Abstract
Intraperitoneal injection of all-trans-retinoic acid (ATRA) results in a reduction of blood pressure in spontaneously hypertensive rats. However, the mechanisms involved in this effect are not clear. We hypothesized that ATRA may relax resistance arteries. In this study, we found that ATRA relaxed phenylephrine-preconstricted mesenteric arterial rings, which were abrogated by the removal of the endothelium. Pretreatment of endothelium-intact arterial rings with an inhibitor of endothelial nitric oxide (NO) synthase, NG-nitro-l-arginine methyl ester (l-NAME), or soluble guanylyl cyclase, 1H-[1,2,4]-oxadiazole-[4,3-α]-quinoxaline-1-one, reduced the vasorelaxant effect of ATRA. Incubation of mesenteric arterial rings with ATRA increased the production of NO and cGMP, which were blocked by NG-nitro-l-arginine methyl ester. The vasorelaxant effect of ATRA was markedly attenuated in the presence of an inhibitor of big conductance calcium-activated potassium channels (charybdotoxin), but not with an inhibitor of voltage-dependent potassium channel (4-aminopyridine) or ATP-sensitive potassium channel (glibenclamide). Activation of retinoic acid receptors (RARs) with CH55 or retinoic X receptors (RXRs) with LGD1069 induced the vasorelaxation of phenylephrine-preconstricted mesenteric arterial rings. The RAR (BMS493) and RXR (UVI3003) antagonists blocked the ATRA-induced vasorelaxation. The vasorelaxant effect ATRA is physiologically relevant because the intravenous infusion of ATRA decreased blood pressure in normotensive rats. We conclude that ATRA relaxes resistance vessels via both RARs and RXRs receptors that are mediated by the endothelium-dependent NO-cGMP pathway, which may participate in the control of blood pressure.
Keywords: all-trans-retinoic acid, mesenteric artery, vasorelaxation, nitric oxide synthase, blood pressure
a biologically active metabolite of vitamin A, all-trans-retinoic acid (ATRA) has anti-inflammatory, anticancer, and immunomodulatory actions (20, 24, 31). ATRA exerts its biological effects by modulating gene transcription through distinct intracellular proteins, including the retinoic acid receptor (RAR) and retinoic X receptor (RXR) (2, 18), and activating some key transcription factors, such as nuclear factor-κB (20).
Recently, the role of ATRA in the development of cardiovascular dysfunctions has gained attention. It has also been shown that ATRA has potent antiproliferative and antioxidant actions (21, 23). A previous study showed that ATRA, given as daily intraperitoneal infusion, lowers blood pressure in spontaneously hypertensive rats (SHRs) (33). However, the mechanisms by which ATRA decreases blood pressure are not clear. To test the hypothesis that ATRA may relax resistance arteries, we studied the vasorelaxant effect of ATRA on phenylephrine (Phe)-preconstricted mesenteric arterial rings from rats. We found that ATRA, via RXR and RAR, induced vasorelaxation via calcium-activated potassium channels that are mediated by endothelium-dependent nitric oxide (NO)-cGMP pathways. The vasorelaxant effect of ATRA is physiologically relevant because the intravenous infusion of ATRA lowered blood pressure in normotensive rats.
MATERIALS AND METHODS
Preparation of rat mesenteric arterial rings.
Male Sprague-Dawley (SD) rats (250–350 g), purchased from Daping Hospital, were anesthetized with pentobarbital sodium (50 mg/kg) and tracheotomized, and blood pressure was determined from the femoral artery. The entire mesenteric bed was carefully removed and placed in ice-cold physiological salt solution (PSS) containing (in mM) 119 NaCl, 4.7 KCl, 2.5 CaCl2·H2O, 1.17 MgSO4·H2O, 25 NaH2CO3, 1.18 KH2PO4, 0.027 EDTA, and 5.5 glucose, adjusted to pH 7.35–7.45. The mesenteric artery was carefully and quickly dissected from the surrounding fat and connective tissues. Third-order branches of the superior mesenteric artery (resting arterial diameter, 250 ± 20 μm) were cut into rings ∼2 mm in length and mounted on 40-μm stainless-steel wires in an isometric Mulvany-Halpern small-vessel myograph (model M610, J. P. Trading, Science Park, Aarhus, Denmark) (19). One wire was attached to a force transducer and the other to a micrometer (19, 25). This arrangement enabled the wall tension to be measured at a predetermined internal circumference. The rings were maintained in PSS at 37°C and continuously bubbled with oxygen (95%) and carbon dioxide (5%) (carbogen). All dissecting procedures were done with extreme care to protect the endothelium from inadvertent damage. In some vessels, the endothelium was removed by pulling a hair along the vessel; successful denudation of the endothelium was confirmed by the absence of relaxation with the addition of acetylcholine (ACh, 10−6 M) (25). All experiments were approved by the Third Military Medical University Animal Use and Care Committee.
Measurement of isometric vascular tone.
Following mounting, the arterial ring was equilibrated in PSS for 1 h at 37°C at a wall tension of 0.1 mN/mm. Based on preliminary data from >100 vessels, we confirmed that a normalized circumference (L0) = 0.9 L100 resulted in maximal active force development. The vessels were studied at L0 in all subsequent protocols. Relaxation induced by ACh (10−6 M) was used to indicate the presence of intact endothelium. After the response to ACh was determined, the vessels were rinsed three times with fresh PSS and allowed to recover to baseline for 15 min. In the first set of experiments, the rings were contracted with Phe (10−5 M) and high-potassium PSS (125 mM) to obtain maximal response. After the maximal response to Phe (10−5 M) plateaued, the response curves to ATRA were measured by a cumulative concentration-dependent protocol (10−8 to 3 × 10−6 M). Response to every single concentration of ATRA was observed for 1 min. The effect of the vehicle for ATRA, dimethylsulfoxide (<1%), was also tested.
