Abstract
Chronic hypoxia (CH)-induced pulmonary hypertension is characterized by vasoconstriction and vascular remodeling, leading to right ventricular dysfunction. Given the role of arterial compliance (Ca) in right ventricular work, a decrease in Ca would add to right ventricular work. Nitric oxide (NO) is a potent vasodilator made by NO synthases from l-arginine (l-Arg). However, little is known of the effect of l-Arg on vascular compliance (Cv) in the lung. We hypothesized that exposure to CH would decrease Ca and that this effect would be reversed by exogenous l-Arg. Sprague-Dawley rats were exposed to either normoxia or CH for 14 days; the lungs were then isolated and perfused. Vascular occlusions were performed and modeled using a three-compliance, two-resistor model. Pressure-flow curves were generated, and a distensible vessel model was used to estimate distensibility and a vascular resistance parameter (R0). Hypoxia resulted in the expected increase in arterial resistance (Ra) as well as a decrease in both Ca and Cv. l-Arg had little effect on Ra, Ca, or Cv in isolated lungs from normoxic animals. l-Arg decreased Ra in lungs from CH rats and redistributed compliance to approximately that found in normoxic lungs. CH increased R0, and l-Arg reversed this increase in R0. l-Arg increased exhaled NO, and inhibition of l-Arg uptake attenuated the l-Arg-induced increase in exhaled NO. These data demonstrate that the CH-induced decrease in Ca was reversed by l-Arg, suggesting that l-Arg may improve CH-induced right ventricular dysfunction.
Keywords: vascular compliance, cationic amino acid transporter, endothelial nitric oxide synthase, isolated perfused lung, right ventricular function
exposure to chronic hypoxia (CH) causes vasoconstriction and vascular remodeling, leading to increased right ventricular (RV) afterload, resulting in RV hypertrophy and eventually to RV dysfunction or cor pulmonale. These effects are caused at least in part by decreases in the activity of endothelial nitric oxide (NO) synthase (eNOS), which results in decreased NO production during hypoxia. Indeed, in patients with pulmonary hypertension, NO production has been shown to be decreased (41). In fact, exogenous NO gas given by inhalation has been used as a therapy for lowering pulmonary vascular resistance in patients with pulmonary hypertension (20, 33). NO is endogenously synthesized from l-arginine (l-Arg) by three isoforms of NOS. Plasma levels of l-Arg have been reported to be low in a rat model of pulmonary hypertension (22) and in patients with pulmonary hypertension (21). Studies (5, 10) have demonstrated an increase in arterial resistance (Ra) in lungs from rats exposed to CH using double occlusion methods. In isolated perfused rat lungs, Emery et al. (6) found that static compliance was decreased after CH, and Vanderpool et al. (41) found that pulmonary arterial compliance (Ca) using microcomputed tomography was decreased after CH. However, to the best of our knowledge, there are no reports of the effects of exogenous l-Arg on vascular compliance after CH in the rat lung using occlusion methods. Since RV work is inversely proportional to Ca (18), understanding the effects of CH on Ca may have important implications for RV function. We therefore tested the hypothesis that CH would result in decreased pulmonary Ca and that this decrease in Ca would be reversed by exogenous l-Arg administration. We used arterial, venous, and double occlusions and a five-compartment model to estimate total pulmonary vascular resistance (RL), Ra, venous resistance (Rv), total pulmonary vascular compliance (CL), Ca, microvascular compliance (Cc), and venous compliance (Cv) as previously described (2, 17, 29). We also examined pressure-flow (P-Q) curves and used a distensible vessel model to estimate distensibility (α) and a vascular resistance parameter (R0) (17, 29, 32). It has been shown that CH leads to increased vasoconstrictor responsiveness (5, 33). Therefore, to examine the effect of vasoconstriction on hemodynamics, we performed experiments with the sequential addition of Nω-nitro-l-arginine methyl ester (l-NAME; a NOS inhibitor), KCl, and diethylenetriamine (DETA)-NONOate (an NO donor). We also examined hemodynamics before and after the addition of l-Arg to the perfusate. l-Lysine (l-Lys) was used as a competitive antagonist of l-Arg uptake. We began to examine if l-citrulline (l-Cit) via the endogenous synthetic pathway for l-Arg would have the same effect as l-Arg in the isolated lung. However, to our surprise, l-Cit had little effect on exhaled NO (exNO) production in the isolated, perfused rat lung, and, therefore, we measured mRNA levels of argininosuccinate synthase (AS) and argininosuccinate lyase (AL) in the whole lung. Finally, we used an in vivo model to examine the effect of l-Cit on exNO production.
MATERIALS AND METHODS
All protocols used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the Research Institute at Nationwide Children's Hospital. Sprague-Dawley rats (Harlan, Indianapolis, IN) were provided with fresh water, food, and clean bedding 3 times/wk. All animals were housed on a 12:12-h light-dark cycle. Rats were placed in either a normoxic (N) or hypobaric hypoxic chamber (pressure: 435 ± 10 mmHg) for 14 days (CH).
Isolation and perfusion of rat lungs.
Lungs from N or CH rats were isolated and perfused as previously described (5, 33). Briefly, on the day of study, rats were euthanized with pentobarbital sodium (100 mg/kg) given intraperitoneally. The trachea was cannulated, and the tracheotomy tube was connected to a piston-type ventilator (Harvard Apparatus, Holleston, MA). Lungs were ventilated with a NO-free gas mixture of 5% CO2-21% O2-balance N2 using a tidal volume of 2.5 ml and a rate of 55 breaths/min. A median sternotomy was performed, and heparin was injected directly into the RV. The pulmonary artery and left ventricle were cannulated. The preparation was immediately perfused with physiological saline solution (PSS) with 4% albumin (wt/vol) and 300 μM meclofenamate (Sigma-Aldrich, St. Louis, MO) at a rate of 30 ml·kg−1·min−1 using a Masterflex roller pump. The perfusion rate was maintained at this rate for the duration of the experiment. The initial perfusion was nonrecirculating until the perfusate exiting the lung was nearly free of blood, at which point recirculating perfusion was established with PSS, and the total volume of the circulation system was ∼40 ml. Lungs were maintained in zone 3 conditions throughout the experimental protocol by maintaining venous pressure (Pv) at 3 mmHg and end-expiratory pressure at 1 mmHg. Pulmonary arterial pressure (Pa), Pv, and airway pressure were recorded continuously using Codas data-acquisition software (CODAS, Dataq Instruments, Akron, OH).
Segmental vascular resistances and vascular compliances.
Three vascular occlusion maneuvers (arterial, venous, and double occlusions) were performed, and the resultant pressure data were modeled using a five-compartment model as previously described (28). Briefly, the occlusion of both the arterial inflow and venous outflow (double occlusion) caused Pa and Pv to rapidly equilibrate at the double occlusion pressure (Pd), which approximates the microvascular pressure. Using these three pressures and the perfusate flow rate (Q), vascular resistances can be calculated using the following equations:
Vascular compliances can be assessed using the vascular resistance data in conjunction with the venous occlusion and arterial occlusion data. CL, Ca, Cc, and Cv were estimated from steady-state Pa and Pv, Q, the area (A2) encompassed by the Pa curve [Pa(t), where t is time] after arterial occlusion, the equilibrium pressure Pd after double occlusion, and the average slope (m) of the Pa(t) and Pv(t) curves after venous occlusion using the following equations:
Vascular volume.
Vascular volume (QL) was measured in isolated perfused lungs using thermodilution. An injector allowed the introduction of 0.35 ml ice-cold saline into the arterial inflow. Serial QL measurements were done at Pv of 1, 4, 8, and 12 mmHg. For these experiments, Q was constant at 20 ml/min. Before the injection, the ventilator was stopped at end expiration, and Pv was adjusted to the desired value. After all measurements were completed, the lungs were removed, and the cannulas were connected directly together to measure the volume of the tubing system alone. The mean transit time (t̄) from the injector to the venous outflow point was calculated as follows:
where C(t) is the amplitude of the thermistor signal at time t, t = 0 is the time of the injection, and a is the time when C(t) had returned to 1% of its peak value. Vascular mean transit time (t̄L) was calculated by subtracting the t̄ obtained after the lungs were removed from the t̄ that was obtained with the lungs in the system. QL was then Q × t̄L. Values of QL measured at different Pv were used to calculate the static vascular compliance (Cst) of the lung from the slope of the QL versus Pv curve (29).
