Abstract
Hemolysis can saturate the hemoglobin (Hb)/heme scavenging system, resulting in increased circulating cell-free Hb (CF-Hb) in hereditary and acquired hemolytic disease. While recent studies have suggested a central role for intravascular hemolysis and CF-Hb in the development of vascular dysfunction, this concept has stimulated considerable debate. This highlights the importance of determining the contribution of CF-Hb to vascular complications associated with hemolysis. Therefore, a novel Hb-binding peptide was synthesized and linked to a small fragment of apolipoprotein E (amino acids 141–150) to facilitate endocytic clearance. Plasma clearance of hE-Hb-b10 displayed a rapid phase t1/2 of 16 min and slow phase t1/2 of 10 h, trafficking primarily through the liver. Peptide hE-Hb-B10 decreased CF-Hb in mice treated with phenylhydrazine, a model of acute hemolysis. Administration of hE-Hb-B10 also attenuated CF-Hb in two models of chronic hemolysis: Berkeley sickle cell disease (SS) mice and mice with severe hereditary spherocytosis (HS). The hemolytic rate was unaltered in either chronic hemolysis model, supporting the conclusion that hE-Hb-B10 promotes CF-Hb clearance without affecting erythrocyte lysis. Interestingly, hE-Hb-B10 also decreased plasma ALT activity in SS and HS mice. Although acetylcholine-mediated facialis artery vasodilation was not improved by hE-Hb-B10 treatment, the peptide shifted vascular response in favor of NO-dependent vasodilation in SS mice. Taken together, these data demonstrate that hE-Hb-B10 decreases CF-Hb with a concomitant reduction in liver injury and changes in vascular response. Therefore, hE-Hb-B10 can be used to investigate the different roles of CF-Hb in hemolytic pathology and may have therapeutic benefit in the treatment of CF-Hb-mediated tissue damage.
Keywords: sickle cell disease, hereditary spherocytosis, hemolysis, mouse models
intravascular hemolysis results in elevated levels of cell-free hemoglobin (CF-Hb) in the plasma compartment. Normally, CF-Hb is rapidly bound by the scavenger protein haptoglobin, maintaining nominal levels of CF-Hb in the plasma (5, 31, 41). However, in chronic hemolytic diseases, this scavenging mechanism becomes saturated, allowing CF-Hb levels to increase in the circulation (5, 38, 49). CF-Hb possesses peroxidase-like activity that contributes to its cytotoxic properties by oxidizing lipids and generating prooxidative and proinflammatory products (5, 36, 48). CF-Hb also reacts directly with nitric oxide (NO), decreasing the bioavailability of NO as well as the vasodilatory and anti-inflammatory properties of this gaseous molecule (23, 49, 50). Together, these findings imply that elevated levels of CF-Hb may have a direct role in the oxidative and inflammatory injuries observed in hemolytic disease.
Indeed, intravascular hemolysis has been linked to pulmonary hypertension (19, 24, 26, 45, 59), systemic vasculopathies (27, 28, 50, 52, 53, 60), and cardiovascular complications (11, 25, 30, 39). Although such studies have suggested that CF-Hb likely plays important roles in vascular disease, the actual contribution of CF-Hb to disease pathology has drawn controversy, particularly in sickle cell disease (SCD) (9, 18). The release of intraerythrocyte arginase (37), erythrocyte and leukocyte adhesion (22, 42, 57), increased coagulant activity (10), and inflammation and reperfusion injury (21) are all potential contributors to the SCD pathology induced or aggravated by hemolysis. Therefore, distinguishing the role of CF-Hb in hemolytic pathology from other potential factors requires the development of agents that specifically target CF-Hb.
An obvious candidate for targeting CF-Hb is haptoglobin. The covalent Hb/haptoglobin complex has long been considered the major pathway for directing CF-Hb clearance (1, 5, 41). Although studies (3, 8, 33) have demonstrated that haptoglobin limits Hb-induced hypertension and renal damage, a recent study (3) showed that haptoglobin did not decrease the half-life (t1/2) of CF-Hb in the circulation or inhibit the ability of CF-Hb to react with NO. Additionally, CF-Hb clearance in haptoglobin-null mice was not altered compared with wild-type mice (32). These findings demonstrate that while haptoglobin likely provides some protection against Hb-induced injury, the capacity of haptoglobin to efficiently clear elevated levels of CF-Hb may be limited. Thus, novel Hb-binding agents that facilitate the clearance of CF-Hb are required to accurately address the role of CF-Hb in hemolytic pathology.
Here, we describe a novel Hb-binding peptide, hE-Hb-B10, that both binds Hb and clears it from the circulation. We examine its potential use as a novel approach to address the role of CF-Hb in hemolytic disease pathology.
MATERIALS AND METHODS
Human subjects.
The use of human subjects was approved by the Institutional Review Boards of Children's Hospital of Wisconsin, Medical College of Wisconsin, and BloodCenter of Wisconsin. Informed consent was obtained from healthy volunteers and individuals with homozygous Hb SS disease and/or guardians for minor children, with assent when appropriate. Individuals with a history of red blood cell transfusion within 2 mo before blood collection were excluded from the study.
Mice.
C57BL/6J mice were purchased from The Jackson Laboratory (stock no. 000664, Bar Harbor, ME). Berkeley SCD mice [Tg(Hu-miniLCRα1Gγ AγδβS) Hba0//Hba0 Hbb0//Hbb0; SS mice] are on the Berkeley mixed genetic background, exclusively express human sickle hemoglobin, and have a phenotype that mimics many features of severe SCD in humans (35, 44). Berkeley HbA mice [Tg(Hu-miniLCRα1Gγ AγδβA) Hba0//Hba0 Hbb0//Hbb0; AA mice] are on the same Berkeley mixed genetic background as SS mice and exclusively express normal human Hb A. WBB6F1-sph/sph mice have severe autosomal recessive hereditary spherocytosis due to a spontaneous single-base deletion in the murine erythroid α-spectrin gene (Spna1) (16, 55). The sph mutation is maintained in the heterozygous state on both the WB/ReJ (WB) and C57BL/6J (B6) backgrounds. F1 hybrid (WBB6F1) mutant mice (sph/sph; HS mice) and normal mice (+/+, sph/+; Ctrl mice) were generated by mating WB and B6 heterozygotes and are genetically identical except at the mutated locus. F1 hybrid mice survive longer than mutant mice on inbred strain backgrounds (16). Mice were cared for according to Association for Assessment and Accreditation of Laboratory Animal Care specifications. Animal experiments were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. Experimental groups contained similar numbers of male and female mice and were 6 wk of age or older.
Peptide identification and synthesis.
