Abstract
Previous studies have determined that premenopausal women exhibit an attenuated metaboreflex; however, little is known about sex specificity of the mechanoreflex. Thus, we sought to determine if sex differences exist in the central and peripheral hemodynamic responses to passive limb movement. Second-by-second measurements of heart rate, stroke volume, cardiac output (CO), mean arterial pressure, and femoral artery blood flow (FBF) were recorded during 3 min of supine passive knee extension in 24 young healthy subjects (12 women and 12 men). Normalization of CO and stroke volume to body surface area, expressed as cardiac index and stroke index, eliminated differences in baseline central hemodynamics, whereas, peripherally, basal FBF and femoral vascular conductance were similar between the sexes. In response to passive limb movement, women displayed significantly attenuated peak central hemodynamic responses compared with men (heart rate: 9.0 ± 1 vs. 14.8 ± 2% change, stroke index: 4.5 ± 0.6 vs. 7.8 ± 1.2% change, cardiac index: 9.6 ± 1 vs. 17.2 ± 2% change, all P < 0.05), whereas movement induced similar increases in peak FBF (167 ± 32 vs. 193 ± 17% change) and femoral vascular conductance (172 ± 31 vs. 203 ± 16% change) in both sexes (women vs. men, respectively). Additionally, there was a significant positive relationship between individual peak FBF and peak CO response to passive movement in men but not in women. Thus, although both sexes exhibited similar movement-induced hyperemia and peripheral vasodilatory function, the central hemodynamic response was blunted in women, implying an attenuated mechanoreflex. Therefore, this study reveals that, as already recognized with the metaboreflex, there is likely a sex-specific attenuation of the mechanoreflex in women.
Keywords: blood flow, mechanoreceptors, afferents, gender
previously, it has been documented that there are sex differences in the metaboreflex (13, 32, 42), contributing to a reduced sympathetic reactivity in women (25); however, there is currently a paucity of data regarding sex differences in the mechanoreflex. Historically, passive stretch (4, 9–11, 14, 17, 18) or movement (36) has been used as a model to activate the mechanoreceptors (40, 41) and the group III afferent-mediated mechanoreflex (23). In support of this, our group has demonstrated, in a study including only men, that the central hemodynamic response to passive limb movement is significantly blunted with the partial pharmacological blockade of group III and IV afferent nerve fibers (57), revealing that a significant portion of this response is mediated by afferent signals. In terms of the sex specificity of this response, only one study (45), to date, has had the potential to measure passive limb movement-induced central hemodynamic responses in men and women, but this study did not actually include such an assessment. Thus, the question remains as to whether sex differences exist in mechanoreflex-evoked increases in the central hemodynamic factors [heart rate (HR), stroke volume (SV), and cardiac output (CO)] that appear to contribute to movement-induced hyperemia (34).
Using traditional active exercise paradigms, such as knee extensor exercise, there is evidence of equal (49) or greater (45) peripheral hemodynamic responses in women compared with men, depending on the experimental approach. Using the passive knee extension model, in the absence of local metabolic perturbation, we (34, 57, 60) and others (19, 24, 46) revealed, again in studies including only men, that passive movement significantly elevates femoral artery blood flow (FBF). Parker et al. (45), in the only passive movement study to include women to date, also measured peripheral hemodynamic responses. However, the researchers focused on steady-state FBF in the third minute of movement, in which, based on a prior work by our group (34), the peak hemodynamic responses are typically transient, with the onset and offset of hyperemia occurring within the first minute. Therefore, using such a low time resolution approach, it was not possible for Parker and colleagues (45) to accurately determine if sex differences exist in the peripheral hemodynamic responses to passive movement. Thus, currently, there is not a valid comparison of the sex-specific peripheral vascular responses to limb movement in the absence of metabolic changes.
Although it is clear that movement typically induces both central and peripheral hemodynamic responses (34), not all agree that these phenomena are mechanistically linked (19). However, as already indicated, work by Trinity et al. (57) revealed that neural blockade, which reduced afferent feedback, blunted the central hemodynamic response and, subsequently, the peripheral hemodynamic response to limb movement, indicating that a central response is an integral component of limb hyperemia. Interestingly, previous studies investigating the link between central and peripheral hemodynamics in men and women at rest (22) and during active exercise (49) have suggested an uncoupling of CO and peripheral hemodynamics in women. However, it is currently unknown if central hemodynamic responses induced by the mechanoreflex are similarly linked to peripheral hemodynamics in both sexes.
Accordingly, the primary goal of the present study was to determine if there are sex-specific central and peripheral hemodynamic responses to passive limb movement, a component of which may be mediated by differences in the mechanoreflex. Specifically, we hypothesized that, at least in part, due to an attenuated mechanoreflex, women would 1) exhibit a reduced central hemodynamic (HR, SV, and CO) response to passive limb movement and 2) as a consequence of this attenuated CO response, passive limb movement-induced changes in FBF would be attenuated compared with their male counterparts.
METHODS
Subjects and General Procedures
Twenty-four recreationally active healthy young men (n = 12) and women (n = 12) participated in this study. The protocol was approved by the Institutional Review Boards of the University of Utah and Salt Lake City Veterans Affairs Medical Center. Written informed consent was obtained from all subjects before their participation in the study. All experiments were performed in a thermoneutral environment (22°C). Subjects reported to the laboratory in a fasted state and without caffeine or alcohol use for 12 and 24 h, respectively. They also had not performed any exercise within the past 24 h. Female subjects were eumenorrheic, not using chemical contraceptives, and were studied during days 1–7 of their menstrual cycle to reduce the impact of the cyclical nature of endogenous female hormones. Menstrual history was determined by questionnaire, and the current menstrual phase was identified by tracking the menstrual cycle for 1 mo before appropriately scheduling the study the following month, referred to as the forward counting method (21, 26). The early follicular phase was chosen, as, previously, plasma estradiol, the primary biologically active estrogen, has been documented to not differ significantly between men and women during this phase (15, 21, 56).