Measurement of cGMP levels in mesenteric artery.
After equilibration of the mesenteric arterial rings for 30 min in PSS with carbogen, endothelium-intact and endothelium-denuded arterial rings were incubated with Phe (10−5 M) for 15 min before the addition of ATRA (10−6 M). Other groups were treated with NG-nitro-l-arginine methyl ester (l-NAME; 10−4 M) for 30 min before the addition of ATRA (10−6 M). The reaction was stopped by freezing the tissues in liquid nitrogen. The tissues were weighed and then homogenized in 6% trichloroacetic acid. The homogenates were centrifuged at 15,000 g for 10 min, and the supernatant was extracted four times with water-saturated diethyl ether and then concentrated in a high-speed refrigerated centrifuge (Neofuge 18R, Heal force). The precipitates were resuspended with 20 mM Tris·HCl buffer (pH 7.4), and the protein concentration was then determined. cGMP content was measured using a cGMP kit (rat cGMP, cGMP ELISA KIT, HuFeng, China). Results were expressed as picomoles of cGMP generated per milligram of protein.
Measurement of NO production in mesenteric artery.
NO production in the mesenteric artery was quantified with the use of 4,5-diaminofluorescein-2 (DAF-2) diacetate (DAF-2DA) as a fluorescent indicator for intracellular NO. (28) Third-order branches of the superior mesenteric artery were removed of connective tissue and fat, as described above, and then cut into rectangular pieces and incubated at 37°C with PSS. The pieces of vessels were loaded with 15 μM DAF-2DA for 30 min and washed with PSS three times for 15 min. DAF-2DA permeates the cell membrane and is converted to DAF-2, which reacts with NO and changes to a highly fluorescent triazole form (DAF-2T) that can be quantified (28). The tissue was incubated with ATRA (10−6 M) for 30 min before recording DAF-2T fluorescence intensity using a microscope (model ECLIPSE Ti-U, Nikon) and a high-speed video system (MHS-200). The fluorescence intensity was analyzed by a Macintosh computer and the National Institutes of Health Image program. Results were expressed as DAF-2T fluorescence.
Immunoblotting.
Mesenteric arterial rings from SD rats were washed three times with cold PSS. Endothelium-intact arterial rings were incubated with ATRA (10−6 M) for 20 min in PSS; those treated with vehicle were considered as controls. The reaction was stopped by freezing the tissues in liquid nitrogen. The tissues were weighed and then homogenized in 6% trichloroacetic acid for 1 h. The homogenates were centrifuged at 15,000 g for 10 min. The supernatant was collected and protein concentration was measured using the bicinchoninic acid method (Pierce, Rockford, IL). The proteins in equal amounts of samples were resolved in 8.0% SDS-polyacrylamide gel and then transferred onto polyvinylidene difluoride membranes. After blocking with 0.5% skim milk, the membranes were incubated with primary antibodies [endothelial NO synthase (eNOS), 1:800 dilution; phospho-eNOS (Ser1177) 1:500 dilution; Akt, 1:800 dilution; and phospho-Akt (Ser473), 1:500 dilution] (Cell Signaling, Beverly, MA) at 4°C overnight. The membrane-bound antibodies were visualized using horseradish peroxidase-conjugated secondary antibodies (1:15,000 dilution, 1 h) and the Odyssey Infrared Imaging System (Li-Cor Bioscience, Bad Homburg). The expression of phosphorylated eNOS and Akt were normalized with total eNOS and Akt, respectively (32).
Materials.
ATRA, ACh chloride, glibenclamide, 4-aminopyridine (4-AP), charybdotoxin, Phe HCl, l-NAME, S-methylisothiourea sulfate (SMT), NG-monomethyl-l-arginine, 1H-[1,2,4]-oxadiazole-[4,3-α]-quinoxaline-1-one (ODQ), and DAF-2DA were obtained from Sigma-Aldrich (St. Louis, MO). LGD1069, CH55, UVI3003, and BMS493 were from Tocris Bioscience (Ellisville, MO). ATRA, ODQ, 4-AP, glibenclamide, LGD1069, CH55, UVI3003, and BMS493 were dissolved into dimethylsulfoxide.
Statistical analysis.
Relaxation responses are expressed as a percent decline from the maximum contractile response to Phe (10−5 M). The results are shown as means ± SE. Comparison within groups was made by repeated-measures ANOVA (or paired t-test when only 2 groups were compared), and comparison among groups (or t-test when only 2 groups were compared) was made by one-way ANOVA with Duncan's test. A value of P < 0.05 was considered significant.
RESULTS
Vasorelaxant effect of ATRA on mesenteric arterial rings preconstricted by Phe.
