Abstract
Chronic inflammation is a salient feature of sickle cell disease (SCD) and transgenic-knockout sickle (BERK) mice. Inflammation is implicated in the activation of hypoxia-inducible factor-1α (HIF-1α) under normoxic conditions. We hypothesize that, in SCD, inflammation coupled with nitric oxide (NO) depletion will induce expression of HIF-1α, a transcription factor with wide-ranging effects including activation of genes for vasoactive molecules. To this end, we have examined the expression of HIF-1α in normoxic BERK mice expressing exclusively human α- and βS- globins, and evaluated the effect of fetal hemoglobin (HbF) in BERK mice (i.e., <1.0%, 20%, and 40% HbF). HbF exerts antisickling and anti-inflammatory effects. Here, we show that HIF-1α is expressed in BERK mice under normoxic conditions, accompanied by increased expression of its vasoactive biomarkers such as VEGF, heme oxygenase-1 (HO-1), and serum ET-1 levels. In BERK mice expressing HbF, HIF-1α expression decreases concomitantly with increasing HbF, commensurately with increased NO bioavailability, and shows a strong inverse correlation with plasma NO metabolites (NOx) levels. Reduced HIF-1α expression is associated with decreased HO-1, VEGF, and ET-1. Notably, arteriolar dilation, enhanced volumetric blood flow, and low blood pressure in normoxic BERK mice all show a trend toward normalization with the introduction of HbF. Also, arginine treatment reduced HIF-1α, as well as VEGF expression in normoxic BERK mice, supporting a role of NO bioavailability in HIF-1α activation. Thus HIF-1α expression in normoxic sickle mice is likely a consequence of chronic inflammation, and HbF exerts an ameliorating effect by decreasing sickling, increasing NO bioavailability, and reducing inflammation.
Keywords: sickle cell disease, hypoxia-inducible factor-1α, fetal hemoglobin, nitric oxide, oxidative stress, inflammation
hemoglobin s (HBS) polymerization under deoxygenated conditions and abnormal red blood cell rheology are central to the pathophysiology of sickle cell disease (SCD), the consequences of which can lead to vaso-occlusion, reperfusion injury, oxidative stress, hemolysis, reduced nitric oxide (NO) bioavailability, and inflammation (15, 19, 25, 41). In SCD, vaso-occlusive events may result in episodes of tissue ischemia. Tissue ischemia is normally associated with the activation of hypoxia-inducible factor-1α (HIF-1α). Under normoxic conditions, HIF-1α expression can be triggered by inflammation (26, 55), a prominent feature of human SCD and transgenic sickle mice (24). Activation of HIF is implicated in cellular adaptive responses to hypoxia and inflammation. HIF can trigger transcription of genes for vasoactive molecules such as vascular endothelial growth factor (VEGF), heme oxygenase-1 (HO-1), and endothelin (29, 52, 58), which are implicated in the pathophysiology of SCD (14, 21, 22, 42, 54).
HIF-1 is a heterodimeric transcription factor consisting of two subunits, i.e, HIF-1α and HIF-1β. HIF-1α is induced during hypoxic (or inflammatory) conditions, while HIF-1β [also known as aryl hydrocarbon nuclear translocator (ARNT)] is constitutively expressed (26, 29, 51). Under normoxic conditions, the oxygen sensor proteins (prolyl hydroxylases or PHDs) and the factor inhibiting HIF-1 (FIH) are active in the degradation (hydroxylation) of HIF-1α. On the other hand, hypoxia inhibits activity of PHDs, resulting in activation of HIF.
Although tissue ischemia is the primary instigator of HIF activation, a number of inflammatory factors/pathways and oxidative stress can potentially induce expression of HIF-1α. Elevated inflammatory cytokines [e.g., interleukin-1β (IL-1β) and tumor necrosis factor (TNF-α)], prostaglandin E2 (PGE2), placental growth factor (PIGF), nuclear factor-κB (NF-κB) activation, and oxidants are implicated not only in inflammatory pathophysiology of human SCD and transgenic sickle mice, but also in HIF-1α activation (11, 31, 33, 38, 44, 47, 55, 60). Among the sources of oxidative stress in SCD and transgenic sickle mice are reperfusion injury, plasma membrane NADPH oxidase (59), increased plasma xanthine oxidase (2), endothelial NO synthase (eNOS) decoupling (28), hemoglobin autoxidation (23), and decreased bioavailability of NO (48).
NO is implicated as a signaling molecule affecting activity of PHDs and HIF-1α expression (8). However, the effect of NO depletion and its replenishment has not been examined under inflammatory conditions in the sickle context. In human SCD and sickle mouse models, intense oxidative stress, hemolysis, and depleted arginine (substrate) levels are implicated in reduced NO bioavailability and inflammatory effects (20, 35, 36, 48). Oxidative stress is significantly alleviated by arginine (13), and NO donors can ameliorate reperfusion injury (27). In contrast, NO synthase inhibitors result in marked inflammation (13, 37).
We hypothesize that, in SCD, chronic inflammation and NO depletion will result in HIF-1α activation. To this end, we have examined the expression of HIF-1α in transgenic-knockout sickle (BERK) mice expressing exclusively human α- and βS- globins. BERK mice exhibit increased oxidative stress, inflammation, hemolysis, and reduced NO bioavailability (12, 35). Notably, BERK mice show induction of non-NO vasodilator enzymes [HO-1 and cyclooxygenase-2 (COX-2)], and elevated PGE2 levels as noted for human SCD (6, 35, 36). COX-2 is induced under inflammatory conditions, and is linked to activation of HIF-1α (55). Induction of non-NO vasodilators in BERK mice is associated with vasodilation and increased blood flow for optimal oxygen delivery in the face of anemia (35). Similarly, human SCD is characterized by hyperperfusion and reduced peripheral resistance (7, 39).
We have previously shown that, in BERK mice, oxidative stress and inflammation are significantly ameliorated with the introduction of antisickling fetal hemoglobin (HbF), and with arginine treatment, which markedly reduce hemolysis and increases NO bioavailability (35, 36). Importantly, increased NO bioavailability leads to marked reduction in HO-1, COX-2 expression, and plasma PGE-2 levels in these mice (35, 36). These observations have prompted us to explore the anti-inflammatory effects of HbF on the expression of HIF-1α and its microvascular and hemodynamic consequences using BERK mouse model.
In the present studies, we have used BERK mice to resolve the following issues: 1) whether the reported oxidative stress and inflammation in BERK model result in the activation of HIF-1α under normoxic conditions; 2) given that anti-sickling HbF results in decreased oxidative stress and inflammation, does it affect HIF-1a expression?; 3) what are the microvascular and hemodynamic consequences of HIF-1α expression and its inhibition?; and 4) does the level of NO bioavailability correlate with HIF-1α expression? We have used two approaches: 1) in vivo studies in transgenic-knockout sickle (BERK) mice expressing varying levels of HbF (i.e., <1%, 20%, and 40%); and 2) arginine treatment of normoxic BERK mice to ascertain the role of NO.
