.066).
Table 1.
Morphologic and baseline echocardiographic parameters in wild-type (WT) and ZmRacD transgenic (TG) mice
| Parameters/Mice | WT | TG |
|---|---|---|
| Morphologic | n = 22 | n = 22 |
| BW, g | 30.7 ± 0.9 | 31.3 ± 1.3 |
| Echocardiographic | n = 12 | n = 10 |
| Heart rate, beats/min | 549 ± 16 | 504 ± 16 |
| LVIDd, mm | 3.82 ± 0.1 | 3.88 ± 0.1 |
| LVIDs, mm | 2.07 ± 0.09 | 2.02 ± 0.16 |
| LVPWd, mm | 1.01 ± 0.05 | 0.94 ± 0.05 |
| LVPWs, mm | 1.64 ± 0.04 | 1.63 ± 0.05 |
| FS, % | 46 ± 1 | 48 ± 2 |
Values are means ± SE; n = no. of animals. BW, body weight; bpm, beats/min; LVIDd, LV internal diameter at diastole; LVIDs, LV internal diameter in systole; LVPWd, LV posterior wall thickness in diastole; LVPWs, LV posterior wall thickness in systole; FS, fractional shortening.
Postischemic myocardial function following in vitro I/R.
Table 2 summarizes baseline cardiac parameters in the isolated hearts. The ages of WT and TG mice used for isolated heart studies were 5.7 ± 0.5 and 5.6 ± 0.2 mo, n = 6/group, respectively. Intrinsic heart rates were comparable between WT and TG hearts (304 ± 11 vs. 296 ± 8 beats/min, n = 6/group); however, LVDP was significantly higher (∼46%) in TG hearts compared with WT hearts (150 ± 9 vs. 103 ± 8 mmHg, P < 0.01, n = 6/group) with identical LV end-diastolic pressure (LVEDP) (3.2 vs. 3.1 mmHg, n = 6/group). Similarly, rate-pressure product (RPP, 10−3 mmHg/min) was significantly higher (∼42%) in TG hearts compared with WT hearts (44 ± 2.5 vs. 31 ± 3.2, P < 0.01, n = 6/group). There was no significant difference in the baseline coronary flow between WT and TG mice (WT vs. TG: 13.4 ± 1.4 vs. 14.7 ± 0.1 ml·min−1·g−1, n = 6/group).
Table 2.
Parameters in the isolated hearts of wild-type (WT) and ZmRacD transgenic (TG) mice before (preischemic) and after 30-min ischemia and 45-min reperfusion
| Parameters/Mice | WT (n = 6) | TG (n = 6) |
|---|---|---|
| Baseline (preischemic) | ||
| Heart rate, beats/min | 304 ± 11 | 296 ± 8 |
| LVDP, mmHg | 103 ± 8 | 150 ± 9** |
| RPP, 10−3 mmHg/min | 31 ± 3.2 | 44 ± 2.5** |
| LVEDP, mmHg | 3.2 ± 0.4 | 3.1 ± 0.5 |
| Reperfusion (45 min) | ||
| Heart rate, beats/min | 289 ± 9 | 241 ± 12‡ |
| LVDP, mmHg | 93 ± 4 | 84 ± 7§ |
| RPP, 10−3 mmHg/min | 27 ± 1.8 | 20 ± 1.9§ |
| LVEDP, mmHg | 11 ± 1§ | 8 ± 2† |
Values are means ± SE. LVDP, left ventricular developed pressure; LVEDP, left ventricular end diastolic pressure; bpm, beats/min; RPP, rate-pressure product; n, number of animals.
P < 0.01 vs. WT;
<0.05,
<0.01,
<0.001 vs. respective baseline value.
