Abstract
Synaptic transmission is amongst the most sophisticated and tightly controlled biological phenomena in higher eukaryotes. In the past few decades, tremendous progress has been made in our understanding of the molecular mechanisms underlying multiple facets of neurotransmission, both pre- and postsynaptically. Brought under the spotlight by pioneer studies in the areas of secretion and signal transduction, phosphoinositides and their metabolizing enzymes have been increasingly recognized as key protagonists in fundamental aspects of neurotransmission. Not surprisingly, dysregulation of phosphoinositide metabolism has also been implicated in synaptic malfunction associated with a variety of brain disorders. In the present chapter, we summarize current knowledge on the role of phosphoinositides at the neuronal synapse and highlight some of the outstanding questions in this research field.
1. INTRODUCTION
Chemical synapses are intercellular junctions through which neurons efficiently transfer electrical signals to target cells. Synapses consist of two juxtaposed structures, the pre- and postsynaptic compartments, which are separated by the synaptic cleft. The presynaptic compartment is specialized for the fast release of neurotransmitters in response to action potentials propagating along axons towards nerve terminals and the opening of voltage-dependent Ca2+ channels. Ca2+-triggered release of neurotransmitters occurs through fast exocytosis of synaptic vesicles (SVs) at specialized sites called the active zones and is generally followed by a slower retrieval of SV membrane by endocytosis (Figure 1). The released neurotransmitters, which are mainly glutamate and gamma-aminobutyric acid (GABA) in the central nervous system, bind to ionotropic and metabotropic postsynaptic receptors. This translates the chemical signal in the form of neurotransmitters into inhibitory and excitatory electrical events as well as into intracellular signaling cascades, thus transmitting action potentials in the target cell. The strength of synaptic transmission can vary across a broad range in a phenomenon called synaptic plasticity, which is typically associated with drastic morphological changes at the postsynapse of excitatory neurons (i.e., dendritic spines) and believed to underlie key neurobehavioral responses, such as learning and memory. The past sixty years has witnessed a worldwide and massive effort to investigate the molecular and cellular bases of synaptic transmission leading to substantial progress in our understanding of how this phenomenon works. However, despite the fact that many aspects of synaptic transmission critically depend on signaling across lipid bilayers and membrane trafficking, less is known about the role of lipids in this process. In the past two decades, lipids such as phosphorylated derivatives of phosphatidylinositol, also called phosphoinositides, have progressively come to center stage due to their growing implication in fundamental aspects of neurotransmission.
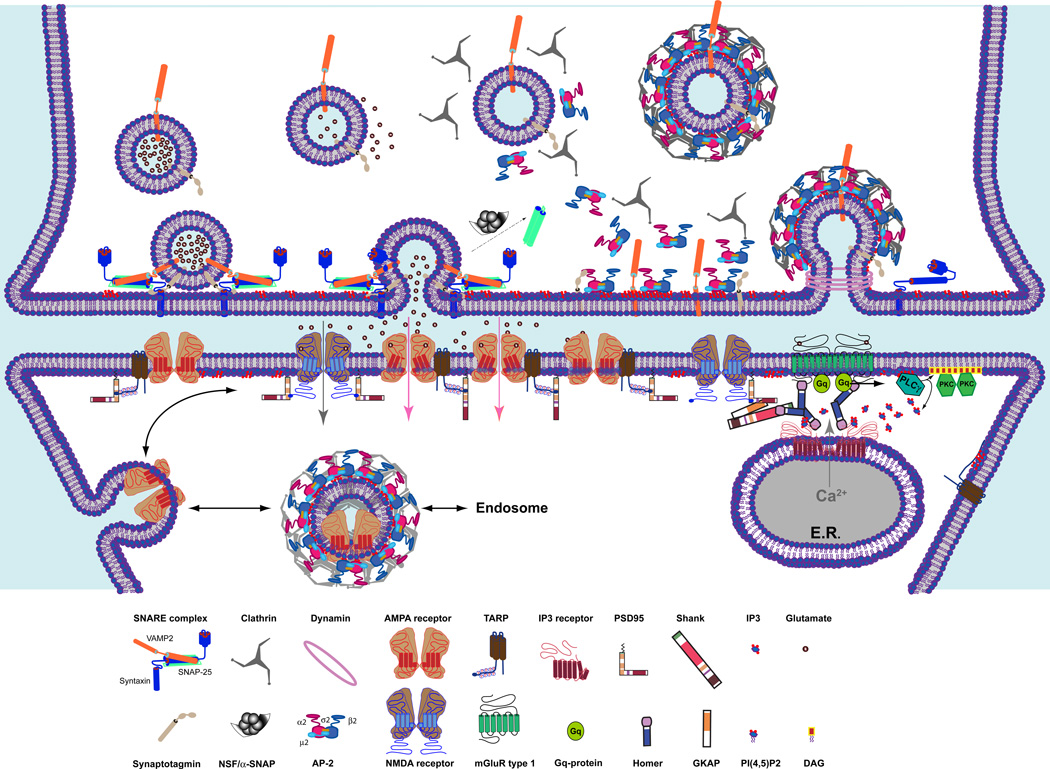
Fig. 1. Neurotransmission at glutamatergic synapses.
Presynatically, synaptic vesicles (SVs) filled with glutamate are localized in proximity to the plasma membrane (PM) and are docked at the active zone after the assembling of the SNARE complex (and other proteins such as Munc18, not depicted here) composed of the PM-associated syntaxin-1, the cytosolic SNAP-25 and the SV membrane-associated synaptobrevin. Upon the arrival of a calcium (Ca2+) influx, the vesicular and the plasma membranes fused and the content of the SV is released in the synaptic cleft. Following SV collapse, the SNARE complex is disassembled by the ATPase N-ethylmaleimide-sensitive factor (NSF) and its adaptor α-SNAP. The fused vesicle is retrieved by CME, which starts by the recruitment of the clathrin adaptors, AP-2 to PM enriched in PtdIns(4,5)P2 and by its interaction with Syt-1. Clathrin molecules assemble into a lattice structure at the endocytic site and, along with a variety of tubulating factors, permit PM invagination and the formation of a CCP. Dynamin oligomers formed a helix that encircles the bud of the pit and triggers the fission and the individuation of a clathrin-coated vesicles. Quickly, the clathrin coat is removed and the AP-2-clathrin complex is disassembled. The SV is refilled with neurotransmitters and can undergo another cycle of exocytosis and endocytosis.
Postsynaptically, released glutamate binds to the ionotropic AMPA and NMDA receptors and to the Gq-coupled metabotropic glutamate receptor. (mGluR) type 1 (the other types of mGluR are not depicted). Gating of the AMPA receptor by glutamate generates a net current influx that depolarizes the postsynaptic PM allowing the gating of glutamate-bound NMDA receptor and a slower influx of sodium and calcium. mGluR type 1 activation releases the Gq proteins and the hydrolysis of PtsIns(4,5)P2 by phospholipase Cγ into diacylglycerol (DAG) and inositol (1,4,5)-trisphosphate (IP3). DAG recruits to the PM and activates the protein kinase C, while IP3 binds to and opens the IP3 receptor (IP3 R), which liberates the calcium store in the endoplasmic reticulum (ER). In the figure, interactions of the receptors with the structural proteins that organized the postsynaptic density (PSD) is emphasized. NMDA binds directly to PSD-95 with a PDZ-binding motif localized at the C-terminal extremity of the receptor. AMPA receptor and mGluR type 1 are indirectly anchored to the PSD-95 complex via the transmembrane AMPA receptor regulatory proteins (TARP) and via Homer/Shank/GKAP, respectively. Homer also interacts with the IP3 R and allows the anchoring of the receptor in proximity to the mGluR type 1. Finally, AMPA receptors are mobile and move traffic between the synaptic and extrasynaptic zone, where they can be internalized by CME and be recycled via the endosome.
Phosphoinositides are quantitatively a minor lipid class in cellular membranes; nevertheless, they play a crucial role in many aspects of cellular physiology, including synaptic function. There are seven known phosphoinositides, one of which, phosphatidylinositol-4,5-bisphosphate [PtdIns(4,5)P2], has been extensively studied due to its abundance and its historical implication in signal transduction as a precursor for second messengers as well as in secretion. This book chapter largely focuses on the role of PtdIns(4,5)P2 metabolism at the neuronal synapse, with particular emphasis on its function in the traffic of SVs at the presynapse. The role of other critical phosphoinositides, such as phosphatidylinositol-3,4,5-trisphosphate [PtdIns(3,4,5)P3], will also be discussed, based on its implication in synaptic plasticity. Phosphoinositides are also important regulators of many ion channels and transporters, such as voltage-gated Ca2+ channels or potassium channels, and this regulation is central to neuronal excitability and synaptic transmission. However, space limitation prevents us from elaborating on this topic, which is covered by a series of recent review articles to which we refer the reader (Hilgemann, 2007, Suh and Hille, 2008, Logothetis et al., 2010). In the present chapter, we describe the main lipid enzymes controlling the metabolism of the two main signaling phosphoinositides at the synapse, PtdIns(4,5)P2 and PtdIns(3,4,5)P3. We then highlight the mechanisms by which these lipids control fundamental aspects of synaptic transmission at the pre- and post-synapse. Finally, we discuss the implications of dysregulation of synaptic phosphoinositide metabolism in disease-related processes, such as in Down syndrome and Alzheimer’s disease.
2. PHOSPHOINOSITIDE METABOLISM AT THE SYNAPSE
While PtdIns(4,5)P2 and PtdIns4P are the most abundant phosphoinositides in cells, the former has been extensively characterized in the past few decades and is now known to play fundamental roles at the plasma membrane. Indeed, PtdIns(4,5)P2 controls both exocytosis and endocytosis, actin dynamics, signal transduction as well as the function of many ion channels and transporters (Di Paolo and De Camilli, 2006, Saarikangas et al., 2008, Suh and Hille, 2008). Although the synaptic plasma membrane contains highly specialized structures, such as the active zone and the post-synaptic density, and a distinct protein and lipid composition, PtdIns(4,5)P2 is known to regulate these very same biological processes at these membranes. Steady-state PtdIns(4,5)P2 levels at the synapse are controlled by a specific set of lipid enzymes, which, at mammalian synapses, include PtdInsP kinases type 1γ (Wenk et al., 2001, Di Paolo et al., 2004) and polyphosphoinositide phosphatase Synj1 (Cremona et al., 1999, Voronov et al., 2008). Additionally, a variety of enzymes, such as phosphoinositide 3-kinases (PI3K), phospholipase C (PLC) and A2, play both critical and pleiotropic roles in phosphoinositide-dependent signaling. Besides PtdIns(4,5)P2, the low abundance phosphoinositide PtdIns(3,4,5)P3 is known to regulate many cellular processes, some of which are critical for synaptic physiology and plasticity. The following section will primarily focus on the enzymes controlling PtdIns(4,5)P2 and PtdIns(4,5)P2 metabolism at the synapse. The precise role of these two phosphoinositides at synapses will be discussed in sections 3 and 4.
2.1 Regulation of PtdIns(4,5)P2 metabolism at the synapse
PtdIns(4,5)P2 can be synthesized by type 1 PtdInsP kinases (PtdInsPK1s), which phosphorylate PtdIns4P on the 5’ position, and by type 2 PtdInsP kinases, which phosphorylate PtdIns5P on the 4’ position (Doughman et al., 2003, Anderson et al., 1999). The activation loop located at the COOH-terminus of the kinase core domain controls the substrate specificity of either class (Kunz et al., 2000). Each type of PtdInsPKs is comprised of three catalytically-active isoforms, α, β, γ (Ishihara et al., 1996, Anderson et al., 1999). A kinase-dead homolog, PtdInsPKH, has also been reported (Chang et al., 2004). Biochemical measurements using immunodepleted or knockout (KO) brain extracts have demonstrated that the main pathway for the synthesis of PtdIns(4,5)P2 in the brain and at the synapse involves the PtdInsPK1γ isoform (Wenk et al., 2001, Di Paolo et al., 2004, Volpicelli-Daley et al., 2010). Its substrate, PtdIns4P, is produced by a PtdIns 4-kinase (PI4K), which was identified as a type IIα enzyme (PI4KIIα), at least in the brain and at neuronal synapses (Guo et al., 2003). In mouse brain, PtdInsPK1γ occurs as 3 splice variants, PtdInsPK1γ635 (or PtdInsPK1γ87), PtdInsPK1γ661 (or PtdInsPK1γ90) and PtdInsPK1γ687 (Ishihara et al., 1998, Giudici et al., 2004). At synapses, PtdInsPK1γ661 is the most abundant isoform and the main kinase for the production of PtdIns(4,5)P2 (Wenk et al., 2001, Di Paolo et al., 2004, Volpicelli-Daley et al., 2010). In mice, the extended COOH-terminal tail of PtdInsPK1γ661 binds to the Four-point-one, Ezrin, Radixin, Moesin (FERM) domain of the head region of talin (Di Paolo et al., 2002, Ling et al., 2002) and with the clathrin adaptor complex protein-2 (AP-2) (Bairstow et al., 2006, Nakano-Kobayashi et al., 2007, Krauss et al., 2006, Kahlfeldt et al., 2010). Three other types of interactors have been identified for PtdInsPK1γ: (i) Rho GTPases (Chatah and Abrams, 2001, Weernink et al., 2004); (ii) Arf GTPases (Krauss and Haucke, 2005, Krauss et al., 2003); and (iii) phospholipase D (Divecha et al., 2000, Jarquin-Pardo et al., 2007, Moritz et al., 1992). Similar to a group of proteins involved in SV endocytosis called the “dephosphins”, PtdInsPK1γ undergoes depolarization-dependent dephosphorylation by calcineurin, a phenomenon known to promote the assembly of endocytic factors (Cousin and Robinson, 2001, Slepnev et al., 1998, Wenk et al., 2001). The phosphorylation of PtdInsPK1γ is mediated at least in part by cyclin-dependent kinase-5 (Cdk-5), a proline-directed serine/ threonine kinase that is critically important for SV trafficking and the control of the recycling pool size (Lee et al., 2005, Kim and Ryan, 2010). Phosphorylation is a primary mechanism by which the activity of PtdInsPK1γ is locally controlled. For instance, phosphorylation of residue S645 in the COOH tail of PtdInsPK1γ661 decreases the binding of the lipid kinase with talin and AP-2 (Lee et al., 2005, Nakano-Kobayashi et al., 2007). Additionally, phosphorylation of the S645 is reduced by phosphorylation of the neighbor residue, Y649, by non-receptor tyrosine kinase Src (Lee et al., 2005).
The main PtdIns(4,5)P2 phosphatase at the mammalian synapse is synaptojanin 1 (Synj1) (McPherson et al., 1996, Cremona et al., 1999). Two different Synj-encoding genes, Synj1 and Synj2, have been reported in mammals, although Synj1 is known to be the predominant activity in the brain (Cremona et al., 1999, Voronov et al., 2008). Additionally, two splice variants for Synj1 have been described, Synj1-145 and Synj1-170 (Ramjaun and McPherson, 1996, Haffner et al., 2000, Haffner et al., 1997, McPherson et al., 1996). However, only the shorter variant, Synj1-145, is highly expressed in mature brain and synapses, while Synj1-170 is ubiquitously expressed but at lower levels. Synj1 contains three protein regions (McPherson et al., 1996, Blero et al., 2007): (i) a central inositol 5-phosphatase domain that can hydrolyze PtdIns(4,5)P2 to release phosphate from the 5’ position of the inositol ring; (ii) an NH2-terminal Sac1 region that can also function as a phosphoinositide phosphatase towards a variety of substrates, including monophosphorylated phosphoinositides and PtdIns(3,5)P2 (Guo et al., 1999); and (iii) a COOH-terminal proline-rich domain (PRD) involved in the binding of Src-homology type 3 (SH3) domain-containing proteins, such as Growth factor Receptor-Bound protein 2 (Grb2), amphiphysin, endophilin and intersectin (Simpson et al., 1999, Yamabhai et al., 1998, Ringstad et al., 1997, de Heuvel et al., 1997, Cestra et al., 1999, Pechstein et al., 2010). It is commonly accepted that the 5-phosphatase activity mediates most of Synj1’s actions, although a recent functional analysis of a Sac1 activity-deficient mutant of Synj1 suggests some roles for this domain in SV recycling (Mani et al., 2007).
As a member of the dephosphin protein group and similar to PtdInsPK1γ, Synj1 is constitutively phosphorylated in nerve terminals by the Cdk-5 and undergoes stimulation-dependent dephosphorylation by calcineurin (Marks and McMahon, 1998, Lee et al., 2004b). Cdk-5 phosphorylates the COOH-terminal tail of Synj1 on a serine residue, which disrupts its interaction with endophilin and amphiphysin (Lee et al., 2004b). Additionally, Synj1 is a substrate of Dyrk1a, a homolog of the fly minibrain kinase (Adayev et al., 2006), and phosphorylation of Synj1 by this kinase regulates its interactions with the SH3 domain of amphiphysin and intersectin, but does not affect its enzymatic activity (Adayev et al., 2006). More relevant to the postsynaptic actions of Synj1, three tyrosine residues in Synj1’s PRD domain can be phosphorylated by the activated EphB receptor, which reduces the interaction between Synj1 and endophilin and affects glutamate receptor internalization (Irie et al., 2005). Finally, in addition to phosphorylation/ dephosphorylation mechanisms, our recent studies have shown that membrane curvature and endophilin control both the recruitment of Synj1 to membranes and its PtdIns(4,5)P2 phosphatase activity (Chang-Ileto et al., 2011).
2.2 Regulation of PtdIns(3,4,5)P3 metabolism of the synapse
The metabolism of PtdIns(3,4,5)P3 is intimately linked to that of PtdIns(4,5)P2, because class I PtdIns 3-kinases (PI3K), which use the latter as a substrate, are believed to be the main enzymes for the synthesis of PtdIns(3,4,5)P3. The phosphatase PTEN (phosphatase and tensin homolog located on chromosome 10) catalyzes the converse reaction by dephosphorylating PtdIns(3,4,5)P3 on the 3’ position. While PtdIns(4,5)P2 has been predominantly studied in its role at the presynapse, PtdIns(3,4,5)P3 has been mostly characterized at the postsynapse.
Class I PI3Ks are heterodimers composed of a regulatory and a catalytical subunit. Four genes encoding catalytic subunits have been reported (p110 or PI3Kα, β, δ and γ). PI3Kα, β, δ compose the class Ia PI3Ks and are mainly activated by receptor tyrosine kinases, while PI3Kγ is the sole member of class Ib (Hawkins et al., 2006, Marone et al., 2008). For the class Ia PI3Ks, several genes have been found to encode for the regulatory subunits, collectively referred to as p85 family members, despite the size diversity. These include pik3r (p85α, p55α, p50α), pik3r2 (p85β) and pik3r3 (p55γ). Two different genes, pik3r5 and pik3r6, code for the p101 and p84 subunits, which form a complex with PI3Kγ. The role of PI3K at the synapse has been mostly assessed using broad specificity pharmacological agents, such as wortmannin (i.e., an inhibitor of class I and III PI3Ks) and LY294002 (i.e., an inhibitor which is fairly specific for class Ia PI3Ks). Generally, little information is known regarding the subtype of PI3K complexes expressed at synapses. Nevertheless, in the brain, the p110β and the p85α seem to be the predominant heterodimer of the class Ia (Geering et al., 2007). At synapses, PI3K activity can also be activated by the complex formed by the GTPase PI3K enhancer-Long (PIKE-L) with Homer 1c and an activated mGluR of class I, which promotes neuronal survival (Rong et al., 2003).
