Abstract
Developing therapies aimed at manipulating microvascular remodeling requires a better understanding of angiogenesis and how angiogenesis relates to other network remodeling processes, such as lymphangiogenesis and neurogenesis. The objective of this study was to develop an angiogenesis model that enables probing of multicellular and multisystem interactions at the molecular level across an intact adult microvascular network. Adult male Wistar rat mesenteric windows were aseptically harvested and cultured in serum-free minimum essential media. Viability/cytotoxicity analysis revealed that cells remain alive for at least 7 days. Immunohistochemical labeling at 3 days for platelet endothelial cell adhesion molecule (PECAM), neuron-glial antigen 2 (NG2), lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), and class III β-tubulin identified endothelial cells, pericytes, lymphatics, and nerves, respectively. Media supplemented with bFGF or VEGF induced an increase in endothelial cell sprouting off existing vessels. Endothelial cell sprouting in both growth factor groups was inhibited by targeting pericytes with NG2 functional blocking antibody. VEGF caused an increase in the number of lymphatic/blood endothelial cell connections compared with media alone or bFGF groups. Finally, the comparison of the same network before and after angiogenesis stimulated by the supplement of media with 20% serum identified the ability of disconnected endothelial segments to reconnect to nearby vessels. The results establish a novel in situ angiogenesis model for investigating the location of capillary sprouting within an intact network, the role of pericytes, lymphatic/blood endothelial cell interactions, and the fate of specific endothelial cell segments. The rat mesentery culture system offers a unique tool for understanding the complex dynamics associated with angiogenesis in an intact adult tissue.
Keywords: microcirculation, capillary sprouting, pericyte, lymphangiogenesis
microvascular remodeling is a common denominator for multiple pathological conditions, including tumor growth, diabetic retinopathy, and myocardial infarction. It is also essential for wound repair and applications in tissue engineering (7, 24). Developing therapies aimed at manipulating microvascular remodeling requires a more integrated understanding of angiogenesis, defined as the growth of new blood capillaries from existing vessels, and how angiogenesis relates to other network remodeling processes, such as lymphangiogenesis and neurogenesis. A critical barrier to advancing our knowledge is the inability to probe specific molecular and cellular interactions across adult microvascular networks in a controlled environment. This study offers a new tissue model system for breaking down this barrier.
Common models include two-dimensional culture systems, three-dimensional culture systems, and ex vivo blood vessel explant assays (i.e. aortic ring assay) (11). A critique of these models is that they do not allow for investigation of mechanistic interactions between multiple cell types in a real microvascular network scenario. For example, consider the aortic ring assay. While capillary sprouting in the aortic ring assay does involve pericytes, fibroblasts and monocytes, endothelial structures originate radially from an aorta slice and not microvessels where angiogenesis normally occurs (11, 19). Another limitation of these models is that they do not allow for the investigating of the interrelationships between microvascular networks and other systems including lymphatic and neural networks.
The objective of this study was to develop an angiogenesis model that enables probing multicellular and multisystem interactions at the molecular level across an intact adult microvascular network. We demonstrate that the rat mesentery culture model can be used to 1) quantify the location of endothelial cell sprouting within a microvascular network during growth factor-induced angiogenesis, 2) investigate the functional role of perivascular cells during endothelial cell sprouting, 3) investigate the effects of growth factor administration on lymphatic-blood endothelial cell interactions, and 4) track specific cellular structures over the time course of microvascular network growth. Our results establish a new tool for angiogenesis research.
MATERIALS AND METHODS
Rat Mesentery Culture Model
All animal experiments were approved by Tulane University's Institutional Animal and Care Use Committee. Prior to harvesting tissue, an aseptic surgical area was prepared and instruments were sterilized. MEM containing Earle's Salts and l-glutamine (MEM, Invitrogen) and 1% PenStrep (Invitrogen), saline and PBS with CaCl2 and MgCl2 were heated to 37°C. Adult male Wistar rats (325–425 g) were anesthetized via intramuscular injection with ketamine (80 mg/kg body wt), xylazine (8 mg/kg body wt), and atropine (0.08 mg/kg body wt). After 5 min, the effect of anesthesia was confirmed and the rat's abdominal hair was shaved and removed. The abdominal skin was sterilized with alternating 70% isopropyl alcohol and iodine wipes. Using a scalpel blade, a (0.75 in.) longitudinal incision was made along the skin, and then the linea alba 1 in. below the sternum. Using cotton tip applicators, the ileum was located as a reference point. Then, the mesentery was gently pulled through a sterile plastic stage, and laid out. Sterile saline at 37°C was continuously dripped onto the tissue to keep it hydrated [see Yang et al. (32) for details on harvesting mesentery tissue and the plastic stage]. Once the mesentery was exposed, the rat was euthanized by intracardiac injection of 0.1–0.2 ml Beuthanasia (Schering-Plough Animal Health).
Mesentery tissues were aseptically harvested from the gut using microscissors and forceps. A mesenteric window was defined as the thin, translucent connective tissue found between artery/vein pairs feeding the small intestine. Minimal fat that bordered each window was cut with each tissue to reduce fat content in the cultures, and to avoid puncturing large blood vessels or the bowel. Tissues were immediately rinsed in sterile PBS with CaCl2 and MgCl2 at 37°C, and immersed in sterile MEM containing 1% PenStrep. After tissues were harvested, they were transported to a sterile culture hood. Tissues were rinsed in sterile PBS and MEM again at 37°C, then transplanted into 12-well culture plates (Invitrogen) containing media for each experimental group. One tissue was placed in each well with 2 ml of media for 3 or 7 days under standard cell culture conditions, and media was changed every 2 days.
Immunohistochemistry
For viability/cytotoxicity, tissues were incubated in Calcien AM and EthD-1 in PBS at 37°C for 10–15 min using the LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes). Then they were rinsed in PBS and spread on slides for microscopy using forceps. For all other immunohistochemistry, tissues were spread on slides, fixed in methanol at −20°C for 30 min, and then labeled with antibodies against platelet endothelial cell adhesion molecule (PECAM; CD31) and neuron-glial antigen 2 (NG2), smooth muscle α-actin (SMαA), lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), class III β-tubulin or bromodeoxyuridine (BrdU). All antibodies were diluted in antibody buffer solution (PBS + 0.1% saponin + 2% BSA). Between antibody incubation periods, tissues were washed with cold PBS with 0.1% saponin for 10 min, three times.
PECAM/NG2.
For PECAM/NG2, the protocol was 1) 1 h incubation at room temperature with 1:200 mouse monoclonal biotinylated CD31 antibody (CD31 antibody, BD Pharmigen) and 1:100 rabbit polyclonal NG2 antibody (Millipore/Chemicon, Billerica, MA) and 5% normal goat serum (NGS, Jackson ImmunoResearch Laboratories); and 2) 1 h incubation with 1:500 CY3-conjugated Streptavidin secondary (Strep-CY3) and 1:100 goat anti-rabbit CY2-conjugated antibody (GAR-CY2) with 5% NGS (15). For NG2 functional studies, NG2 targeting was confirmed by labeling tissues with only the secondary antibody.