P-Q curve.
P-Q curves were constructed as previously described (17, 29). Briefly, Q was decreased to 4.5 ml/min, and the height of the venous reservoir was adjusted to maintain Pv at 3 mmHg, the ventilator was turned off, Pa was recorded, and the ventilator was then turned on. This procedure was repeated with Q at 8, 12, 18, and 25 ml/min, and the Pa value with Pv maintained at 3 mmHg at each Q point was recorded. After the P-Q data were collected, Q was returned to the standard flow rate.
Two parameters, α (which represents the fractional change in vessel diameter per mmHg change in pressure) and R0 (which represents the vascular resistance that would exist if the resistance vessels were at their respective diameters obtained when the vascular pressure were zero), were estimated using a distensible vessel model as previously described (17, 29). Briefly, P-Q curves were fit with the following equation using nonlinear regression:
exNO measurement.
In the isolated, perfused lung, exNO was measured as previously described (3, 30). Briefly, 25 min after the equilibration of the system, the baseline exhaled gas was collected for 5 min into a mylar balloon attached to the ventilator exhaust port. The gas collected in the mylar balloon was analyzed using a chemilluminescence NO analyzer (model 280i, Sievers, Boulder, CO). The analyzer was calibrated using a standard curve generated daily with authentic NO (1 ppm in N2, Matheson, Chicago, IL) mixed with NO-free nitrogen using precision flow meters to obtain concentrations ranging from 0 to 500 ppb (vol/vol). The NO detection limit was 0.5 ppb. After baseline gas collection, l-Arg was added into the perfusate reservoir to achieve the desired final concentration, and the exhaled gas was collected after 25 min for 5 min for NO measurement.
In whole animals, exNO was measured as previously described (5). Briefly, rats were anesthetized with 50 mg/kg ip pentobarbital, intubated, and mechanically ventilated using a NO-free gas mixture of 21% O2-balance N2 with a tidal volume of 2.5 ml and a respiratory rate of 55 breaths/min. The carotid artery was cannulated using polyethylene-50 tubing and connected to a blood pressure transducer (Columbus Instruments, Columbus, OH). Blood pressure was continuously monitored (Cardiomax, Columbus Instruments). The jugular vein was cannulated for the administration of medications. The baseline exhaled gas was collected for 5 min into a mylar balloon attached to the ventilator exhaust port after a 25-min equilibration period. The collected exhaled gas was analyzed using a chemilluminescence NO analyzer as described above. After the baseline gas collection, treatment was given intravenously, and the exhaled gas was collected after 25 min for NO measurement as described above.
Protein isolation.
Lung tissue was collected at the end of the experiment and frozen at −80°C. Lungs were homogenized in ice-cold Dulbecco's PBS (pH 7.4) containing protease inhibitors and phosphatase inhibitors. Samples were centrifuged at 12,000 g for 15 min, and the supernatants were collected and analyzed for total protein content using the Bradford assay (Bio-Rad, Hercules, CA). Supernatants were stored at −80°C for further study.
Immunoblot analysis.
Tissue homogenates were assayed by Western blot analysis as previously described (5, 30) using the following antibodies: eNOS (1:1,000, BD Transduction, San Jose, CA), neuronal NOS (nNOS; 1:500, BD Transduction), inducible NOS (1:500, BD Transduction), AS (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), AL (1:200, Santa Cruz Biotechnology), and β-actin (1:5,000, Sigma-Aldrich).
RT-PCR.
To measure the mRNA expression levels of AS1 and AL in the rat lung, liver, and kidney, RT-PCR was performed as previously described, and levels were normalized to GAPDH (4). The following primers were used: AS1, forward 5′-TTTGTTCGCCACTGCATCGACAAG-3′ and reverse 5′-TCTATTTGGCGGTGACCTTGCTCT-3′; AL, forward 5′-GGGCCCAAGGCATCTTCAAATTGT-3′ and reverse 5′-AACAGGAACTCAGCCACGAAGTCT-3′; and GAPDH, forward 5′-TGATGCTGGTGCTGAGTATGTCGT-3′ and reverse 5′-TTGTCATTGAGAGCAATGCCAGCC-3′.
Statistical analysis.
Values are means ± SE. Unpaired t-tests were used to compare data between N and CH groups with the same treatment. A paired t-test was used to compare data before and after the addition of l-Arg or l-Cit when only one concentration was used. One-way repeated-measures ANOVA was used to compare data among treatment groups within the N or CH groups. Two-way ANOVA was used for exNO data with increasing l-Arg and l-NAME concentrations to compare the effects of concentration and exposure. In Fig. 2, the slopes of the lines were compared using analysis of covariance. Differences were considered significant when P < 0.05.
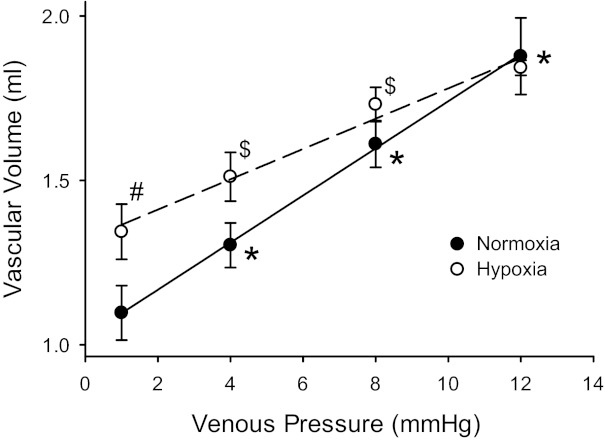
Fig. 2.
Static vascular compliance was greater in lungs from N rats than in those from CH rats. Vascular volume (QL) was measured by thermodilution at four different venous pressures (Pv) at a constant flow rate of 20 ml/min in isolated lungs from N (n = 7) and CH (n = 6) rats. QL increased in N lungs with each increase in Pv. Although the QL at a Pv of 1 mmHg was greater in CH rats, there was a smaller change in QL for each Pv increase, and the change in Pv from 8 to 12 mmHg did not significantly increase QL. The slopes of the lines (static vascular compliance) were different (P < 0.01) by analysis of covariance (0.070 ± 0.009 ml/mmHg in N vs. 0.046 ± 0.007 ml/mmHg in CH). #CH different from N at the same Pv (P < 0.001); *QL different from previous Pv in N lungs (P < 0.001); $QL different from previous Pv in CH lungs (P < 0.05).
RESULTS
Vascular resistances.
To evaluate the effect of CH exposure on RL and CL, lungs from N or CH rats were isolated and perfused. The baseline venous, arterial, and double occlusion maneuvers were performed after a 25-min equilibration period. To assess vasoreactivity, isolated perfused lungs were treated sequentially with l-NAME (final perfusate concentration: 3 mM), KCl (final perfusate concentration: 30 mM), and DETA-NONOate (final perfusate concentration: 100 μM), and each condition lasted 30 min. RL was greater in lungs from CH animals than in those from N animals (Table 1). Ra was ∼80% greater and Rv was ∼50% greater in lungs from CH animals than in lungs from N animals (Table 1). The addition of l-NAME to the perfusate had little effect on RL in either N or CH lungs. The addition of KCl to the perfusate resulted in robust vasoconstriction in both N and CH lungs; however, RL in CH lungs was nearly threefold greater than in N lungs (Table 1). The addition of DETA-NONOate to the perfusate significantly decreased RL in both groups, although the values for RL did not return all the way to basal levels (Table 1). The main contributor to the KCl-induced increase in vascular resistance was Ra in both N and CH lungs, although the KCl-induced increase in Ra was substantially greater in CH lungs than in N lungs (Table 1). Although lower in absolute magnitude than in Ra, the additon of KCl significantly increased Rv in both N and CH lungs, and again the increase in Rv was greater in CH lungs than in N lungs (Table 1). The addition of the NO donor DETA-NONOate significantly decreased both Ra and Rv, although Ra and Rv remained above basal levels in both N and CH lungs (Table 1).
Table 1.