PTEN is a 403 amino-acid protein composed of two large domains, the phosphatase domain and a C2 domain. The phosphatase domain is flanked at the NH2 -terminus by a short PtdIns(4,5)P2-binding domain composed of several basic amino acids and the C2 domain is flanked at the COOH-terminus by a PDZ-binding Thr-Lys-Val motif (Bonifant et al., 2007). PTEN is the only 3-phosphatase identified that can mediate the PtdIns(3,4,5)P3-to-PtdIns(4,5)P2 conversion, thereby arresting PI3K-dependent signaling. PTEN is widely expressed in mouse brain, and preferentially in neurons, particularly Purkinje cells, olfactory mitral and large pyramidal neurons, where it is present in dendrites and spines (Perandones et al., 2004, Chang et al., 2007).
3. PRESYNAPTIC ROLES OF PHOSPHOINOSITIDES
Phosphoinositides are known to play a multitude of roles in cell physiology, including multiple aspects of neuronal function. Because changes in the metabolism of these lipids were originally studied in the context of secretion, the first clues that these lipids may play a critical role in secretion and particularly, secretory granule exocytosis, were obtained from work carried out in chromaffin and PC12 cells (Martin, 2003, Di Paolo and De Camilli, 2006). However, the identification of Synj as a major regulator of SV recycling contributed to the remarkable expansion of our knowledge on the role of Synj’s main substrate, PtdIns(4,5)P2, in endocytosis. There is now robust molecular, physiological and genetic evidence indicating that PtdIns(4,5)P2, controls both the exocytic and endocytic limbs of SV trafficking, and that perturbation of its metabolism alters presynaptic function in neurons. The following section highlights the functional consequences of phosphoinositide perturbations on the SV cycle as well as the molecular basis underlying this process.
3.1 Synaptic vesicle exocytosis
Release of neurotransmitters is triggered by the fusion of SVs with the presynaptic plasma membranes in response to action potentials and Ca2+ entry (Figure 1). In the past two decades, the field has achieved a remarkable understanding of this phenomenon through the identification and characterization of proteins mediating the fusion process using molecular, biochemical, structural and genetic approaches (Sudhof and Malenka, 2008). The fusion machinery consists of three or four SNARE proteins and one sec1-Munc18-like protein (or SM protein) that are controlled by a variety of accessory factors, including members of the synaptotagmin (Syt) family. These proteins serve as the Ca2+-sensors that mediate the bulk of neurotransmitter release under normal stimulation conditions in all synapses. There is now increasing evidence that phosphoinositides play an important modulatory role in several aspects of the fusion of SVs, as hinted by seminal studies on secretory granule exocytosis twenty years ago.
3.1.1 Exocytic defects in PtdIns(4,5)P2-deficient synapses and neurosecretory cells
Studies from the groups of Holz and Martin in the early 1990s showed a critical role for inositol lipids and PtdIns(4,5)P2 in the exocytosis of secretory granules in broken (or permeabilized) chromaffin or PC12 cells (Eberhard et al., 1990, Hay et al., 1995, Martin, 1997). Importantly, a phosphatidylinositol transfer protein (Hay and Martin, 1993) and a PtdInsP kinase (Hay et al., 1995) were identified as factors required for the ATP- and Ca2+-dependent priming step in the release of large dense core vesicles (LDCVs) in PC12 cells, while a PtdIns 4-kinase activity was shown to be essential for secretion in chromaffin cells (Wiedemann et al., 1996). Remarkably, these early studies suggested that PtdIns(4,5)P2 plays a role in LDCV exocytosis as an intact molecule rather than as a cleavage product of PLC, a biochemical reaction previously implicated in secretion (Osborne et al., 2006). Subsequently, a series of exocytic factors were found to bind PtdIns(4,5)P2, demonstrating the importance of this lipid as an intact molecule in the fusion process. These include Syt (Schiavo et al., 1996, Bai et al., 2004), rabphilin (Chung et al., 1998), Ca2+-dependent activator protein for secretion (CAPS) (Loyet et al., 1998), SNARE proteins (Lam et al., 2008), and secretory carrier membrane protein 2 (SCAMP2) (Liao et al., 2007).
While a role for PtdIns(4,5)P2 in the fusion of LDCV had been well established by the Holz and Martin studies, a reliance of SV exocytosis on this lipid has only been suggested by a couple of studies. In the first study, reducing the synthesis of PtdIns4P, the main precursor of PtdIns(4,5)P2, by phenylarside oxide was shown to decrease the release of glutamate from depolarized synaptosomes (Wiedemann et al., 1998). The second study involved the functional characterization of mice lacking PtdInsPK1γ, the main PtdIns(4,5)P2-synthesizing enzyme at the synapse (Di Paolo et al., 2004). Indeed, deletion of PtdInsPK1γ was shown to produce defects in neurotransmitter release in cultured cortical neurons, based on the reduced frequency of miniature EPSPs and inhibitory postsynaptic potentials and the smaller readily-releasable pool (RRP) observed upon brief applications of hypertonic solutions (Di Paolo et al., 2004). Consistent with a smaller RRP, genetic ablation of PtdInsPK1γ also enhanced rapid depression during prolonged high-frequency stimulation. It was hypothesized that these exocytic defects may be accounted for, in part, by a delay in the replacement of docked and primed vesicles that have undergone exocytosis with new fusion-competent vesicles (Di Paolo et al., 2004). Defects in SV recycling also occur in mutant synapses and are discussed below.
A follow-up study on primary chromaffin cells lacking PtdInsPK1γ confirmed that reduced levels of PtdIns(4,5)P2 decrease the size of the RRP of LDCVs and its refilling rate. Together with electron microscopy data showing an increase in morphologically-docked vesicles, data from this study indicated that PtdInsPK1γ controls the priming of LDCVs (Gong et al., 2005). Finally, amperometry measurements in mutant chromaffin cells revealed a delay in the expansion of the fusion pore, thus implicating PtdIns(4,5)P2 in the regulation of LDCV fusion pore dynamics (Gong et al., 2005). This study confirms the notion that PtdIns(4,5)P2 controls the priming step in the exocytosis of LDCVs (Eberhard et al., 1990, Hay et al., 1995) and is in partial agreement with an independent study in PC12 cells in which acute manipulations of PtdIns(4,5)P2 levels were shown to affect the rate of fusion of LDCVs (Milosevic et al., 2005).
3.1.2 Molecular basis for the actions of PtdIns(4,5)P2 in exocytosis
Since the discovery that PtdIns(4,5)P2 promotes exocytosis independently of its metabolic conversion to diacylglycerol (DAG) and inositol-1,4,5-trisphosphate [Ins(1,4,5)P3] by PLC (Eberhard 1990, Hay 1995), the idea that PtdIns(4,5)P2 effectors may mediate the fusion process gained in popularity, particularly in light of the identification of peptides or protein modules binding to this lipid with high selectivity and affinity. Two such PtdIns(4,5)P2 effectors were extensively characterized in this context, CAPS and Syt, with significant implications for the priming and Ca2+-sensing stages of the exocytic process.
CAPS and the priming step
CAPS was originally discovered as a 145 KDa cytosolic factor promoting Ca2+-dependent exocytosis of LDCVs (Walent et al., 1992). Following the identification of CAPS as a homolog of Unc-31 (Ann et al., 1997), studies on Unc-31 and on mammalian CAPS in semi-broken synaptosomes confirmed the involvement of this gene family in LDCV exocytosis, but not in the fusion of SVs (Speese et al., 2007, Tandon et al., 1998, Gracheva et al., 2007). Although synaptic transmission at the fly and worm neuromuscular junction (NMJ) was found to be altered in CAPS mutants, these phenotypes were interpreted as secondary to defects in LDCV exocytosis (Speese et al., 2007, Renden et al., 2001, Gracheva et al., 2007). In the mouse, ablation of CAPS-1 was shown to perturb the uptake of catecholamines into LDCVs and the exocytosis of LDCVs (Speidel et al., 2005), while deletion of CAPS-2 interferes with the development of the cerebellum and short-term plasticity (Sadakata et al., 2007). A role for CAPS in SV exocytosis was unambiguously shown in synapses derived from mice lacking CAPS-1 and 2 (Jockusch et al., 2007). The double KO mice exhibited dramatic reduction in the number of fusion-competent SVs concomitantly with a normal number of vesicles docked at the plasma membrane, thus implying an essential role of CAPS in the priming step in excitatory neurons. Further supporting this view, deficits in the mutant mice were rescued, at least transiently, by hyperactivation of Munc-13, a well-established priming factor (see below) (Jockusch et al., 2007, Augustin et al., 1999, Varoqueaux et al., 2002, Siksou et al., 2009) but see also (Siksou et al., 2009) for a role in Munc-13 in SV docking. CAPS also binds to membranes in a Ca2+-dependent manner via its C2 domain and to phosphoinositides, such as PtdIns4P, PtdIns(4,5)P2 and PtdIns(3,4)P2, via its PH domain (Loyet et al., 1998, James et al., 2008). Binding of CAPS to PtdIns(4,5)P2 appears to be critical for the fusion-promoting action of CAPS because it decreases the intrinsic ability of the inverted cone shape of PtdIns(4,5)P2 to block SNARE-mediated fusion and may directly affect the fusogenic properties of syntaxin/ Soluble NSF Attachment Protein-25 (SNAP-25) (Grishanin et al., 2004, James et al., 2008). Syntaxin itself can directly bind to phospholipids, particularly “cone shape” lipid phosphatidic acid (PA), which is fusogenic, and PtdIns(4,5)P2, via a polybasic juxtamembrane region. Indeed, in PC12 cells, PtdIns(4,5)P2 co-clusters with syntaxin at sites of exocytosis (Aoyagi et al., 2005). Since mutations of the juxtamembrane domain decrease the fusion pore size in PC12 cells and this effect is mediated by PA, it was suggested that this syntaxin-1 domain clusters PA at the site of fusion (Lam et al., 2008).
The function of priming factor Munc-13 heavily relies on the ability of this family of proteins to bind to DAG via their C1 domain, suggesting the interesting possibility that PtdIns(4,5)P2 hydrolysis by PLC also regulates the priming step of SV exocytosis (Rhee et al., 2002, Lou et al., 2008). Additionally, recent work has shown that Munc-13 also binds phosphoinositides in a Ca2+-dependent manner via its central C2B domain and that this property allows Munc13 to potentiate SV exocytosis during repeated action potentials, thereby minimizing synaptic depression induced by SV depletion (Shin et al., 2010).
Role of synaptotagmin in Ca2+-sensing and synaptic vesicle fusion
Synaptotagmin is considered the main Ca2+ sensor for Ca2+-triggered (fast) synchronous release of neurotransmitter at central synapses, but not for the (slow) asynchronous release (Pang and Sudhof, 2010, Perin et al., 1991, Brose et al., 1992, Geppert et al., 1994, Chapman, 2008). Originally identified as a 65 KDa protein enriched in SVs and LDCV (Matthew et al., 1981, Chapman, 2008), synaptotagmin-1 (Syt-1) is the founding member of a large family that includes seventeen other proteins (Chapman, 2008). Syt-1 is the most extensively studied Syt member and is believed to be the main isoform controlling SV exocytosis. Work carried out in the past fifteen years, including genetic studies in various species and recent cell-free fusion assays, suggest a complex involvement of Syt-1 in the fusion process, which can be summarized as follows: Syt-1 is believed to act as a pre-fusion “clamp” in the absence of Ca2+ (Martens et al., 2007, Chicka et al., 2008) and to accelerate SNARE-mediated fusion in the presence of the divalent cation, in part by regulating fusion pore dynamics and the final steps of fusion (Stein et al., 2007, Chapman, 2008).
Since the primary sequence of Syt-1 revealed, in addition to the single NH2-terminal transmembrane domain anchored to the SV membrane, a large cytodomain consisting of tandem C2 domains, C2A and C2B, separated by a 9-residue linker, the idea that Syt may bind and “sense” Ca2+ was put forward (Brose et al., 1992, Geppert et al., 1994). Indeed, while the C2A domain can bind to three Ca2+ ions, the C2B domain binds to two ions, in agreement with the previous notion that the Ca2+ sensor for synchronous release exhibits an apparent cooperativity of five Ca2+ ions (Bollmann et al., 2000, Schneggenburger and Neher, 2000). In addition to its role in Ca2+ sensing, SV-bound Syt-1 has been shown to play a crucial role in docking the vesicles to the plasma membrane via its interaction with SNAP-25, presumably as part of an assembled Munc18-syntaxin1/SNAP-25 acceptor complex (de Wit et al., 2009). C2 domains often bind phospholipids, thus suggesting a relationship between Ca2+ binding and membrane interactions. Indeed, the C2B domain of Syt-1 was shown to bind to phosphoinositides and the specificity of interaction of this domain for PtdIns(4,5)P2 or PtdIns(3,4,5)P3 depends on intracellular levels of Ca2+ (Schiavo et al., 1996). The relationship between the C2 domains and phospholipids has been extensively dissected by several groups using biochemical, structural and functional assays in combination with site-directed mutagenesis of the C2 domains. Indeed, in the presence of Ca2+, the C2A domain of Syt-1 interacts with anionic phospholipids, such as phosphatidylserine (PS), which triggers the insertion of the Ca2+-binding loops of C2A into lipid bilayers. It was shown that the C2B domain alone also binds weakly to PS, but binds preferentially to PtdIns(4,5)P2. C2B does not penetrate into the bilayer as a result of this interaction, it was thus suggested that the C2B domain may simply serve to tether Syt-1 to the plasma membrane (Bai et al., 2004). However, dimers of the C2A and C2B domains primarily mediate the Ca2+-dependent binding of Syt-1 to PtdIns(4,5)P2- or PtdIns4P-containing membranes and the Ca2+-dependent evoked release (Hui et al., 2009). After its insertion into the plasma membrane, bending of the membrane by Syt-1 is dependent on the C2B domain. The positive membrane curvature induced by Ca2+-bound Syt-1 facilitates the fusion of SVs to the plasma membrane by reducing the distance between the membranes and by reducing the energy barrier for their fusion (Shin et al., 2010).
3.2 Synaptic vesicle recycling
Sustained release of neurotransmitter is crucial for neuronal communication. To prevent SV depletion and a rundown of neurotransmission, SVs must be efficiently recycled in nerve terminals, particularly during prolonged stimulations. Several recycling pathways have been described, including those involving ‘kiss-and-run’ mechanisms and clathrin-mediated endocytosis (CME), which has been extensively characterized at the molecular level. This latter pathway occurs at the periphery of the active zone (also called periactive zone) and is typically triggered upon full collapse of SVs with the plasma membrane during the release process. An important implication of full fusion of SVs with the plasma membrane is that SV-associated components undergo rapid diffusion at the synaptic membrane. Thus, precise and efficient molecular mechanisms must be in place to ensure the appropriate capture and sorting of SV-associated components into endocytic vesicles in order to preserve the molecular identity and functionality of SVs.
Work carried out in the past decade has provided overwhelming evidence showing that phosphoinositides and specifically PtdIns(4,5)P2 regulate multiple aspects of SV recycling. In particular, studies on Synj1 and PtdInsP kinase type 1γ, have established that both an excess and a deficiency of PtdIns(4,5)P2 interfere with the SV cycle. At the molecular level, dysregulation of PtdIns(4,5)P2 metabolism at the synapse alters the function of a large variety of proteins that control multiple aspects of SV trafficking, including the recycling of SVs via the pathway of CME. The following section highlights how a loss of the main PtdIns(4,5)P2-metabolizing enzymes affects this process and the physiology of nerve terminals. This followed by a discussion of the mechanistic aspects underlying the pleiotropic roles of PtdIns(4,5)P2 in the process of SV recycling.
3.2.1 Synaptic vesicle recycling defects upon PtdIns(4,5)P2 imbalance
Consequences of Synj loss on nerve terminal function
The identification of Synj1 by De Camilli et al. as a partner for the SH3 domain of Grb2 (and amphiphysin), along with the endocytic fission factor dynamin, indirectly suggested an involvement of this enzyme in SV recycling (McPherson et al., 1994, McPherson et al., 1996). Conclusive evidence for a direct role of Synj in this process emanated from a genetic study in the mouse, where a null mutant was shown to produce defects in synaptic vesicle recycling (Cremona et al., 1999). At the Synj1−/− synapse, increased PtdIns(4,5)P2 levels correlated with an excess of clathrin-coated vesicles (CCVs), suggesting a delay in the uncoating reaction (Cremona et al., 1999). In agreement with defects of recycling, a deeper depression of neurotransmission was observed in hippocampal slices as well as in primary cortical cultures from null mice during a prolonged stimulation (Cremona et al., 1999, Luthi et al., 2001). Fluorescent dye (FM1-43) uptake and release assays in cultured neurons confirmed that newly endocytosed vesicles recycle with slower kinetics in nerve terminals, likely reflecting the accumulation of CCVs (Cremona et al., 1999, Kim et al., 2002, Mani et al., 2007). Strikingly, endocytic defects resulting from inactivation of Synj function were also observed in other species, such as the budding yeast (Singer-Kruger et al., 1998, Stefan et al., 2002), the worm (Harris et al., 2000) and the fly (Dickman et al., 2005, Verstreken et al., 2003) Importantly, nerve terminals from lower organisms lacking the only Synj ortholog showed pleiotropic defects in SV recycling, including an accumulation of coated and, in some cases, even non-coated pits at various stages of invagination (Verstreken et al., 2003, Harris et al., 2000). Consistent with the abovementioned studies, an experimental manipulation of the lamprey reticulospinal (giant) synapse showed an accumulation of clathrin-coated pits (CCPs) and free CCVs upon blockade of the interaction of Synj with the SH3 domain of its main interactor, endophilin, utilizing a proline-rich peptide derived from the COOH-terminal tail of mammalian Synj1 (Gad et al., 2000). Additionally, in the zebrafish nrc mutant, defects in SV trafficking were observed in the ribbon synapses of fish photoreceptors as a result of a stop mutation the Synj1 gene, although in this case, an accumulation of coated intermediates was not reported (Van Epps et al., 2004). Instead, nrc cone photoreceptor pedicle exhibited unanchored ribbons as well as a reduction in SV number and an abnormal distribution of these organelles. A more recent electron tomography study from De Camilli et al. not only showed striking evidence for an accumulation of CCVs in Synj1−/− synapse, but also highlighted a stronger requirement for Synj1 in GABAergic neurons, at least based on a morphological assessment (Hayashi et al., 2008). In summary, the endocytic function of Synj appears to be largely conserved across evolution, although the precise actions of the phosphoinositide phosphatase in this process depend on the cell or synapse type, perhaps reflecting the pleiotropic role of phosphoinositides in cell physiology.
