Abstract
Consumption of the dietary flavanol (−)-epicatechin (EPI) is associated with enhanced endothelial function and augmented skeletal muscle capillarity and mitochondrial volume density. The potential for EPI to improve peripheral vascular function and muscle oxygenation during exercise is unknown. We tested the hypothesis that EPI administration in healthy rats would improve treadmill exercise performance secondary to elevated skeletal muscle blood flow and vascular conductance [VC, blood flow/mean arterial pressure (MAP)] and improved skeletal muscle microvascular oxygenation. Rats received water (control, n = 12) or 4 mg/kg EPI (n = 12) via oral gavage daily for 24 days. Exercise endurance capacity and peak O2 uptake (V̇o2 peak) were measured via treadmill runs to exhaustion. MAP (arterial catheter) and blood flow (radiolabeled microspheres) were measured and VC was calculated during submaximal treadmill exercise (25 m/min, 5% grade). Spinotrapezius muscle microvascular O2 pressure (Po2mv) was measured (phosphorescence quenching) during electrically induced twitch (1 Hz) contractions. In conscious rats, EPI administration resulted in lower (↓∼5%) resting (P = 0.03) and exercising (P = 0.04) MAP. There were no differences in exercise endurance capacity, V̇o2 peak, total exercising hindlimb blood flow (control, 154 ± 13; and EPI, 159 ± 8 ml·min−1·100 g−1, P = 0.68), or VC (control, 1.13 ± 0.10; and EPI, 1.24 ± 0.08 ml·min−1·100 g−1·mmHg−1, P = 0.21) between groups. Following anesthesia, EPI resulted in lower MAP (↓∼16%) but did not impact resting Po2mv or any kinetics parameters (P > 0.05 for all) during muscle contractions compared with control. EPI administration (4 mg·kg−1·day−1) improved modestly cardiovascular function (i.e., ↓MAP) with no impact on exercise performance, total exercising skeletal muscle blood flow and VC, or contracting muscle microvascular oxygenation in healthy rats.
Keywords: vasodilation, oxygen delivery, exercise performance, flavanols, supplements
flavanoids are a subclass of dietary polyphenols that impact a vast array of biological and physiological functions (28). High dietary consumption of flavanoids, specifically flavanols (or flavan-3-ols), through, for example, cocoa plant-based dark chocolate, is associated with reduced risk and prevalence of cardiovascular disease and cardiovascular-related mortality (26). Importantly, the beneficial cardiovascular effects of flavanol-rich cocoa are mimicked by oral consumption of the flavanol (−)-epicatechin (EPI), which provides the experimental advantage of avoiding the confounding nutrient array of complex food matrixes (40). Specifically, Schroeter et al. (40) identified that peak improvements in endothelial function occur concomitant with peak plasma EPI metabolite levels.
EPI administration improves endothelial function (19) and promotes vasodilation (12, 34) via upregulation of nitric oxide (NO)-mediated function (12, 37, 40). Within the peripheral vasculature, NO constitutes a critical modulator of active skeletal muscle blood flow distribution (22) and the skeletal muscle O2 delivery-to-O2 utilization [O2 uptake (V̇o2)] ratio, which sets the microvascular partial pressure of O2 (Po2mv), and represents the pressure head for capillary-myocyte O2 flux (11). Reduced NO bioavailability (as found in many chronic disease conditions such as diabetes, chronic heart failure, peripheral artery disease, etc.) is associated with compromised peripheral vascular function and exercise intolerance (14). Conversely, augmenting NO-mediated function through interventions such as exercise training induces a preferential blood flow redistribution within and among active skeletal muscle (2) and elevates Po2mv following the onset of muscle contractions [i.e., slows the Po2mv kinetics fall (20)].
Recent experimental evidence in healthy middle-aged (12 mo old) mice supports that 15 days of EPI administration increases exercise capacity secondary to increases in skeletal muscle capillarity, mitochondrial volume density, and isolated muscle contractile function (33). It is unknown, however, whether EPI administration enhances peripheral vascular function and skeletal muscle oxygenation during exercise. Enhanced vascular function and augmented O2 delivery may represent a mechanistic link between the EPI-induced elevations in mitochondrial oxidative capacity and improvements in exercise capacity observed in mice (33). This information would constitute a crucial first step in identifying the potential for EPI consumption to enhance peripheral vascular function in patient populations where reduced NO bioavailability underlies, at least, in part, peripheral vascular dysfunction and exercise intolerance.
The purpose of the present investigation was to test the hypotheses that EPI administration (4 mg/kg for 24 days total) in healthy rats would 1) elevate skeletal muscle blood flow and vascular conductance (VC) during submaximal locomotory exercise, 2) elevate skeletal muscle oxygenation (i.e., higher Po2mv) during muscle contractions, and 3) that such augmentations would mechanistically underlie greater exercise endurance capacity and V̇o2 peak.
MATERIALS AND METHODS
Animal Care and Assignment
A total of 24 young adult (initial age, ∼4 mo old) male Sprague-Dawley rats (Charles River, Boston, MA) were used in this investigation. Rats were housed two per cage and kept on a 12-h:12-h light-dark cycle in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Food and water were available ad libitum. All experimental procedures described herein were approved by Institutional Animal Care and Use Committee of Kansas State University and conducted in accordance with National Institutes of Health guidelines.
All rats were acclimatized to running on a custom-built motor-driven treadmill (5 days, 5 min/day, up to 25 m/min). Rats were weighed and required to perform a treadmill run to exhaustion to determine exercise endurance capacity (see below for protocol details). Rats were then randomized into control (n = 12) or EPI (n = 12) groups such that initial body mass (control, 322 ± 14; and EPI, 333 ± 14 g, P = 0.26) and exercise endurance capacity (control, 52.6 ± 3.2; and EPI, 44.8 ± 2.3 min; P = 0.07) were not different between groups. Rats in the EPI group were administered 2 mg/kg EPI (>96% pure, Sigma-Aldrich, St. Louis, MO) via oral gavage twice daily (morning/afternoon, 4 mg·kg−1·day−1 total) for 21 days whereas rats in the control group were gavaged with water twice daily over the identical time frame (see Experimental Considerations for further discussion regarding dosing).
Assessment of Exercise Performance
Following the initial 21 day gavage period, rats performed a treadmill run to exhaustion to determine exercise endurance capacity and a maximal treadmill test to determine V̇o2 peak. Exercise tests were completed in random order and with at least 24 h between tests. Rats continued to receive EPI or water via oral gavage twice daily on the days of the exercise performance assessments.
Endurance capacity.
