Abstract
PKA-mediated phosphorylation of contractile proteins upon β-adrenergic stimulation plays an important role in the regulation of cardiac performance. Phosphorylation of the PKA sites (Ser23/Ser24) of cardiac troponin (cTn)I results in a decrease in myofilament Ca2+ sensitivity and an increase in the rate of relaxation. However, the relation between the level of phosphorylation of the sites and the functional effects in the human myocardium is unknown. Therefore, site-directed mutagenesis was used to study the effects of phosphorylation at Ser23 and Ser24 of cTnI on myofilament function in human cardiac tissue. Serines were replaced by aspartic acid (D) or alanine (A) to mimic phosphorylation and dephosphorylation, respectively. cTnI-DD mimics both sites phosphorylated, cTnI-AD mimics Ser23 unphosphorylated and Ser24 phosphorylated, cTnI-DA mimics Ser23 phosphorylated and Ser24 unphosphorylated, and cTnI-AA mimics both sites unphosphorylated. Force development was measured at various Ca2+ concentrations in permeabilized cardiomyocytes in which the endogenous troponin complex was exchanged with these recombinant human troponin complexes. In donor cardiomyocytes, myofilament Ca2+ sensitivity (pCa50) was significantly lower in cTnI-DD (pCa50: 5.39 ± 0.01) compared with cTnI-AA (pCa50: 5.50 ± 0.01), cTnI-AD (pCa50: 5.48 ± 0.01), and cTnI-DA (pCa50: 5.51 ± 0.01) at ∼70% cTn exchange. No effects were observed on the rate of tension redevelopment. In cardiomyocytes from idiopathic dilated cardiomyopathic tissue, a linear decline in pCa50 with cTnI-DD content was observed, saturating at ∼55% bisphosphorylation. Our data suggest that in the human myocardium, phosphorylation of both PKA sites on cTnI is required to reduce myofilament Ca2+ sensitivity, which is maximal at ∼55% bisphosphorylated cTnI. The implications for in vivo cardiac function in health and disease are detailed in the discussion in this article.
Keywords: myofilament function, protein phosphorylation, cardiomyocyte, troponin I
during stress and exercise, sympathetic activation of the heart increases heart rate and stroke volume to meet the demands of the body. This is mediated via the stimulation of β1-adrenergic receptors, which leads to the activation of a downstream kinase, PKA. PKA enhances cardiomyocyte contraction and relaxation by phosphorylation of proteins involved in Ca2+ handling and myofilament proteins such as cardiac troponin (cTn)I, cardiac myosin-binding protein-C (cMyBP-C), and titin (for reviews, see Refs. 1 and 37).
PKA-mediated phosphorylation of myofilament proteins is thought to exert a positive lusitropic effect, which enables the heart to relax more rapidly when heart rate increases. This positive lusitropic effect may be induced by a decrease in myofilament Ca2+ sensitivity (32, 35, 51) and by enhanced cross-bridge cycling kinetics (11, 20, 33). It is well established (mainly from studies in rodents) that phosphorylation of cTnI at the PKA sites Ser23 and Ser24 leads to a decrease in myofilament Ca2+ sensitivity, through a conformational change of the troponin complex. This structural change reduces the affinity of Ca2+ binding to cTnC (16, 30). The role of phosphorylation of cTnI at the PKA sites as a regulator of cross-bridge cycling is less clear. Some studies (11, 20, 36) have reported an increase in cross-bridge kinetics via phosphorylation of cTnI. However, others (7, 33) have attributed an increase in cross-bridge kinetics to phosphorylation of cMyBP-C independent of cTnI phosphorylation, whereas several studies (8, 15, 17, 44) did not find an effect of PKA on cross-bridge kinetics at all. In the present study, we aimed to study the effect of site-specific phosphorylation of cTnI on myofilament Ca2+ sensitivity and cross-bridge kinetics in human cardiomyocytes since insights into the functional effects of cTnI phosphorylation and the relation between the level of phosphorylation and the functional effects in the human myocardium are lacking.
The effects of PKA-mediated phosphorylation on the contractile function of cardiomyocytes are challenging to study. First, Ca2+-handling and myofilament proteins are simultaneously phosphorylated by PKA. Triton permeabilization of cardiomyocytes eliminates the effects of Ca2+-handling proteins, which makes it possible to study the specific effects on myofilament proteins. Second, incubation of triton-permeabilized cardiomyocytes with exogenous PKA does not reveal the functional effects of site-specific cTnI phosphorylation as PKA phosphorylates multiple myofilament proteins. The collective effects are important for in vivo function, but to understand the effects in both healthy and diseased myocardium, information is required of each of the contributing factors. Finally, theoretically, four different PKA-phosphorylated cTnI forms could coexist: one unphosphorylated, two monophosphorylated (either on Ser23 or Ser24), and one bisphosphorylated (18, 34). A previous study (52) in rodents suggested that bisphosphorylation of cTnI is required for the reduction in myofilament Ca2+ sensitivity, but knowledge of the functional consequences of monophosphorylated cTnI also is important since recent studies (48, 50) in human postmortem control hearts and fresh donor transplant hearts with normal cardiac function revealed that ∼40% of cTnI is monophosphorylated at Ser23. Moreover, differences in the level of monophosphorylated cTnI have been reported between donor and end-stage failing hearts (41, 50).
We therefore studied site-specific functional effects of phosphorylation at both PKA sites separately and in combination in human cardiomyocytes. To this end, myofilament force development was measured at various Ca2+ concentrations in triton-permeabilized cardiomyocytes in which the endogenous troponin complex was exchanged with exogenous recombinant whole human troponin complexes. These cTn complexes contained unphosphorylated PKA sites, pseudo-monophosphorylated cTnI, and pseudo-bisphosphorylated cTnI. Ser23 and/or Ser24 were mutated into aspartic acid and into alanine to mimic phosphorylation and dephosphorylation, respectively. This approach allowed us to study the effect of cTnI phosphorylation on active [maximal force (Fmax)] and passive isometric force (Fpas), the force-Ca2+ relation, and the rate of force redevelopment (ktr; a measure of cross-bridge kinetics). In addition, cardiomyocytes from idiopathic dilated cardiomyopathic (IDCM) tissue were used for titration of the effect of pseudo-bisphosphorylated cTnI as these cardiomyocytes have a low level of cTnI bisphosphorylation at baseline (13, 41).
The results of our study suggests that phosphorylation of both PKA sites on cTnI is required to reduce Ca2+ sensitivity in human cardiomyocytes as no change in Ca2+ sensitivity was observed upon exchange with cTn complexes containing pseudo-monophosphorylated cTnI. The maximal reduction in myofilament Ca2+ sensitivity was reached at ∼55% bisphosphorylated cTnI. Fmax, Fpas, the steepness of the force-Ca2+ relation [Hill coefficient (nHill)], and cross-bridge kinetics were not significantly altered by pseudo-phosphorylation of cTnI at Ser23 and/or Ser24. The implications of these findings for in vivo cardiac function in health and disease are detailed in the discussion.
MATERIALS AND METHODS
Expression and purification of recombinant troponin subunits.
Four different cTnI forms were made via site-directed mutations of Ser23 and/or Ser24 into aspartic acid (D) to mimic phosphorylation or into alanine (A) to mimic dephosphorylation: pseudo-bisphosphorylated cTnI (cTnI-DD), pseudo-phosphorylated cTnI at only Ser23 (cTnI-DA) or only Ser24 (cTnI-AD), and unphosphorylated cTnI (cTnI-AA). cDNA encoding human cardiac isoforms [cTnC, cTnT, myc tag-labeled cTnT (cTnT-myc), cTnI, cTnI-AA, cTnI-AD, cTnI-DA, and cTnI-DD] were transformed in Esherichia coli Rosetta2 (27) and cultured under carbenicillin/chloramphenicol selection in Overnight Express TB medium (EMD Biosciences). Cultures were harvested by centrifugation, resuspended in PBS, and centrifuged at 10,000 g. Pellets were stored at −80°C until use.
Troponin subunits were purified using fast protein liquid chromatography (AKTA-FPLC System, Amersham Biosciences) essentially as previously described (27).
Reconstitution of troponin complexes.