Vascular resistance
| Baseline | l-NAME | KCl | DETA-NONOate | |
|---|---|---|---|---|
| Normoxia | ||||
| RL | 29 ± 4 | 26 ± 4 | 170 ± 12‡ | 59 ± 5‡ |
| Ra | 17 ± 3 | 14 ± 3 | 121 ± 8‡ | 39 ± 5‡ |
| Rv | 12 ± 1 | 12 ± 1 | 49 ± 7‡ | 19 ± 2‡ |
| Hypoxia | ||||
| RL | 49 ± 6* | 46 ± 7* | 466 ± 35†‡ | 112 ± 9†‡ |
| Ra | 31 ± 6* | 28 ± 5* | 367 ± 46†‡ | 83 ± 11†‡ |
| Rv | 18 ± 1* | 18 ± 2* | 99 ± 6†‡ | 27 ± 3*‡ |
Values are mean ± SE. Vascular resistances are in units of mmHg·ml−1·s−1. l-NAME, Nω-nitro-l-arginine methyl ester; DETA-NONOate, diethylenetriamine NONOate; RL, total pulmonary vascular resistance; Ra, arterial resistance; Rv, venous resistance.
Hypoxia different from normoxia (P < 0.05);
hypoxia different from normoxia (P < 0.001);
different from the previous condition with the same exposure (P < 0.001).
Vascular compliance.
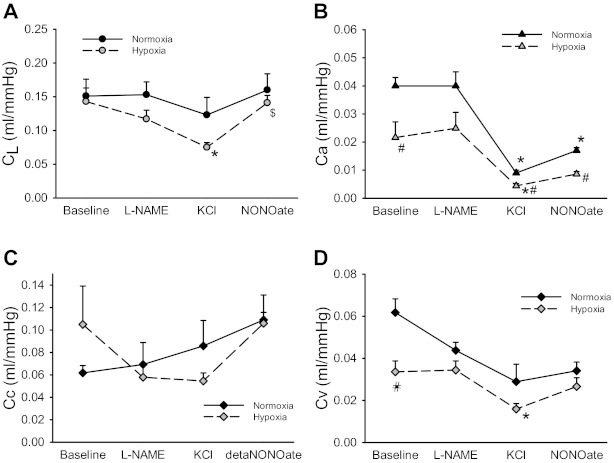
The vascular occlusion data were also used to calculate CL and its distribution among Ca, Cc, and Cv. During baseline conditions, CL did not differ between isolated lungs from N and CH animals (Fig. 1A). The addition of l-NAME had little effect on CL in either N or CH lungs. However, CL was significantly lower in CH lungs after KCl-induced vasoconstriction, and the addition of DETA-NONOate increased CL in CH lungs to baseline levels (Fig. 1A). Ca was significantly lower in isolated lungs from CH animals compared with N animals (Fig. 1B). The addition of l-NAME had little effect on Ca in either N or CH lungs, whereas the addition of KCl resulted in a significant decrease in Ca in both N and CH lungs (Fig. 1B). The addition of DETA-NONOate resulted in a modest increase in Ca in N lungs, with no significant change in Ca in CH lungs (Fig. 1B). The majority of the total vascular compliance was in Cc in both N and CH lungs under all conditions (Fig. 1C). There were no significant effects of any of the vasoactive agents on Cc in either N or CH lungs (Fig. 1C). Cv was significantly lower in isolated lungs from CH animals than in those from N animals (Fig. 1D). The addition of l-NAME had little effect on Cv, whereas the addition of KCl significantly decreased Cv only in lungs from CH animals. The addition of DETA-NONOate had little effect on Cv in either N or CH lungs (Fig. 1D).
Fig. 1.
Chronic hypoxia (CH) had little effect on the total pulmonary vascular compliance (CL) but resulted in lower arterial compliance (Ca) and venous compliance (Cv). Nitric oxide (NO) resulted in a significant increase in compliance in CH lungs after KCl-induced vasoconstriction. Vascular compliance was estimated from vascular occlusion maneuvers in isolated perfused lungs using a five-compartment model that included CL (A), Ca (B), Cv (C), and microvascular complicance (Cc; D). Vascular occlusions were performed under four conditions, each lasting 30 min: baseline (BL), 3 mM Nω-nitro-l-arginine methyl ester (l-NAME), 30 mM KCl, and 100 μM diethylenetriamine (DETA)-NONOate. *Different from BL in the same group (P < 0.05); $different from 30 mM KCl in the same group (P < 0.05); #CH different from normoxia (N) with the same perfusate (P < 0.05).
The CL measured during occlusions is a measure of dynamic vascular compliance. To determine the effect of hypoxia on Cst in the isolated rat lung, total QL in the lungs was measured at various outflow pressures (Pv), including 1, 4, 8, and 12 mmHg. QL at a Pv of 1 mmHg was greater in isolated lungs from CH animals than in those from N animals (Fig. 2). In N lungs, QL increased significantly with each increase in Pv, whereas in CH lungs, the QL increase with Pv was smaller than in N lungs and only increased with the first two Pv steps (Fig. 2). Cst, the slope of the QL versus Pv curve, was significantly lower in isolated lungs from CH animals than in those from N animals (Fig. 2).
Lung P-Q curves.
To further examine the effect of CH on pulmonary hemodynamics, P-Q curves were constructed using values from isolated perfused lungs from N and CH rats. R0 and α were calculated from P-Q curves (Table 2). No obvious differences in α were detected between N and CH lungs at baseline. α in N lungs did not change upon the addition of l-NAME, KCl, or DETA-NONOate. However, KCl-induced vasoconstriction resulted in a significant decrease in α in CH lungs, and the addition of DETA-NONOate caused an increase in α back to basal levels (Table 2). Similar to what was found with RL, isolated lungs from CH animals had significantly greater R0 at baseline and under all vasoactive conditions studied than isolated lungs from N animals under the same conditions (Table 2). The addition of l-NAME had little effect on R0 in either N or CH lungs. The addition of KCl resulted in ∼14-fold and ∼21-fold increases in R0 in N and CH lungs, respectively (Table 2). The addition of DETA-NONOate decreased R0 in both N and CH lungs but did not return values to baseline levels (Table 2).
Table 2.
Pressure-flow results
| α, %/mmHg |
R0,mmHg·ml−1·s−1 |
|||
|---|---|---|---|---|
| Normoxia | Hypoxia | Normoxia | Hypoxia | |
| Baseline | 3.7 ± 1.0 | 4.4 ± 1.3 | 0.94 ± 0.15 | 2.31 ± 0.59* |
| l-NAME | 3.1 ± 0.6 | 4.9 ± 0.9 | 0.74 ± 0.11 | 2.53 ± 0.65† |
| KCl | 3.8 ± 2.2 | 1.4 ± 0.3‡ | 10.12 ± 1.17‡ | 53.03 ± 3.73†‡ |
| DETA-NONOate | 3.0 ± 0.6 | 4.4 ± 0.2 | 2.32 ± 0.27‡ | 8.51 ± 3.25*‡ |
Values are means ± SE. α, distensibility; R0, vascular resistance parameter.
Hypoxia different from normoxia (P < 0.05);
hypoxia different from normoxia (P < 0.005);
different from the previous condition with the same exposure (P < 0.05).
Lung NO production.
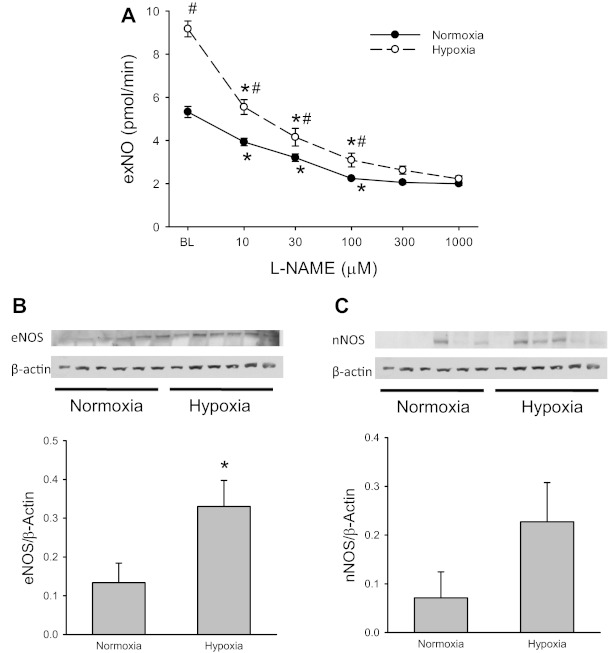
To determine the effect of CH on exNO production and lung levels of eNOS and nNOS protein, lungs from N and CH rats were isolated, perfused, and ventilated with a normoxic gas mixture. exNO was measured at baseline and then 30 min after each subsequent addition of l-NAME to the perfusate (final concentrations of 10, 30, 100, 300, and 1,000 μM, respectively). The lungs were then frozen in liquid nitrogen and stored at −80°C until used for protein extraction for eNOS and nNOS with immunoblot analysis. exNO production from isolated perfused lungs from CH rats was significantly greater than from lungs of N rats (Fig. 3A). l-NAME inhibited exNO production in isolated lungs from both N and CH rats in a concentration-dependent manner, reaching a maximal effect at ∼100 μM in both N and CH lungs (Fig. 3A). There was significantly more eNOS protein in lungs from CH rats than in lungs from N rats (Fig. 3B). Although there was a trend to greater nNOS expression in CH lungs, there was no statistically significant difference in lung nNOS protein levels between N and CH rats (Fig. 3C).