Since the absolute baseline LVDP and RPP were different between the two groups, postischemic data were normalized to their respective preischemic values (PI or baseline, 100%). Figure 2, A and B, shows time course for the recovery of LV systolic pressure (LVSP) and LVEDP in WT and TG hearts subjected to 20-min global ischemia and 45-min reperfusion. With reperfusion, postischemic LVSP was significantly impaired in TG hearts compared with WT hearts. However, recovery of LVEDP (an index of LV relaxation) was not affected in TG hearts, and LVEDP in WT and TG hearts after 45-min reperfusion was 11 ± 1 and 8 ± 2 mmHg, respectively, n = 6/group. Importantly, postischemic recovery of heart rate was markedly reduced in TG hearts compared with WT hearts from their respective PI value. At 45-min reperfusion, postischemic heart rate was significantly reduced to 81 ± 2% (P < 0.001; n = 6) in TG hearts compared with 95 ± 2% (P < 0.05; n = 6) in WT hearts. Impaired recovery of global contractile function was consistently seen in TG hearts with significantly lower postischemic left ventricular developed pressure (LVDP; Fig. 2C) and RPP (Fig. 2D). Postischemic coronary flow at 45-min reperfusion in both WT and TG mice was not significantly different from respective baseline value, and it was 16.7±1.1 and 14.7±0.7 ml·min−1·g−1 in WT and TG hearts, respectively.
Fig. 2.
Postischemic myocardial function in isolated hearts subjected to 20-min global ischemia and 45-min reperfusion. Time course for the recovery of postischemic left ventricular (LV) systolic pressure (LVSP), LV end-diastolic pressure (LVEDP), LV developed pressure (LVDP), and cardiac contractile index as rate-pressure product (RPP) in WT and TG hearts. LVSP (A), LVDP (C), and RPP (D) are expressed as percentage of preischemic (PI) value, and LVEDP (B) is expressed as mmHg. IS, 20-min ischemia. Values are means ± SE. The differences between WT and TG hearts are highly significant with *P < 0.05. n = 6/group.
Myocardial infarction following in vivo I/R.
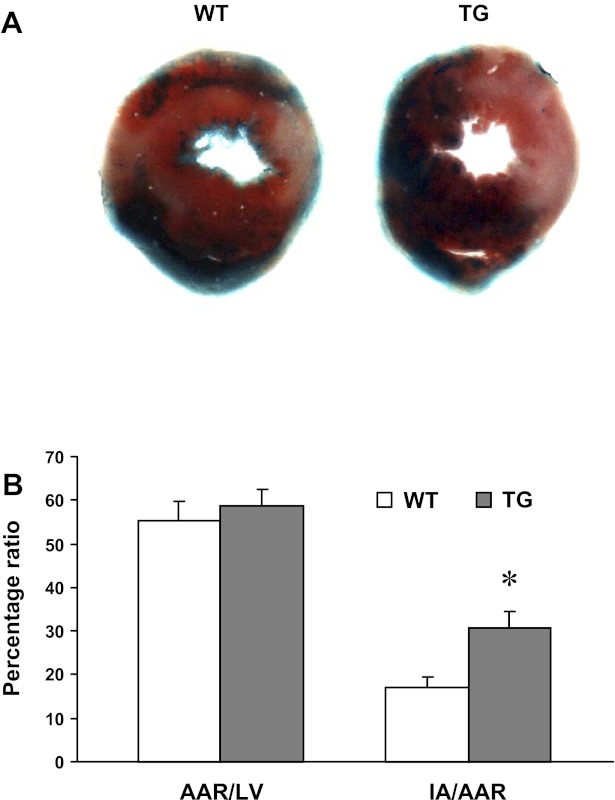
To correlate ZmRacD overexpression with irreversible myocardial injury, myocardial infarct size was assessed following 30-min LAD ligation and 24-h reperfusion (Fig. 3). The ages of WT and TG mice used for isolated hearts were 6.6 ± 0.7 and 6.0 ± 0.6 mo, n = 5/group, respectively. While both WT and TG hearts exhibited comparable values for AAR/LV (Fig. 3B), TG hearts had much larger infarct area (IA) compared with WT hearts (Fig. 3). The calculated IA/AAR was 17 ± 2% in WT hearts and 31 ± 4% in TG hearts (P < 0.05, n = 5/group).
Fig. 3.
Measurements of infarct size in hearts subjected to 30-min in vivo regional myocardial ischemia and 24-h reperfusion. A: blue dye was infused to visualize the nonrisk area and TTC-staining was performed to visualize the infarcted area (IA). B: percentages of LV area at risk (AAR) over LV (AAR/LV) and IA over the LV AAR (IA/AAR). Values are means ± SE. *P < 0.05 vs. WT. n = 5/group.
Effects of ZmRacD expression on cardiac proteins.
One important function of ZmRacD gene is to regulate the assembly and activation of the NADPH oxidase enzyme complex enhancing O2·− production when expressed in mammalian tissues (19, 28). Our results showed significantly increased (∼1.8-fold) expression of NADPH oxidase subunit gp91phox in the hearts of ZmRacD-TG mice compared with WT mice (Fig. 4A).
