Abstract
Although elevated levels of H2O2 have been implicated to play important roles in the pathogenesis of various cardiovascular diseases, the underlying mechanisms remain unclear. This study aims to examine the effect of H2O2 on endothelial nitric oxide (NO) production in intact venules, and elucidate the role and mechanisms of NO in H2O2-induced increases in microvessel permeability. Experiments were conducted on individually perfused rat mesenteric venules. Microvessel permeability was determined by measuring hydraulic conductivity (Lp), and endothelial [Ca2+]i was measured on fura-2-loaded vessels. Perfusion of H2O2 (10 μM) caused a delayed and progressively increased endothelial [Ca2+]i and Lp, a pattern different from inflammatory mediator-induced immediate and transient response. Under the same experimental conditions, measuring endothelial NO via DAF-2 and the spatial detection of cell apoptosis by fluorescent markers revealed that H2O2 induced two phases of NO production followed by caspase activation, intracellular Ca2+ accumulation, and vascular cell apoptosis. The initial NO production was correlated with increased endothelial NO synthase (eNOS) Ser1177 phosphorylation in the absence of elevated endothelial [Ca2+]i, whereas the second phase of NO depended on increased [Ca2+]i and was associated with Thr495 dephosphorylation without increased Ser1177 phosphorylation. Inhibition of NOS prevented H2O2-induced caspase activation, cell apoptosis, and increases in endothelial [Ca2+]i and Lp. Our results indicate that H2O2 at micromolar concentration is able to induce a large magnitude of NO in intact venules, causing caspase activation-mediated endothelial Ca2+ accumulation, cell apoptosis, and increases in permeability. The mechanisms revealed from intact microvessels may contribute to the pathogenesis of oxidant-related cardiovascular diseases.
Keywords: hydrogen peroxide, nitric oxide, cell apoptosis, microvessel permeability
increased production of reactive oxygen species (ROS) has been shown to cause increases in microvessel permeability, resulting in tissue damage and organ dysfunctions (2, 7, 42). We had previously demonstrated that superoxide and inflammatory mediators induce immediate and transient increases in endothelial cell (EC) intracellular Ca2+ concentration ([Ca2+]i) and microvessel permeability, whereas perfusion of hydrogen peroxide (H2O2) induces delayed and progressively increased EC [Ca2+]i and hydraulic conductivity (Lp) (42). The different patterns of response suggest that the mechanisms involved in H2O2-induced permeability increases are different from those induced by inflammatory mediators.
Currently, it has been recognized that inflammatory mediator-induced endothelial nitric oxide synthase (eNOS) activation and excessive nitric oxide (NO) production are essential for increasing microvessel permeability (22, 38, 39). Blockade of NO production by nonspecific nitric oxide synthase (NOS) inhibitor or specific eNOS inhibitor, caveolin-1 scaffolding domain, attenuates platelet activating factor (PAF)-induced immediate and transient increases in the microvessel permeability in intact venules (39, 43). Studies conducted in large arteries and arterioles indicated that H2O2 acts as an important vasodilator through the activation of eNOS and increased NO production (3, 36). However, whether H2O2 induces NO production in intact venules and the functional roles of H2O2-induced NO production in the regulation of microvessel permeability remain to be identified.
Previous cell culture studies reported that exogenously supplied large amounts of NO by S-nitrosyl-N-acetylpenicillamine (SNAP, NO donor) induced venous EC apoptosis (29), and increased eNOS activity was found in H2O2-exposed thoracic aortic ECs (30). Our previous study demonstrated that the H2O2-induced delayed and progressive increases in EC [Ca2+]i and Lp were partially reversed after perfusion of H2O2 at 100 μM for 1 h, and were not reversible after 1 h of H2O2 perfusion at 500 μM, suggesting a potential cell damage at microvascular walls (42). Human plasma H2O2 levels are moderately elevated under disease conditions, such as hypertension and cancer, ranging from 3.4 to 6.2 μM (1, 21). However, most of the experimental studies have used much higher concentrations of H2O2 (near or at millimolar range). The effect of H2O2 at a near-pathological concentration (10 μM) on intact microvessels has not been investigated and the causal relationship between H2O2-induced NO production, vascular cell apoptosis, and increases in microvessel permeability remains to be revealed.