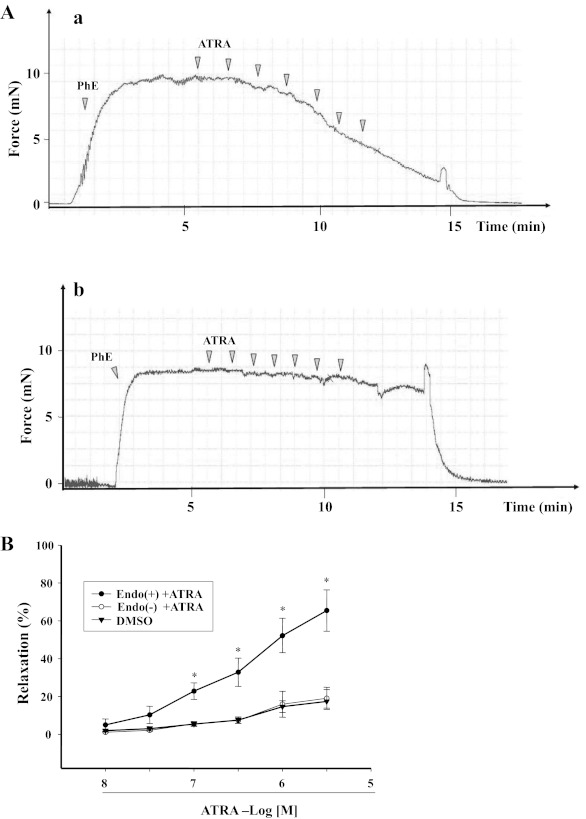
ATRA (1 × 10−8–3 × 10−6 M), by itself, had no vasoconstrictor effect but relaxed arterial rings preconstricted with Phe in a concentration-dependent manner (Fig. 1, A and B). The maximal relaxant effect of ATRA was 65.5 ± 10.9%. To determine the role of endothelium on the vasorelaxant effect of ATRA, the endothelium was denuded, which prevented the vasorelaxant effect of ATRA. The graph of the ATRA-induced vasodilation in endothelium-intact arteries is shown in subsequent graphs for comparison with other experiments. Representative tracings of the vasorelaxant effect of ATRA in endothelium-intact and endothelium-denuded arterial rings are shown in Fig. 1A, a and b, respectively.
Fig. 1.
Concentration-response curves of all-trans-retinoic acid (ATRA)-induced relaxation of phenylephrine (Phe)-preconstricted rat mesenteric arterial rings. A: representative tracings: endothelium-intact artery (Endo+; a) and endothelium-denuded artery (Endo−; b). B: Endo+ (n = 8) and Endo− (n = 5) rat mesenteric arterial rings were preconstricted with Phe and then treated with different concentrations of ATRA (10−8–3 × 10−6 M). Each value represents the mean ± SE. *P < 0.01 vs. ATRA (10−8 M), Endo− + ATRA, or DMSO.
Role of NO-cGMP on the vasorelaxant effect of ATRA on mesenteric arterial rings preconstricted by Phe.
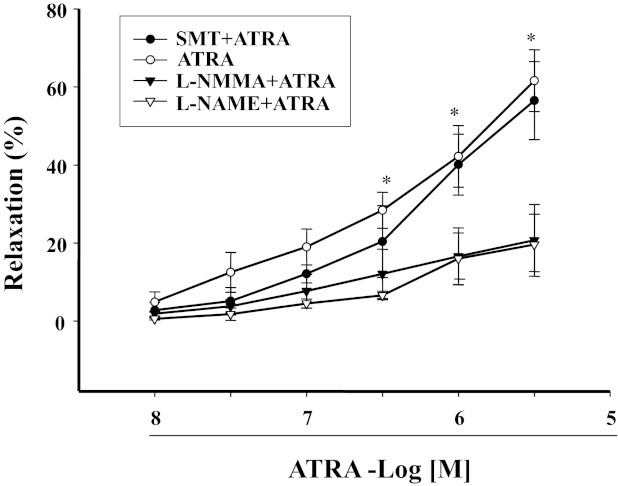
Because ATRA has been reported to increase NO production in endothelial cells (1, 28), we next studied the role of NO on the vasorelaxant effect of ATRA (10−8–3 × 10−6 M). In the presence of a NO synthase (NOS) inhibitor NG-monomethyl-l-arginine (10−5 M), the vasorelaxant effect of ATRA was significantly reduced (Fig. 2). To determine the specific NOS isoform involved, we used specific NOS isoenzyme inhibitors, i.e., l-NAME, an eNOS inhibitor, and SMT, an inducible NOS (iNOS) inhibitor. We found that the vasorelaxant effect of ATRA was blocked by l-NAME (10−4 M) but not by SMT (10−5 M) (12), indicating that eNOS, but not iNOS, was involved in the ATRA-mediated vasorelaxation (Fig. 2). DAF-2T fluorescence studies showed that ATRA induced NO production in a time-dependent manner, which was maximal at 15 min and lasted for at least 1 h (Fig. 3A).
Fig. 2.
Role of nitric oxide (NO) synthase (NOS) in ATRA-induced vasorelaxation. Phe-preconstricted mesenteric arterial rings were treated with ATRA (10−8–3×10−6 M) in the presence of NG-monomethyl-l-arginine (l-NMMA; 10−5 M, n = 4), a NOS inhibitor; NG-nitro-l-arginine methyl ester (l-NAME; 10−4 M, n = 5), an endothelial NOS (eNOS) inhibitor; or S-methylisothiourea sulfate (SMT; 10−5 M, n = 5), an inducible NOS inhibitor. Each value represents the mean ± SE. *P < 0.01 vs. l-NMMA + ATRA or l-NAME + ATRA.
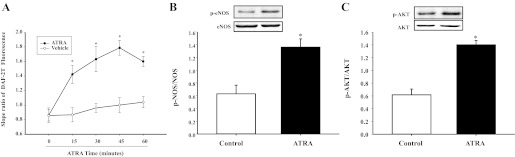
Fig. 3.