The exercise endurance capacity protocol consisted of a progressive exercise test in which rats initially ran at a speed of 25 m/min up a 5% grade for 15 min. Thereafter, the treadmill grade was held constant while the speed was increased by 5 m/min every 15 min until the rat was unable/unwilling to maintain pace with the treadmill belt (i.e., unable to come off the back of the treadmill lane for >3 s) despite manual bursts of high-pressure air aimed at the hindlimbs. Endurance capacity was measured to the nearest second. The presence of a consistent running gait and pattern throughout the test (i.e., not repeatedly stopping, sprinting, stopping, etc.) and confirmation of exhaustion as indicated via an attenuation of the rats' righting reflex following the test were required for a valid exercise endurance capacity measurement. Two rats from the EPI group did not meet this criteria even after repeat tests resulting in a final posttreatment/control exercise endurance capacity sample of 12 control and 10 EPI rats. Our laboratory has previously demonstrated that this protocol elicits highly reproducible within-animal times to exhaustion (7).
V̇o2 peak.
Rats were placed in a custom-made metabolic chamber designed to fit into one stall on the treadmill. V̇o2 peak measurement uses standard techniques described by Brooks and White (6) and Musch et al. (31) for determining V̇o2 and carbon dioxide production (V̇co2). Gas analysis measurements were made in real time via regularly calibrated CO2 and O2 analyzers (CO2, model CD-3A; and O2, model S-3A/I; AEI Technologies, Pittsburgh, PA) set in series. Each rat initially ran in the metabolic chamber at a speed of 25 m/min (5% grade) for 2 min. Subsequently, the speed of the treadmill was increased to 40 m/min for an additional 2 min. Thereafter, the treadmill speed was increased progressively in a ramp-like manner by ∼5–10 m/min every minute until the rat was unable or unwilling to keep pace with the treadmill belt. V̇o2 peak was recorded as the V̇o2 at which the rat was no longer able/willing to run, at which point the test was immediately terminated to avoid injury. Criteria for a successful test was the observation of a consistent running gait preceding obvious physical exertion at the end of the test (i.e., lowering of the hindlimbs and elevation of the snout) and/or no further increase in V̇o2 despite continued increases in treadmill speed in combination with a respiratory exchange ratio (RER; V̇co2/V̇o2) ≥ 1.0 (average RER = 1.05 ± 0.01, and RER range = 1.01–1.09). This protocol elicits highly reproducible within-animal V̇o2 peak measurements (7). Technical problems with O2 and CO2 analyzers during our experimental protocol resulted in a final sample of seven control and seven EPI rats for V̇o2 peak.
Surgical Instrumentation
Rats were weighed and gavaged (24 days total of EPI or water gavage) on the morning of the final experimental protocols. Rats were subsequently anesthetized with 5% isoflurane-O2 gas mixture. While being maintained on a 2 to 3% isoflurane-O2 mixture, one catheter [polyethylene (PE)-10 tubing connected to PE-50, Clay Adams Brand, Sparks, MD] was placed in the aortic arch via the right carotid artery and another was placed in the caudal (tail) artery, as previously described (32). Both catheters were tunneled subcutaneously to the dorsal aspect of the cervical region and exteriorized through a puncture wound in the skin. The incisions were then closed, anesthesia was terminated, and the animal was given >1 h to recover (13).
Protocol 1: Measurement of Hindlimb Skeletal Muscle Blood Flow
Following the recovery from anesthesia, each rat was placed into a single lane on the treadmill. The tail artery catheter was connected to a 1-ml plastic syringe that was connected to a Harvard infusion/withdrawal pump (model 907, South Nattick, MA). The carotid artery catheter was connected to a pressure transducer set at the same level as the rat for continuous measurement of mean arterial pressure (MAP) and heart rate (HR). Exercise was initiated, and the speed of the treadmill was increased progressively during the next 30 s to a speed of 25 m/min [5% grade, ∼65% V̇o2 peak (30)]. The rat then exercised steadily for another 2.5 min. After 3 min of total exercise time, blood withdrawal from the tail artery catheter was initiated at a rate of 0.25 ml/min. Simultaneously, MAP and HR were measured via the carotid artery catheter. The carotid artery catheter was then disconnected from the pressure transducer, and ∼6–7 × 105 microspheres (15 μm diameter, 57Co or 85Sr, in random order, Perkin Elmer Life and Analytical Sciences, Waltham, MA) were injected into the aortic arch via the carotid artery catheter to determine regional blood flow. Following microsphere infusion, a blood sample (∼0.3 ml) was taken from the carotid artery catheter for measurement of blood gases, pH, and blood [lactate]. Subsequently (∼30 s after the microsphere infusion), blood withdrawal from the tail artery catheter was stopped and exercise was terminated.
After a >30 min recovery period, MAP and HR were measured as the rat quietly sat on the treadmill. A second microsphere infusion (differently labeled from the first infusion) and blood sampling procedure were then performed exactly as described above during exercise to determine resting hindlimb muscle blood flow. This strategy (exercise followed by rest) minimizes the potential for blood sampling to affect the exercise response and facilitates resting measurements that do not reflect the preexercise anticipatory response (1).
Protocol 2: Measurement of Spinotrapezius Muscle Po2mv
Following the second (resting) microsphere infusion, rats were anesthetized with diluted pentobarbital sodium (administered intra-arterially into the caudal artery to effect) and continuously monitored via the toe pinch and blink reflexes with anesthesia supplemented as necessary and placed on a heating pad to maintain core temperature at 38°C (measured via rectal probe). Overlying skin and fascia were then carefully reflected from the middorsal-caudal region of each rat, and the right spinotrapezius muscle was exposed in a manner that ensured the integrity of the vascular and neural supply to the muscle (3). The spinotrapezius muscle was used to examine the effects of EPI on muscle microcirculatory function given its mixed muscle fiber-type composition and oxidative enzyme concentration that is analogous to human quadriceps muscle (8, 29). Silver wire electrodes were then sutured (6-0 silk) to the rostral (cathode) and caudal (anode) regions of the muscle. The exposed spinotrapezius muscle was continuously superfused with a warmed (38°C) Krebs-Henseleit bicarbonate-buffered solution equilibrated with 5% CO2-95% N2, and the surrounding exposed tissue was covered with Saran wrap (Dow Brands, Indianapolis, IN). The phosphorescent probe palladium meso-tetra (4-carboxyphenyl) porphyrin dendrimer (R2, 15–20 mg/kg dissolved in saline; Oxygen Enterprises, Philadelphia, PA) was infused via the carotid artery catheter. After an ∼15-min stabilization period, the carotid artery catheter was connected to a pressure transducer for continuous monitoring of HR and MAP and Po2mv measurement (see below) were initiated. Once Po2mv baseline was stable for at least 30 s, 1-Hz contractions (∼5–8 V, 2-ms pulse duration) were initiated and then terminated after 3 min. Po2mv measurements were stopped, and rats were euthanized with a pentobarbital sodium overdose (>50 mg/kg ia into the carotid artery catheter). Surgical complications in two control and two EPI rats resulted in a final Po2mv measurement sample of 10 control and 10 EPI rats. Power analysis based on known sample variability of Po2mv measurement and anticipated treatment effects (33) indicate that eight rats per group is sufficient to detect significant differences at the P < 0.05 level when they exist.