Fractions containing equal ratios of cTnT, cTnC, and cTnI subunits were pooled and finally dialyzed against 10 mM imidazole, 200 mM KCl, 5 mM MgCl2, 2.5 mM EGTA, 1 mM DTT, and 0.1 mM PMSF (pH 6.9, 2 times, 1 liter each) before the complexes were concentrated to a final concentration of >2 mg/ml by centrifugation using Centriprep YM-10 centrifugal filters (Millipore).
Exchange of human troponin complex.
Exchange of recombinant cTn in human cardiomyocytes was done as previously described (5, 27) with minor modifications. Cardiomyocytes were isolated from three donor hearts and from an IDCM heart, which were obtained during heart transplantation surgery. Tissue was collected in cardioplegic solution and stored in liquid nitrogen. Samples were obtained after informed consent and with approval of the local Human Research Ethics Committee of The University of Sydney (no. 7326). This investigation conformed with the principles outlined in the Declaration of Helsinki (1997). The human cardiac samples have been extensively characterized (force characteristics and cTnI phosphorylation) in a previous study (13).
Single cardiomyocytes were mechanically isolated with a glass tissue homogenizer as previously described (27). Cardiomyocytes were subsequently incubated overnight at 4°C in exchange solution containing the appropriate concentration of recombinant human cTn complex (1.0 or 2.0 mg/ml in donor cells or a range between 0.0625 to 2.0 mg/ml in IDCM cardiomyocytes) with the addition of 4 mM CaCl2, 4 mM DTT, 5 μl/ml protease inhibitor cocktail (P8340, Sigma), 10 μl/ml phosphatase inhibitor cocktails 1 and 2 (P2850 and P5726, Sigma), and 50 nM calyculin A (C5552, Sigma) (pH 6.9). The next day, cardiomyocytes were washed twice in rigor solution and finally in relaxing solution (5.95 mM Na2ATP, 6.04 mM MgCl2, 2 mM EGTA, 139.6 mM KCl, and 10 mM imidazole, pH 7.0). It has been previously demonstrated that this method results in a homogenous distribution of recombinant cTn complex within the exchanged cardiomyocyte.
Determination of the degree of troponin exchange.
To determine the degree of cTn exchange and to assess the protein phosphorylation status, half of the suspension of cells was treated with a 2D-clean-up kit (GE Healthcare) as described by manufacturer's protocol after overnight cTn exchange. After treatment, tissue pellets were homogenized in sample buffer containing 15% glycerol, 62.5 mM Tris (pH 6.8), 1% (wt/vol) SDS, and 2% (wt/vol) DTT. Protein concentrations were measured with a RCDC Protein Assay Kit II (Bio-Rad) and ranged between 2 and 4 mg/ml.
Immunoblot analysis was used to determine the degree of exchange of endogenous cTn by the recombinant cTn complex. Therefore, recombinant cTnT was labeled with a myc tag to allow discrimination between endogenous and recombinant cTn complexes. Proteins were separated on a one-dimensional 13% SDS-polyacrylamide gel and blotted onto a nitrocellulose membrane (Hybond) using the protocol supplied by the manufacturer in 1 h at 75 V. A specific monoclonal antibody against cTnT (clone JLT-12, Sigma, dilution: 1:1,250) was used to detect endogenous and recombinant cTnT by enhanced chemiluminescence (Amersham Biosciences).
Myofilament protein phosphorylation.
Phosphorylation levels of sarcomeric proteins were determined before and after cTn exchange using ProQ diamond-stained one-dimensional gels, as previously described (49). Phosphorylation signals were normalized to the intensities of SYPRO ruby-stained myosin light chain (MLC)2 bands to correct for small differences in protein loading. To correct for differences in staining between gels, the PeppermintStick phosphoprotein marker (Molecular Probes) was used. The ratio of the intensities of the ProQ diamond- and SYPRO ruby-stained ovalbumin band was used to correct for intergel differences.
The distribution of endogenous phosphorylated species of cTnI in the donor hearts and in IDCM cardiomyocytes was analyzed using Phos-tag acrylamide gels (FMS Laboratory, Hiroshima University, Japan) as previously described (13, 24).
Isometric force measurements.
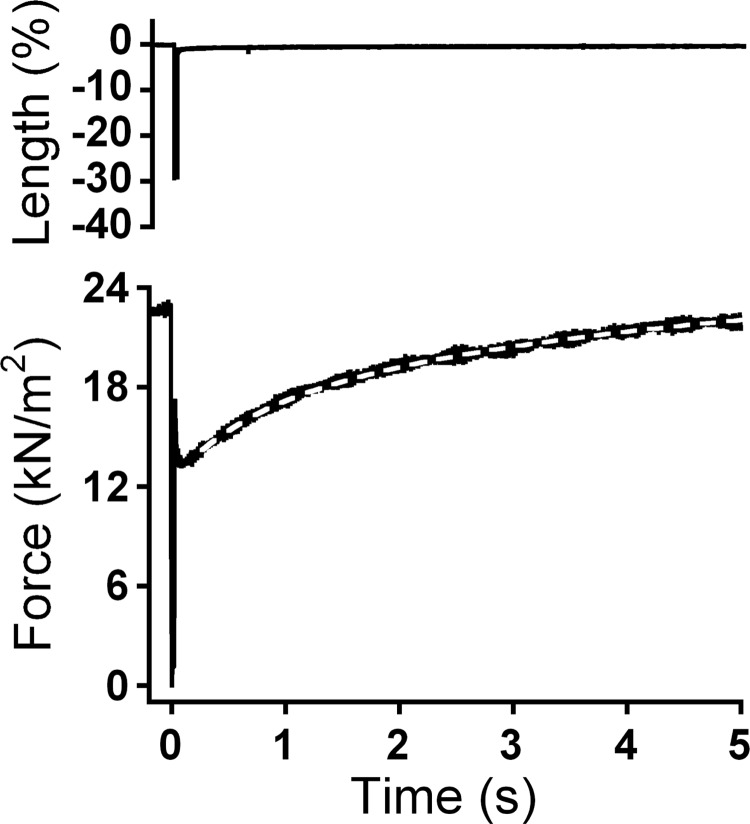
Force measurements in cardiomyocytes exchanged with pseudo-phosphorylated cTn complexes were performed as previously described (27). Isometric force was measured at 15°C and at a sarcomere length of 2.2 μm (40). The following parameters were determined: Fmax at pCa 4.5, Fpas at pCa 9.0, the Ca2+ sensitivity of force development (pCa50), nHill, and ktr at maximal and submaximal Ca2+ concentrations. ktr was determined using a slack-restretch test. Thereafter, after the cell had reached steady-state force in activating solution, it was shortened within 1 ms to 70% of its original length and restretched after 30 ms. As a result of this intervention, force first dropped to zero and then quickly redeveloped to the original steady-state level. A single exponential was fitted to force redevelopment to determine ktr.
Data analysis.
Data analysis was performed as previously described using the following Hill equation to fit force-Ca2+ relations: F(Ca2+)/F0 = [Ca2+]nHill/(Ca50nHill + [Ca2+]nHill), where F is steady-state force, F0 is the steady-state force at saturating Ca2+ concentration, nHill is the steepness of the relationship, and Ca50 (or pCa50) is the midpoint of the relation. Data in donor and failing cardiomyocytes were compared using one-way ANOVA followed by a Bonferroni post hoc test. Values are means ± SE; n is the number of myocytes.
RESULTS
Quantification of troponin exchange in human cardiomyocytes.