Human Hb (10 nM, Sigma-Aldrich, St. Louis, MO) was coated onto a 96-well plate overnight followed by blocking with BSA and incubation with phage using phage display library PhD12 and following the manufacturer's guidelines (New England Biolabs, Ipswich, MA). Unbound phage were washed away, and bound phage were harvested by elution with 1 μM Hb and amplified for further screening. Three rounds of selection were performed with increasingly stringent conditions by washing with 0.1, 0.2, and 0.3% Tween-20 at each successive round to enrich for phage with higher binding affinity for hemoglobin. We then amplified phage from single plaques for DNA sequencing and Hb-binding verification by ELISA-based assay where a microtiter plate was coated with hemoglobin, blocked with BSA, 105 to 1012 virons added to each well, and the binding virons detected by HRP-conjugated anti-M13 antibody. We selected peptide Hb-B10 as our lead peptide. Hb-B10 was NH2-terminally coupled to LRKLRKRLLR, corresponding to amino acid residues 141–150 of human apolipoprotein E (ApoE), creating hE-Hb-B10 (13, 14). Peptides were synthesized using standard protocols on an ABI 433 (Applied Biosystems, Carlsbad, CA). The peptide resin was cleaved, precipitated, and lyophilized, followed by HPLC purification. The mass of the final product was verified by MALDI-TOF mass spectrometry analysis.
Binding kinetics.
The NH2-terminus of Hb-B10 was biotinylated with EZ-Link NHS-LC-biotin (Thermo Fisher Scientific, Rockford, IL) and loaded onto streptavidin-coated biosensors at 25 μg/ml (Octet RED96 System, ForteBio, Menlo Park, CA). Sensors were equilibrated in kinetic buffer (1×, ForteBio) and transferred to a solution of oxyHb (5–50 μM) to allow association between Hb-B10 and Hb. Sensors were incubated in kinetic buffer without Hb to measure the dissociation of Hb from Hb-B10. Dissociation constants were calculated with data analysis software (version 6.3) from ForteBio.
NO scavenging assay.
Washed streptavidin-coated magnetic beads (2.4 nmol/mg binding capacity, Solulink, San Diego, CA) were blocked in 10% normal human plasma in PBS for 20 min at 25°C. After being blocked, beads were incubated with plasma from individuals with SCD plus biotinylated Hb-B10 at 25°C for 10 min. Beads were pelleted from the sample using a magnet, and the plasma supernatant was used in NO consumption experiments performed as previously described (58) with minor modifications. Briefly, 50 mM dipropylenetriamine NONOate (Cayman Chemical, Ann Arbor, MI) was added to 5 ml PBS and equilibrated in a purge vessel connected to a NO chemiluminescence analyzer. The chemiluminescence signal, reflective of NO within the chamber, was allowed to reach a baseline (100–150 mV), and samples (10 μl) were injected into the solution. A decrease in the chemiluminescent signal indicated consumption of NO by oxyHb or other plasma components. NO consumption by SCD plasma samples was quantified by comparing changes in the chemiluminescent signal to those generated by known concentrations of oxyHb.
Peptide clearance measurements.
For plasma clearance experiments, hE-Hb-B10 was conjugated to 5,6-carboxyfluorescein (FAM; Anaspec, Fremont, CA). C57BL/6J mice were injected with FAM-labeled hE-Hb-B10 (70 μg/mouse ip). For each time point, plasma was obtained from two deeply anesthetized mice as described below, and the fluorescence within the plasma was measured using a Wallac VICTOR counter (Perkin-Elmer Wallac, Waltham, MA, excitation: 488 nm/emission: 520 nm). Background fluorescence was determined in plasma from nontreated mice, and this value was subtracted. Arbitrary fluorescence values were normalized to a standard curve of FAM-labeled hE-Hb-B10 in mouse plasma to obtain micrograms per milliliter of peptide. These values were then fitted to a double exponential equation to determine t1/2 values of hE-Hb-B10 in plasma (Sigma Plot software, Systac Software, San Jose, CA) (17, 20).
Peptide tissue distribution.
Mice were treated with FAM-labeled hE-Hb-B10 (70 µg/mouse ip) and harvested as described below. After perfusion of the mouse with PBS, the liver, kidney, spleen, lung, aorta, and brain were dissected and fixed in 4% paraformaldehyde for 24 h followed by cryoprotection in 30% sucrose at 4°C for 72 h. Tissues were mounted in OCT embedding media (Tissue-Tech, Torrance, CA) on dry ice and stored at −80°C. Frozen tissue sections on slides were fixed in 4% paraformaldehyde, washed in PBS, and stained with 4′,6-diamidino-2-phenylindole for 1 min (Invitrogen, Grand Island, NY). Stained slides were washed with PBS and mounted in fluorescence mounting medium (Vector Laboratories, Burlingame, CA). Slides were analyzed using an Olympus Fluoview FV1000 MPE multiphoton laser scanning microscope at ×20 or ×60 magnification (Olympus, Center Valley, PA).
Phenylhydrazine and peptide treatments.
To induce acute hemolysis, C57BL/6J mice were given a single intraperitoneal injection of 4 mg/mouse phenylhydrazine (PHZ; Sigma) or PBS (32). PHZ-injected mice received hE-Hb-B10 (40 μg/mouse ip) or PBS at 10 and 21 h after PHZ. SS and HS mice received daily treatments with hE-Hb-B10 (10–20 μg·mouse−1·day−1 ip) or PBS for 3 wk. All mice were harvested within 1 h of the final hE-Hb-B10 or PBS injection.
Whole blood and plasma assays.
Human blood samples were collected into 3.8% sodium citrate (volume: 1:9). Murine blood was obtained from deeply anesthetized mice by cardiac puncture and drawn into anticoagulant (sodium citrate or heparin). Blood cells were separated from plasma by centrifugation at 2,000 g for 10 min. Plasma was further clarified by centrifugation at 8,100 g for 10 min and then aliquoted for storage at −80°C (16). A complete blood count was determined using an automated veterinary blood counter (Heska, Fort Collins, CO). Reticulocyte counts were determined by flow cytometry using thiazole orange (BD Biosciences, San Jose, CA) based on the percentage of thiazole orange-positive cells within the erythrocyte gate (35). CF-Hb levels in plasma were measured using reagents from Catachem (Oxford, CT). Plasma lactate dehydrogenase (LDH) activity was assayed using a kit from BioAssay Systems (Hayward, CA). Plasma alanine aminotransferase (ALT) activity was quantified using a kit from Bioo Scientific (Austin, TX).
Facialis artery vasodilation studies.
Facialis arteries (180 to 250 µm) were removed under deep anesthesia, cannulated, and connected to appropriate buffers for vasodilation experiments as previously described (31, 43). Vessels were preconstricted with the thromboxane A2 agonist U-46619 (10−9 to 10−8 mol/l), and the vasodilation that occurred in response to acetylcholine (ACh, 10−7 to 10−4 mol/l) in the presence and absence of Nω-nitro-l-arginine methyl ester (l-NAME), 100 µmol/l) was recorded (16, 43).