Effect of ATRA on NO production in mesenteric arterial tissue. A: effect of ATRA on NO production in mesenteric arterial tissue. Mesenteric arterial tissues were treated with ATRA (10−6 M, n = 3) at the indicated duration. NO production was quantified by measuring 4,5-diaminofluorescein-2 triazole (DAF-2T) high fluorescence. Each value represents the mean ± SE. *P < 0.01 vs. control (0 min) or vehicle. B and C: effect of ATRA on the phosphorylation of eNOS (p-eNOS; B) and Akt (p-Akt; C) in mesenteric arterial tissue. Mesenteric arterial tissues were treated with ATRA (10−6 M) for 20 min; total and p-eNOS and p-Akt were quantified by immunoblotting. The mean ± SE of the ratio of phosphorylated and total eNOS or Akt is shown. *P < 0.01 vs. control; n = 5.
A previous study showed that ATRA increased NO production in vascular endothelial cells by phosphorylation of eNOS through the phosphatidylinositol 3-kinase/Akt pathway (28). In our current study, using mesenteric arterial rings, we also found that ATRA (10−6 M/20 min) increased the phosphorylation of eNOS and Akt, indicating that Akt-eNOS was involved in the ATRA-mediated increase in NO production (Fig. 3, B and C).
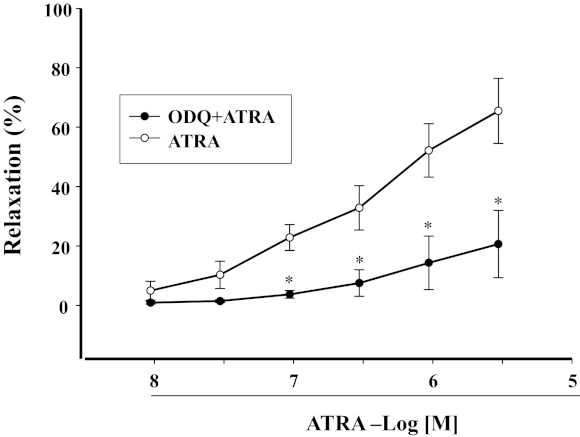
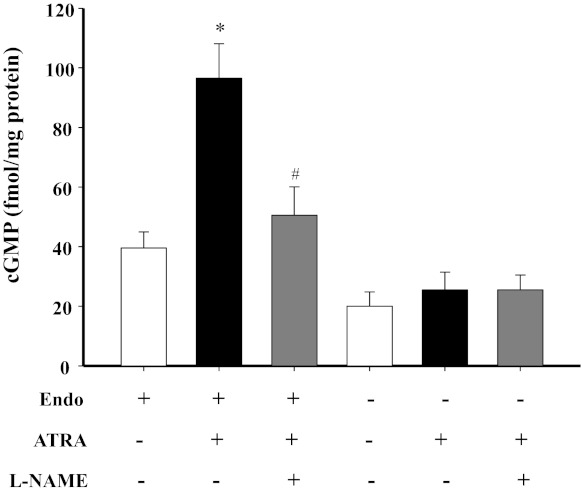
As a downstream signaling molecule of NO (7), we studied the role of cGMP on the vasorelaxant effect of ATRA. We found that ODQ (10−5 M), a selective inhibitor of soluble guanylyl cyclase (sGC), markedly decreased the vasorelaxant effect of ATRA (10−6 M) (Fig. 4). Incubation of mesenteric arterial rings with ATRA (10−6 M) increased cGMP production that was blocked by l-NAME (10−4 M). Moreover, the stimulatory effect of ATRA on cGMP production also disappeared in the endothelium-denuded mesenteric arterial rings (Fig. 5).
Fig. 4.
Effect of 1H-[1,2,4]-oxadiazole-[4,3-α]-quinoxaline-1-one (ODQ), an inhibitor of soluble guanylyl cyclase, on ATRA-induced relaxation of Phe-preconstricted mesenteric arterial rings. Phe-preconstricted mesenteric arterial rings were incubated with ODQ (10−5 M, n = 5) for 30 min and then treated with ATRA (1 × 10−8–3 × 10−6 M) (n = 8 for the ATRA alone). Each value represents the mean ± SE. *P < 0.01 vs. ATRA.
Fig. 5.
Effect of l-NAME on ATRA-induced increase in cGMP levels in mesenteric arterial rings with intact or denuded endothelium. Mesenteric arterial rings with or without endothelium were incubated with ATRA (10−6 M) in the presence or absence of l-NAME (10−4 M), an NOS inhibitor. Each value represents the mean ± SE. *P < 0.01 vs. control (Endo+, ATRA−, and l-NAME−). #P < 0.01 vs. ATRA group (Endo+, ATRA+, and l-NAME−); n = 6.
To determine whether or not prostanoids were involved in the vasorelaxant effect of ATRA, we studied the vasorelaxant effect of ATRA in the presence of the cyclooxygenase inhibitor indomethacin (10−5 M). It resulted that the vasorelaxant effect of ATRA was not influenced by indomethacin (data not shown).
Role of potassium channels in the vasorelaxant effect of ATRA.