Fig. 3.
Exhaled NO (exNO) production was greater from lungs isolated from CH animals than from lungs isolated from N animals, and the protein abundance of endothelial NO synthase (eNOS) was significantly greater in CH lungs than in N lungs. A: exNO production from CH and N lungs with increasing quanitites of the NOS inhibitor l-NAME added to the perfusate. #CH different from N at the same additive concentration (P < 0.05); *different from the previous additive concentration under the same condition (P < 0.05). B: abundance of eNOS protein in N and CH lungs. *CH different from N (P < 0.05). C: abundance of neuronal NOS (nNOS) protein in N and CH lungs. It should be pointed out that under the conditions of our Western blot analysis, inducible NOS protein expression was undetectable in both N and CH lungs.
l-Arg and pulmonary hemodynamics.
The effects of exogenous l-Arg on hemodynamics were determined in isolated perfused lungs. Vascular occlusions were performed at baseline and after the addition of l-Arg to the perfusate to achieve a final concentration of 3 mM. The addition of l-Arg decreased RL only in lungs from CH rats (Table 3). This decrease in RL was due to a decrease in Ra with the addition of l-Arg with little effect on Rv (Table 3). The addition of exogenous l-Arg had little effect on the distribution of vascular resistance in lungs from N rats. However, the addition of l-Arg changed the distribution of vascular resistance in CH lungs so that it was essentially equally distributed between arteries and veins and not different from that seen in N lungs (Table 3).
Table 3.
Hemodynamics after l-arginine
| Normoxia |
Hypoxia |
|||
|---|---|---|---|---|
| Baseline | l-arginine | Baseline | l-arginine | |
| RL, mmHg·ml−1·s−1 | 28 ± 4 | 26 ± 3 | 53 ± 10* | 38 ± 4† |
| Ra, mmHg·ml−1·s−1 | 15 ± 2 | 13 ± 1 | 32 ± 7* | 19 ± 2† |
| Rv, mmHg·ml−1·s−1 | 14 ± 2 | 13 ± 2 | 21 ± 3 | 19 ± 2 |
| Ra, % of total RL | 53 ± 2 | 51 ± 2 | 60 ± 1* | 51 ± 2† |
| Rv, % of total RL | 47 ± 2 | 49 ± 2 | 40 ± 1* | 49 ± 2† |
| CL, ml/mmHg | 0.15 ± 0.01 | 0.13 ± 0.02 | 0.13 ± 0.01 | 0.12 ± 0.01 |
| Ca, ml/mmHg | 0.041 ± 0.004 | 0.040 ± 0.004 | 0.020 ± 0.005* | 0.030 ± 0.007 |
| Cc, ml/mmHg | 0.061 ± 0.006 | 0.055 ± 0.008 | 0.089 ± 0.010* | 0.058 ± 0.013 |
| Cv, ml/mmHg | 0.047 ± 0.007 | 0.038 ± 0.008 | 0.024 ± 0.005* | 0.029 ± 0.006 |
| Ca, % of total CL | 28 ± 2 | 31 ± 3 | 15 ± 4* | 26 ± 4† |
| Cc, % of total CL | 41 ± 5 | 42 ± 4 | 67 ± 7* | 49 ± 8† |
| Cv, % of total CL | 31 ± 3 | 27 ± 5 | 18 ± 3* | 25 ± 4† |
Values are means ± SE. CL, total pulmonary vascular compliance; Ca, arterial compliance; Cv, venous compliance; Cc, microvascular compliance.
Hypoxia different from normoxia (P < 0.05);
different from the previous condition with the same exposure (P < 0.05).
There were no differences in CL between lungs isolated from N or CH rats, and the addition of l-Arg had no effect on CL in either N or CH lungs (Table 3). However, Ca and Cv in lungs from CH rats were significantly lower than in lungs from N rats, and the addition of l-Arg had little effect on Ca or Cv in either N or CH lungs (Table 3). Again, Cc was significantly greater in CH lungs than in N lungs, and the addition of l-Arg to the perfusate eliminated the differences in Ca, Cv, and Cc between N and CH lungs (Table 3). The majority of CL in the lungs was in Cc for both N and CH animals, although the percentage of the vascular compliance contributed by Cc was significantly greater in lungs from CH rats than in lungs from N rats (Table 3). In lungs from CH animals, the addition of l-Arg to the perfusate decreased the percentage of the vascular compliance contributed by Cc, such that the distribution of vascular compliance in CH lungs after the addition of 3 mM l-Arg resembled that seen in lungs from N rats (Table 3).
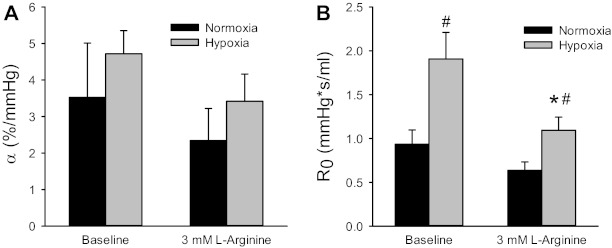
To further examine the effect of exogenous l-Arg on pulmonary hemodynamics, P-Q curves were constructed in isolated perfused lungs from N and CH rats at baseline and after the addition of l-Arg to the perfusate to achieve a final concentration of 3 mM. The addition of l-Arg to the perfusate in N lungs had little effect on the P-Q curve; however, the addition of l-Arg to the perfusate of CH lungs resulted in vascular pressures at each flow rate that were not different from baseline values in N lungs (data not shown). α was not significantly different between lungs from N and CH rats, and the addition of l-Arg to the perfusate had little effect on α (Fig. 4A). R0 was significantly greater in lungs from CH rats than in lungs from N rats, and the addition of l-Arg to the perfusate significantly decreased R0 in CH lungs (Fig. 4B). Indeed, R0 values in CH lungs with l-Arg added to the perfusate were not significantly different from R0 values in N lungs at baseline (Fig. 4B).
Fig. 4.
Addition of l-arginine (l-Arg) to achieve a final perfusate concentration of 3 mM decreased vascular resistance in CH lungs such that it was not different from that in N lungs. Using a distensible vessel model for the pressure-flow (P-Q)data generated values for vascular distensibility (α; A) and a vascular resistance parameter (R0; B). There were no differences in α between N (n = 7) and CH (n = 6) lungs, and the addition of l-Arg had little effect on α. R0 was greater in isolated perfused lungs from CH rats than from N rats, and l-Arg significantly decreased R0 in CH lungs. #CH different from N under the same condition (P < 0.05); *CH + l-Arg different from CH (P < 0.05).
Effect of exogenous l-Arg on exNO production in isolated perfused lungs.