Fig. 4.
Quantification of myocardial proteins in WT and TG hearts. Representative Western blots and densitometric ratios for gp91phox (A) and PAK1 (B) in total cardiac homogenates of WT and TG mice. Each blot was normalized to internal control (GAPDH), and then expressed as percentage of WT where WT was assigned as 100%. Values are means ± SE. *P < 0.05, **P < 0.01 vs. WT. n = 6/group.
Because Rac1 can modulate myocardial contractility by activating PAK1 (41), we investigated the relation of ZmRacD overexpression on endogenous PAK1 protein levels. Interestingly, there was a ∼1.6-fold increase in the in situ expression of myocardial PAK1 proteins in TG mice compared with WT mice (Fig. 4B).
DISCUSSION
The major goal of this study was to determine whether cardiomyocyte-specific overexpression of Rac has any effect on the susceptibility and outcome of myocardial recovery following acute I/R. In order to examine this question, we used a novel ZmRacD TG mouse model (19) without any overt cardiac phenotype, in which expression of total Rac protein is ∼2-fold higher than WT hearts. The most striking finding is that when TG hearts were subjected to a sublethal in vitro I/R, they failed to recover and developed much more postischemic contractile dysfunction compared with the near-complete recovery in WT hearts. Importantly, in a clinically relevant model of acute in vivo regional myocardial I/R, TG mice were more susceptible to postischemic injury with significantly larger myocardial infarction. Our results showed that constitutive expression of ZmRacD in the hearts results in concomitant increased expression of NADPH subunit gp91phox, O2·−, and increased expression of PAK1. This is the first report that demonstrates the deleterious effects of increased myocardial Rac expression where it converts reversible in vitro myocardial stress into irreversible persistent myocardial dysfunction and exacerbates in vivo myocardial injury following an acute lethal I/R. Thus under pathophysiological conditions of increased Rac activity even without an overt cardiac phenotype, the heart might be at the risk of increased I/R injury.
Effects of small GTP-binding protein expression on the heart.
The small GTP-binding protein Rac1 of the Rho subfamily has been implicated in various cardiovascular disorders including vascular dysfunction, hypertension, and cardiac hypertrophy (12, 27, 29, 35, 55). Increased expression and/or activity of Rac1 were demonstrated in the blood vessels of diabetic rats (55) and in human saphenous vein smooth muscle cells secondary to thromboxane A2 analogue (38). Importantly, Rac1 has been reported to play an important role in platelet aggregation stimulated by atherosclerotic plaque in humans (18). In patients with ischemic or dilated cardiomyopathies (37) and atrial fibrillation (2), increased NADPH oxidase-dependent O2·− is reported to be coupled with increased myocardial Rac1 expression and Rac1-GTPase activity. Recently, genome-wide association analysis of coronary heart disease has implicated Rac1 as a potentially important gene in the pathophysiology of the disease (14).
We have also shown that the constitutive overexpression of Rac1 in smooth muscle cells results in increased vascular O2·− generation, hypertension and mild cardiac hypertrophy in transgenic mice (27). However, our novel ZmRacD-TG mouse model (19, 20) is unique compared with Rac1 TG model (49), because, ZmRacD TG mice gradually develop cardiac phenotype with aging and provide an executable window to investigate the pathological conditions involved in the progression of Rac-induced cardiovascular diseases. At basal levels of transgene expression, increased myocardial Rac expression, Rac-GTPase activity and oxidative stress were present in all transgenic mice (19); but only the older mice (>18 mo of age) exhibited cardiac hypertrophy and a moderate decrease in systolic function. This cardiac phenotype in older mice compared with young adult mice (8 mo) was attributed to the additional age-associated stress in the form of enhanced expression of transgenic as well as endogenous Rac (19). Because in the ZmRacD TG mouse model elevated levels of Rac expression induce cardiac hypertrophy, LV dilation, and systolic dysfunction only after 18 mo of age (19), we chose to study young adult mice (5–8 mo of age) for the effect of increased myocardial Rac on acute myocardial I/R injury.