The objective of this study is to examine the cellular and molecular mechanisms of H2O2-induced increases in microvesssel permeability by evaluating the effect and cellular mechanisms of H2O2 at a near-pathological concentration on eNOS activation and NO production in intact venules, and further characterizing the functional roles and mechanisms of NO in the regulation of H2O2-induced permeability increases. Experiments were conducted on individually perfused rat mesenteric venules with intact surrounding circulation. We first evaluated the EC [Ca2+]i and permeability responses to H2O2 at 10 μM. Under the same experimental conditions, the direct effects of H2O2 on NO production and the signaling mechanisms of H2O2-induced eNOS activation and cell apoptosis were also examined. Microvessel permeability was determined by measuring the Lp. EC [Ca2+]i and NO were measured using fura-2 and DAF-2 with fluorescence imaging. Vascular cell apoptosis with spatial resolution on the microvascular walls was evaluated by fluorescent markers using confocal microscopy. The causal relationships between NO, caspase activation, vascular cell apoptosis, endothelial Ca2+ accumulation, and permeability increases were investigated using pharmacological inhibitors.
MATERIALS AND METHODS
Animal preparation.
Experiments were performed in venular microvessels in rat mesenteries with diameters ranging between 35 and 50 μm. Female Sprague-Dawley rats (2–3 mo old, 220–250 g, Hilltop Laboratory Animal, Scottdale, PA) were anesthetized with pentobarbital sodium (65 mg/kg body wt) administered subcutaneously. A midline surgical incision (1.5–2 cm) was made in the abdominal wall and the mesentery was gently taken out from the abdominal cavity and spread over a glass coverslip attached to an animal tray for fluorescence imaging studies. The upper surface of the mesentery was continuously superfused with mammalian Ringer solution at 37°C. Each experiment was performed on one microvessel per animal to avoid any potential effect of the applied reagents on subsequent vessel studies. All procedures and animal use were approved by the Animal Care and Use Committee at West Virginia University.
Measurement of Lp in individually perfused rat mesenteric microvessels.
All measurements were based on the modified Landis technique, which measures the volume flux of water across the microvessel wall. The assumptions and limitations of the original method and its application to mammalian microvessels have been evaluated in detail elsewhere (6, 20). Briefly, a single microvessel was cannulated with a micropipette and perfused with albumin-Ringer solution (control) containing 1% (vol/vol) hamster red blood cells as markers. A known hydrostatic pressure (40–60 cmH2O), controlled by a water manometer, is applied through the micropipette to the vessel lumen, which allows the perfusate to continuously flow through the vessel. Water flux was measured when the downstream of the vessel was briefly occluded. Lp was calculated as the slope of the relationship between the initial water flow per unit area and the pressure difference across the microvessel wall. In each experiment, the baseline Lp and the Lp after application of testing solutions were measured in the same vessel, and the changes in Lp were expressed as the ratio of Lptest/Lpcontrol. The testing agent including H2O2 and any inhibitor used in the experiments was added to the perfusate and delivered into the vessel lumen through the cannulation pipette. To prevent the red blood cell marker from interacting with H2O2 in the perfusate, marker cells were not present during the H2O2 perfusion period. Only during Lp measurements after perfusion of H2O2 for 1 or 2 h was a small amount of red blood cells added to the perfusate by recannulation of the vessel.
Measurements of EC [Ca2+]i.
EC [Ca2+]i was measured in individually perfused microvessels using the fluorescent Ca2+ indicator fura 2-AM. Experiments were performed on a Nikon Diaphod 300 microscope equipped with a Nikon photometry system. In each experiment, a venular microvessel was cannulated and perfused first with albumin-Ringer solution that contained 10 μM of fura 2-AM for 45 min. The vessel was then recannulated and perfused with albumin-Ringer solution for 10 min to remove fura 2-AM from the vessel lumen. A segment of fura 2-AM-loaded vessel at least 100 μm away from the cannulation site was then positioned within the measuring window which covered ∼50 endothelial cells forming the vessel wall. The excitation wavelengths for fura 2-AM were selected by two narrow-band interference filters (340 ± 5 and 380 ± 5 nm; Oriel), and the emission was separated with a dichroic mirror (DM400) and a wide-band interference filter (500 ± 35 nm; Oriel). The corresponding fluorescence intensity (FI) values (FI340 and FI380, respectively) were collected with a 0.25-s exposure at each wavelength. At the end of the experiment, the microvessel was superfused with a modified Ringer solution (5 mM of Mn2+ without Ca2+) and perfused with the same solution that contained ionomycin (10 μM) to bleach the Ca2+-sensitive form of fura 2. The background FI due to unconverted fura 2-AM and other Ca2+-insensitive forms of fura 2 was subtracted from FI340 and FI380 values. The ratios of the two FI values were converted to Ca2+ concentrations using an in vitro calibration curve (14).