Passive Exercise Protocol
Before the experimental protocols, a blood sample was collected by standard venipuncture technique from the antecubital vein for the analysis of fasting glucose, blood lipids (total cholesterol, HDL, LDL, and triglycerides), blood chemistry [creatinine, urea nitrogen, and ions (K+, Na+, Cl−, etc.)], and a complete blood count (hemoglobin, white blood cell, neutrophils, lymphocytes, and monocytes). After blood sampling, subjects rested in the supine position for 20 min before the start of data collection. While subjects remained supine, the initial protocol consisted of a 60-s resting baseline followed by a 3-min bout of passive knee extension. During baseline measurements, the passive leg was supported at 180° by a member of the research team. One minute before the start of baseline measurements, a pneumatic cuff, placed at the level of the tibial plateau on the passively moved leg, was inflated to 250 mmHg. The cuff, which remained inflated during the remainder of the passive movement protocol, eliminated potential fluctuations in blood flow to the lower leg as a consequence of movement-related changes in gravitational and centrifugal forces. Cuffing had no effect on basal hemodynamics, and we (34) have previously documented that passive movement-induced changes in central hemodynamics were similar with or without cuffing. The cuff was tolerated well by all subjects.
All passive movement was performed by the same member of the research team moving the subjects' lower leg through the range of motion defined by 90 and 180° knee joint angles at 1 Hz (where the fully extended knee joint is defined as 180°). Movement in which the ultrasound Doppler assessment was performed was minimized by the researcher moving the leg holding the knee brace on the thigh. Real-time feedback was provided by a position sensor to ensure a consistent range of motion and a metronome to maintain cadence. Before the start and throughout the protocol, subjects were encouraged to remain passive and resist any urge to assist with leg movement. In the rare instance that a subject assisted or resisted the movement, the protocol was terminated and repeated after at least 10 min of recovery. Throughout the protocol, the control leg remained supported on the table in a fully extended position (180° of knee extension).
Measurements
Central variables.
HR, CO, and mean arterial pressure (MAP) were determined with a Finometer (Finapress Medical Systems, Amsterdam, The Netherlands). SV was calculated using the modelflow method (59), which includes age, sex, height, and weight in its algorithm (Beatscope version 1.1, Finapres Medical Systems) and has previously been documented to accurately track SV at rest and during exercise (8, 20, 39, 48). Although potential differences may exist in absolute pressure and/or SV associated with the modelflow method (3), focusing on the change from baseline allows accurate tracking across time (5). The CO coefficient of variation for repeated trials in this model was, in our hands, 5%. CO was then calculated as the product of HR and SV. To account for differences in body size, CO and SV were normalized to body surface area, which was calculated using the formula developed by Mosteller (38a), and these data were then expressed as cardiac index (CI) and stroke index (SI), respectively (27).
FBF.
Measurements of femoral arterial blood velocity and vessel diameter were performed in the passively moved leg and contralateral leg distal to the inguinal ligament and proximal to the bifurcation of the superficial and deep femoral arteries with a Logic 7 and Logic e ultrasound systems (General Electric Medical Systems, Milwaukee, WI) operated by a trained technician. The Logic 7 and Logic e systems were equipped with linear array transducers operating at an imaging frequencies of 14 and 12 MHz, respectively. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area. Blood velocity was obtained using the same transducers with a Doppler frequency of 5 MHz. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel based on real-time ultrasound visualization. Arterial diameter was measured and angle corrected, and intensity-weighted mean velocity (Vmean) values were then calculated using commercially available software (General Electric Medical Systems). Using arterial diameter and Vmean, FBF was calculated as follows: FBF = Vmean × π (vessel diameter/2)2 × 60, where blood flow is in milliliters per minute. To account for potential differences in MAP, femoral vascular conductance (FVC) was calculated as follows: FVC = FBF/MAP.
Knee joint angle.
During each protocol, the knee joint angle of the passive leg was continuously recorded using a Vishay Spectrol 360° Smart Position Sensor (Vashay, Malvern, PA) attached to a knee brace worn by the subjects.
Data acquisition.
Throughout the protocol, signals reflecting HR, SV, CO, MAP, and knee joint angle underwent analog-to-digital conversion and were simultaneously acquired (200 Hz) using commercially available data-acquisition software (AcqKnowledge, Biopac Systems, Goleta, CA). In addition, audio antegrade and retrograde signals from the Doppler ultrasound system were acquired (10,000 Hz) to serve as qualitative indicators of blood velocity changes and to ensure accurate temporal alignment of blood velocity measurements obtained from this system and the other variables collected (i.e., HR, SV, CO, and MAP as well as the knee joint angle documenting the range of the passive movement).
Data Analysis
From the velocity and femoral artery diameter, net blood flow was calculated on a second-by-second basis for the passively moved leg for the first minute and 12-s averages thereafter, as the peak response typically occurs within the first 40 s and, thus, such high-fidelity assessments are no longer necessary after the first minute. Before analysis, all second-by-second data were smoothed using a 3-s rolling average. To identify sex-specific responses across time, two-way (sex × time) repeated-measures ANOVAs were used. As the responses to passive movement are transient and vary in terms of time between individuals, a peak or nadir response was determined for each variable on an individual basis. Using individual data, baseline values, maximal, absolute, and relative changes, and time to maximal responses were identified for each measured variable. These values were then compared between sexes by independent t-tests. Additionally, to provide insight into the relationship between CO and the FBF response, a linear regression analysis between the peaks of these two variables was performed. α was set at 0.05 for all comparisons. All data are presented as means ± SE.