PECAM/SMαA.
For PECAM/SMαA, the protocol was 1) 1 h incubation at room temperature with 1:200 CD31 antibody; and 2) 1 h incubation with CY2-conjugated streptavidin secondary (Jackson ImmunoResearch Laboratories) and 1:200 CY3 conjugated mouse monoclonal SMαA antibody (Sigma-Aldrich) (15).
PECAM/LYVE-1.
For PECAM/LYVE-1, the protocol was 1) 1 h incubation at room temperature with 1:200 CD31 antibody and 1:100 rabbit polyclonal LYVE-1 antibody (AngioBio) and 5% NGS; and 2) 1 h incubation with 1:500 Strep-CY3 and 1:100 GAR-CY2 with 5% NGS (26).
PECAM/class III β-tubulin.
For PECAM/class III β-tubulin, the protocol was 1) 36 h incubation at 4°C with 1:200 mouse monoclonal class III β-tubulin antibody (2G10, Abcam) with 5% NGS; 2) 36 h incubation at 4°C with 1:100 goat anti-mouse CY2-conjugated secondary antibody (GAM-CY2, Jackson ImmunoResearch Laboratories) and 5% NGS; 3) 1 h incubation at room temperature with 5% NGS; 4) 1 h incubation at room temperature with 1:200 CD31 antibody; and 5) 1 h incubation with 1:500 Strep-CY3 (28).
BrdU/PECAM.
For BrdU/PECAM, the protocol was as follows. Prior to fixation, tissue was incubated in media with BrdU (Sigma-Aldrich) for 2 h under standard culture conditions. After tissues were spread on slides and fixed in MeOH, they were incubated in 6 mol/l HCl for 1 h to denature the DNA. Tissues were then washed in PBS + 0.1% saponin, and incubated with antibodies against PECAM and BrdU: 1) 1 h incubation at room temperature with 1:100 mouse monoclonal anti-BrdU (Dako) and 5% NGS; 2) 1 h incubation at room temperature with 1:100 GAM-CY2 Fab fragments (Jackson ImmunoResearch Laboratories) and 5% NGS; 3) 1 h incubation at room temperature with 1:200 CD31 antibody; and 4) 1 h incubation with 1:500 Strep-CY3 (12).
Quantification of NG2 Expression
Eight mesenteric tissues from four different rats were harvested and immediately labeled for PECAM/NG2, representing preculture expression of NG2. Eight mesenteric tissues from four different rats were harvested and cultured for 7 days in MEM + 1% PenStrep. Feeding and draining arterioles and venules were visually assessed as NG2 positive or negative in three tissues from each rat (n = 12) at each time point. Capillary expression of NG2 was assessed in characteristic 4× network images from precultured and 3-day cultured tissues.
Functional Studies: Stimulation of Angiogenesis and NG2 Inhibition
To stimulate angiogenesis, media was supplemented with 60 ng/ml of recombinant human bFGF (Invitrogen) or 200 ng/ml of recombinant rat VEGF164 (R&D Systems). NG2 function was blocked by supplementing media with 10 μg/ml of rabbit polyclonal NG2 function-blocking antibody (Millipore/Chemicon). Rabbit IgG (Jackson ImmunoResearch Laboratories) at the same concentration as NG2 antibody (10 μg/ml) was added to growth factor supplemented media as an additional control group for nonspecific rabbit-derived antibody binding. Each growth factor (GF) study included four experimental groups: 1) control (MEM alone); 2) MEM + GF; 3) MEM + GF + NG2 antibody; and 4) MEM + GF + rabbit IgG. For both studies, 12 tissues from four rats were harvested and split evenly into the experimental groups (n = 12 per group). As an immunohistochemical control for NG2 targeting, tissues cultured with VEGF + rabbit IgG were incubated with GAR CY2-conjugated antibody alone.
Quantification of Angiogenesis
Endothelial sprouting and capillary density were quantified per tissue from 4× images of randomly selected networks in each tissue. For each tissue, up to five randomly selected areas were imaged to represent all microvascular networks. At least 1 network was imaged in each tissue. In the case that a tissue had only one network less than five fields of view in size, the entire network was imaged. A network was defined as having a feeding arteriole, draining venule, and multiple branches. Data were collected from 4× images using the Java-based NIH ImageJ image processing software version 1.43u. The number of capillary sprouts was quantified per three groups: capillary sprouts originating from arterioles larger than 20 μm, capillary sprouts originating from venules larger than 20 μm, and capillary sprouts originating from capillaries. Capillary sprouts were defined as blind ended PECAM positive endothelial cell segments. Sprouting was quantified from arterioles and venules larger than 20 μm in order to demonstrate the ability to examine the locations of sprouting across the hierarchy of a network. Twenty microns was selected because, for this diameter threshold, arterial versus venous identities are clearly distinguishable. Arterioles versus paired venules exhibit smaller diameters and have more elongated cells (15, 29, 31). Additionally, arterioles in this diameter range differentially label for NG2 compared to venules (16).
Quantification of Lymphatic/Blood Endothelial Cell Connections
Lymphatic/blood endothelial cell connections were quantified per overlapping lymphatic/blood vessel area. Direct connections between lymphatic and blood endothelial cells in rat mesenteric microvascular networks were previously characterized by Robichaux et al. (26). Consistent with this previous study, lymphatic/blood endothelial cell connections in this study were identified based on 1) overlap of PECAM staining, 2) PECAM staining in the same focal plane, and 3) a shared cellular or staining morphology. PECAM positive lymphatic vessel were distinguished from blood vessels by their larger diameter, more irregular diameter, decreased labeling intensity, and endothelial cell morphology (14). Lymphatic/blood endothelial cell connections were identified based on continuous PECAM labeling and a shared cellular morphology (26). Data were collected from the representative images from each tissue using the Java-based NIH ImageJ image processing software version 1.43u.
Microscopy
Images were acquired using 4× (dry, NA = 0.1), 10× (dry, NA = 0.3), 20× (oil, NA = 0.8; or air, NA = 0.75), and 60× (0il, NA = 1.4) objectives on an inverted microscope (Olympus IX70) coupled with a Photometrics CoolSNAP EZ camera. Network images quantified for vascular data were captured using the 4× objective. Confocal microscopy of lymphatic/blood endothelial cell connections were captured with a 63× (oil, NA = 1.4) objective on an inverted microscope coupled with a Zeiss LSM 510 confocal system. Twenty images were captured at 0.5-μm intervals (9.5 μm depth) with an optical thickness less than 0.7 μm. Lymphatic vessel filopodia images were acquired using a 60× (oil, NA = 1.4) objective and the Olympus IX70-CoolSNAP microscope-camera combination. Images were captured in 0.5-μm intervals through an 8-μm depth, and processed through Nikon Extended Depth of Focus (EDF) software to generate all-in-focus images. Time lapse images were captured using the 4× (dry, NA = 0.1) and 10× (dry, NA = 0.3) objective.