Measurement of endothelial NO production.
Endothelial NO was quantified at cellular levels in individually perfused microvessels using a fluorescence imaging system and 4,5-diaminofluorescein diacetate (DAF-2 DA). The experimental rigs were the same as that used for Ca2+ measurements, except that a 12-bit digital, cooled, charge-coupled device camera (ORCA; Hamamatsu) was used for image acquisition. The excitation wavelength for DAF-2 DA was selected by an interference filter (480/40 nm), and emission was separated by a dichroic mirror (505 nm) and a band-pass barrier (535/50 nm). To minimize photo bleaching, a neutral density filter (0.5 N) was positioned in front of the interference filter and the exposure time was minimized to 0.12 s at 1-min intervals. All the images were acquired and analyzed using Metafluor software (Universal Imaging). During the experiment, each vessel was continuously perfused with albumin-Ringer solution containing DAF-2 DA (5 μM). NO production was measured after the DAF-2 loading reached the steady state (40). Basal NO was measured for 10 min before H2O2 perfusion. All images were collected from a group of endothelial cells located in the same focal plane of the vessel wall.
Quantitative analysis was conducted at the individual endothelial cell level using manually selected regions of interest (ROIs) along the vessel wall. Each ROI covers the area of one individual cell as indicated by the fluorescence outline. The tissue autofluorescence was subtracted from all of the measured FIs. The changes in FIDAF upon addition of test reagents were expressed as the net changes in FIDAF (ΔFIDAF), which was the difference between the maximum FI before the increased FI rate fell back to the basal level and the FI right before the application of test reagents. FI was expressed in arbitrary units (AU) measured with identical instrumental settings. The rate of FIDAF change was derived by the first differential conversion of cumulative FIDAF over time.
Detection of cell apoptosis in intact venules.
Cell apoptosis in intact venules was determined by perfusing vessels with Alexa488-annexin-V, which preferentially binds with translocated phosphatidylserine (PS) on the out leaflet of cell membrane during cell apoptosis. Vybrant FAM Caspases-3/7 assay kit that contains the fluorescently labeled inhibitor of caspases (FLICA) was applied to examine the activated caspase 3/7 in H2O2 perfused vessels. During the experiment, each vessel was perfused with H2O2 at 10 or 100 μM for certain periods of time, followed by perfusion with Alexa488-annexin-V (1:20 dilution from the stock solutions) and FLICA (1:150 dilution from the stock solution provided by the company), respectively. Cell viability on the vascular wall was detected by perfusion of vessels with CellTrace calcein red-orange AM (1 μM) for 10 min. A Leica TCS SL confocal microscope was used for collecting the images. Stacks of images were obtained from each vessel by optical sectioning at successive X-Y focal planes with a vertical step at 0.5 μm, using a Leica objective ×20 (HC PL APO, NA 0.7) with ×3 electronic zoom. Image analysis and FI quantification were performed using Leica confocal software. Identical imaging settings were applied to each group of experiments.
The quantitative analysis of FLICA staining was conducted on the stacks of images obtained from the bottom half of each vessel. The ROIs were defined by the outline of each vessel segment. The total FI of FLICA (the intensity value of all pixels of vessel volume) was calculated as area × depth × mean intensity per pixel, where the area is the pixel number of the selected ROI, the depth is the total number of images at z-dimension, and the mean intensity per pixel is the mean fluorescence intensity after subtraction of background signal. The total FI was normalized by the vascular surface area (FI/A).
Fluorescent immunostaining and confocal imaging.