Statistical analysis.
For in vitro functional analyses, statistical significance was determined using a paired t-test (Microsoft Excel, Microsoft, Redmond, WA). For in vivo experiments, unpaired t-tests were used for data that were distributed normally; Welch's correction was applied when comparing populations with unequal SDs. For data that did not pass tests for normality, nonparametric Mann-Whitney U-tests were used for comparisons (GraphPad InStat 3, GraphPad, La Jolla, CA). Statistical comparison of vessel dilation in the absence and presence of l-NAME was done using two-way ANOVA (GraphPad Prism 5).
RESULTS
Peptide development.
We combined the methods of phage display and ELISA to identify peptides that recognize and bind human Hb. From these experiments, we identified the amino acid sequence CHNLLPTPWWCA as our lead Hb-binding peptide and termed this peptide Hb-B10. Using biolayer interferometry (Octet RED96 System), we determined the binding affinity of Hb-B10 for oxyHb to be 21 ± 4 μmol/l (mean ± SD).
In vitro functional analysis.
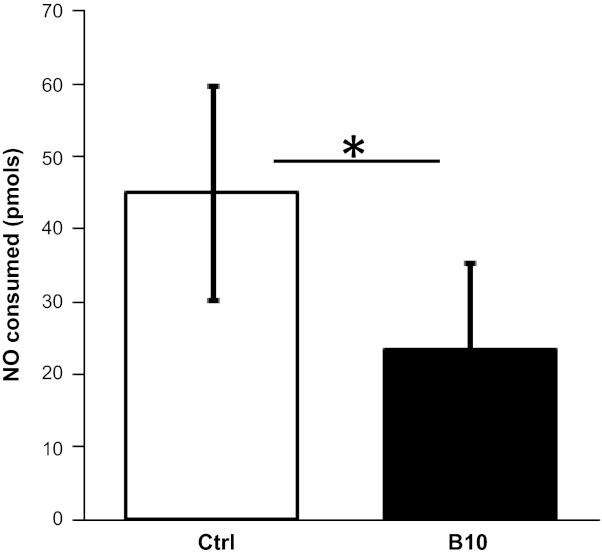
OxyHb is an effective scavenger of NO with a rate constant of 4.5 × 107 M−1·s−1 (15). Plasma from individuals with SCD consumes NO due to the elevated levels of oxyCF-Hb (49). To determine whether Hb-B10 inhibits NO scavenging by plasma with elevated CF-Hb, human SCD plasma was incubated with biotinylated Hb-B10 and streptavidin-coated magnetic beads followed by the removal of magnetic beads and associated Hb-B10/CF-Hb. Consistent with previous findings (58), control SCD plasma incubated with streptavidin-coated magnetic beads alone decreased the chemiluminescent signal, indicative of NO consumption. However, the addition of Hb-B10-coated magnetic beads to SCD plasma attenuated NO consumption (Fig. 1). These data demonstrate that Hb-B10 binds and efficiently promotes the removal of a factor in SCD plasma, which contributes to NO consumption. Since we have shown that Hb-B10 specifically binds CF-Hb and oxyHb is known to consume NO (15), these data indicate that Hb-B10 effectively removes CF-Hb from plasma in vitro.
Fig. 1.
Hb-B10 inhibits the nitric oxide (NO) scavenging capacity of plasma. The amount of NO consumed by plasma from individuals with sickle cell disease (SCD) was measured after an incubation in the absence [control (Ctrl)] or presence of 30 μM Hb-B10. Data are means ± SE; n = 4. *P < 0.05.
In vivo clearance and tissue distribution of hE-Hb-B10.
For Hb-B10 to decrease CF-Hb in the plasma in vivo requires that Hb-B10 both bind to and clear CF-Hb from the circulation. To accomplish both functions in vivo, Hb-B10 was coupled to a fragment of ApoE (hE; LRKLRKRLLR, residues 141–150), which has been shown to effectively clear lipoproteins from the circulation when linked to 18A, a well-characterized class A amphipathic helix that binds lipoproteins (13, 14, 17, 20).
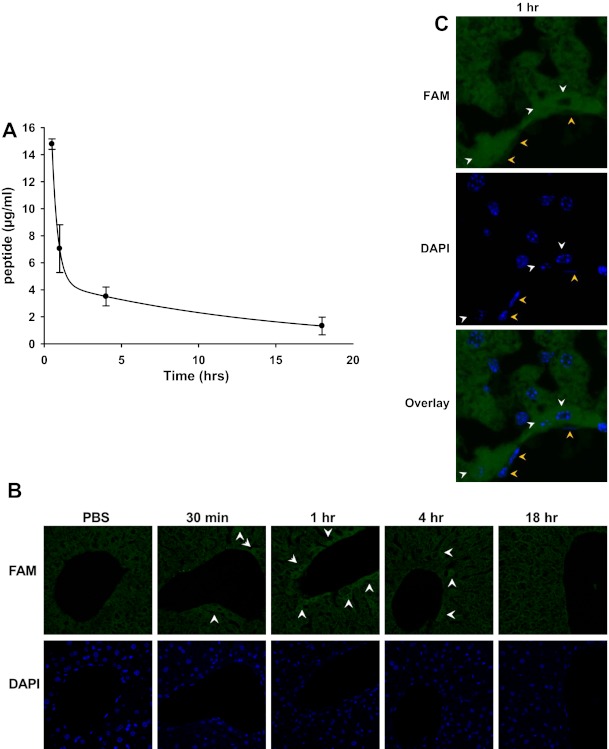
To determine the peptide clearance rate, C57BL/6J mice were injected with FAM-labeled hE-Hb-B10, and fluorescence within the plasma was measured. The pharmacokinetic data from this study fitted a double-exponential equation, suggesting that hE-Hb-B10 is cleared from the plasma in two phases: a rapid phase with a t1/2 of 15.62 min and a slow phase with a t1/2 of 9.96 h (Fig. 2A). These clearance data are similar to those reported in previous studies (17, 20), where the hE peptide was linked to 18A to form the lipoprotein-clearing peptide hE-18A. Additionally, it has been previously demonstrated that peptides containing the hE sequence traffic to the liver (40). Consistent with these findings, FAM-labeled hE-Hb-B10 was detected in the livers of treated mice by fluorescent histological examination (Fig. 2, B and C). The FAM-labeled peptide was most prominent in perivascular hepatic cells at 1 h (Fig. 2, B and C, white arrows). Diffuse fluorescence within the liver at the 30-min and 4-h time points likely reflects the two phases of hE-Hb-B10 clearance (Fig. 2B, white arrows). Fluorescence in other tissues (the aorta, brain, lung, kidney, and spleen) was indistinguishable from background fluorescence. This suggests that hE-Hb-B10 likely traffics CF-Hb to the liver for clearance, similar to other hE-linked peptides (17, 40).
