Abstract
Human cytomegalovirus (HCMV) is associated with vascular diseases in both immunosuppressed and immunocompetent individuals. CMV infections cycle between active and latent phases throughout life. We and others have shown vascular dysfunction during active mouse CMV (mCMV) infections. Few studies have examined changes in physiology during latent CMV infections, particularly vascular responses or whether the negative effects of aging on vascular function and fertility will be exacerbated under these conditions. We measured vascular responses in intact mesenteric and uterine arteries dissected from young, mid-aged, and aged latently mCMV-infected (mCMV genomes are present but infectious virus is undetectable) and age-matched uninfected mice using a pressure myograph. We tested responses to the α1-adrenergic agonist phenylephrine, the nitric oxide donor sodium nitroprusside, and the endothelium-dependent vasodilator methacholine. In young latently mCMV-infected mice, vasoconstriction was increased and vasodilation was decreased in mesenteric arteries, whereas both vasoconstriction and vasodilation were increased in uterine arteries compared with those in age-matched uninfected mice. In reproductively active mid-aged latently infected mice, mesenteric arteries showed little change, whereas uterine arteries showed greatly increased vasoconstriction. These vascular effects may have contributed to the decreased reproductive success observed in mid-aged latently mCMV-infected compared with age-matched uninfected mice (16.7 vs. 46.7%, respectively). In aged latently infected mice, vasodilation is increased in mesenteric and uterine arteries likely to compensate for increased vasoconstriction to mediators other than phenylephrine. The novel results of this study show that even when active mCMV infections become undetectable, vascular dysfunction continues and differs with age and artery origin.
Keywords: uterine arteries, mesenteric arteries, infertility, vascular function, pressure myograph
human cytomegalovirus (HCMV) is a member of the β-Herpesviridae family (50). In the human population, it infects 40 to 80% of individuals (10). Although in the general population it is thought to be mostly asymptomatic, infection has been associated with tumorigenesis, transplant rejection, vascular diseases such as atherosclerosis and restenosis, and increased mortality and hypertension (9, 19, 23, 26, 33, 62, 64). The mechanisms whereby HCMV infections result in vasculopathy are still unclear.
The prevalence of systemic cardiovascular diseases increases dramatically after age 50 (5, 8). This is accompanied by increased vascular tone, endothelial dysfunction, and hypertension (35). Moreover, vasodilation is decreased and vasoconstriction is increased. Production, bioavailability, and smooth muscle sensitivity to nitric oxide (NO), a potent vasodilator, typically decrease, whereas prostaglandin H synthase (PGHS)-1/2 expression and production of vasoconstrictor prostanoids are increased with age (32, 55, 56). Infertility also greatly increases as women age; between 30 to 45 years of age, the fertility rate drops by >30% (21). Potential causes of reduced fertility before menopause include chromosomal abnormalities, hormonal imbalances, uterine and ovarian malformations, and hypertensive complications, all of which increase with age (21, 51). Hypertensive complications, such as increased vascular resistance and decreased endothelium-dependent vasodilation, may be increased in HCMV-infected individuals (16, 19).
Like other herpesviruses, CMV is not cleared from the host following primary infection. CMV maintains a lifelong infection through cycles of latency and reactivation (46). Latency is defined as the presence of viral DNA in the absence of detection of infectious, replicating virus (46), although it is likely that low levels of virus persist during chronic infections (15). Reactivation of a latent CMV infection, and thus production of infectious, replicating virus, occurs in different tissues in response to stimuli such as stress, inflammation, drug treatments, and pregnancy (7, 47, 58). The number of reactivation cycles an individual undergoes in a lifetime is difficult to estimate because of the varied stimuli that likely contribute to viral reactivation. Viral replication and the subsequent immune responses are likely necessary for the establishment or acceleration of vasculopathy (60) and atherosclerosis (16). However, diseases such as inflammatory bowel disease may be exacerbated during latent CMV infections (40). Khoretonenko et al. (28) recently showed that endothelial-dependent vasodilation was reduced in small cremaster muscle arterioles using intravital microscopy in mice at 9 and 12 wk of persistent mouse CMV (mCMV) infection. Previously, we have shown that active mCMV infections impair vascular responses in both mesenteric and uterine arteries isolated from young nonpregnant and late pregnant mice and mounted on a pressure myograph system. These effects likely occur by both direct viral infection of cells within the vascular wall and indirect, systemic effects of the immune system (13, 14). What is not yet known is whether vascular changes in these vascular beds are maintained once the active infection becomes latent, particularly during aging.
Our objectives were to investigate the effect of latent CMV infections on vascular function and fertility with age. Vascular resistance and systemic blood pressure are impacted by the mesenteric vasculature. In our previous studies we showed an overall increase in vasodilation in mesenteric arteries isolated from mice actively infected with mCMV (14), which may be to compensate for increased vasoconstrictory activity (6, 12, 13). It is unknown whether these effects in mesenteric arteries are maintained during mCMV latency and whether the additional stress of aging would reduce the mCMV-mediated increase in vasodilation allowing vasoconstrictory effects to increase vascular tone. We hypothesized that the oldest animals infected with CMV at a young age will experience the greatest vascular dysfunction compared with age-matched uninfected controls and all other animal groups. Uterine artery vascular responses are important to reproductive function and we had previously shown decreased vasodilation and increased vasoconstriction in uterine arteries from mCMV-infected pregnant mice (14). Pregnancy and aging are both stress factors for development of vascular dysfunction particularly in the presence of a second stressor such as an infection. Since we saw that vascular responses in the uterine arteries were impaired in actively infected pregnant mice, we decided to examine uterine artery function and reproductive success during the dual stress of aging and a latent mCMV infection. We hypothesized that uterine arteries from mid-aged but not young latently infected mice will show decreased vasodilation compared with age-matched uninfected controls and that this will be associated with increased infertility. Moreover, we hypothesized that independent of infection, mesenteric and uterine arteries from aged uninfected mice will have a greater reduction in NO-mediated vasodilation compared with arteries from young or mid-aged uninfected mice and therefore show a greater impairment of methacholine (ME)-induced vasodilation. We therefore measured vasoconstriction and vasodilation responses in mesenteric and uterine arteries isolated from young, mid-aged, and aged mCMV-infected female mice, where infectious virus was undetectable but mCMV DNA was present, compared with age-matched uninfected mice.
METHODS
Animals.