The mesentery bearing each perfused vessel was fixed with paraformaldehyde, followed by permeabilization with 0.1% Triton X-100 before exposure to the anti-eNOS and anti-phosphorylated eNOS at Ser1177 (Abcam) or Thr495 (Cell Signaling Technology) antibody. The tissue was then incubated with Alexa 488-conjugated secondary antibody (Invitrogen) at room temperature for 3 h. DRAQ5 (Biostatus) was used for nuclei staining. Confocal images were obtained using a Leica objective ×63 (HCX PL APO, NA 1.2) with ×1.5 electronic zoom, and the vertical step was 0.3 μm. The mean FI of each stack of ROIs that cover the area of individual endothelial cells was quantified using Leica confocal software. A mean of the FI averaged from four ROIs of each vessel segment was calculated and the ratio of the FI over control value was calculated to represent the changes in the protein content.
Solutions and reagents.
Mammalian Ringer solution (14) was used for dissecting mesenteries, superfusing tissues, and preparing perfusion solutions. The composition of the mammalian Ringer solution was (in mM) 132 NaCl, 4.6 KCl, 2 CaCl2, 1.2 MgSO4, 5.5 glucose, 5.0 NaHCO3, 20 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), and Na-HEPES. All perfusates used for control and test perfusion contained BSA (10 mg/ml). Fura-2 AM, Alexa488-annexin-V, Vybrant FAM Caspase-3/7 kit and CellTrace calcein red-orange AM were purchased from Invitrogen. H2O2 (30%), DAF-2 DA, and LaCl3 were purchased from Sigma. All the fluorescent dyes except for Alexa488-annexin-V and Vybrant FAM Caspase-3/7 were prepared in DMSO for stock solution, and at least 1:1,000 dilution was made for the final working solutions. All of the perfusates containing the test reagents were freshly prepared before each cannulation.
Data analysis and statistics.
All values are presented as means ± SE. Each “N” represents the number of vessels. Paired t-test was used for paired data analysis. ANOVA was used to compare the data between groups. A probability value of P < 0.05 was considered as statistically significant. In summary figures, the asterisk indicates a significant increase from the baseline control, and the single dagger indicates a significant decrease from the control or the H2O2-induced responses.