Based on studies of Synj function in various organisms as well as of the role of PtdIns(4,5)P2 in multiple experimental systems, a main function of Synj appears to be the elimination of PtdIns(4,5)P2 from membranes during the endocytic process (Stefan et al., 2002, Cremona and De Camilli, 2001, Cremona et al., 1999, Di Paolo and De Camilli, 2006). However, Synj family members also contain an NH2-terminal Sac1 domain, which is also present in other proteins and has been shown to dephosphorylate phosphoinositides other than PtdIns(4,5)P2. These include PtdIns3P, PtdIns4P, PtdIns5P and PtdIns(3,5)P2 (Guo et al., 1999, Hughes et al., 2000). Although the original study by Cremona et al only shows an increase in PtdIns(4,5)P2 in Synj1−/− primary cortical neurons, only two phosphoinositides, namely the most abundant, were analyzed: PtdIns(4,5)P2 and PtdIns4P. Consequently, changes in the levels of other Synj1 substrates cannot be ruled out and PtdIns(4,5)P2-independent phenotypes in synapses lacking Synj cannot be excluded either. Supporting this idea, the Sac1 domain of yeast Synj-like protein 2 (Sjl2) and 3 (Sjl3) was shown to hydrolyze PtdIns3P in a physiological context, although concomitant deletion of myotubularin ortholog Ymr1p (i.e., an inositol 3-phosphatase) was required to unmask this function (Parrish et al., 2004). Importantly, a more recent study on Synj1−/− cultured neurons expressing various Synj1 mutant constructs confirmed the essential nature of the inositol 5-phosphatase domain of this enzyme, suggesting that PtdIns(4,5)P2 dephosphorylation is central to its function [although PtdIns(3,4,5)P3 dephosphorylation may also play a role]. This study also unmasked a role for the Sac1 domain of Synj1 in SV internalization, but only for brief stimuli (Mani et al., 2007). However, the physiological substrate(s) of Synj1’s Sac1 domain at synapses is (are) still undetermined. Finally, the role of the other Synj isoform, Synj2, has not been addressed at synapses.
Consequences of PtdInsPK1γ loss on nerve terminal function
Studies on Synj rapidly became a driving force to further explore the role of PtdIns(4,5)P2 at the synapse and specifically, to identify the main enzymatic source of PtdIns(4,5)P2 in this compartment. Of the three type 1 PtdIns kinase isoforms known, PtdInsPK1γ was shown to represent the main activity in the brain and was thus further characterized (Wenk et al., 2001). Evidence for a role of this enzyme in synaptic function originated from a mouse genetic study, where ablation of PtdInsPK1γ was shown to cause a deficiency of CCVs upon stimulation and an increase in surface area of horseradish peroxidase-laden endosome-like structures (likely corresponding to bulk invaginations of the plasma membrane)(Di Paolo et al., 2004). Functional correlates of these morphological phenotypes were a decrease in the rate of SV endocytosis, as measured by the synaptopHluorin technology (Sankaranarayanan and Ryan, 2000, Miesenbock et al., 1998), as well as a reduced rate of SV recycling, based on FM1-43 dye uptake and release assays (Di Paolo et al., 2004). Similarly to Synj1−/− synapses, basal synaptic transmission and short-term plasticity were preserved after ablation of PtdInsPK1γ. However, faster depression of inhibitory postsynaptic currents was observed during the first hundred action potentials of a train of stimuli (Di Paolo et al., 2004). Other electrophysiological changes consistent with exocytic defects were described in section 3.1.1.
Further suggesting an important role of PtdInsPK1γ in SV recycling was a study in the lamprey giant synapse showing that blocking the interaction between the COOH-terminal tail of this lipid kinase and the FERM domain of talin with a PtdInsPK1γ peptide (Di Paolo et al., 2002), caused the appearance of aberrant CCPs and a decrease in synaptic F-actin upon prolonged stimulation (Morgan et al., 2004). In contrast to the case of Synj, there are no studies on lower organisms indicating a role for PtdInsPK in SV trafficking. There is however a fly mutant (tweek) which is associated with lower synaptic levels and aberrant distribution of PtdIns(4,5)P2 as well as with defects in SV trafficking (Verstreken et al., 2009). Because the underlying gene does not encode a PIP kinase, the link with PtdIns(4,5)P2 metabolism has remained elusive.
3.2.2 Molecular basis for the actions of PtdIns(4,5)P2 in synaptic vesicle recycling
A major function of PtdIns(4,5)P2 metabolism is to control the process of CME. While this phenomenon is far from being specific to nerve terminals, most of our understanding of the role of PtdIns(4,5)P2 originates from studies of clathrin-mediated recycling of SVs at the synapse. Another key function of this lipid is to regulate actin dynamics. We summarize the current knowledge on how PtdIns(4,5)P2 regulates these two processes at the presynapse with a focus on molecular details.
Clathrin coat recruitment
Several studies in the late 1990’s indicated that clathrin coat components (e.g. AP-2) as well as other endocytic proteins, such as dynamin, bind to PtdIns(4,5)P2 (Gaidarov and Keen, 1999, Jost et al., 1998). However, the physiological significance of these interactions was best highlighted by a mouse genetic study on Synj1−/− synapse, which showed that increased PtdIns(4,5)P2 levels facilitate clathrin coat assembly in vitro and conversely, delay coat shedding in vivo (Cremona et al., 1999). Furthermore, ablation of the main PtdIns(4,5)P2-synthesizing enzyme at the synapse, PtdInsPK1γ, leads to a decreased association of the clathrin coat proteins with membranes in cell-free assays (Wenk et al., 2001) and a reduced number of CCVs in stimulated cultured neurons (Di Paolo et al., 2004). As a result of these studies, a model emerged in which PtdIns(4,5)P2 is a key parameter controlling the affinity of coat proteins for synaptic membranes and thus the efficacy and kinetics of SV recycling.
The clathrin coat includes both the heavy and light chains of clathrin as wells as adaptor proteins, which are essential for the recruitment of clathrin to membranes (in addition to mediating the sorting of cargo proteins into CCPs). The clathrin coat is organized into an assembly of triskelia, which consist of three heavy chains (CHC) and three light chains (CLC), at a stoichiometry of 1:1. Triskelia self-assemble into a basket-like polyhedral protein lattice of pentagons and hexagons that coat the endocytic vesicle (Kirchhausen, 2000). The initiation of CME at the plasma membrane occurs by the membrane association of a variety of adaptor proteins, all of which bind PtdIns(4,5)P2 and clathrin. The main clathrin adaptors at the synapse are discussed below, with emphasis on the best-characterized adaptor at the molecular/structural level, namely AP-2.
AP-2 is a heterotetramer consisting of α, β2, μ2, and σ2 subunits (also called adaptins) and plays a central role in clathrin-mediated internalization of multiple cargoes (Robinson, 2004). Indeed, AP-2 is considered a ‘’hub” in the endocytic network due to its multiple interactions with clathrin, accessory factors and transmembrane protein cargoes. Although several studies have indicated a critical role for AP-2 in clathrin mediated-receptor internalization, its implication in SV endocytosis was first demonstrated in a fly mutant in the α-adaptin gene (Gonzalez-Gaitan and Jackle, 1997). However, more recent studies in the worm (Gu et al., 2008) and dissociated hippocampal neurons showed that ablation/silencing of the μ2 subunit of AP-2 fails to eliminate cargo retrieval and SV recycling, although slower endocytic kinetics are observed in AP-2 depleted hippocampal synapses (Dittman and Ryan, 2009, Kim and Ryan, 2010).
In the current model, PtdIns(4,5)P2–containing membranes play a pivotal role in switching AP-2 from a closed (or locked) conformation to an open, ligand-bound conformation (Jackson et al., 2010b, Kelly et al., 2008, Collins et al., 2002). The first step in AP-2 activation involves its recruitment to the plasma membrane through an interaction of basic residues from its α and β2 subunits with PtdIns(4,5)P2. These electrostatic interactions facilitate the binding of μ2’s COOH terminus with the membrane, which subsequently allows AP-2 to adopt an open conformation and interact with sorting signals from transmembrane cargo proteins (Jackson et al., 2010b). A key effect of this activation is to dislodge the β2 subunit, which acts as a “latch” and can no longer block the two peptide ligand-binding sites in the active conformation. These in turn can freely bind to YxxF or [ED]xxxL[LI] motifs that belong to cargo proteins, resulting in high affinity (i.e., low nM) interactions of the AP-2 complex with the plasma membrane (Jackson et al., 2010b). Further underscoring the importance of PtdIns(4,5)P2 in the activation of AP-2, this clathrin adaptor has been shown to directly bind to PtdInsP K1γ, suggesting the occurrence of a positive feedback loop controlling PtdIns(4,5)P2 production in proximity to sites of AP-2 recruitment. Specifically, PtdInsPK1γ was shown to bind to the μ2 subunit of AP-2, and this binding stimulates its kinase activity (Krauss et al., 2006, Bairstow et al., 2006). Additionally, the long isoform of PtdInsPK1γ (specify which one here) also interacts with the appendage domain of the β2 subunit of AP-2, promoting its catalytic activity only its COOH-terminal extension is in a dephosphorylated state (Nakano-Kobayashi et al., 2007), This same binding platform of β2 also interacts with the heavy chain of clathrin in a mutually exclusive manner (Thieman et al., 2009). It is of note that the other two PtdInsPK1 isoforms, α and β, also interact with AP-2, suggesting that the ability to interact with the clathrin coat is a general feature of PtdInsPK1s (Krauss et al., 2006).
Recruitment of clathrin also occurs via monomeric adaptors, such as AP180 and Clathrin Assembly Lymphoid Myeloid (CALM), which both harbor an AP180 N-terminal homology (ANTH) domain. This NH2-terminal module contains a series of surface basic residues that interact with phosphoinositides, including PtdIns(4,5)P2, while the central and COOH-terminal portions of AP180 bind to CHC and AP-2, via a variety of peptide motifs. Functional evidence for a role of AP180/CALM in SV recycling has so far been only obtained in the worm and the fly, where mutations in the only ortholog result in an alteration of the size of CCVs and SVs as well as missorting and higher cell surface levels of SV protein, synaptobrevin (Harel et al., 2008, Dittman and Kaplan, 2006, Zhang et al., 1998, Nonet et al., 1999). Thus, AP180/CALM likely regulate both cargo recruitment and the size of the membrane area destined to be internalized at synapses. Other clathrin adaptors, such as epsins, are endowed with membrane deforming properties and will be discussed in section 5.2.
Finally, the F-BAR domain–containing Fer/Cip4 homology domain-only proteins 1 and 2 (FCHo1/2) may play an important role in the regulation of the number of endocytic sites. These proteins possess an NH2-terminal F-BAR domain (see section 5.2) and a COOH-terminal μ2-homology domain. Remarkably, FCHo1/2 are recruited to CCPs concomitantly with Eps15, intersectin and AP-2, but prior to clathrin. Their F-BAR domain is extended with a region that is enriched in basic residues and preferentially interacts with PtdIns(4,5)P2, targeting these proteins to the plasma membrane (Henne et al., 2010).
Membrane curvature generation and stabilization
Generation of membrane curvature during the endocytic process is believed to be driven by clathrin assembly per se and by the action of various families of proteins endowed with membrane deforming capacity. This latter property was originally shown for the fission factor dynamin (Takei et al., 1995) and its SH3 domain-containing interactor amphiphysin, a Bin/ Amphiphysin/ Rvs (BAR) protein (Takei et al., 1999). However, the past decade has witnessed an expansion in the catalog of endocytic proteins with similar properties and share the ability to bind PtdIns(4,5)P2, but with differential selectivity and affinity.
The discovery of the membrane deforming property of BAR proteins, such as amphiphysin (Takei et al., 1999) and subsequently endophilin (Farsad et al., 2001), preceded the structure determination of the BAR domain (Peter et al., 2004). However, the latter not only provided a structural basis for the observed effects of this domain on lipid bilayers, but it also introduced the concept of curvature sensing/stabilization for BAR proteins. Indeed, the BAR domain of amphiphysin was originally identified as a crescent-shaped dimer with positively charged residues lining its concave surface. This banana shape of the BAR domain was shown to prefer highly curved substrate liposomes and primarily uses electrostatic forces to bind negatively charged lipids, such as PS or PtdIns(4,5)P2 (Peter et al., 2004). The BAR family of proteins has now been expanded and can be subdivided into several classes, based on differing structural features: (i) N-BAR proteins (e.g., amphiphysin, endophilin); (ii) F-BAR proteins (e.g., syndapin); (iii) I-BAR proteins; and (iv) PX-BAR proteins represented by several isoforms of the Sorting Nexin (SNX) family (SNX 1,2,5,6 and 9) (Shimada et al., 2007, Frost et al., 2008, Weissenhorn, 2005, Gallop et al., 2006, Peter et al., 2004, Frost et al., 2009). Members in each of these classes have been implicated in membrane trafficking and/or endocytic processes. The N-BAR proteins, in particular, play an important role in phosphoinositide metabolism not only because their N-BAR domain binds these lipids, but also because well-characterized members of this subfamily, such as endophilin and amphiphysin, possess a COOH-terminal SH3 domain that physically interacts with Synj (Chang-Ileto et al., 2011, Gad et al., 2000, Ringstad et al., 1997). Importantly, several studies have established the functional significance of the Synj1-endophilin interaction in various species, including the worm (Jorgensen et al., 1995), the fly (Verstreken et al., 2003); the lamprey (Gad et al., 2000) and the mouse (Mani et al., 2007).
Although endophilin’s N-BAR domain binds to PtdIns(4,5)P2, it interacts in vitro with various acidic phospholipids via the concave face of this module, which is lined with basic residues (Chang-Ileto et al., 2011, Weissenhorn, 2005, Mattila et al., 2007, Tsujita et al., 2006, Itoh et al., 2005, Peter et al., 2004, Gallop et al., 2006). Since the interaction with membranes is largely electrostatic in nature and that the plasma membrane has an overall negative surface charge [in part because of the greater content in PtdIns(4,5)P2], endophilin primarily tubulates the plasma membrane (Chang-Ileto et al., 2011). While the relatively late recruitment of endophilin to CCPs (i.e., at a time immediately preceding the fission process) argue against a primary role of the tubulating activity of its N-BAR domain in the budding process, it may be involved in stabilizing membrane curvature at sites of endocytosis, potentially in concert with other BAR proteins and/or the fission factor, dynamin (Perera et al., 2006). A key function of endophilin, however, appears to be the recruitment of Synj to sites of endocytosis, thus controlling PtdIns(4,5)P2 elimination during the endocytic process. This idea is supported by biochemical, morphological and physiological data from various species and research groups (Micheva et al., 1997, Gad et al., 2000, Verstreken et al., 2003, Schuske et al., 2003, Dickman et al., 2005, Chang-Ileto et al., 2011, Perera et al., 2006). Ablation of endophilin recaptitulates many of the features observed upon ablation/ inactivation of Synj, including the accumulation of CCPs at various stages, including free CCVs. The interaction between endophilin 1 and Synj1 is regulated by phosphorylation/ dephosphorylation of Synj1 by the protein kinase Cdk-5 and the phosphatase calcineurin. Additionally, endophilin stimulates the PtdIns(4,5)P2 5-phosphatase activity of Synj1 (Chang-Ileto et al., 2011, Lee et al., 2004b) and this phenomenon is enhanced by small liposomes (i.e., 50 nm in diameter), suggesting that robust stimulation of Synj1 by endophilin 1 may occur at sites of high curvature, namely the neck (or perhaps the bud) of the endocytic pit (Chang-Ileto et al., 2011) (Figure 2). Endophilin may also have additional functions at nerve terminals which are unrelated to endocytosis, as suggested by a recent study (Bai et al., 2010). Other BAR proteins, such as PX-BAR member SNX9, also bind to phosphoinositides, although both PX and BAR domains are involved in these interactions with lipids and are required for the proper localization of SNX9 to CCPs (Yarar et al., 2008).
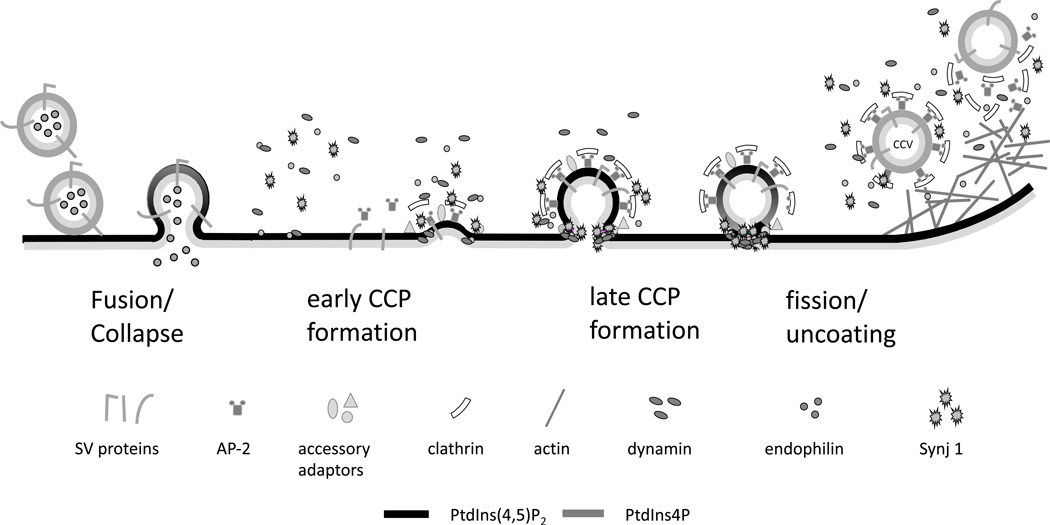
Fig. 2. PtdIns(4,5)P2 role in endocytosis.
The PtdIns4P-enriched membrane of the SV fused and merged with the PtdIns(4,5)P2-enriched PM liberating the neurotransmitter extracellularly. AP-2 is recruited by PtdIns(4,5)P2 with other accessory clathrin adaptors to the surface of the PM. The PM buds and invaginates to form a Ω-shaped pit covered by clathrin lattice and that comprised the SV proteins. Fission factor, dynamin gets recruited to the bud with endophilin and synaptojanin. Dynamin mediates the scission of its neck to release a free clathrin-coated vesicle (CCV), possibly facilitated by the hydrolysis of PtdIns(4,5)P2 by synaptojanin1 (Synj1). The PtdIns(4,5)P2 present on the CCV is dephosphorylated to PI(4)P by Synj1, thereby promoting the shading of the adaptors from the membrane and the uncoating reaction.
In addition to BAR proteins, epsin (Eps15 interacting protein) family members may also contribute to curvature generation/stabilization at sites of endocytosis, particularly epsin 1. Epsins interact with the CHC, the appendage domain of α-adaptin and the EH domain of Eps15. Epsins also harbor ubiquitin-interacting motifs, which may help to internalize ubiquitinated cargo proteins (Shih et al., 2002). Importantly, the NH2-terminal ENTH domain of epsins, which is distinct from the ANTH domain of AP180/ CALM, contains an unstructured NH2-terminal sequence that folds into an α-helix upon interaction with PtdIns(4,5)P2. This α-helix inserts itself into lipid bilayers, thus providing ENTH domains with the ability to deform membranes in vitro, a function that may be important for the budding of CCPs in physiological contexts (Ford et al., 2002, Itoh et al., 2001). Consistent with this idea, inactivation of epsin by antibody microinjection into the lamprey giant synapse produces a stimulation-dependent accumulation of large CCPs (Jakobsson et al., 2008). However, loss of epsin in the fly mutant “Liquid Facets” does not seem to alter SV recycling (Bao et al., 2008) and it is unclear whether epsin null mice have SV endocytic defects (Chen et al., 2009).
In conclusion, membrane curvature needed for CCV formation is likely to be generated through clathrin assembly as well as via the coordinated action of several proteins containing ENTH, F-BAR and N-BAR modules. PtdIns(4,5)P2 not only plays an important role in the targeting of these proteins to the cell surface, but also, in the case of epsins, in altering the conformation of these proteins/modules, which in turn facilitate curvature generation.