RESULTS
Subject Characteristics
Both male and female subject characteristics are shown in Table 1. Height, weight, and body mass index were all lower in women compared with men, and women also had a lower concentration of red blood cells, hemoglobin, and hematocrit (all P < 0.05). Blood lipids and blood chemistry were similar between the sexes.
Table 1.
Subject characteristics
| Men | Women | |
|---|---|---|
| Age, yr | 24 ± 1 | 22 ± 1 |
| Height, cm | 181 ± 2 | 165 ± 2* |
| Weight, kg | 83 ± 3 | 57 ± 2* |
| Body mass index, kg/m2 | 25 ± 1 | 21 ± 1* |
| Body surface area, m2 | 2.0 ± 0.3 | 1.6 ± 0.3* |
| Glucose, mg/dl | 77 ± 4 | 75 ± 3 |
| Cholesterol, mg/dl | 146 ± 13 | 147 ± 8 |
| Triglyceride, mg/dl | 79 ± 12 | 78 ± 11 |
| HDL, mg/dl | 46 ± 4 | 55 ± 2 |
| LDL, mg/dl | 94 ± 13 | 82 ± 6 |
| Cholesterol-to-HDL ratio | 3.3 ± 0.4 | 2.7 ± 0.1 |
| White blood cells, k/μl | 5.3 ± 0.3 | 6.2 ± 0.4 |
| Red blood cells, M/μl | 5.1 ± 0.2 | 4.5 ± 0.1* |
| Hemoglobin, g/dl | 15 ± 0.4 | 13 ± 0.3* |
| Hematocrit, % | 45 ± 0.9 | 40 ± 0.8* |
Values are means ± SE; n = 12 subjects/group.
P < 0.05 vs. men.
Central Hemodynamics
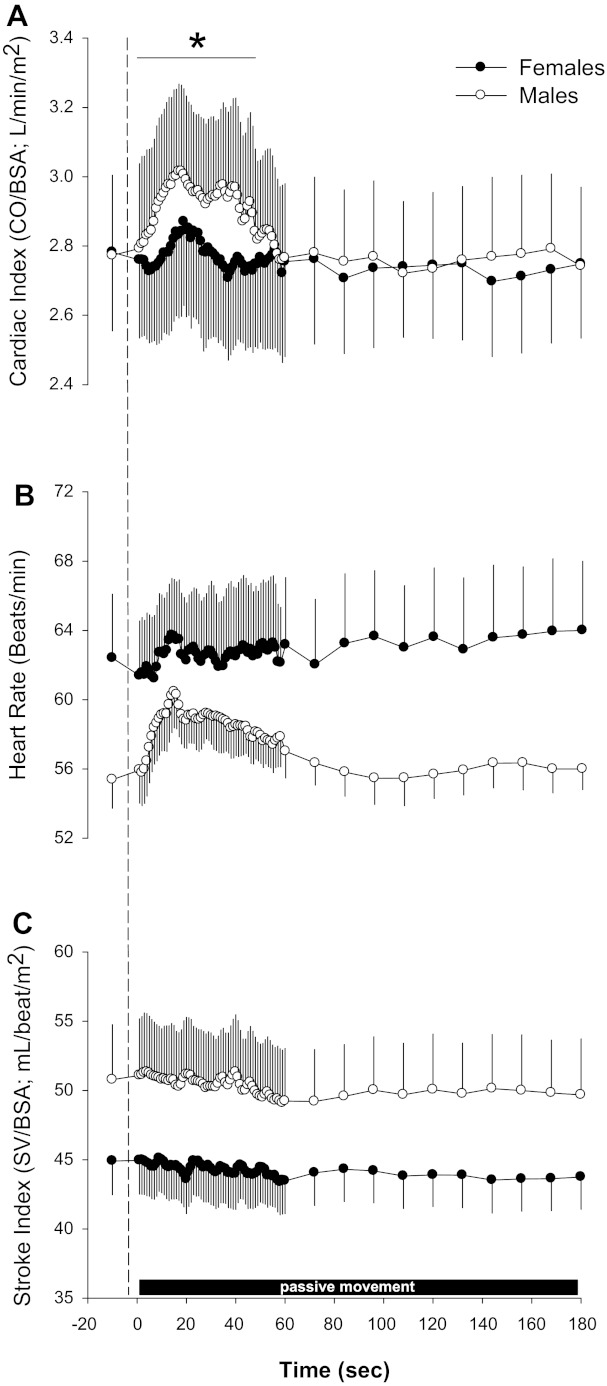
Women had significantly lower baseline values for CO (women: 4.5 ± 0.3 l/min and men: 5.7 ± 0.5 l/min) and SV (women: 73 ± 4 ml/beat and men: 104 ± 9 ml/beat) compared with men (both P < 0.05). However, when expressed as CI (women: 2.8 ± 0.2 l·min−1·m−2 and men: 2.8 ± 0.2 l·min−1·m−2) and SI (women: 45 ± 3 ml·beat−1·m−2 and men: 51 ± 4 ml·beat−1·m−2), women had similar baseline values compared with men (Fig. 1). Baseline HR tended to be greater in women (62 ± 4 beats/min) than men (55 ± 2 beats/min), but this did not achieve statistical significance (P > 0.05). In response to passive limb movement, a significant sex × time interaction was evident for CI (P < 0.05), such that women displayed an attenuated CI response to passive movement (Fig. 1A) compared with men. This sex × time interaction also held true if absolute CO values were used for the analysis (data not shown). There was also a tendency for women to exhibit less tachycardia (Fig. 1B), but this interaction effect did not reach significance (P > 0.05). ANOVA did indicate a significant main effect for time for HR, SI, and CI in both men and women, revealing a significant increase in these variables over time in response to passive movement (P < 0.05).