The present study reveals HIF-1α expression in normoxic BERK mice, which are characterized by chronic inflammation. Importantly, we show that HIF-1α expression decreases commensurate with an increase in NO bioavailability as a consequence of increase in HbF levels. Arteriolar dilation, enhanced volumetric blood flow and low blood pressure, observed in normoxic BERK mice, all show a trend toward normalization with the introduction of HbF. Also, arginine supplementation of normoxic BERK mice, which are arginine deficient, reduces HIF-1α expression, supporting a role of NO in modulation of HIF-1α expression under inflammatory conditions.
MATERIAL AND METHODS
Sickle Mice
Transgenic-knockout sickle (BERK) mice, expressing exclusively human α- and βS-globins and generated by Paszty et al. (46), were bred at Albert Einstein College of Medicine as described (46, 49). C57BL/6J mice were used as controls. BERK mice were backcrossed for eight or more generations with C57BL/6J mice. BERK mice exhibit several features of human SCD, i.e., hemolysis, reticulocytosis, low hematocrit, and extensive multiple organ damage (46, 49).
Two different γ-constructs generated by Gilman et al. (18) (γM and γH) as previously described were used for generation of HbF expressing BERKγM and BERKγH mice. The γM (G203) construct was identical to the γH construct except that it included a PCR-generated 4.3-kb fragment (HumHBB 912 to 5200) containing HS 3 and 4 of the LCR instead of HS4. Injection of these constructs into fertilized mouse oocytes resulted in production of several founder mice. To breed mice on a day-to-day basis the following schemes are used: 1) the BERKγH mice (γH construct) breed successfully with each other; 2) for BERKγM (γM or G203 construct) mice, fully knocked out healthy females expressing neutral hemoglobins such as HbA (miniLCRA) (Hba0//Hba0 Hbb0//Hbb0) or HbC and a BERKγM male in which the γM is hemizygous were used. The globin composition in adult transgenic-knockout mice used for experimentation was determined by HPLC as described (16). The percent HbF was calculated as the percent of all β-like chains. Mice were maintained in a pathogen-free facility at Albert Einstein College of Medicine. These knockout mice were extensively backcrossed (an average of 8 generations) onto C57BL/6J background.
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Albert Einstein College of Medicine. To determine the effect of HbF, we used normoxic BERK mice (4–6 mo old) expressing >1.0%, ∼20%, and ∼40% HbF (termed BERK, BERKγM, and BERKγH mice, respectively). Hematological characteristics of these mice have been described (16, 35). Control C57BL mice were maintained on a standard diet and water ad libitum. Sickle mice were maintained on “sickle chow” developed by Paszty et al. (46) without added arginine (Purina Mills, St. Louis, MO). In another series of experiments, BERK mice were supplemented with 5% arginine in mouse chow (Harlan Teklad, Madison, WI) for 15 days and comparisons were made with parameters measured in untreated BERK mice.
Western Blots
HIF-1α expression was detected by Western blotting in rapidly excised whole cremaster muscle preparation and its cytoplasmic and nuclear extracts. Nuclear and cytoplasmic extracts were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL) according to manufacturer's instructions. Lysates were prepared using a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN), and protein concentration determined at 320 nm. Western blots were performed using 7.5% SDS-PAGE (HIF-1α) and 10% SDS-PAGE (HO-1 and VEGF). The membranes were incubated in blocking buffer with antibodies to HIF-1α (Chemicon International, Temecula, CA; 1:500), HO-1 (Stressgene, Victoria, BC, Canada; 1:3,000), and VEGF (R&D Systems; 1:1,000) . Incubation with a primary antibody was followed by horseradish peroxidase-conjugated secondary antibody, as described (36). To ascertain equal loading of samples, the stripped membranes were reincubated for 1 h with goat anti-actin antibody followed by incubation with anti-goat IgG-horseradish peroxidase secondary antibody. The specific proteins were detected by enhanced chemiluminescence (ECL, Dupont, Wilmington, DE). The protein bands on the developed film were scanned and quantified by ImageQuant TL 7.0 software (GE Healthcare Biosciences, Piscataway, NJ).
Endothelin-1 (ET-1) Immunoassay
Serum endothelin-1 (ET-1) levels were monitored in C57BL, BERK, BERKγM, BERKγH, and arginine-treated BERK mice. The samples were measured in duplicate using the mouse-reactive Quantikine ET-1 Immunoassay kit (R&D Systems, Minneapolis, MN) according to manufacturer's instructions as described (50). This assay employs the quantitative sandwich enzyme immunoassay technique. Using the Quantikine immunoassay kit, the lower limit for detection for ET-1 ranges from 0.031 to 0.207 pg/ml (mean = 0.087 pg/ml).
Microcirculatory Studies
Mice were anesthetized intraperitoneally with 10% urethane and 2% α-chloralose in saline (5 ml/kg), and tracheostomized and the left carotid artery was cannulated for monitoring arterial pressure. In vivo microcirculatory observations were made in the transilluminated open cremaster muscle preparation, prepared according to the method of Baez (4) as modified for the suffusion and maintenance for the mouse preparation (34). Briefly, the preparation was suffused with a bicarbonate Ringer's solution (mmol/l: 135.0 NaCl, 5.0 KCl, 27.0 NaHCO3, and 0.64 MgCl2, and 11.6 glucose), pH of the solution adjusted to 7.35–7.4 by continuous bubbling with 94.6% N2 and 5.4% CO2 and osmolarity adjusted to 330 mOsm, which is comparable to that of mouse plasma (10). The temperature of the solution (flow rate, 5–6 ml/min) was maintained at 34.5–35°C, and monitored by a telethermometer (YSI, Yellow Springs, OH). Microscopic observations were carried out using a Nikon microscope (model E400; Morrell Instrument, Melville, NY) equipped with a Dage-MTI CCD television camera (model CCD-300T-RC, Dage-MTI, Michigan City, IN) and a Sony U-matic video recorder (model VO9600; Sony, Teaneck, NJ).
Microvascular parameters were measured on-line after 45-min stabilization of the open cremaster muscle under constant suffusion with Ringer's solution. Vessel luminal diameter (D) was measured on-line in A2 and A3 arterioles using an image-shearing device (model 907, Instruments for Physiology and Medicine, San Diego, CA). Wall shear rates and volumetric blood flow (Q) were calculated from vessel diameters and mean flow velocity (centerline red blood cell velocity/1.6) as described (35).
Arterial Hemoglobin Oxygen Saturation (SpO2)
Arterial hemoglobin oxygen saturation (SpO2) was monitored using Ohmeda oximeter and oxytip probe (model 3770; Ohmeda, Madison, WI). Oximeter probe was placed on the thigh. Before the probe was placed, the thighs were shaved and thoroughly cleaned with isopropyl alcohol and gauze pad. Measurements were made in both right and left thighs of mice and the values from both the thighs were averaged.
Measurement of Intravascular Sickling, Hemolysis, and Plasma NO Metabolites (NOx)
To determine intravascular sickling, blood samples were drawn from the tail vein into airtight syringes containing 2.5% glutaraldehyde solution in 0.1 mol/l cacodylate buffer, pH 7.4 as described (12). For plasma free hemoglobin, blood samples were drawn from the abdominal aorta, and determinations made using a tetramethyl-benzidine-based assay kit (Catachem, Bridgeport, CT) that measures plasma free hemoglobin and other heme-containing proteins present in plasma. Hematocrit (Hct) was measured using microhematocrit centrifuge (MicroHematocrit, Damon/IEF Division, Needham Heights, MA). Total NOx concentration in plasma was determined by nitrate/nitrite colorimetric assay kit (Cayman Chemical, Ann Arbor, MI) using manufacturer's instructions.