C57BL/6J female mice purchased from Jackson Laboratories were housed in Health Sciences Laboratory Services at the University of Alberta. They were infected with mCMV containing a LacZ insertion in the nonessential immediate early 2 gene (RM427+; gift from E. Mocarski, Stanford University, Stanford, CA) (57). RM427+ was propagated in mouse fibroblasts as previously described (14). An active mCMV infection was detected by staining for β-galactosidase protein, expressed from the LacZ insertion. Young female mice were injected with 106 plaque forming units of RM427+ intraperitoneally and euthanized via cervical dislocation within 1 to 2 wk postinfection (acute mCMV infection) or 2 to 3 mo postinfection (latent mCMV infection). Young infected and uninfected mice ranged from 4–6 mo of age. Mid-aged latently mCMV-infected and uninfected mice (7–10 mo of age) were euthanized 5–8 mo postinfection, and aged latently mCMV-infected and uninfected mice (18–30 mo of age) were euthanized 16–28 mo postinfection. Some mid-aged latently mCMV-infected and uninfected female mice were bred to young uninfected males. Tissues including the heart, kidney, liver, lungs, spleen, uteri, and the mesentery were collected from each animal. A latent infection was defined as the presence of viral DNA in the lung, spleen, liver, or kidney as assessed by PCR, but the absence of detectable infectious mCMV as assessed by lack of detection of β-galactosidase protein established in each animal a minimum of 2 mo after receiving 106 plaque forming units of mCMV intraperitoneally. All animal studies were conducted in accordance with the Canadian Council on animal care guidelines and policies with approval from the Health Sciences Animal Care and Use Committee for the University of Alberta.
Immunofluorescence.
The heart, kidney, liver, lungs, and spleen were immediately embedded in optimum cutting temperature (Tissue-Tek) and snap frozen. Tissues in optimum cutting temperature were cryosectioned into 7-μm slices, mounted onto slides, dried overnight, and stored at −80°C. Slides were thawed for 1 to 2 h at RT, fixed in cold methanol for 10 min at −20°C, and washed with phosphate-buffered saline (PBS) three times for 10 min each. Tissue sections on the slides were circled with a PAP pen and blocked with 10% normal goat serum (NGS; Cedarlane) for 1 h. After blocking, chicken anti-β-galactosidase primary antibody (1 μg/ml; AbCam) diluted in 10% NGS was added to two of the three sections on the slide, with the remaining section receiving only 10% NGS as the negative control. The slides were incubated at 4°C overnight. Following three 5-min washes with PBS, Alexa Fluor-488 goat anti-chicken (4 μg/ml) secondary antibody was added to each section and incubated for 45 min at room temperature. After three 10-min washes in PBS, 4′,6-diamidino-2-phenylindole (DAPI, 0.915 mg/ml; Invitrogen) was added for 15 min at room temperature and washed again three times for 5 min each with PBS. Vectashield H:1000 (Vector, Burlington, CA) was applied, and the section was then sealed with a coverslip and stored in the dark at 4°C. Stained sections were viewed with an Olympus ×81 fluorescent microscope (Olympus, Ontario, Canada) using Slidebook 2, 3-D Timelapse Imaging Software to normalize the images (Intelligent Imaging Innovations).
DNA extraction.
DNA was basically extracted as described in Schang et al. (52). Finely chopped kidneys and spleens were incubated in 2 ml TNES buffer, consisting of 10 mM Tris (pH 8.0), 0.4 M NaCl, 100 mM EDTA, and 0.6% SDS and 30 μl of proteinase K (20 mg/ml, Fermentas) for ∼48 h at 55°C and then extracted with 1 volume of 1:1 phenol:chloroform. The aqueous layer was chloroform extracted thrice, the DNA was precipitated with 1 volume of 2-propanol overnight at −20°C and pelleted by centrifugation at 10,000 g for 25 min at 4°C (Beckman Coulter Avanti J-E centrifuge; JA-14 rotor). The pellet was resuspended in 5 ml of TE buffer, consisting of 10 mM Tris (pH 8.0) and 1 mM EDTA, as well as 1:1 phenol:chloroform extracted once and chloroform extracted thrice. The DNA pellet was resuspended in 5 ml double-distilled water (ddH2O), precipitated with 1 volume of 2-propanol overnight at −20°C, rinsed with 2 ml of −20°C 70% ethanol, air dried, and dissolved in 100 μl sterile ddH2O.
Finely chopped lungs and livers were digested in 2.5 ml of STES buffer, consisting of 0.2 M Tris, 0.5 M NaCl, 0.01 M EDTA, and 0.5% SDS and 50 μl of proteinase K (20 mg/ml, Fermentas) for ∼48 h at 55°C. For lungs, the volume was brought up to 3 ml with sterile ddH2O, the samples were extracted thrice with phenol:chloroform, and the aqueous layer was then extracted twice with 1 volume of chloroform. The DNA was precipitated with 1 volume of 2-propanol, pelleted by centrifugation, resuspended in 6 ml of ddH2O, and reprecipitated with 5 ml of 2-propanol at −20°C overnight. The final pellet was rinsed with 2 ml of −20°C 70% ethanol, air dried, and dissolved in 100 μl of ddH2O.
Liver samples were extracted once with phenol, thrice with phenol:chloroform, and once with chloroform. The DNA was precipitated with 2 ml of 2-propanol. The resuspended DNA was extracted twice with 1 volume of chloroform, and reprecipitated with 5 ml of 2-propanol at 4°C for 2 h. The final pellet was air dried and dissolved in 200 μl of ddH2O.
DNA concentrations were determined by optical density at 260 to 280 nm.
Polymerase chain reaction.
The ie1/ie3 promoter region of mCMV was amplified using the primers described by Tang and Maul (59) (forward, 5′-GTA CAA AAG GTC AAT AGG GG-3′; and reverse, 5′-GTA CCG ACG CTG GTC GCG CC-3′). Spleen (300 ng) or lung (1,000 ng) DNA, where concentration permitted, were amplified with 1.25 U Platinum Pfx DNA polymerase (Invitrogen) in 18 mM (NH4)2SO4 and 60 mM Tris-SO4 (pH 8.9) (Pfx amplification buffer), 0.5 mM MgSO4, 0.3 μM of each primer, and 0.3 mM each dNTP. The initial denaturation step was at 96°C for 4.5 min. DNA was amplified for 40 cycles of denaturation at 96°C for 1 min, annealing at 62°C for 1 min and extension at 68°C for 1 min. A final extension step of 5 min at 68°C was performed after the 40 cycles. DNA from mice that did not test positive for mCMV in any tissue in the original amplification was reamplified with Taq polymerase, using one twentieth of the product of the first amplification as the template.
Kidney (200 ng) or liver (1,000 ng) DNA, where concentration permitted, was amplified with 2.5 U Taq polymerase (Invitrogen) in 20 mM Tris·HCl (pH 8.4) and 50 mM KCl (Taq amplification buffer), 1.5 mM MgCl2, 0.3 μM of each primer, and 0.3 mM each dNTP. The initial denaturation was at 94°C for 3 min. DNA was amplified for 40 cycles of denaturation at 94°C for 45 s, annealing at 63.4°C for 45 s, and extension at 72°C for 90 s. A final extension step of 10 min at 72°C was performed after the 40 cycles.
Southern blot transfer.
The PCR products were resolved by electrophoresis in 2% agarose gels in TAE buffer, consisting of 40 mM Tris-acetate and 50 mM EDTA (pH 8.5). Agarose gels were rinsed in ddH2O and incubated in 5 M HCl for 45 min and then in alkaline transfer buffer (0.4 M NaOH, 1 M NaCl) for 15 min, all with gentle agitation. Buffer was replaced with fresh alkaline transfer buffer for an additional 15-min wash. The gels were next incubated in neutralization buffer consisting of 1 M Tris (pH 7.4) and 2 M NaCl, twice for 15 min each. Meanwhile, a nylon membrane cut to fit the gel was wetted in distilled water and incubated in 10× SSC (1× SSC: 150 mM NaCl, 15 mM sodium citrate) for 15 min. The blotting apparatus was assembled and DNA was capillary transferred with 10× SSC for ∼48 h. Membranes were then incubated for 1 min each in 0.4 N NaOH and then 1× SSC/0.2 M Tris (pH 7.0).