Time Lapse Imaging of Endothelial Cell Dynamics
After mesentery tissues were aseptically harvested from adult male Wistar rats, rinsed in PBS and immersed in sterile MEM, they were transferred to individual wells in a 6-well culture plate (Invitrogen). Each tissue was quickly spread out on the bottom of a well and secured in place with a commercially available 1-μm polycarbonate filter insert (CellCrown; Sigma-Aldrich). On day 0, 2 ml media containing FITC-BSI (1:40; Sigma-Aldrich), 20% fetal bovine serum (FBS, Invitrogen), and 1% PenStrep was added to each well and incubated for 30 min under standard culture conditions. Lectin supplemented media was then removed, and excess lectin was washed with lectin-free media. After tissues were labeled and washed, 2 ml of MEM with 20% FBS and 1% PenStrep was added to each well, and media was changed daily. This concentration was used because 20% FBS caused dramatic increases in vessel density during preliminary experiments, optimizing vessel remodeling for time-lapse imaging. Labeling with FITC-BSI (Sigma-Aldrich) identified blood endothelial cell segments, including vascular islands, and lymphatic endothelial cells present along intact microvascular networks before angiogenesis. Vascular islands are defined as endothelial cell segments disconnected from nearby networks (12, 26). After 3 days of culture in MEM plus 20% FBS, tissues were again labeled with FITC-BSI, and the same networks were reimaged.
Statistical Analysis
Mann-Whitney rank sum tests were used to compare the percentage of NG2-positive arterioles and venules between pre- and postculture groups. A Student's t-test accounting for unequal variances was used to compare the percentage of NG2-positive capillaries between pre- and postculture groups. Capillary sprouting metrics were compared across experimental groups using one-way ANOVA followed by pairwise comparisons with the Student-Newman-Keuls method. Lymphatic/blood endothelial cell connection densities were compared between the growth factor-stimulated groups and respective control groups using a Student's t-test. For all tests, statistical significance was accepted when P < 0.05. Statistical tests were run on SigmaStat 3.5 software.
RESULTS
Microvascular Networks Remain Intact and Viable in Culture
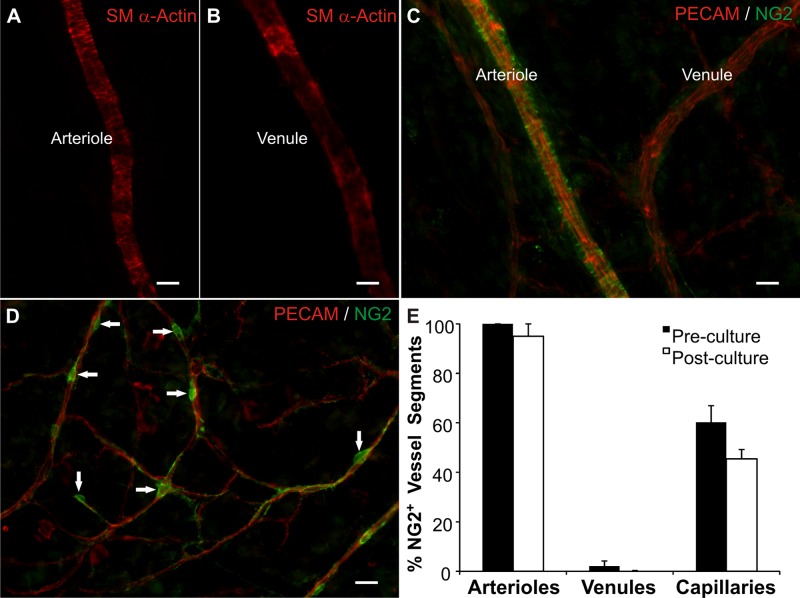
Microvascular networks displayed hierarchical branching patterns after being cultured for 3 days. These networks consisted of arterioles, capillaries, and venules. PECAM, SM α-actin, and NG2 labeling identified the presence of endothelial cells, smooth muscle cells and pericytes, respectively (Fig. 1). Arterioles compared with paired venules displayed increased SM α-actin cell wrapping (Fig. 1, A and B). NG2 labeling of smooth muscle cells along larger vessels has been previously shown to be arterial versus venule specific (15). In the cultured mesenteric tissues, we also observed arterial specific NG2 smooth muscle cell labeling (Fig. 1C). These observations provide an example of differential arterial/venous identity in an adult microvascular network in the absence of hemodynamic forces and motivate future investigations aimed at elucidating the functional role of NG2 as an arterial/venous marker. NG2-positive pericytes along capillaries exhibited a typical elongated morphology (Fig. 1D). After 3 days in culture the percentages of NG2-positive vessel segments per vessel type (arterioles, venules, or capillaries) were not statistically different (Fig. 1E), indicating that perivascular cells remain differentiated for at least 3 days.
Fig. 1.
Vascular cells remain present and maintain morphological and phenotypic characteristics in the rat mesentery tissues cultured in serum free media for 3 days. After 3 days in culture, arterial (A) and venous smooth muscle cells (B) are identified by smooth muscle α-actin immunolabeling and display respective arterial and venous morphologies. C: neuron-glial antigen 2 (NG2) and platelet endothelial cell adhesion molecule (PECAM) labeling of a paired arteriole and venule. NG2 labeling displayed an arterial-specific smooth muscle cell identity. D: NG2 and PECAM labeling along capillaries. NG2-positive cells exhibited typical pericyte morphology (arrows). E: quantitative comparison of the percentage of vessels with NG2-positive cells in freshly harvested tissues (preculture) and tissues cultured in MEM for 3 days (postculture). Values are means ± SE. Scale bars = 25 μm.
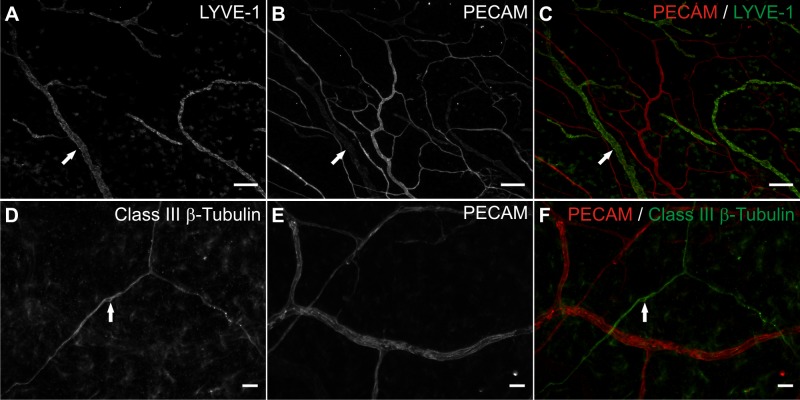
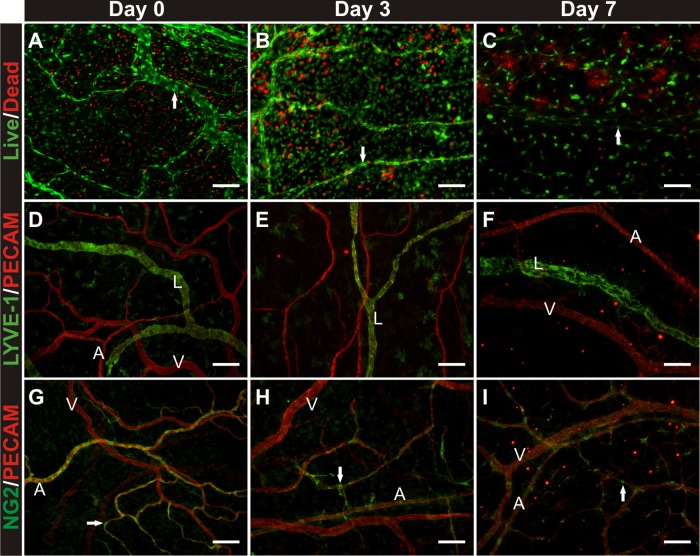
Lymphatic networks and nerves also remain present. Lymphatic versus blood vessel identity was confirmed based on endothelial cell morphology, PECAM labeling intensity, and labeling with LYVE-1 (Fig. 2, A–C). Nerves were distinguished from blood vessels based on morphology and labeling with class III β-tubulin (Fig. 2, D–F). Tissues remained viable after being cultured in serum-free MEM for 7 days (Fig. 3, A–C). Live cells were present throughout the tissue and along branching structures consistent with microvessels. Colocalization of live cells with microvessels was confirmed by colabeling with calcein and BSI-lectin (data not shown). After 7 days in culture, LYVE-1-positive lymphatic vessels remain present (Fig. 3, D–F), yet the number of NG2-positive perivascular cells is reduced, indicating the potential for cell dedifferentiation during culture experiments longer than 3 days (Fig. 3, G–I).