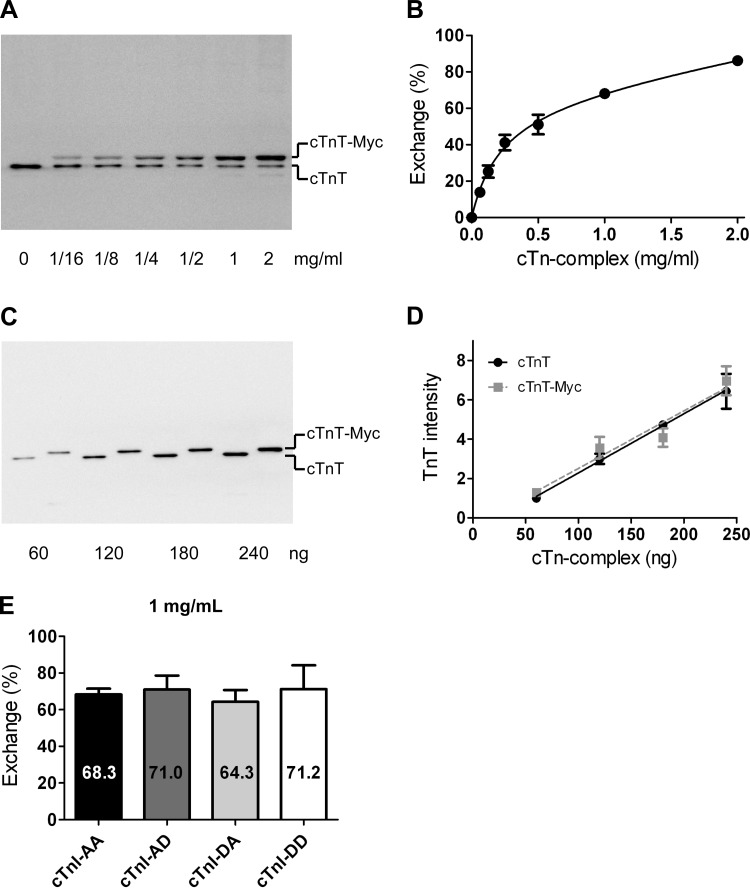
Figure 1A shows a representative immunoblot loaded with samples of cardiomyocytes incubated overnight with increasing concentrations (range: 0–2 mg/ml) of recombinant cTn. cTnT-myc migrated more slowly through the gel compared with endogenous cTnT, and, therefore, two cTnT bands were found. The percentage of cTn exchange was calculated from the ratio of cTnT-myc and the total amount of cTnT. In Fig. 1B, the percentage of exchanged cTn was plotted against the cTn concentration in the exchange solution. The results shown in Fig. 1, C and D, indicate that the affinity of the cTnT antibody was the same for cTnT compared with cTnT-myc and that cTnT loading (ranging from 60 to 240 ng cTn/lane) was within the linear range.
Fig. 1.
Quantification of troponin exchange in cardiomyocytes by immunoblotting. A: immunoblot stained with an antibody against cardiac troponin (cTn)T that recognizes both endogenous cTnT (bottom band) and recombinant myc tag-labeled cTnT (cTnT-myc; top band). The example shows a suspension of cardiomyocytes exchanged with increasing concentrations of recombinant cTn. B: average percentages of cTn exchange obtained during all exchange experiments plotted against the cTn concentration in the exchange solution. The following double-exponential curve was fitted to the data points: y = A[1 − exp(−x/k1)] + (100 − A)[1 − exp(−x/k2)], yielded an A value of 68.9%, a k1 value of 0.13 mg/ml, and a k2 value of 1.29 mg/ml. Error bars are shown when larger than symbol size. C: immunoblot of recombinant cTn containing cTnT without (lanes 1, 3, 5, and 7) or with (lanes 2, 4, 6, and 8) the Myc tag label. D: average cTnT intensities of four blots plotted against the cTn amount (in ng) loaded per lane for cTnT- and cTnT-myc-containing cTn complexes. Linear regression analysis indicated that the slopes and intercepts did not significantly differ. E: average percentages of cTn exchange in cardioymyocytes after an overnight incubation in exchange solution with 1 mg/ml cTn containing the different pseudo-phosphorylated cTnI species (average values represent cTn exchange experiments in cardiomyocytes isolated from 3 donor hearts). No significant differences were found in exchange percentages between the various cTnI complexes. cTnI-AA, pseudo-dephosphorylated cTnI; cTnI-AD, pseudo-monophosphorylation of Ser24; cTnI-DA, pseudo-monophosphorylation of Ser23; cTnI-DD, pseudo-bisphosphorylated cTnI.
No significant differences in the percentages of cTn exchange were found between cardiomyocytes with the different recombinant cTn complexes. This indicates that the different pseudo-phosphorylated cTn complexes incorporated similarly in the myofilaments. The percentage of exchange with the four pseudo-phosphorylated cTn complexes at a concentration of 1 mg/ml in three donor samples was, on average, 68.7 ± 1.6% (Fig. 1E). At a concentration of 2 mg/ml cTn during overnight incubation, the average percentage of exchange amounted to 86.2 ± 3.5% in cardiomyocytes isolated from three donor samples.
cTnI phosphorylation after exchange.
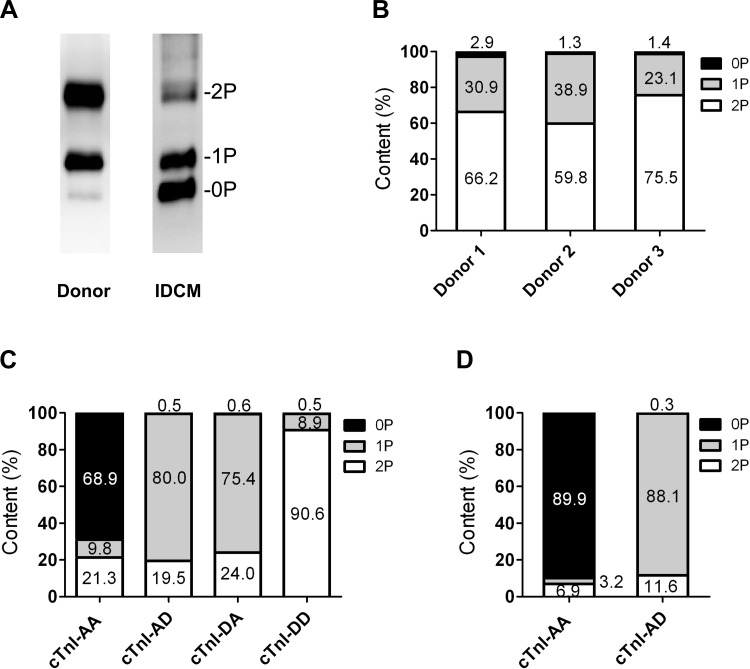
The endogenous cTnI phosphorylation as assessed by Phos tag analysis (Fig. 2, A and B) showed an average of 67.2 ± 4.6% bisphosphorylated cTnI, 31.0 ± 4.6% monophosphorylated cTnI, and 1.8 ± 0.5% unphosphorylated cTnI in the three donor hearts used in the exchange experiments (Fig. 2B), whereas unphosphorylated (60.3%) and monophosphorylated (33.1%) cTnI were the most abundant forms in the IDCM sample used (Fig. 2A). Figure 2, C (1 mg/ml) and D (2 mg/ml), shows the percentages of unphosphorylated (endogenous unphosphorylated cTnI plus recombinant cTnI-AA), monophosphorylated (endogenous monophosphorylated cTnI plus recombinant cTnI-AD or DA), and bisphosphorylated (endogenous bisphosphorylated cTnI plus recombinant cTnI-DD) cTnI species after exchange calculated on the basis of the cTn exchange percentage determined with immunoblot analyses (Fig. 1E). ProQ Diamond-stained gels demonstrated that no significant differences in the level of phosphorylation of other myofilament proteins (cMyBP-C, desmin, and MLC2) were induced upon exchange with the various cTn complexes (Table 1).
Fig. 2.
cTnI (pseudo-)phosphorylation levels after exchange. A: Phos tag-stained gel showing cTn phosphorylation at baseline (i.e., before exchange). B: distribution of phosphospecies of the three donor samples used in the exchange experiments. C (1 mg/ml) and D (2 mg/ml): percentages of unphosphorylated (0P; endogenous unphosphorylated cTnI plus recombinant cTnI-AA), monophosphorylated (1P; endogenous monophosphorylated cTnI plus recombinant cTnI-AD/DA), and bisphosphorylated (2P; endogenous bisphosphorylated cTnI plus recombinant cTnI-DD) cTnI species after exchange calculated with the cTn exchange percentages determined with immunoblot analyses (as shown in Fig. 1E). IDCM, ideopathic dilated cardiomyopathic sample.
Table 1.