RESULTS
H2O2 induces delayed and progressive increases in microvessel Lp and EC [Ca2+]i.
We had previously demonstrated that H2O2 at concentrations of 100 and 500 μM induces delayed and progressively increased EC [Ca2+]i and microvessel Lp (42). To study the mechanisms of H2O2-induced increases in microvessel permeability, we further evaluated the changes in Lp and EC [Ca2+]i in response to a lower level of H2O2 (10 μM).
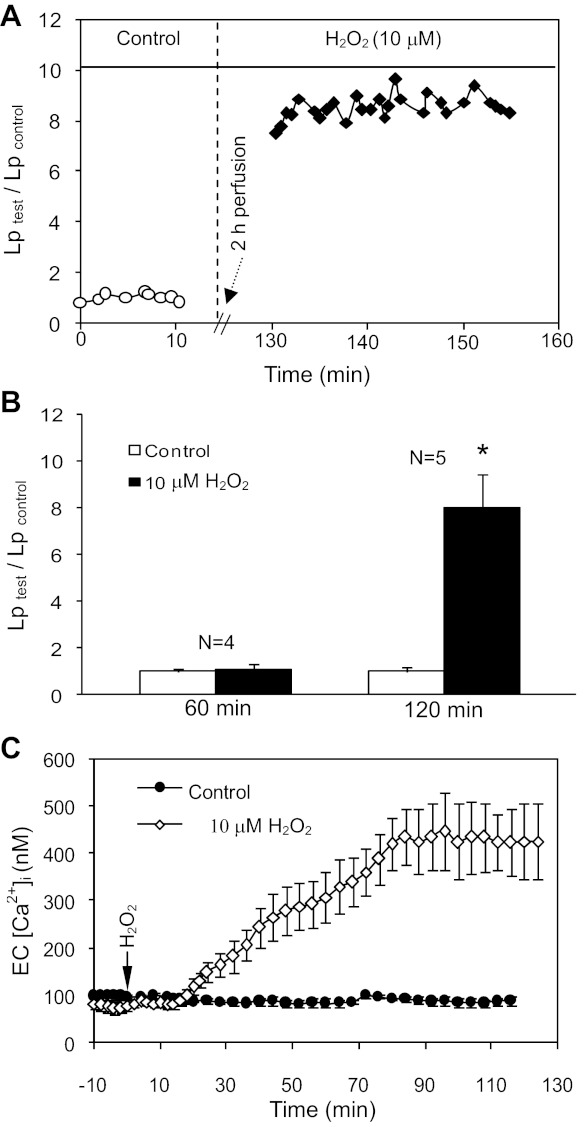
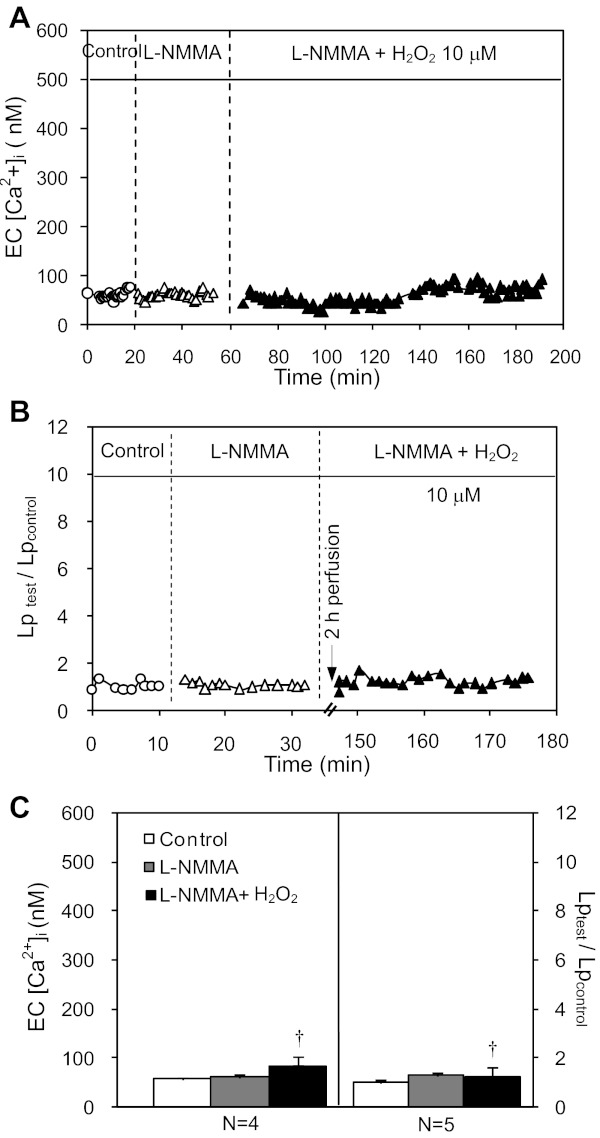
Lp was measured in 12 vessels. The mean baseline Lp was 1.9 ± 0.2 × 10−7 cm·s−1·cmH2O−1. Unlike the results with higher concentrations of H2O2 (100 and 500 μM) that increased Lp at 1- and 0.5-h perfusion, respectively (42), 10 μM of H2O2 did not increase Lp up to 1-h perfusion (N = 7, P > 0.05), but significantly increased Lp to 8.0 ± 0.87 times the control value at 2-h perfusion (N = 5, P < 0.01). Figure 1A shows the Lp measurements in one individual experiment, and the summarized results are presented in Fig. 1B.
Fig. 1.
H2O2 induces delayed and progressive increases in microvessel hydraulic conductivity (Lp) and EC intracellular calcium concentration ([Ca2+]i). A: representative Lp measurements from one individual experiment after 2-h perfusion with H2O2 (10 μM). B: summarized Lp data showing the time-dependent Lp response to H2O2. *P < 0.05, significant increase from control. C: the pooled time course of changes in EC [Ca2+]i in H2O2 (N = 5) or albumin-Ringer solution (N = 3) perfused vessels.
EC [Ca2+] was measured in five vessels. The mean baseline EC [Ca2+]i was 74 ± 5.5 nM. EC [Ca2+]i did not increase up to 18 ± 5.5 min of H2O2 perfusion and then gradually increased to a plateau level of 417 ± 45.0 nM at 92 ± 3.1 min of H2O2 perfusion. Control experiments showed no increases in EC [Ca2+]i during a 2-h period of albumin-Ringer perfusion (N = 3). Figure 1C shows the pooled time course of changes in EC [Ca2+]i in H2O2 or albumin-Ringer perfused vessels.