Statistical Analysis
Comparisons were made using Student's t-test. Where tests for normality failed, or Bartlett's test for homogeneity of variance showed significant difference in the standard deviations, nonparametric tests such as Kruskal-Wallis test for ANOVA or the Wilcoxon two-sample test were used. P < 0.05 was considered significant. Regression analysis was done using linear model Y = a + bX. Statistical analysis was performed using Statgraphics plus 5.0 program for Windows (Manugistics, Rockville, MD). Plasma NOx calculation was performed using the analysis spread sheet provided by Cayman Chemical at http://www.caymanchem.com/app/template/Home.vm.
RESULTS
Arterial Hemoglobin Oxygen Saturation (%SpO2)
Arterial hemoglobin oxygen saturation (%SpO2) showed no significant differences among normoxic C57BL mice (n = 4) and BERK mouse lines expressing varying levels of HbF (n=4–6; %SpO2 range: 96.3–97.8) (Table 1). These values are comparable with %SpO2 values reported for normoxic mouse (30). Previous studies have shown that %SpO2 is a valid predictor of %hemoglobin oxygen saturation (%HbO2) as it shows high correlation with %HbO2 (3, 40, 45).
Table 1.
Hematological parameters, arterial hemoglobin oxygen saturation (SpO2), intravascular sickling, and hemolysis (cell-free plasma hemoglobin) in knockout sickle (BERK) mice expressing varying levels of HbF
| Mouse | HbF, % | Hematocrit, % | SpO2, % | Intravascular Sickling, % | Plasma Hb, μmol Heme |
|---|---|---|---|---|---|
| C57BL/6J | 0 | 47.1 ± 1.5 (5)* | 97.8 ± 0.9 (4) | 0 | 2.6 ± 0.3(5)* |
| BERK | <1.0 | 18.7 ± 0.8 (5)* | 96.3 ± 1.1 (4) | 20.6 ± 1.0 (4)* | 8.0 ± 0.7 (5)* |
| BERKγM | 21.0 | 36.3 ± 1.1 (5)* | 96.3 ± 1.0 (4) | 14.5 ± 1.3 (4)* | 5.7 ± 0.4 (5)* |
| BERKγH | 40.0 | 40.9 ± 1.3 (5)+ | 96.5 ± 0.4 (6) | 5.4 ± 0.7 (5)* | 5.2 ± 0.15 (4)+ |
Values are means ± SE; nos. in parentheses represent the number of mice used in these measurements. BERK, transgenic knockout sickle mice; Hb, hemoglobin.
P < 0.05 among the groups indicated; +P < 0.05 vs. C57BL and BERK mice (multiple comparisons by ANOVA).
Expression of HIF-1α in Normoxic Transgenic-Knockout Sickle (BERK) Mice
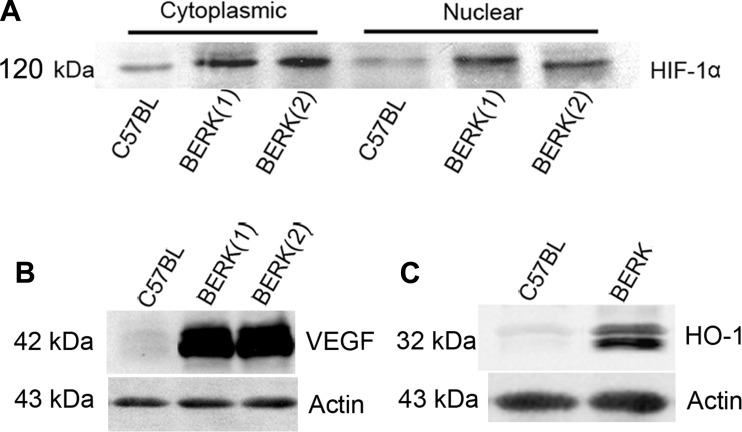
Western blotting of cytoplasmic and nuclear extracts of rapidly excised cremaster muscle from normoxic BERK mice showed distinct bands of HIF-1α in cytoplasmic and nuclear extracts (Fig. 1A). In contrast, control C57BL showed weakly positive bands. HIF-1α expression in cytoplasmic and nuclear extracts of BERK mice showed average increases of 1.6- and 2.3-fold, respectively, compared with C57BL mice (P < 0.05 and P < 0.01, n = 4 each; Fig. 1A), suggesting translocation of the HIF-1 dimer to the nucleus. These findings show that HIF-1α is expressed in BERK mice under normoxic conditions.
Fig. 1.
A: Western blots of HIF-1α in cytoplasmic and nuclear extracts of cremaster muscle from normoxic BERK mice. B and C: HIF-1α activation in normoxic BERK mice was associated with pronounced induction of VEGF (B) and HO-1 (C).
The induction of HIF-1α in normoxic BERK mice was associated with upregulation of VEGF, a target molecule for HIF-1α (Fig. 1B). Furthermore, HIF-1α activation and hemolysis in normoxic BERK mice was accompanied by marked upregulation of HO-1, a vasodilator enzyme (Fig. 1C), which is in accord with our previous studies (39). In contrast, normoxic control C57BL revealed weakly positive or no expression of VEGF and HO-1 (Fig. 1, B and C).
Introduction of Fetal Hemoglobin (HbF) in BERK Mice Decreases HIF-1α Expression
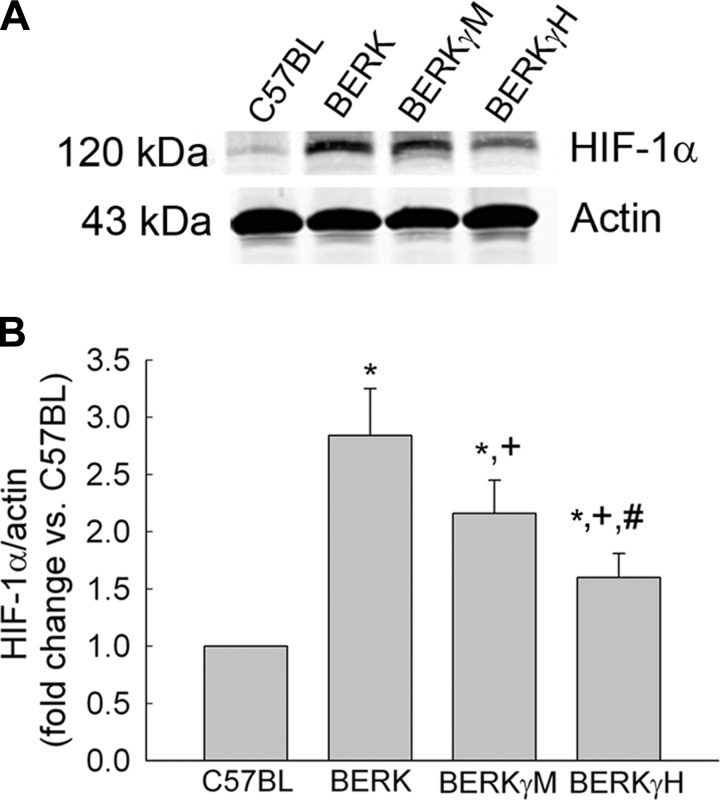
Next, we investigated if inhibition of sickling by genetic manipulation to enhance antisickling HbF levels in BERK mice will have a modulating effect on HIF-1α expression. To this end, we performed Western blotting of whole cremsater lysates to examine the effect of HbF on expression of HIF-1α in BERK mice expressing varying levels of HbF, i.e., BERK (<1% HbF), BERKγM (20% HbF), and BERKγH (40% HbF).