Hybridization.
Membranes were prehybridized in 7 ml of rapid hybrid buffer (Amersham Biosciences, Pistcataway, NJ) for 1 h at 67°C. An mCMV DNA-specific oligo (5′-GGT CGC GCC TCT TAT ACC CAC G) was synthesized by Integrated DNA Technologies through the University of Alberta Institute for Biomolecular Design. The probe was end-labeled with T4 kinase (Invitrogen) as per the manufacturer's instructions. Briefly, 5 pmol of probe were incubated with 50 μCi [γ-32P]-ATP (PerkinElmer, Boston, MA) and 5 U T4 kinase in 250 mM imidazole-HCl pH 6.4, 60 mM MgCl, 5 mM mercaptoethanol, and 350 μM ADP (exchange reaction buffer) for 1 h at 37°C. The probe and blocker (3.25 μg Vero DNA) were incubated in a boiling water bath for 10 min, cooled on ice for 5 min, added to 5 ml of rapid hybrid buffer prewarmed to 67°C, and then added to the membranes for hybridization for 3 h at 67°C. Membranes were washed twice for 20 min each in 2× SSC/0.1% SDS at room temperature and then in 0.5× SSC/0.5% SDS for 15 min at 52°C. The membranes containing the PCR products from reamplications were further washed in 0.5× SSC/0.5% SDS for an additional 15 min at 60°C. Membranes were exposed to FischerBiotech Intensifying Screens.
Myograph studies.
The second-order mesenteric and main uterine arteries were dissected free of adipose and connective tissue in HEPES-buffered physiological saline solution (HEPES-PSS), consisting of (in mM) 10 HEPES, 1.56 CaCl, 142 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4, and 5.5 glucose at pH 7.5 and immediately used in myograph studies in a dual-chamber arteriograph (Living Systems Instrumentation). In each chamber, one end of an artery was mounted and tied onto a glass cannula (80–100 μm diameter), connected to a pressure transducer to modulate intraluminal pressure, as previously described (20, 22). Residual blood was removed from the artery with a low flow (10 μl/min) of HEPES-PSS, and the other end of the vessel was mounted and tied onto the second cannula. The vessel was then pressurized to 60 (mesenteric arteries) or 50 (uterine arteries) mmHg for 30 min (18, 41, 61). Arteries unable to maintain pressure were discarded and not included in the data sets. In this situation, new arteries from the same animal were mounted. Each 2.5-ml HEPES-PSS bath containing the vessel was kept at a constant temperature of 37°C.
Experimental design.
Following equilibration, a CCD video camera module (Sony) connected to a compound microscope was used to measure the initial lumen diameter with a video dimension analyzer as previously described (14, 20). Vasoconstriction in mesenteric and uterine arteries was assessed following increasing concentrations of the α1-adrenergic agonist phenylephrine (PE; 10 nM to 10 μM; Sigma). Vasodilation was assessed to increasing concentrations of ME (1 nM to 10 μM; Sigma) after 50% preconstriction to PE with or without pretreatment with the NO inhibitor NG-nitro-l-arginine methyl ester (100 μM; Calbiochem) and/or the PGHS-1/2 inhibitor meclofenamate (1 μM; Sigma). Endothelium-independent vasodilation was measured after adding the NO donor sodium nitroprusside (SNP; 0.1 nM to 100 μM; Sigma) to preconstricted arteries. The percent vasoconstriction was calculated as 1 − L2/L1 × 100, where L1 is the initial lumen diameter and L2 is the arterial lumen diameter following drug addition. The percent vasodilation was calculated as L2 − L1/L1 × 100, which was normalized to the artery diameter when fully relaxed. This was attained by measuring the passive lumen diameter following a thorough washout with Ca2+-free EGTA PSS, consisting of (in mM) 10 HEPES, 142 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4, and 2 EGTA, and a 10-min incubation with 100 μM of papaverine (Sigma). After incubation in Ca2+-free EGTA PSS, a distensibility curve was performed on arteries with step increases in pressure from 4 to 170 mmHg. The lumen diameter was unable to be read at 0 mmHg (collapsed vessel), and, therefore, pressure readings began at 4 mmHg as previously described (12). Distensibility was calculated as D2 − D1/D2 × 100, where D1 is the initial diameter and D2 is the lumen diameter post-pressure change.
Statistics.
Results were averaged by group (mCMV-infected, uninfected, mesentery, and uterine) and treatment and reported as means ± SE. Values for the PE, ME, and SNP dose-response curves were compared for arteries from mCMV-infected and uninfected mice with the repeated-measures two-way ANOVA to determine significance between curves. This was followed by Holm-Sidak's post hoc analysis to determine significance between points within the curves. The EC50 was calculated for each set of sigmoidal-shaped curves for the uterine arteries. The EC50 values for mesenteric arteries could not be calculated since these curves were not sigmoidal. EC50 values were compared using a Student's t-test. Significance was accepted at P < 0.05.
RESULTS
Detection of latent mCMV infection in young, mid-aged, and aged mice.
To evaluate the status of infection in active versus latently infected mice, we assessed β-galactosidase protein and mCMV DNA in the heart, kidney, liver, lung, and spleen. Detection of β-galactosidase produced from the LacZ gene insertion into the nonessential immediate early 2 gene in the mCMV RM427+ virus represents the presence of active replicating virus. In contrast, a latent infection was defined as undetectable β-galactosidase protein expression with positive detection of mCMV DNA in mCMV-infected mice. In the young, acute, mCMV-infected mice, β-galactosidase was detected in all five tissues (active infection) (Fig. 1A). In contrast, no tissues tested from the uninfected or mCMV-infected young mice 2-mo postinfection (latent infection) expressed β-galactosidase (Fig. 1A). Similarly, tissues from mid-aged (data not shown) or aged (Fig. 1B) uninfected or latently mCMV-infected mice did not express β-galactosidase. In contrast, all infected mice tested positive for mCMV DNA in at least one organ. Three of five acutely infected mice tested positive in all organs but one, one tested positive in all organs, and one in only one organ. Most latently infected mice tested positive in one (all young and aged mice and one mouse mid-aged) to two organs (most mid-aged), except for one mid-aged mouse that tested positive in three organs. As expected (2, 45), mCMV DNA was detected most frequently in kidneys during acute infection or in lungs and spleens during latency.
Fig. 1.
Immunofluorescent staining for β-galactosidase protein expression in tissues from young and aged latently mouse cytomegalovirus (mCMV)-infected and uninfected mice. Heart, kidney, liver, lung, and spleen tissues from young (A) and aged (B) mice that were uninfected or infected at 1 to 2 mo of age were collected 2 wk postinfection (acute infection; A) or >2 mo postinfection (latent infection: young, A; and aged, B). Tissues were stained for β-galactosidase protein expression (green) to test for active infection. Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI, blue).