H2O2 induces Ca2+ influx dependent and independent NO production in the ECs of intact venules.
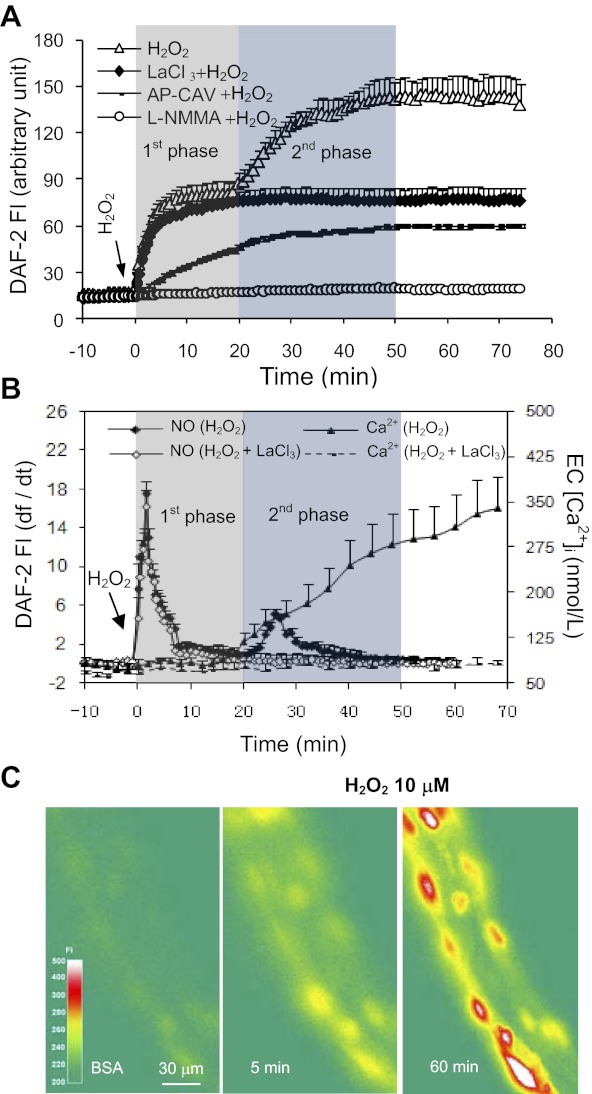
The effect of H2O2 (10 μM) on NO production in the ECs was investigated in five vessels. The mean basal NO production rate was 0.1 ± 0.01 AU/min. Perfusion of H2O2 induced an immediate increase in NO production. The initial peak rate reached 17.5 ± 1.34 AU/min within 2 min and fell to 0.8 ± 0.56 AU/min at 20 ± 2.3 min of H2O2 perfusion (Fig. 2B). The net increase in FIDAF (ΔFIDAF) was 68 ± 8.3 AU. With continuous H2O2 perfusion, a second peak of NO production occurred at ∼26 min with a peak rate at 5.2 ± 0.60 AU/min and fell to 0.12 ± 0.06 AU/min at 50 min of H2O2 perfusion (Fig. 2B). The second phase ΔFIDAF was 54 ± 4.1 AU. Representative images are presented in Fig. 2C.
Fig. 2.
H2O2 (10 μM) induces Ca2+ influx-independent and -dependent endothelial NO production. A: pooled cumulative FI curve of DAF-2 upon H2O2 perfusion in the absence (N = 5) or presence of LaCl3 (N = 4), AP-CAV (N = 4), and NG-monomethyl-l-arginine (l-NMMA) (N = 4). B: the time course of NO production rate (df/dt, left Y-axis) superimposed with the changes in EC [Ca2+]i (right Y-axis) in H2O2-perfused vessels in the absence or presence of LaCl3 (pooled data). The shaded areas represent the two phases of NO production. C: representative DAF-2 fluorescence images from one individual experiment, showing the changes in FIDAF over time after H2O2 perfusion. The color scale shows the FIDAF in arbitrary unit.