Fig. 2.
Lymphatics and nerves remain present in the rat mesentery culture model in serum free media for 3 days. A–C: lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) and PECAM immunolabeling identified intact lymphatic networks (arrows) after 3 days of culture in serum free media. D–F: class III β-tubulin and PECAM immunolabeling. After 3 days of culture in serum free media. Positive class III β-tubulin immunolabeling identified nerves (arrows). Scale bars = 200 μm (A–C), 25 μm (D–F).
Fig. 3.
Evaluation of tissue viability and vascular cell phenotypes preculture, and 3 and 7 days in culture with serum-free media. A–C: cell viability/cytotoxicity labeling. Arrows indicate apparent live vascular structures. D–F: LYVE-1 and PECAM immunolabeling. G–I: NG2 and PECAM immunolabeling. Arrows indicate pericytes. By day 7, the majority of arterioles lose NG2-positive cell coverage. NG2-positive cells were still observable along capillaries, yet the percentage of capillaries with pericytes decreases. “A” indicates arteriole. “V” indicates venule. “L” indicates lymphatic. Scale bars = 50 μm.
Stimulation of Angiogenesis Allows for Quantification of Capillary Sprouting at Specific Vessel Locations
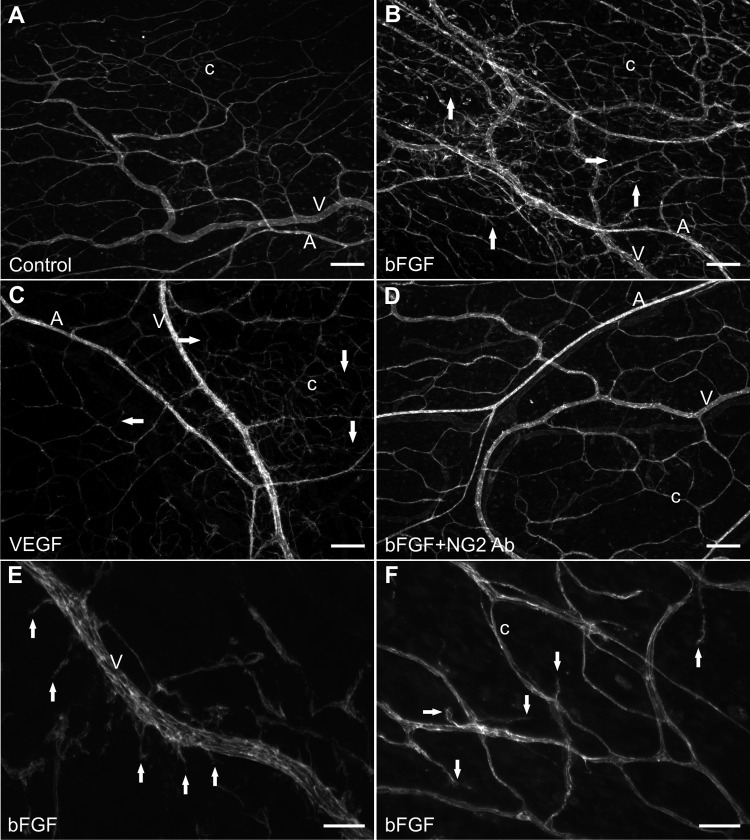
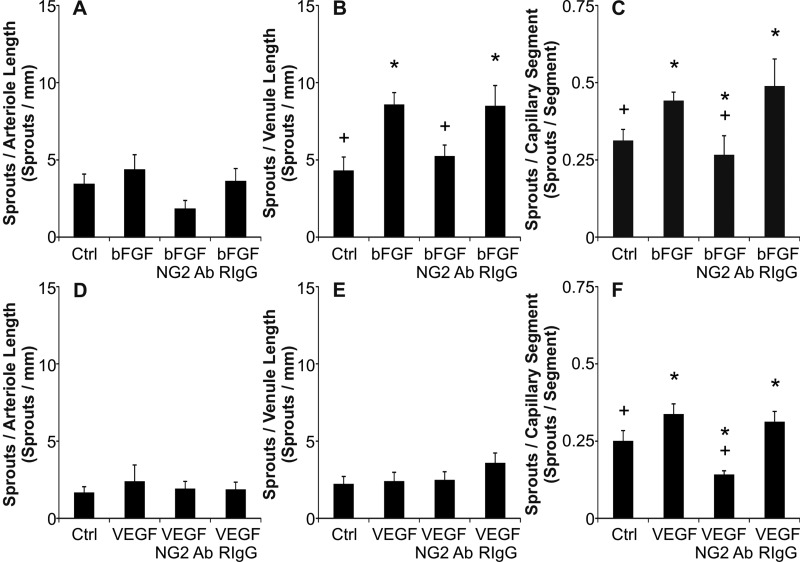
Culturing rat mesenteric tissues in MEM plus bFGF or MEM plus VEGF for 3 days stimulates angiogenesis. Compared with the respective MEM-alone control groups, the angiogenic groups displayed an increase in capillary sprouting (Fig. 4). Capillary sprouting was associated with endothelial cell proliferation (data not shown). Both the presence of bFGF (Fig. 5C) and VEGF (Fig. 5F) caused an increase in the number of capillary sprouts per capillary segment. However, only bFGF caused a statistically significant increase in the number of capillary sprouts off venules greater than 20 μm (Fig. 5, B and E). Per growth factor, a significant increase in capillary sprouting along arterioles was not observed (Fig. 5, A and D). These results demonstrate that capillary sprouting in this model preferentially occurs along specific vessel types (capillaries and venules), a phenomenon consistent with the in vivo observations (25). The number of arterioles, venules, and capillary segments normalized per vascular area was not significantly different across MEM alone and MEM plus growth factor groups (data not shown).