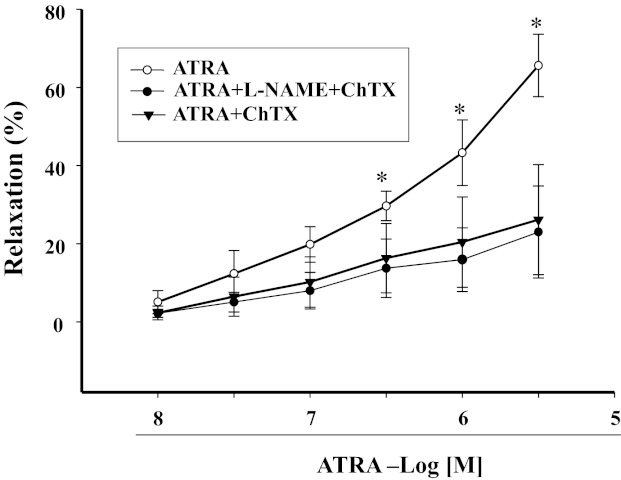
Because the vasorelaxant effect of cGMP could be via potassium channels (27, 29), we studied the effect of different potassium channel blockers: glibenclamide (10−5 M) (14), an ATP-sensitive potassium channel blocker; 4-AP (10−4 M) (14), a voltage-dependent potassium channel blocker; and charybdotoxin (10−5 M), a calcium-activated potassium channel blocker. In the presence of charybdotoxin (10−5 M), the vasorelaxant effect of ATRA in arterial rings preconstricted with Phe was blocked (Fig. 6), indicating the importance of calcium-activated potassium channels in the vasorelaxant effect of ATRA. ATRA could not relax arterial rings preconstricted with high potassium chloride (125 mM) (data not shown), further indicating the importance of potassium channels in the vasorelaxant effect of ATRA. Moreover, neither glibenclamide nor 4-AP blocked the vasorelaxant effect of ATRA in Phe-preconstricted mesenteric arterial rings (data not shown), indicating that ATP-sensitive potassium channel and voltage-dependent potassium channel were not involved in the vasorelaxant effect of ATRA.
Fig. 6.
Effect of l-NAME and/or charybdotoxin (ChTX), a calcium-activated potassium channel blocker, on ATRA-induced relaxation in Phe-preconstricted mesenteric arterial rings. Phe-preconstricted mesenteric arterial rings were incubated with l-NAME (10−4 M) with or without ChTX (10−5 M) for 30 min and then treated with ATRA (1 × 10−8–3 × 10−6 M). Each value represents the mean ± SE. *P < 0.01, ATRA vs. other groups; n = 5.
Involvement of ATRA receptor subtypes in ATRA-mediated vasorelaxation of mesenteric arterial rings preconstricted by Phe.
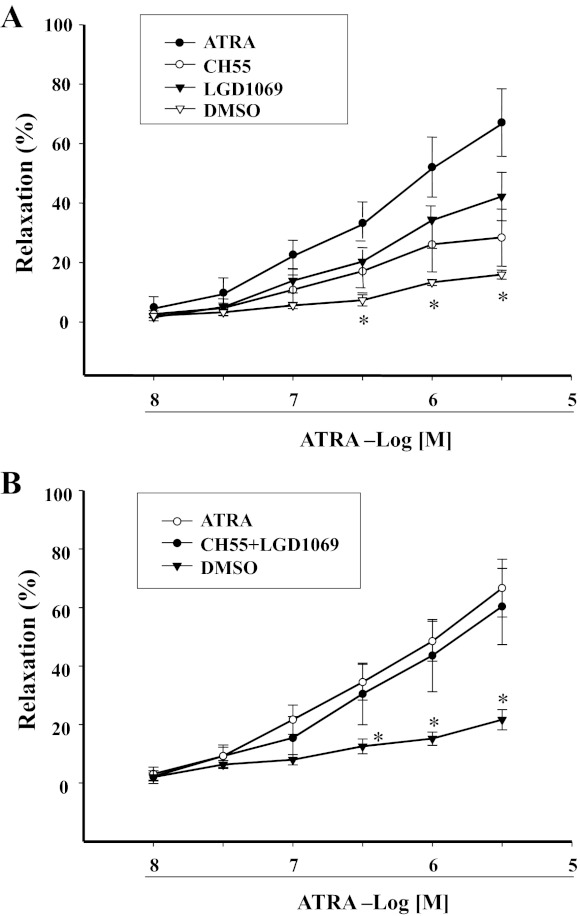
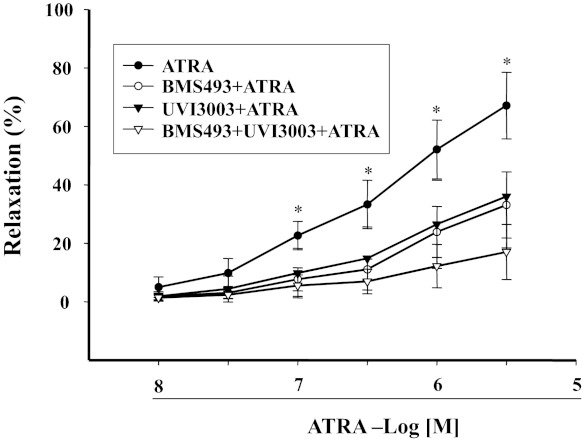
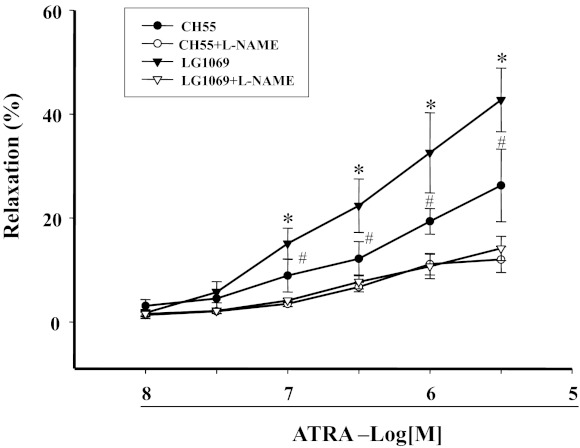
Retinoid signals are transduced by the RAR and RXR. We found that CH55 (10−8 M–3 × 10−6 M), an RAR agonist, relaxed mesenteric arterial rings preconstricted by Phe, but to a lesser extent than ATRA. Similar results were obtained with an RXR agonist LGD1069 (10−8 M–3 × 10−6 M). However, simultaneous stimulation of RXR and RAR with CH55 and LGD1069 produced a vasorelaxant effect similar to ATRA (Fig. 7, A and B), indicating that both RAR and RXR can contribute to the relaxation produced by ATRA. This interpretation was confirmed by our study in which the combination of RAR antagonist (BMS493) and RXR antagonist (UV13003) completely blocked, whereas either antagonist only partially blocked, the ATRA-induced vasorelaxation in mesenteric arterial rings preconstricted by Phe (Fig. 8). The role of NO on the RAR- and RXR-mediated vasodilation was studied in the presence of the eNOS inhibitor l-NAME (10−4 M), which blocked the vasodilatory effect mediated by LG1069 (10−8–3 × 10−6 M) or CH55 (10−8–3 × 10−6 M) (Fig. 9), indicating that NO was involved in both RAR- and RXR-mediated vasodilation.