In agreement with our previous studies (19, 20), there was no difference in BW between young adult WT and TG mice, and in vivo baseline cardiac function measured by echocardiography was similar in both strains (Table 1). However, in vitro perfused TG hearts displayed an increased baseline cardiac contraction (Table 2). Of note, markedly increased LVDP with similar perfusate calcium has been reported in phospholamban knockout (13) and SERCA1a overexpressing (52) hearts compared with WT hearts. Interestingly, phospholamban knockout mice were susceptible to myocardial I/R injury (13), whereas SERCA1a overexpressing mice were resistant to myocardial I/R injury (52). While we cannot rule out that unrecognized adaptations have occurred in our TG mice, the increased cardiac contraction in isolated hearts is likely related to the intrinsic effects of myocardial ZmRacD gene. Recent evidence suggests that P21-activated kinase (PAK) is an important effector of Rac1 (6) and PAK regulates cell contractility via myofilament activity and Ca2+ fluxes through distinct mechanisms (35, 46). Rac1-activated PAK1 has been reported to increase the Ca2+ sensitivity of cardiac muscle fiber bundles in rats involving phosphorylation of troponin I (11). TG hearts had increased levels of PAK1 (Figure 4B) concurrent with increased myocardial total Rac1 (Figure 1B); thus, it is possible that increased baseline cardiac contraction in isolated TG hearts, in the absence of neurohormonal influence, is related to PAK1-mediated increased Ca2+ sensitivity and myocardial contraction.
Small GTP-binding proteins and I/R injury.
I/R injury still remains a major concern in acute MI, cardiac transplantation, balloon angioplasty, and coronary artery bypass surgery (10, 56). Small GTPases of Rho family have been reported to modulate oxidant production in response to both in vitro and in vivo hypoxia/reoxygenation in various noncardiac cells or organs (25, 31, 41). Myocardial I/R triggers an extreme situation of cardiac oxidative stress (16, 60). Our data demonstrate that even with a reversible model of I/R insult (stunning), postischemic recovery of both cardiac inotropy and chronotropy was markedly attenuated in TG mice (Fig. 2 and Table 1), i.e., these mice showed increased susceptibility to I/R injury. Here, it is noteworthy that PAK1 activity is increased by hypoxia/reoxygenation in cultured cardiomyocytes (43) and PAK1 can blunt β-adrenergic pacemaker activity in myocardial cells (30). Increased susceptibility with stunning was consistently observed in a clinically relevant model of acute in vivo I/R where MI was significantly larger in TG mice (Fig. 3). Thus these striking differences between WT and TG hearts with both stunning and infarction models clearly indicate that increased expression of ZmRacD renders the heart more susceptible to stressful conditions.
Myocardial stunning is a reversible phenomenon where postischemic myocardial function can recover to near preischemic levels upon reperfusion. The pathogenesis of myocardial stunning includes two principal theories, oxyradical hypothesis and calcium hypothesis (7, 8, 24). In TG hearts, the recovery of LVSP, LVDP, and RPP were significantly reduced with a reversible myocardial insult (Fig. 2), and the postischemic heart rate was markedly lower compared with WT hearts. The mechanisms of myocardial contraction include intracellular Ca2+ mobilization and Ca2+ concentration, Ca2+ binding to troponin C triggering cross-bridge cycling, Ca2+ sensitivity of the myofilaments and maximum Ca2+-activated force (5, 21, 34). There was no impairment in the diastolic function (LVEDP) between two strains, an indication that intracellular Ca2+ mobilization and Ca2+ load was not impaired, indicating that the reduced postischemic contractile function might be due to altered myofilament responsiveness to calcium. The role of oxidant stress as the principal initiator of myocardial stunning is well reported with a decrease in Ca2+-induced activation of the myofilaments (32, 44). Recently, it has been reported that I/R-induced increase in myocardial Rac1 activity in mice was associated with increased NADPH oxidase activity and ROS generation (44). Deficiency of myocardial Rac1 or an inhibitor of Rac1 decreased NADPH oxidase activity and ROS generation in parallel with the improvement of postischemic function and myocardial infarction (44). Importantly, Rac1-induced activation of myocardial NADPH oxidase has been shown to increase mitochondrial ROS production and myocardial dysfunction (47, 50). With increased myocardial Rac (Fig. 1), TG mice exhibited increased myocardial Nox2 (gp91phox) and ROS (Fig. 4); and under stress conditions, these may lead to increased myocardial oxidative stress and I/R injury as reported for diabetic mice with increased myocardial Rac1 activity (44). Thus postischemic inotropic and chronotropic dysfunction observed in TG hearts may involve diverse signaling pathways including oxidative stress and/or dysregulation of PAK1-mediated myocardial contractility.