Fig. 2.
Plasma clearance of hE-Hb-B10 (B10) is biphasic and occurs primarily in the liver. A: the concentration of 5,6-carboxyfluorescein (FAM)-labeled hE-Hb-B10 in the plasma of C57BL/6J mice at various time points after peptide treatment (70 μg/mouse ip). Plasma fluorescence was fitted to a biexponential equation, as previously described for hE-linked peptides. Data are means ± SD. B: the time-dependent fluorescence of FAM-labeled hE-Hb-B10 was detected (green, white arrows) in the liver. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue), ×20 magnification. C: detection of FAM-labeled hE-Hb-B10 in perivascular hepatocytes (white arrows, round nuclei) and absence of FAM-labeled hE-Hb-B10 in endothelial cells (orange arrows, elongated nuclei), ×60 magnification.
Effect of hE-Hb-B10 on CF-Hb in a murine model of acute hemolysis.
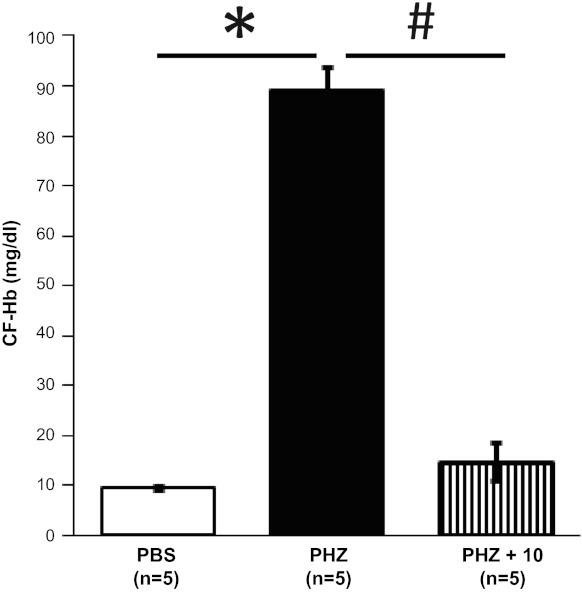
To assess whether hE-Hb-B10 clears CF-Hb from the circulation in vivo, mice were treated with PHZ to induce acute hemolysis (32). As expected, PHZ increased circulating CF-Hb in the plasma of these mice (Fig. 3). Administration of hE-Hb-B10 at 10 and 21 h after PHZ treatment markedly reduced CF-Hb to levels that were comparable with plasma CF-Hb in vehicle-treated (nonhemolyzed) mice (Fig. 3).
Fig. 3.
hE-Hb-B10 decreases cell-free Hb (CF-Hb) in a mouse model of acute hemolysis. Plasma CF-Hb levels were measured in C57BL/6 mice treated intraperitoneally with PBS, phenylhydrazine (PHZ; 4 μg/mouse), or PHZ followed by hE-Hb-B10 (PHZ + B10). Data are means ± SE. *P < 0.0001, PBS vs. PHZ; #P = 0.008, PHZ vs. PHZ + B10.
Effects of hE-Hb-B10 in murine models of chronic hemolysis.
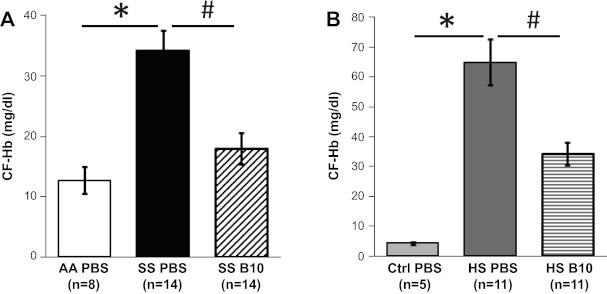
Next, we investigated whether hE-Hb-B10 reduced CF-Hb in two murine models of chronic hemolysis: SS mice and HS mice (16, 44). Similar to previous studies (16, 35), PBS-treated SS and HS mice had increased concentrations of CF-Hb compared with AA and Ctrl mice, respectively (Fig. 4, A and B). The higher hemolytic rate in HS mice was reflected in the elevated reticulocyte count compared with SS mice (see Table 1). Importantly, hE-Hb-B10 reduced CF-Hb in the plasma of both SS and HS mice after 3 wk of daily treatment (Fig. 4, A and B). Together, these results suggest that hE-Hb-B10 effectively reduces CF-Hb in murine models of both acute and chronic hemolysis.
Fig. 4.
hE-Hb-B10 decreases CF-Hb in mouse models of chronic hemolysis. A: plasma CF-Hb levels in AA and SS mice were measured after daily intraperitoneal treatment with PBS or hE-Hb-B10 for 3 wk. B: plasma CF-Hb levels in Ctrl and HS mice were measured after daily intraperitoneal treatment with PBS or hE-Hb-B10 for 3 wk. Data are means ± SE. *P < 0.0001, control mice (AA and Ctrl mice) compared with their respective mutant mice (SS or HS mice); #P ≤ 0.003, PBS-treated compared with hE-Hb-B10-treated mutant mice.
Table 1.
Effect of hE-Hb-B10 on the hemolytic rate in SS and HS mice
| Whole Blood Hb, g/dl | Reticulocyte Count, percentage of red blood cells | Plasma Lactate Dehydrogenase Activity, IU/l | |
|---|---|---|---|
| SS mice | |||
| PBS | 7.0 ± 0.7 (n = 9) | 53 ± 14 (n = 9) | 211 ± 48 (n = 9) |
| hE-Hb-B10 | 6.3 ± 0.9 (n = 7) | 55 ± 12 (n = 7) | 173 ± 42 (n = 7) |
| HS mice | |||
| PBS | 5.3 ± 0.7 (n = 14) | 94 ± 3 (n = 7) | 860 ± 173 (n = 13) |
| hE-Hb-B10 | 4.7 ± 0.8 (n = 11) | 93 ± 4 (n = 11) | 963 ± 256 (n = 12) |
Values are means ± SD; n, no. of mice/group. SS mice, mice with sickle cell disease; HS mice, mice with hereditary spherocytosis.
The reduction in CF-Hb in the above experiments could be the result of either increased clearance of CF-Hb or an overall reduction in hemolysis. To determine if hE-Hb-B10 altered the hemolytic rate in chronic hemolysis models, we measured the complete blood count and reticulocyte count in vehicle- and peptide-treated mice. The stable total Hb level and reticulocyte count in both SS and HS mice suggest there were no overall changes in steady-state red blood cell destruction and the compensatory bone marrow response in treated mice (Table 1). These data provide strong support for the conclusion that hE-Hb-B10 reduces CF-Hb in vivo by promoting CF-Hb clearance from the circulation, rather than by reducing hemolytic rate. In addition, hE-Hb-B10 had no effect on total plasma LDH activity in either SS or HS mice (Table 1). As plasma LDH activity increases in response to both hemolysis and tissue damage (12), the high hemolytic rates in both models may limit our ability to detect subtle changes in LDH activity that might occur as a result of changes in tissue injury.