Western blots and densitometric analysis (Fig. 2, A and B) of the whole cremaster lysates of normoxic BERK mice showed a 2.8-fold increase in HIF-1α expression (P < 0.003 vs. C57BL, n = 4 each). As shown in Fig. 2, A and B, increasing the expression of HbF in BERK mice resulted in a progressive decrease in HIF-1α expression in BERKγM and BERKγH mice with the latter showing a 43% decrease in HIF-1α expression compared with BERK mice (P < 0.01, n = 4 each).
Fig. 2.
Effect of HbF on HIF-1α expression in normoxic BERK mice. A and B: a progressive decrease in HIF-1α expression in the whole cremaster lysates with an increase in HbF levels in BERKγM and BERKγH mice (A) was confirmed by densitometric analysis (B). *P < 0.05–0.003 vs. C57BL; +P < 0.05–0.01 vs. BERK; #P < 0.04 vs. BERKγM.
HIF-1α Reduction Is Associated with Decreased Expression of HO-1 and VEGF, and Reduced ET-1 Levels
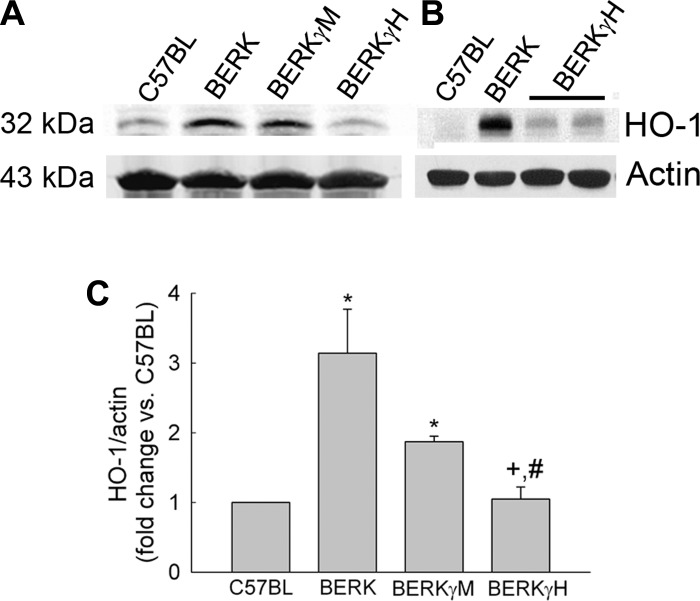
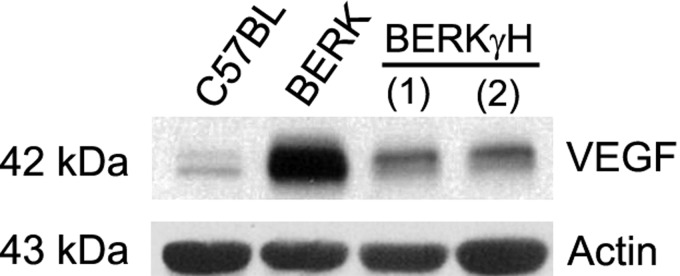
Normoxic BERK mice showed >3-fold increase in HO-1 expression compared with C57BL mice (P < 0.0001, i = 4 each; Fig. 3, A and B). With increase in HbF levels to 40% in BERKγH mice (n = 5), HO-1 expression was reduced as compared with BERK mice (Fig. 3, A and B) as evidenced by an average 66% decease (P < 0.001; Fig. 3C), suggesting an inhibitory effect of decreased HIF-1α expression, reduced intravascular sickling, and hemolysis (Table 1). Also, the marked 5.3-fold increase observed in VEGF expression in normoxic BERK mice as compared with C57BL mice (P < 0.0001, n = 3), showed a distinct ∼57% decrease that correlated with reduced HIF-1α expression in BERKγH mice (40% HbF) (P < 0.0001 vs. BERK, n = 3 each) (Fig. 4).
Fig. 3.
Effect of HbF on HO-1 expression in normoxic BERK mice. A: Western blots of HO-1 in the whole cremsater lysates from BERKγM and BERKγH showed a progressive decrease in the expression of HO-1 expression with maximal decrease in the BERKγH mice as is also evident in B, and confirmed by densitometric analysis (C). *P < 0.025–0.0003 vs. C57BL; +P < 0.015 vs. BERK; #P < 0.03 vs. BERKγM.
Fig. 4.
Effect of HbF on VEGF expression in normoxic BERK mice. Western blots of whole cremaster muscle lysates showed markedly reduced VEGF expression in BERKγH mice.
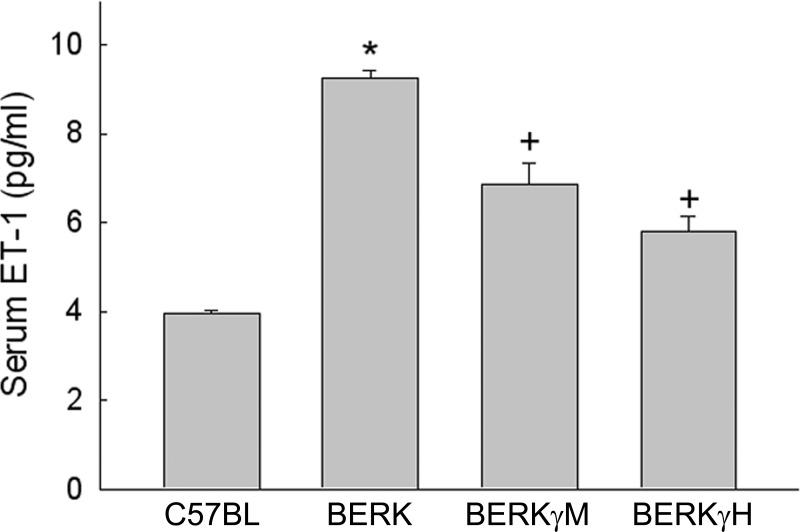
Serum levels of ET-1, another target vasoactive molecule of HIF-1α, were elevated by 2.3-fold in normoxic BERK mice compared with C57BL controls (P < 0.0001, n = 3 each) (Fig. 5). With increase in HbF levels to 20% (BERKγM) and 40% (BERKγH), the corresponding ET-1 levels showed significant ∼26% and ∼40% decreases compared with normoxic BERK mice (P < 0.01 and P < 0.0005).
Fig. 5.
Effect of HbF on serum ET-1 levels in normoxic BERK mice. ET-1 levels showed marked 2.3-fold increase in BERK mice, which was significantly reduced in BERKγM and BERKγH mice. *P < 0.0001 vs. C57BL; +P < 0.01–0.0005 vs. BERK.