Measurement of Po2mv and Curve Fitting
The Stern-Volmer relationship allows the calculation of Po2mv through the direct measurement of a phosphorescence lifetime via the following equation (39):
where kQ is the quenching constant and τ° and τ are the phosphorescence lifetimes in the absence of O2 and the ambient O2 concentration, respectively. For R2, kQ is 409 mmHg−1·s−1 and τ° is 601 μs (30), and these characteristics do not change over the physiological range of pH and temperature in the rat in vivo, and therefore the O2 pressure is determined directly from the phosphorescence lifetime (30, 39).
The R2 phosphorescent probe binds to albumin and consequently is uniformly distributed throughout the plasma. A previous study from our laboratory systematically investigated the compartmentalization of R2 and confirmed that it remains within the microvasculature of exposed muscle over the duration considered in the present experiments, thereby ensuring a valid Po2mv measurement (35). Po2mv was determined with a PMOD 5000 frequency-domain phosphorometer (Oxygen Enterprises). The common end of the light guide was placed ∼2–4 mm superficial to the dorsal surface of the right spinotrapezius muscle. The muscle field was chosen to be devoid of large blood vessels, and hence the Po2mv measurement principally sampled capillary blood. Po2mv was continuously measured and recorded at 2-s intervals throughout the duration of the contraction periods.
Curve fitting of the measured Po2mv responses was performed with commercially available software (SigmaPlot 11.2, Systat Software, San Jose, CA), and the data were fit with either a one- or two-component model as described below:
where Po2mv (t) represents the Po2mv at any given time t; Po2mv (BL) corresponds to the precontracting resting baseline Po2mv; Δ1 and Δ2 are the amplitudes for the first and second component, respectively; TD1 and TD2 are the time delays for each component; and τ1 and τ2 are the time constants (i.e., time to 63% of the final response value) for each component. Goodness of fit was determined using the following criteria: 1) the coefficient of determination, 2) sum of the squared residuals, and 3) visual inspection and analysis of the model fits to the data and the residuals. The mean response time (MRT) of the kinetics response was calculated for the first component to provide an index of the overall principal kinetics response according to the following equation:
where TD1 and τ1 are as described above.
Determination of Blood Flow and VC
Following euthanasia, the thorax was opened and the placement of the carotid artery catheter into the aortic arch was confirmed by anatomical dissection. Organs of the splanchnic region, the kidneys, and 28 individual skeletal muscles, and muscle parts of the hindlimbs were identified and removed. The tissues were blotted, weighed, and placed immediately into counting vials.
The radioactivity of each tissue was determined on a gamma scintillation counter (Packard Auto Gamma Spectrometer, model 5230, Downers Grove, IL). Accounting for cross talk between isotopes, blood flows to each tissue were determined using the reference sample method (32) and expressed as milliliters per minute per 100 g of tissue. Adequate mixing of the microspheres was determined by the demonstration of a <15% difference between blood flow to the right and left kidneys and/or to the right and left hindquarter musculature. Blood flow was normalized to MAP to calculate VC (in ml·min−1·100 g−1·mmHg−1). Poor microsphere mixing (blood flow differences > 15% between right and left kidneys) occurred in two rats, resulting in a final blood flow sample of 11 control and 11 EPI rats. Power analysis based on known sample variability of blood flow and VC and anticipated treatment effects (33) indicates that 8–10 rats per group is sufficient to detect significant differences at the P < 0.05 level when they exist.
Statistical Analysis
Blood [lactate], MAP, HR, blood flow, VC, and the Po2mv-to-MAP ratio were compared between (control vs. EPI) and within (rest vs. exercise or precontractions vs. during contractions) groups via mixed two-way ANOVAs (Student-Newman-Keuls post hoc test). Body mass, exercise endurance capacity, V̇o2 peak, and Po2mv kinetics parameters were compared via unpaired Student's t-tests. Significance was accepted at P < 0.05.
RESULTS
Body Mass, Endurance Capacity, and V̇o2 Peak
Body mass following the treatment period was not different between groups (control, 384 ± 13; and EPI, 399 ± 20 g, P = 0.48). There were no differences in posttreatment exercise endurance capacity (control, 43.4 ± 2.3; and EPI, 36.8 ± 2.9 min, P = 0.06), the change in endurance capacity (post-pretreatment, control, −9.2 ± 3.0; and EPI, −8.6 ± 2.3 min, P = 0.86), or V̇o2 peak (control, 84 ± 2; and EPI, 82 ± 4 ml·kg−1·min−1, P = 0.70) between groups.
Protocol 1: Blood flow and VC at Rest and During Exercise
Blood gases, blood [lactate], MAP, and HR.
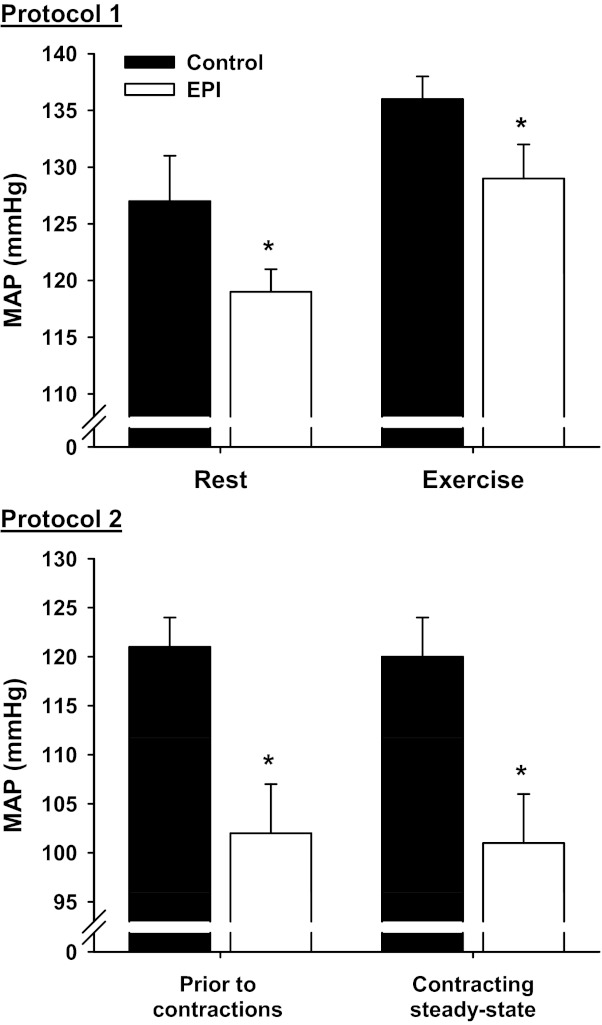
There were no differences in arterial blood pH, Po2, or Pco2 between control and EPI groups at rest or during exercise (data not shown, P > 0.05 for all). Resting (control, 0.6 ± 0.1; and EPI, 0.7 ± 0.1 mmol/l, P = 0.08) and exercising (control, 3.2 ± 0.5; and EPI, 3.3 ± 0.8 mmol/l, P = 0.81) arterial blood [lactate] were not different between groups. MAP was lower in the EPI group compared with control both at rest (P = 0.03) and during exercise (P = 0.04, Fig. 1). HR at rest (control, 427 ± 11; and EPI, 444 ± 14 beats/min, P = 0.38) and during exercise (control, 530 ± 12; and EPI, 550 ± 14 beats/min, P = 0.29) were not different between groups.