Pregnancy success rate in mid-aged latently mCMV-infected and uninfected mice.
Mid-aged latently mCMV-infected and age-matched uninfected mice were bred. Seven of fifteen mid-aged uninfected mice (46.7%) but only three of eighteen mid-aged latently mCMV-infected mice (16.7%) carried their pregnancies to term after being visibly plugged. In comparison, young uninfected mice and young actively mCMV-infected mice completed 83.3% (10 of 12) and 90.0% (9 of 10) of their pregnancies, respectively, after visible plugging.
PE-induced vasoconstriction in arteries from young, mid-aged, and aged latently mCMV-infected and age-matched uninfected mice.
Vasoconstriction to the α1-adrenergic agonist PE in mesenteric arteries significantly increased (P < 0.05) in young latently mCMV-infected compared with uninfected mice (Fig. 2A). No differences were seen in mesenteric arteries from mid-aged and aged latently infected mice compared with age-matched uninfected mice (Fig. 2, B and C). Similar to mesenteric arteries, PE-induced vasoconstriction was significantly increased (P < 0.05) in uterine arteries from young latently mCMV-infected compared with young uninfected mice (Fig. 2D). Overall, PE-induced vasoconstriction was significantly more sensitive (P < 0.001) in uterine arteries from mid-aged latently mCMV-infected mice (EC50, 88.9 ± 5.8 nM) compared with those from mid-aged uninfected mice (EC50, 706 ± 79 nM) (Fig. 2E). PE-induced vasoconstriction did not differ between uterine arteries from aged latently mCMV-infected and uninfected mice (Fig. 2F).
Fig. 2.
Responses to phenylephrine (PE) by mesenteric and uterine arteries. PE-induced vasoconstriction was measured in mesenteric (A–C) and uterine (D–F) arteries from young (A and D), mid-aged (B and E), and aged (C and F) latently mCMV-infected and age-matched uninfected mice. Results for each curve were summarized and expressed as means ± SE percent decrease in lumen diameter compared at each PE concentration with the initial equilibrated diameter. Significant differences between the curves was calculated using a repeated-measures 2-way ANOVA (P < 0.05). This was followed by Holm-Sidak's post hoc analysis to determine significance between points within the curves (P < 0.05). *P < 0.05, significant differences; n = number of animals.
Endothelium-dependent vasodilation in arteries from young, mid-aged, and aged latently mCMV-infected and age-matched uninfected mice.
Vasodilation curves significantly differed overall (P < 0.01) in mesenteric arteries from young and aged mice; however, vasodilation at specific doses of the endothelium-dependent vasodilator ME in mesenteric arteries from latently mCMV-infected and uninfected mice significantly differed only in mid-aged and aged mice (Fig. 3, A–C). In mid-aged latently mCMV-infected mice, vasodilation was slightly but significantly decreased (P < 0.05) only at the maximum ME concentration. In contrast, ME-induced vasodilation was significantly increased (P < 0.05) at several ME concentrations, including the maximum in mesenteric arteries from aged latently mCMV-infected compared with age-matched uninfected mice (Fig. 3, B and C). In uterine arteries, vasodilation overall was significantly greater in uterine arteries from young, mid-aged, and aged mCMV-infected compared with uninfected mice when comparing entire curves (P < 0.05) (Fig. 3, D–F). However, only uterine arteries from young latently mCMV-infected (EC50, 74.0 ± 2.77 nM) compared with young uninfected mice (EC50, 111 ± 7.13 nM) showed a significantly increased sensitivity to ME (P < 0.001) (Fig. 3D). Uterine arteries from mid-aged and aged latently mCMV-infected and uninfected mice did not differ at specific doses of ME (Fig. 3, E and F).
Fig. 3.
Responses to methacholine (ME) by mesenteric and uterine arteries. ME-induced vasodilation was measured in mesenteric (A–C) and uterine (D–F) arteries from young (A and D), mid-aged (B and E), and aged (C and F) latently mCMV-infected and age-matched uninfected mice. Results for each curve were summarized and expressed as means ± SE percent increase in lumen diameter compared with the initial preconstricted diameter and normalized to the passive lumen diameter. Significance was assessed as for Fig. 2. *P < 0.05, significant differences; n = number of animals.
Contribution of NO or prostaglandins to endothelium-dependent vasodilation in arteries from young, mid-aged, and aged latently mCMV-infected and age-matched uninfected mice.
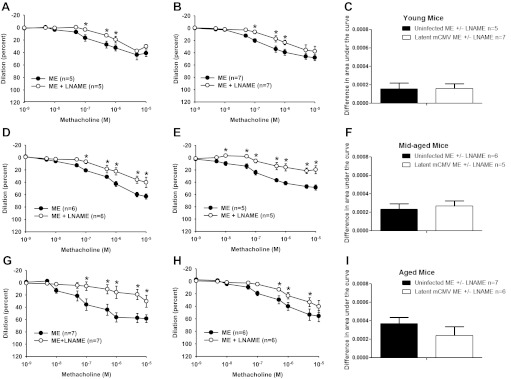
To determine whether changes in the contribution of NO or prostaglandins to ME-induced vasodilation could explain the results in Fig. 3, we measured the difference between arteries from latently mCMV-infected and uninfected mice to ME-induced vasodilation in the presence or absence of single or combined inhibitors to NO or PGHS-1/2. Although all arteries showed that NO contributed to ME-induced vasodilation, there were no differences in that contribution in mesenteric arteries from latently mCMV-infected compared with uninfected mice at any age (Fig. 4). In addition, PGHS-1/2 inhibition or the combined inhibition of NO and PGHS-1/2 did not alter ME-induced vasodilation in mesenteric arteries in any group (data not shown). Similar results were found in uterine arteries (data not shown).
Fig. 4.
Nitric oxide contribution to ME-induced vasodilation in mesenteric arteries from young, mid-aged, and aged uninfected and latently mCMV-infected mice. ME-induced vasodilation was measured in mesenteric arteries from young (A–C), mid-aged (D–F), and aged (G–I) latently mCMV-infected (B, E, and H) and uninfected (A, D, and G) mice in the presence and absence of NG-nitro-l-arginine methyl ester (l-NAME). Results for each curve were expressed as means ± SE, and the dose-response curves were compared as in Fig. 2. The area under each curve was calculated, and the difference between 2 curves on the same graph was obtained. These differences in area under the curve were compared between latently mCMV-infected and uninfected groups (C, F, and I) with a Student's t-test. *P < 0.05, significant differences; n = number of animals.
Endothelium-independent vasodilation in arteries from young, mid-aged, and aged latently mCMV-infected and age-matched uninfected mice.
Mesenteric arteries from young latently mCMV-infected mice had a slight but significantly increased vasodilation to the NO donor SNP at only one physiologically relevant dose (0.5 μM; P < 0.05) compared with age-matched uninfected mice (Fig. 5A). Mesenteric arteries from mid-aged latently mCMV-infected mice had significantly decreased SNP-induced vasodilation (P < 0.05) compared with age-matched uninfected mice at the highest concentrations (Fig. 5B). Mesenteric arteries from aged latently mCMV-infected and uninfected mice did not differ in response to SNP (Fig. 5C). In contrast to mesenteric arteries, uterine arteries from young and mid-aged latently mCMV-infected mice did not differ in response to SNP compared with age-matched uninfected mice (Fig. 5, D and E). In uterine arteries from aged latently mCMV-infected mice, however, SNP-induced vasodilation was significantly decreased overall (P < 0.05) compared with age-matched uninfected mice (Fig. 5F).