Endocytic Fission
The fission of CCPs during the process of SV endocytosis is believed to be mediated by the large GTPase dynamin. This protein was first identified as critical in neuronal function in temperature-sensitive mutants in the fly (“Shibire”)(Chen et al., 1991, van der Bliek and Meyerowitz, 1991, Grigliatti et al., 1973, Suzuki et al., 1971) and purified through its association with microtubules in vitro (Obar et al., 1990, Shpetner and Vallee, 1989). Dynamin contains an NH2-terminal GTPase domain, a PH domain that binds to PtdIns(4,5)P2, a middle GTPase effector domain (GED) that is important for oligomerization, and a COOH-terminal proline-rich domain, which interacts with the SH3 domain of several endocytic proteins, including endophilin, amphiphysin, intersectin, syndapin and SNX9 (Slepnev and De Camilli, 2000). Dynamin has been established as an endocytic factor critical for membrane fission based on the expression of dominant-negative mutants (Yamashita et al., 2005, Newton and Messing, 2006, Koenig and Ikeda, 1999). However, deletion of dynamin1, the major dynamin isoform in the brain only partially impairs the retrieval of SVs during strong stimulation (Ferguson et al., 2007). SV endocytosis occurs after its cessation or during mild stimulation, most likely due to the low expression of dynamin 3, which is relocalized at the synapse in absence of dynamin 1 (Ferguson et al., 2007).
The relationships between dynamin and phophoinositide metabolism are three-fold. First, as mentioned above, the PH domain of dynamin binds to PtdIns(4,5)P2 and this interaction is critical for dynamin’s function, at least in part because it allows dynamin to interact with the plasma membrane (Roux et al., 2006, Lee et al., 1999). Second, PtdIns(4,5)P2 stimulates the GTPase activity of dynamin (Zheng et al., 1996). Finally, our recent study has shown that acutely-induced PtdIns(4,5)P2 dephosphorylation of endophilin-coated tubules by the 5-phosphatase domain of Synj1 triggers their fragmentation in a dynamin-dependent fashion, suggesting that PtdIns(4,5)P2 hydrolysis facilitates membrane fission (Chang-Ileto et al., 2011). While the precise molecular basis for this phenomenon is unclear, we have hypothesized that an acute loss of PtdIns(4,5)P2 may help to disassemble dynamin scaffolds from membranes. This disassembly of dynamin, which is dependent on dynamin’s own GTPase activity, appears to permit membrane fission by destabilizing the underlying constricted bilayer membranes (Pucadyil and Schmid, 2008, Bashkirov et al., 2008). In addition, rapid depletion of PtdIns(4,5)P2 by Synj1 selectively at endocytic sites [(presumably at the bud neck (Sundborger et al.)] may produce a transient gradient of this lipid between the globular part of the bud and its neck, which in turn may cause lipid phase separation between these compartments (Liu et al., 2006). The predicted outcome of this rapidly-induced heterogeneity in lipid composition is the generation of interfacial forces at the CCP-bud neck interface, resulting in the squeezing of the lipid domain boundary and facilitating membrane fission (Liu et al., 2010).
Clathrin coat shedding
Once a CCV has been released by the fission process, the free vesicle undergoes uncoating in order to recycle clathrin coat components (as well as other endocytic factors) and to produce a new SV. The uncoating of the clathrin coat is triggered by the concerted action of the molecular chaperone Hsc70 and the DNA-J factor auxilin, which mediates the recruitment of the ATPase to the CCV (Eisenberg and Greene, 2007, Yim et al., 2010). A major breakthrough in the field of SV endocytosis was the original characterization of nerve terminals from Synj1−/− mouse neurons showing an increased number of CCVs. Drawing upon studies showing that clathrin adaptor AP-2 and dynamin bind to PtdIns(4,5)P2 (Jost et al., 1998, Gaidarov and Keen, 1999), the hypothesis that PtdIns(4,5)P2 elimination by Synj1 may facilitate the shedding of clathrin adaptors was proposed (Cremona et al., 1999). Subsequent studies have confirmed this phenotype, although loss of Synj1 (or its orthologs in other genetic models) is now known to produce pleiotropic defects in SV recycling, as testified by the report of aberrant numbers of clathrin-coated structures at multiple stages of invagination in affected synapses. This is consistent with the complexity and multitude of PtdIns(4,5)P2 actions in the recycling process.
While the hydrolysis of PtdIns(4,5)P2 may suffice to destabilize the clathrin coat, it may not be sufficient to trigger its complete disassembly, partly because clathrin spontaneously forms triskelia together with its adaptors. As mentioned above, Hsc70 facilitates the uncoating of clathrin alongside auxilin. Interestingly, auxilin contains an inactive PTEN-homology domain that is necessary for its targeting to clathrin-coated membranes and binds to phosphoinositides, most notably to PtdIns4P and, to a lower extent, PtdIns(4,5)P2 (Guan et al., 2010, Massol et al., 2006). A burst of recruitment of auxilin to the CCVs occurs after the peak of recruitment of dynamin at the CCVs via the binding of the PTEN-homology domain to membranes (Massol et al., 2006). Because Synj1 is recruited to CCPs concomitantly with dynamin (Perera et al., 2006), it is tempting to speculate that the PtdIns(4,5)P2-to-PtdIns4P conversion mediated by Synj (following its recruitment by endophilin) may not only facilitate membrane fission (Chang-Ileto et al., 2011), but also represent a signal for the recruitment and activation of auxilin, and thus, the uncoating process (Guan et al., 2010). This mechanism would also allow for a tight coupling between the fission and uncoating steps of CCVs.
Actin dynamics
It has long been known that phosphoinositides are primary regulators of actin dynamics, largely because a significant number of actin regulatory proteins bind to these lipids, PtdInds(4,5)P2 in particular (Saarikangas et al., 2008, Yin and Janmey, 2003). A commonly accepted view is that PtdInds(4,5)P2 predisposes actin for polymerization by the nucleation of F-actin by the actin-related protein 2 and 3 (Arp2/3) complex, the removal of capping proteins and the dissociation of actin monomer-profilin complexes (Saarikangas et al., 2008, Yin and Janmey, 2003). Actin plays fundamental roles in multiple aspects of neuronal function, including neurite outgrowth and pathfinding, organelle trafficking, and dendritic spine morphogenesis. It also plays an important role presynaptically, where it functions in the structural organization of the active zone. At the subcellular level, F-actin localizes predominantly around the pool of releasable SVs and in the endocytic (periactive) zones, at least in some large synapses (Gaffield et al., 2006, Richards et al., 2004, Schafer, 2002, Morgan et al., 2004, Bloom et al., 2003). Accordingly, several key endocytic proteins, such as amphiphysin (Yamada et al., 2009), either interact with actin regulators or are actin regulators themselves (review by (Schafer, 2002). Remarkably, acute manipulations of actin dynamics in mammalian hippocampal synapses with actin drugs have relatively minor consequences on the SV cycle (Dittman and Ryan, 2009, Sankaranarayanan et al., 2003). Indeed, the only phenotype caused by a treatment with the actin depolymerizing drug latrunculin-A in this model system is a slight acceleration of the rate of SV exocytosis, with no effects on the rate of SV internalization (Sankaranarayanan et al., 2003). However, a clear role for actin in SV recycling was demonstrated at other types of synapses, such as the lamprey giant synapse, where injections of phalloidin (i.e., an F-actin stabilizing drug) and the Clostridium botulinum C2 toxin (i.e., an actin ADP-ribosylation factor that prevents the polymerization of actin) were shown to cause the accumulation of CCPs with wide neck and the expansion of the plasma membrane (Shupliakov et al., 2002). It is thus not surprising that in such synapses with a strong requirement for actin a role for PtdIns(4,5)P2 was also demonstrated. Indeed, injection of a peptide that competes for the binding of PtdInsPK1γ to the FERM domain of talin, an adaptor between integrin and the actin cytoskeleton (Di Paolo et al., 2002), results in the disorganization of the actin network in the periactive zone and produces both an increase in the number of unconstricted CCPs and a depletion of SVs upon stimulation (Morgan et al., 2004). Furthermore, in recent work at the drosophila neuromuscular junction, PtdIns(4,5)P2 was shown to restrict the size of nerve terminals by controlling the localization of WASP, a stimulator of actin nucleation by the Arp2/3 complex (Khuong et al., 2010). Finally, consistent with the ability of this lipid to also promote actin polymerization (Di Paolo and De Camilli, 2006, Yin and Janmey, 2003), abnormally high levels of actin-like cytomatrix were found in nerve terminals from Synj1−/− mice (Cremona et al., 1999) and in the giant synapse from the lamprey following a microinjection of inhibitory antibodies against Synj (Gad et al., 2000), although these studies did not directly address the consequences of such cytoskeletal anomalies on the traffic of SVs.
4. POSTSYNAPTIC ROLES OF PHOSPHOINOSITIDES AT EXCITATORY SYNAPSES
Although PtdIns(4,5)P2 is enriched at the dendritic spines (Horne and Dell'Acqua, 2007), little is known about its metabolism and the role of this lipid at the postsynapse. PtdIns(4,5)P2 has been mostly characterized in light of its role in signal transduction as a PLC substrate downstream of stimulated metabotropic receptors (Rebecchi and Pentyala, 2000). However, as at the presynapse, PtdIns(4,5)P2 metabolism controls actin dynamics, which, at the postsynapse, mediates changes in dendritic spine morphology, as well the traffic of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors. Critical for some paradigms of synaptic plasticity is the phosphorylation of PtdIns(4,5)P2 to PtdIns(3,4,5)P3 by PI3Ks and the converse reaction by PTEN. These aspects are discussed in this section.
4.1 Regulation of spine morphology and actin assembly
As mentioned above, PtdIns(4,5)P2 is a critical regulator of actin dynamics as most actin regulatory proteins interact with and are controlled by this lipid. Dendritic spines are sites of intense actin dynamics, which, together with intracellular and synaptic membrane remodeling, contribute to changes in spine morphology. A major regulator of spine dynamics appears to be Myristoylated-Alanine-Rich C Kinase Substrate (MARCKS), which is an F-actin and Ca2+/Calmodulin (CaM)-binding protein that interacts with the plasma membrane via the dual actions of a hydrophobic, myristoylated NH2 terminus and a polybasic stretch mediating electrostatic interactions with anionic phospholipids (McLaughlin et al., 2002). Through these interactions, MARCKS can laterally sequester PtdIns(4,5)P2 molecules within the membrane. Several serine residues of the effector domain of MARCKS can be phosphorylated by the protein kinase C (PKC), which decreases the electrostatic interaction with phosphoinositides and triggers the translocation of MARCKS into the cytosol (Arbuzova et al., 2002). Interfering with MARCKS expression and function through RNAi or expression of dominant-negative mutants results in destabilization of the spines and disruption of the actin cytoskeleton (Calabrese and Halpain, 2005). A model arising is that synaptic activity-induced activation of PKC leads to the phosphorylation and relocation of MARCKS into the cytosol. This in turns causes the release of PtdIns(4,5)P2 and the reorganization of the actin skeleton, possibly via a pathway involving the N-WASP-Cdc42-Arp2/3 complex and forming new F-actin branches on mother filaments. Decrease of Arp2/3 by silencing the p34 subunit of this complex leads to a reduction in the number of spines and the elongation of the remaining ones. A similar phenotype is observed by silencing N-WASP or Cdc42 (Wegner et al., 2008). Other postsynaptic PtdIns(4,5)P2 -binding proteins, such as AKAP79/150, may operate through mechanisms similar to those controlling MARCKS function (Gomez et al., 2002, Dell'Acqua et al., 1998, Horne and Dell'Acqua, 2007).
Among many actin- and phosphoinositide-binding proteins, Cosediment with Filamentous Actin (cofilin, also known as actin depolymerizing factor) has been extensively studied in the postsynapse. Cofilin family members bind to ADP-actin (in the G- or F forms) and promote the depolymerization at the pointed ends of actin filament (Ono, 2007). They also sever actin filament in a Ca2+-independent manner, which increases the number of filament ends (dos Remedios et al., 2003). The actin-binding domains of cofilin also interacts with PtdIns(4,5)P2 (Yonezawa et al., 1991, Kusano et al., 1999) and thus the actin-binding ability of cofilin is inhibited by this lipid (Yonezawa et al., 1991). LIM kinase and the phosphatase Slingshot phopshorylate/dephosphorylate cofilin, which leads to its inactivation or its activation, respectively (Niwa et al., 2002, Yuen et al., 2010). During long-term potentiation (LTP), cofilin is inactivated by phosphorylation, which promotes actin polymerization (Fukazawa et al., 2003). In contrast, during long-term depression (LTD), dephosphorylation of cofilin is required for spine shrinkage (Zhou et al., 2004) and for the depression of N-Methyl-D-aspartate (NMDA) receptor-dependent current (Morishita et al., 2005). During chemically-induced synaptic potentiation, cofilin is first dephosphosylated, which coincides with insertion of AMPA receptors to the synaptic membrane and an increase in the number of actin barbed ends. When cofilin is re-phosphorylated, enlargement of the spines is observed (Gu et al., 2010). Forebrain-specific ablation of cofilin in the mouse has recently been show to cause an impairment of associative learning, demonstrating genetically the role of actin dynamics during memory processes (Rust et al., 2010).
4.2 PtdIns(4,5)P2 in signaling mechanisms downstream of metabotropic glutamate receptors
Neurotransmitters regulate post-synaptic elements with different kinetics depending on whether they activate ionotropic receptors or metabotropic receptors. Generally, the latter types are receptors coupled to heteromeric G-proteins and characterized by seven α-helical transmembrane domains. Those receptors mediate their effects primarily by the activation of different small G-proteins and a large variety of downstream effectors. Class I metabotropic glutamate receptors (mGluR), which include mGluR1 and mGluR5, are key modulators of synaptic transmission and plasticity (Ferraguti et al., 2008). They localize to the periphery of the post-synaptic density (PSD), in part through an interaction of their proline-rich COOH-terminus with scaffolding proteins Homer, which in turn interacts with Shank and PSD-95 (Brakeman et al., 1997, Tu et al., 1999) (Figure 1). At excitatory synapses, type 1 mGluRs mainly exert their actions through stimulation of PLCβ, which follows the release and activation of Gq proteins (Ferraguti et al., 2008). PLCβ, similar to other phosphoinositide-specific PLC isoforms, hydrolyzes PtdIns(4,5)P2 to generate DAG and Ins(1,4,5)P3. These two second messengers initiate distinct signal transduction pathways through activation of PKC (as well as other C1 domain-containing DAG effectors) and of intracellular Ca2+ release via activation of the Ins(1,4,5)P3 receptor, respectively. Other metabotropic receptors, such as the muscarinic acetylcholine receptors, also operate via the Gαq/PLC pathway and are important for the modulation of synaptic transmission (Seol et al., 2007, Giessel and Sabatini, 2010). At some synapses, LTD is not induced when PLC is blocked pharmacologically or upon genetic ablation of PLCβ1 (Choi et al., 2005, Reyes-Harde and Stanton, 1998). Importantly, activation of type 1 mGluRs and the NMDA receptor can independently stimulate PLCs (with strong stimulation of NMDA receptor activating the Ca2+-sensitive isoform PLCδ), thus leading to the stimulation of PKC in dendrites (Codazzi et al., 2006). PLC is also important for NMDA-induced spine shrinkage and synaptic depression via the depletion of PtdIns(4,5)P2 and the decrease of F-actin (Horne and Dell'Acqua, 2007).
A fundamental aspect of GPCR regulation involves their desensitization following their stimulation by ligands (DeWire et al., 2007). The mechanism of ligand-dependent silencing or desensitization of the GPCR signaling is highly conserved and occurs via the phosphorylation of COOH-terminal serine/threonine residues via G-protein receptor kinases, followed by the recruitment of phosphoinositide binding protein β-arrestin. Binding of β-arrestin occludes the sites of the activated receptor that interact with G proteins and therefore limits the responsiveness of the GPCRs to repeated stimulations. Importantly, the binding of β-arrestin to the GPCRs exposes the β-arrestin C-terminus, which can then bind to both clathrin and the β-subunit of AP-2 and trigger the internalization of the phospho-GPCRs via CME. Similar to other endocytic adaptors for clathrin, β-arrestin interacts with phosphoinositides and PtdIns(4,5)P2 and PtdIns(3,4,5)P3 in particular. Mutation of the phosphoinositide-binding pocket of β-arrestin does not affect its interaction with clathrin or GPCRs, but it prevents the internalization of the activated β-adrenergic receptors (Gaidarov et al., 1999). Moreover, β-arrestins do not only interact with phosphoinositides but they also directly recruit PIP5K1α to sites of GPCR endocytosis (Nelson et al., 2008). While much less studied compared to the β-adrenergic receptor, the agonist-induced internalization of mGluR1 likely occurs via the β-arrestin and dynamin pathway in neurons, as shown in heterologous systems (Dale et al., 2001, Mundell et al., 2001). In addition, β-arrestin might be responsible for the termination of DAG signal by recruiting a diacylglycerol kinase in a mechanism similar to that observed after activation of the Gq protein-coupled M1 receptor (Nelson et al., 2007).
4.3 Role of PtdIns(4,5)P2 in the trafficking of AMPA receptors
At excitatory synapses, fast response to glutamate is mainly mediated by the AMPA receptor. The second ionotropic glutamate receptor, NMDA receptor, is activated secondarily to AMPA-dependent depolarization and permits Ca2+ influx that has an important role in modulating synaptic function. There are four AMPA receptor genes (GluA1–4), whose products form a heterotetrameric cationic channel consisting generally of two different subunits (e.g., GluA1/GluA2 and GluA2/GluA3 are the most abundant in mature synapses (Wenthold et al., 1992, Lu et al., 2009). The COOH-terminal domains of the four subunits are divergent in length and sequence and are the main regions by which the AMPA receptor binds to a variety of auxiliary and regulatory proteins (Fukata et al., 2005) These proteins control key aspects of the AMPA receptor function, including its trafficking and signaling properties.
Role of phosphoinositides in the control of cell surface levels of the AMPA receptor
Because the AMPA receptor exclusively operates at the postsynaptic plasma membrane, a key aspect of its regulation involves the control of its cell surface levels via exo-endocytosis (Newpher and Ehlers, 2008). The internalization of the AMPA receptor largely occurs through clathrin- and dynamin-mediated endocytosis (Lee et al., 2004a, Morishita et al., 2005, Kastning et al., 2007) and thus the molecular machinery mediating this process shares many features in common with that controlling SV endocytosis. Just like endocytic zones are segregated from the exocytic (or active) zones at the presynapse, the postsynaptic sites of endocytosis appear to be extra-synaptic as CCPs are mostly observed outside of the PSD (Blanpied et al., 2002). Constitutive endocytosis of the AMPA receptor in proximity to the PSD is required to recapture the receptors that have “escaped” from the PSD through lateral diffusion (Lu et al., 2007). The COOH-terminal domain of the short-tailed subunit, GluA2, directly binds to the μ2 subunit of the AP-2 complex and this interaction is required for AMPA receptor internalization (Kastning et al., 2007, Lee et al., 2004a). Consistent with the principles governing the clathrin-mediated retrieval of SVs, a role for PtdIns(4,5)P2 was demonstrated by studies on Synj1. While the first study showed a role for Synj1 downstream of ephrinB-EphB signaling in the internalization of AMPA receptor (Irie et al., 2005), the second, more recent study utilized Synj1 KO neurons to show enhanced amplitudes of AMPA responses as well as a decrease in NMDA-induced internalization of AMPA receptor in mutant synapses (Gong and De Camilli, 2008).