Fig. 4.
Microvascular networks can be stimulated to undergo angiogenesis while in culture for 3 days. Networks after 3 day culture in serum-free MEM (A), MEM plus bFGF (B), MEM plus VEGF (C), and MEM plus bFGF and NG2 antibody (D). E and F: higher magnification images of capillary sprouts (arrows) off a venule and existing capillaries. “A” indicates arteriole. “V” indicates venule. “c” indicates capillary. Scale bars = 200 μm (A–D), 50 μm (E and F).
Fig. 5.
Quantification of capillary sprouting per vessel type after bFGF (A–C) and VEGF stimulation (D–F). For each growth factor stimulation experiment the numbers of sprouts per vessel type length after 3 days in culture were compared across experimental groups: control (MEM alone), MEM + growth factor (GF), MEM + GF + NG2 antibody, and MEM + GF + Rabbit IgG. The rabbit IgG groups controlled for potential nonspecific antibody binding effects. *Significance against the control group (P < 0.05). +Significance against the growth factor group (P < 0.05). Values are means ± SE.
Capillary Sprouting is Regulated by Endothelial Cell-Pericyte Interactions
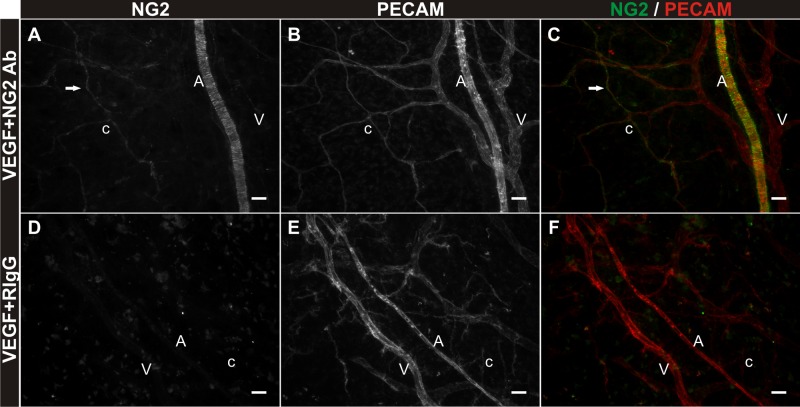
Angiogenesis involves both endothelial cells and pericytes (9). As an example, NG2, a membrane spanning chondroitin sulfate proteoglycan expressed by pericytes, has recently been shown to be a regulator of endothelial cell proliferation and migration associated with capillary sprouting (8, 23). The inclusion of NG2 antibody in the bFGF supplemented MEM prevented an increase in capillary sprouting in the mesentery culture model (Figs. 4D and 5, B and C). The density of capillary sprouts off venules and capillaries was decreased for the bFGF plus NG2 antibody group versus bFGF alone and the bFGF plus rabbit IgG groups. The density of capillary sprouts off capillaries was also decreased for the VEGF plus NG2 antibody group (Fig. 5F). NG2-positive perivascular cell targeting was confirmed by secondary only labeling at the end of the experiment (Fig. 6). Tissues were labeled for PECAM and a secondary antibody to target the rabbit-derived NG2 antibody. The lack of secondary labeling in the RIgG + VEGF group serves as a control for NG2 Ab specificity and antibody targeting.
Fig. 6.
Confirmation of antibody targeting NG2-positive cells over the culture time course. A–C: NG2 and PECAM labeling of rat mesenteric tissues post 3 days in culture in MEM + VEGF + NG2 antibody. Anti-rabbit IgG secondary labeling at day 3 identifies NG2-positive smooth muscle cells tightly wrapped around PECAM-positive arterioles and pericytes (arrows) elongated along PECAM-positive capillaries. D–F: NG2 and PECAM labeling in tissues cultured in MEM + VEGF + rabbit IgG antibody. Secondary antibody labeling targeting bound rabbit-derived protein is absent in field of views containing PECAM-positive vessels. “A” indicates arteriole. “V” indicates venule. “c” indicates capillary. Scale bars = 25 μm.
Lymphatic/Blood Endothelial Cell Interactions Increase During Angiogenesis
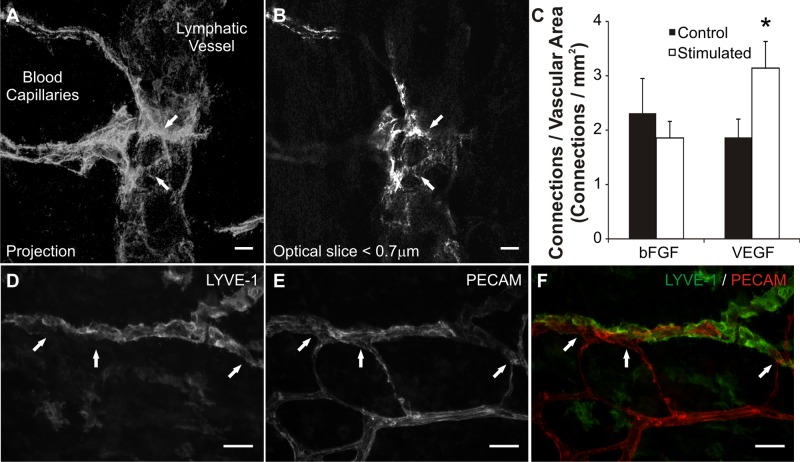
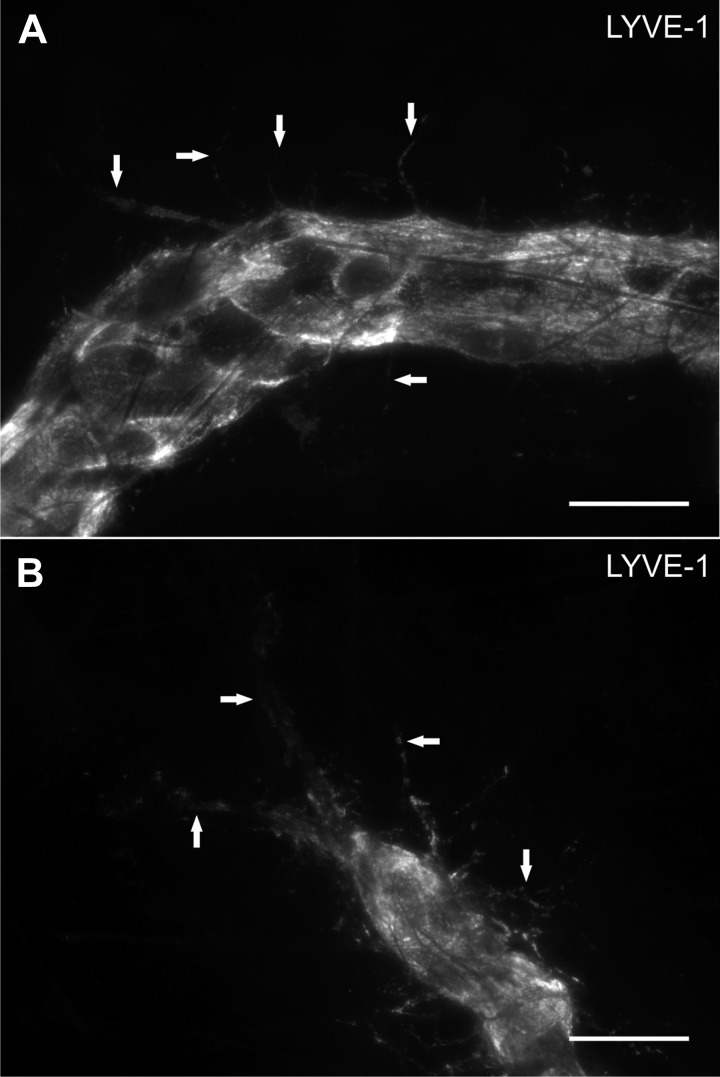
The rat mesentery culture model also enables the investigation of lymphatic/blood endothelial cell interactions during angiogenesis. Angiogenesis and lymphangiogenesis are known to share common signaling cues (1, 2). Our laboratory discovered the presence of physical lymphatic/blood endothelial cell connections in adult rat mesenteric microvascular networks (26). In the present study, we show that the occurrence of these physical interactions can be influenced by specific growth factors (Fig. 7). VEGF caused an increase in the number of lymphatic/blood endothelial cell connections per vascular area compared with the MEM-alone control group, while bFGF did not (Fig. 7C). Observation of sub-0.7-μm optical sections using confocal microscopy confirmed PECAM labeling continuity along endothelial cells at connections sites (Fig. 7, A and B). Lymphatic vessel identity at sites of connection was verified with LYVE-1 labeling (Fig. 7, D–F). Evidence for lymphangiogenesis in our model is supported by the observation of LYVE-1-positive endothelial cell extensions in tissues cultured for 3 days in MEM plus bFGF (Fig. 8).