Fig. 7.
Concentration-response curves of retinoic acid receptor (RAR) and retinoic X receptor (RXR) agonists on Phe-preconstricted endothelium-intact mesenteric arterial rings. Phe-preconstricted mesenteric arterial rings were incubated with CH55 (10−8–3 × 10−6 M, n = 6), an RAR agonist, or LGD1069 (10−8–3 × 10−6 M, n = 7), an RXR agonist (n = 8 for ATRA alone) (A) or the combination of CH55 and LGD1069 (n = 4) (B) for 1 min. Each value represents the mean ± SE. *P < 0.05, DMSO (vehicle) vs. other group.
Fig. 8.
Concentration-response curves of RAR and RXR antagonists on the ATRA-induced vasorelaxation in Phe-preconstricted mesenteric arterial rings. Phe-preconstricted mesenteric arterial rings were incubated with BMS493 (10−7 M), an RAR antagonist; UVI3003 (10−7 M), an RXR receptor antagonist; or the combination of BMS493 and UV13003 for 30 min and then incubated with ATRA. Each value represents the mean ± SE. *P < 0.01 vs. other groups; n = 8.
Fig. 9.
Effects of eNOS inhibitor l-NAME on RAR- or RXR-induced vasorelaxation: l-NAME (10−4 M, n = 4), LG1069 (10−8–3 × 10−6 M, n = 4), and CH55 (10−8–3 × 10−6 M, n = 4). Each value represents the mean ± SE. *P < 0.05 vs. l-NAME + LG1069 group; #P < 0.05 vs. l-NAME + CH55 group.
Effect of ATRA on blood pressure in SD rats.
To determine the physiological significance of our findings, we infused ATRA intravenously (5 μg·kg−1·min−1) in SD rats. We found that systolic, diastolic, and mean arterial blood pressures tended to decrease after 10 min of infusion of ATRA and significantly decreased after 20 min. The decrease in blood pressure was accompanied by a slight decrease in the heart rate (Table 1).
Table 1.
Effect of ATRA on blood pressure and HR in SD rats
| ATRA |
|||
|---|---|---|---|
| Parameter | Basal | 10 min | 20 min |
| SBP, mmHg | 124.3 ± 6.6 | 113.0 ± 11.2 | 104.3 ± 10.3* |
| DBP, mmHg | 97.3 ± 8.5 | 87.3 ± 9.6 | 81.3 ± 6.1* |
| MAP, mmHg | 108.8 ± 7.6 | 99.0 ± 9.3 | 90.3 ± 8.7* |
| HR, beats/min | 362.3 ± 5.3 | 357.0 ± 3.7 | 346.0 ± 3.1* |
Values are means ± SE; n = 4 Sprague-Dawley (SD) rats. ATRA, all-trans-retinoic acid; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial blood pressure; HR, heart rate.
P < 0.01 vs. basal.
DISCUSSION
Retinoids are a group of potent natural or synthetic molecules that exert important roles in angiogenesis and the embryonic development of the cardiovascular system (6, 16). Retinoids also inhibit cellular proliferation, increase elastin synthesis, and stimulate metalloproteinase inhibitor production by fibroblasts that may maintain the stability of atherosclerotic plaques (30). A previous study (33) demonstrated that the daily intraperitoneal injection of retinoic acid for 1 mo reduced the elevated blood pressure and attenuated the myocardial damage in the SHR. We hypothesized that the reduction of blood pressure in the SHR with retinoic acid may be mediated, in part, by a direct relaxant effect on systemic arterial resistance vessels. The results of this study suggest a series of signaling pathways from the ATRA receptor to Akt-eNOS, resulting in the generation of NO. NO, generated by the endothelial cells, diffuses into vascular smooth muscle cells and activates sGC, which increases intracellular cGMP concentrations (15, 17). cGMP, acting as a second messenger, produces a wide range of effects, including the activation of protein kinase G that opens of big conductance calcium-activated potassium channels, resulting in vasorelaxation.