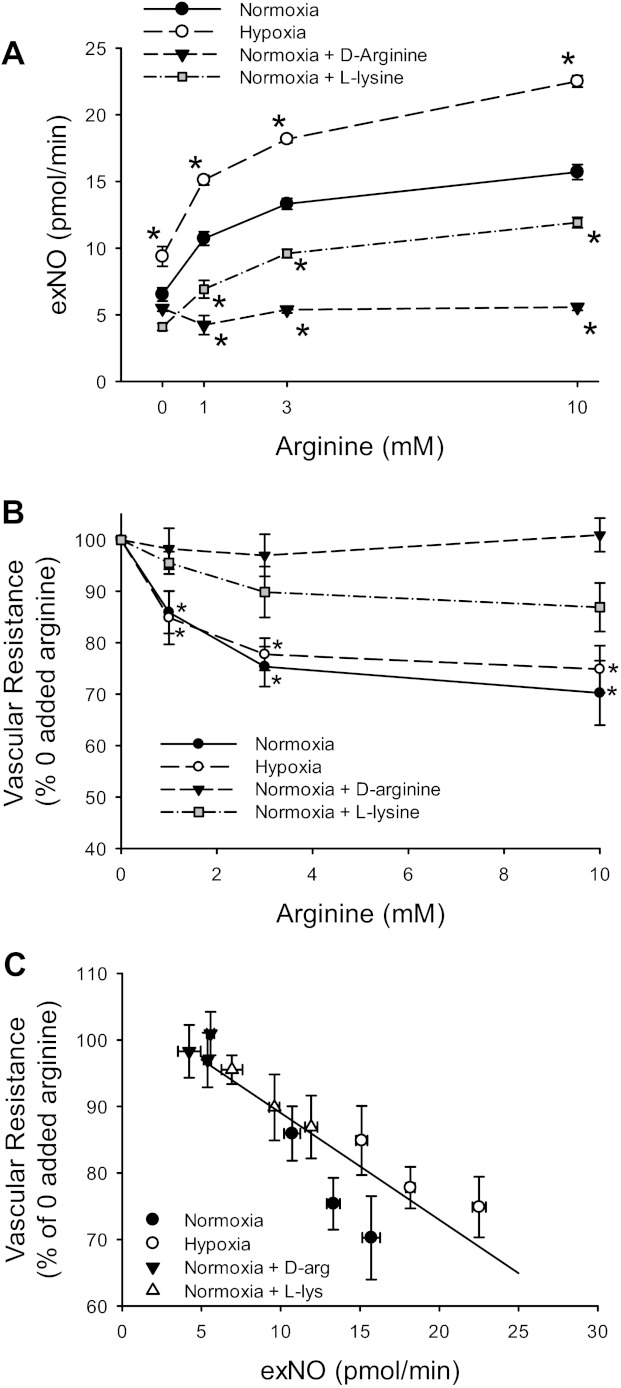
The effect of exogenous l-Arg on exNO production from isolated perfused lungs from N and CH rats was determined at baseline and after the addition of l-Arg to the perfusate to achieve final perfusate concentrations of 1, 3, and 10 mM. The production of exNO increased in an l-Arg concentration-dependent manner in lungs from both N and CH rats, and lungs from CH animals produced more exNO than did lungs from N animals (Fig. 5A). In one set of experiments, d-Arg, which is not actively taken up by the lung, was substituted for l-Arg. The addition of d-Arg to the perfusate had no effect on exNO production at any of the concentrations studied (Fig. 5A). To demonstrate that the l-Arg effect on exNO production was due to active l-Arg uptake, l-Lys, a competitive inhibitor of l-Arg transporters, was added to the perfusate in a set of N lungs. Since 10 mM l-Arg was the largest concentration studied, we chose a concentration of 30 mM l-Lys. exNO production in the presence of l-Lys was significantly lower at all l-Arg concentrations studied (Fig. 5A). In three lungs, the effect of 30 mM d-Lys, which is not transported by cationic amino acid transporters (CATs), on exNO production in the presence of increasing l-Arg concentrations was studied. d-Lys had no effect on the l-Arg-induced increase in exNO production in the isolated perfused rat lung (data not shown).
Fig. 5.
l-Arg caused a concentration-dependent increase in exNO production that was dependent on l-Arg transport. A: exNO measured in isolated perfused lungs from N (n = 19) and CH (n = 12) rats with increasing concentrations of l-Arg. d-Arg was used in N lungs since it is not actively transported, and 30 mM l-lysine (l-Lys), a competitive inhibitor of l-Arg transport, was added in N lungs. *Different from N at the same concentration of l-Arg (P < 0.005). Two-way ANOVA using N and CH data showed a significant effect of both l-Arg concentration and exposure on exNO production (P < 0.001 for both). B: l-Arg caused a concentration-dependent decrease in vascular resistance. Vascular resistance is shown as a percentage of vascular resistance with no added l-Arg in isolated perfused lungs from A. *Different from no added l-Arg (P < 0.01). C: vascular resistance was negatively correlated with exNO production. The change in vascular resistance was plotted against exNO production (R = −0.89, P < 0.001).
The exNO production served as a surrogate marker for vasoactive NO production in these isolated lungs, since RL fell with increasing doses of l-Arg in lungs from both N and CH rats (Fig. 5B). The addition of d-Arg had no effect on RL in isolated perfused lungs (Fig. 5B). The l-Arg-induced decrease in RL was attenuated in N lungs when 30 mM l-Lys was added to the perfusate (Fig. 5B). When the change in RL was plotted against the exNO production from all conditions studied (Fig. 5C), there was a significant negative correlation (R = −0.89, P < 0.001).
Effect of acute hypoxia on exNO production in isolated rat lungs.
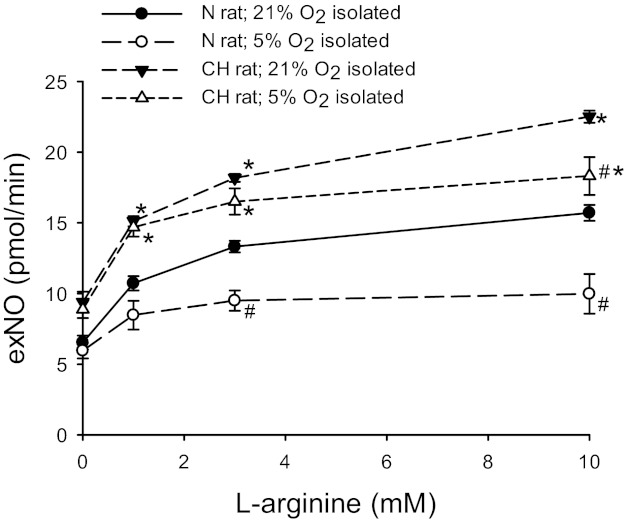
To determine the effect of acute hypoxia on exNO production in rat lungs, isolated lungs from N or CH rats were ventilated with a NO-free gas mixture of 5% CO2-21% O2-balance N2 for 30 min, and the ventilating gas mixture was then changed to 5% CO2-5% O2-balance N2. After a 30-min equilibration period, l-Arg was added to the perfusate to achieve final concentrations of 1, 3, and 10 mM. The data shown in Fig. 5 for N and CH lungs ventilated with 21% O2 was used for comparison. Isolated lungs ventilated with 5% O2 had significantly lower exNO production than lungs ventilated with 21% O2, although lungs from CH rats ventilated with 5% O2 had significantly greater exNO production than lungs isolated from N rats ventilated with 5% O2 (Fig. 6).
Fig. 6.
Exposure to acute hypoxic resulted in lower l-Arg-induced exNO production in isolated lungs from both N and CH rats. Lungs from either N (n = 6) or CH (n = 6) rats were isolated and perfused with the ventilating gas mixture of 5% O2-5% CO2-balance N2. The solid symbols show data from Fig. 5, i.e., isolated lungs from N or CH rats ventilated with 21% O2-5% CO2-balance N2. The open symbols are exNO production from isolated perfused lungs from N or CH rats ventilated with 5% O2-5% CO2-balance N2. *CH different from N lungs in the same ventilation group (P < 0.005); #5% O2 different from 21% O2 in the same exposure group (P < 0.05).
Effect of l-Cit on exNO production in isolated lungs.