Fig. 1.
Central hemodynamic responses to passive limb movement between women and men. A–C: cardiac index (CI; A), heart rate (HR; B), and stroke index (SI; C) responses to passive movement in women and men. CO, cardiac output; BSA, body surface area; SV, stroke volume. Note: these data are temporally aligned and thus underrepresent true individual peak responses. Data are expressed as means ± SE. *P < 0.05, sex × time interaction.
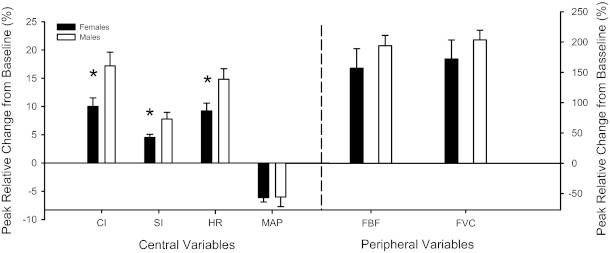
Analysis of the individual peak central responses (Fig. 2) revealed that changes in SI (women: 4.5 ± 0.6% change and men: 7.8 ± 1.2% change), HR (women: 9.0 ± 1% change and men: 14.8 ± 2% change), and CI (women: 9.6 ± 1% change and men: 17.2 ± 2% change) were all lower (P < 0.05) in women compared with men. There were no sex differences in terms of the time at which the individual peak responses occurred for CI (women: 21 ± 4 s and men: 23 ± 3 s), HR (women: 15 ± 3 s and men: 23 ± 4 s), or SI (women: 18 ± 3 s and men: 21 ± 4 s) (all P > 0.05). It should be noted that due to the temporal alignment and group averaging of the data shown in Figs. 1 and 3, the individual responses are underrepresented; thus, the responses shown in Fig. 2 are of a greater magnitude as they represent individual peak responses.
Fig. 2.
Individual peak relative changes in central and peripheral hemodynamic of women and men. MAP, mean arterial pressure; FBF, femoral blood flow; FVC, forearm vascular conductance. Note: these data represent individual peak responses, which are underrepresented in Figs. 1 and 3. Data are expressed as means ± SE. *P < 0.05, men vs. women.
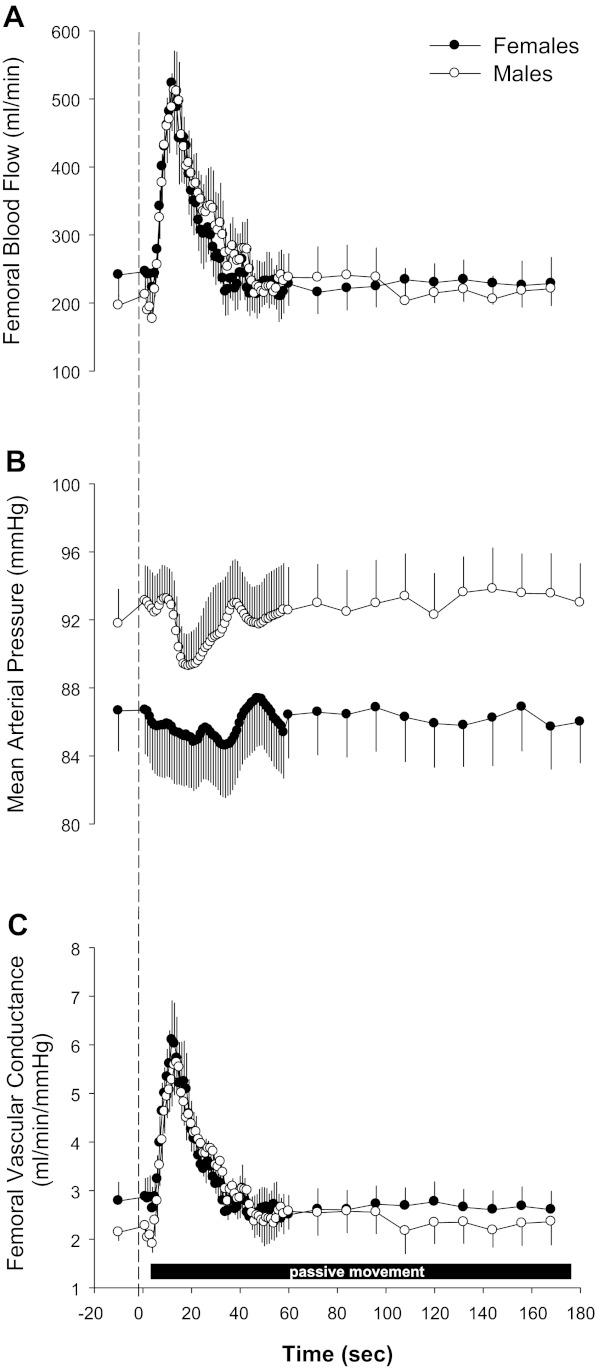
Fig. 3.
Peripheral hemodynamic responses to passive limb movement in women and men. A–C: absolute FBF (A), MAP (B), and FVC (C) in response to passive movement in women and men. Note: these data are temporally aligned and thus underrepresent true individual peak responses. Data are expressed as means ± SE.
Peripheral Hemodynamics
At rest, FBF between the sexes was not statistically different in the passive leg (women: 241 ± 36 ml/min and men: 196 ± 15 ml/min, P > 0.05; Fig. 3A) or contralateral limb (women: 290 ± 47 ml/min and men: 260 ± 46 ml/min, P > 0.05) nor different between legs within each group (both P > 0.05). Baseline MAP tended to be lower in women (87 ± 2 mmHg) compared with men (92 ± 2 mmHg, P > 0.05). However, there were no differences in resting FVC in the passive leg (women: 2.7 ± 1.4 ml·min−1·mmHg−1 and men: 2.1 ± 0.6 ml·min−1·mmHg−1, P > 0.05; Fig. 3C) or contralateral leg (women: 3.3 ± 0.5 ml·min−1·mmHg−1 and men: 3.6 ± 1.0 ml·min−1·mmHg−1, P > 0.05). Two-way ANOVA revealed no significant sex × time interactions for either the passive or contralateral limb FBF, MAP, or FVC but did indicate a significant main effect for time and therefore a significant increase in FBF and FVC and a decrease in MAP in response to passive movement (Fig. 3).