Fig. 1.
Effects of (−)-epicatechin (EPI) administration on mean arterial pressure (MAP). Protocol 1 (top): MAP measured at rest and during submaximal treadmill exercise. Within control and EPI conditions, exercise MAP was significantly different from rest (P < 0.05 for both: control, n = 11; and EPI, n = 11). Protocol 2 (bottom): MAP measured before and during the steady state of electrically induced spinotrapezius muscle contractions. There were no differences between MAP measurement time points within control and EPI groups (P > 0.05). Data are means ± SE; control, n = 10; and EPI, n = 10. *P < 0.05 vs. control.
Blood flow and VC.
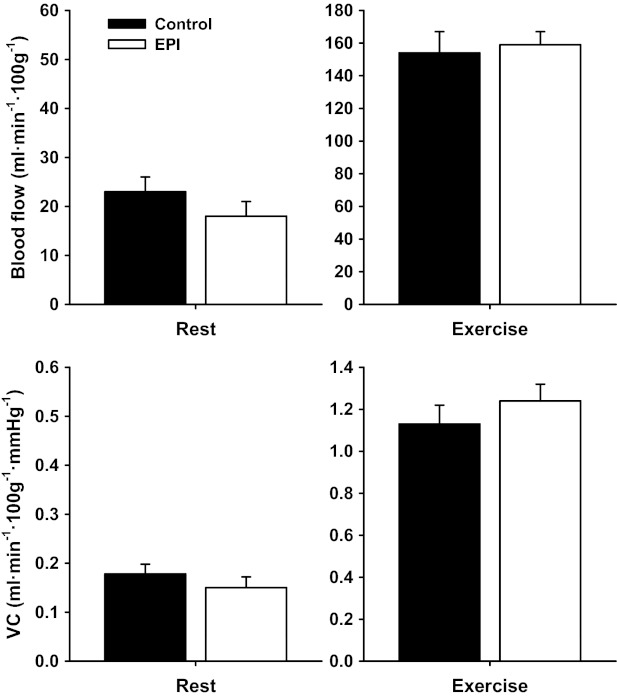
There were no differences in resting or exercising total hindlimb skeletal muscle blood flow (rest, P = 0.67; and exercise, P = 0.68) or VC (rest, P = 0.76; and exercise, P = 0.21) between control and EPI groups (Fig. 2). Neither were there differences in resting blood flow or VC (Table 1) or exercising blood flow (Table 2) in any of the 28 individual hindlimb muscles or muscle parts between groups. Exercising VC was greater (P < 0.05) in 5 of 28 individual muscles or muscle parts (tibialis posterior, red portion of the tibialis anterior, biceps femoris posterior, semitendinosus, and gracilis) in EPI rats compared with control (Table 2).
Fig. 2.
Total hindlimb muscle blood flow and vascular conductance (VC) at rest and during submaximal treadmill exercise for control and EPI rats. Within control and EPI groups, exercising blood flow and VC were significantly different (P < 0.05 for all) from rest. There were no differences between control and EPI groups. Note different y-axis scales for rest and exercise graphs. Data are means ± SE; control, n = 11; and EPI: n = 11.
Table 1.
Effects of EPI on resting blood flow and VC to the individual muscles or muscle parts of the rat hindlimb
| Blood Flow, ml·min−1·100 g−1 |
VC, ml·min−1·100 g−1·mmHg−1 |
|||
|---|---|---|---|---|
| Control | EPI | Control | EPI | |
| Ankle extensors | ||||
| Soleus | 138 ± 13 | 93 ± 22 | 1.09 ± 0.10 | 0.81 ± 0.20 |
| Plantaris | 26 ± 6 | 12 ± 3 | 0.18 ± 0.04 | 0.10 ± 0.02 |
| Gastrocnemius, red | 46 ± 9 | 34 ± 8 | 0.36 ± 0.06 | 0.28 ± 0.06 |
| Gastrocnemius, white | 12 ± 1 | 10 ± 1 | 0.09 ± 0.01 | 0.08 ± 0.01 |
| Gastrocnemius, mixed | 19 ± 3 | 14 ± 3 | 0.15 ± 0.02 | 0.12 ± 0.02 |
| Tibialis posterior | 21 ± 3 | 20 ± 5 | 0.17 ± 0.02 | 0.17 ± 0.04 |
| Flexor digitorum longus | 25 ± 6 | 20 ± 3 | 0.20 ± 0.05 | 0.16 ± 0.03 |
| Flexor halicus longus | 17 ± 3 | 12 ± 2 | 0.13 ± 0.02 | 0.10 ± 0.02 |
| Ankle flexors | ||||
| Tibialis anterior, red | 28 ± 5 | 25 ± 6 | 0.22 ± 0.04 | 0.22 ± 0.05 |
| Tibialis anterior, white | 21 ± 3 | 14 ± 3 | 0.16 ± 0.02 | 0.12 ± 0.02 |
| Extensor digitorum longus | 16 ± 2 | 17 ± 4 | 0.13 ± 0.01 | 0.15 ± 0.03 |
| Peroneals | 18 ± 3 | 18 ± 3 | 0.14 ± 0.02 | 0.15 ± 0.03 |
| Knee extensors | ||||
| Vastus intermedius | 125 ± 26 | 87 ± 20 | 0.97 ± 0.20 | 0.75 ± 0.17 |
| Vastus medialis | 31 ± 8 | 22 ± 7 | 0.24 ± 0.06 | 0.18 ± 0.05 |
| Vastus lateralis, red | 78 ± 18 | 53 ± 11 | 0.62 ± 0.14 | 0.45 ± 0.10 |
| Vastus lateralis, white | 16 ± 2 | 12 ± 2 | 0.12 ± 0.02 | 0.10 ± 0.02 |
| Vastus lateralis, mixed | 26 ± 5 | 17 ± 4 | 0.21 ± 0.04 | 0.14 ± 0.03 |
| Rectus femoris, red | 39 ± 11 | 18 ± 5 | 0.31 ± 0.09 | 0.16 ± 0.05 |
| Rectus femoris, white | 20 ± 4 | 13 ± 3 | 0.16 ± 0.03 | 0.11 ± 0.03 |
| Knee flexors | ||||
| Biceps femoris, anterior | 12 ± 2 | 9 ± 2 | 0.09 ± 0.01 | 0.08 ± 0.02 |
| Biceps femoris, posterior | 13 ± 1 | 12 ± 3 | 0.10 ± 0.01 | 0.10 ± 0.02 |
| Semitendinosus | 15 ± 2 | 21 ± 4 | 0.12 ± 0.02 | 0.18 ± 0.04 |
| Semimembranosus, red | 18 ± 3 | 18 ± 3 | 0.14 ± 0.02 | 0.15 ± 0.03 |
| Semimembranosus, white | 13 ± 1 | 10 ± 2 | 0.10 ± 0.01 | 0.08 ± 0.01 |
| Thigh adductors | ||||
| Adductor longus | 162 ± 12 | 155 ± 15 | 1.28 ± 0.09 | 1.29 ± 0.11 |
| Adductor magnus & brevis | 21 ± 3 | 16 ± 3 | 0.16 ± 0.02 | 0.14 ± 0.02 |
| Gracilis | 16 ± 2 | 21 ± 4 | 0.13 ± 0.02 | 0.17 ± 0.03 |
| Pectinius | 37 ± 5 | 39 ± 8 | 0.29 ± 0.04 | 0.32 ± 0.06 |
Values are means ± SE; control, n = 11; and (−)-epicatechin (EPI), n = 11. There were no differences between control and EPI for any muscle or muscle part. VC, vascular conductance.