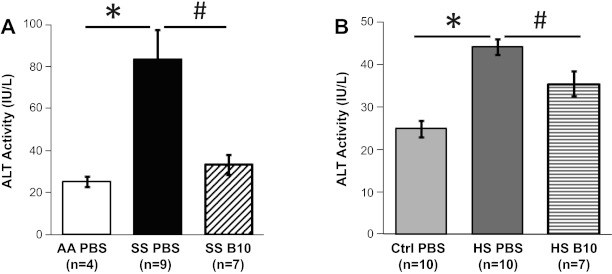
To ensure that the clearance of CF-Hb in our chronic hemolysis models via the liver did not result in liver toxicity, we measured plasma ALT activity in mice treated with PBS or hE-Hb-B10 (2, 46). Both SS and HS mice are known to have baseline chronic liver injury (2, 16, 35, 44, 56), which is reflected by elevated ALT activity in the plasma of both SS and HS mice compared with activity levels in their respective controls (Fig. 5, A and B). It is important to note that treatment with hE-Hb-B10 did not cause further liver toxicity as measured by plasma ALT activity. In fact, hE-Hb-B10 treatment actually lowered plasma ALT activity in both SS and HS mice (Fig. 5, A and B). These data are consistent with the notion that the reduction of CF-Hb by hE-Hb-B10 concomitantly decreased liver injury in these murine models of chronic hemolytic disease.
Fig. 5.
hE-Hb-B10 reduces plasma alanine aminotransferase (ALT) activity in mouse models of chronic hemolytic anemia. A: plasma ALT activity in AA and SS mice was measured after daily intraperitoneal treatment with PBS or hE-Hb-B10 for 3 wk. B: plasma ALT activity in Ctrl and HS mice was measured after daily intraperitoneal treatment with PBS or hE-Hb-B10 for 3 wk. Data are means ± SE. *P = 0.003, AA mice compared with SS mice, and P < 0.0001, Ctrl mice compared with HS mice; #P ≤ 0.02, PBS-treated compared with B10-treated mutant mice.
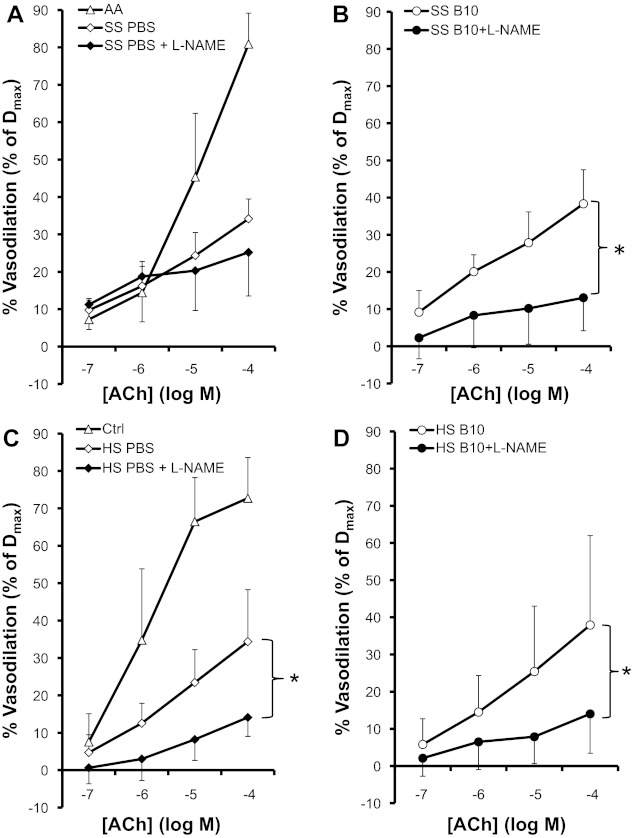
Effect of hE-Hb-B10 on nitric-oxide dependent vascular function.
Facialis artery dilation in response to acetylcholine is NO-dependent in normal mice (43) and is attenuated in both SS and HS mice relative to respective control mice (Fig. 6, A and C) (16, 43). We found that the overall acetylcholine-induced facialis artery dilation is not improved in hE-Hb-B10-treated compared to PBS-treated SS or HS mice (Fig. 6, open diamonds vs. open circles). Interestingly, the acetylcholine-mediated vasodilation was inhibited by l-NAME (Fig. 6B), an inhibitor of NO synthesis, in hE-Hb-B10-treated but not PBS-treated SS mice (Fig. 6A). These results suggest that in SS mice, treatment with hE-Hb-B10 shifts the facialis artery response to acetylcholine in favor of NO-dependent vasodilation. In HS mice, the NO dependence of facialis artery vasodilation remains NO dependent at baseline (Fig. 6C) and this is unaffected by hE-Hb-B10 treatment (Fig. 6D).
Fig. 6.
hE-Hb-B10 restores Nω-nitro-l-arginine methyl ester (l-NAME) inhibition of vasodilation in response to ACh in SS mice. Facialis arteries (180–250 μm) were isolated from SS and HS mice after daily intraperitoneal treatment with PBS or hE-Hb-B10 for 3 wk. Vasodilation in response to ACh was measured in the absence or presence of the NO synthase inhibitor l-NAME. A: comparison of vasodilation of arteries from representative AA mice versus SS mice treated with PBS in the absence and presence of l-NAME. B: comparison of vasodilation of arteries from SS mice treated with hE-Hb-B10 in the absence or presence of l-NAME. C: comparison of vasodilation of arteries from representative Ctrl mice versus HS mice treated with PBS in the absence and presence of l-NAME. D: comparison of vasodilation of arteries from HS mice treated with hE-Hb-B10 in the absence or presence of l-NAME. Dmax, maximum diameter. Data are means ± SD; n = 9 vessels from 5 mice for SS mice treated with PBS with or without l-NAME, 9 vessels from 6 mice for SS mice treated with hE-Hb-B10 with or without l-NAME, 13 vessels from 9 mice for HS mice treated with PBS, 10 vessels from 6 mice for HS mice treated with PBS with l-NAME, 12 vessels from 9 mice for HS mice treated with hE-Hb-B10, and 8 vessels from 5 mice for HS mice treated with hE-Hb-B10 with l-NAME. *P < 0.05, vessel dilation without l-NAME compared with vessel dilation with l-NAME. Representative data from AA mice (5 vessels from 3 mice) and Ctrl mice (7 vessels from 4 mice) are for comparison only and were not included in the statistical analyses.