The COOH-terminal tail of GluA2 and GluA3 also interacts with PDZ domain-containing proteins, such as PICK1 (Protein Interacting with C Kinase 1) and GRIP1/2 (Glutamate Receptor Interacting Protein 1 and 2). The former was shown to interact with phosphoinositides (Jin et al., 2006). PICK1 and GRIP1/2 antagonistically control the association of AMPA receptors with the PSD and the cell surface with fundamental implications for synaptic plasticity. While GRIP1/2 stabilizes the AMPA receptor at the synaptic membrane (Osten et al., 2000) or facilitates the reinsertion at the surface of internalized AMPA receptor via the exocyst complex (Mao et al., 2010), PICK1 facilitates the removal of the receptor from the cell surface and maintains it in an intracellular compartment (Lin and Huganir, 2007). A switch from GRIP1/2 to PICK1 and a resulting untethering of GluA2 from the PSD occurs when the serine 880 of the receptor is phosphorylated by protein kinases, such as PKCα(Chung et al., 2000, Seidenman et al., 2003). Conversely, PICK1 blocks the reinsertion of internalized AMPA receptor at the PSD (Citri et al., 2010), a process that is important for NMDA-dependent LTD (Terashima et al., 2008). The interaction of GluA2 with GRIP or PICK is also important for the replacement of GluA2-lacking receptor newly inserted in the perisynaptic region with GluA2-containing receptor (Yang et al., 2010) and with the maintenance of Ca2+-permeable AMPA receptor at sensory synapses (Clem et al., 2010). PICK1 contains an NH2-terminal PDZ domain and a central BAR domain, both of which bind to phosphoinositides. The BAR domain preferentially interacts with monophosphorylated phosphoinositides, such as PtdIns3P, via a series of acidic residues on the concave side of the crescent-like domain (Jin et al., 2006). The BAR domain is crucial for the localization of PICK1 (Jin et al., 2006) and for the induction of LTD (Jin et al., 2006, Steinberg et al., 2006). Similarly, the PDZ domain of PICK1 binds to PtdIns(4,5)P2 and PtdIns(3,4,5)P3 via three basic residues and a neighboring hydrophobic Cys-Pro-Cys motif. Mutation of the two cysteine residues into glycines impairs the formation of PICK1 clusters in non-neuronal cells and at excitatory synapses (Pan et al., 2007).
Phosphoinositide-dependent extrasynaptic clustering of TARP-AMPA receptor complexes
TARPs (Transmembrane AMPA Receptor Regulatory Proteins) are auxiliary subunits that regulate the trafficking and the kinetic properties of AMPA receptors (Milstein and Nicoll, 2008). Genetic deletion of TARPs, such as γ2 (stargazin) and γ8, results in the reduction of glutamatergic neurotransmission (Hashimoto et al., 1999, Rouach et al., 2005). Phospholipids, such as phosphoinositides, play a central role in this regulation. TARPs bind to the transmembrane region and the extracellular portions of all the AMPA receptor subunits and these interactions are required for the proper trafficking of the AMPA receptor from the endoplasmic reticulum to the synapse. The last four amino-acids of the cytosolic COOH-terminus (TTPV) of TARPs bind to the PDZ domain of PSD-95, a master regulator of the PSD, and this interaction is necessary (but not sufficient) for efficient targeting of the AMPA receptors to the PSD (Cuadra et al., 2004). The COOH-terminus of stargazin binds to phosphoinositides and PA via conserved arginine residues and this interaction is competed by PSD-95 (Sumioka et al., 2010) or disrupted by Calcium/Calmodulin-dependent Kinase II (CamKII)- or PKC-mediated phosphorylation of adjacent serine residues. Phosphorylation of this tail reduces the electrostatic interaction of stargazin with the anionic phospholipids within the membranes, thus permitting the diffusion and the binding of TARP with the postsynaptic scaffolding proteins, such as PSD-95. As a result, AMPA receptors are both immobilized and activated at the PSD (Opazo and Choquet, 2010). In summary, the regulation of the interaction between AMPA receptor and TARPs by phosphoinositides and phosphorylation-based mechanisms appears to be a key method to control the mobility of AMPA receptors in or out of the synapses during synaptic plasticity.
4.4 Role of PtdIns(3,4,5)P3 in synaptic plasticity
Class 1 PI3Ks and their downstream effectors are present at the postsynapses (Raymond et al., 2002) and are part of the complex organized around the AMPA receptor (Man et al., 2003, Peineau et al., 2007). In fact, recent work has shown that a constant production of PtdIns(3,4,5)P3 is essential for the maintenance of AMPA receptor at the synapse. Both in hippocampal slices and in cultured hippocampal neurons, manipulations of the metabolism of PtdIns(3,4,5)P3 alter AMPA receptor-dependent responses. Expression of constitutively active (i.e., membrane-bound) PI3K increases AMPA currents, while inhibition of PI3K or sequestering PtdIns(3,4,5)P3 with the PH domain of General Receptor for Phosphoinositides 1 (GRP1) reduces the amplitude of evoked currents (Arendt et al., 2010). Mechanistically, basal PtdIns(3,4,5)P3 levels appear to control the stability of PSD-95 in the spine and the mobility of AMPA receptor at the PSD. Depleting PtdIns(3,4,5)P3 promotes the relocation and the accumulation of the AMPA receptor in the extrasynaptic zones of the membrane (Arendt et al., 2010).
Similar to CamKII, PI3K is also an essential regulator of synaptic plasticity. Pharmacological inhibition of PI3K prevents the formation of LTP at various synapses, such as at the CA3-CA1 synapse (Raymond et al., 2002, Sanna et al., 2002), at cortical synapses in the lateral amygdala (Lin et al., 2001) and at parallel fibers-Prukinje cell synapses (Jackson et al.). LTP is also impaired after sequestering PtdIns(3,4,5)P3 with overexpression of PH-GRP1 (Arendt et al., 2010). These results are consistent with the impairment in learning and memory observed in mice treated with PI3K inhibitors (Lin et al., 2001, Chen et al., 2005). During LTP, PI3K activity is increased by the small GTPase Ras (Qin et al., 2000), a pathway that is also impaired in the Fragile X syndrome, which is associated with mental retardation (Hu et al., 2008). During glycine-induced LTP, the activity of PI3K associated with the AMPA-receptor is increased, which regulates the insertion of AMPA receptor at the synaptic membrane (Man et al., 2003). PI3Ks are also involved in some forms of LTD through their association with the mGluR5-Homer complex, which mediates synaptic plasticity in the nucleus accumbens during alcohol consumption (Cozzoli et al., 2009) or is important to limit LTD at the synapse receiving the conditioning stimulus (Daw et al., 2002).
The downstream effectors of the PI3K pathway involved in synaptic plasticity have also been investigated. It is well accepted that PtdIns(3,4,5)P3 generated by PI3Ks recruits to membranes phosphoinositide-dependent kinase 1 (PDK1) and Akt (or PKB) via their PH domain (Pearce et al., 2010). Downstream of phosphorylated Akt, Glycogen synthase kinase-3β (GSK3β) has a central role in the balance between NMDA-dependent LTD and LTP. Indeed, the LTP induced by activation of PI3K/Akt promotes the phosphorylation of Ser9 of GSK3β, thereby inhibiting its activity and the induction LTD for a period of an hour. During LTD, activation of phosphatase PP1 and/or inhibition of Akt result in the dephosphorylation of Ser9 and the activation of GSK3β, which is necessary for LTD induction (Peineau et al., 2007).
Downstream of PI3K also lies the mTOR/p70S6K pathway, which plays an important role at the postsynapse. mTOR activation during LTP requires the coincident activation of the PI3K pathway with the Erk pathway (Tsokas et al., 2007). mTOR regulates the transcription of mRNAs encoding proteins involved in protein translation, primarily cap-dependent translation initiation. Maintenance of LTP for more than 2–3 hours requires protein synthesis (Fonseca et al., 2006, Huang et al., 1996), a phase that is inhibited by rapamycin, an inhibitor of mTOR, when applied during the induction of LTP (Tang et al., 2002, Cammalleri et al., 2003). Gene deletion of FKBP12, an inhibitor of mTOR, increases mTOR activity, which promotes LTP and enhances contextual fear memory but alters the capacity of the transgenic mice to remodel memory (Hoeffer et al., 2008). Similarly, mGluR-dependent LTD required de novo synthesis of protein, the initiation of seems to be dependent on PI3K and mTOR (Hou and Klann, 2004).
PTEN is the enzyme that counterbalances PI3K by removing the 3-phosphate from PtdIns(3,4,5)P3 (Maehama and Dixon, 1999, Sulis and Parsons, 2003). Conditional ablation of PTEN in a discrete number of mature neurons in the cortex results in a tremendous impairment of social interaction among other behavioral abnormalities in mice (Kwon et al., 2006). Morphologically, ablation or silencing of PTEN deletion induces an increase in axonal and dendritic growth, thus phenocopying the consequences of Pi3K overexpression and hyperactivation of mTOR (Jaworski et al., 2005, Chow et al., 2009). Abnormal synapses with a higher number of SVs and a higher density of spines have also been reported. In the mutant mice, all the downstream targets of Akt, including GSK3β, were found to be hyperphosphorylated (Chow et al., 2009). A similar phenotype is observed in a PTEN haploinsufficient mice and this phenotype is exacerbated by the haploinsufficiency of the serotonin transporter Slca4 gene (Page et al., 2009). PTEN can be recruited to the spine by binding to the PDZ domain of PSD-95 after NMDA receptor activation and regulates the expression of LTD by affecting AMPA receptor localization (Jurado et al., 2010). PTEN may also directly bind to extrasynaptic NMDA receptors and positively regulate signaling downstream of this glutamate receptor (Ning et al., 2004).
5. PHOSPHOINOSITIDE IMBALANCE IN SYNAPTIC DYSFUNCTION AND BRAIN DISORDERS
Due to the pleiotropic roles of phosphoinositides, their metabolism is subject to tight regulation. Not surprisingly, several phosphoinositide-metabolizing enzymes have been implicated in a number of human diseases, including genetic disorders (Di Paolo and De Camilli, 2006, Pendaries et al., 2003). Recently, our group has linked PtdIns(4,5)P2 metabolism and in particular Synj1 to Down syndrome (DS) and Alzheimer’s disease (AD).
5.1 Synaptojanin1 overexpression in Down syndrome
Phosphoinositides have been shown to be directly or indirectly linked to several conditions associated with mental retardation (MR), including the Fragile-X and DS. As described by J. Lejeune in the 1950’s, DS is the result of the trisomy of the chromosome 21 and occurs in 1 birth out of 800. One of the stringent phenotypes of DS is MR, which reflects the dosage imbalance of genes expressed in the brain. The gene encoding Synj1 is located on human chromosome 21 (mapped on 21q22.2) and the presence of 3 copies in individuals with DS results in a higher expression of Synj1 in DS brain (Arai et al., 2002). A similar result was also observed in the partial trisomy mouse Ts65Dn (Voronov et al., 2008), which has a partial triplication of mouse chromosome 16, which is highy syntenic with human chromosome 21 (Reeves et al., 1995). We have used the Ts65Dn mice, which recapitulates many features of individuals with DS, and a BAC transgenic mouse that overexpresses Synj1 [Tg(Synj1)] uniquely due to 3 gene copies (Voronov et al., 2008). In the brain of both mouse models, the PtdIns(4,5)P2 5-phosphatase activity was increased and the levels of PtdIns(4,5)P2 were reduced. Removal of one copy of Synj1 from the Ts65Dn background rescued the defects in PtdIns(4,5)P2 metabolism (Voronov et al., 2008). While the Ts65Dn mice have important cognitive deficits (Seregaza et al., 2006), the Tg(Synj1) mice were shown to perform poorly in the Morris-water maze task, which assesses spatial learning. However, as expected, cognitive deficits resulting from Synj1 overexpression are milder than those observed in Ts65Dn, further suggesting that multiple genes contribute to MR in DS.
5.2 PtdIns(4,5)P2 dysregulation in Alzheimer’s disease
AD is the most common form of late-onset dementia. It is characterized by an accumulation of amyloid-beta (Aβ) and neurofibrillary tangles and results in age-dependent cognitive decline. Several studies on animals models of AD described defects in excitatory neurotransmission (Hsieh et al., 2006), and synaptic plasticity (Walsh et al., 2002, Venkitaramani et al., 2007), in synaptic loss (Hsieh et al., 2006, Lue et al., 1999) and a neuron network imbalance with an increased synchronic activity (Palop et al., 2007, Busche et al., 2008, Palop and Mucke, 2010). Recent studies have suggested that phosphoinositide imbalance plays an important role in the pathophysiological processes involving Aβ. Presenilin-1 is the catalytic subunit of the proteolytic complex γ-secretase that cleaves the COOH-terminal part of the transmembrane domain of the amyloid precursor protein (APP) producing peptides of different sizes but particularly Aβ40 and in lower amount Aβ42, which is more aggregate prone and synaptotoxic (Haass, 2004). Mutations of presenilin-1 observed in familial forms of AD cause an imbalance in PtdIns(4,5)P2 metabolism as observed with the reduction of the calcium-permeable transient receptor potential melastatin 7-associated magnesium-inhibited cation current, that is positively regulated by PtdIns(4,5)P2. The current downregulated by mutated presenilin1 alterations in cellular cation homeostasis. PtdIns(4,5)P2 levels variation induced by altering PLC activity correlates negatively with the levels of Aβ42 (Landman et al., 2006). Furthermore, incubation of cortical cultures with submicromolar concentration of oligomeric Aβ42 was shown to reduce the levels of PtdIns(4,5)P2 in a NMDA- and PLC-dependent manner. Compensating for this deficiency of PtdIns(4,5)P2 by deleting a copy of Synj1 rescued Aβ-induced LTP defects in hippocampal slices (Berman et al., 2008). Our findings suggest a hypothesis whereby PtdIns(4,5)P2 dyshomeostasis underlies early neurotoxic effects of Aβ at synapses and that Synj1 haploinsufficiency protects against the actions of this peptide. More generally, we hypothesize that Aβ-induced synaptic dysfunction and cognitive deficits can be ameliorated by treatments that prevent PtdIns(4,5)P2 downregulation in AD. Finally, as mentioned earlier, a key feature of DS is MR, whose onset typically starts in early childhood (Roizen and Patterson, 2003). Superimposed to these deficits is the progressive development of AD pathology, which leads to further cognitive decline in middle-aged individuals with DS (Lott and Head, 2001). A key causative agent for AD in DS is the overexpression of chromosome 21-linked APP, the precursor of Aβ. Our studies point to the occurrence in DS of a “dual hit” on PtdIns(4,5)P2, accounted for by Synj1 trisomy and by Aβ elevation, which could potentiate AD-linked cognitive decline in middle-aged adults with DS.
6. CONCLUSIONS AND PERSPECTIVES
As highlighted in this book chapter, phosphoinositides mediate a multitude of biological functions at the neuronal synapse, both pre- and postsynaptically. Emphasis was put on PtdIns(4,5)P2 and PtdIns(3,4,5)P3 because these two lipids have been extensively studied and shown to regulate important synaptic functions. Their enrichment at the plasma membrane, along with a rich set of effectors and metabolizing enzymes operating at this compartment (or in close proximity thereof), predisposes them for specialized functions occurring at the cell surface, including the fusion and internalization of SVs, the control of cell surface levels of ion channels, signaling of ligand-bound receptors and regulation of actin dynamics. A fundamental question in the field (and one that is pertinent to the broad field of phosphoinositide research) is how one or two lipids is/are able to control so many critical functions within a single membrane compartment. A partial answer proposed by several investigators is that phosphoinositide metabolism may be controlled locally, in micro- or perhaps even nanodomains, by tightly regulated recruitment and/or activation of lipid enzymes, by lipid sequestration/release mechanisms or by changes in membrane properties (e.g, curvature). The fast diffusion rates reported for membrane lipids, including PtdIns(4,5)P2, are difficult to reconcile with the concept of local control of phosphoinositide metabolism, unless mechanisms that significantly slow down diffusion rates or bona fide “fences” exist. In this respect, the ability to visualize lipids with genetically-encoded fluorescent probes, such as PH domains, in combination with super resolution fluorescence microscopy will be informative.
While PtdIns(4,5)P2 and PtdIns(3,4,5)P3 have been extensively characterized, little is known about the role of the other five phosphoinositides at synapses, and particularly, the low abundance ones, such as PtdIns(3,4)P2, PtdIns(3,5)P2, and PtdIns5P. Answers may come from the characterization of various KO or mutant animals that are defective for enzymes involved in their metabolism, although in some cases, the development of better probes for low abundance phosphoinositides and identification of synapse-specific effectors may be required to have a better understanding of what these lipids do at the neuronal synapse and elsewhere.
ACKNOWLEDGMENTS
Work on phosphoinositides in the Di Paolo lab is funded by NIH grants R01 NS056049, R01 R01HD05547 and R03 AG033212 and by the McKnight Endowment Fund. Belle Chang-Ileto is funded by the NIH (F31 NS058096).