Microvascular Parameters
Arteriolar diameters.
In accord with our previous studies (36), BERK mice (n = 5) showed a pronounced ∼50% dilation of cremasteric arterioles (branching orders: A2 and A3) compared with control C57BL mice (n = 6) (P < 0.001, Fig. 6A). Increasing the HbF levels resulted in a progressive decrease in arteriolar diameters in BERKγM and BERKγH mice (n = 4 each) with BERKγH (40% HbF) mice showing maximal decrease in the diameter (P < 0.0001 vs. BERK mice); the arteriolar diameters in BERKγH mice were not significantly different from those in control C57BL mice (Fig. 6A).
Fig. 6.
Effect of HbF on arteriolar diameter (branching orders: A2 and A3) and flow parameters in A2 arterioles in the cremaster muscle microcirculation. A: compared with C57BL, BERK mice show marked ∼50% and 45% increase in A2 and A3 diameters, respectively. Increasing the HbF levels resulted in a progressive decrease in arteriolar diameters in BERKγM and BERKγH mice; the resulting diameters in BERKγH (40% HbF) were not significantly different from the diameters in C57BL mice. B: commensurate with the increase in %HbF, BERK mice showed a progressive decrease in wall shear rates. C: the reduced A2 diameter in HbF expressing BERK mice was accompanied by reduced volumetric blood flow (Q). *P < 0.0001 vs. C57BL; +P < 0.022–0.0001 vs. BERK; #P < 0.02 vs. BERKγM.
Microhemodynamic parameters.
The effect of HbF on microvascular flow parameters was examined in A2 arterioles. BERK mice (n = 5) showed 40% decrease in the wall shear rate compared with C57BL mice (n = 6) (P < 0.023) (Fig. 6B). In contrast, the progressive reduction in A2 diameters in BERKγM and BERKγH mice (each n = 4) resulted in marked 43% and 69% increases in the wall shear rate, respectively, compared with BERK mice (P < 0.022 and P < 0.012) (Fig. 6B). As previously reported (36), BERK mice showed greater than 2-fold increase in volumetric blood flow (Q) in A2 arterioles compared with C57BL mice (Fig. 6C). In marked contrast, Q showed a progressive decrease in BERKγM and BERKγH mice with the latter showing ∼40% reduction compared with BERK mice (P < 0.01). Importantly, the resulting wall shear rates and Q in BERKγH mice were not significantly different from those for C57BL mice.
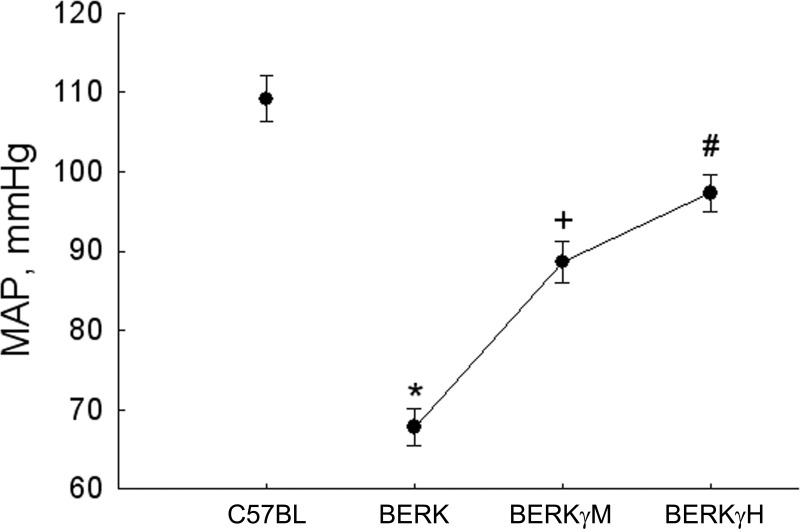
Mean arterial pressure (MAP).
As shown in Fig. 7, the arteriolar dilation in BERK mice was associated with a marked decline in MAP (67.8 ± 2.3 vs. 109.2 ± 2.9 mmHg in C57BL; P < 0.00001). In contrast, the decrease in A2 and A3 arterioles (resistance vessels) in BERKγM and BERKγH mice was accompanied by a progressing increase in MAP, with BERKγH mice showing MAP values of 97.3 ± 2.3 mmHg (P < 0.0001 vs. BERK mice).
Fig. 7.
Effect of HbF on the mean arterial pressure (MAP) in normoxic BERK mice. The arteriolar dilation in BERK mice was associated with marked decrease in MAP. In contrast, the decrease in arteriolar diameters was accompanied by a progressive decrease in MAP in BERKγM and BERKγH mice. *P < 0.0001 vs. C57BL; +P < 0.001 vs. BERK; #P < 0.05 vs. BERKγM.
Correlates of HIF-1α Expression
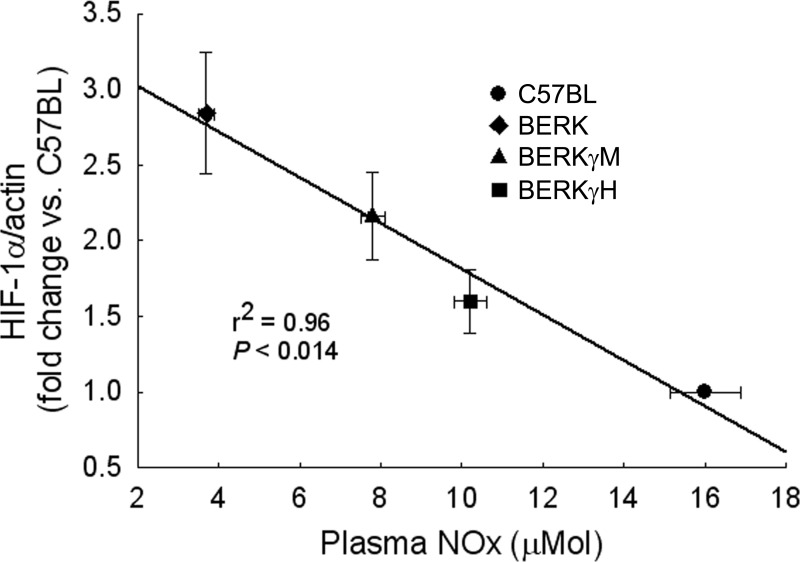
The data from the present experiments show that increasing HbF levels in BERK mice were associated with decreased intravascular sickling and hemolysis (plasma Hb levels) (Table 1), as also noted in our previous studies (12). HIF-1α expression showed a strong correlation with plasma Hb levels (r2 = 0.97, P < 0.015). Importantly, linear regression analysis revealed a strong relationship between plasma NOx levels and HIF-1α expression (Fig. 8) as HIF-1α expression level decreased commensurate with an increase in plasma NOx levels in BERKγM and BERKγH mice. Thus, 2.8-fold higher HIF-1α expression in normoxic BERK mice (<1.0% HbF) compared with C57BL mice is associated with lower plasma NOx levels (3.7 ± 0.2 vs. 16.0 ± 0.9 μmol in C57BL), while 43% decrease in HIF-1α expression in BERKγH (40% HbF) mice are accompanied by higher NOx levels (10.4 ± 0.4 μmol).