Statistical analysis of the individual peak responses revealed that passive limb movement induced similar increases in peak FBF (women: 167 ± 32% change and men: 193 ± 17% change, P > 0.05) and peak FVC (women: 172 ± 31% change and men: 203 ± 16% change, P > 0.05) of the passively moved leg (Fig. 2). In the contralateral limb, again, passive movement resulted in similar increases in peak FBF (women: 105 ± 34% change and men: 137 ± 17% change, P > 0.05) and peak FVC (women: 104 ± 32% change and men: 145 ± 57% change, P > 0.05), although the response was attenuated compared with the passively moved leg. In terms of the nadir for MAP, there were no differences in absolute MAP (81 ± 3 vs. 86 ± 3 mmHg, P > 0.05), absolute change (−6 ± 2 vs. −6 ± 1 mmHg, P > 0.05), or relative change (−6.6 ± 2 vs. −7.2 ± 1% change, P > 0.05; Fig. 2) in pressure. Finally, there were no sex differences in terms of the time at which the individual peak responses occurred for FBF (women: 10 ± 2 s and men: 14 ± 3 s), MAP (women: 18 ± 5 s and men: 14 ± 4 s), or FVC (women: 10 ± 2 s and men: 16 ± 3 s) (all P > 0.05) in the passively moved leg.
Relationship Between Central and Peripheral Hemodynamics
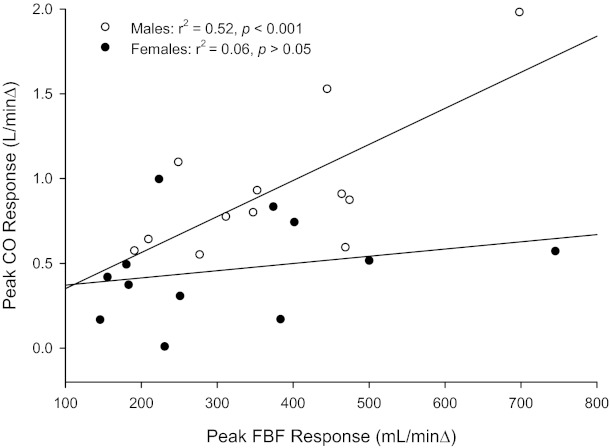
Linear regression analyses of the peak blood flow and CO responses revealed a significant relationship in men (y = 0.0021x + 0.1381, r2 = 0.52, P < 0.05) but not in women (y = 0.0004x + 0.3288, r2 = 0.06, P > 0.05; Fig. 4).
Fig. 4.
Relationship between peak FBF and peak CO responses to passive movement in women and men.
DISCUSSION
This study aimed to elucidate sex-specific differences in central and peripheral hemodynamic responses to passive movement, a component of which is mediated by the mechanoreflex. Basal FBF, MAP, and FVC were not different between the sexes. However, in response to passive movement, women displayed attenuated HR, SV, and CO responses, which held true even when normalized to body size (CI), and yet achieved a similar FBF response. Although we cannot definitively rule out the potential role of other factors, these data suggest that women display an attenuated mechanoreflex, as evidenced by the blunted CI response. However, despite this clear sex difference in terms of central hemodynamic responses, the attainment of a similar FBF in both men and women indicates that women may rely more on local vasodilation to promote movement-induced hyperemia than do their male counterparts.
Sex Specificity in Central Hemodynamics
The exercise pressor reflex results from group III and IV afferent nerve activation and contributes to an increase in HR, SV, and sympathetic nerve activity (SNA), acting in concert to increase CO, redistribute blood volume, and maintain MAP (1, 29, 37). Previous studies (13, 28, 32, 42) investigating sex differences in hemodynamics have determined that women display an attenuated exercise pressor reflex compared with men. Jarvis et al. (28) determined that while there were clear MAP differences between sexes during handgrip exercise and postexercise cuff occlusion, there were no sex differences in the pressure response to the cold pressor test. The results from Jarvis et al. (28) suggest that the sex differences during handgrip and postexercise cuff occlusion are likely a consequence of sex-specific differences in afferent ergoreceptor signaling (ensemble group III and IV afferent nerve fibers) and not cardiovascular control center processing.
In support of this reduced afferent signaling in women, the present findings suggest that women display attenuated group III afferent nerve or mechanoreceptor sensitivity, as evidenced by a lower CO response to passive limb movement. Although the mechanism responsible for this attenuation was not elucidated in the present study, previous work (51–54) performed using an animal model has determined that estrogen, either endogenously or via spinal application, can attenuate the exercise pressor reflex in both male and female rats. This implies a significant role of estrogen in modulating afferent nerve signaling and the subsequent exercise pressor reflex (51–54). However, recent data from humans has suggested that a sex-specific effect of the exercise pressor reflex persists across the menstrual cycle, where endogenous hormones (estrogen and progesterone) oscillate from low to high concentrations, casting doubt on the estrogen dependence of this phenomenon (28). Furthermore, in agreement with the results of Jarvis et al. (28), the present findings may also not depend on estrogen per se, as these experiments were performed in the early follicular phase of the menstrual cycle, where estrogen is at low levels, similar to that of men (15, 21, 56). Schmitt et al. (53) indicated that, while the exercise pressor reflex can be suppressed by estrogen, there appears to be cross-talk between the estrogen and opioid systems, suggesting an indirect effect of estrogen. Therefore, in combination, the results from present and previous studies suggest that through either direct or indirect mechanisms, the presence of estrogen and/or other factors associated with being female appear to blunt both group III (mechanosensitive) and group IV (metabosensitive) afferent feedback and subsequent cardioacceleration.