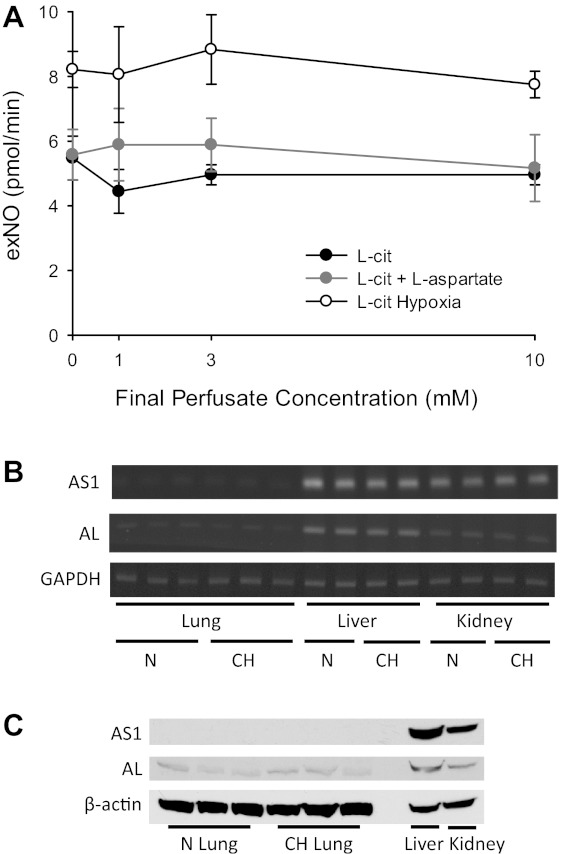
l-Cit and l-aspartate are metabolized to l-argininosuccinate via AS, and l-argininosuccinate is metabolized to l-Arg with fumarate as a coproduct by AL. It has been suggested that in endothelial cells, AS and AL are involved in a complex with eNOS, such that l-Arg derived from l-Cit is preferentially metabolized by eNOS (9, 37). Therefore, we measured exNO in isolated lungs from N and CH rats at final perfusate concentrations of 0, 1, 3, and 10 mM l-Cit. There were no detectable changes in exNO levels with increasing concentrations of l-Cit (Fig. 7A). We then added both l-Cit and l-aspartate at equal final perfusate concentrations of 0, 1, 3, and 10 mM while measuring exNO production in lungs from N rats. The simultaneous addition of l-Cit and l-aspartate had no discernable effect on exNO production (Fig. 7A). Given these somewhat surprising findings, we measured mRNA levels of AS and AL in lung, liver, and kidney homogenates from N and CH rats using RT-PCR. We found very low levels of AS1 and AL mRNA in the lungs, whereas there were easily detectable mRNA levels of AS1 and AL in the liver and kidney (Fig. 7B). We also measured protein levels of AS1 and AL in lung, liver, and kidney homogenates from N and CH rats and found no discernable AS1 and faint AL bands in the lungs, whereas the liver and kidney had strong bands for AS1 and AL (Fig. 7C).
Fig. 7.
The production of exNO was unaffected by the addition of l-citrulline (l-Cit) to the perfusate in isolated rat lungs. A: exNO production in isolated perfused lungs from N rats (n = 6) with increasing final perfusate concentrations of l-Cit. In a second group (n = 5), both l-Cit and l-aspartate were added at equimolar final perfusate concentrations. In a third group (n = 6), l-Cit was added with increasing final perfusate concentrations to isolated perfused lungs from CH rats. B: RT-PCR results of argininosuccinate synthase (AS)1 and argininosuccinate lyase (AL) mRNA levels in the lungs, liver, and kidneys of N and CH rats. GAPDH from the same tissues served as a loading control. C: AS1 and AL protein levels in the lungs, liver, and kidneys of N and CH rats. β-Actin from the same tissues served as a loading control.
Effect of l-Arg or l-Cit on exNO production in vivo.
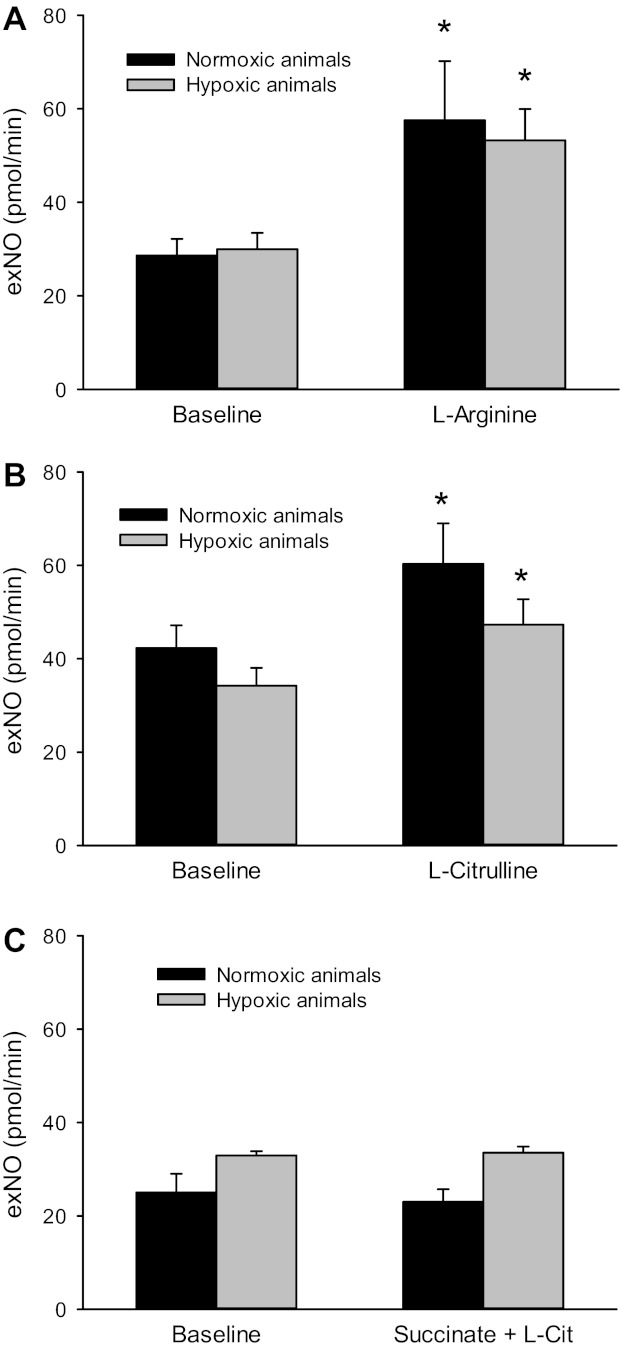
Since l-Cit had little effect on exNO production in isolated lungs from N rats, and given that the liver and kidney had high levels of AS and AL, we determined the effect of l-Arg and l-Cit on exNO in vivo in N or CH rats. After 14 days of exposure, rats were anesthetized with pentobarbital and instrumented as described in materials and methods. After a 25-min equilibration period, the exhaled gas was collected for the determination of exNO. Animals were then given either l-Arg (125 mg/kg iv) or l-Cit (125 mg/kg iv), and, 25 min later, the exhaled gas was collected for exNO measurement. We based our doses of l-Arg and l-Cit on the circulating blood volume of a rat (16) to try and achieve an initial plasma concentration of ∼10 mM. In intact anesthetized animals, there were no differences in basal exNO production between N and CH animals (Fig. 8). Treatment of the intact anesthetized rats with l-Arg resulted in an increase in exNO production in both N and CH animals (Fig. 8A). In intact anesthetized animals, treatment with l-Cit resulted in an increase in exNO production in both N and CH animals (Fig. 8B). The effect of l-Cit was due to its conversion to l-Arg, as demonstrated by the lack of effect of l-Cit on exNO in the intact anesthetized rat when it was given succinate, a putative inhibitor of AS, before l-Cit (Fig. 8C).
Fig. 8.
Both l-Arg and l-Cit increased exNO production in anesthetized rats. A: exNO production in anesthetized rats after 14 days of N (n = 6) or CH (n = 4) exposure. After a 30-min equilibration period, animals were given l-Arg (125 mg/kg) via a jugular venous catheter. *Different from BL in the same exposure group (P < 0.05). B: exNO production in anesthetized N (n = 6) and CH (n = 6) rats given l-Cit (125 mg/kg). *Different from BL in the same exposure group (P < 0.05). C: exNO production in anesthetized N (n = 6) and CH (n = 6) rats given l-Cit and succinate (125 mg/kg), a putative inhibitor of AS. *Different from BL in the same exposure group (P < 0.05).
DISCUSSION
The major new findings in this study were that 1) CH decreased Ca and Cv while increasing Cc, 2) CH resulted in greater QL at low Pv, 3) CH decreased Cst, 4) l-Arg reversed CH-induced alterations in vascular compliance, 5) l-Arg also decreased CH-induced vascular resistance by decreasing Ra, and 6) the l-Arg-induced reversal of hemodynamic parameters in CH lungs was due to augmented NO production and depended on active transport of l-Arg from the vascular space. These results support our hypothesis that exogenous l-Arg results in increased NO production and reverses CH-induced alterations in hemodynamics in the lung.