Fig. 8.
Relationship between HIF-1α expression and plasma NOx levels. HIF-1α expression showed a strong correlation with the levels of plasma NOx.
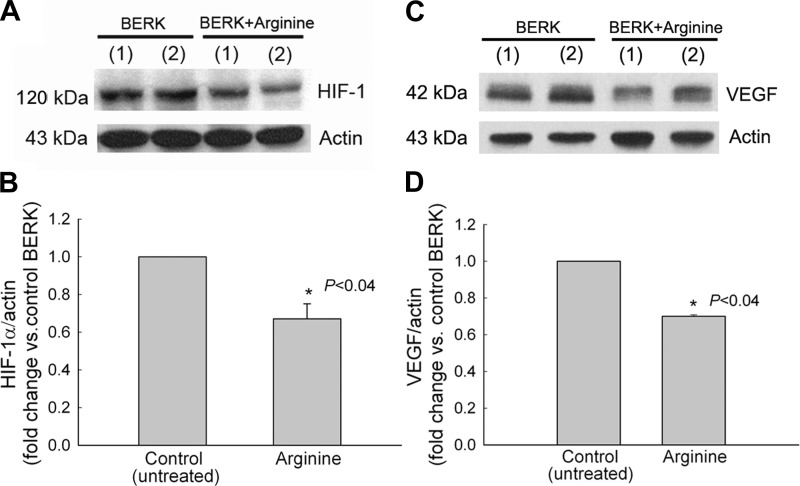
Arginine Reduces HIF-1α Expression in BERK Mice
Since increased NOx levels in BERK expressing HbF were associated with reduced HIF-1α expression and because BERK mice show decreased plasma arginine levels (36), we examined if promoting NO production in BERK mice by arginine supplementation would lead to a similar effect on HIF-1α expression. As shown in Fig. 9A, BERK mice treated with 5% arginine in mouse chow (15 days) showed a significant >30% reduction in HIF-1α expression (P < 0.04 vs. untreated BERK control, n = 4 each), indicating an attenuating effect of increased NO bioavailability on HIF-1α expression. Moreover, the reduced HIF-1α expression was associated with ∼30% decrease in VEGF expression (P < 0.4, n = 3 each, Fig. 9B). Of relevance are our previous findings showing upregulation of plasma NOx levels and downregulation of HO-1 in BERK mice with arginine treatment (36).
Fig. 9.
Arginine treatment reduces expression of HIF-1α and VEGF in normoxic BERK mice. Western blots of whole cremaster muscle lysates, and their densitometric analysis, showed 30% decreases in HIF-1α (A and B) and VEGF expression (C and D) in arginine-treated BERK mice compared with untreated BERK controls (each P < 0.04).
DISCUSSION
The present study demonstrates that HIF-1α is expressed in normoxic transgenic-knockout sickle (BERK) mice at normal arterial HbO2 saturation. This finding is novel in the context of SCD but not totally unexpected as sickle patients and BERK mice show marked inflammation that is implicated in the activation of HIF-1α under normoxic conditions (26, 55). Second, in BERK mice, HIF-1α expression decreases concomitantly with increasing HbF, commensurately with increased NO bioavailability, and shows a strong inverse correlation with plasma NO metabolites (NOx) levels. Third, we show amelioration of vasodilation and a trend toward normalization of hemodynamic parameters in BERK mice expressing HbF. Finally, the results show that arginine treatment of normoxic BERK mice (deficient in arginine substrate) reduces HIF-1α expression by increasing NOx production.
We show that HIF-1α expression in normoxic BERK mice is associated with induction of VEGF, HO-1, and elevated serum ET-1 levels. Upregulation of HO-1, a vasodilator enzyme, is consistent with previous observations in BERK mice (6, 36). These observations are also consistent with the reported upregulation of VEGF and HO-1 in sickle patients (22, 32, 42). While VEGF induction has long been associated with hypoxia and HIF-1α activation (52, 53), HO-1 is induced in response to HIF-1α activation, hemolysis, and oxidative stress (43). The elevated levels of ET-1 in BERK mice are reminiscent of higher ET-1 levels encountered in sickle patients particularly during painful vaso-occlusive episodes (21).
We propose that the observed activation of HIF-1α in normoxic BERK mice is plausibly a consequence of intense oxidative stress and inflammation in these mice. There is overwhelming evidence of intense oxidative stress and inflammation in BERK mice. The intense oxidative stress in BERK mice is evidenced by increased superoxide (O2·−) generation, extensive peroxidation of membrane lipids, peoxynitrite (NOO−) formation, and a depletion of antioxidants, including glutathione (GSH), superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx) (12). Furthermore, along with oxidative stress, elevated inflammatory cytokines, as reported in sickle patients and transgenic sickle mice and mentioned in the introduction, are implicated in the activation of HIF-1α under normoxic conditions (17, 33). Inflammatory cytokines can mediate upregulation of HIF-1α via an NF-kB/COX-2/PGE2 pathway independent of hypoxia (26, 33, 55). In fact, COX-2 expression and PGE2 levels are markedly elevated in BERK mice (36).
We have previously shown that intense oxidative stress, COX-2/PGE2 upregulation, and inflammation in BERK mice are markedly reduced by antisickling HbF and increase in NO bioavailability (12, 13, 35, 36). The present studies show that the expression of HIF-1α and its vasoactive biomarkers such as VEGF, HO-1, and ET-1 is significantly reduced in BERK mice expressing antisickling and anti-inflammatory HbF. HbF also reduces endothelial NO synthase expression in BERK mice as previously shown using Western blotting and immunohistochemistry (35, 36). Also, in agreement with our previous studies (12), we find that increasing HbF levels in BERK mice are associated with decreased intravascular sickling and hemolysis (plasma Hb levels), while increased NOx levels (see Table 1) are likely the result of reduced hemolysis and oxidative stress (2, 48). Moreover, we find a strong correlation between HIF-1α expression and plasma NOx levels in normoxic BERK mice expressing HbF, i.e., the higher the NOx levels the lower the HIF-1α expression (see Fig. 8). We have previously shown that a decrease in hemolysis and oxidative stress in BERK mice expressing HbF or by arginine supplementation is associated with increased NO bioavailability and markedly reduced inflammation (12, 36), suggesting a relationship between NO bioavailability and inflammation in SCD. The anti-inflammatory effect of HbF in BERK mice is comparable to that observed in sickle patients, treated with hydroxyurea, a standard current therapy to raise the level of antisickling HbF (9, 56). Also, in BERK mice, introduction of HbF significantly reduces global oxidative stress accompanied by marked threefold decrease in peripheral leukocyte counts (i.e., from 35,064 ± 3,255 counts/μl in BERK mice to 11,542 ± 1,085 counts/μl in BERKγH mice) (12), demonstrating a pronounced anti-inflammatory effect of HbF. Taken together, these findings indicate that increased NO bioavailability not only reduces inflammation in sickle mice, but is also associated with reduced HIF-1α expression.