Overview of myofilament protein phosphorylation before and after exchange
| Cardiac Myosin-Binding Protein-C | Desmin | Myosin Light Chain 2 | |
|---|---|---|---|
| Before exchange | 0.60 ± 0.07 | 0.27 ± 0.02 | 0.25 ± 0.04 |
| Control exchange | 0.58 ± 0.07 | 0.26 ± 0.03 | 0.31 ± 0.01 |
| cTnI-AA | 0.56 ± 0.04 | 0.24 ± 0.06 | 0.31 ± 0.03 |
| cTnI-AD | 0.54 ± 0.05 | 0.24 ± 0.05 | 0.28 ± 0.02 |
| cTnI-DA | 0.58 ± 0.11 | 0.29 ± 0.04 | 0.27 ± 0.02 |
| cTnI-DD | 0.55 ± 0.03 | 0.23 ± 0.06 | 0.25 ± 0.02 |
Values are means ± SE of the ProQ/SYPRO intensity ratio. Shown is myofilament protein phosphorylation determined before and after exchange in three donor samples via ProQ diamond staining. Control exchange cardiomyocytes were kept overnight in exchange solution without recombinant cardiac troponin (cTn)I added. No significant effect was found of the control exchange protocol on myofilament protein phosphorylation compared with before exchange (by Student's t-test). cTn exchange at 1 mg/ml also did not affect myofilament protein phosphorylation (by one-way ANOVA comparing control exchange and the four cTnI exchange groups). A, alanine substitution; D, aspartic acid substitution.
Comparison of functional parameters after exchange with cTn with or without the myc tag.
To test whether the myc tag, added to cTnT as a tool to quantify cTn exchange, interfered with myofilament function, recombinant cTn complex containing cTnT or cTnT-myc was exchanged in donor cardiomyocytes. No significant differences were found between cells (2 donors, 6–8 cells) exchanged with these complexes (Table 2), demonstrating that the myc tag did not influence any of the functional parameters studied.
Table 2.
Force measurements in cardiomyocytes after exchange with recombinant cTnI
| Fmax | Fpas | pCa50 | nHill | ktr-max | |
|---|---|---|---|---|---|
| 1 mg/ml cTn | |||||
| cTnI-Wt | 20.6 ± 1.7 | 3.3 ± 0.5 | 5.54 ± 0.02 | 2.6 ± 0.2 | 0.59 ± 0.04 |
| cTnI-Wt (myc) | 20.0 ± 1.8 | 3.3 ± 0.4 | 5.51 ± 0.02 | 3.0 ± 0.1 | 0.63 ± 0.03 |
| cTnI-AA (myc) | 24.6 ± 1.5 | 3.2 ± 0.2 | 5.50 ± 0.01 | 3.2 ± 0.1 | 0.59 ± 0.03 |
| cTnI-AD (myc) | 23.3 ± 1.8 | 3.3 ± 0.3 | 5.48 ± 0.01 | 3.1 ± 0.1 | 0.50 ± 0.04 |
| cTnI-DA (myc) | 22.3 ± 1.4 | 3.2 ± 0.3 | 5.51 ± 0.01 | 3.3 ± 0.1 | 0.55 ± 0.02 |
| cTnI-DD (myc) | 20.9 ± 1.3 | 3.2 ± 0.4 | 5.39 ± 0.01*** | 3.6 ± 0.3 | 0.60 ± 0.06 |
| 2 mg/ml cTn | |||||
| cTnI-AA (myc) | 24.4 ± 1.0 | 2.6 ± 0.2 | 5.48 ± 0.01 | 3.3 ± 0.2 | 0.55 ± 0.02 |
| cTnI-AD (myc) | 22.7 ± 1.2 | 3.2 ± 0.2 | 5.47 ± 0.02 | 3.0 ± 0.1 | 0.52 ± 0.04 |
Values are means ± SE. Recombinant wild-type (Wt) cTn complexes with or without the myc tag were exchanged at 1 mg/ml in two donor samples (6–8 myocytes/group). No significant differences were found in the force parameters between cTn complexes with or without the myc tag label. Myc tag-labeled cTnI mutated at Ser23 and Ser24 into alanine or aspartic acid was exchanged at 1 mg/ml (13 myocytes/cTnI mutant) in three donor samples. The midpoint of the Ca2+ sensitivity of force development (pCa50) was significantly lower in cTnI-DD compared with cTnI-AA, cTnI-AD, or cTnI-DA. Myc tag-labeled cTn-AA and cTn-AD were exchanged at 2 mg/ml (11 myocytes/cTnI mutant) in three donor samples. No significant differences were found in the maximal rate of force development (ktr-max; in s−1) at saturating Ca2+ concentration (pCa 4.5) between cTn-AA and cTn-AD (as analyzed by a Student's t-test). Fmax, maximal force (in kN/m2); Fpas, passive force (in kN/m2); nHill, Hill coefficient (steepness of the force-pCa curve).
P < 0.0001 (by one-way ANOVA followed by a Bonferroni post hoc test).
Effects of pseudo-phosphorylated cTn on myofilament Ca2+ sensitivity.
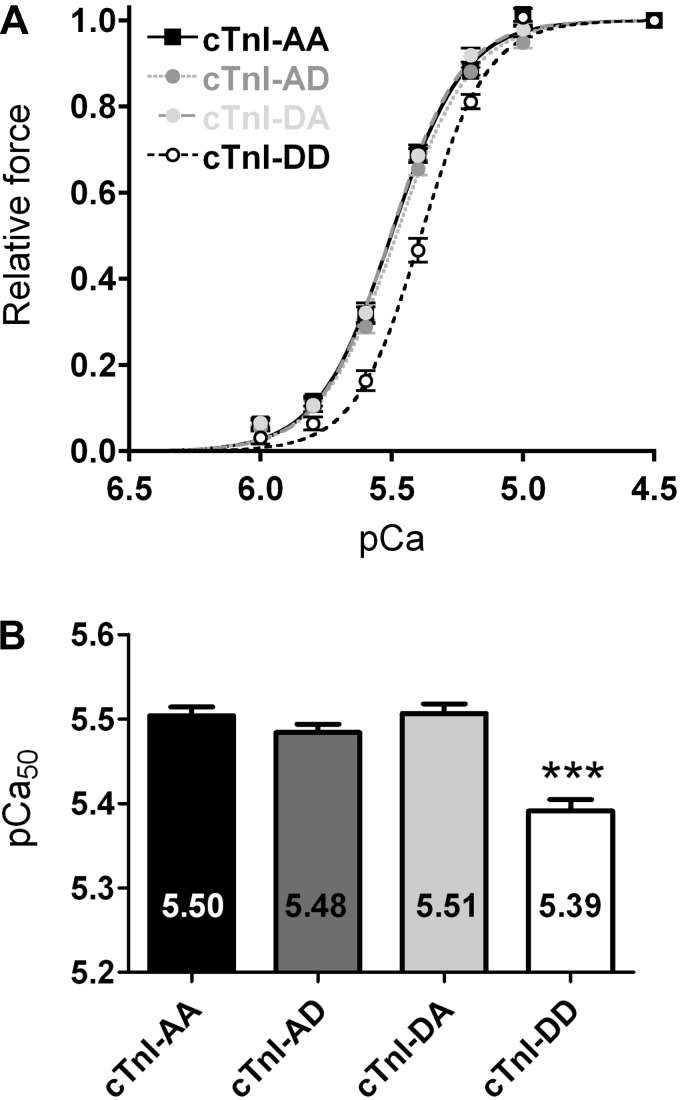
The effect of site-specific pseudo-phosphorylation of PKA sites Ser23 and Ser24 on pCa50 was measured at various Ca2+ concentrations in permeabilized donor cardiomyocytes (each complex: 3 donor hearts, 13 cells) in which the endogenous cTn complex was partially exchanged with recombinant cTn complexes (1 mg/ml cTn complex; Figs. 1E and 2C). Endogenous cTnI phosphorylation was largely removed by exchange with nonphosphorylated recombinant cTn complex (Fig. 2C) and enabled us to compare the functional effects of the four different (un)phosphorylated cTn complexes in donor myocardium. Compared with unphosphorylated cTnI (cTnI-AA), myofilament Ca2+ sensitivity was significantly lower after exchange with pseudo-phosphorylated cTnI at both sites (cTnI-DD), as evident from the rightward shift of the force-pCa curve for cTnI-DD compared with cTnI-AA (Fig. 3, A and B). It can also be seen in Fig. 3 that the curves after cTn-DA, cTn-AD, and cTn-AA exchange were indistinguishable. This indicates that pseudo-monophosphorylation of the PKA sites did not influence Ca2+ sensitivity (Fig. 3, A and B, and Table 2) and suggests that bisphosphorylation is required to cause a change in Ca2+ sensitivity. The difference in pCa50 in donor cardiomyocytes upon exchange with cTnI-DD compared with the averaged pCa values of cells exchanged with cTnI-AA, cTnI-AD, or cTnI-DA was 0.11 ± 0.01 pCa units. In a previous study from our group (27), it has been shown that PKA treatment of human donor cardiomyocytes after exchange with wild-type unphosphorylated cTn decreased Ca2+ sensitivity to a similar extent (0.08 pCa units). The similar decrease in myofilament Ca2+ sensitivity observed in the present study of 0.11 pCa units reflects a change in EC50 of 0.91 μM between cTn-DD (pCa50: 5.39 and EC50: 4.07 μM) and nonphosphorylated cTnI-AA (pCa50: 5.50 and EC50: 3.16 μM; Fig. 3B), which is likely to be of functional significance. Note that myofilament Ca2+ sensitivity after exchange with unphosphorylated cTnI-AA and unphosphorylated wild-type cTn complex were the same (Table 2). This indicates that cTnI-AA exchange mimics the effects of exchange using the unphosphorylated Ser23- and Ser24-containing cTn complex. In addition, experiments were performed using 2 mg/ml cTn-AA and cTn-AD (3 donors, 11 cells) to increase the extent of cTn exchange (86.2 ± 4%) and thereby increase the level of (pseudo-)monophosphorylated cTnI to 88.1% (Fig. 2D). Also, at higher cTn exchange levels, pseudo-monophosphorylation of Ser24 (cTnI-AD) did not lower myofilament Ca2+ sensitivity compared with unphosphorylated cTnI (cTnI-AA; Table 2). This strengthens the observations at 1 mg/ml cTn that pseudo-monophosphorylation has no effect on Ca2+ sensitivity.