Fig. 7.
The effect of growth factor stimulation on lymphatic/blood endothelial cell connections. A: confocal projection of sub-0.7-μm slices displays lymphatic/blood endothelial cell connections (arrows) identified by continuous PECAM labeling across cell types. Continuous PECAM labeling was confirmed by observation of each individual optical slice (B) throughout the thickness of the vessels at the connection site. C: quantification of connections per vascular area for bFGF and VEGF stimulated networks. VEGF stimulated an increase in connection density compared with control tissues. The density of connections did not change between unstimulated and bFGF stimulated tissues. *Significance against the control group (P < 0.05). D–F: LYVE-1 and PECAM labeling of lymphatic and blood vessels at connection sites (arrows). Scale bars = 10 μm (A, B), 50 μm (D–F).
Fig. 8.
Examples of LYVE-1-positive filopodia (arrows) extending from lymphatic vessels at different locations within a lymphatic network stimulated by 3-day culture in MEM plus bFGF. A: extensions off a lymphatic vessel segment. B: extensions off a blind ended lymphatic vessel. Scale bars = 25 μm.
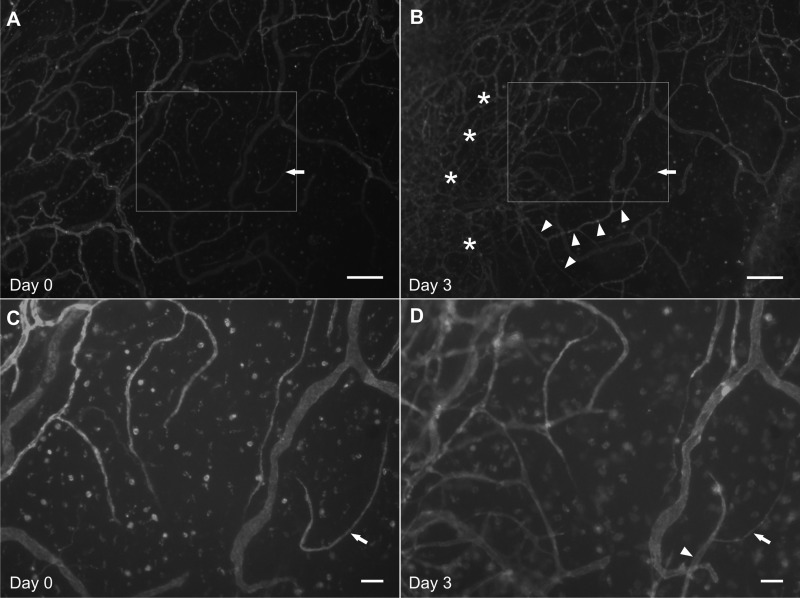
Time Lapse Imaging Identifies Capillary Sprout Formation and Vascular Island Reincorporation
Modification of physical culture conditions to 1) maintain the position of the mesenteric tissue on the bottom of the culture well and 2) ensure that the tissue remains spread out, enables time lapse imaging of the same microvascular network. Figure 9 shows pre- and postimages for a microvascular network cultured for 3 days in MEM plus 20% serum. Twenty percent serum was selected as the stimulus because it produces a dramatic increase in capillary density indicative of network growth. Comparison of the paired before and after angiogenesis images identifies the location of new capillaries and capillary sprouts. Images before and after angiogenesis in the same network show the model’s potential for use in future cell lineage studies. Our laboratory has previously reported that disconnected endothelial segments, termed vascular islands, are present in unstimulated networks (12). We have shown that during angiogenesis these vascular islands are associated with proliferation and their number decrease as network size increases (12). However, evidence for their direct involvement in angiogenesis was limited by the inability to track the fate of these segments over time. Here we demonstrate the value of our model by using it to confirm that vascular islands are able to connect to existing networks (Fig. 6). These results provide direct evidence for a new mode of endothelial cell dynamics during angiogenesis and support the application of this model for single cell lineage studies.
Fig. 9.
Time-lapse imaging of cultured mesentery vascular networks allows for tracking of endothelial cell dynamics. A and C: day 0. Pre-angiogenesis images of a intact microvascular network immunolabeled with BSI-lectin. Arrow indicates a vascular island defined as an endothelial segment disconnected from the network. B and D: day 3. Postangiogenesis images of the same microvascular network relabeled with BSI-lectin. Arrow identifies the vascular island indicated in A. Connection of the originally disconnected vascular island was confirmed by continuous labeling with the network (arrowheads). Angiogenesis at day 3 is evident by local regions of increased vessel density (*). C and D: higher magnification images of the rectangular regions identified in the corresponding pre- or postangiogenesis images. Scale bars = 200 μm (A and B), 50 μm (C and D).
DISCUSSION
This study establishes the rat mesentery culture model as a tissue culture system that enables investigators to study adult microvascular growth and remodeling within an intact microvascular network. The rat mesentery culture model is advantageous because it 1) contains the hierarchy of microvessels including arterioles, venules, and capillaries; 2) contains endothelial cells, smooth muscle cells, and pericytes; 3) can be used to probe mechanistic cell-cell interactions at specific locations within a microvascular network during angiogenesis; 4) can be used to investigate the interrelationships between angiogenesis, lymphangiogenesis and neurogenesis; and 5) can be used to track the fate of cellular structures during microvascular network remodeling.