We have previously shown that, in BERK mice, NO depletion by oxygen radicals, cell-free plasma hemoglobin, and arginine (substrate) deficiency is compensated by upregulation of non-NO vasodilators such as COX-2 that produces PGE2 and HO-1 that produces carbon monoxide (CO) (36). In this study, we show that HIF-1α activation in BERK mice is associated with HO-1 induction. As mentioned in the introduction, HO-1 is a target molecule of HIF-1α and is also induced by hemolysis and oxidative stress (43). HO-1 catalyzes degradation of heme to biliverdin/bilirubin and CO, and exerts a vasodilatory effect via generation of CO (43). One potential mechanism for the induction of HO-1 in response to oxidative stress may involve activation of the redox-sensitive transcription factor, NF-E2-regulated factor 2 (Nrf2) that binds antioxidant response element (ARE) of HO-1 gene (and other antioxidant genes) and initiates transcription of HO-1 (1, 5, 57). Nrf2/ARE pathway and its relationship with HIF-1α need to be explored in the context of oxidative stress and its amelioration in sickle cell disease.
Together with COX-2, HO-1 expression will contribute to the pronounced vasodilation, increased volumetric blood flow (Q), and low mean arterial pressure (MAP) in normoxic BERK mice. These hemodynamic adaptations (i.e., vasodilation and increased Q) are likely a compensatory response to provide adequate oxygen delivery in BERK mice, which is consistent with the observed normal arterial hemoglobin oxygen saturation (%SpO2) levels in these mice even at a low hematocrit level (see Table 1). Furthermore, consistent with the reduction in HIF-1α and HO-1 expression in BERK mice with an increase in %HbF, we find a progressive decrease in arteriolar diameters and volumetric flow, and an increase in MAP (see Figs. 6 and 7). The present studies also show that, in BERK mice expressing HbF, the observed decreases in arteriolar diameters and flow are associated with increased hematocrit levels, which would maintain normal arterial %SpO2 levels during steady state (Table 1). However, we cannot rule out the possibility that markedly reduced intravascular sickling in BERKγH mice would also reduce HIF-1α activation by minimizing sickling-induced localized hypoxic episodes.
It is noteworthy that, in BERK mice, NO depletion coupled with pronounced vasodilation is also implicated in impaired vasoreactivity to NO-mediated vasodilators such as acetylcholine and sodium nitroprusside (35, 36). In contrast, BERK mice expressing HbF or treated with arginine show increased NO bioavailability and reduced non-NO vasodilators (i.e., COX-2/PGE2 and HO-1), resulting in enhanced vasoreactivity to NO-mediated vasodilators (35, 36). These studies reinforce the notion that NO bioavailability influences vascular tone and reactivity in these mice.
The role of NO is further supported by arginine experiments in BERK mice. Arginine not only reduces HIF-1α and VEGF expression as shown in this study, but also reduces COX-2 and HO-1 expression and improves hemodynamic parameters as shown previously (36). Also, we have previously shown that arginine supplementation of BERK mice, which are arginine deficient (36), results in >2-fold increase in plasma NOx levels accompanied by a distinct reduction in oxidative stress and inflammation (13, 36). Future studies are warranted to examine tetrabiohydrobiopterin (BH4) deficiency in BERK mice and its contribution to NO bioavailability.
Although many previous studies have examined the role of NO in the regulation of HIF-1α expression using different lines of cells under culture conditions, no previous study has addressed the role of NO in HIF-1α regulation in vivo under inflammatory conditions that characterize SCD. The present in vivo studies show that reduced inflammation and increased NO production in normoxic BERK mice expressing HbF are distinctly associated with suppression of HIF-1α activation and inhibition of vasodilators, resulting in improved microvascular and hemodynamic parameters in the BERK model of sickle cell disease. Future studies will be required to further explore the role of HIF-1 and its target molecules in the pathophysiology of SCD. The unique feature of inflammation in SCD is that it can be ameliorated by increased HbF, thereby coupling HbS polymerization/sickling to NO depletion, HIF-1α expression, and inflammation in this disease.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-074007 (to D. K. Kaul) and HL-092183 (to M. E. Fabry) and American Heart Association Grant-in-Aid 11GRNT5750019 (to D. K. Kaul).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.K.K. conception and design of research; S.S. and X.Z. performed experiments; D.K.K., M.E.F., and X.Z. analyzed data; D.K.K. interpreted results of experiments; D.K.K. and X.Z. prepared figures; D.K.K. drafted manuscript; D.K.K. and M.E.F. edited and revised manuscript; D.K.K. approved final version of manuscript.
REFERENCES
- 1. Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 274: 26071–26078, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA 98: 15215–15220, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aughey K, Hess D, Eitel D, Bleecher K, Cooley M, Ogden C, Sabulsky N. An evaluation of pulse oximetry in prehospital care. Ann Emerg Med 20: 887–891, 1991 [DOI] [PubMed] [Google Scholar]
- 4. Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc Res 5: 384–394, 1973 [DOI] [PubMed] [Google Scholar]
- 5. Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J 371: 887–895, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest 116: 808–816, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belhassen L, Pelle G, Sediame S, Bachir D, Carville C, Bucherer C, Lacombe C, Galacteros F, Adnot S. Endothelial dysfunction in patients with sickle cell disease is related to selective impairment of shear stress-mediated vasodilation. Blood 97: 1584–1589, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Brune B, Zhou J. Hypoxia-inducible factor-1alpha under the control of nitric oxide. Methods Enzymol 435: 463–478, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Charache S, Barton FB, Moore RD, Terrin ML, Steinberg MH, Dover GJ, Ballas SK, McMahon RP, Castro O, Orringer EP. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine 75: 300–326, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Chen D, Kaul DK. Rheologic and hemodynamic characteristics of red cells of mouse, rat and human. Biorheology 31: 103–113, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Critchley HOD, Osei J, Henderson TA, Boswell L, Sales KJ, Jabbour HN, Hirani N. Hypoxia-inducible factor-1alpha expression in human endometrium and its regulation by prostaglandin E-series prostanoid receptor 2 (EP2). Endocrinology 147: 744–753, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Dasgupta T, Fabry ME, Kaul DK. Antisickling property of fetal hemoglobin enhances nitric oxide bioavailability and ameliorates organ oxidative stress in transgenic-knockout sickle mice. Am J Physiol Regul Integr Comp Physiol 298: R394–R402, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dasgupta T, Hebbel RP, Kaul DK. Protective effect of arginine on oxidative stress in transgenic sickle mouse models. Free Radic Biol Med 41: 1771–1780, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ergul S, Brunson CY, Hutchinson J, Tawfik A, Kutlar A, Webb RC, Ergul A. Vasoactive factors in sickle cell disease: in vitro evidence for endothelin-1-mediated vasoconstriction. Am J Hematol 76: 245–251, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Fabry ME, Nagel RL. The effect of deoxygenation on red cell density: significance for the pathophysiology of sickle cell anemia. Blood 60: 1370–1377, 1982 [PubMed] [Google Scholar]