Fig. 3.
Ca2+ sensitivity of force development (pCa) is reduced only after phosphorylation of both Ser23 and Ser24 on cTnI. A: myofilament force development measured at various Ca2+ concentrations in permeabilized donor cardiomyocytes in which the endogenous cTn complex was partially exchanged (68.7 ± 2%) with 1 mg/ml of recombinant myc tag-labeled cTn complexes (13 cardiomyocytes from 3 donor hearts in all groups). B: compared with unphosphorylated cTnI (cTnI-AA), pCa derived from the midpoint of the force-pCa relationship (pCa50) was significantly reduced after exchange with pseudo-phosphorylated cTnI at both PKA sites (cTnI-DD). Exchange with cTnI-DA or cTnI-AD did not alter Ca2+ sensitivity compared with unphosphorylated cTnI. The reduced Ca2+ sensitivity upon exchange with cTnI-DD is evident from the rightward shift of the force-pCa curve in cTnI-DD-exchanged cardiomyocytes compared with cells exchanged with cTnI-AA, cTnI-AD, or cTnI-DA. ***P < 0.0001, cTnI-DD vs. all other complexes (by posttest Bonferroni analyses of one-way ANOVA).
Saturation of the effect of cTnI bisphosphorylation on myofilament Ca2+ sensitivity.
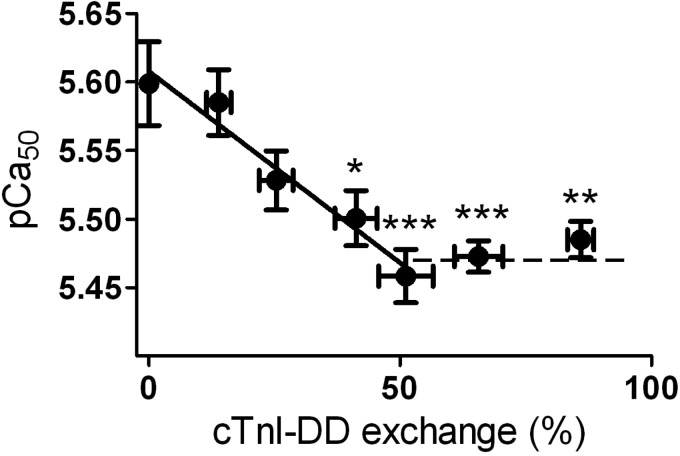
To test how much PKA-mediated cTnI bisphosphorylation is necessary to maximally reduce myofilament Ca2+ sensitivity, cTnI-DD was exchanged in IDCM cardiomyocytes. This was done at cTn concentrations between 0 and 2 mg/ml to vary the degree of exchange (4–5 cells/concentration). IDCM cardiomyocytes were used in these experiments because the endogenous levels of cTnI Ser23/Ser24 bisphosphorylation in the end-stage failing human myocardium are low compared with donor tissue. The results shown in Fig. 2A demonstrate, in agreement with previous studies (13, 41), that cTnI Ser23/Ser24 bisphosphorylation was low (∼7%) in the IDCM cardiomyocytes used. Comparison of the phosphorylation levels of other myofilament proteins in IDCM tissue (cMyBP-C: 0.39 ± 0.03, desmin: 0.23 ± 0.04, and MLC2: 0.21 ± 0.03) with the values in donor tissue (Table 1) also indicates that, in addition, cMyBP-C phosphorylation was lower in IDCM tissue compared with donor tissue. Cardiomyocytes incubated overnight in exchange solution without complex served as controls. cTnI-DD exchange caused a gradual decline in pCa50 saturating at 51 ± 5% of cTnI-DD (Fig. 4). The maximal decrease in pCa50 was 0.13 ± 0.02 units. This value corresponds well with the difference in pCa50 between cTnI-AA and cTnI-DD in donor cells. Assuming a linear decline in Ca2+ sensitivity (slope: 0.0028) between 0% and 51% of cTnI-DD exchange, it can be calculated that 3.6% of cTnI-DD is sufficient to reduce Ca2+ sensitivity by 0.01 pCa units.
Fig. 4.
Ca2+ sensitivity decreased maximally at ∼55% of bisphosphorylated cTnI Ser23/Ser24 in end-stage failing cardiomyocytes. Shown is the relation between bisphosphorylated cTnI and myofilament Ca2+ sensitivity in IDCM cardiomyocytes (4–5 cells) exchanged with different concentrations of recombinant cTn-DD (concentrations of 0.06125, 0.125, 0.25, 0.5, 1.0, and 2.0 mg/ml of cTnI-DD were used). Cardiomyocytes immersed in exchange solution without complex during the overnight exchange served as controls (0% cTn exchange). cTnI-DD exchange caused a gradual decline in pCa50 of 0.13 ± 0.03, saturating at 51 ± 5% of troponin exchange. *P < 0.05, **P < 0.01, and ***P < 0.001, control vs. cTnI-DD (by posttest Bonferroni analyses of one-way ANOVA). Solid line: pCa50 = 5.608 − 0.0028 × exchange percentage(cTnI-DD).
Effects of pseudo-phosphorylated cTn on Fmax, Fpas, and nHill.
Fmax, Fpas, and nHill were determined in permeabilized donor cardiomyocytes (3 donors, 13 cells at 1 mg/ml and 11 cells at 2 mg/ml) in which the endogenous cTn complex was exchanged with recombinant human cTn complexes. Fmax, Fpas, and nHill (Table 2) were not significantly different after exchange with cTn containing the mono- and bisphosphorylated cTnI species.
Effects of pseudo-phosphorylated cTn on ktr.