ABBREVIATIONS
- Aβ
Amyloid Beta
- AMPA
Alpha-amino-3-hydroxy-5-Methyl-4- isoxazole-Propionic Acid
- ANTH
AP180 N-Terminal Homology
- AP-2
Adaptor complex Protein-2
- APP
Amyloid Precursor Protein
- Arp2/3
Actin-related protein 2 and 3
- ATP
Adenosine Trisphosphate
- BAR
Bin/ Amphiphysin/ Rvs
- CamKII
Calcium/Calmodulin-dependent Kinase II
- CALM
Clathrin Assembly Lymphoid Myeloid
- Ca2+
Calcium
- CAPS
Calcium-dependent Activator Protein for Secretion
- CaM
Calcium/Calmodulin
- CCV
Clathrin-Coated Vesicles
- CCP
Clathrin-Coated Pits
- Cdk-5
Cyclin-Dependent Kinase-5
- CHC
Clathrin Heavy Chains
- CLC
Clathrin Light Chains
- CME
Clathrin-Mediated Endocytosis
- Cofilin
Cosediment with Filamentous Actin
- DAG
Diacylglycerol
- DS
Down Syndrome
- Dyrk
Dual specificity Tyrosine phosphorylation Regulated Kinase
- ENTH
Epsin N-Terminal Homology
- EphB
EphrinB receptor
- EPSP
Excitatory Postsynaptic Potential
- Epsin
Eps15-Interacting protein
- ERK
Extracellular signal-Regulated Kinase
- FERM
Four-point-one, Ezrin, Radixin, Moesin
- GABA
γ Aminobutyric Acid
- GED
GTPase Effector Domain
- Grb2
Growth factor Receptor-Bound protein 2
- GRIP1
Glutamate Receptor-Interacting Protein 1
- GRP1
General Receptor for Phosphoinositide 1
- GSK3β
Glycogen Synthase Kinase 3 Beta
- GTPase
Guanosine Triphosphate Phosphatase
- Hsc70
Heat shock cognate protein of 70 KDa
- KO
Knock Out
- LDCV
Large Dense Core Vesicle
- LTD
Long-Term Depression
- LTP
Long-Term Potentiation
- MARCKS
Myristoylated-Alanine-Rich C Kinase Substrate
- mGluR
Metabotropic Glutamate Receptor
- MR
Mental Retardation
- NMJ
Neuromuscular Junction
- NMDA
N-Methyl-D-aspartate
- NSF
N-ethylmaleimide-sensitive factor
- PA
Phosphatidic Acid
- PC12
Pheochromocytoma 12
- PDZ
PSD-95/ Drosophila disc large tumor suppressor/ Zonula occludens-1 protein
- PH
Pleckstrin Homology
- PI3K
phosphoinositide 3-kinase
- PICK1
Protein Interacting with C Kinase 1
- PIKE-L
PI3K Enhancer-Long
- PKC
Protein Kinase C
- PLC
phospholipase C
- PRD
Proline-Rich Domain
- PS
Phosphatidyl Serine
- PSD
PostSynaptic Density
- PtdInsPK
Phosphatidylinositol phosphate kinase
- PtdIns3/4/5P
Phosphatidylinositol 3/4/5 monophosphate
- PtdIns(4,5)P2
Phosphatidylinositol 4,5 bisphosphate
- PtdIns(3,4,5)P3
Phosphatidylinositol 3,4,5 trisphosphate
- PTEN
Phosphatase and TENsin homolog located on chromosome 10
- RRP
Readily Releasable Pool
- Sac1
Suppressor of Actin 1
- SH3
Src-Homology type 3
- SNAP
Soluble NSF Attachment Protein
- SNARE
SNAP receptor
- Sjl
Synaptojanin-like protein
- SNX
Sorting Nexin proteins
- Synj
Synaptojanin
- Syt
Synaptotagmin
- TARP
Transmembrane AMPA receptor Regulatory Proteins
- WASP
Wiskott-Aldrich Syndrome Protein
- Ymr1p
Yeast myotubularin-related phosphatase
REFERENCES
- Adayev T, Chen-Hwang MC, Murakami N, Wang R, Hwang YW. MNB/DYRK1A phosphorylation regulates the interactions of synaptojanin 1 with endocytic accessory proteins. Biochem Biophys Res Commun. 2006;351:1060–1065. doi: 10.1016/j.bbrc.2006.10.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Boronenkov IV, Doughman SD, Kunz J, Loijens JC. Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J Biol Chem. 1999;274:9907–9910. doi: 10.1074/jbc.274.15.9907. [DOI] [PubMed] [Google Scholar]
- Ann K, Kowalchyk JA, Loyet KM, Martin TF. Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. J Biol Chem. 1997;272:19637–19640. doi: 10.1074/jbc.272.32.19637. [DOI] [PubMed] [Google Scholar]
- Aoyagi K, Sugaya T, Umeda M, Yamamoto S, Terakawa S, Takahashi M. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J Biol Chem. 2005;280:17346–17352. doi: 10.1074/jbc.M413307200. [DOI] [PubMed] [Google Scholar]
- Arai Y, Ijuin T, Takenawa T, Becker LE, Takashima S. Excessive expression of synaptojanin in brains with Down syndrome. Brain Dev. 2002;24:67–72. doi: 10.1016/s0387-7604(01)00405-3. [DOI] [PubMed] [Google Scholar]
- Arbuzova A, Schmitz AA, Vergeres G. Cross-talk unfolded: MARCKS proteins. Biochem J. 2002;362:1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt KL, Royo M, Fernandez-Monreal M, Knafo S, Petrok CN, Martens JR, Esteban JA. PIP3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane. Nat Neurosci. 2010;13:36–44. doi: 10.1038/nn.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Bai J, Hu Z, Dittman JS, Pym EC, Kaplan JM. Endophilin functions as a membrane-bending molecule and is delivered to endocytic zones by exocytosis. Cell. 2010;143:430–441. doi: 10.1016/j.cell.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat Struct Mol Biol. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- Bairstow SF, Ling K, Su X, Firestone AJ, Carbonara C, Anderson RA. Type Igamma661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J Biol Chem. 2006;281:20632–20642. doi: 10.1074/jbc.M601465200. [DOI] [PubMed] [Google Scholar]
- Bao H, Reist NE, Zhang B. The Drosophila epsin 1 is required for ubiquitin-dependent synaptic growth and function but not for synaptic vesicle recycling. Traffic. 2008;9:2190–2205. doi: 10.1111/j.1600-0854.2008.00832.x. [DOI] [PubMed] [Google Scholar]
- Bashkirov PV, Akimov SA, Evseev AI, Schmid SL, Zimmerberg J, Frolov VA. GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell. 2008;135:1276–1286. doi: 10.1016/j.cell.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- Blero D, Payrastre B, Schurmans S, Erneux C. Phosphoinositide phosphatases in a network of signalling reactions. Pflugers Arch. 2007;455:31–44. doi: 10.1007/s00424-007-0304-5. [DOI] [PubMed] [Google Scholar]
- Bloom O, Evergren E, Tomilin N, Kjaerulff O, Low P, Brodin L, Pieribone VA, Greengard P, Shupliakov O. Colocalization of synapsin and actin during synaptic vesicle recycling. J Cell Biol. 2003;161:737–747. doi: 10.1083/jcb.200212140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Brose N, Petrenko AG, Sudhof TC, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48:77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Cammalleri M, Lutjens R, Berton F, King AR, Simpson C, Francesconi W, Sanna PP. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci U S A. 2003;100:14368–14373. doi: 10.1073/pnas.2336098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestra G, Castagnoli L, Dente L, Minenkova O, Petrelli A, Migone N, Hoffmuller U, Schneider-Mergener J, Cesareni G. The SH3 domains of endophilin and amphiphysin bind to the proline-rich region of synaptojanin 1 at distinct sites that display an unconventional binding specificity. J Biol Chem. 1999;274:32001–32007. doi: 10.1074/jbc.274.45.32001. [DOI] [PubMed] [Google Scholar]
- Chang JD, Field SJ, Rameh LE, Carpenter CL, Cantley LC. Identification and characterization of a phosphoinositide phosphate kinase homolog. J Biol Chem. 2004;279:11672–11679. doi: 10.1074/jbc.M309721200. [DOI] [PubMed] [Google Scholar]
- Chang N, El-Hayek YH, Gomez E, Wan Q. Phosphatase PTEN in neuronal injury and brain disorders. Trends Neurosci. 2007;30:581–586. doi: 10.1016/j.tins.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Chang-Ileto B, Frere SG, Chan RB, Voronov SV, Roux A, Di Paolo G. Synaptojanin 1-Mediated PI(4,5)P(2) Hydrolysis Is Modulated by Membrane Curvature and Facilitates Membrane Fission. Dev Cell. 2011;20:206–218. doi: 10.1016/j.devcel.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- Chatah NE, Abrams CS. G-protein-coupled receptor activation induces the membrane translocation and activation of phosphatidylinositol-4-phosphate 5-kinase I alpha by a Rac- and Rho-dependent pathway. J Biol Chem. 2001;276:34059–34065. doi: 10.1074/jbc.M104917200. [DOI] [PubMed] [Google Scholar]
- Chen H, Ko G, Zatti A, Di Giacomo G, Liu L, Raiteri E, Perucco E, Collesi C, Min W, Zeiss C, De Camilli P, Cremona O. Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin 1 and epsin 2 in mice. Proc Natl Acad Sci U S A. 2009;106:13838–13843. doi: 10.1073/pnas.0907008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- Chen X, Garelick MG, Wang H, Lil V, Athos J, Storm DR. PI3 kinase signaling is required for retrieval and extinction of contextual memory. Nat Neurosci. 2005;8:925–931. doi: 10.1038/nn1482. [DOI] [PubMed] [Google Scholar]
- Chicka MC, Hui E, Liu H, Chapman ER. Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+ Nat Struct Mol Biol. 2008;15:827–835. doi: 10.1038/nsmb.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Chang J, Jiang B, Seol GH, Min SS, Han JS, Shin HS, Gallagher M, Kirkwood A. Multiple receptors coupled to phospholipase C gate long-term depression in visual cortex. J Neurosci. 2005;25:11433–11443. doi: 10.1523/JNEUROSCI.4084-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow DK, Groszer M, Pribadi M, Machniki M, Carmichael ST, Liu X, Trachtenberg JT. Laminar and compartmental regulation of dendritic growth in mature cortex. Nat Neurosci. 2009;12:116–118. doi: 10.1038/nn.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Song WJ, Kim K, Bednarski JJ, Chen J, Prestwich GD, Holz RW. The C2 domains of Rabphilin3A specifically bind phosphatidylinositol 4,5-bisphosphate containing vesicles in a Ca2+-dependent manner. In vitro characteristics and possible significance. J Biol Chem. 1998;273:10240–10248. doi: 10.1074/jbc.273.17.10240. [DOI] [PubMed] [Google Scholar]
- Citri A, Bhattacharyya S, Ma C, Morishita W, Fang S, Rizo J, Malenka RC. Calcium binding to PICK1 is essential for the intracellular retention of AMPA receptors underlying long-term depression. J Neurosci. 2010;30:16437–16452. doi: 10.1523/JNEUROSCI.4478-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Anggono V, Huganir RL. PICK1 regulates incorporation of calcium-permeable AMPA receptors during cortical synaptic strengthening. J Neurosci. 2010;30:6360–6366. doi: 10.1523/JNEUROSCI.6276-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codazzi F, Di Cesare A, Chiulli N, Albanese A, Meyer T, Zacchetti D, Grohovaz F. Synergistic control of protein kinase Cgamma activity by ionotropic and metabotropic glutamate receptor inputs in hippocampal neurons. J Neurosci. 2006;26:3404–3411. doi: 10.1523/JNEUROSCI.0478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 2002;109:523–535. doi: 10.1016/s0092-8674(02)00735-3. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona O, De Camilli P. Phosphoinositides in membrane traffic at the synapse. J Cell Sci. 2001;114:1041–1052. doi: 10.1242/jcs.114.6.1041. [DOI] [PubMed] [Google Scholar]
- Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, McCormick DA, De Camilli P. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- Cuadra AE, Kuo SH, Kawasaki Y, Bredt DS, Chetkovich DM. AMPA receptor synaptic targeting regulated by stargazin interactions with the Golgi-resident PDZ protein nPIST. J Neurosci. 2004;24:7491–7502. doi: 10.1523/JNEUROSCI.1255-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LB, Bhattacharya M, Seachrist JL, Anborgh PH, Ferguson SS. Agonist-stimulated and tonic internalization of metabotropic glutamate receptor 1a in human embryonic kidney 293 cells: agonist-stimulated endocytosis is beta-arrestin1 isoform-specific. Mol Pharmacol. 2001;60:1243–1253. doi: 10.1124/mol.60.6.1243. [DOI] [PubMed] [Google Scholar]
- Daw MI, Bortolotto ZA, Saulle E, Zaman S, Collingridge GL, Isaac JT. Phosphatidylinositol 3 kinase regulates synapse specificity of hippocampal long-term depression. Nat Neurosci. 2002;5:835–836. doi: 10.1038/nn903. [DOI] [PubMed] [Google Scholar]
- de Heuvel E, Bell AW, Ramjaun AR, Wong K, Sossin WS, McPherson PS. Identification of the major synaptojanin-binding proteins in brain. J Biol Chem. 1997;272:8710–8716. doi: 10.1074/jbc.272.13.8710. [DOI] [PubMed] [Google Scholar]
- de Wit H, Walter AM, Milosevic I, Gulyas-Kovacs A, Riedel D, Sorensen JB, Verhage M. Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25 acceptor complexes. Cell. 2009;138:935–946. doi: 10.1016/j.cell.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Dell'Acqua ML, Faux MC, Thorburn J, Thorburn A, Scott JD. Membrane-targeting sequences on AKAP79 bind phosphatidylinositol-4, 5-bisphosphate. EMBO J. 1998;17:2246–2260. doi: 10.1093/emboj/17.8.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan TA, De Camilli P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Pellegrini L, Letinic K, Cestra G, Zoncu R, Voronov S, Chang S, Guo J, Wenk MR, De Camilli P. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature. 2002;420:85–89. doi: 10.1038/nature01147. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Horne JA, Meinertzhagen IA, Schwarz TL. A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell. 2005;123:521–533. doi: 10.1016/j.cell.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Dittman J, Ryan TA. Molecular circuitry of endocytosis at nerve terminals. Annu Rev Cell Dev Biol. 2009;25:133–160. doi: 10.1146/annurev.cellbio.042308.113302. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Kaplan JM. Factors regulating the abundance and localization of synaptobrevin in the plasma membrane. Proc Natl Acad Sci U S A. 2006;103:11399–11404. doi: 10.1073/pnas.0600784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divecha N, Roefs M, Halstead JR, D'Andrea S, Fernandez-Borga M, Oomen L, Saqib KM, Wakelam MJ, D'Santos C. Interaction of the type Ialpha PIPkinase with phospholipase D: a role for the local generation of phosphatidylinositol 4, 5-bisphosphate in the regulation of PLD2 activity. Embo J. 2000;19:5440–5449. doi: 10.1093/emboj/19.20.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J Membr Biol. 2003;194:77–89. doi: 10.1007/s00232-003-2027-7. [DOI] [PubMed] [Google Scholar]
- Eberhard DA, Cooper CL, Low MG, Holz RW. Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem J. 1990;268:15–25. doi: 10.1042/bj2680015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E, Greene LE. Multiple roles of auxilin and hsc70 in clathrin-mediated endocytosis. Traffic. 2007;8:640–646. doi: 10.1111/j.1600-0854.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- Farsad K, Ringstad N, Takei K, Floyd SR, Rose K, De Camilli P. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol. 2001;155:193–200. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Brasnjo G, Hayashi M, Wolfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, O'Toole E, Flavell R, Cremona O, Miesenbock G, Ryan TA, De Camilli P. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Crepaldi L, Nicoletti F. Metabotropic glutamate 1 receptor: current concepts and perspectives. Pharmacol Rev. 2008;60:536–581. doi: 10.1124/pr.108.000166. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nagerl UV. A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron. 2006;52:239–245. doi: 10.1016/j.neuron.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, De Camilli P, Unger VM. Structural basis of membrane invagination by F-BAR domains. Cell. 2008;132:807–817. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell. 2009;137:191–196. doi: 10.1016/j.cell.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Tzingounis AV, Trinidad JC, Fukata M, Burlingame AL, Nicoll RA, Bredt DS. Molecular constituents of neuronal AMPA receptors. J Cell Biol. 2005;169:399–404. doi: 10.1083/jcb.200501121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Gad H, Ringstad N, Low P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, Crun J, Ellisman MH, De Camilli P, Shupliakov O, Brodin L. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron. 2000;27:301–312. doi: 10.1016/s0896-6273(00)00038-6. [DOI] [PubMed] [Google Scholar]
- Gaffield MA, Rizzoli SO, Betz WJ. Mobility of synaptic vesicles in different pools in resting and stimulated frog motor nerve terminals. Neuron. 2006;51:317–325. doi: 10.1016/j.neuron.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Keen JH. Phosphoinositide-AP-2 Interactions Required for Targeting to Plasma Membrane Clathrin-coated Pits. J Cell Biol. 1999;146:755–764. doi: 10.1083/jcb.146.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarov I, Krupnick JG, Falck JR, Benovic JL, Keen JH. Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J. 1999;18:871–881. doi: 10.1093/emboj/18.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallop JL, Jao CC, Kent HM, Butler PJ, Evans PR, Langen R, McMahon HT. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 2006;25:2898–2910. doi: 10.1038/sj.emboj.7601174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering B, Cutillas PR, Nock G, Gharbi SI, Vanhaesebroeck B. Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc Natl Acad Sci U S A. 2007;104:7809–7814. doi: 10.1073/pnas.0700373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Giessel AJ, Sabatini BL. M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK channels. Neuron. 2010;68:936–947. doi: 10.1016/j.neuron.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudici ML, Emson PC, Irvine RF. A novel neuronal-specific splice variant of Type I phosphatidylinositol 4-phosphate 5-kinase isoform gamma. Biochem J. 2004;379:489–496. doi: 10.1042/BJ20031394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez LL, Alam S, Smith KE, Horne E, Dell'Acqua ML. Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin. J Neurosci. 2002;22:7027–7044. doi: 10.1523/JNEUROSCI.22-16-07027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong LW, De Camilli P. Regulation of postsynaptic AMPA responses by synaptojanin 1. Proc Natl Acad Sci U S A. 2008;105:17561–17566. doi: 10.1073/pnas.0809221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong LW, Di Paolo G, Diaz E, Cestra G, Diaz ME, Lindau M, De Camilli P, Toomre D. Phosphatidylinositol phosphate kinase type I gamma regulates dynamics of large dense-core vesicle fusion. Proc Natl Acad Sci U S A. 2005;102:5204–5209. doi: 10.1073/pnas.0501412102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gaitan M, Jackle H. Role of Drosophila alpha-adaptin in presynaptic vesicle recycling. Cell. 1997;88:767–776. doi: 10.1016/s0092-8674(00)81923-6. [DOI] [PubMed] [Google Scholar]
- Gracheva EO, Burdina AO, Touroutine D, Berthelot-Grosjean M, Parekh H, Richmond JE. Tomosyn negatively regulates CAPS-dependent peptide release at Caenorhabditis elegans synapses. J Neurosci. 2007;27:10176–10184. doi: 10.1523/JNEUROSCI.2339-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigliatti TA, Hall L, Rosenbluth R, Suzuki DT. Temperature-sensitive mutations in Drosophila melanogaster. XIV. A selection of immobile adults. Mol Gen Genet. 1973;120:107–114. doi: 10.1007/BF00267238. [DOI] [PubMed] [Google Scholar]
- Grishanin RN, Kowalchyk JA, Klenchin VA, Ann K, Earles CA, Chapman ER, Gerona RR, Martin TF. CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron. 2004;43:551–562. doi: 10.1016/j.neuron.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, Zheng JQ. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci. 2010;13:1208–1215. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Schuske K, Watanabe S, Liu Q, Baum P, Garriga G, Jorgensen EM. Mu2 adaptin facilitates but is not essential for synaptic vesicle recycling in Caenorhabditis elegans. J Cell Biol. 2008;183:881–892. doi: 10.1083/jcb.200806088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Dai H, Harrison SC, Kirchhausen T. Structure of the PTEN-like region of auxilin, a detector of clathrin-coated vesicle budding. Structure. 2010;18:1191–1198. doi: 10.1016/j.str.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Wenk MR, Pellegrini L, Onofri F, Benfenati F, De Camilli P. Phosphatidylinositol 4-kinase type IIalpha is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc Natl Acad Sci U S A. 2003;100:3995–4000. doi: 10.1073/pnas.0230488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Stolz LE, Lemrow SM, York JD. SAC1-like domains of yeast SAC1, INP52, and INP53 and of human synaptojanin encode polyphosphoinositide phosphatases. J Biol Chem. 1999;274:12990–12995. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- Haass C. Take five--BACE and the gamma-secretase quartet conduct Alzheimer's amyloid beta-peptide generation. EMBO J. 2004;23:483–488. doi: 10.1038/sj.emboj.7600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner C, Di Paolo G, Rosenthal JA, De Camilli P. Direct interaction of the 170 kDa isoform of synaptojanin 1 with clathrin and with the clathrin adaptor AP-2. Curr Biol. 2000;10:471–474. doi: 10.1016/s0960-9822(00)00446-2. [DOI] [PubMed] [Google Scholar]