Whenever MAP is perturbed, as in the present study where both males and females experienced an equal decrease in MAP, a potential role of the baroreflex cannot be overlooked. However, prior work by Kim et al. (30) indicated that the baroreflex-mediated pressor response to carotid hypotension was similar between men and women, suggesting similar baroreflex function between the sexes in response to hypotensive stimuli. Additionally, work by Shoemaker et al. (55) revealed that in response to hypotensive stress, women actually exhibited an equal CO response to men. Finally, work by our group, in which passive movement responses with and without cuff occlusion of the leg, which prevented the hyperemia and the small decrease in MAP, resulted in similar changes in HR, SV, and CO, highlighting that these hemodynamic responses are likely the result of evoking the mechanoreflex and not the baroreflex. Taken together, these previous findings suggest that impaired baroreflex function is an unlikely explanation for the reduced CO response in women in the present study and that other mechanisms must be involved (e.g., the mechanoreflex).
Sex Specificity of Peripheral Hemodynamics
Potential sex differences in muscle blood flow at rest and during exercise have received relatively little attention, as the majority of studies have focused on men. Ridout et al. (49) documented that, in absolute terms (l/min), peak FBF during knee extensor exercise was not different between men and women, despite women having a lower quadriceps muscle mass and lower peak work rate. When normalized by either quadriceps muscle mass or perfusion pressure, peak FVC tended to be higher in women but was not statistically different (49). A likely contributor to the greater blood flows in women is their lower hemoglobin levels (Table 1), as decreased hemoglobin has been documented to increase blood flow to compensate for lower arterial O2 content, ultimately allowing O2 delivery to remain constant (31).
While total leg mass or muscle mass was not assessed in this study, and thus hyperemia could not be expressed per unit of muscle, the subject characteristics (e.g., stature, weight, body mass index, and body surface area) suggest that the women were, on average, smaller than the men. Thus, normalizating a similar level of blood flow, already elevated by reduced hemoglobin levels, to a smaller muscle mass/volume would yield an even greater muscle mass-specific blood flow at rest and during passive movement in women compared with men, although the relative change from baseline would likely remain similar. Such inferences certainly support the conclusion that women achieved at least an equal, if not greater, movement-induced hyperemia (Fig. 3).
Previous research by Parker and colleagues (45) investigated sex differences in the blood flow response to passive movement (45). In agreement with their findings, the data from the present study revealed that baseline FBF and FVC were not different between men and women. However, as already recognized, the study by Parker et al. (45) assessed hemodynamic changes in the third minute of passive movement and, therefore, likely missed the largest passive movement-induced hyperemia in both sexes. Despite our higher time resolution and the subsequent capability to accurately assess the transient hyperemic response in men and women, there were still no apparent sex differences in peak FBF (Fig. 3). Given the similar peak FBF responses but disparate changes in CO, it appears that there is a sex-specific difference in the ability to achieve this hyperemic response to passive limb movement in the face of an attenuated central hemodynamic change. Specifically, men appeared to produce a greater CO response to passive limb movement, which has been documented to play a significant role in increasing peripheral blood flow (34, 57), whereas women appeared to be more capable of eliciting a greater local vasodilatory response, offsetting the attenuated increase in CO. Interestingly, Ridout et al. (49) found that, when examining at sex differences in central and peripheral hemodynamics during knee extension exercise, there was a significant positive relationship between peak FBF and peak CO in men, whereas no such relationship was evident in women. Therefore, the results from the work of Ridout et al. (49) support the concept that FBF in women is less dependent on CO than their male counterparts. Using a similar analytic approach (Fig. 4), the present study also revealed that, for a given hyperemic response, women require less of a change in CO. Given Ohm's law, this equal peripheral hyperemia with a lower CO response in women would suggest an increase in resistance elsewhere. However, examination of the contralateral (nonmoving) limb, one site in which there could be such a reduction in vascular conductance, FBF and FVC were not different between men and women. Thus, it is likely that resistance increased to a greater extent in women than in men in another vascular bed, such as the mesenteric, or perhaps women experienced greater venous pooling in the legs, neither of which was examined in the present study and would require further study to elucidate the contribution of each.
Although even the fundamental mechanisms contributing to exercise-induced hyperemia in men and women remain elusive, we speculate that those specific to movement, such as ATP release (38), mechanically induced vasodilation, myogenic regulation (7), or other local factors, could be responsible for the equal blood flow response in men and women despite the attenuated CO increase in women. One such local factor could be muscle SNA or norepinephrine release. It is relatively well accepted that women typically have lower basal SNA (16, 33, 42, 43, 55), although it is unknown if differences remain during passive limb movement. Welsh and Segal (58) found that muscle lengthening itself was capable of eliciting local action potentials and subsequent norepinephrine-induced vasoconstriction of feed arteries and arterioles. Given the inverse relationship between muscle SNA and blood flow (44), muscle shortening and lengthening associated with passive movement (35) could result in a local sympathetic response, a process that could be attenuated in women and warrants further investigation.