Implications of small GTPase modulation in postischemic myocardial recovery.
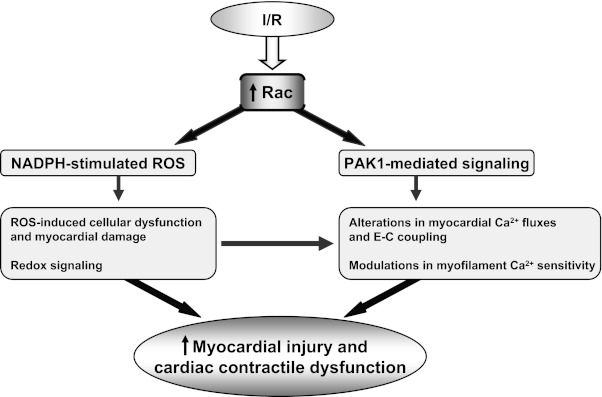
Involvement of small GTP-binding proteins has been consistently reported in endothelial dysfunction, atherosclerosis, hypertension, and cardiovascular events in both animals and humans (2, 9, 12, 27, 35–37, 49, 55). Importantly, cardiac-specific overexpression of a constitutively active form of human Rac1 in transgenic mice resulted in cardiac hypertrophy and demonstrated rapid-onset of cardiac phenotype and high postnatal mortality rate within weeks of birth (49). The ZmRacD mouse model (19) is unique compared to the Rac1 model (49), because it shows normal cardiac function at low workload but develops severe cardiac dysfunction with stress. The ZmRacD mouse model (19) has increased myocardial Nox2 expression, and increased Nox2 expression has been reported in human cardiomyocytes following acute MI (33). Moreover, in line with the established role of the catalytic unit gp91phox and Rac1 in the activation of NADPH-oxidase activity, both gp91phox and ZmRacD expressions were increased in our model. Importantly, the ZmRacD mouse model has the advantage as it provides a platform to investigate the role of increased Rac protein in different pathophysiological conditions in the absence of overt cardiac phenotype; and so far, this is the first study to demonstrate that hearts with increased Rac expression are at greater risk to develop postischemic cardiac injury that in the clinical setting would be expected to lead to greater morbidity and mortality. Myocardial NADPH activity is reported to be high in the post-MI hearts of animals and humans (2, 22, 33); thus, it is possible that conditions with preexisting increased myocardial Rac would lead to aggravation of post-MI function and remodeling. Of note, increased expression and activity of Rac1-GTPase activity is reported in patients with atrial fibrillation (2) and HF (37); hence, the results of the current study would suggest these patients to be susceptible to more severe I/R injury. While the underlying cellular and/or molecular mechanisms of increased Rac-mediated postischemic myocardial dysfunction, injury and remodeling remain to be fully delineated in a long-term follow-up study, based on the current study, the possible cascade of events linking activation of Rac signaling pathways following myocardial I/R to the deleterious effects on postischemic myocardium can be considered as depicted in Fig. 5.
Fig. 5.
Cascade of events by which ischemia-perfusion (I/R)-induced stimulation of Rac-mediated signaling pathways may influence postischemic myocardial contractile function and infarction. E-C coupling, excitation-contraction coupling.
In conclusion, the present study with a novel animal model provides clear direct evidence that overexpression of Rac protein, independent of any cardiac phenotype, renders the heart more susceptible to subsequent postischemic myocardial dysfunction and infarction, and thus, indicates a potential molecular link between increased Rac activity and myocardial susceptibility to acute I/R injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-63744, HL-65608, and HL-38324 to J. L. Zweier.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.H.T. and J.L.Z. conception and design of research; M.H.T., M.T.E., F.Y., Y.N., M.A.A., M.V., and H.H.H. performed experiments; M.H.T., M.T.E., F.Y., Y.N., M.V., and H.H.H. analyzed data; M.H.T., M.T.E., F.Y., M.V., H.H.H., and J.L.Z. interpreted results of experiments; M.H.T., M.T.E., F.Y., and H.H.H. prepared figures; M.H.T. and J.L.Z. drafted manuscript; M.H.T., M.T.E., H.H.H., and J.L.Z. edited and revised manuscript; M.H.T., M.T.E., F.Y., Y.N., M.A.A., M.V., H.H.H., and J.L.Z. approved final version of manuscript.