- Haffner C, Takei K, Chen H, Ringstad N, Hudson A, Butler MH, Salcini AE, Di Fiore PP, De Camilli P. Synaptojanin 1: localization on coated endocytic intermediates in nerve terminals and interaction of its 170 kDa isoform with Eps15. FEBS Lett. 1997;419:175–180. doi: 10.1016/s0014-5793(97)01451-8. [DOI] [PubMed] [Google Scholar]
- Harel A, Wu F, Mattson MP, Morris CM, Yao PJ. Evidence for CALM in directing VAMP2 trafficking. Traffic. 2008;9:417–429. doi: 10.1111/j.1600-0854.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- Harris TW, Hartwieg E, Horvitz HR, Jorgensen EM. Mutations in synaptojanin disrupt synaptic vesicle recycling. J Cell Biol. 2000;150:589–600. doi: 10.1083/jcb.150.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Fukaya M, Qiao X, Sakimura K, Watanabe M, Kano M. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J Neurosci. 1999;19:6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans. 2006;34:647–662. doi: 10.1042/BST0340647. [DOI] [PubMed] [Google Scholar]
- Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, Martin TF. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- Hay JC, Martin TF. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca(2+)-activated secretion. Nature. 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Raimondi A, O'Toole E, Paradise S, Collesi C, Cremona O, Ferguson SM, De Camilli P. Cell- and stimulus-dependent heterogeneity of synaptic vesicle endocytic recycling mechanisms revealed by studies of dynamin 1-null neurons. Proc Natl Acad Sci U S A. 2008;105:2175–2180. doi: 10.1073/pnas.0712171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, McMahon HT. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328:1281–1284. doi: 10.1126/science.1188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann DW. Local PIP(2) signals: when, where, and how? Pflugers Arch. 2007;455:55–67. doi: 10.1007/s00424-007-0280-9. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Tang W, Wong H, Santillan A, Patterson RJ, Martinez LA, Tejada-Simon MV, Paylor R, Hamilton SL, Klann E. Removal of FKBP12 enhances mTOR-Raptor interactions LTP, memory, and perseverative/repetitive behavior. Neuron. 2008;60:832–845. doi: 10.1016/j.neuron.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne EA, Dell'Acqua ML. Phospholipase C is required for changes in postsynaptic structure and function associated with NMDA receptor-dependent long-term depression. J Neurosci. 2007;27:3523–3534. doi: 10.1523/JNEUROSCI.4340-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Qin Y, Bochorishvili G, Zhu Y, Van Aelst L, Zhu JJ. Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. J Neurosci. 2008;28:7847–7862. doi: 10.1523/JNEUROSCI.1496-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Nguyen PV, Abel T, Kandel ER. Long-lasting forms of synaptic potentiation in the mammalian hippocampus. Learn Mem. 1996;3:74–85. doi: 10.1101/lm.3.2-3.74. [DOI] [PubMed] [Google Scholar]
- Hughes WE, Cooke FT, Parker PJ. Sac phosphatase domain proteins. Biochem J. 2000;350(Pt 2):337–352. [PMC free article] [PubMed] [Google Scholar]
- Hui E, Johnson CP, Yao J, Dunning FM, Chapman ER. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell. 2009;138:709–721. doi: 10.1016/j.cell.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie F, Okuno M, Pasquale EB, Yamaguchi Y. EphrinB-EphB signalling regulates clathrin-mediated endocytosis through tyrosine phosphorylation of synaptojanin 1. Nat Cell Biol. 2005;7:501–509. doi: 10.1038/ncb1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H, Shibasaki Y, Kizuki N, Katagiri H, Yazaki Y, Asano T, Oka Y. Cloning of cDNAs encoding two isoforms of 68-kDa type I phosphatidylinositol-4-phosphate 5-kinase. J Biol Chem. 1996;271:23611–23614. doi: 10.1074/jbc.271.39.23611. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Shibasaki Y, Kizuki N, Wada T, Yazaki Y, Asano T, Oka Y. Type I phosphatidylinositol-4-phosphate 5-kinases. Cloning of the third isoform and deletion/substitution analysis of members of this novel lipid kinase family. J Biol Chem. 1998;273:8741–8748. doi: 10.1074/jbc.273.15.8741. [DOI] [PubMed] [Google Scholar]
- Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, De Camilli P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell. 2005;9:791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science. 2001;291:1047–1051. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- Jackson C, Welch HC, Bellamy TC. Control of cerebellar long-term potentiation by P-Rex-family guanine-nucleotide exchange factors and phosphoinositide 3-kinase. PLoS One. 2010a;5:e11962. doi: 10.1371/journal.pone.0011962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LP, Kelly BT, McCoy AJ, Gaffry T, James LC, Collins BM, Honing S, Evans PR, Owen DJ. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell. 2010b;141:1220–1229. doi: 10.1016/j.cell.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson J, Gad H, Andersson F, Low P, Shupliakov O, Brodin L. Role of epsin 1 in synaptic vesicle endocytosis. Proc Natl Acad Sci U S A. 2008;105:6445–6450. doi: 10.1073/pnas.0710267105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DJ, Khodthong C, Kowalchyk JA, Martin TF. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol. 2008;182:355–366. doi: 10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarquin-Pardo M, Fitzpatrick A, Galiano FJ, First EA, Davis JN. Phosphatidic acid regulates the affinity of the murine phosphatidylinositol 4-phosphate 5-kinase-Ibeta for phosphatidylinositol-4-phosphate. J Cell Biochem. 2007;100:112–128. doi: 10.1002/jcb.21027. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. Control of dendritic arborization by the phosphoinositide-3'-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci. 2005;25:11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Ge WP, Xu J, Cao M, Peng L, Yung W, Liao D, Duan S, Zhang M, Xia J. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J Neurosci. 2006;26:2380–2390. doi: 10.1523/JNEUROSCI.3503-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch WJ, Speidel D, Sigler A, Sorensen JB, Varoqueaux F, Rhee JS, Brose N. CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell. 2007;131:796–808. doi: 10.1016/j.cell.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Jorgensen EM, Hartwieg E, Schuske K, Nonet ML, Jin Y, Horvitz HR. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature. 1995;378:196–199. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- Jost M, Simpson F, Kavran JM, Lemmon MA, Schmid SL. Phosphatidylinositol-4,5-bisphosphate is required for endocytic coated vesicle formation. Curr Biol. 1998;8:1399–1402. doi: 10.1016/s0960-9822(98)00022-0. [DOI] [PubMed] [Google Scholar]
- Jurado S, Benoist M, Lario A, Knafo S, Petrok CN, Esteban JA. PTEN is recruited to the postsynaptic terminal for NMDA receptor-dependent long-term depression. EMBO J. 2010;29:2827–2840. doi: 10.1038/emboj.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlfeldt N, Vahedi-Faridi A, Koo SJ, Schafer JG, Krainer G, Keller S, Saenger W, Krauss M, Haucke V. Molecular basis for association of PIPKI gamma-p90 with clathrin adaptor AP-2. J Biol Chem. 2010;285:2734–2749. doi: 10.1074/jbc.M109.074906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastning K, Kukhtina V, Kittler JT, Chen G, Pechstein A, Enders S, Lee SH, Sheng M, Yan Z, Haucke V. Molecular determinants for the interaction between AMPA receptors and the clathrin adaptor complex AP-2. Proc Natl Acad Sci U S A. 2007;104:2991–2996. doi: 10.1073/pnas.0611170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BT, McCoy AJ, Spate K, Miller SE, Evans PR, Honing S, Owen DJ. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 2008;456:976–979. doi: 10.1038/nature07422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuong TM, Habets RL, Slabbaert JR, Verstreken P. WASP is activated by phosphatidylinositol-4,5-bisphosphate to restrict synapse growth in a pathway parallel to bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2010;107:17379–17384. doi: 10.1073/pnas.1001794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ryan TA. CDK5 serves as a major control point in neurotransmitter release. Neuron. 2010;67:797–809. doi: 10.1016/j.neuron.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WT, Chang S, Daniell L, Cremona O, Di Paolo G, De Camilli P. Delayed reentry of recycling vesicles into the fusion-competent synaptic vesicle pool in synaptojanin 1 knockout mice. Proc Natl Acad Sci U S A. 2002;99:17143–17148. doi: 10.1073/pnas.222657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. Clathrin. Annu Rev Biochem. 2000;69:699–727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Contribution of active zone subpopulation of vesicles to evoked and spontaneous release. J Neurophysiol. 1999;81:1495–1505. doi: 10.1152/jn.1999.81.4.1495. [DOI] [PubMed] [Google Scholar]
- Krauss M, Haucke V. Functional assay of effectors of ADP ribosylation factor 6 during clathrin/AP-2 coat recruitment to membranes. Gtpases Regulating Membrane Dynamics. 2005;404:388–398. doi: 10.1016/S0076-6879(05)04034-6. [DOI] [PubMed] [Google Scholar]
- Krauss M, Kinuta M, Wenk MR, De Camilli P, Takei K, Haucke V. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Igamma. J Cell Biol. 2003;162:113–124. doi: 10.1083/jcb.200301006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M, Kukhtina V, Pechstein A, Haucke V. Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mu-cargo complexes. Proc Natl Acad Sci U S A. 2006;103:11934–11939. doi: 10.1073/pnas.0510306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J, Wilson MP, Kisseleva M, Hurley JH, Majerus PW, Anderson RA. The activation loop of phosphatidylinositol phosphate kinases determines signaling specificity. Mol Cell. 2000;5:1–11. doi: 10.1016/s1097-2765(00)80398-6. [DOI] [PubMed] [Google Scholar]
- Kusano K, Abe H, Obinata T. Detection of a sequence involved in actin-binding and phosphoinositide-binding in the N-terminal side of cofilin. Mol Cell Biochem. 1999;190:133–141. [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AD, Tryoen-Toth P, Tsai B, Vitale N, Stuenkel EL. SNARE-catalyzed fusion events are regulated by Syntaxin1A-lipid interactions. Mol Biol Cell. 2008;19:485–497. doi: 10.1091/mbc.E07-02-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman N, Jeong SY, Shin SY, Voronov SV, Serban G, Kang MS, Park MK, Di Paolo G, Chung S, Kim TW. Presenilin mutations linked to familial Alzheimer's disease cause an imbalance in phosphatidylinositol 4,5-bisphosphate metabolism. Proc Natl Acad Sci U S A. 2006;103:19524–19529. doi: 10.1073/pnas.0604954103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Frank DW, Marks MS, Lemmon MA. Dominant-negative inhibition of receptor-mediated endocytosis by a dynamin-1 mutant with a defective pleckstrin homology domain. Curr Biol. 1999;9:261–264. doi: 10.1016/s0960-9822(99)80115-8. [DOI] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004a;43:221–236. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Lee SY, Voronov S, Letinic K, Nairn AC, Di Paolo G, De Camilli P. Regulation of the interaction between PIPKI gamma and talin by proline-directed protein kinases. J Cell Biol. 2005;168:789–799. doi: 10.1083/jcb.200409028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc Natl Acad Sci U S A. 2004b;101:546–551. doi: 10.1073/pnas.0307813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Ellena J, Liu L, Szabo G, Cafiso D, Castle D. Secretory carrier membrane protein SCAMP2 and phosphatidylinositol 4,5-bisphosphate interactions in the regulation of dense core vesicle exocytosis. Biochemistry. 2007;46:10909–10920. doi: 10.1021/bi701121j. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu KT, Leu TH, Chang WC, Gean PW. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron. 2001;31:841–851. doi: 10.1016/s0896-6273(01)00433-0. [DOI] [PubMed] [Google Scholar]
- Lin DT, Huganir RL. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27:13903–13908. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature. 2002;420:89–93. doi: 10.1038/nature01082. [DOI] [PubMed] [Google Scholar]
- Liu J, Kaksonen M, Drubin DG, Oster G. Endocytic vesicle scission by lipid phase boundary forces. Proc Natl Acad Sci U S A. 2006;103:10277–10282. doi: 10.1073/pnas.0601045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Sun Y, Oster GF, Drubin DG. Mechanochemical crosstalk during endocytic vesicle formation. Curr Opin Cell Biol. 2010;22:36–43. doi: 10.1016/j.ceb.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis DE, Petrou VI, Adney SK, Mahajan R. Channelopathies linked to plasma membrane phosphoinositides. Pflugers Arch. 2010;460:321–341. doi: 10.1007/s00424-010-0828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott IT, Head E. Down syndrome and Alzheimer's disease: a link between development and aging. Ment Retard Dev Disabil Res Rev. 2001;7:172–178. doi: 10.1002/mrdd.1025. [DOI] [PubMed] [Google Scholar]
- Lou X, Korogod N, Brose N, Schneggenburger R. Phorbol esters modulate spontaneous and Ca2+-evoked transmitter release via acting on both Munc13 and protein kinase C. J Neurosci. 2008;28:8257–8267. doi: 10.1523/JNEUROSCI.0550-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyet KM, Kowalchyk JA, Chaudhary A, Chen J, Prestwich GD, Martin TF. Specific binding of phosphatidylinositol 4,5-bisphosphate to calcium-dependent activator protein for secretion (CAPS), a potential phosphoinositide effector protein for regulated exocytosis. J Biol Chem. 1998;273:8337–8343. doi: 10.1074/jbc.273.14.8337. [DOI] [PubMed] [Google Scholar]
- Lu J, Helton TD, Blanpied TA, Racz B, Newpher TM, Weinberg RJ, Ehlers MD. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–889. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, Di Paolo G, Cremona O, Daniell L, De Camilli P, McCormick DA. Synaptojanin 1 contributes to maintaining the stability of GABAergic transmission in primary cultures of cortical neurons. J Neurosci. 2001;21:9101–9111. doi: 10.1523/JNEUROSCI.21-23-09101.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 1999;9:125–128. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D'Souza S, Wong TP, Taghibiglou C, Lu J, Becker LE, Pei L, Liu F, Wymann MP, Macdonald JF, Wang YT. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/s0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Mani M, Lee SY, Lucast L, Cremona O, Di Paolo G, De Camilli P, Ryan TA. The dual phosphatase activity of synaptojanin1 is required for both efficient synaptic vesicle endocytosis and reavailability at nerve terminals. Neuron. 2007;56:1004–1018. doi: 10.1016/j.neuron.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Takamiya K, Thomas G, Lin DT, Huganir RL. GRIP1 and 2 regulate activity-dependent AMPA receptor recycling via exocyst complex interactions. Proc Natl Acad Sci U S A. 2010;107:19038–19043. doi: 10.1073/pnas.1013494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- Martin TF. Phosphoinositides as spatial regulators of membrane traffic. Curr Opin Neurobiol. 1997;7:331–338. doi: 10.1016/s0959-4388(97)80060-8. [DOI] [PubMed] [Google Scholar]
- Martin TF. Tuning exocytosis for speed: fast and slow modes. Biochim Biophys Acta. 2003;1641:157–165. doi: 10.1016/s0167-4889(03)00093-4. [DOI] [PubMed] [Google Scholar]
- Massol RH, Boll W, Griffin AM, Kirchhausen T. A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc Natl Acad Sci U S A. 2006;103:10265–10270. doi: 10.1073/pnas.0603369103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew WD, Tsavaler L, Reichardt LF. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J Cell Biol. 1981;91:257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PK, Pykalainen A, Saarikangas J, Paavilainen VO, Vihinen H, Jokitalo E, Lappalainen P. Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism. J Cell Biol. 2007;176:953–964. doi: 10.1083/jcb.200609176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- McPherson PS, Garcia EP, Slepnev VI, David C, Zhang X, Grabs D, Sossin WS, Bauerfeind R, Nemoto Y, De Camilli P. A presynaptic inositol-5-phosphatase. Nature. 1996;379:353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- McPherson PS, Takei K, Schmid SL, De Camilli P. p145, a major Grb2-binding protein in brain, is co-localized with dynamin in nerve terminals where it undergoes activity-dependent dephosphorylation. J Biol Chem. 1994;269:30132–30139. [PubMed] [Google Scholar]
- Micheva KD, Kay BK, McPherson PS. Synaptojanin forms two separate complexes in the nerve terminal.Interactions with endophilin and amphiphysin. J Biol Chem. 1997;272:27239–27245. doi: 10.1074/jbc.272.43.27239. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Milosevic I, Sorensen JB, Lang T, Krauss M, Nagy G, Haucke V, Jahn R, Neher E. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J Neurosci. 2005;25:2557–2565. doi: 10.1523/JNEUROSCI.3761-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein AD, Nicoll RA. Regulation of AMPA receptor gating and pharmacology by TARP auxiliary subunits. Trends Pharmacol Sci. 2008;29:333–339. doi: 10.1016/j.tips.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Di Paolo G, Werner H, Shchedrina VA, Pypaert M, Pieribone VA, De Camilli P. A role for talin in presynaptic function. J Cell Biol. 2004;167:43–50. doi: 10.1083/jcb.200406020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita W, Marie H, Malenka RC. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat Neurosci. 2005;8:1043–1050. doi: 10.1038/nn1506. [DOI] [PubMed] [Google Scholar]
- Moritz A, de Graan PN, Gispen WH, Wirtz KW. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J Biol Chem. 1992;267:7207–7210. [PubMed] [Google Scholar]
- Mundell SJ, Matharu AL, Pula G, Roberts PJ, Kelly E. Agonist-induced internalization of the metabotropic glutamate receptor 1a is arrestin- and dynamin-dependent. J Neurochem. 2001;78:546–551. doi: 10.1046/j.1471-4159.2001.00421.x. [DOI] [PubMed] [Google Scholar]
- Nakano-Kobayashi A, Yamazaki M, Unoki T, Hongu T, Murata C, Taguchi R, Katada T, Frohman MA, Yokozeki T, Kanaho Y. Role of activation of PIP5Kgamma661 by AP-2 complex in synaptic vesicle endocytosis. EMBO J. 2007;26:1105–1116. doi: 10.1038/sj.emboj.7601573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CD, Kovacs JJ, Nobles KN, Whalen EJ, Lefkowitz RJ. Beta-arrestin scaffolding of phosphatidylinositol 4-phosphate 5-kinase Ialpha promotes agonist-stimulated sequestration of the beta2-adrenergic receptor. J Biol Chem. 2008;283:21093–21101. doi: 10.1074/jbc.M800431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CD, Perry SJ, Regier DS, Prescott SM, Topham MK, Lefkowitz RJ. Targeting of diacylglycerol degradation to M1 muscarinic receptors by beta-arrestins. Science. 2007;315:663–666. doi: 10.1126/science.1134562. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PM, Messing RO. Intracellular signaling pathways that regulate behavioral responses to ethanol. Pharmacol Ther. 2006;109:227–237. doi: 10.1016/j.pharmthera.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Ning K, Pei L, Liao M, Liu B, Zhang Y, Jiang W, Mielke JG, Li L, Chen Y, El-Hayek YH, Fehlings MG, Zhang X, Liu F, Eubanks J, Wan Q. Dual neuroprotective signaling mediated by downregulating two distinct phosphatase activities of PTEN. J Neurosci. 2004;24:4052–4060. doi: 10.1523/JNEUROSCI.5449-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- Nonet ML, Holgado AM, Brewer F, Serpe CJ, Norbeck BA, Holleran J, Wei L, Hartwieg E, Jorgensen EM, Alfonso A. UNC-11, a Caenorhabditis elegans AP180 Homologue, Regulates the Size and Protein Composition of Synaptic Vesicles. Mol Biol Cell. 1999;10:2343–2360. doi: 10.1091/mbc.10.7.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar RA, Collins CA, Hammarback JA, Shpetner HS, Vallee RB. Molecular cloning of the microtubule-associated mechanochemical enzyme dynamin reveals homology with a new family of GTP-binding proteins. Nature. 1990;347:256–261. doi: 10.1038/347256a0. [DOI] [PubMed] [Google Scholar]