Maximal ktr at pCa 4.5 (ktr-max) after exchange with pseudo-unphosphorylated cTn did not differ from ktr-max after exchange with pseudo-monophosphorylated or pseudo-bisphosphorylated cTn (Table 2). However, because ktr-max showed a tendency to decrease in cTnI-AD, additional experiments were done at even higher exchange percentages (cTn concentration: 2 mg/ml) where cTnI-AA was compared with cTnI-AD (Table 2). In agreement with the results at 1 mg/ml exchange, ktr-max did not significantly differ between cTnI-AA and cTnI-AD. In addition, no significant differences in ktr values measured at submaximal Ca2+ concentrations were found after exchange with cTn containing mono- and bisphosphorylated cTnI species (data not shown). Note that ktr-max after exchange using cTnI-AA was the same as in the exchange using unphosphorylated wild-type cTnI (Table 2). The effect of pseudo-phosphorylation at the PKA sites on force redevelopment was also analyzed with a double-exponential equation (Fig. 5), as previously described by Caremani et al. (6). A fivefold difference in the rate constants of the fast phase (1.12 ± 0.07 s−1, n = 52) and slow phase (0.22 ± 0.03 s−1, n = 52) were observed. In agreement with the results of the monoexponential fit, no differences in the parameter values were observed between groups. On the basis of these data, it can be concluded that pseudo-phosphorylation at Ser23 and/or Ser24 did not affect ktr.
Fig. 5.
Determination of the rate of tension redevelopment (ktr). Maximal ktr at pCa 4.5 (0.57 s−1) was determined from a single exponential curve fit of force redevelopment after a slack-restretch test in activating solution. This example includes the results of a second-order curve fit (slow component of 0.19 s−1 and fast component of 1.50 s−1). The first-order and second-order curve fit overlapped, and, therefore, only the second-order fit is shown.
DISCUSSION
Both Ser23 and Ser24 need to be phosphorylated to reduce Ca2+ sensitivity.
The present study is the first to examine the effects of site-specific phosphorylation of cTnI- Ser23 and/or Ser24 in human cardiomyocytes. Our study suggests that (mono)phosphorylation of either Ser23 or Ser24 does not alter myofilament Ca2+ sensitivity and that both serines need to be phosphorylated to reduce the Ca2+ sensitivity of myofilament force. These results are compatible with a model proposed on the basis of biochemical studies (29) indicating that monophosphorylation and consecutive bisphosphorylation exert a stepwise effect on the affinity of binding between cTnI and cTnT as well as cTnC, whereas bisphosphorylation of cTnI causes a reduction in the affinity of cTnC for Ca2+. A more recent study (16) has provided insights on the conformational transitions within the cTn complex upon bisphosphorylation, but alternative models have been proposed as well (for a recent review, see Ref. 31).
In a previous study by Zhang et al. (52), a chimera of a mouse-bovine cTn complex with alanine mutations was exchanged in skinned porcine cardiac myocytes to mimic dephosphorylation at Ser23 and/or Ser24. PKA treatment of porcine cells containing alanine-mutated cTn complex did not reduce Ca2+ sensitivity, whereas PKA did reduce Ca2+ sensitivity in cells exchanged with the wild-type complex. This indicated that Ser23 and Ser24 were required for the PKA-mediated decrease in Ca2+ sensitivity but did not rule out the possibility that the decrease depended on the phosphorylation of other target proteins, such as cMyBP-C and titin. To circumvent this problem, Dohet et al. (10) mutated both Ser23 and Ser24 of cTnI to aspartic acid or alanine and exchanged human cTn mutants in porcine cardiac muscle fibers. This study showed that bisphosphorylated cTn complex (cTnI-DD) lowered Ca2+ sensitivity compared with the nonphosphorylated cTn complex (cTnI-AA). Our study extends these previous studies in that human recombinant proteins were exchanged in human tissue and demonstrates that phosphorylation of both Ser23 and Ser24 of cTnI is required and necessary to reduce Ca2+ sensitivity in the human myocardium.
This conclusion relies on the assumption that alanine and aspartic acid mimic dephosphorylated and phosphorylated serine, respectively. Previous studies have indicated that this is indeed the case with respect to interactions between the cTn subunits (12, 29), Ca2+ dissociation rates from cTnC (45), and pCa50 (10). Moreover, it can be noted that we observed similar functional parameters in cells exchanged with dephosphorylated cTnI mimicked with alanine substitutions at Ser23 and Ser24 and unphosphorylated wild-type cTnI (Table 2). Therefore, it appears safe to assume that alanine and aspartic acid substitutions at Ser23 and Ser24 of cTnI indeed reflect the structural and functional consequences of Ser23 and Ser24 (de)phosphorylation.
Maximal reduction in myofilament Ca2+ sensitivity at ∼55% of bisphosphorylated cTnI.
To study how much Ser23/Ser24 bisphosphorylation is required to maximally decrease Ca2+ sensitivity, exchange experiments with cTnI-DD were done at different concentrations in cardiomyocytes from IDCM tissue, in which Ser23/Ser24 bisphosphorylation is low (∼7%; Fig. 2A) (13, 41). The maximal decrease in Ca2+ sensitivity was reached at 51 ± 5% of cTnI-DD exchange (Fig. 4). Because of the low percentage of endogenous bisphosphorylation in IDCM cardiomyocytes remaining after exchange (∼3.5%), we conclude that ∼55% bisphosphorylated cTnI Ser23/Ser24 is required to maximally decrease myofilament Ca2+ sensitivity.
The fact that approximately half of the cTn units along the myofilament need to be phosphorylated to cause a maximal decrease in Ca2+ sensitivity implies that the "phosphorylation signal' is spread along the thin filament. However, we did not find a significant change in cooperativity (nHill) of myofilament force development upon cTnI-DD exchange, and pseudo-monophosphorylation also did not affect the cooperativity of myofilament force development (Table 2). Dohet et al. (10) also did not find a difference in nHill after exchange with cTnI-DD compared with cTnI-AA in porcine cardiac muscle fibers. Moreover, nHill was not significantly different in myofibrils from wild-type mice compared with transgenic mice with both serine residues mutated to aspartic acid (22, 35). Thus, although nHill itself does not appear to be changed by phosphorylation of the NH2-terminal extension of cTnI, some form of communication along the thin filament needs to be present to explain why partial cTnI phosphorylation maximally reduces the activation state of all participating troponin units.
No effect of exchange of pseudo-phosphorylated cTnI at Ser23 and Ser24 on maximal and passive force.
Our results indicated that pseudo-phosphorylation of Ser23 and/or Ser24 of cTnI did not affect Fmax at saturating Ca2+ levels of human cardiomyocytes. This is in line with previous studies (4, 39) showing no effect of PKA incubation on Fmax at saturating Ca2+ in human skinned cardiomyocytes. In addition, no change was found in Fpas upon exchange with either pseudo-monophosphorylated or pseudo-bisphosphorylated cTnI. These results are consistent with a previous study (47) indicating that PKA reduces passive tension in cardiomyocytes via phosphorylation of titin's cardiac-specific N2B domain.
ktr is not affected by cTnI phosphorylation at Ser23 and Ser24.
A small but statistically insignificant trend for a decrease in ktr-max at 1 mg/ml cTn exchange in cTnI-AD compared with cTnI-AA was found (Table 2). Additional experiments with higher cTn concentrations (2 mg/ml) did not show any difference in ktr-max (Table 2). In accordance with our findings, it has been demonstrated that PKA treatment did not affect cross-bridge cycling in skinned trabeculae (8, 17) and cardiomyocytes (15) from rat hearts and in human myofibrils (44). Other studies (11, 20, 36) in rodents showed that PKA-mediated phosphorylation of cTnI did increase cross-bridge kinetics. More recently, it has been shown that PKA-mediated phosphorylation of cMyBP-C increases cross-bridge kinetics in transgenic mice, independent of cTnI phosphorylation (7, 33). The reasons for these differences are unclear, but our data indicate that in the human heart, PKA-mediated phosphorylation of cTnI may induce positive lusitropic effects by affecting Ca2+ sensitivity, whereas no effect was found on ktr, which represents the sum of the apparent rates of cross-bridge attachment and detachment under isometric conditions. However, it cannot be excluded that cross-bridge kinetics are affected by phosphorylation of cTnI in the in vivo situation because Layland and Kentish (21) observed, in line with our findings, in isolated sarcoplasmic reticulum-inhibited cardiac trabeculae no relaxant effect of isoprenaline during isometric contractions, but, on the other hand, an increase in the rate of relaxation during work-loop contractions (which mimic the in vivo situation).