ACKNOWLEDGMENT
This study was in progress when the ZmRac-D mouse model article (19) was in the review process.
REFERENCES
- 1. Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353: 668–670, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Adam O, Frost G, Custodis F, Sussman MA, Schäfers HJ, Böhm M, Laufs U. Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol 50: 359–367, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Ambrosio G, Zweier JL, Becker LC. Apoptosis is prevented by administration of superoxide dismutase in dogs with reperfused myocardial infarction. Basic Res Cardiol 93: 94–96, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiarello M, Flaherty JT. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem 268: 18532–18541, 1993 [PubMed] [Google Scholar]
- 5. Blinks JR, Endoh M. Modification of myofibrillar responsiveness to Ca2+ as an inotropic mechanism. Circulation 73: III85–III98, 1986 [PubMed] [Google Scholar]
- 6. Bokock GM. Biology of the p21-activated kinases. Annu Rev Biochem 72: 743–781, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Bolli R, Jeroudi MO, Patel BS, Aruoma OI, Halliwell B, Lai EK, McCay PB. Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion. Evidence that myocardial “stunning” is a manifestation of reperfusion injury. Circ Res 65: 607–622, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Bolli R, Marbán E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79: 609–634, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame. A decade of hypertrophic signaling hits. Circ Res 98: 730–742, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol 14: 170–175, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Buscemi N, Foster DB, Neverova I, Van Eyk JE. p21-activated kinase increases the calcium sensitivity of rat triton-skinned cardiac muscle fiber bundles via a mechanism potentially involving novel phosphorylation of troponin I. Circ Res 91: 509–516, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Clerk A, Sugden PH. Small guanine nucleotide-binding proteins and myocardial hypertrophy. Circ Res 86: 1019–1023, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Cross HR, Kranias EG, Murphy E, Steenbergen C. Ablation of PLB exacerbates ischemic injury to a lesser extent in female than male mice: protective role of NO. Am J Physiol Heart Circ Physiol 284: H683–H690, 2003 [DOI] [PubMed] [Google Scholar]
- 14. de Las Fuentes L, Yang W, Dávila-Román VG, Charles Gu C. Pathway-based genome-wide association analysis of coronary heart disease identifies biologically important gene sets. Eur J Hum Genet 20: 1168–1173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doanes AM, Irani K, Goldschmidt-Clermont PJ, Finkel T. A requirement for rac1 in the PDGF-stimulated migration of fibroblasts and vascular smooth cells. Biochem Mol Biol Int 45: 279–287, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Downey JM. Free radicals and their involvement during long-term myocardial ischemia and reperfusion. Annu Rev Physiol 52: 487–504, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Duilio C, Ambrosio G, Kuppusamy P, DiPaula A, Becker LC, Zweier JL. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. Am J Physiol Heart Circ Physiol 280: H2649–H2657, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Dwivedi S, Pandey D, Khandoga AL, Brandl R, Siess W. Rac1-mediated signaling plays a central role in secretion-dependent platelet aggregation in human blood stimulated by atherosclerotic plaque. J Transl Med 8: 128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elnakish MT, Awad MM, Hassona MD, Alhaj MA, Kulkarni A, Citro LA, Sayyid M, Abouelnaga ZA, El-Sayed O, Kuppusamy P, Moldovan L, Khan M, Hassanain HH. Cardiac remodeling caused by transgenic overexpression of a corn Rac gene. Am J Physiol Heart Circ Physiol 301: H868–H880, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Elnakish MT, Hassona MD, Alhaj MA, Moldovan L, Janssen PML, Khan M, Hassanain HH. Rac-induced left ventricular dilation in thyroxine-treated ZmRacD transgenic mice: role of cardiomyocyte apoptosis and myocardial fibrosis. PLos One 7: e42500, 2012. doi:10.1371/journal.pone.0042500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Endoh M. Cardiac Ca2+ signaling, Ca2+ sensitizers. Circ J 72: 1915–1925, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Fukui T, Yoshiyama M, Hanatani A, Omura T, Yoshikawa J, Abe Y. Expression of p22-phox and