Smooth muscle cells undergo vasorelaxation that is mediated by the endothelium through a variety of direct and indirect pathways, including hyperpolarization and opening of potassium-sensitive channels in smooth muscle cells or endothelial cells (8, 26). Our current study shows that removal of the endothelium abolishes the vasorelaxation caused by ATRA, indicating the necessity of an intact endothelium in this effect. The endothelium responds to various neurohumoral and physical stimuli by releasing endothelium-dependent vasoconstrictor and vasorelaxant factors, such as endothelium-derived relaxing factor (11) and prostacyclin (9). To determine the mechanism by which the endothelium mediates the vasorelaxant effect of ATRA, we studied the effects of NOS inhibitors on the ATRA-induced vasorelaxation. Pretreatment of mesenteric arterial rings with the eNOS inhibitor l-NAME, but not the iNOS inhibitor SMT, abolishes the vascular relaxant effect. Previous studies have demonstrated that sGC, an NO-dependent and constitutively active enzyme, is also responsible for the conversion of GTP to the second messenger, cGMP (15, 17). In our study, pretreatment with the sGC inhibitor ODQ reduces the ATRA-induced relaxation and ATRA-induced increase in cGMP production, indicating the importance of the NO-cGMP pathway in the vasorelaxation of the mesenteric artery caused by ATRA. In our study, the vasorelaxation induced by ATRA is abolished by high concentration of potassium chloride or charybdotoxin indicating that activation of potassium channels, specifically, big conductance calcium-activated potassium channels in vascular smooth cells, which are presumably downstream of NO-cGMP, is important in ATRA-mediated vasorelaxation. Due to the nonselective property of charybdotoxin on intermediate and big conductance calcium-activated potassium channels, whether or not the intermediate channel is involved in the signal pathway is not clear, which needs to be confirmed in the future.
Retinoid signals are transduced by the RAR and RXR. These receptors, belonging to the nuclear hormone receptor superfamily (3, 10, 22), may mediate the effects of retinoic acid mentioned above, i.e., cell growth, differentiation, and apoptosis. Haxsen et al. (13) reported that retinoic acid could inhibit the proliferative effect of angiotensin II on vascular smooth muscle cells via RAR- and RXR-dependent pathways. RAR and RXR are also involved in preventing angiotensin II- and stretch-induced reactive oxygen species generation and stretch-induced apoptosis in cardiomyocytes. However, in endothelial cells, the retinoic acid-induced prostaglandin I synthase is mediated by RAR and not RXR (4). In our study, we used retinoid receptor-selective agonists and antagonists to determine the retinoic receptor involved in the ATRA-mediated vascular relaxation. An RAR- or an RXR-specific agonist partially reproduces the concentration-dependent vasorelaxant effect of ATRA in rat mesenteric arterial rings preconstricted by Phe. The vasorelaxant effect of ATRA is only partially blocked by either the RAR or RXR antagonist. In contrast, the concurrent use of both RAR and RXR agonists fully reproduces the vasorelaxant effect of ATRA, whereas the concurrent use of both RAR and RXR antagonists completely blocks the vasorelaxant effect of ATRA. Thus both RXR and RAR are involved in the vasorelaxant effect of ATRA.
The vasorelaxant effect of ATRA shown in vitro is physiologically relevant because the intravenous infusion of ATRA decreases blood pressure in SD rats, consistent with the reported hypotensive effect of ATRA (33). However, the hypotensive effect of ATRA was noted only after 3 wk of ATRA administration in the SHR but not in the normotensive Wistar-Kyoto rats (33). The reason for the discrepancy between the report of Zhong et al. and our current study may be related to the method of ATRA delivery. Zhong et al. administered ATRA by intraperitoneal injection instead of intravenous infusion. The vehicle (50% intralipid, 45% saline, and 5% ethanol) used to dissolve ATRA may have slowed down or decreased the absorption of ATR, and thus the hypotensive effect may not have been immediately obvious or not sufficient enough to decrease the blood pressure in normotensive rats.
In conclusion, ATRA relaxes resistance vessels through an endothelium-dependent NO-cGMP pathway that is dependent on both RAR and RXR. The vasorelaxant effect ATRA is physiologically relevant because the intravenous infusion of ATRA could lower blood pressure.
GRANTS
These studies were supported in part by grants from the National Basic Research Program of China (973 Program) Grants 2008CB517308, 2013CB531100, and 2012CB517801; Natural Science Foundation Project of CQ CSTC Grant CSTC, 2009BA5044; National Natural Science Foundation of China Grants 30925018 and 31130029; and National Heart, Lung, and Blood Institute Grant R01-HL-092196.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.W., Y.H., J.Y., Z.W., L.L., W.W., L.Z., D.W., X.T., C.F., and C.Z. performed experiments; Y.W. and C.Z. analyzed data; P.A.J. and C.Z. drafted manuscript; C.Z. edited and revised manuscript.