DISCUSSION
The direct role of CF-Hb in the pathobiology of hemolytic disease, particularly in SCD, is both poorly understood and controversial (9, 18), in part due to the lack of tools that directly target CF-Hb in the setting of active hemolysis. We have developed a novel Hb-binding peptide to further address this issue: Hb-B10. During peptide synthesis, Hb-B10 was covalently linked to a small fragment of ApoE (hE, residues 141–150) that has been shown to facilitate both the uptake of lipoproteins by small peptides and drug transport by liposomes via endocytic clearance through the ubiquitous heparan sulfate proteoglycan-associated pathway (14, 47, 51). Although modification of any small peptide might affect function, we found that the pharmacokinetic profile and liver localization of hE-Hb-B10 were very similar to previous reports for other hE-conjugated peptides (17, 20, 40).
Peptide hE-Hb-B10 is able to effectively attenuate the acute increase in CF-Hb concentrations in the PHZ-treated mice to nearly basal levels within 24 h. This rapid clearance of CF-Hb in PHZ-treated mice via hE-Hb-B10 is similar to the rapid reduction of plasma cholesterol observed in animals treated with hE-18A (17, 20). In addition, both hE-Hb-B10 and hE-18A are taken up by the liver, and the plasma clearance kinetics of both peptides are similar (17, 40). These data imply a common uptake and clearance mechanism for hE18A and hE-Hb-B10 via the heparan-sulfate proteoglycan-associated pathway of the liver (17, 20).
Importantly, hE-Hb-B10 effectively decreases CF-Hb in two distinct murine models of chronic hemolysis with strikingly different hemolytic rates (refer to Table 1). Furthermore, even though Hb-B10 was selected for optimal binding to normal human Hb, hE-Hb-B10 effectively promoted the clearance of human sickle Hb (SS mice) and mouse Hb (PHZ and HS mice) (16, 44). These data suggest that hE-Hb-B10 may be very useful for examining the pathology of CF-Hb in a variety animal models and potentially human subjects with hemolytic diseases of varying severities.
SS and HS mice have baseline chronic liver injury indicated by histopathology and elevated plasma ALT activity (2, 16, 35, 44, 56). Thus potential hepatoxicity of hE-Hb-B10 in the chronic hemolysis models is of concern. Therefore, we measured plasma ALT activity to ensure that the transport of CF-Hb by hE-Hb-B10 did not potentiate liver injury in either model. In fact, we found that hE-Hb-B10 actually attenuated plasma ALT activity in both murine models. Thus reducing circulating levels of CF-Hb with hE-Hb-B10 appears to improve markers of liver injury in the SS and HS mice. The fact that hE-Hb-B10 treatment decreased plasma ALT activity in both SS and HS mice suggests that, in addition to not inducing liver injury, hE-Hb-B10 treatment may actually improve baseline liver function through efficient clearance of CF-Hb. It is possible that CF-Hb directly contributes to liver injury by inducing oxidative tissue damage through lipid oxidation (5, 36, 48). Thus the clearance of CF-Hb could lesson liver oxidative injury. In addition, CF-Hb inhibits the protective mechanisms of NO by rapidly reacting with this molecule and decreasing its bioavailability (23, 49, 50). Therefore, lowering CF-Hb with hE-Hb-B10 could inhibit these direct effects of CF-Hb. Another explanation for the decrease in ALT in hE-Hb-B10-treated mice is that increased cellular uptake of hemoglobin/heme/iron may induce protective/anti-inflammatory mechanisms in the liver. For example, the cytoprotective enzyme heme oxygenase-1 (HO-1) is induced by its substrate (heme) (34, 54). Others have demonstrated that potentiation of HO-1 expression or administration of HO-1 enzymatic products is protective in SS mice (4, 6, 7). Further studies are required to elucidate the mechanisms by which hE-Hb-B10 reduces liver injury as well as to identify any additional potential therapeutic effects of this peptide for treatment of hemolytic anemia.
It is interesting that plasma ALT activity is higher in SS mice compared to HS mice. These differences develop despite of the fact that SS mice have a much lower hemolytic rate than HS mice. Even though hemolysis and heme-induced oxidative injury likely contribute to liver damage in both mouse models, the marked increase in plasma ALT in the SS mice may result from tissue injury induced by vascular occlusion because of erythrocyte sickling. Another divergence of the two hemolytic murine models used in this study is the vasodilatory response of the facialis artery to acetylcholine in the presence of the NOS inhibitor l-NAME. Vessels from HS mice still appear to rely on NO production for vasodilation, albeit attenuated compared with Ctrl mice. In contrast, vessels from SS mice still have a measurable vasodilation that occurs in the presence of l-NAME. Thus vasooclusion due to erythrocyte sickling may also contribute to vessel injury in SS mice, requiring compensatory pathways to maintain some amount of vascular tone. Potential mediators of facialis artery vasodilation in SS mice include the cyclooxygenase 2 or HO-1 pathways (29), as well as endothelial-independent vasodilation by cAMP (23). The finding that hE-Hb-B10 returns vessels from SS mice to a more NO-dependent phenotype suggests hemoglobin/heme trafficking to the liver induces cytoprotective mechanisms that improve vascular function. Belcher et al. (7) have shown that potentiating the expression of HO-1 in the liver of SS mice improved vascular stasis in areas distal to the liver (dorsal skin folds). It is also possible that decreasing CF-Hb improved NO bioavailability, resulting in NO-mediated cytoprotection to combat occlusion-induced vessel injury. Future studies are needed to define the mechanism(s) by which hE-Hb-B10 alters vessel function in SS mice.
In summary, these data demonstrate that hE-Hb-B10 is a novel and effective tool to investigate fundamental questions about the role(s) of CF-Hb in the complex pathobiology of vascular and organ injury in hemolytic anemia. These findings also indicate that hE-Hb-B10 may have therapeutic benefit by suppressing Hb-mediated tissue injury. Future studies are required to determine both the mechanisms of hE-Hb-B10-mediated CF-Hb clearance by the liver as well as the associated reductions in plasma ALT activity and alterations in vessel function.
GRANTS
This work was supported by American Heart Association Grant 0530073N (to N. J. Wandersee), National Institutes of Health Grants HL-071214 (to K. A. Pritchard, Jr.), HL-081139 (to K. A. Pritchard, Jr., and C. A. Hillery), HL-102836 (to K. A. Pritchard, Jr., and C. A. Hillery), HL-090503 (to N. Hogg, C. A. Hillery, and N. J. Wandersee), NS-070711 (to C. A. Hillery and N. J. Wandersee), 5-T32-HL-007209-33 (to M. S. Hanson), 5-F31-HL-092773 (to T. C. Flewelen), and EB-001980 (to the National Biomedical Electron Paramagnetic Resonance Center), the Department of Pediatric Surgery (to H. Xu), and the Midwest Athletes Against Childhood Cancer Fund (to N. J. Wandersee).