Fig. 5.
Responses to sodium nitroprusside (SNP) by mesenteric and uterine arteries. SNP-induced vasodilation was measured in mesenteric (A–C) and uterine (D–F) arteries from young (A and D), mid-aged (B and E), and aged (C and F) latently mCMV-infected and age-matched uninfected mice. Results were summarized and presented as means ± SE, and significant differences were assessed as for Fig. 2. *P < 0.05, significant differences; n = number of animals.
Distensibility of arteries from young, mid-aged, and aged latently mCMV-infected and age-matched uninfected mice.
Mesenteric arteries from young latently mCMV-infected mice had significantly decreased passive distensibility (P < 0.05) compared with young uninfected mice (Fig. 6A). Mesenteric arteries from mid-aged and aged latently mCMV-infected mice (Fig. 6, B and C) and uterine arteries from young, mid-aged, and aged latently mCMV-infected mice showed no differences in passive distensibility compared with age-matched controls (Fig. 6, D–F).
Fig. 6.
Distensibility curves for mesenteric and uterine arteries. Changes in lumen diameter were measured after stepwise increases in intraluminal pressure in Ca2+-free EGTA physiological saline solution in the presence of papaverine in mesenteric (A–C) and uterine (D–F) arteries from young (A and D), mid-aged (B and E), and aged (C and F) latently mCMV-infected and age-matched uninfected mice. Results were expressed as means ± SE percent increase in lumen diameter compared with the initial diameter at 4 mmHg. Significant differences were assessed as for Fig. 2. Where error bars are not visible, the errors are too small to be seen at this scale. *P < 0.05, significant differences; n = number of animals.
Comparison of vascular responses in arteries from uninfected mice during aging.
Mesenteric and uterine arteries from young, mid-aged, and aged uninfected mice did not differ in response to PE-induced vasoconstriction (Fig. 7, A–D). Although mesenteric arteries also did not differ in response to ME (Fig. 7B), uterine arteries from mid-aged uninfected mice (EC50, 63.4 ± 2.60 nM) were significantly more sensitive (P < 0.001) to ME than both young (EC50, 110 ± 7.13 nM) and aged (EC50, 153 ± 16.6 nM) uninfected mice (Fig. 7E). Mesenteric arteries from mid-aged and aged uninfected mice had significantly increased endothelium-independent SNP-induced vasodilation (P < 0.05) compared with young uninfected mice (Fig. 7C). Uterine arteries from young, mid-aged, and aged uninfected mice did not differ in response to SNP (Fig. 7F). There were no differences in distensibility for either mesenteric or uterine arteries from uninfected mice among any of these age groups (data not shown).
Fig. 7.
Comparison of vascular responses in mesenteric and uterine arteries from young, mid-aged, and aged uninfected mice. PE-induced vasoconstriction for mesenteric (A) and uterine (D) arteries, ME-induced vasodilation for mesenteric (B) and uterine (E) arteries, and SNP-induced vasodilation for mesenteric (C) and uterine (F) arteries were measured and compared among the 3 age groups. Results were summarized and are presented as means ± SE. Significant differences between the curves were calculated using a repeated-measures 2-way ANOVA with Holm-Sidak's post hoc analysis (P < 0.05). Significant differences: *P < 0.05, mid-aged compared with young; #P < 0.05, aged compared with young.
DISCUSSION
Active infections with HCMV, a β-Herpesviridae virus, are associated with tumorigenesis, transplant rejection, vascular diseases such as atherosclerosis and restenosis, increased mortality, and hypertension (9, 19, 23, 26, 33, 62, 64). Our study shows for the first time that vascular dysfunction in mesenteric and uterine arteries from mice infected with mCMV occurs even after the active infection has progressed to the latent stage, in which infectious virus is undetectable. However, the specific vascular changes depended on the vascular bed and in some cases worsened with age. In young mice, mesenteric and uterine arteries from latently mCMV-infected mice showed increased vasoconstriction and uterine arteries showed increased endothelial-dependent vasodilation compared with arteries from age-matched uninfected mice. In mid-aged latently infected mice, vasoconstriction was increased in uterine arteries, which may in part have contributed to the reduced fertility observed in these mice compared with age-matched uninfected mice. Finally, in aged latently mCMV-infected mice, both mesenteric and uterine arteries showed increased endothelial-dependent vasodilation and the uterine arteries also showed a decreased vasodilation response to an NO donor.
Viral latency has been operationally defined as the absence of infectious, replicating virus with maintenance of the viral genome in the tissues of the host (46). Most organs in the body can be sites of both active and latent CMV infections; only lymphocytes and neutrophils do not support CMV replication (42). Whereas viral latency may exist in one organ or cell type, viral replication (i.e., reactivation) may be occurring in another organ or cell type within the same host (2). It is also difficult to predict when reactivation of a latent CMV infection will occur (37, 49). In the murine model, the lung is a predominant site of latency where viral genome load is high and reactivation is frequent (2). In our study, we detected mCMV DNA in lungs, spleens, livers, and kidneys. However, we did not detect a reporter of productive infection, β-galactosidase, in any of five different organs, including the lung, at any time in our latently mCMV-infected mouse model. Therefore, although we cannot fully discard that viral replication had occurred elsewhere in the latently infected mice, the presence of viral DNA with the lack of detectable productive infection in five organs, together with evidence from many other studies, implies latent infections (63).
Mesenteric arteries from young latently mCMV-infected mice showed increased PE-induced vasoconstriction with small changes in endothelial-dependent and -independent vasodilation that offset each other. These results are consistent with other studies showing that angiotensin II (6) and bradykinin (17) are up- or downregulated during an active CMV infection, respectively, and induce a hypertensive phenotype (6). Mesenteric arteries from young and mid-aged latently mCMV-infected mice had minimally but significantly reduced ME-induced endothelium-dependent vasodilation similar to previous findings in cremaster arteries in a model of persistent mCMV infection (28). Here the authors found a greater reduction in vasodilation using the intravital system in which circulating mediators remain in contact with the intact arteries unlike the pressure myograph system used in this study in which arteries are dissected and infused with physiological saline. Other circulating vasoconstrictors likely impact the dilation responses during intravital microscopy. In contrast and contrary to our hypothesis, mesenteric arteries from aged latently mCMV-infected mice showed increased ME-induced vasodilation similar to our previous studies in young actively infected mice. We and others have shown that aging (22, 56) and CMV infections (6, 14, 17, 19, 28, 31) are associated with increased blood pressure, vascular stiffness, and vascular dysfunction. The systemic vasculature in aging may compensate for the combined insults of aging and infection with increased endothelium-dependent vasodilation, even in the presence of a latent, not active, mCMV infection (12). Even though there does not appear to be an increased contribution of NO synthase (NOS) activity or increased sensitivity of smooth muscle cells to NO in mesenteric arteries from aged infected mice, it is possible in an in vivo situation that conditions of increased oxidative stress as found in aging could lead to overall reduced NO bioavailability and reduced vasodilation as found using intravital miscroscopy (28).