Implications of a Reduced Mechanoreflex
It is well known that there are some rather overt differences in basic physiology as well as cardiovascular disease prevalence between men and women. In their premenopausal years, women appear to be at a reduced risk for cardiovascular disease, a relative immunity that appears to be negated or even reversed postmenopause (6). In parallel, it has been well established that chronic elevation of SNA is likely a major contributor to the development of hypertension (12, 50) and that young premenopausal females tend to have lower basal SNA (16, 33, 42, 43, 55). In addition, women have suppressed metaboreceptor responsiveness or a reduced sympathetic response to afferent metabolic stimuli (13, 32, 42) and, as revealed in the present study, an attenuated mechanoreflex. We speculate that this reduction in afferent nerve sensation or signaling may contribute significantly to the reduced sympathetic reactivity in women and ultimately the reduced incidence of hypertension and cardiovascular disease during the premenopausal years (47).
Experimental Considerations
As physical activity levels were not directly assessed in the present study, the possibility exists that differences in physical fitness may have played a role in the response to passive limb movement. However, an assumption of this study was that potential variations in fitness level in recreationally active people would be evenly distributed between men and women. Additionally, while the role of the baroreflex has been studied by other research groups (30, 55) and determined to be similar between men and women in response to hypotensive stimuli, the role of the baroreflex in mediating the responses observed in the subjects in the present study cannot be completely ruled out. Of note, we observed a reduction in MAP, a response that is at odds with the notion that the mechanoreflex contributes to the exercise pressor reflex. This observation is likely due to the reductionist approach used, where leg vasodilation offsets any increase in pressure that would have occurred, which is in contrast to the passive stretch model, where no such vasodilation occurs and thus MAP increases (4, 9–11, 14, 17, 18). The decrement in MAP observed in the present study would not likely occur if both central command and the metaboreflex were engaged, as in the case of traditional active exercise, and does not negate the role of the mechanoreflex in the present experimental paradigm. In support of this notion, previous work from our group (34) demonstrated that using a cuff to prevent the leg vasodilation associated with passive movement resulted in similar central hemodynamic responses and a rise in MAP. This highlights that, while not a pure model, the use of passive movement does result in mechanosensitive afferent feedback, which acts to increase central hemodynamics.
Conclusions
Passive knee extension, a model devoid of metabolic perturbation, evoked a blunted CI response in women compared with men; however, despite this reduced central hemodynamic response, women achieved equal movement-induced hyperemia. Although we cannot completely rule out the role of other factors, the attenuated central hemodynamic response in women is likely mediated by a reduced mechanoreflex compared with men, while the impact of this on peripheral hemodynamics is unremarkable, perhaps as a consequence of augmented dilatory mechanisms in women.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant P01-HL-091830 as well as Advanced Fellowships in Geriatrics (to S. J. Ives and M. A. H. Witman) from the Department of Veterans Affairs and Veterans Affairs Merit Grant E6910R (to R. S. Richardson). J. McDaniel was supported by Career Development Award CDA2-E7560W.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.J.I., J.M., and R.S.R. conception and design of research; S.J.I., J.M., M.A.H.W., and R.S.R. performed experiments; S.J.I., J.M., M.A.H.W., and R.S.R. analyzed data; S.J.I., J.M., M.A.H.W., and R.S.R. interpreted results of experiments; S.J.I., J.M., and M.A.H.W. prepared figures; S.J.I., J.M., M.A.H.W., and R.S.R. drafted manuscript; S.J.I., J.M., M.A.H.W., and R.S.R. edited and revised manuscript; S.J.I., J.M., M.A.H.W., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the volunteers for participation in the study.
REFERENCES
- 1. Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Azabji Kenfack M, Lador F, Licker M, Moia C, Tam E, Capelli C, Morel D, Ferretti G. Cardiac output by Modelflow method from intra-arterial and fingertip pulse pressure profiles. Clin Sci (Lond) 106: 365–369, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Baum K, Selle K, Leyk D, Essfeld D. Comparison of blood pressure and heart rate responses to isometric exercise and passive muscle stretch in humans. Eur J Appl Physiol 70: 240–245, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Celermajer D, Sorensen K, Spiegelhalter D, Georgakopoulos D, Robinson J, Deanfield J. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol 572: 561–567, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Critchley LA, Lee A, Ho AMH. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg 111: 1180–1192, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Cui J, Mascarenhas V, Moradkhan R, Blaha C, Sinoway LI. Effects of muscle metabolites on responses of muscle sympathetic nerve activity to mechanoreceptor(s) stimulation in healthy humans. Am J Physiol Regul Integr Comp Physiol 294: R458–R466, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Drew RC, Bell MPD, White MJ. Modulation of spontaneous baroreflex control of heart rate and indexes of vagal tone by passive calf muscle stretch during graded metaboreflex activation in humans. J Appl Physiol 104: 716–723, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Drew RC, McIntyre DB, Ring C, White MJ. Local metabolite accumulation augments passive muscle stretch-induced modulation of carotid-cardiac but not carotid-vasomotor baroreflex sensitivity in man. Exp Physiol 93: 1044–1057, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Esler M. The sympathetic nervous system through the ages: from Thomas Willis to resistant hypertension. Exp Physiol 96: 611–622, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX, Sinoway LI. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol 80: 245–251, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Fisher JP, Bell MPD, White MJ. Cardiovascular responses to human calf muscle stretch during varying levels of muscle metaboreflex activation. Exp Physiol 90: 773–781, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Fu MhH, Maher AC, Hamadeh MJ, Ye C, Tarnopolsky MA. Exercise, sex, menstrual cycle phase, and 17β-estradiol influence metabolism-related genes in human skeletal muscle. Physiol Genomics 40: 34–47, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 587: 2019–2031, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gladwell VF, Coote JH. Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol 540: 1095–1102, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gladwell VF, Fletcher J, Patel N, Elvidge LJ, Lloyd D, Chowdhary S, Coote JH. The influence of small fibre muscle mechanoreceptors on the cardiac vagus in humans. J Physiol 567: 713–721, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. González-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol 586: 2405–2417, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci 97: 291–301, 1999 [PubMed] [Google Scholar]