The rat mesentery has been previously cultured to examine proliferation and cytochemical activity of fibroblasts and mesothelial cells during wound healing (22). During culture mast cells, blood vessels and lymphatic vessels remained intact (22). In the current study, we show that cultured mesenteric microvascular networks can be stimulated to undergo angiogenesis. An advantage of the rat mesentery culture model is that angiogenesis can be evaluated at different locations within an intact microvascular network. Another ex vivo model that offers this capability is the retinal explant assay. Yet, for the retinal explant assay, the ability to investigate either pericyte-endothelial or blood endothelial-lymphatic endothelial cell interactions has not been demonstrated. The serum free media culture conditions used in the current study are similar to those used for the aortic ring assay (19) and are sufficient to keep resident cells alive during endothelial sprouting. The culture conditions allowed the vasculature to maintain typical arterial/venous identities and endothelial cell, smooth muscle cell, and pericyte morphologies observed in vivo (15, 17, 32). Compared with the aortic ring and retinal explant assays, an advantage of our culture method is its simplicity. In the rat mesentery culture model, tissues can be allowed to freely float in media while in culture and require minimal preparation. Since vessel growth occurs within the intact tissue, the model system is self-contained and does not require embedding into a matrix. Similar to the aortic ring and retinal explant assays, the supplement of growth factors served to increase capillary sprouting in the mesentery culture model (13, 18, 33).
In other ex vivo models, angiogenesis is evaluated on an avascular background, resulting in essentially binary angiogenic responses compared with controls. For example, bFGF supplemented media (identical concentration as our model) induced approximately a 275% increase compared with controls in the aortic ring assay over 7 days (30). In our model, bFGF supplemented media resulted in a 99% increase in capillary sprouting off of venules and a 41% increase in capillary sprouting off of capillaries. While the nominal angiogenic effect in our study is less, it is more in line with the in vivo response percentages for rat mesenteric microvascular networks stimulated by intraperitoneal injections of bFGF (21). These comparisons suggest that cultured rat mesentery responses might be more comparable to in vivo responses rather than those reported for other ex vivo models. The rat mesentery culture model is also characterized by a high variation in responses. The combination of smaller nominal changes and variation in responses suggests that the statistical considerations made for in vivo experiments should also be made with the rat mesentery culture model.
In addition, we show that cultured mesentery tissues contain initial lymphatic networks that maintain their branching architecture and LYVE-1 lymphatic marker identity. Nerves are also present and label positively for class III β-tubulin, which is an established nerve marker in this tissue type (28). A critical issue for the use of the rat mesentery culture model is cell differentiation and function. Three days was selected for our study because at this time point we confirmed NG2 arterial/venous differentiation, the presence of NG2-positive pericytes along capillaries, and the presence of intact lymphatic networks. By 7 days, the number of NG2-positive perivascular cells is reduced. These qualitative observations at 7 days motivate future studies to evaluate the maintenance of specific cell phenotype and function.
Capillary sprouting in bFGF stimulated networks preferentially occurred off capillaries and venules rather than arterioles. The spatial location of sprouts within a microvascular network is consistent with in vivo observations (25). bFGF and VEGF have been previously documented as potent angiogenic stimulators (18, 21). We show that both growth factors are capable of inducing capillary sprouting in the rat mesentery culture model. It should be noted that sprouting in the MEM-alone control groups for each growth factor experiment were not the same. This difference highlights the potential for results to be dependent on network heterogeneity commonly observed in different rat littermates. For the respective growth factor experiments, tissues were harvested from the same littermates. We also show that both bFGF- and VEGF-stimulated angiogenesis can be inhibited by targeting NG2-positive pericytes. For the growth factor and inhibition studies, media was changed every 2 days to replenish the supply of supplement. While our results do show that changes every 2 days are sufficient, future experiments will be needed to compare whether replenishing the supplement daily versus every 2 days influences the tissue response.
Our observation of a VEGF-specific increase in apparent lymphatic/blood endothelial cell connections serves an example of using the rat mesentery culture model for investigating interrelationships between lymphatic and blood vessel systems. Our results suggest that connections across systems are dynamic and can be influenced by the local environment. In our laboratory's original characterization of these connections in unstimulated adult rat mesenteric networks, connections were shown to be nonluminal. The lack of perfusion in the culture model limits the ability to evaluate whether the connections stimulated by VEGF become functional. Still, the observations of apparent interactions between lymphatic and blood endothelial cells supports the potential for the rat mesentery culture model to be used for future studies aimed at understanding how angiogenesis and lymphangiogenesis are related. Recently, a lymphatic analog to the aortic ring assay was introduced as a tool for identifying lymphatic molecular mechanisms in vitro (6). In contrast to both lymphatic and aortic ring assays, the rat mesentery culture model enables simultaneous investigation of both systems along intact networks.
Images from the same network before and after 20% serum stimulation demonstrates 1) the use of the model for cell tracking experiments and 2) the ability for vascular islands to connect to a nearby, growing network. Twenty percent serum was selected as the stimulus because it produces a dramatic increase in vessel density (i.e., the formation of new vessel segments) indicative of network growth as compared with the growth factor stimulations. Over the 3-day culture time course, both bFGF and VEGF caused an increase in capillary sprouting, but not necessarily an increase in the vessel density. Comparison of BSI-lectin labeled networks before and after angiogenesis identifies new vessel formation and the fate of initially disconnected endothelial cell segments, termed vascular islands. We show that vascular islands can connect to nearby growing vascular networks. This result confirms a novel cellular dynamic involved in angiogenesis and indicates the potential for this model to be used for time lapse observation of single cell dynamics.
Characterization of rat mesenteric tissues chronically stimulated to undergo angiogenesis in vivo has proven valuable for identifying cellular dynamics during capillary sprout formation (3, 29). The rat mesentery tissue is 20- to 40-μm thick and offers an en face view of intact microvascular networks down to the single cell level (20). In the current study, we demonstrate that this tissue can be cultured and stimulated to undergo angiogenesis over a 3-day time course. Compared with chronic in vivo experiments, our model allows for investigation of multiple angiogenic scenarios and real time imaging in the same tissue, within a controllable environment free of systemic side effects.
A current limitation of this model is that we do not know how long the microvascular network structure can be maintained in culture. However, we do show that structure can be maintained for at least 3 days, a duration sufficient for the induction of angiogenesis. Another limitation is that the rat mesentery culture model lacks the presence of blood flow and related shear stresses, which have been implicated in the regulation and guidance of angiogenesis (3, 11, 27). The absence of shear stress is similarly a limitation of common in vitro and ex vivo culture assays such as 2D endothelial cell cultures, 3D matrix systems, and the aortic ring assay, all of which have been proven extremely valuable for studying angiogenesis (5, 10, 19). The model also requires rat mesenteric connective tissue eliminating the potential use of murine-derived mesenteric tissues derived from knockout animals. Unfortunately, based on our experience and reports from others, the same connective tissue in the mouse is mostly avascular (20). The question whether growth can be stimulated in mouse mesenteric windows remains and requires future trials with different mouse strains and stimuli. Anghelina et al. (4) reports the ability of intraperitoneal injections of VEGF and mechanical irritation to stimulate capillary ingrowth into otherwise avascular mesenteric windows (4).