Uterine arteries from latently mCMV-infected mice responded differently to PE, SNP, and ME than mesenteric arteries. In uterine arteries from young latently mCMV-infected compared with uninfected mice, the vasoconstriction induced by PE and the sensitivity to ME were both increased. The increase in both vasodilation and vasoconstriction may be explained by changes to artery composition and receptor expression. We previously reported changes in distensibility (12), also observed in the current study in arteries from young latently infected mice, which can impact both vasodilation and vasoconstriction. CMV also increases smooth muscle cell proliferation (62), which may contribute to increased vasoconstriction. Furthermore, inflammation and damage of the endothelium have also been associated with a CMV infection, both of which are important to the development of vascular diseases (3). Further evidence illustrates that an infection with CMV increases cellular cGMP (1) and induces the transcription factor, NF-κB, which can lead to the activation of the muscarinic 3 receptor (43). This receptor is the primary muscarinic receptor responsible for vasodilation in uterine arteries (44). NF-κB activation is also increased during inflammation, and persistent or latent CMV infections are associated with increased inflammation and endothelial cell activation (28). Thus a latent or persistent CMV infection may also contribute to increased vasodilation in uterine arteries. Further studies must be performed with other vasoconstrictory drugs to determine whether the vasoconstriction responses we observe in either the presence or absence of a CMV infection are a generalized phenomenon or specific to α1-adrenergic agonists.
In uterine arteries from mid-aged latently mCMV-infected mice, the dramatic increase in sensitivity to PE-induced vasoconstriction in the absence of a comparable compensatory increase in vasodilation could lead to reduced uterine blood flow. This could contribute to the reduced number of completed pregnancies in mid-aged latently mCMV-infected compared with age-matched uninfected mice (16.7 vs. 46.7%, respectively). Although C57BL/6J mice are fertile until 12 mo of age, the frequency of gestational complications, including embryo resorptions, increases with age (24). Thus the reduced fertility in the mid-aged uninfected animals is not unexpected (46.7 compared with 83.3% in young uninfected mice) (21). However, it is surprising that vascular dysfunction and reduced fertility appear to be exacerbated in mid-aged latently mCMV infected mice, where an active infection was not detected. These results in addition to recent studies describing the importance of viral infections in recurrent spontaneous abortion and stillbirths (25, 39) emphasize the importance for further investigation into the adverse effects of HCMV infection on fertility and pregnancy outcomes in addition to its devastating and well-known effects in congenital infections.
In aged mice, uterine arteries from latently mCMV-infected and uninfected mice did not differ in response to PE. However, there was an overall increase in endothelial-dependent vasodilation that was not due to changes in NOS activity along with a decrease in smooth muscle sensitivity to NO in uterine arteries from aged latently mCMV-infected mice. This suggests that vasodilation mechanisms may be reduced in vivo in uterine arteries compared with mesenteric arteries with age. As aged mice are no longer reproductively active (24), increased uterine artery blood flow is less important than in young and mid-aged mice. In contrast, it is essential to compensate for vascular dysfunction in systemic (mesenteric) vascular beds that regulate blood pressure and cardiovascular function (36). The increase in ME-dependent vasodilation in mesenteric arteries from aged latently infected mice does not appear to be dependent on NOS or prostaglandin activity or an increase in smooth muscle sensitivity to NO, similar to findings in cremaster arteries from young persistently CMV-infected mice (31). It is therefore likely to be due to changes in other endothelium-derived hyperpolarizing factors that may contribute to ME-induced vasodilation (4). This is in contrast to our previous study in young active mCMV-infected mice (12).
CMV latency is established in the host as a lifelong infection. Reactivated CMV is associated with several vascular diseases that occur with age, including atherosclerosis (34). During active CMV infections, CMV-specific T cells resolve active infections and maintain latent infections in part through the production of several cytokines such as interferon-γ (IFN-γ) (46, 60). Effectively reducing viral load as occurs in fully immunocompetent hosts accelerates the establishment of latency and reduces the incidence of reactivation (49). Cytokine production is reduced in immunosuppressed individuals, who are highly susceptible to CMV disease (11). IFN-γ also inhibits reactivation of mCMV and sustains latency (46). Interestingly, IFN-γ causes vascular dysfunction itself (53). It reduces sensitivity to NO, decreases endothelial NOS, and increases inducible NOS expression in human coronary artery allografts (29). Platelet adhesion, a marker of inflammation, was increased in HCMV-infected human pulmonary artery endothelial cells (48) and in postcapillary venules from mice with a persistent mCMV infection (28). Therefore, immune response mediators that increase during active or latent mCMV infections likely contribute to the vascular dysfunction and vasculopathies. In support of this, we have recently shown that an active in vivo mCMV infection has greater vascular effects than those seen after viral attachment or early viral entry (13, 14). Measuring circulating levels and testing the effects of several inflammatory cytokines, including IFN-γ and TNF-α, on vascular function to compare the direct and indirect effects of a CMV infection will be important.
Finally, it is perhaps surprising that we found few differences in vascular responses when comparing young, mid-aged, and aged mice, independent of mCMV infection. Moreover, aging appeared to increase vasodilation, which is opposite to the decrease in vasodilation that might be expected given the previously published decreases in NO production, bioavailability, and smooth muscle sensitivity with age alone (27, 54–56). The increased uterine and mesenteric vasodilation to endothelial-dependent and independent vasodilators, respectively, may be compensatory mechanisms in this animal model to deal with the increased vascular dysfunction that occurs with normal aging (30). Increased capacity for vasodilation could perhaps explain the finding that myogenic tone is reduced in mesenteric arteries from both male and female mid-aged and aged mice compared with young mice (18). Although there were no differences observed in PE-induced vasoconstriction with aging alone in this study, in contrast to increases in vasoconstriction in the rat model (38), our findings are consistent with other C57BL/6 mouse studies (18).
We are the first to show that both active and latent mCMV infections cause vascular dysfunction in two different types of arteries at several different ages. Determining what mediators are involved in vascular dysfunction during CMV infections will be very important in targeting viral-induced factors that exacerbate vascular-specific complications that occur with age. Understanding CMV-induced vascular dysfunction will increase our knowledge of the development of cardiovascular diseases associated with a CMV infection, and the vascular complications that may contribute to infertility in mid-aged CMV-infected women. Taken together, these results and those of our combined studies (12–14) emphasize the importance of both direct and indirect effects of both active and latent mCMV infections on vascular responses in pregnancy and aging.