gp91-phox, essential components of NADPH oxidase, increases after myocardial infarction. Biochem Biophys Res Commun 281: 1200–1206, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Gregg D, Rauscher FM, Goldschmidt-Clermont PJ. Rac regulates cardiovascular superoxide through diverse molecular interactions: more than a binary GTP switch. Am J Physiol Cell Physiol 285: C723–C734, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Gross GJ, Kersten JR, Warltier DC. Mechanisms of postischemic contractile dysfunction. Ann Thorac Surg 68: 1898–1904, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Harada N, Iimuro Y, Nitta T, Yoshida M, Uchinami H, Nishio T, Hatano E, Yamamoto N, Yamamoto Y, Yamaoka Y. Inactivation of the small GTPase Rac1 protects the liver from ischemia/reperfusion injury in the rat. Surgery 134: 480–491, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Hassanain HH, Goldschmidt-Clermont PJ. Rac, superoxide and signal transduction. In: Antioxidant and Redox Regulation of Genes, edited by Sen CK, Sies H, Baeuerle PA. San Diego, CA: Academic, chapt. 3, p. 47–79, 1999 [Google Scholar]
- 27. Hassanain HH, Gregg D, Marcelo ML, Zweier JL, Souza HP, Selvakumar B, Ma Q, Moustafa-Bayoumi M, Binkley PF, Flavahan NA, Morris M, Dong C, Goldschmidt-Clermont PJ. Hypertension caused by transgenic overexpression of Rac1. Antioxid Redox Signal 9: 91–100, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Hassanain HH, Sharma YK, Moldovan L, Khramtsov V, Berliner LJ, Duvick JP, Goldschmidt-Clermont PJ. Plant rac proteins induce superoxide production in mammalian cells. Biochem Biophys Res Commun 272: 783–788, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275: 1649–1652, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Ke Y, Lei M, Collins TP, Rakovic S, Mattick PA, Yamasaki M, Brodie MS, Terrar DA, Solaro RJ. Regulation of L-type calcium channel and delayed rectifier potassium channel activity by p21-activated kinase-1 in guinea pig sinoatrial node pacemaker cells. Circ Res 100: 1317–1327, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Kim KS, Takeda K, Sethi R, Pracyk JB, Tanaka K, Zhou YF, Yu ZX, Ferrans VJ, Bruder JT, Kovesdi I, Irani K, Goldschmidt-Clermont P, Finkel T. Protection from reoxygenation injury by inhibition of rac1. J Clin Invest 101: 1821–1826, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim SJ, Depre C, Vatner SF. Novel mechanisms mediating stunned myocardium. Heart Fail Rev 8: 143–153, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Krijnen PAJ, Meischl C, Hack CE, Meijer CJLM, Visser CA, Ross D, Niessen HWM. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J Clin Pathol 56: 194–199, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kusuoka H, Koretsune Y, Chacko VP, Weisfeldt ML, Marban E. Excitation-contraction coupling in postischemic myocardium. Does failure of activator Ca2+ transients underlie stunning? Circ Res 66: 1268–1276, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Lezoualc'h F, Métrich M, Hmitou I, Duquesnes N, Morel E. Small GTP-binding proteins and their regulators in cardiac hypertrophy. J Mol Cell Cardiol 44: 623–632, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Li JM, Gall NP, Grieve DJ, Chen M, Shah AM. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension 40: 477–484, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Maack C, Kartes T, Kilter H, Schäfers HJ, Nickenig G, Böhm M, Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation 108: 1567–1574, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Muzaffar S, Shukla N, Bond M, Sala-Newby G, Angelini GD, Newby AC, Jeremy JY. Acute inhibition of superoxide formation and Rac1 activation by nitric oxide and iloprost in human vascular smooth muscle cells in response to the thromboxane A2 analogue, U46619. Prostaglandins Leukot Essent Fatty Acids 78: 247–255, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagase M, Ayuzawa N, Kawarazaki W, Ishizawa K, Ueda K, Yoshida S, Fujita T. Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: role of small GTPase Rac1. Hypertension 59: 500–506, 2012 [DOI] [PubMed] [Google Scholar]