By comparing H2O2-induced NO production with the time course of increases in EC [Ca2+]i, we found that the initial increase in NO production occurred in the absence of increased EC [Ca2+]i, and only the second phase of NO production was correlated with the delayed increases in EC [Ca2+]i (Fig. 2B). We then examined the role of Ca2+ influx in H2O2-induced NO production. We first confirmed that the preperfusion of lanthanum chloride (LaCl3, 50 μM, a nonselective divalent cationic channel blocker on cell membrane) for 20 min blocked the H2O2-induced increases in EC [Ca2+]i from 417 ± 45.0 to 95 ± 7.2 nM (N = 4, P < 0.05, Fig. 2B). Under the same experimental conditions, we measured the H2O2-induced NO production. LaCl3 did not affect the H2O2-induced initial NO production, but blocked the second NO increase (N = 4, Fig. 2, A and B), indicating that the H2O2-induced initial NO did not require increased Ca2+ influx, but the second phase of NO production was initiated by the increased EC [Ca2+]i.
To examine whether the H2O2-induced NO production is a result of NOS activation, NO was measured when each vessel was perfused with NG-monomethyl-l-arginine (l-NMMA) (2 mM) and H2O2 (N = 4). Preperfusion of vessels with l-NMMA for 30 min completely abolished H2O2-induced NO production (Fig. 2A).
Roles of Ca2+/calmodulin, serine phosphorylation, and threonine dephosphorylation in H2O2-induced eNOS activation and NO production.
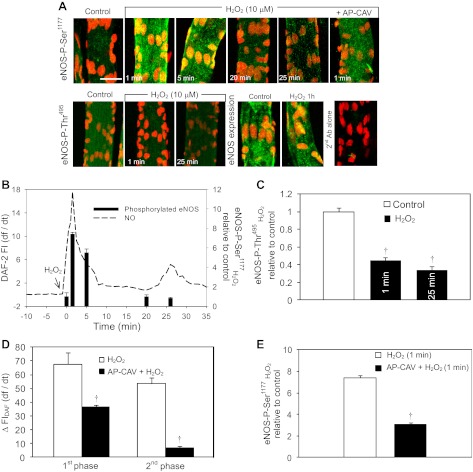
Both the binding of Ca2+/calmodulin (CaM) complex to eNOS and the phosphorylation modification of eNOS are demonstrated to regulate eNOS activities (9). To understand the mechanisms of H2O2-induced eNOS activation, we first examined the roles of serine phosphorylation at 1177 (Ser1177) and threonine dephosphorylation at 495 (Thr495) in H2O2-induced eNOS activation. Using immunostaining and confocal imaging, we observed basal levels of eNOS phosphorylation at Ser1177 and Thr495 in albumin-Ringer perfused vessels (Fig. 3A, N = 3 per group). Upon H2O2 perfusion, the eNOS phosphorylation at Ser1177 increased to 7.4 ± 0.21 times the basal value at 1 min of H2O2 perfusion, and declined to 5.5 ± 0.40, 1.0 ± 0.20, and 0.8 ± 0.10 times that of the control at 5, 20, and 25 min of H2O2 perfusion, respectively (Fig. 3, A and B; N = 3 per group). The expression of eNOS did not change before and after H2O2 perfusion for 1 h (Fig. 3A). The transient increases in eNOS-Ser1177 phosphorylation was found to be closely correlated with the time course of H2O2-induced initial NO production, which occurred in the absence of increased EC [Ca2+]i. However, no increased Ser1177 phosphorylation was observed in the second phase of NO production (Fig. 3B) that depended on the increased EC [Ca2+]i (Fig. 2, A and B). In contrast, the eNOS-Thr495 phosphorylation significantly decreased to 0.4 ± 0.03 and 0.3 ± 0.04 times of the baseline level at 1 and 25 min of H2O2 perfusion, respectively (Fig. 3, A and C, P < 0.05, N = 3 per group), indicating the eNOS-Thr495 dephosphorylation was associated with the peak rate of H2O2-induced NO production in both phases.
Fig. 3.