DISCLOSURES
C. A. Hillery is a consultant for Bayer Pharmaceuticals.
AUTHOR CONTRIBUTIONS
Author contributions: M.S.H., H.X., T.C.F., A.C.F., K.A.P., C.A.H., N.H., and N.J.W. conception and design of research; M.S.H., H.X., T.C.F., S.L.H., D.R., D.W.J., and A.C.F. performed experiments; M.S.H., H.X., T.C.F., S.L.H., D.R., D.W.J., A.C.F., and N.J.W. analyzed data; M.S.H., H.X., T.C.F., D.R., D.W.J., A.C.F., K.A.P., C.A.H., N.H., and N.J.W. interpreted results of experiments; M.S.H., H.X., T.C.F., and N.J.W. prepared figures; M.S.H., H.X., and N.J.W. drafted manuscript; M.S.H., H.X., T.C.F., S.L.H., D.R., A.C.F., K.A.P., C.A.H., N.H., and N.J.W. edited and revised manuscript; M.S.H., H.X., T.C.F., S.L.H., D.R., D.W.J., A.C.F., K.A.P., C.A.H., N.H., and N.J.W. approved final version of manuscript.
ACKNOWLEDGMENTS
hE-Hb-B10 was designed through the efforts of Translational Vascular Biology Program. The authors greatly appreciate the technical assistance of Weiling Wang, Thomas Foster, and Trudy Holyst.
REFERENCES
- 1. Ascenzi P, Bocedi A, Visca P, Altruda F, Tolosano E, Beringhelli T, Fasano M. Hemoglobin and heme scavenging. IUBMB Life 57: 749–759, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA 98: 15215–15220, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baek JH, D'Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest 122: 1444–1458, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beckman JD, Belcher JD, Vineyard JV, Chen C, Nguyen J, Nwaneri MO, O'Sullivan MG, Gulbahce E, Hebbel RP, Vercellotti GM. Inhaled carbon monoxide reduces leukocytosis in a murine model of sickle cell disease. Am J Physiol Heart Circ Physiol 297: H1243–H1253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belcher JD, Beckman JD, Balla G, Balla J, Vercellotti G. Heme degradation and vascular injury. Antioxid Redox Signal 12: 233–248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest 116: 808–816, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belcher JD, Vineyard JV, Bruzzone CM, Chen C, Beckman JD, Nguyen J, Steer CJ, Vercellotti GM. Heme oxygenase-1 gene delivery by Sleeping Beauty inhibits vascular stasis in a murine model of sickle cell disease. J Mol Med (Berl) 88: 665–675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boretti FS, Buehler PW, D'Agnillo F, Kluge K, Glaus T, Butt OI, Jia Y, Goede J, Pereira CP, Maggiorini M, Schoedon G, Alayash AI, Schaer DJ. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest 119: 2271–2280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF, Ware RE. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood 116: 687–692, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Cappellini MD. Coagulation in the pathophysiology of hemolytic anemias. Hematology Am Soc Hematol Educ Program 74–78, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Cogliandro T, Derchi G, Mancuso L, Mayer MC, Pannone B, Pepe A, Pili M, Bina P, Cianciulli P, De S, V, Maggio A. Guideline recommendations for heart complications in thalassemia major. J Cardiovasc Med (Hagerstown) 9: 515–525, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Cohen JA, Brecher ME, Bandarenko N. Cellular source of serum lactate dehydrogenase elevation in patients with thrombotic thrombocytopenic purpura. J Clin Apher 13: 16–19, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Datta G, Chaddha M, Garber DW, Chung BH, Tytler EM, Dashti N, Bradley WA, Gianturco SH, Anantharamaiah GM. The receptor binding domain of apolipoprotein E, linked to a model class A amphipathic helix, enhances internalization and degradation of LDL by fibroblasts. Biochemistry (Mosc) 39: 213–220, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Datta G, Garber DW, Chung BH, Chaddha M, Dashti N, Bradley WA, Gianturco SH, Anantharamaiah GM. Cationic domain 141–150 of apoE covalently linked to a class A amphipathic helix enhances atherogenic lipoprotein metabolism in vitro and in vivo. J Lipid Res 42: 959–966, 2001 [PubMed] [Google Scholar]
- 15. Doyle MP, Hoekstra JW. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem 14: 351–358, 1981 [DOI] [PubMed] [Google Scholar]
- 16. Frei AC, Guo Y, Jones DW, Pritchard KA, Jr., Fagan KA, Hogg N, Wandersee NJ. Vascular dysfunction in a murine model of severe hemolysis. Blood 112: 398–405, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garber DW, Handattu S, Aslan I, Datta G, Chaddha M, Anantharamaiah GM. Effect of an arginine-rich amphipathic helical peptide on plasma cholesterol in dyslipidemic mice. Atherosclerosis 168: 229–237, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Gladwin MT, Barst RJ, Castro OL, Gordeuk VR, Hillery CA, Kato GJ, Kim-Shapiro DB, Machado R, Morris CR, Steinberg MH, Vichinsky EP. Pulmonary hypertension and NO in sickle cell. Blood 116: 852–854, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gladwin MT, Kato G. Mechanism of Disease: Hemolysis associated endothelial dysfunction and pulmonary hypertension; an emerging cause of death in the hemoglobinopathies. Adv Pulm Hypertension 23–30, 2007 [Google Scholar]
- 20. Gupta H, White CR, Handattu S, Garber DW, Datta G, Chaddha M, Dai L, Gianturco SH, Bradley WA, Anantharamaiah GM. Apolipoprotein E mimetic Peptide dramatically lowers plasma cholesterol and restores endothelial function in watanabe heritable hyperlipidemic rabbits. Circulation 111: 3112–3118, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation 11: 129–151, 2004 [PubMed] [Google Scholar]
- 22. Hebbel RP, Yamada O, Moldow CF, Jacob HS, White JG, Eaton JW. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest 65: 154–160, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood 109: 3088–3098, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu W, Jin R, Zhang J, You T, Peng Z, Ge X, Bronson RT, Halperin JA, Loscalzo J, Qin X. The critical roles of platelet activation and reduced NO bioavailability in fatal pulmonary arterial hypertension in a murine hemolysis model. Blood 116: 1613–1622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jais X, Till SJ, Cynober T, Ioos V, Garcia G, Tchernia G, Dartevelle P, Simonneau G, Delaunay J, Humbert M. An extreme consequence of splenectomy in dehydrated hereditary stomatocytosis: gradual thrombo-embolic pulmonary hypertension and lung-heart transplantation. Hemoglobin 27: 139–147, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Jison ML, Gladwin MT. Hemolytic anemia-associated pulmonary hypertension of sickle cell disease and the nitric oxide/arginine pathway. Am J Respir Crit Care Med 168: 3–4, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Kato GJ, Hsieh M, Machado R, Taylor J, Little J, Butman JA, Lehky T, Tisdale J, Gladwin MT. Cerebrovascular disease associated with sickle cell pulmonary hypertension. Am J Hematol 81: 503–510, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kato GJ, McGowan V, Machado RF, Little JA, Taylor J, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM, Jr., Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood 107: 2279–2285, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaul DK, Zhang X, Dasgupta T, Fabry ME. Arginine therapy of transgenic-knockout sickle mice improves microvascular function by reducing non-nitric oxide vasodilators, hemolysis, and oxidative stress. Am J Physiol Heart Circ Physiol 295: H39–H47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knobloch K, Zardo P, Gohrbandt B, Fischer S, Leyh RG, Tiede A, Ganser A, Schubert J. Cardiac surgery in a patient with paroxysmal nocturnal hemoglobinuria. Haematologica 87: ECR29, 2002 [PubMed] [Google Scholar]