Table 2.
Exercising individual hindlimb skeletal muscle and muscle part blood flow and VC for control and EPI groups
| Blood Flow, ml·min−1·100 g−1 |
VC, ml·min−1·100 g−1·mmHg−1 |
|||
|---|---|---|---|---|
| Control | EPI | Control | EPI | |
| Ankle extensors | ||||
| Soleus | 340 ± 23 | 326 ± 33 | 2.50 ± 0.17 | 2.53 ± 0.27 |
| Plantaris | 317 ± 24 | 284 ± 16 | 2.33 ± 0.17 | 2.21 ± 0.13 |
| Gastrocnemius, red | 490 ± 45 | 421 ± 32 | 3.59 ± 0.32 | 3.27 ± 0.26 |
| Gastrocnemius, white | 79 ± 24 | 72 ± 15 | 0.57 ± 0.17 | 0.58 ± 0.13 |
| Gastrocnemius, mixed | 211 ± 20 | 194 ± 12 | 1.55 ± 0.14 | 1.51 ± 0.10 |
| Tibialis posterior | 186 ± 23 | 223 ± 20 | 1.37 ± 0.16 | 1.73 ± 0.16* |
| Flexor digitorum longus | 121 ± 31 | 154 ± 26 | 0.88 ± 0.22 | 1.22 ± 0.21 |
| Flexor halicus longus | 112 ± 20 | 116 ± 6 | 0.82 ± 0.14 | 0.91 ± 0.05 |
| Ankle flexors | ||||
| Tibialis anterior, red | 424 ± 34 | 489 ± 46 | 3.14 ± 0.28 | 3.82 ± 0.38* |
| Tibialis anterior, white | 149 ± 15 | 159 ± 15 | 1.12 ± 0.12 | 1.25 ± 0.13 |
| Extensor digitorum longus | 92 ± 13 | 84 ± 8 | 0.68 ± 0.10 | 0.66 ± 0.07 |
| Peroneals | 177 ± 23 | 187 ± 15 | 1.29 ± 0.16 | 1.46 ± 0.12 |
| Knee extensors | ||||
| Vastus intermedius | 461 ± 44 | 481 ± 41 | 3.39±.032 | 3.73 ± 0.32 |
| Vastus medialis | 240 ± 22 | 241 ± 26 | 1.77 ± 0.17 | 1.88 ± 0.21 |
| Vastus lateralis, red | 471 ± 44 | 453 ± 39 | 3.48 ± 0.33 | 3.50 ± 0.29 |
| Vastus lateralis, white | 46 ± 10 | 35 ± 4 | 0.34 ± 0.07 | 0.28 ± 0.04 |
| Vastus lateralis, mixed | 234 ± 33 | 200 ± 17 | 1.71 ± 0.23 | 1.55 ± 0.13 |
| Rectus femoris, red | 339 ± 29 | 320 ± 31 | 2.48 ± 0.20 | 2.48 ± 0.24 |
| Rectus femoris, white | 157 ± 18 | 136 ± 12 | 1.15 ± 0.13 | 1.06 ± 0.10 |
| Knee flexors | ||||
| Biceps femoris anterior | 79 ± 22 | 57 ± 10 | 0.57 ± 0.15 | 0.45 ± 0.08 |
| Biceps femoris posterior | 122 ± 16 | 148 ± 14 | 0.89 ± 0.12 | 1.16 ± 0.13* |
| Semitendinosus | 76 ± 8 | 94 ± 10 | 0.56 ± 0.06 | 0.74 ± 0.09* |
| Semimembranosus, red | 171 ± 22 | 177 ± 16 | 1.25 ± 0.16 | 1.39 ± 0.15 |
| Semimembranosus, white | 47 ± 9 | 59 ± 8 | 0.34 ± 0.06 | 0.47 ± 0.08 |
| Hip adductos | ||||
| Adductor longus | 350 ± 36 | 365 ± 23 | 2.57 ± 0.25 | 2.84 ± 0.20 |
| Adductor magnus & brevis | 121 ± 12 | 123 ± 9 | 0.89 ± 0.09 | 0.96 ± 0.08 |
| Gracilis | 57 ± 10 | 75 ± 10 | 0.42 ± 0.07 | 0.60 ± 0.09* |
| Pectinius | 83 ± 22 | 97 ± 14 | 0.60 ± 0.16 | 0.76 ± 0.12 |
Values are means ± SE; control, n = 11; and EPI, n = 11. Within control and EPI groups, exercising blood flow and VC were significantly elevated above rest for all 28 individual hindlimb muscles and muscle parts (P > 0.05 for all).
P < 0.05 vs. control.
There were no differences in renal blood flow or VC at rest or during exercise between control and EPI groups (P > 0.05 for all, Table 3). There were no differences in blood flow or VC at rest or during exercise in the majority of the organs of the splanchnic region (Table 3). The exceptions were: 1) resting blood flow and VC were lower in the large intestine and liver, and 2) exercising blood flow was lower in the large intestine only for EPI versus control.
Table 3.