- 40. Ozaki M, Deshpande SS, Angkeow P, Suzuki S, Irani K. Rac1 regulates stress-induced, redox-dependent heat shock factor activation. J Biol Chem 275: 35377–35383, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Ozaki M, Deshpande SS, Angkeow P, Bellan J, Lowenstein CJ, Dinauer MC, Goldschmidt-Clermont PJ, Irani K. Inhibition of the Rac1 GTPase protects against nonlethal ischemia/reperfusion-induced necrosis and apoptosis in vivo. FASEB J 14: 418–429, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Piper HM, García-Dorado D. Prime causes of rapid cardiomyocyte death during reperfusion. Ann Thorac Surg 68: 1913–1919, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Seko Y, Takahashi N, Tobe K, Kadowaki T, Yazaki Y. Hypoxia and hypoxia/reoxygenation activate p65PAK, p38 mitogen-activated protein kinase (MAPK), and stress-activated protein kinase (SAPK) in cultured rat cardiac myocytes. Biochem Biophys Res Commun 239: 840–844, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Shan L, Li J, Wei M, Ma J, Wan L, Zhu W, Li Y, Zhu H, Arnold JM, Peng T. Disruption of Rac1 signaling reduces ischemia-reperfusion injury in the diabetic heart by inhibiting calpain. Free Radic Biol Med 49: 1804–1814, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Shattock MJ. Do we know the mechanism of myocardial stunning? Basic Res Cardiol 93: 145–149, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Sheehan KA, Ke Y, Solaro RJ. p21-Activated kinase-1 and its role in integrated regulation of cardiac contractility. Am J Physiol Regul Integr Comp Physiol 293: R963–R973, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Shen E, Li Y, Li Y, Shan L, Zhu H, Feng Q, Arnold JM, Peng T. Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes 58: 2386–2395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sulciner DJ, Irani K, Yu ZX, Ferrans VJ, Goldschmidt-Clermont P, Finkel T. Rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-kappaB activation. Mol Cell Biol 16: 7115–7121, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sussman MA, Welch S, Walker A, Klevitsky R, Hewett TE, Price RL, Schaefer E, Yager K. Altered focal adhesion regulation correlates with cardiomyopathy in mice expressing constitutively active rac1. J Clin Invest 105: 875–786, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Syed I, Kyathanahalli CN, Jayaram B, Govind S, Rhodes CJ, Kowluru RA, Kowluru A. Increased phagocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: role of Rac1-JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet. Diabetes 60: 2843–2852, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Talukder MA, Kalyanasundaram A, Zuo L, Velayutham M, Nishijima Y, Periasamy M, Zweier JL. Is reduced SERCA2a expression detrimental or beneficial to postischemic cardiac function and injury? Evidence from heterozygous SERCA2a knockout mice. Am J Physiol Heart Circ Physiol 294: H1426–H1434, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Talukder MA, Kalyanasundaram A, Zhao X, Zuo L, Bhupathy P, Babu GJ, Cardounel AJ, Periasamy M, Zweier JL. Expression of SERCA isoform with faster Ca2+ transport properties improves postischemic cardiac function and Ca2+ handling and decreases myocardial infarction. Am J Physiol Heart Circ Physiol 293: H2418–H2428, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Talukder MA, Yang F, Nishijima Y, Chen CA, Kalyanasundaram A, Periasamy M, Zweier JL. Reduced SERCA2a converts sub-lethal myocardial injury to infarction, and affects postischemic functional recovery. J Mol Cell Cardiol 46: 285–287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thompson-Gorman SL, Zweier JL. Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. J Biol Chem 265: 6656–6663, 1990 [PubMed] [Google Scholar]
- 55. Vecchione C, Aretini A, Marino G, Bettarini U, Poulet R, Maffei A, Sbroggiò M, Pastore L, Gentile MT, Notte A, Iorio L, Hirsch E, Tarone G, Lembo G. Selective Rac-1 inhibition protects from diabetes-induced vascular injury. Circ Res 98: 218–225, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Verma S, Fedak PW, Weisel RD, Butany J, Rao V, Maitland A, Li RK, Dhillon B, Yau TM. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation 105: 2332–2336, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol 48: 765–772, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci USA 84: 1404–1407, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zweier JL, Kuppusamy P, Williams R, Rayburn BK, Smith D, Weisfeldt ML, Flaherty JT. Measurement and characterization of postischemic free radical generation in the isolated perfused heart. J Biol Chem 264: 18890–18895, 1989 [PubMed] [Google Scholar]
- 60. Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res 70: 181–190, 2006 [DOI] [PubMed] [Google Scholar]