- 16. Fabry ME, Suzuka SM, Weinberg RS, Lawrence C, Factor SM, Gilman JG, Costantini F, Nagel RL. Second generation knockout sickle mice: the effect of HbF. Blood 97: 410–418, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Frede S, Berchner-Pfannschmidt U, Fandrey J. Regulation of hypoxia-inducible factors during inflammation. Methods Enzymol 435: 405–419, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Gilman J. Developmental changes of human Gγ and Aγ and mouse embryonic ϵγ1ϵγ2 and βη in transgenic mice with HS4-Gγ-Aγ. Blood 86: 648a, 1995 [Google Scholar]
- 19. Gladwin MT, Kato GJ. Cardiopulmonary complications of sickle cell disease: role of nitric oxide and hemolytic anemia. Hematology Am Soc Hematol Educ Program 51–57, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gladwin MT, Schechter AN, Ognibene FP, Coles WA, Reiter CD, Schenke WH, Csako G, Waclawiw MA, Panza JA, Cannon RO., 3rd Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation 107: 271–278, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Graido-Gonzalez E, Doherty JC, Bergreen EW, Organ G, Telfer M, McMillen MA. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood 92: 2551–2555, 1998 [PubMed] [Google Scholar]
- 22. Gurkan E, Tanriverdi K, Baslamisli F. Clinical relevance of vascular endothelial growth factor levels in sickle cell disease. Ann Hematol 84: 71–75, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Hebbel RP, Eaton JW, Balasingam M, Steinberg MH. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest 70: 1253–1259, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation 11: 129–151, 2004 [PubMed] [Google Scholar]
- 25. Hebbel RP, Vercellotti GM. The endothelial biology of sickle cell disease. J Lab Clin Med 129: 288–293, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Hirota SA, Beck PL, MacDonald JA. Targeting hypoxia-inducible factor-1 (HIF-1) signaling in therapeutics: implications for the treatment of inflammatory bowel disease. Recent Pat Inflamm Allergy Drug Discov 3: 1–16, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Hoffman A, Goldstein S, Samuni A, Borman JB, Schwalb H. Effect of nitric oxide and nitroxide SOD-mimic on the recovery of isolated rat heart following ischemia and reperfusion. Biochem Pharmacol 66: 1279–1286, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood 109: 3088–3098, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang LE, Bunn HF. Hypoxia-inducible factor and its biomedical relevance. J Biol Chem 278: 19575–19578, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Ikegami M, Weaver TE, Grant SN, Whitsett JA. Pulmonary surfactant surface tension influences alveolar capillary shape and oxygenation. Am J Respir Cell Mol Biol 41: 433–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang H, Zhu YS, Xu H, Sun Y, Li QF. Inflammatory stimulation and hypoxia cooperatively activate HIF-1alpha in bronchial epithelial cells: involvement of PI3K and NF-kappaB. Am J Physiol Lung Cell Mol Physiol 298: L660–L669, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Jison ML, Munson PJ, Barb JJ, Suffredini AF, Talwar S, Logun C, Raghavachari N, Beigel JH, Shelhamer JH, Danner RL, Gladwin MT. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood 104: 270–280, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J 17: 2115–2117, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Kaul DK, Fabry ME, Costantini F, Rubin EM, Nagel RL. In vivo demonstration of red cell-endothelial interaction, sickling and altered microvascular response to oxygen in the sickle transgenic mouse. J Clin Invest 96: 2845–2853, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaul DK, Liu XD, Chang HY, Nagel RL, Fabry ME. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. J Clin Invest 114: 1136–1145, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaul DK, Zhang X, Dasgupta T, Fabry ME. Arginine therapy of transgenic-knockout sickle mice improves microvascular function by reducing non-nitric oxide vasodilators, hemolysis, and oxidative stress. Am J Physiol Heart Circ Physiol 295: H39–H47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA 88: 4651–4655, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu XH, Kirschenbaum A, Lu M, Yao S, Dosoretz A, Holland JF, Levine AC. Prostaglandin E2 induces hypoxia-inducible factor-1alpha stabilization and nuclear localization in a human prostate cancer cell line. J Biol Chem 277: 50081–50086, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Lonsdorfer J, Bogui P, Otayeck A, Bursaux E, Poyart C, Cabannes R. Cardiorespiratory adjustments in chronic sickle cell anemia. Bull Eur Physiopath Respir 19: 339–344, 1983 [PubMed] [Google Scholar]
- 40. Martin D, Powers S, Cicale M, Collop N, Huang D, Criswell D. Validity of pulse oximetry during exercise in elite endurance athletes. J Appl Physiol 72: 455–458, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Nagel RL. The challenge of painful crisis in sickle cell disease. JAMA 286: 2152–2153, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, Hebbel RP. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol 158: 893–903, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nath KA, Katusic ZS, Gladwin MT. The perfusion paradox and vascular instability in sickle cell disease. Microcirculation 11: 179–193, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Oh YT, Lee JY, Yoon H, Lee EH, Baik HH, Kim SS, Ha J, Yoon KS, Choe W, Kang I. Lipopolysaccharide induces hypoxia-inducible factor-1 alpha mRNA expression and activation via NADPH oxidase and Sp1-dependent pathway in BV2 murine microglial cells. Neurosci Lett 431: 155–160, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Ortiz FO, Aldrich TK, Nagel RL, Benjamin LJ. Accuracy of pulse oximetry in sickle cell disease. Am J Respir Crit Care Med 159: 447–451, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease [see comments]. Science 278: 876–878, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Patel N, Gonsalves CS, Malik P, Kalra VK. Placenta growth factor augments endothelin-1 and endothelin-B receptor expression via hypoxia-inducible factor-1 alpha. Blood 112: 856–865, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 8: 1383–1389, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Ryan TM, Ciavatta DJ, Townes TM. Knockout-transgenic mouse model of sickle cell disease. Science 278: 873–876, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Said N, Smith S, Sanchez-Carbayo M, Theodorescu D. Tumor endothelin-1 enhances metastatic colonization of the lung in mouse xenograft models of bladder cancer. J Clin Invest 121: 132–147, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 13: 167–171, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, Yu A. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol 475: 123–130, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359: 843–845, 1992 [DOI] [PubMed] [Google Scholar]
- 54. Solovey A, Gui L, Ramakrishnan S, Steinberg MH, Hebbel RP. Sickle cell anemia as a possible state of enhanced anti-apoptotic tone: survival effect of vascular endothelial growth factor on circulating and unanchored endothelial cells. Blood 93: 3824–3830, 1999 [PubMed] [Google Scholar]
- 55. Stasinopoulos I, O'Brien DR, Bhujwalla ZM. Inflammation, but not hypoxia, mediated HIF-1alpha activation depends on COX-2. Cancer Biol Ther 8: 31–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Steinberg MH, Lu ZH, Barton FB, Terrin ML, Charache S, Dover GJ. Fetal hemoglobin in sickle cell anemia: determinants of response to hydroxyurea. Multicenter Study of Hydroxyurea. Blood 89: 1078–1088, 1997 [PubMed] [Google Scholar]
- 57. Ueda K, Ueyama T, Yoshida K, Kimura H, Ito T, Shimizu Y, Oka M, Tsuruo Y, Ichinose M. Adaptive HNE-Nrf2-HO-1 pathway against oxidative stress is associated with acute gastric mucosal lesions. Am J Physiol Gastrointest Liver Physiol 295: G460–G469, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem 268: 21513–21518, 1993 [PubMed] [Google Scholar]
- 59. Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB J 19: 989–991, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 217: 674–685, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]