Renal and splanchnic organ blood flow and VC at rest and during exercise for control and EPI groups
| At Rest |
During Exercise |
|||||||
|---|---|---|---|---|---|---|---|---|
| Blood flow, ml·min−1·100 g−1 |
VC, ml·min−1·100 g−1·mmHg−1 |
Blood flow, ml·min−1·100 g−1 |
VC, ml·min−1·100 g−1·mmHg−1 |
|||||
| Control | EPI | Control | EPI | Control | EPI | Control | EPI | |
| Kidney | 619 ± 33 | 635 ± 35 | 4.94 ± 0.31 | 5.37 ± 0.36 | 416 ± 50† | 421 ± 61† | 3.06 ± 0.37† | 3.23 ± 0.45† |
| Stomach | 142 ± 10 | 170 ± 17 | 1.16 ± 0.09 | 1.44 ± 0.15 | 71 ± 8† | 97 ± 20† | 0.52 ± 0.06† | 0.75 ± 0.15† |
| Spleen | 418 ± 61 | 316 ± 46 | 3.29 ± 0.48 | 2.65 ± 0.39 | 64 ± 12† | 46 ± 10† | 0.47 ± 0.09† | 0.35 ± 0.08† |
| Pancreas | 184 ± 14 | 262 ± 95 | 1.47 ± 0.13 | 2.17 ± 0.76 | 155 ± 25 | 134 ± 18 | 1.15 ± 0.19 | 1.04 ± 0.14† |
| Small intestine | 477 ± 34 | 455 ± 48 | 3.81 ± 0.31 | 3.84 ± 0.43 | 279 ± 34† | 261 ± 34† | 2.04 ± 0.23† | 2.00 ± 0.25† |
| Large intestine | 243 ± 19 | 170 ± 21* | 1.94 ± 0.17 | 1.43 ± 0.18* | 154 ± 19† | 96 ± 16*† | 1.14 ± 0.15† | 0.74 ± 0.11† |
| Liver‡ | 26 ± 6 | 13 ± 3* | 0.20 ± 0.04 | 0.11 ± 0.02* | 23 ± 4† | 21 ± 3† | 0.17 ± 0.31 | 0.17 ± 0.02† |
Values are means ± SE; control, n = 11; and EPI, n = 11.
Denotes arterial, not portal, blood flow.
P < 0.05 vs. control;
P < 0.05 vs. rest.
Protocol 2: Spinotrapezius Muscle Po2mv
MAP and HR.
MAP was lower in the EPI group compared with control before (P = 0.007) and during spinotrapezius muscle contractions (P = 0.007, Fig. 1). HR before (control, 380 ± 20; and EPI, 381 ± 13 beats/min, P = 0.97) and during contractions (control, 382 ± 20; and EPI, 374 ± 6 beats/min, P = 0.74) was not different between groups.
Po2mv kinetics parameters.
There were no differences in resting baseline Po2mv, Po2mv kinetics parameters following the onset of contractions (i.e., speed and amplitude of the response), or contracting steady-state Po2mv between control and EPI rats (Table 4, Fig. 3). There were no differences in the Po2mv-to-MAP ratio at rest (control, 0.28 ± 0.02; and EPI, 0.34 ± 0.04, P = 0.14) or during the contracting steady-state (control, 0.17 ± 0.02; and EPI, 0.23 ± 0.03, P = 0.12) between groups.
Table 4.
Spinotrapezius muscle Po2mv resting baseline and kinetics parameters for control and EPI groups
| Control | EPI | |
|---|---|---|
| Po2mv(BL), mmHg | 33.2 ± 2.5 | 33.4 ± 2.6 |
| Δ1Po2mv, mmHg | 15.1 ± 1.1 | 14.4 ± 1.3 |
| Δ2Po2mv, mmHg | 4.5 ± 0.8 | 4.3 ± 0.9 |
| ΔtotalPo2mv, mmHg | 18.2 ± 1.9 | 19.4 ± 3.2 |
| Po2mv(steady state), mmHg | 21.3 ± 2.0 | 20.0 ± 2.8 |
| TD1, s | 7.9 ± 1.3 | 7.8 ± 1.1 |
| TD2, s | 41.1 ± 3.6 | 57.3 ± 11.4 |
| τ1, s | 17.4 ± 2.6 | 14.0 ± 3.2 |
| τ2, s | 53.0 ± 12.3 | 41.0 ± 7.5 |
| MRT1, s | 25.2 ± 2.8 | 22.0 ± 2.2 |
Values are means ± SE; control, n = 7 of 10; and EPI, n = 7 of 10. Where second component model averages are shown, the value reflects only those rats where a two-component model was applied to describe the microvascular O2 pressure (Po2mv) data. Po2mv(BL), precontracting Po2mv; Δ1Po2mv, amplitude of the first component; Δ2Po2mv, amplitude of the second component; ΔtotalPo2mv, overall amplitude regardless of one- or two-component model fit; Po2mv(steady-state), contracting steady-state Po2mv; TD1, time delay for the first component; TD2, time delay for the second component; τ1, time constant for the first component; τ2, time constant for the second component; MRT1, mean response time describing the kinetics response of the primary component. There were no differences between control and EPI for any kinetics parameter.
Fig. 3.
Average microvascular O2 pressure (Po2mv) profiles during electrically induced contractions of the spinotrapezius muscle in control and EPI rats. There were no differences between control and EPI at any time point. The onset of contractions at time 0 is indicated by the vertical dashed line. Data are means ± SE; control, n = 10; and EPI, n = 10.
DISCUSSION
EPI administration (4 mg·kg−1·day−1) reduced MAP in healthy rats at rest, during treadmill exercise, and before and during electrically induced muscle contractions. Despite these significant reductions in systemic driving pressure for bulk O2 delivery, EPI did not impair skeletal muscle blood flow at rest or during exercise (and actually resulted in higher exercising VC in 5 of 28 individual hindlimb muscles or muscle parts) or alter resting or contracting muscle Po2mv. In this regard, our data are consistent with the burgeoning literature demonstrating the blood pressure-lowering effects of EPI consumption. However, despite these implications for improvements in global cardiovascular function and contrary to our original hypotheses, EPI administration did not augment skeletal muscle O2 delivery or muscle microvascular oxygenation during exercise or muscle contractions, respectively, and did not enhance exercise endurance capacity or V̇o2 peak.
EPI Administration, Cardiovascular Hemodynamics, and Muscle Oxygenation (Po2mv)
The bulk of the existing literature supports the blood pressure-lowering effects of high-flavanoid cocoa consumption (18, 19, 24, 25, 28). Although cocoa-based supplements and dark chocolate typically contain several different classes of flavanoids, cocoa-induced reductions in blood pressure have been specifically attributed to the quantity of EPI consumption (9, 24, 40). The reduced MAP reported presently was likely the result of slight improvements in peripheral vascular function given that similar HRs and skeletal muscle blood flows between control and EPI groups suggest unchanged cardiac outputs. Furthermore, resting VC was not elevated in any tissue measured (and actually reduced in the large intestine and liver), suggesting that reductions in systemic vascular resistance (inversely proportional to VC) may have occurred in unmeasured vascular beds such as within trunk/front limb muscles, skin, and/or bone, etc. During exercise, the ∼5% lower MAP in the EPI group is likely attributable, in part, to the elevated VC found in 5 of 28 muscles or muscle parts (accounting for ∼25% of the total hindlimb musculature). To our knowledge, the present investigation is the first to report lower MAP following EPI administration during locomotory running exercise. This has significant clinical implications since EPI administration may reduce the risk of acute exercise-induced cardiovascular ischemic events (i.e., myocardial infarction, stroke, etc.).