There were no differences between CH and N lungs in CL by occlusions or in α from the P-Q data. However, the vascular occlusion data revealed a significant redistribution of pulmonary vascular compliance after CH; Ca and Cv decreased, whereas Cc increased. The CH-induced redistribution of vascular compliance from arteries and veins to the microvasculature resulted in an increase in lung blood volume at resting Pv. However, the lung vessels were stiffer, such that the increase in QL for a given increase in Pv was lower after CH. A larger blood volume at low Pv that is mainly accommodated in the microvasculature may represent a physiological compensatory mechanism to maintain gas exchange under hypoxic conditions. The increase in Cc would be consistent with angiogenesis during exposure to CH, as has been previously described (10, 12). The redistribution of vascular compliance could also be due to the larger arteries and veins becoming stiffer after CH, which would be a consequence of the vascular remodeling that is a hallmark of CH (5, 15, 35, 42). We (2) have previously reported that in neonatal pigs, chronic pulmonary overcirculation induced by placement of an aorta-to-lobar pulmonary artery shunt resulted in significant pulmonary arterial remodeling that was associated with a significant reduction in Ca. However, it is of interest to note that in hypoxic lungs, the acute addition of l-Arg redistributed vascular compliance to resemble the vascular compliance distribution found in N lungs, suggesting that rather than vascular remodeling the decrease in Ca was due to vasoconstriction, since it is unlikely that changes in vessel structure would occur in the time course of these isolated lung experiments. This concept is consistent with a recent study by Vanderpool et al. (41), wherein acute Rho kinase inhibition after CH exposure in mice essentially normalized pulmonary vascular resistance while the proximal arterial thickening and stiffening persisted.
Traditionally, pulmonary Pa and pulmonary vascular resistance are assessed as the markers of pulmonary hypertension. However, since blood flow is pulsatile, Ca will also affect pulmonary hemodynamics and RV afterload. The total energy that the RV must use to propel the stroke volume is inversely proportional to the vascular compliance (18). This may have clinical ramifications given that Mahapatra et al. (19) found that Ca (estimated by the investigators as the stroke volume divided by the pulse pressure during right heart catheterization) was inversely correlated with survival in patients with pulmonary arterial hypertension.
We found that eNOS protein expression and exNO production was higher in lungs from CH rats than in those from N rats. This finding is consistent with previous reports using the CH rat model (5, 15, 33), and LeCras et al. (15) found that it was CH and not alterations in lung blood flow that led to the increase in eNOS expression in the rat. We also found that acute hypoxia resulted in decreased exNO production in isolated lungs from both CH and N rats. This finding is consistent with a study by Sato et al. (35) in isolated lungs from hypoxic rats ventilated with either a normoxic or hypoxic gas mixture. This decrease in exNO production could be secondary to the decreased production of NO by eNOS, or it could be secondary to decreased bioavailability of NO within the lung. For example, Jernigan et al. (13) found that the production of ROS was greater in CH lungs than in N lungs and that treatment with ROS scavengers improved endothelium-derived NO-dependent vasodilation. Taken together, we speculate that the elevated eNOS expression in lungs from hypoxic rats represents a compensatory response to the decreased NO production and/or bioavailability caused by hypoxia.
l-Arg resulted in a concentration-dependent increase in exNO production in isolated lungs from both N and CH rats. The l-Arg-induced increase in exNO production depended on l-Arg transport into the lung, since d-Arg did not increase exNO production and l-Lys, but not d-Lys, attenuated the l-Arg-induced increase in exNO production. In the lung, the majority of l-Arg uptake is due to the activity of CATs, specifically CAT-1 and CAT-2, which are encoded by the genes slc7a1 and slc7a2 (20). The l-Arg-induced increase in exNO was associated in these isolated lungs with a decrease in total pulmonary vascular resistance, demonstrating that exNO is a measure of vasoactive NO production in the isolated perfused lung. It has been found that the administration of exogenous l-Arg to rats during CH exposure can attenuate the resultant pulmonary hypertension (7, 39). Our results demonstrate that l-Arg can also lower pulmonary vascular resistance in established CH-induced pulmonary hypertension. In human studies (22, 27, 31), it has been reported that exogenous l-Arg causes systematic vasodilation in healthy subjects, and in one study (26), the short-term administration of l-Arg improved pulmonary artery pressure and pulmonary vascular resistance in patients with pulmonary hypertension. Thus, augmenting l-Arg uptake may represent a potential therapy for pulmonary hypertension that may increase the production of NO and thereby reduce pulmonary vascular resistance. It is of interest to note that a study by Howell et al. (11) found that the administration of l-Arg during CH exposure in rats ameliorated pulmonary hypertension and promoted angiogenesis, suggesting another possible mechanism whereby l-Arg may benefit patients with pulmonary hypertension.
In pulmonary vascular endothelial cells, it has been suggested that the enzymes required for recycling of l-Cit to l-Arg, AS and AL, are essential for eNOS-dependent NO production (9, 37). These findings have led to the notion that exogenous l-Cit may be a more efficient way of increasing NO production by eNOS (9). There have been two small studies examining l-Cit in patients with pulmonary hypertension (1, 36). Given the effect of l-Arg on hemodynamics and exNO production in the isolated rat lung, we repeated our experiments using l-Cit. To our surprise, there was little effect of exogenous l-Cit on exNO production in the isolated perfused rat lung. However, we found low levels of expression of AS1 and very low levels of expression of AL in the rat lung, with relatively robust expression in the kidney and liver, a finding that is consistent with a report in Wistar rats (25). Given these findings regarding the organ-specific distribution of AS and AL in the rat, we sought to determine if supplementing the whole animal would result in an increase in exNO production. When we administered either l-Arg or l-Cit to anesthetized rats, we found a significant increase in exNO production in both N and CH animals. Taken together, these data suggest that l-Cit can be used in rats to increase lung NO production via its conversion to l-Arg in organs other than the lung.
In summary, this study shows that CH resulted in higher pulmonary Ra and lower pulmonary Ca. The effects of hypoxia on pulmonary Ca may have important implications for right heart function in pulmonary hypertension. We found that l-Arg administration to isolated lungs from CH-exposed rats resulted in a redistribution of vascular compliance such that it was not different from the vascular compliance distribution found in N lungs, suggesting that the alterations in vascular compliance seen with CH are due to vasoconstriction. l-Arg administration caused a concentration-dependent increase in exNO production in the lung that depended on active l-Arg transport, and this l-Arg transport-dependent increase in NO production was associated with reductions in pulmonary vascular resistance. Taken together, our findings suggest that interventions to increase l-Arg uptake by the lung will increase NO production, resulting in improved Ca, and the improved vascular compliance may have beneficial effects on RV function.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-075261.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute.
AUTHOR CONTRIBUTIONS
Author contributions: Y.J., B.C., L.G.C., Y.L., and L.D.N. conception and design of research; Y.J., T.J.C., and L.D.N. performed experiments; Y.J., B.C., T.J.C., L.G.C., Y.L., and L.D.N. analyzed data; B.C., L.G.C., Y.L., and L.D.N. interpreted results of experiments; T.J.C. and L.D.N. prepared figures; L.D.N. drafted manuscript; Y.J., B.C., T.J.C., L.G.C., Y.L., and L.D.N. edited and revised manuscript; Y.J., B.C., T.J.C., L.G.C., Y.L., and L.D.N. approved final version of manuscript.