- Ono S. Mechanism of depolymerization and severing of actin filaments and its significance in cytoskeletal dynamics. Int Rev Cytol. 2007;258:1–82. doi: 10.1016/S0074-7696(07)58001-0. [DOI] [PubMed] [Google Scholar]
- Opazo P, Choquet D. A three-step model for the synaptic recruitment of AMPA receptors. Mol Cell Neurosci. 2010;46:1–8. doi: 10.1016/j.mcn.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Osborne SL, Wen PJ, Meunier FA. Phosphoinositide regulation of neuroexocytosis: adding to the complexity. J Neurochem. 2006;98:336–342. doi: 10.1111/j.1471-4159.2006.03892.x. [DOI] [PubMed] [Google Scholar]
- Osten P, Khatri L, Perez JL, Kohr G, Giese G, Daly C, Schulz TW, Wensky A, Lee LM, Ziff EB. Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron. 2000;27:313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]
- Page DT, Kuti OJ, Prestia C, Sur M. Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proc Natl Acad Sci U S A. 2009;106:1989–1994. doi: 10.1073/pnas.0804428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Synaptic depression and aberrant excitatory network activity in Alzheimer's disease: two faces of the same coin? Neuromolecular Med. 2010;12:48–55. doi: 10.1007/s12017-009-8097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Wu H, Shen C, Shi Y, Jin W, Xia J, Zhang M. Clustering and synaptic targeting of PICK1 requires direct interaction between the PDZ domain and lipid membranes. EMBO J. 2007;26:4576–4587. doi: 10.1038/sj.emboj.7601860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish WR, Stefan CJ, Emr SD. Essential role for the myotubularin-related phosphatase Ymr1p and the synaptojanin-like phosphatases Sjl2p and Sjl3p in regulation of phosphatidylinositol 3-phosphate in yeast. Mol Biol Cell. 2004;15:3567–3579. doi: 10.1091/mbc.E04-03-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- Pechstein A, Shupliakov O, Haucke V. Intersectin 1: a versatile actor in the synaptic vesicle cycle. Biochem Soc Trans. 2010;38:181–186. doi: 10.1042/BST0380181. [DOI] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Pendaries C, Tronchere H, Plantavid M, Payrastre B. Phosphoinositide signaling disorders in human diseases. FEBS Lett. 2003;546:25–31. doi: 10.1016/s0014-5793(03)00437-x. [DOI] [PubMed] [Google Scholar]
- Perandones C, Costanzo RV, Kowaljow V, Pivetta OH, Carminatti H, Radrizzani M. Correlation between synaptogenesis and the PTEN phosphatase expression in dendrites during postnatal brain development. Brain Res Mol Brain Res. 2004;128:8–19. doi: 10.1016/j.molbrainres.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Perera RM, Zoncu R, Lucast L, De Camilli P, Toomre D. Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc Natl Acad Sci U S A. 2006;103:19332–19337. doi: 10.1073/pnas.0609795104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin MS, Brose N, Jahn R, Sudhof TC. Domain structure of synaptotagmin (p65) J Biol Chem. 1991;266:623–629. [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Schmid SL. Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell. 2008;135:1263–1275. doi: 10.1016/j.cell.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjaun AR, McPherson PS. Tissue-specific alternative splicing generates two synaptojanin isoforms with differential membrane binding properties. J Biol Chem. 1996;271:24856–24861. doi: 10.1074/jbc.271.40.24856. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Redman SJ, Crouch MF. The phosphoinositide 3-kinase and p70 S6 kinase regulate long-term potentiation in hippocampal neurons. Neuroscience. 2002;109:531–536. doi: 10.1016/s0306-4522(01)00500-0. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT, Davisson MT. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- Renden R, Berwin B, Davis W, Ann K, Chin CT, Kreber R, Ganetzky B, Martin TF, Broadie K. Drosophila CAPS is an essential gene that regulates dense-core vesicle release and synaptic vesicle fusion. Neuron. 2001;31:421–437. doi: 10.1016/s0896-6273(01)00382-8. [DOI] [PubMed] [Google Scholar]
- Reyes-Harde M, Stanton PK. Postsynaptic phospholipase C activity is required for the induction of homosynaptic long-term depression in rat hippocampus. Neurosci Lett. 1998;252:155–158. doi: 10.1016/s0304-3940(98)00496-0. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, Hesse D, Sudhof TC, Takahashi M, Rosenmund C, Brose N. Beta phorbol ester-and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- Richards DA, Rizzoli SO, Betz WJ. Effects of wortmannin and latrunculin A on slow endocytosis at the frog neuromuscular junction. J Physiol. 2004;557:77–91. doi: 10.1113/jphysiol.2004.062158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstad N, Nemoto Y, De Camilli P. The SH3p4/Sh3p8/SH3p13 protein family: binding partners for synaptojanin and dynamin via a Grb2-like Src homology 3 domain. Proc Natl Acad Sci U S A. 1997;94:8569–8574. doi: 10.1073/pnas.94.16.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Roizen NJ, Patterson D. Down's syndrome. Lancet. 2003;361:1281–1289. doi: 10.1016/S0140-6736(03)12987-X. [DOI] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- Rouach N, Byrd K, Petralia RS, Elias GM, Adesnik H, Tomita S, Karimzadegan S, Kealey C, Bredt DS, Nicoll RA. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- Roux A, Uyhazi K, Frost A, De Camilli P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–531. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- Rust MB, Gurniak CB, Renner M, Vara H, Morando L, Gorlich A, Sassoe-Pognetto M, Banchaabouchi MA, Giustetto M, Triller A, Choquet D, Witke W. Learning, AMPA receptor mobility and synaptic plasticity depend on n-cofilin-mediated actin dynamics. EMBO J. 2010;29:1889–1902. doi: 10.1038/emboj.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarikangas J, Zhao H, Lappalainen P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol Rev. 2008;90:259–289. doi: 10.1152/physrev.00036.2009. [DOI] [PubMed] [Google Scholar]
- Sadakata T, Kakegawa W, Mizoguchi A, Washida M, Katoh-Semba R, Shutoh F, Okamoto T, Nakashima H, Kimura K, Tanaka M, Sekine Y, Itohara S, Yuzaki M, Nagao S, Furuichi T. Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release. J Neurosci. 2007;27:2472–2482. doi: 10.1523/JNEUROSCI.2279-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, Atluri PP, Ryan TA. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat Neurosci. 2003;6:127–135. doi: 10.1038/nn1002. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Cammalleri M, Berton F, Simpson C, Lutjens R, Bloom FE, Francesconi W. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J Neurosci. 2002;22:3359–3365. doi: 10.1523/JNEUROSCI.22-09-03359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA. Coupling actin dynamics and membrane dynamics during endocytosis. Curr Opin Cell Biol. 2002;14:76–81. doi: 10.1016/s0955-0674(01)00297-6. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Gu QM, Prestwich GD, Sollner TH, Rothman JE. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc Natl Acad Sci U S A. 1996;93:13327–13332. doi: 10.1073/pnas.93.23.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Schuske KR, Richmond JE, Matthies DS, Davis WS, Runz S, Rube DA, van der Bliek AM, Jorgensen EM. Endophilin is required for synaptic vesicle endocytosis by localizing synaptojanin. Neuron. 2003;40:749–762. doi: 10.1016/s0896-6273(03)00667-6. [DOI] [PubMed] [Google Scholar]
- Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci. 2003;23:9220–9228. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seregaza Z, Roubertoux PL, Jamon M, Soumireu-Mourat B. Mouse models of cognitive disorders in trisomy 21: a review. Behav Genet. 2006;36:387–404. doi: 10.1007/s10519-006-9056-9. [DOI] [PubMed] [Google Scholar]
- Shih SC, Katzmann DJ, Schnell JD, Sutanto M, Emr SD, Hicke L. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat Cell Biol. 2002;4:389–393. doi: 10.1038/ncb790. [DOI] [PubMed] [Google Scholar]
- Shimada A, Niwa H, Tsujita K, Suetsugu S, Nitta K, Hanawa-Suetsugu K, Akasaka R, Nishino Y, Toyama M, Chen L, Liu ZJ, Wang BC, Yamamoto M, Terada T, Miyazawa A, Tanaka A, Sugano S, Shirouzu M, Nagayama K, Takenawa T, Yokoyama S. Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell. 2007;129:761–772. doi: 10.1016/j.cell.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Shin OH, Lu J, Rhee JS, Tomchick DR, Pang ZP, Wojcik SM, Camacho-Perez M, Brose N, Machius M, Rizo J, Rosenmund C, Sudhof TC. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat Struct Mol Biol. 2010;17:280–288. doi: 10.1038/nsmb.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpetner HS, Vallee RB. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989;59:421–432. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- Shupliakov O, Bloom O, Gustafsson JS, Kjaerulff O, Low P, Tomilin N, Pieribone VA, Greengard P, Brodin L. Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc Natl Acad Sci U S A. 2002;99:14476–14481. doi: 10.1073/pnas.212381799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siksou L, Triller A, Marty S. An emerging view of presynaptic structure from electron microscopic studies. J Neurochem. 2009;108:1336–1342. doi: 10.1111/j.1471-4159.2009.05888.x. [DOI] [PubMed] [Google Scholar]
- Simpson F, Hussain NK, Qualmann B, Kelly RB, Kay BK, McPherson PS, Schmid SL. SH3-domain-containing proteins function at distinct steps in clathrin-coated vesicle formation. Nat Cell Biol. 1999;1:119–124. doi: 10.1038/10091. [DOI] [PubMed] [Google Scholar]
- Singer-Kruger B, Nemoto Y, Daniell L, Ferro-Novick S, De Camilli P. Synaptojanin family members are implicated in endocytic membrane traffic in yeast. J Cell Sci. 1998;111(Pt 22):3347–3356. doi: 10.1242/jcs.111.22.3347. [DOI] [PubMed] [Google Scholar]
- Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci. 2000;1:161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- Slepnev VI, Ochoa GC, Butler MH, Grabs D, De Camilli P. Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science. 1998;281:821–824. doi: 10.1126/science.281.5378.821. [DOI] [PubMed] [Google Scholar]
- Speese S, Petrie M, Schuske K, Ailion M, Ann K, Iwasaki K, Jorgensen EM, Martin TF. UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J Neurosci. 2007;27:6150–6162. doi: 10.1523/JNEUROSCI.1466-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speidel D, Bruederle CE, Enk C, Voets T, Varoqueaux F, Reim K, Becherer U, Fornai F, Ruggieri S, Holighaus Y, Weihe E, Bruns D, Brose N, Rettig J. CAPS1 regulates catecholamine loading of large dense-core vesicles. Neuron. 2005;46:75–88. doi: 10.1016/j.neuron.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Stefan CJ, Audhya A, Emr SD. The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4,5)-bisphosphate. Mol Biol Cell. 2002;13:542–557. doi: 10.1091/mbc.01-10-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A, Radhakrishnan A, Riedel D, Fasshauer D, Jahn R. Synaptotagmin activates membrane fusion through a Ca2+-dependent trans interaction with phospholipids. Nat Struct Mol Biol. 2007;14:904–911. doi: 10.1038/nsmb1305. [DOI] [PubMed] [Google Scholar]
- Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S, Jin W, Thomas GM, Linden DJ, Huganir RL. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49:845–860. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Malenka RC. Understanding synapses: past, present, and future. Neuron. 2008;60:469–476. doi: 10.1016/j.neuron.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulis ML, Parsons R. PTEN: from pathology to biology. Trends Cell Biol. 2003;13:478–483. doi: 10.1016/s0962-8924(03)00175-2. [DOI] [PubMed] [Google Scholar]
- Sumioka A, Yan D, Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66:755–767. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundborger A, Soderblom C, Vorontsova O, Evergren E, Hinshaw JE, Shupliakov O. An endophilin-dynamin complex promotes budding of clathrin-coated vesicles during synaptic vesicle recycling. J Cell Sci. 2010;124:133–143. doi: 10.1242/jcs.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki DT, Grigliatti T, Williamson R. Temperature-sensitive mutations in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 1971;68:890–893. doi: 10.1073/pnas.68.5.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, McPherson PS, Schmid SL, De Camilli P. Tubular membrane invaginations coated by dynamin rings are induced by GTP-gamma S in nerve terminals. Nature. 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- Takei K, Slepnev VI, Haucke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nature Cell Biology. 1999;1:33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- Tandon A, Bannykh S, Kowalchyk JA, Banerjee A, Martin TF, Balch WE. Differential regulation of exocytosis by calcium and CAPS in semi-intact synaptosomes. Neuron. 1998;21:147–154. doi: 10.1016/s0896-6273(00)80522-x. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A, Pelkey KA, Rah JC, Suh YH, Roche KW, Collingridge GL, McBain CJ, Isaac JT. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron. 2008;57:872–882. doi: 10.1016/j.neuron.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieman JR, Mishra SK, Ling K, Doray B, Anderson RA, Traub LM. Clathrin regulates the association of PIPKIgamma661 with the AP-2 adaptor beta2 appendage. J Biol Chem. 2009;284:13924–13939. doi: 10.1074/jbc.M901017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Ma T, Iyengar R, Landau EM, Blitzer RD. Mitogen-activated protein kinase upregulates the dendritic translation machinery in long-term potentiation by controlling the mammalian target of rapamycin pathway. J Neurosci. 2007;27:5885–5894. doi: 10.1523/JNEUROSCI.4548-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujita K, Suetsugu S, Sasaki N, Furutani M, Oikawa T, Takenawa T. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol. 2006;172:269–279. doi: 10.1083/jcb.200508091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- Van Epps HA, Hayashi M, Lucast L, Stearns GW, Hurley JB, De Camilli P, Brockerhoff SE. The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring. J Neurosci. 2004;24:8641–8650. doi: 10.1523/JNEUROSCI.2892-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaramani DV, Chin J, Netzer WJ, Gouras GK, Lesne S, Malinow R, Lombroso PJ. Beta-amyloid modulation of synaptic transmission and plasticity. J Neurosci. 2007;27:11832–11837. doi: 10.1523/JNEUROSCI.3478-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Koh TW, Schulze KL, Zhai RG, Hiesinger PR, Zhou Y, Mehta SQ, Cao Y, Roos J, Bellen HJ. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Ohyama T, Haueter C, Habets RL, Lin YQ, Swan LE, Ly CV, Venken KJ, De Camilli P, Bellen HJ. Tweek, an evolutionarily conserved protein, is required for synaptic vesicle recycling. Neuron. 2009;63:203–215. doi: 10.1016/j.neuron.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Lucast L, Gong LW, Liu L, Sasaki J, Sasaki T, Abrams CS, Kanaho Y, De Camilli P. Phosphatidylinositol-4-phosphate 5-kinases and phosphatidylinositol 4,5-bisphosphate synthesis in the brain. J Biol Chem. 2010;285:28708–28714. doi: 10.1074/jbc.M110.132191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronov SV, Frere SG, Giovedi S, Pollina EA, Borel C, Zhang H, Schmidt C, Akeson EC, Wenk MR, Cimasoni L, Arancio O, Davisson MT, Antonarakis SE, Gardiner K, De Camilli P, Di Paolo G. Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down's syndrome. Proc Natl Acad Sci U S A. 2008;105:9415–9420. doi: 10.1073/pnas.0803756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walent JH, Porter BW, Martin TF. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 1992;70:765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Weernink PA, Meletiadis K, Hommeltenberg S, Hinz M, Ishihara H, Schmidt M, Jakobs KH. Activation of type I phosphatidylinositol 4-phosphate 5-kinase isoforms by the Rho GTPases, RhoA, Rac1, and Cdc42. J Biol Chem. 2004;279:7840–7849. doi: 10.1074/jbc.M312737200. [DOI] [PubMed] [Google Scholar]
- Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ. N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J Biol Chem. 2008;283:15912–15720. doi: 10.1074/jbc.M801555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenhorn W. Crystal structure of the endophilin-A1 BAR domain. J Mol Biol. 2005;351:653–661. doi: 10.1016/j.jmb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, Arioka M, Martin TF, De Camilli P. PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron. 2001;32:79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Yokotani N, Doi K, Wada K. Immunochemical characterization of the non-NMDA glutamate receptor using subunit-specific antibodies. Evidence for a hetero-oligomeric structure in rat brain. J Biol Chem. 1992;267:501–507. [PubMed] [Google Scholar]
- Wiedemann C, Schafer T, Burger MM. Chromaffin granule-associated phosphatidylinositol 4-kinase activity is required for stimulated secretion. EMBO J. 1996;15:2094–2101. [PMC free article] [PubMed] [Google Scholar]
- Wiedemann C, Schafer T, Burger MM, Sihra TS. An essential role for a small synaptic vesicle-associated phosphatidylinositol 4-kinase in neurotransmitter release. J Neurosci. 1998;18:5594–5602. doi: 10.1523/JNEUROSCI.18-15-05594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamabhai M, Hoffman NG, Hardison NL, McPherson PS, Castagnoli L, Cesareni G, Kay BK. Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J Biol Chem. 1998;273:31401–31407. doi: 10.1074/jbc.273.47.31401. [DOI] [PubMed] [Google Scholar]
- Yamada H, Padilla-Parra S, Park SJ, Itoh T, Chaineau M, Monaldi I, Cremona O, Benfenati F, De Camilli P, Coppey-Moisan M, Tramier M, Galli T, Takei K. Dynamic interaction of amphiphysin with N-WASP regulates actin assembly. J Biol Chem. 2009;284:34244–34256. doi: 10.1074/jbc.M109.064204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Hige T, Takahashi T. Vesicle endocytosis requires dynamin-dependent GTP hydrolysis at a fast CNS synapse. Science. 2005;307:124–127. doi: 10.1126/science.1103631. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Zhou Q. Perisynaptic GluR2-lacking AMPA receptors control the reversibility of synaptic and spines modifications. Proc Natl Acad Sci U S A. 2010;107:11999–12004. doi: 10.1073/pnas.0913004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarar D, Surka MC, Leonard MC, Schmid SL. SNX9 activities are regulated by multiple phosphoinositides through both PX and BAR domains. Traffic. 2008;9:133–146. doi: 10.1111/j.1600-0854.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- Yim YI, Sun T, Wu LG, Raimondi A, De Camilli P, Eisenberg E, Greene LE. Endocytosis and clathrin-uncoating defects at synapses of auxilin knockout mice. Proc Natl Acad Sci U S A. 2010;107:4412–4417. doi: 10.1073/pnas.1000738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- Yonezawa N, Homma Y, Yahara I, Sakai H, Nishida E. A short sequence responsible for both phosphoinositide binding and actin binding activities of cofilin. J Biol Chem. 1991;266:17218–17221. [PubMed] [Google Scholar]
- Yuen EY, Liu W, Kafri T, Van Praag H, Yan Z. Regulation of AMPA receptor channels and synaptic plasticity by cofilin phosphatase Slingshot in cortical neurons. J Physiol. 2010;588:2361–2371. doi: 10.1113/jphysiol.2009.186353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Koh YH, Beckstead RB, Budnik V, Ganetzky B, Bellen HJ. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21:1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]
- Zheng J, Cahill SM, Lemmon MA, Fushman D, Schlessinger J, Cowburn D. Identification of the binding site for acidic phospholipids on the pH domain of dynamin: implications for stimulation of GTPase activity. J Mol Biol. 1996;255:14–21. doi: 10.1006/jmbi.1996.0002. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]