- 21. Harris RA, Tedjasaputra V, Zhao J, Richardson RS. Premenopausal women exhibit an inherent protection of endothelial function following a high-fat meal. Reprod Sci 19: 221–228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance. Hypertension 53: 571–576, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol 99: 1891–1896, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Hellsten Y, Rufener N, Nielsen JJ, Høier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294: R975–R982, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR. Gender differences in sympathetic nervous system regulation. Clin Exp Pharmacol Physiol 26: 122–126, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Ives S, Blegen M, Coughlin M, Redmond J, Matthews T, Paolone V. Salivary estradiol, interleukin-6 production, and the relationship to substrate metabolism during exercise in females. Eur J Appl Physiol 111: 1649–1658, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Jarvis SS, Florian JP, Curren MJ, Pawelczyk JA. Sex differences in vasoconstrictor reserve during 70 deg head-up tilt. Exp Physiol 95: 184–193, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Jarvis SS, VanGundy TB, Galbreath MM, Shibata S, Okazaki K, Reelick MF, Levine BD, Fu Q. Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol 301: R193–R200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Kim A, Deo SH, Vianna LC, Balanos GM, Hartwich D, Fisher JP, Fadel PJ. Sex differences in carotid baroreflex control of arterial blood pressure in humans: relative contribution of cardiac output and total vascular conductance. Am J Physiol Heart Circ Physiol 301: H2454–H2465, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koskolou MD, Roach RC, Calbet JAL, Rådegran G, Saltin B. Cardiovascular responses to dynamic exercise with acute anemia in humans. Am J Physiol Heart Circ Physiol 273: H1787–H1793, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Maixner W, Humphrey C. Gender differences in pain and cardiovascular responses to forearm ischemia. Clin J Pain 9: 16–25, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol Regul Integr Comp Physiol 275: R1600–R1604, 1998 [DOI] [PubMed] [Google Scholar]
- 34. McDaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. J Appl Physiol 108: 76–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McDaniel J, Ives SJ, Richardson RS. Human muscle length-dependent changes in blood flow. J Appl Physiol 112: 560–565, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, MacLellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol 287: H1937–H1943, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983 [DOI] [PubMed] [Google Scholar]
- 38. Mortensen SP, Thaning P, Nyberg M, Saltin B, Hellsten Y. Local release of ATP into the arterial inflow and venous drainage of human skeletal muscle: insight from ATP determination with the intravascular microdialysis technique. J Physiol 589: 1847–1857, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 317: 1098–1098, 1987 [DOI] [PubMed] [Google Scholar]
- 39. Mukkamala R, Xu D. Continuous and less invasive central hemodynamic monitoring by blood pressure waveform analysis. Am J Physiol Heart Circ Physiol 299: H584–H599, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakamoto T, Matsukawa K. Muscle mechanosensitive receptors close to the myotendinous junction of the Achilles tendon elicit a pressor reflex. J Appl Physiol 102: 2112–2120, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Nakamoto T, Matsukawa K. Muscle receptors close to the myotendinous junction play a role in eliciting exercise pressor reflex during contraction. Auton Neurosci 138: 99–107, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Ng A, Callister R, Johnson D, Seals D. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21: 498, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298: H1128–H1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Herr MD, Proctor DN. Sex differences in leg vasodilation during graded knee extensor exercise in young adults. J Appl Physiol 103: 1583–1591, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Radegran G, Saltin B. Muscle blood f low at onset of dynamic exercise in humans. Am J Physiol Heart Circ Physiol 274: H314–H322, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension 37: 1199–1208, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Reisner AT, Xu D, Ryan KL, Convertino VA, Rickards CA, Mukkamala R. Monitoring non-invasive cardiac output and stroke volume during experimental human hypovolaemia and resuscitation. Br J Anaesth 106: 23–30, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ridout SJ, Parker BA, Smithmyer SL, Gonzales JU, Beck KC, Proctor DN. Age and sex influence the balance between maximal cardiac output and peripheral vascular reserve. J Appl Physiol 108: 483–489, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sander M, Hansen J, Victor RG. The sympathetic nervous system is involved in the maintenance but not initiation of the hypertension induced by Nω-nitro-l-arginine methyl ester. Hypertension 30: 64–70, 1997 [DOI] [PubMed] [Google Scholar]
- 51. Schmitt PM, Gohil K, Kaufman MP. Spinal estrogen attenuates the exercise pressor reflex but has little effect on the expression of genes regulating neurotransmitters in the dorsal root ganglia. J Appl Physiol 100: 958–964, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Schmitt PM, Kaufman MP. Estrogen attenuates the exercise pressor reflex in female cats. J Appl Physiol 95: 1418–1424, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Schmitt PM, Kaufman MP. Estrogen's attenuating effect on the exercise pressor reflex is more opioid dependent in gonadally intact than in ovariectomized female cats. J Appl Physiol 98: 633–639, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Schmitt PM, Kaufman MP. High concentrations of 17β-estradiol attenuate the exercise pressor reflex in male cats. J Appl Physiol 94: 1431–1436, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol 281: H2028–H2035, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Timmons B, Hamadeh M, Devries M, Tarnopolsky M. Influence of gender, menstrual phase, and oral contraceptive use on immunological changes in response to prolonged cycling. J Appl Physiol 99: 979, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O'Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299: H1693–H1700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Welsh DG, Segal SS. Muscle length directs sympathetic nerve activity and vasomotor tone in resistance vessels of hamster retractor. Circ Res 79: 551–559, 1996 [DOI] [PubMed] [Google Scholar]
- 59. Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566–2573, 1993 [DOI] [PubMed] [Google Scholar]
- 60. Wray D, Donato A, Uberoi A, Merlone J, Richardson R. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol 565: 1053, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]