REFERENCES
- 1. Barr FE, Tirona RG, Taylor MB, Rice G, Arnold J, Cunningham G, Smith HA, Campbell A, Canter JA, Christian KG, Drinkwater DC, Scholl F, Kavanaugh-McHugh A, Summar ML. Pharmacokinetics and safety of intravenously administered citrulline in children undergoing congenital heart surgery: potential therapy for postoperative pulmonary hypertension. J Thorac Cardiovasc Surg 134: 319–326, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bousamra M, Rossi R, Jacobs E, Parviz M, Busch C, Nelin LD, Haworth S, Dawson CA. Systemic lobar shunting induces advanced pulmonary vasculopathy. J Thorac Cardiovasc Surg 120: 88–98, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Carter BW, Jr, Chicoine LG, Nelin LD. l-Lysine decreases nitric oxide production and increases vascular resistance in lungs isolated from lipopolysaccharide-treated neonatal pigs. Pediatr Res 55: 979–987, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Chang R, Chicoine LG, Cui H, Kanagy NL, Walker BR, Liu Y, English BK, Nelin LD. Cytokine-induced arginase activity in pulmonary endothelial cells depends on Src family tyrosine kinase activity. Am J Physiol Lung Cell Mol Physiol 295: L688–L697, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chicoine LG, Paffett ML, Metroupolus M, Resta TC, Nelin LD, Walker BR. Maturational changes in the regulation of pulmonary vascular tone by nitric oxide in neonatal rats. Am J Physiol Lung Cell Mol Physiol 293: L1261–L1270, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Emery CJ, Bee D, Barer GR. Mechanical properties and reactivity of vessels in isolated perfused lungs of chronically hypoxic rats. Clin Sci (Lond) 61: 569–580, 1981 [DOI] [PubMed] [Google Scholar]
- 7. Fagan JM, Rex SE, Hayes-Licitra SA, Waxman L. l-Arginine reduces right heart hypertrophy in hypoxia-induced pulmonary hypertension. Biochem Biophys Res Commun 254: 100–103, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Fagan KA, Fouty BW, Tyler RC, Morris KG, Hepler LK, Sato K, LeCras TD, Abman SH, Weinberger HD, Huang PL, McMurtry IF, Rodman DM. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest 103: 291–299, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flam BR, Eichler DC, Solomonson LR. Endothelial nitric oxide production is tightly coupled to the citrulline-NO cycle. Nitric Oxide 17: 115–121, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Howell K, Preston RJ, McLoughlin P. Chronic hypoxia causes angiogenesis in addition to remodeling in the adult rat pulmonary circulation. J Physiol 547: 133–145, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howell K, Costello CM, Sands M, Dooley I, McLoughlin P. l-Arginine promotes angiogenesis in the chronically hypoxic lung: a novel mechanism ameliorating pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 296: L1042–L1050, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 97: 185–191, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Jernigan NL, Walker BR, Resta TC. Endothelium-derived reactive oxygen species and endothelin-1 attenuate NO-dependent pulmonary vasodilation following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L801–L808, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Jin Y, Calvert TJ, Chicoine LG, Chen B, Joshi M, Bauer JA, Liu Y, Nelin LD. Mice deficient in Mkp-1 develop more severe pulmonary hypertension and greater lung protein levels of arginase in response to chronic hypoxia. Am J Physiol Heart Circ Physiol 298: H1518–H1528, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. LeCras TD, Tyler RC, Horan MP, Morris KG, Tuder RM, McMurtry IF, Johns RA, Abman SH. Effects of chronic hypoxia and altered hemodynamics on endothelial nitric oxide synthase expression in the adult rat lung. J Clin Invest 101: 795–801, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med 25: 72–76, 1985 [PubMed] [Google Scholar]
- 17. Linehan JH, Haworth ST, Nelin LD, Krenz GS, Dawson CA. A simple distensible vessel model for interpreting pulmonary vascular pressure-flow curves. J Appl Physiol 73: 987–994, 1992 [DOI] [PubMed] [Google Scholar]
- 18. Linehan JH, Dawson CA, Rickaby DA, Bronikowski TA. Pulmonary vascular compliance and viscoelasticity. J Appl Physiol 61: 1802–1824, 1986 [DOI] [PubMed] [Google Scholar]
- 19. Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 47: 799–803, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev 83: 183–252, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Matsumoto A, Momomura S, Hirata Y, Aoyagi T, Sugiura S, Omata M. Inhaled nitric oxide and exercise capacity in congestive heart failure. Lancet 349: 999–1000, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Mehta S, Stewart DJ, Langleben D, Levy RD. Short-term pulmonary vasodilation with l-arginine in pulmonary hypertension. Circulation 92: 1539–1545, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Mitani Y, Maruyama K, Sakurai M. Prolonged administration of l-arginine ameliorates chronic pulmonary hypertension and pulmonary vascular remodeling in rats. Circulation 96: 689–697, 1997 [PubMed] [Google Scholar]
- 24. Moinard C, Cynober L. Citrulline: a new player in the control of nitrogen homeostasis. J Nutr 137: 1621S–1625S, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Nagasaki A, Gotoh T, Takeya M, Yu Y, Takiguchi M, Matsuzaki H, Takatsuki K, Mori M. Co-induction of nitric oxide synthase, argininosuccinate synthetase, and argininosuccinate lyase in lipopolysaccharide-treated rats. J Biol Chem BiolChem 271: 2658–2662, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Nagaya N, Uematsu M, Oya H, Sato N, Sakamaki F, Kyotani S, Ueno K, Nakanishi N, Yamagishi M, Miyatake K. Short-term oral administration of l-arginine improves hemodynamics and exercise capacity in patients with precapillary pulmonary hypertension. Am J Respir Crit Care Med 163: 887–891, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Nakaki T, Hishikawa K, Suzuki H, Saruta T, Kato R. l-Arginine-induced hypotension. Lancet 336: 696–699, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Nelin LD, Rickaby DA, Linehan JH, Dawson CA. The vascular site of action of hypoxia in the neonatal pig lung. Pediatr Res 35: 25–29, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Nelin LD, Krenz GS, Rickaby DA, Linehan JH, Dawson CA. A distensible vessel model applied to hypoxic pulmonary vasoconstriction in the neonatal pig. J Appl Physiol 74: 2049–2056, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Nelin LD, Thomas CJ, Dawson CA. Effect of hypoxia on nitric oxide production in neonatal pig lung. Am J Physiol Heart Circ Physiol 271: H8–H14, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Panza JA, Casino PR, Badar DM, Quyyumi AA. Effect of increased availability of endothelium-derived nitric oxide precursor on endothelium-dependent vascular relaxation in normal subjects and in patients with essential hypertension. Circulation 87: 1475–1481, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol 288: L419–L425, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Resta TC, Chicoine LG, Omdahl JL, Walker BR. Maintained upregulation of pulmonary eNOS gene and protein expression during recovery from chronic hypoxia. Am J Physiol Heart Circ Physiol 276: H699–H708, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Roger N, Barbara JA, Roca J, Rovira I, Gomez FP, Rodriguez-Roizin R. Nitric oxide inhalation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 156: 800–806, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Sato K, Rodman DM, McMurtry IF. Hypoxia inhibits the increased ETB receptor-mediated NO synthesis in hypertensive rat lungs. Am J Physiol Lung Cell Mol Physiol 276: L571–L581, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Smith HA, Canter JA, Christian KG, Drinkwater DC, Scholl FG, Christman BW, Rice GD, Barr FE, Summar ML. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. J Thorac Cardiovasc Surg 132: 58–65, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Solomonson LP, Flam BR, Pendleton LC, Goodwin BL, Eichler DC. The caveolar nitric oxide synthase/arginine regeneration system for NO production in endothelial cells. J Exp Biol 206: 2083–2087, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Steudel W, Scherrer-Crosbie M, Bloch KD, Weimann J, Huang PL, Jones RC, Picard MH, Zapol WM. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J Clin Invest 101: 2468–2477, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sumou IK, Du JB, Wei B, Zhang CY, Qi JG, Tang CS. Effect of l-arginine on pulmonary artery smooth muscle cell apoptosis in rats with hypoxic pulmonary vascular structural remodeling. Acta Biochem Biophys Sinica 38: 15–21, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Toby IT, Chicoine LG, Cui H, Chen B, Nelin LD. Hypoxia-induced proliferation of human pulmonary microvascular endothelial cells depends on epidermal growth factor receptor tyrosine kinase activation. Am J Physiol Lung Cell Mol Physiol 298: L600–L606, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vanderpool RR, Kim AR, Molthen R, Chesler NC. Effects of acute Rho kinase inhibition on chronic hypoxia-induced changes in proximal and distal pulmonary arterial structure and function. J Appl Physiol 110: 188–198, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu W, Kanebo FT, Zheng S, Comhair SAA, Janocha AJ, Goggans T, Thunnissen EB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004 [DOI] [PubMed] [Google Scholar]