GRANTS
Funding support from Natural Sciences and Engineering Research Council of Canada (to D. G. Hemmings and studentship for R. B. Gombos), Women and Children's Health Research Institute (to D. G. Hemmings), Mazankowski Alberta Heart Institute (studentship for R. B. Gombos), Burroughs Wellcome Fund (to L. M. Schang), and Canadian Institutes of Health Research (to D. G. Hemmings and L. M. Schang) is acknowledged. L. M. Schang is a Burroughs Wellcome Fund investigator in the pathogenesis of infectious disease.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.B.G., L.M.S., and D.G.H. conception and design of research; R.B.G., J.C.B., J.T., R.L.G., and K.L.C. performed experiments; R.B.G., J.T., R.L.G., and K.L.C. analyzed data; R.B.G., J.C.B., R.L.G., K.L.C., L.M.S., and D.G.H. interpreted results of experiments; R.B.G., J.C.B., J.T., and D.G.H. prepared figures; R.B.G. drafted manuscript; R.B.G., L.M.S., and D.G.H. edited and revised manuscript; R.B.G., J.C.B., J.T., R.L.G., K.L.C., L.M.S., and D.G.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. E. Mocarski (Stanford University) for the gift of RM427+, the mCMV virus used in our studies.
REFERENCES
- 1. Albrecht T, Boldogh I, Fons M, AbuBakar S, Deng CZ. Cell activation signals and the pathogenesis of human cytomegalovirus. Intervirology 31: 68–75, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Balthesen M, Messerle M, Reddehase MJ. Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol 67: 5360–5366, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolovan-Fritts CA, Spector SA. Endothelial damage from cytomegalovirus-specific host immune response can be prevented by targeted disruption of fractalkine-CX3CR1 interaction. Blood 111: 175–182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49: 590–596, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention Prevalence of heart disease—United States, 2005. MMWR Morb Mortal Wkly Rep 56: 113–118, 2007 [PubMed] [Google Scholar]
- 6. Cheng J, Ke Q, Jin Z, Wang H, Kocher O, Morgan JP, Zhang J, Crumpacker CS. Cytomegalovirus infection causes an increase of arterial blood pressure. PLoS Pathog 5: e1000427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheung WW, Tse E, Leung AY, Yuen KY, Kwong YL. Regular virologic surveillance showed very frequent cytomegalovirus reactivation in patients treated with alemtuzumab. Am J Hematol 82: 108–111, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Chow CM, Donovan L, Manuel D, Johansen H, Tu JV. Regional variation in self-reported heart disease prevalence in Canada. Can J Cardiol 21: 1265–1271, 2005 [PubMed] [Google Scholar]
- 9. Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 62: 3347–3350, 2002 [PubMed] [Google Scholar]
- 10. Demmler GJ. Infectious Diseases Society of America and Centers for Disease Control. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev Infect Dis 13: 315–329, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, Ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood 101: 2686–2692, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Gombos RB, Hemmings DG. Differential effects on nitric oxide-mediated vasodilation in mesenteric and uterine arteries from cytomegalovirus-infected mice. Am J Physiol Heart Circ Physiol 299: H1124–H1134, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Gombos RB, Teefy J, Lee A, Hemmings DG. Impact of local endothelial challenge with cytomegalovirus or glycoprotein B on vasodilation in intact pressurized arteries from nonpregnant and pregnant mice. Biol Reprod 87: 83, 2012 [DOI] [PubMed] [Google Scholar]
- 14. Gombos RB, Wolan V, McDonald K, Hemmings DG. Impaired vascular function in mice with an active cytomegalovirus infection. Am J Physiol Heart Circ Physiol 296: H937–H945, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Goodrum F, Caviness K, Zagallo P. Human cytomegalovirus persistence. Cell Microbiol 14: 644–655, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grahame-Clarke C. Human cytomegalovirus, endothelial function and atherosclerosis. Herpes 12: 42–45, 2005 [PubMed] [Google Scholar]
- 17. Grahame-Clarke C, Chan NN, Andrew D, Ridgway GL, Betteridge DJ, Emery V, Colhoun HM, Vallance P. Human cytomegalovirus seropositivity is associated with impaired vascular function. Circulation 108: 678–683, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Gros R, Van Wert R, You X, Thorin E, Husain M. Effects of age, gender, and blood pressure on myogenic responses of mesenteric arteries from C57BL/6 mice. Am J Physiol Heart Circ Physiol 282: H380–H388, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Haarala A, Kahonen M, Lehtimaki T, Aittoniemi J, Jylhava J, Hutri-Kahonen N, Taittonen L, Laitinen T, Juonala M, Viikari J, Raitakari OT, Hurme M. Relation of high cytomegalovirus antibody titres to blood pressure and brachial artery flow-mediated dilation in young men: the Cardiovascular Risk in Young Finns Study. Clin Exp Immunol 167: 309–316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Halpern W, Osol G, Coy GS. Mechanical behavior of pressurized in vitro prearteriolar vessels determined with a video system. Ann Biomed Eng 12: 463–479, 1984 [DOI] [PubMed] [Google Scholar]
- 21. Heffner LJ. Advanced maternal age—how old is too old? N Engl J Med 351: 1927–1929, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Hemmings DG, Xu Y, Davidge ST. Sphingosine 1-phosphate-induced vasoconstriction is elevated in mesenteric resistance arteries from aged female rats. Br J Pharmacol 143: 276–284, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hirabayashi Y, Ishii T, Kodera T, Fujii H, Munakata Y, Sasaki T. Acute cytomegalovirus infection and transient carotid intimal-medial thickening in a young, otherwise healthy woman. J Clin Microbiol 41: 3978–3980, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holinka CF, Tseng YC, Finch CE. Reproductive aging in C57BL/6J mice: plasma progesterone, viable embryos and resorption frequency throughout pregnancy. Biol Reprod 20: 1201–1211, 1979 [DOI] [PubMed] [Google Scholar]
- 25. Iwasenko JM, Howard J, Arbuckle S, Graf N, Hall B, Craig ME, Rawlinson WD. Human cytomegalovirus infection is detected frequently in stillbirths and is associated with fetal thrombotic vasculopathy. J Infect Dis 203: 1526–1533, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Kalil AC, Florescu DF. Prevalence and mortality associated with cytomegalovirus infection in nonimmunosuppressed patients in the intensive care unit. Crit Care Med 37: 2350–2358, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Karaki H, Nakagawa H, Urakawa N. Age-related changes in the sensitivity to verapamil and sodium nitroprusside of vascular smooth muscle of rabbit aorta. Br J Pharmacol 85: 223–228, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khoretonenko MV, Leskov IL, Jennings SR, Yurochko AD, Stokes KY. Cytomegalovirus infection leads to microvascular dysfunction and exacerbates hypercholesterolemia-induced responses. Am J Pathol 177: 2134–2144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koh KP, Wang Y, Yi T, Shiao SL, Lorber MI, Sessa WC, Tellides G, Pober JS. T cell-mediated vascular dysfunction of human allografts results from IFN-gamma dysregulation of NO synthase. J Clin Invest 114: 846–856, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Labinskyy N, Csiszar A, Veress G, Stef G, Pacher P, Oroszi G, Wu J, Ungvari Z. Vascular dysfunction in aging: potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr Med Chem 13: 989–996, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leskov IL, Whitsett J, Vasquez-Vivar J, Stokes KY. NAD(P)H oxidase and eNOS play differential roles in cytomegalovirus infection-induced microvascular dysfunction. Free Radic Biol Med 51: 2300–2308, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matz RL, de Sotomayor MA, Schott C, Stoclet JC, Andriantsitohaina R. Vascular bed heterogeneity in age-related endothelial dysfunction with respect to NO and eicosanoids. Br J Pharmacol 131: 303–311, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maussang D, Verzijl D, van Walsum M, Leurs R, Holl J, Pleskoff O, Michel D, van Dongen GA, Smit MJ. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci USA 103: 13068–13073, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Melnick JL, Adam E, DeBakey ME. Cytomegalovirus and atherosclerosis. Bioessays 17: 899–903, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Mittelmark MB, Psaty BM, Rautaharju PM, Fried LP, Borhani NO, Tracy RP, Gardin JM, O'Leary DH. Prevalence of cardiovascular diseases among older adults. The Cardiovascular Health Study. Am J Epidemiol 137: 311–317, 1993 [DOI] [PubMed] [Google Scholar]