- 31. Koshida R, Ou J, Matsunaga T, Chilian WM, Oldham KT, Ackerman AW, Pritchard KA., Jr Angiostatin: a negative regulator of endothelial-dependent vasodilation. Circulation 107: 803–806, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Lim SK, Kim H, Lim SK, bin AA, Lim YK, Wang Y, Chong SM, Costantini F, Baumman H. Increased susceptibility in Hp knockout mice during acute hemolysis. Blood 92: 1870–1877, 1998 [PubMed] [Google Scholar]
- 33. Lim YK, Jenner A, Ali AB, Wang Y, Hsu SI, Chong SM, Baumman H, Halliwell B, Lim SK. Haptoglobin reduces renal oxidative DNA and tissue damage during phenylhydrazine-induced hemolysis. Kidney Int 58: 1033–1044, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Loboda A, Jazwa A, Grochot-Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, Jozkowicz A, Dulak J. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 10: 1767–1812, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood 107: 1651–1658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller YI, Altamentova SM, Shaklai N. Oxidation of low-density lipoprotein by hemoglobin stems from a heme-initiated globin radical: antioxidant role of haptoglobin. Biochemistry (Mosc) 36: 12189–12198, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Jr., Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 294: 81–90, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood 32: 811–815, 1968 [PubMed] [Google Scholar]
- 39. Murali B, Drain A, Seller D, Dunning J, Vuylsteke A. Pulmonary thromboendarterectomy in a case of hereditary stomatocytosis. Br J Anaesth 91: 739–741, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Nayyar G, Handattu SP, Monroe CE, Chaddha M, Datta G, Mishra VK, Keenum TD, Palgunachari MN, Garber DW, Anantharamaiah GM. Two adjacent domains (141–150 and 151–160) of apoE covalently linked to a class A amphipathic helical peptide exhibit opposite atherogenic effects. Atherosclerosis 213: 449–457, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nielsen MJ, Moestrup SK. Receptor targeting of hemoglobin mediated by the haptoglobins: roles beyond heme scavenging. Blood 114: 764–771, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Okpala I. The intriguing contribution of white blood cells to sickle cell disease - a red cell disorder. Blood Rev 18: 65–73, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Ou J, Ou Z, Jones DW, Holzhauer S, Hatoum OA, Ackerman AW, Weihrauch DW, Gutterman DD, Guice K, Oldham KT, Hillery CA, Pritchard KA., Jr L-4F, an apolipoprotein A-1 mimetic, dramatically improves vasodilation in hypercholesterolemia and sickle cell disease. Circulation 107: 2337–2341, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science 278: 876–878, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Qin X, Hu W, Song W, Blair P, Wu G, Hu X, Song Y, Bauer S, Feelisch M, Leopold JA, Loscalzo J, Halperin JA. Balancing role of nitric oxide in complement-mediated activation of platelets from mCd59a and mCd59b double-knockout mice. Am J Hematol 84: 221–227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramaiah SK. Preclinical safety assessment: current gaps, challenges, and approaches in identifying translatable biomarkers of drug-induced liver injury. Clin Lab Med 31: 161–172, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Re F, Cambianica I, Zona C, Sesana S, Gregori M, Rigolio R, La FB, Nicotra F, Forloni G, Cagnotto A, Salmona M, Masserini M, Sancini G. Functionalization of liposomes with ApoE-derived peptides at different density affects cellular uptake and drug transport across a blood-brain barrier model. Nanomedicine 7: 551–559, 2011 [DOI] [PubMed] [Google Scholar]
- 48. Reeder BJ, Wilson MT. Hemoglobin and myoglobin associated oxidative stress: from molecular mechanisms to disease States. Curr Med Chem 12: 2741–2751, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 8: 1383–1389, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 293: 1653–1662, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Sauer I, Dunay IR, Weisgraber K, Bienert M, Dathe M. An apolipoprotein E-derived peptide mediates uptake of sterically stabilized liposomes into brain capillary endothelial cells. Biochemistry (Mosc) 44: 2021–2029, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Scheuerle AF, Serbecic N, Beutelspacher SC. Paroxysmal nocturnal hemoglobinuria may cause retinal vascular occlusions. Int Ophthalmol 29: 187–190, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Schilling RF, Gangnon RE, Traver MI. Delayed adverse vascular events after splenectomy in hereditary spherocytosis. J Thromb Haemost 6: 1289–1295, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Takahashi T, Morita K, Akagi R, Sassa S. Heme oxygenase-1: a novel therapeutic target in oxidative tissue injuries. Curr Med Chem 11: 1545–1561, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Wandersee NJ, Birkenmeier CS, Gifford EJ, Mohandas N, Barker JE. Murine recessive hereditary spherocytosis, sph/sph, is caused by a mutation in the erythroid alpha-spectrin gene. Hematol J 1: 235–242, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Wandersee NJ, Lee JC, Kaysser TM, Bronson RT, Barker JE. Hematopoietic cells from -spectrin-deficient mice are sufficient to induce thrombotic events in hematopoietically ablated recipients. Blood 92: 4856–4863, 1998 [PubMed] [Google Scholar]
- 57. Wandersee NJ, Olson SC, Holzhauer SL, Hoffmann RG, Barker JE, Hillery CA. Increased erythrocyte adhesion in mice and humans with hereditary spherocytosis and hereditary elliptocytosis. Blood 103: 710–716, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Wang X, Tanus-Santos JE, Reiter CD, Dejam A, Shiva S, Smith RD, Hogg N, Gladwin MT. Biological activity of nitric oxide in the plasmatic compartment. Proc Natl Acad Sci U S A 101: 11477–11482, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Y, Qui Y, Zhu J, Gao D. Pulmonary hypertension associated with autoimmune hemolytic anemia: a case report. Int J Cardiol 115: e1–e2, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Ziakas PD, Poulou LS, Pomoni A. Thrombosis in paroxysmal nocturnal hemoglobinuria at a glance: a clinical review. Curr Vasc Pharmacol 6: 347–353, 2008 [DOI] [PubMed] [Google Scholar]