Despite the reduced MAP presently reported, there was no impairment in hindlimb skeletal muscle O2 delivery, which is generally consistent with improved peripheral vascular function. We originally hypothesized that EPI administration would enhance exercising blood flow and VC. These hypotheses were based on widespread reports that EPI enhances vasodilatory responses to endothelial agonists or NO donors (16, 17), augments reactive hyperemia (40), and the importance of NO in mediating increased blood flow and O2 delivery during exercise (22). Moreover, EPI administration in healthy middle-aged mice elevated skeletal muscle mitochondrial volume density and the capillary-to-fiber ratio (33), and direct arterial EPI infusion in healthy rats induced marked femoral artery vasodilation (34). The lack of elevations in blood flow and higher exercising VC in only 5 of 28 individual muscles or muscle parts presently found was therefore surprising and may reflect the exquisite O2 delivery/V̇o2 matching present during exercise in healthy subjects (27) or, more likely, only minimal effects of EPI administration on vascular function during exercise in healthy rats. Another consideration is that potential EPI-induced elevations in NO bioavailability may have disrupted crucial reactive oxygen species-mediated vasodilatory signals (i.e., hydrogen peroxide) (21), thereby masking improvements in NO-mediated vascular control. There is also the potential for augmented NO bioavailability to suppress reactive oxygen species-mediated intracellular adaptations (15), although these possibilities in regard to EPI administration have not yet been investigated.
The Po2mv baseline and kinetics parameters presently reported are consistent with those previously established for healthy rats in our laboratory (4). EPI administration did not reduce microvascular oxygenation (i.e., the Po2mv profile) despite significantly lower MAP before and during contractions. Similar to treadmill exercise, however, the lower pressures and preserved Po2mv did not translate to significant improvements in the Po2mv-to-MAP ratio (conceptually similar to VC). Therefore, these data are consistent with the resting and exercising blood flow data herein and support that EPI administration may provide modest cardiovascular benefits (i.e., reduced MAP and elevated exercising VC in 5 of 28 individual muscles and muscle parts) but does not enhance skeletal muscle O2 delivery in healthy rats. Presently, given the lack of elevated O2 delivery during exercise/muscle contractions, it is not surprising that exercise endurance capacity and V̇o2 peak were not different between control and EPI groups. The ∼18% decrease in exercise endurance capacity for both groups between pre- and posttreatment/control tests is likely attributable to the ∼20% increase in body mass over the same time period. Presently, this lack of EPI-induced effects on exercise endurance capacity is contrary to the demonstration that 15 days of EPI administration (2 mg/kg daily) elevates treadmill running time-to-exhaustion in mice (33), which may be attributable to animal (age and species) and/or protocol differences [i.e., exhaustion elicited in ∼40 min presently vs. ∼13–15 min in Nogueira et al. (33)].
Experimental Considerations
The present EPI dose (4 mg·kg−1·day−1 for 24 days) is greater than the dose that enhanced exercise endurance capacity, muscle fatigue resistance, and skeletal muscle mitochondrial volume density and capillarity in mice (2 mg·kg−1·day−1 for 15 days) as reported by Nogueira et al. (33). EPI (10 mg·kg−1·day−1) has been administered in rat models of hypertension where chronic pharmacological NO synthase inhibition (NG-nitro-l-arginine methyl ester) was concurrently administered (16, 17). Presently, considering the use of healthy rats with intact NO synthase function, we elected to match more closely the EPI dose of Nogueira et al. (33) who reported dramatic EPI-induced improvements in skeletal muscle oxidative capacity and exercise performance in healthy (albeit middle aged) mice. Indeed, preliminary studies in our laboratory (n = 4) matched the EPI dose administered to mice (2 mg·kg−1·day−1 for 15 days) by Nogueira and colleagues (33), and these rats demonstrated no differences in MAP, blood flow, VC, or Po2mv compared with control rats (S. W. Copp, T. Inagaki, D. M. Hirai, D. C. Poole, and T. I. Musch, unpublished observations). Therefore, we elected to administer the higher dose (4 mg·kg−1·day−1) for 21 days as reported herein.
We examined the effects of EPI administration on exercising vascular control in healthy young adult rats. It is possible that significant EPI-induced improvements in exercising vascular control and contracting muscle microvascular oxygenation would manifest in rat models that demonstrate peripheral vascular dysfunction and exercise intolerance due, at least in part, to reduced NO bioavailability. These models include, for example, senescent (33), type-2 diabetic (5), and/or heart failure (10, 22) rats. Indeed, age differences may account for the disparities between the present data (∼4- to 5-mo-old rats) and that of Nogueira et al. [12-mo-old mice (33)], although caged and sedentary rodent models may more closely represent a model of physical inactivity versus a healthy physiological model (38). Investigating the effects of EPI in models in which vascular dysfunction is present is particularly appealing given that EPI consumption may reduce the proinflammatory cytokines IL-1β and TNF-α, plasma endothelin-1, and NADPH oxidase activity in vascular tissue (16, 17), signaling pathways that have been associated with vascular dysfunction in various pathological conditions (36, 41).
Conclusions
EPI administration (4 mg·kg−1·day−1 for 24 days) in healthy young adult rats resulted in a lower resting and exercising MAP compared with control rats. Despite the lower driving pressure for bulk O2 delivery (i.e., MAP), resting and exercising skeletal muscle blood flow and Po2mv were not impaired. However, contrary to our hypothesis, the lack of changes in blood flow and microvascular oxygenation during exercise/muscle contractions with EPI administration were associated with unimproved exercise endurance capacity and V̇o2 peak. In this regard, the present data demonstrate that direct EPI administration provides general cardiovascular benefits (i.e., lower resting and exercising MAP) but does not impact skeletal muscle O2 delivery or exercise performance in the healthy rat at least under the conditions evaluated herein.
GRANTS
S. W. Copp is supported by an American Heart Association Midwest Affiliate Predoctoral Fellowship. Experiments were funded by an American College of Sports Medicine Foundation Doctoral grant (to S. W. Copp); Kansas State University SMILE award (to T. I. Musch); and American Heart Association Midwest Affiliate Grant 10GRNT4350011 and National Heart, Lung, and Blood Institute Grant HL-108328 (D. C. Poole).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.W.C., T.I., D.M.H., D.C.P., and T.I.M. conception and design of research; S.W.C., T.I., M.J.W., D.M.H., S.K.F., C.T.H., G.E.S., and T.I.M. performed experiments; S.W.C. and T.I. analyzed data; S.W.C., T.I., M.J.W., D.M.H., S.K.F., C.T.H., G.E.S., D.C.P., and T.I.M. interpreted results of experiments; S.W.C. prepared figures; S.W.C. drafted manuscript; S.W.C., T.I., D.M.H., S.K.F., C.T.H., G.E.S., D.C.P., and T.I.M. edited and revised manuscript; S.W.C., T.I., M.J.W., D.M.H., S.K.F., C.T.H., G.E.S., D.C.P., and T.I.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank K. Sue Hageman and Hunter Jewett for excellent technical assistance.