Residual force after the shortening restretch protocol used to determine ktr ranged between 30% and 50%. This residual force is most likely mainly caused by cross-bridge reattachment during restretch (duration: 2 ms). However, the ktr values obtained did not depend on the level of residual force, and the average residual force within the experimental groups after exchange did not differ. Moreover, the ktr values after the exchange protocol were comparable with values reported in previous studies (40, 46) in cardiomyocytes without prior treatment. Therefore, we are confident that the comparison of ktr in the different experimental groups was valid.
Implications of cTnI phosphorylation at Ser23 and Ser24 in health and disease.
In the present study, we provided evidence that bisphosphorylation of PKA sites Ser23 and Ser24 of human cTnI reduces myofilament Ca2+ sensitivity and that the functional range lies between 0% and 55% of bisphosphorylation. The level of phosphorylation of these two serines is determined by the balance between kinase and phosphatase activity at the myofilaments. PKA phosphorylates Ser23 and Ser24 upon stimulation of the β-adrenergic receptor pathway with different affinities (18). In addition, PKC (34), PKD (14), and PKG (2) are all known to phosphorylate cTnI at Ser23 and Ser24. Although in vitro studies (14, 18, 26, 28, 52) have demonstrated that PKA preferentially phosphorylates Ser24 over Ser23, it has been recently demonstrated using top-down mass spectrometry that cTnI in human cardiac tissue is only monophosphorylated at Ser23 (48, 50). This surprising finding may be explained by preferential dephosphorylation of Ser24 by phosphatases (9, 19, 46). Indeed, it has been demonstrated that protein phosphatase 2A has a preference for Ser24 (18). It cannot be excluded that the level of bisphosphorylation needed to maximally reduce Ca2+ sensitivity depends on the phosphorylation status of other myofilament proteins and thus might vary with disease state.
In the donor samples used in this study, the level of endogenous bisphosphorylation amounted to 67.2%, which is above the level of saturation of Ser23/Ser24 bisphosphorylation. Recent studies (48, 50) have reported relatively low levels of bisphosphorylation of cTnI Ser23/Ser24 (∼15%) and a relatively large fraction of monophosphorylated cTnI at Ser23 (∼40%) in human postmortem control hearts and in fresh transplant donor hearts. This suggests that the healthy human heart has a reserve to decrease myofilament Ca2+ sensitivity during β-adrenergic receptor stimulation. In addition, several studies have demonstrated lower cTnI phosphorylation of the PKA sites (3, 23–25, 41, 50) and higher myofilament Ca2+ sensitivity in end-stage failing hearts relative to donor hearts (25, 41), which has been ascribed to downregulation and desensitization of the β-adrenergic receptor pathway. Comparison of human cardiac samples from heart failure patients with different disease severity [ranging from New York Heart Association (NYHA) class I to IV] showed increased Ca2+ sensitivity only in the end stage (NYHA class IV) of cardiac disease (38), which suggests that the detrimental effects of reduced cTnI phosphorylation may only become evident at the end stage of heart failure. However, recent studies in patients with obstructive familial hypertrophic cardiomyopathy and normal systolic but impaired diastolic function (NYHA class III) showed increased myofilament Ca2+ sensitivity (43) and lower cTnI phosphorylation (24, 42) in familial hypertrophic cardiomyopathic myocarium compared with nonfailing myocardium. In addition, a recent study (50) showed that the level of cTnI bisphosphorylation in postmortem hearts with mild hypertrophy was significantly lower (4.1%) compared with control levels (18.4%). Collectively, these studies indicate that cTnI bisphosphorylation and the associated impact on Ca2+ sensitivity depends on the stage of heart failure (NYHA class) as well as on etiology.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-063038.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.J.M.W., A.M.M., G.J.M.S., and J.v.d.V. conception and design of research; P.J.M.W., D.B.F., A.L.T., and A.H.F. performed experiments; P.J.M.W., G.J.M.S., and J.v.d.V. analyzed data; P.J.M.W., G.J.M.S., and J.v.d.V. interpreted results of experiments; P.J.M.W. prepared figures; P.J.M.W. drafted manuscript; P.J.M.W., G.J.M.S., and J.v.d.V. edited and revised manuscript; P.J.M.W., D.B.F., A.L.T., A.H.F., C.d.R., A.M.M., G.J.M.S., and J.v.d.V. approved final version of manuscript.
REFERENCES
- 1. Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Blumenthal DK, Stull JT, Gill GN. Phosphorylation of cardiac troponin by guanosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem 253: 324–326, 1978 [PubMed] [Google Scholar]
- 3. Bodor GS, Oakeley AE, Allen PD, Crimmins DL, Ladenson JH, Anderson PA. Troponin I phosphorylation in the normal and failing adult human heart. Circulation 96: 1495–1500, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJM, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation 111: 774–781, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Brenner B, Kraft T, Yu LC, Chalovich JM. Thin filament activation probed by fluorescence of N-((2-(iodoacetoxy)ethyl)-N-methyl)amino-7-nitrobenz-2-oxa-1,3-diazole-labeled troponin I incorporated into skinned fibers of rabbit psoas muscle. Biophys J 77: 2677–2691, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caremani M, Dantzig J, Goldman YE, Lombardi V, Linari M. Effect of inorganic phosphate on the force and number of myosin cross-bridges during the isometric contraction of permeabilized muscle fibers from rabbit psoas. Biophys J 95: 5798–5808, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen PP, Patel JR, Rybakova IN, Walker JW, Moss RL. Protein kinase A-induced myofilament desensitization to Ca2+ as a result of phosphorylation of cardiac myosin-binding protein C. J Gen Physiol 136: 615–627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Tombe PP, Stienen GJM. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ Res 76: 734–741, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Deshmukh PA, Blunt BC, Hofmann PA. Acute modulation of PP2a and troponin I phosphorylation in ventricular myocytes: studies with a novel PP2a peptide inhibitor. Am J Physiol Heart Circ Physiol 292: H792–H799, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Dohet C, al-Hillawi E, Trayer IP, Ruegg JC. Reconstitution of skinned cardiac fibres with human recombinant cardiac troponin-I mutants and troponin-C. FEBS Lett 377: 131–134, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, Martin AF, Solaro RJ, Moss RL, Leiden JM. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol 517: 143–157, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finley N, Abbott MB, Abusamhadneh E, Gaponenko V, Dong W, Gasmi-Seabrook G, Howarth JW, Rance M, Solaro RJ, Cheung HC, Rosevear PR. NMR analysis of cardiac troponin C-troponin I complexes: effects of phosphorylation. FEBS Lett 453: 107–112, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Hamdani N, Borbely A, Veenstra SP, Kooij V, Vrydag W, Zaremba R, Dos Remedios CG, Niessen HW, Michel MC, Paulus WJ, Stienen GJM, van der Velden J. More severe cellular phenotype in human idiopathic dilated cardiomyopathy compared to ischemic heart disease. J Muscle Res Cell Motil 31: 289–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haworth RS, Cuello F, Herron TJ, Franzen G, Kentish JC, Gautel M, Avkiran M. Protein kinase D is a novel mediator of cardiac troponin I phosphorylation and regulates myofilament function. Circ Res 95: 1091–1099, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Hofmann PA, Lange JH., III Effects of phosphorylation of troponin I and C protein on isometric tension and velocity of unloaded shortening in skinned single cardiac myocytes from rats. Circ Res 74: 718–726, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Howarth JW, Meller J, Solaro RJ, Trewhella J, Rosevear PR. Phosphorylation-dependent conformational transition of the cardiac specific N-extension of troponin I in cardiac troponin. J Mol Biol 373: 706–722, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Janssen PM, de Tombe PP. Protein kinase A does not alter unloaded velocity of sarcomere shortening in skinned rat cardiac trabeculae. Am J Physiol Heart Circ Physiol 273: H2415–H2422, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Jaquet K, Thieleczek R, Heilmeyer LM., Jr Pattern formation on cardiac troponin I by consecutive phosphorylation and dephosphorylation. Eur J Biochem 231: 486–490, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Jideama NM, Crawford BH, Hussain AK, Raynor RL. Dephosphorylation specificities of protein phosphatase for cardiac troponin I, troponin T, and sites within troponin T. Int J Biol Sci 2: 1–9, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res 88: 1059–1065, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Layland J, Kentish JC. Myofilament-based relaxant effect of isoprenaline revealed during work-loop contractions in rat cardiac trabeculae. J Physiol 544: 171–182, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu QW, Hinken AC, Patrick SE, Solaro RJ, Kobayashi T. Phosphorylation of cardiac troponin I at protein kinase C site threonine 144 depresses cooperative activation of thin filaments. J Biol Chem 285: 11810–11817, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McConnell BK, Moravec CS, Bond M. Troponin I phosphorylation and myofilament calcium sensitivity during decompensated cardiac hypertrophy. Am J Physiol Heart Circ Physiol 274: H385–H396, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Messer AE, Gallon CE, McKenna WJ, Dos Remedios CG, Marston SB. The use of phosphate-affinity SDS-PAGE to measure the cardiac troponin I phosphorylation site distribution in human heart muscle. Proteomics Clin Appl 3: 1371–1382, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Messer AE, Jacques AM, Marston SB. Troponin phosphorylation and regulatory function in human heart muscle: dephosphorylation of Ser23/24 on troponin I could account for the contractile defect in end-stage heart failure. J Mol Cell Cardiol 42: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Mittmann K, Jaquet K, Heilmeyer LM., Jr Ordered phosphorylation of a duplicated minimal recognition motif for cAMP-dependent protein kinase present in cardiac troponin I. FEBS Lett 302: 133–137, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Narolska NA, Piroddi N, Belus A, Boontje NM, Scellini B, Deppermann S, Zaremba R, Musters RJ, Dos Remedios CG, Jaquet K, Foster DB, Murphy AM, van Eyk JE, Tesi C, Poggesi C, van der Velden J, Stienen GJM. Impaired diastolic function after exchange of endogenous troponin I with C-terminal truncated troponin I in human cardiac muscle. Circ Res 99: 1012–1020, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Quirk PG, Patchell VB, Gao Y, Levine BA, Perry SV. Sequential phosphorylation of adjacent serine residues on the N-terminal region of cardiac troponin-I: structure-activity implications of ordered phosphorylation. FEBS Lett 370: 175–178, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Reiffert SU, Jaquet K, Heilmeyer LM, Jr, Herberg FW. Stepwise subunit interaction changes by mono- and bisphosphorylation of cardiac troponin I. Biochemistry 37: 13516–13525, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J Biol Chem 257: 260–263, 1982 [PubMed] [Google Scholar]
- 31. Solaro RJ, Kobayashi T. Protein phosphorylation and signal transduction in cardiac thin filaments. J Biol Chem 286: 9935–9940, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solaro RJ, Moir AJ, Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature 262: 615–617, 1976 [DOI] [PubMed] [Google Scholar]
- 33. Stelzer JE, Patel JR, Walker JW, Moss RL. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ Res 101: 503–511, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Swiderek K, Jaquet K, Meyer HE, Schachtele C, Hofmann F, Heilmeyer LM., Jr Sites phosphorylated in bovine cardiac troponin T and I. Characterization by 31P-NMR spectroscopy and phosphorylation by protein kinases. Eur J Biochem 190: 575–582, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Takimoto E, Soergel DG, Janssen PM, Stull LB, Kass DA, Murphy AM. Frequency- and afterload-dependent cardiac modulation in vivo by troponin I with constitutively active protein kinase A phosphorylation sites. Circ Res 94: 496–504, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Turnbull L, Hoh JF, Ludowyke RI, Rossmanith GH. Troponin I phosphorylation enhances crossbridge kinetics during beta-adrenergic stimulation in rat cardiac tissue. J Physiol 542: 911–920, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van der Velden J. Diastolic myofilament dysfunction in the failing human heart. Pflügers Arch 462: 155–163, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van der Velden J, Klein LJ, Zaremba R, Boontje NM, Huybregts MA, Stooker W, Eijsman L, de Jong JW, Visser CA, Visser FC, Stienen GJM. Effects of calcium, inorganic phosphate, and pH on isometric force in single skinned cardiomyocytes from donor and failing human hearts. Circulation 104: 1140–1146, 2001 [DOI] [PubMed] [Google Scholar]
- 39. van der Velden J, Narolska NA, Lamberts RR, Boontje NM, Borbely A, Zaremba R, Bronzwaer JG, Papp Z, Jaquet K, Paulus WJ, Stienen GJM. Functional effects of protein kinase C-mediated myofilament phosphorylation in human myocardium. Cardiovasc Res 69: 876–887, 2006 [DOI] [PubMed] [Google Scholar]
- 40. van der Velden J, Papp Z, Boontje NM, Zaremba R, de Jong JW, Janssen PM, Hasenfuss G, Stienen GJM. The effect of myosin light chain 2 dephosphorylation on Ca2+ -sensitivity of force is enhanced in failing human hearts. Cardiovasc Res 57: 505–514, 2003 [DOI] [PubMed] [Google Scholar]
- 41. van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, Burton PB, Goldmann P, Jaquet K, Stienen GJM. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res 57: 37–47, 2003 [DOI] [PubMed] [Google Scholar]
- 42. van Dijk SJ, Dooijes D, Dos Remedios CG, Michels M, Lamers JM, Winegrad S, Schlossarek S, Carrier L, ten Cate FJ, Stienen GJM, van der Velden J. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation 119: 1473–1483, 2009 [DOI] [PubMed] [Google Scholar]
- 43. van Dijk SJ, Paalberends ER, Najafi A, Michels M, Sadayappan S, Carrier L, Boontje NM, Kuster DW, van Slegtenhorst M, Dooijes D, Dos Remedios CG, ten Cate FJ, Stienen GJM, van der Velden J. Contractile dysfunction irrespective of the mutant protein in human hypertrophic cardiomyopathy with normal systolic function. Circ Heart Fail 5: 36–46, 2012 [DOI] [PubMed] [Google Scholar]
- 44. Walker JS, Walker LA, Margulies K, Buttrick P, de Tombe PP. Protein kinase A changes calcium sensitivity but not crossbridge kinetics in human cardiac myofibrils. Am J Physiol Heart Circ Physiol 301: H138–H146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ward DG, Brewer SM, Gallon CE, Gao Y, Levine BA, Trayer IP. NMR and mutagenesis studies on the phosphorylation region of human cardiac troponin I. Biochemistry 43: 5772–5781, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Wijnker PJM, Boknik P, Gergs U, Muller FU, Neumann J, dos Remedios CG, Schmitz W, Sindermann JR, Stienen GJM, van der Velden J, Kirchhefer U. Protein phosphatase 2A affects myofilament contractility in non-failing but not in failing human myocardium. J Muscle Res Cell Motil 32: 221–233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res 90: 1181–1188, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Zabrouskov V, Ge Y, Schwartz J, Walker JW. Unraveling molecular complexity of phosphorylated human cardiac troponin I by top down electron capture dissociation/electron transfer dissociation mass spectrometry. Mol Cell Proteomics 7: 1838–1849, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Zaremba R, Merkus D, Hamdani N, Lamers JMJ, Paulus WJ, Dos Remedios CG, Duncker DJ, Stienen GJM, van der Velden J. Quantitative analysis of myofilament protein phosphorylation in small cardiac biopsies. Proteomics Clin Appl 1: 1285–1290, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Zhang J, Guy MJ, Norman HS, Chen YC, Xu Q, Dong X, Guner H, Wang S, Kohmoto T, Young KH, Moss RL, Ge Y. Top-down quantitative proteomics identified phosphorylation of cardiac troponin I as a candidate biomarker for chronic heart failure. J Proteome Res 10: 4054–4065, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ Res 76: 1028–1035, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Zhang R, Zhao J, Potter JD. Phosphorylation of both serine residues in cardiac troponin I is required to decrease the Ca2+ affinity of cardiac troponin C. J Biol Chem 270: 30773–30780, 1995 [DOI] [PubMed] [Google Scholar]