REFERENCES
- 1. Achan V, Tran CT, Arrigoni F, Whitley GS, Leiper JM, Vallance P. All-trans-retinoic acid increases nitric oxide synthesis by endothelial cells: a role for the induction of dimethylarginine dimethylaminohydrolase. Circ Res 90: 764–769, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res 43: 1773–1808, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Botling J, Castro DS, Oberg F, Nilsson K, Perlmann T. Retinoic acid receptor/retinoid X receptor heterodimers can be activated through both subunits providing a basis for synergistic transactivation and cellular differentiation. J Biol Chem 272: 9443–9449, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Camacho M, Rodríguez C, Salazar J, Martínez-González J, Ribalta J, Escudero JR, Masana L, Vila L. Retinoic acid induces PGI synthase expression in human endothelial cells. J Lipid Res 49: 1707–1714, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Choudhary R, Baker KM, Pan J. All-trans retinoic acid prevents angiotensin II- and mechanical stretch-induced reactive oxygen species generation and cardiomyocyte apoptosis. J Cell Physiol 215: 172–181, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Colbert MC, Hall DG, Kimball TR, Witt SA, Lorenz JN, Kirby ML, Hewett TE, Klevitsky R, Robbins J. Cardiac compartment-specific overexpression of a modified retinoic acid receptor produces dilated cardiomyopathy and congestive heart failure in transgenic mice. J Clin Invest 100: 1958–1968, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta 1411: 334–350, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Dora KA. Coordination of vasomotor responses by the endothelium. Circ J 74: 226–232, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Dusting GJ, Chapple DJ, Hughes R, Moncada S, Vane JR. Prostacyclin (PGI2) induces coronary vasodilatation in anaesthetised dogs. Cardiovasc Res 12: 720–730, 1978 [PubMed] [Google Scholar]
- 10. Egea PF, Rochel N, Birck C, Vachette P, Timmins PA, Moras D. Effects of ligand binding on the association properties and conformation in solution of retinoic acid receptors RXR and RAR. J Mol Biol 307: 557–576, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 12. Gocan NC, Scott JA, Tyml K. Nitric oxide produced via neuronal NOS may impair vasodilatation in septic rat skeletal muscle. Am J Physiol Heart Circ Physiol 278: H1480–H1489, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Haxsen V, Adam-Stitah S, Ritz E, Wagner J. Retinoids inhibit the actions of angiotensin II on vascular smooth muscle cells. Circ Res 88: 637–644, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Kitamura K, Kamouchi M. K channel openers activate different K channels in vascular smooth muscle cells. Cardiovasc Drugs Ther 3: 539–546, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Knowles RG, Moncada S. Nitric oxide as a signal in blood vessels. Trends Biochem Sci 17: 399–402, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Lansink M, Koolwijk P, van Hinsbergh V, Kooistra T. Effect of steroid hormones and retinoids on the formation of capillary-like tubular structures of human microvascular endothelial cells in fibrin matrices is related to urokinase expression. Blood 92: 927–938, 1998 [PubMed] [Google Scholar]
- 17. Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52: 375–414, 2000 [PubMed] [Google Scholar]
- 18. Marill J, Idres N, Capron CC, Nguyen E, Chabot GG. Retinoic acid metabolism and mechanism of action: a review. Curr Drug Metab 4: 1–10, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41: 19–26, 1977 [DOI] [PubMed] [Google Scholar]
- 20. Na SY, Kang BY, Chung SW, Han SJ, Ma X, Trinchieri G, Im SY, Lee JW, Kim TS. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFκB. J Biol Chem 274: 7674–7680, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Neuville P, Yan Z, Gidlöf A, Pepper MS, Hansson GK, Gabbiani G, Sirsjö A. Retinoic acid regulates arterial smooth muscle cell proliferation and phenotypic features in vivo and in vitro through an RARα-dependent signaling pathway. Arterioscler Thromb Vasc Biol 19: 1430–1436, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Pogenberg V, Guichou JF, Vivat-Hannah V, Kammerer S, Pérez E, Germain P, de Lera AR, Gronemeyer H, Royer CA, Bourguet W. Characterization of the interaction between retinoic acid receptor/retinoid X receptor (RAR/RXR) heterodimers and transcriptional coactivators through structural and fluorescence anisotropy studies. J Biol Chem 280: 1625–1633, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Ramya D, Siddikuzzaman Manjamalai A, Berlin Grace VM. Chemoprotective effect of all-trans retinoic acid (ATRA) on oxidative stress and lung metastasis induced by benzo(a)pyrene. Immunopharmacol Immunotoxicol 34: 317–325, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Salazar MD, Ratnam M, Patki M, Kisovic I, Trumbly R, Iman M, Ratnam M. During hormone depletion or tamoxifen treatment of breast cancer cells the estrogen receptor apoprotein supports cell cycling through the retinoic acid receptor α1 apoprotein. Breast Cancer Res 13: R18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schäfer A, Burkhardt M, Vollkommer T, Bauersachs J, Münzel T, Walter U, Smolenski A. Endothelium-dependent and -independent relaxation and VASP serines 157/239 phosphorylation by cyclic nucleotide-elevating vasodilators in rat aorta. Biochem Pharmacol 65: 397–405, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Somani A, Steiner ME, Hebbel RP. The dynamic regulation of microcirculatory conduit function: features relevant to transfusion medicine. Transfus Apher Sci 43: 61–68, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Standen NB, Quayle JM. K+ channel modulation in arterial smooth muscle. Acta Physiol Scand 1998 164: 549–557, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Uruno A, Sugawara A, Kanatsuka H, Kagechika H, Saito A, Sato K, Kudo M, Takeuchi K, Ito S. Upregulation of nitric oxide production in vascular endothelial cells by all-trans retinoic acid through the phosphoinositide 3-kinase/Akt pathway. Circulation 112: 727–736, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Waldron GJ, Cole WC. Activation of vascular smooth muscle K+ channels by endothelium-derived relaxing factors. Clin Exp Pharmacol Physiol 26: 180–184, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Wiegman PJ, Barry WL, McPherson JA, McNamara CA, Gimple LW, Sanders JM, Bishop GG, Powers ER, Ragosta M, Owens GK, Sarembock IJ. All-trans-retinoic acid limits restenosis after balloon angioplasty in the focally atherosclerotic rabbit: a favorable effect on vessel remodeling. Arterioscler Thromb Vasc Biol 20: 89–95, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Yang Y, Vacchio MS, Ashwell JD. 9-cis-retinoic acid inhibits activation-driven T-cell apoptosis: implications for retinoid X receptor involvement in thymocyte development. Proc Natl Acad Sci USA 90: 6170–6174, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res 99: 494–500, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Zhong JC, Huang DY, Yang YM, Li YF, Liu GF, Song XH, Du K. Upregulation of angiotensin-converting enzyme 2 by all-trans retinoic acid in spontaneously hypertensive rats. Hypertension 44: 907–912, 2004 [DOI] [PubMed] [Google Scholar]