REFERENCES
- 1. Armstrong RB, Hayes DA, Delp MD. Blood flow distribution in rat muscles during preexercise anticipatory response. J Appl Physiol 67: 1855–1861, 1989 [DOI] [PubMed] [Google Scholar]
- 2. Armstrong RB, Laughlin MH. Exercise blood flow patterns within and among rat muscles after training. Am J Physiol Heart Circ Physiol 246: H59–H68, 1984 [DOI] [PubMed] [Google Scholar]
- 3. Bailey JK, Kindig CA, Behnke BJ, Musch TI, Schmid-Schoenbein GW, Poole DC. Spinotrapezius muscle microcirculatory function: effects of surgical exteriorization. Am J Physiol Heart Circ Physiol 279: H3131–H3137, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Behnke BJ, Kindig CA, Musch TI, Koga S, Poole DC. Dynamics of microvascular oxygen pressure across the rest-exercise transition in rat skeletal muscle. Respir Physiol 126: 53–63, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol 511: 53–64, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Brooks GA, White TP. Determination of metabolic and heart rate responses of rats to treadmill exercise. J Appl Physiol 45: 1009–1015, 1978 [DOI] [PubMed] [Google Scholar]
- 7. Copp SW, Davis RT, Poole DC, Musch TI. Reproducibility of endurance capacity and V̇o2 peak in male Sprague-Dawley rats. J Appl Physiol 106: 1072–1078, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Ellinger S, Reusch A, Stehle P, Helfrich HP. Epicatechin ingested via cocoa products reduces blood pressure in humans: a nonlinear regression model with a Bayesian approach. Am J Clin Nutr 95: 1365–1377, 2012 [DOI] [PubMed] [Google Scholar]
- 10. Ferreira LF, Hageman KS, Hahn SA, Williams J, Padilla DJ, Poole DC, Musch TI. Muscle microvascular oxygenation in chronic heart failure: role of nitric oxide availability. Acta Physiol (Oxf) 188: 3–13, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Ferreira LF, Padilla DJ, Williams J, Hageman KS, Musch TI, Poole DC. Effects of altered nitric oxide availability on rat muscle microvascular oxygenation during contractions. Acta Physiol (Oxf) 186: 223–232, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Fisher ND, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens 21: 2281–2286, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Flaim SF, Nellis SH, Toggart EJ, Drexler H, Kanda K, Newman ED. Multiple simultaneous determinations of hemodynamics and flow distribution in conscious rat. J Pharmacol Methods 11: 1–39, 1984 [DOI] [PubMed] [Google Scholar]
- 14. Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44: 126–131, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Gomez-Guzman M, Jimenez R, Sanchez M, Romero M, O'Valle F, Lopez-Sepulveda R, Quintela AM, Galindo P, Zarzuelo MJ, Bailon E, Delpon E, Perez-Vizcaino F, Duarte J. Chronic (−)-epicatechin improves vascular oxidative and inflammatory status but not hypertension in chronic nitric oxide-deficient rats. Br J Nutr 106: 1337–1348, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Gomez-Guzman M, Jimenez R, Sanchez M, Zarzuelo MJ, Galindo P, Quintela AM, Lopez-Sepulveda R, Romero M, Tamargo J, Vargas F, Perez-Vizcaino F, Duarte J. Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension. Free Radic Biol Med 52: 70–79, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr 81: 611–614, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, Desideri G, Blumberg JB, Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 46: 398–405, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Hirai DM, Copp SW, Ferguson SK, Holdsworth CT, McCullough DJ, Behnke BJ, Musch TI, Poole DC. Exercise training and muscle microvascular oxygenation: functional role of nitric oxide. J Appl Physiol 113: 557–565, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirai DM, Copp SW, Schwagerl PJ, Musch TI, Poole DC. Acute effects of hydrogen peroxide on skeletal muscle microvascular oxygenation from rest to contractions. J Appl Physiol 110: 1290–1298, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol 77: 1288–1293, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Hirai T, Zelis R, Musch TI. Effects of nitric oxide synthase inhibition on the muscle blood flow response to exercise in rats with heart failure. Cardiovasc Res 30: 469–476, 1995 [PubMed] [Google Scholar]
- 24. Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 95: 740–751, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 88: 38–50, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Janszky I, Mukamal KJ, Ljung R, Ahnve S, Ahlbom A, Hallqvist J. Chocolate consumption and mortality following a first acute myocardial infarction: the Stockholm Heart Epidemiology Program. J Intern Med 266: 248–257, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Joyner MJ, Wilkins BW. Exercise hyperaemia: is anything obligatory but the hyperaemia? J Physiol 583: 855–860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal 15: 2779–2811, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leek BT, Mudaliar SR, Henry R, Mathieu-Costello O, Richardson RS. Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 280: R441–R447, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Lo LW, Vinogradov SA, Koch CJ, Wilson DF. A new, water soluble, phosphor for oxygen measurements in vivo. Adv Exp Med Biol 428: 651–656, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Musch TI, Bruno A, Bradford GE, Vayonis A, Moore RL. Measurements of metabolic rate in rats: a comparison of techniques. J Appl Physiol 65: 964–970, 1988 [DOI] [PubMed] [Google Scholar]
- 32. Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol 262: H411–H419, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Nogueira L, Ramirez-Sanchez I, Perkins GA, Murphy A, Taub PR, Ceballos G, Villarreal FJ, Hogan MC, Malek MH. (−)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J Physiol 589: 4615–4631, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ottaviani JI, Momma TY, Heiss C, Kwik-Uribe C, Schroeter H, Keen CL. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free Radic Biol Med 50: 237–244, 2011 [DOI] [PubMed] [Google Scholar]
- 35. Poole DC, Behnke BJ, McDonough P, McAllister RM, Wilson DF. Measurement of muscle microvascular oxygen pressures: compartmentalization of phosphorescent probe. Microcirculation 11: 317–326, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol 302: H1050–H1063, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F. (−)-Epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension 55: 1398–1405, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roberts MD, Company JM, Brown JD, Toedebusch RG, Padilla J, Jenkins NT, Laughlin MH, Booth FW. Potential clinical translation of juvenile rodent inactivity models to study the onset of childhood obesity. Am J Physiol Regul Integr Comp Physiol 303: R247–R258, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rumsey WL, Vanderkooi JM, Wilson DF. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science 241: 1649–1651, 1988 [DOI] [PubMed] [Google Scholar]
- 40. Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Nat Acad Sci USA 103: 1024–1029, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thorin E, Webb DJ. Endothelium-derived endothelin-1. Pflügers Arch 459: 951–958, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]