- 36. Mohammed MM, Myers DS, Sofola OA, Hainsworth R, Drinkhill MJ. Vasodilator effects of leptin on canine isolated mesenteric arteries and veins. Clin Exp Pharmacol Physiol 34: 771–774, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Morita-Hoshi Y, Heike Y, Kawakami M, Sugita T, Miura O, Kim SW, Mori SI, Fukuda T, Tanosaki R, Tobinai K, Takaue Y. Functional analysis of cytomegalovirus-specific T lymphocytes compared with tetramer assay in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 41: 515–521, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 282: H1843–H1854, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Nigro G, Mazzocco M, Mattia E, Di Renzo GC, Carta G, Anceschi MM. Role of the infections in recurrent spontaneous abortion. J Matern Fetal Neonatal Med 24: 983–989, 2011 [DOI] [PubMed] [Google Scholar]
- 40. Onyeagocha C, Hossain MS, Kumar A, Jones RM, Roback J, Gewirtz AT. Latent cytomegalovirus infection exacerbates experimental colitis. Am J Pathol 175: 2034–2042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Osol G, Celia G, Gokina N, Barron C, Chien E, Mandala M, Luksha L, Kublickiene K. Placental growth factor is a potent vasodilator of rat and human resistance arteries. Am J Physiol Heart Circ Physiol 294: H1381–H1387, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pancholi P, Wu F, Della-Latta P. Rapid detection of cytomegalovirus infection in transplant patients. Expert Rev Mol Diagn 4: 231–242, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Paula FM, Barbosa HC, Carneiro EM, Persaud SJ, Gagliardino JJ, Boschero AC, Souza KL. Requirement of NF-kappaB signalling pathway for modulation of the cholinergic muscarinic M3 receptor expression by INGAP-PP in insulin-producing cells. Eur J Pharmacol 642: 37–46, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Pesic S, Grbovic L, Stoiljkovic M, Nikolic V, Djokic J. Functional characterization of the muscarinic receptors involved in endothelium-dependent relaxation in isolated canine uterine artery. J Vet Pharmacol Ther 32: 109–115, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Pollock JL, Virgin HW., 4th Latency, without persistence, of murine cytomegalovirus in the spleen and kidney. J Virol 69: 1762–1768, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Presti RM, Pollock JL, Dal Canto AJ, O'Guin AK, Virgin HW., 4th Interferon gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J Exp Med 188: 577–588, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Prosch S, Wendt CE, Reinke P, Priemer C, Oppert M, Kruger DH, Volk HD, Docke WD. A novel link between stress and human cytomegalovirus (HCMV) infection: sympathetic hyperactivity stimulates HCMV activation. Virology 272: 357–365, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Rahbar A, Soderberg-Naucler C. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J Virol 79: 2211–2220, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reddehase MJ, Balthesen M, Rapp M, Jonjic S, Pavic I, Koszinowski UH. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J Exp Med 179: 185–193, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roizmann B, Desrosiers RC, Fleckenstein B, Lopez C, Minson AC, Studdert MJ. The family Herpesviridae: an update. The Herpesvirus Study Group of the International Committee on Taxonomy of Viruses. Arch Virol 123: 425–449, 1992 [DOI] [PubMed] [Google Scholar]
- 51. Roupa Z, Polikandrioti M, Sotiropoulou P, Faros E, Koulouri A, Wozniak G, Gourni M. Causes of infertility in women at reproductive age. Health Sci J 3: 80–87, 2009 [Google Scholar]
- 52. Schang LM, Kutish GF, Osorio FA. Correlation between precolonization of trigeminal ganglia by attenuated strains of pseudorabies virus and resistance to wild-type virus latency. J Virol 68: 8470–8476, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Skaro AI, Liwski RS, Zhou J, Vessie EL, Lee TD, Hirsch GM. CD8+ T cells mediate aortic allograft vasculopathy by direct killing and an interferon-gamma-dependent indirect pathway. Cardiovasc Res 65: 283–291, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Smith AR, Visioli F, Frei B, Hagen TM. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell 5: 391–400, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Smith AR, Visioli F, Hagen TM. Plasma membrane-associated endothelial nitric oxide synthase and activity in aging rat aortic vascular endothelia markedly decline with age. Arch Biochem Biophys 454: 100–105, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol 101: 1751–1759, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Stoddart CA, Cardin RD, Boname JM, Manning WC, Abenes GB, Mocarski ES. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J Virol 68: 6243–6253, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tanaka A, Hirota K, Takahashi K, Numazaki Y. Suppression of cell mediated immunity to cytomegalovirus and tuberculin in pregnancy employing the leukocyte migration inhibition test. Microbiol Immunol 27: 937–943, 1983 [DOI] [PubMed] [Google Scholar]
- 59. Tang Q, Maul GG. Mouse cytomegalovirus immediate-early protein 1 binds with host cell repressors to relieve suppressive effects on viral transcription and replication during lytic infection. J Virol 77: 1357–1367, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tu W, Potena L, Stepick-Biek P, Liu L, Dionis KY, Luikart H, Fearon WF, Holmes TH, Chin C, Cooke JP, Valantine HA, Mocarski ES, Lewis DB. T-cell immunity to subclinical cytomegalovirus infection reduces cardiac allograft disease. Circulation 114: 1608–1615, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Veerareddy S, Cooke CL, Baker PN, Davidge ST. Vascular adaptations to pregnancy in mice: effects on myogenic tone. Am J Physiol Heart Circ Physiol 283: H2226–H2233, 2002 [DOI] [PubMed] [Google Scholar]
- 62. Yonemitsu Y, Kaneda Y, Komori K, Hirai K, Sugimachi K, Sueishi K. The immediate early gene of human cytomegalovirus stimulates vascular smooth muscle cell proliferation in vitro and in vivo. Biochem Biophys Res Commun 231: 447–451, 1997 [DOI] [PubMed] [Google Scholar]
- 63. Yuhasz SA, Dissette VB, Cook ML, Stevens JG. Murine cytomegalovirus is present in both chronic active and latent states in persistently infected mice. Virology 202: 272–280, 1994 [DOI] [PubMed] [Google Scholar]
- 64. Zhang M, Yang Y, Yang X, Cai J. Human cytomegalovirus infection is a novel etiology for essential hypertension. Med Hypotheses 76: 682–684, 2011 [DOI] [PubMed] [Google Scholar]