In conclusion, the rat mesentery culture model offers an innovative tool for investigating angiogenesis. It provides an assay for observing cell dynamics involved in capillary sprouting at specific locations within a microvascular network and for functional studies in an intact tissue. Use of the rat mesentery culture model could provide valuable insight into the cellular interactions involved in angiogenesis and motivates a new line of experiments aimed at understanding the interrelationships between angiogenesis, lymphangiogenesis, and neurogenesis.
GRANTS
This work was supported by the Tulane Center for Aging funded by National Institutes of Health (NIH) Grant P20GM103629-01A1 (PI: M. Jazwinski), the Tulane Hypertension and Renal Center of Excellence funded by NIH Grant P20RR017659-10 (PI: L. Gabriel Navar) and the Board of Regents of the State of Louisiana LEQSF(2009-12)-RD-A-19 (PI: W. L. Murfee).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.C.S., M.S.A., and W.L.M. conception and design of research; P.C.S. and M.S.A. performed experiments; P.C.S. analyzed data; P.C.S. and W.L.M. interpreted results of experiments; P.C.S. prepared figures; P.C.S. and W.L.M. drafted manuscript; P.C.S., M.S.A., T.A., and W.L.M. edited and revised manuscript; P.C.S., M.S.A., T.A., and W.L.M. approved final version of manuscript.
REFERENCES
- 1. Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8: 464–478, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature 438: 946–953, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Anderson CR, Hastings NE, Blackman BR, Price RJ. Capillary sprout endothelial cells exhibit a CD36 low phenotype: regulation by shear stress and vascular endothelial growth factor-induced mechanism for attenuating anti-proliferative thrombospondin-1 signaling. Am J Pathol 173: 1220–1228, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anghelina M, Moldovan L, Moldovan NI. Preferential activity of Tie2 promoter in arteriolar endothelium. J Cell Mol Med 9: 113–121, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bayless KJ, Kwak HI, Su SC. Investigating endothelial invasion and sprouting behavior in three-dimensional collagen matrices. Nat Protoc 4: 1888–1898, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Bruyere F, Melen-Lamalle L, Blacher S, Roland G, Thiry M, Moons L, Frankenne F, Carmeliet P, Alitalo K, Libert C, Sleeman JP, Foidart JM, Noel A. Modeling lymphangiogenesis in a three-dimensional culture system. Nat Methods 5: 431–437, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Carmeliet P. Angiogenesis in life, disease and medicine. Nature 438: 932–936, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol Biol Cell 15: 3580–3590, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 314: 15–23, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Goodwin AM. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc Res 74: 172–183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaunas R, Kang H, Bayless KJ. Synergistic regulation of angiogenic sprouting by biochemical factors and wall shear stress. Cell Mol Bioeng 4: 547–559, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelly-Goss MR, Winterer ER, Stapor PC, Yang M, Sweat RS, Stallcup WB, Schmid-Schonbein GW, Murfee WL. Cell proliferation along vascular islands during microvascular network growth. BMC Physiol 12: 7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murakami T, Suzuma K, Takagi H, Kita M, Ohashi H, Watanabe D, Ojima T, Kurimoto M, Kimura T, Sakamoto A, Unoki N, Yoshimura N. Time-lapse imaging of vitreoretinal angiogenesis originating from both quiescent and mature vessels in a novel ex vivo system. Invest Ophthalmol Vis Sci 47: 5529–5536, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Murfee WL, Rappleye JW, Ceballos M, Schmid-Schonbein GW. Discontinuous expression of endothelial cell adhesion molecules along initial lymphatic vessels in mesentery: the primary valve structure. Lymphat Res Biol 5: 81–89, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Murfee WL, Rehorn MR, Peirce SM, Skalak TC. Perivascular cells along venules upregulate NG2 expression during microvascular remodeling. Microcirculation 13: 261–273, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Murfee WL, Skalak TC, Peirce SM. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation 12: 151–160, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol 113: 147–154, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nicosia RF, Nicosia SV, Smith M. Vascular endothelial growth factor, platelet-derived growth factor, and insulin-like growth factor-1 promote rat aortic angiogenesis in vitro. Am J Pathol 145: 1023–1029, 1994 [PMC free article] [PubMed] [Google Scholar]
- 19. Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest 63: 115–122, 1990 [PubMed] [Google Scholar]
- 20. Norrby K. In vivo models of angiogenesis. J Cell Mol Med 10: 588–612, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Norrby K. Basic fibroblast growth factor and de novo mammalian angiogenesis. Microvasc Res 48: 96–113, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Norrby K, Franzen L. A tissue model for the study of cell proliferation in vitro. In Vitro 16: 31–37, 1980 [DOI] [PubMed] [Google Scholar]
- 23. Ozerdem U, Stallcup WB. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis 7: 269–276, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peirce SM, Skalak TC. Microvascular remodeling: a complex continuum spanning angiogenesis to arteriogenesis. Microcirculation 10: 99–111, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Rhodin JA, Fujita H. Capillary growth in the mesentery of normal young rats. Intravital video and electron microscope analyses. J Submicrosc Cytol Pathol 21: 1–34, 1989 [PubMed] [Google Scholar]
- 26. Robichaux JL, Tanno E, Rappleye JW, Ceballos M, Stallcup WB, Schmid-Schonbein GW, Murfee WL. Lymphatic/Blood endothelial cell connections at the capillary level in adult rat mesentery. Anat Rec (Hoboken) 293: 1629–1638, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song JW, Munn LL. Fluid forces control endothelial sprouting. Proc Natl Acad Sci USA 108: 15342–15347, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stapor PC, Murfee WL. Spatiotemporal distribution of neurovascular alignment in remodeling adult rat mesentery microvascular networks. J Vasc Res 49: 299–308, 2012 [DOI] [PubMed] [Google Scholar]
- 29. Stapor PC, Murfee WL. Identification of class III beta-tubulin as a marker of angiogenic perivascular cells. Microvasc Res 83: 257–262, 2012 [DOI] [PubMed] [Google Scholar]
- 30. Villaschi S, Nicosia RF. Angiogenic role of endogenous basic fibroblast growth factor released by rat aorta after injury. Am J Pathol 143: 181–190, 1993 [PMC free article] [PubMed] [Google Scholar]
- 31. Yang M, Aragon M, Murfee WL. Angiogenesis in mesenteric microvascular networks from spontaneously hypertensive versus normotensive rats. Microcirculation 18: 574–582, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang M, Stapor PC, Peirce SM, Betancourt AM, Murfee WL. Rat mesentery exteriorization: a model for investigating the cellular dynamics involved in angiogenesis. J Vis Exp 2012. May 20 (63): e3954 doi:10.3791/3954, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu WH, Iurlaro M, MacIntyre A, Fogel E, Nicosia RF. The mouse aorta model: influence of genetic background and aging on bFGF- and VEGF-induced angiogenic sprouting. Angiogenesis 6: 193–199, 2003 [DOI] [PubMed] [Google Scholar]