H2O2-induced changes in endothelial nitric oxide synthase (eNOS) phosphorylation at Ser1177 and Thr495 and the roles of Ca2+/CaM in H2O2-induced eNOS activation. A: representative confocal images of fluorescent immunostaining of eNOS, phosphorylated eNOS at Ser1177 (top panel) and Thr495 (bottom panel) in control (albumin-Ringer solution) and H2O2 perfused vessels. The time labeled on each image represents the duration of H2O2 perfusion. The fluorescence intensity quantifications of each group of images are shown in B–E. The eNOS staining showed no significant change in eNOS expression before and after H2O2 perfusion. The last image (far right of bottom panel) is the negative control with second antibody alone. The nuclei were stained with DRAQ5 (red). Each image is the projection of the lower half of the vessel and represents the pattern of multiple segments of three vessels. The scale bar represents 30 μm. B: temporal correlations between the changes in NO production rate (left Y-axis) and eNOS phosphorylation at Ser1177 (right Y-axis). A transient increase in Ser1177 phosphorylation is correlated with the initial NO production with the peak at 1 min of H2O2 perfusion and then declined with time. No increase in Ser1177 phosphorylation occurred at the second phase of NO production (25 min of H2O2 perfusion). C: changes in eNOS phosphorylation at Thr495. Thr495 phosphorylation showed a modest decrease from the control level at the initial peak NO (1 min of H2O2 perfusion) and a more significant decrease at the second phase of NO (25 min of H2O2 exposure). D: effects of AP-CAV (10 μM) on H2O2-induced NO production (N = 3). Preperfusion of vessels with AP-CAV attenuated the H2O2-induced initial NO and abolished the second phase of NO production. E: effect of AP-CAV on H2O2-induced eNOS phosphorylation at Ser1177 (N = 3 per group). AP-CAV attenuated the H2O2-induced initial increase in Ser1177 phosphorylation (a representative image is shown in A, far right top panel). †P < 0.05, significant decrease from control (C); significant decrease from H2O2 responses (D and E); N = 3 per group.
The role of Ca2+/CaM in H2O2-induced NO production was examined using a CaM-binding antagonist, the scaffolding domain of caveolin-1 (AP-CAV) (39). The mean basal NO production rate of three vessels was 0.14 ± 0.02 AU/min. Perfusion of AP-CAV (10 μM) for 30 min attenuated the H2O2-induced initial NO production from 68 ± 8.3 to 37 ± 1.2 AU (ΔFIDAF) and completely blocked the second phase of NO production (Figs. 2A and 3D). Under the same experimental conditions, eNOS-Ser1177 phosphorylation at 1 min of H2O2 perfusion was reduced from 7.4 ± 0.21 to 3.1 ± 0.18 times that of the control (N = 3, Fig. 3D).
H2O2 induces caspase 3/7 activation and vascular cell apoptosis in intact venules.
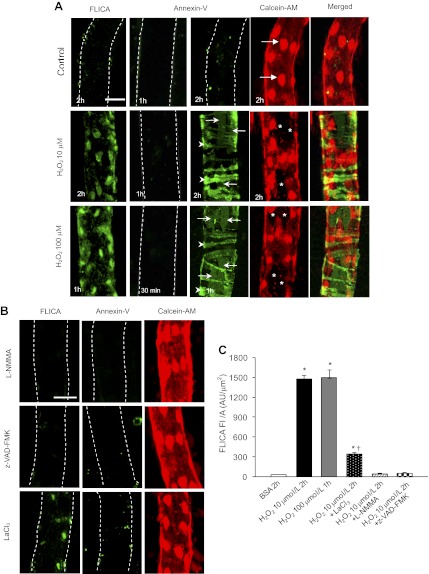
We examined cell apoptosis in H2O2 (10, 100 μM)-perfused vessels using FLICA, an indicator for activated caspase 3/7, and Alexa488-Annexin-V to label the externalized phosphatidylserine (PS). Control experiments were conducted in nine vessels. The perfusion of albumin-Ringer solution for 2 h showed negative staining for either FLICA or Annexin-V, and all ECs were well labeled with Calcein-AM (live-cell dye), suggesting that the cells were intact and viable (Fig. 4A, top panel). The effect of H2O2 at 10 μM on vascular cell apoptosis was investigated in 11 vessels. Annexin-V staining remained negative after 1 h of H2O2 perfusion (N = 3). After 2 h of H2O2 perfusion, the majority of pericytes (arrowheads) and 25 ± 5.1% of the ECs showed positive Annexin-V staining (arrows, Fig. 4A, middle panel, N = 4). Calcein-AM and Annexin-V double staining showed that ECs with positive Annexin-V staining cannot retain the live-cell dye and were negative for Calcein-AM staining (Fig. 4A, middle panel). Over the same time course, we observed an extensive FLICA positive staining in the vascular cells (Fig. 4A, middle panel, N = 4). The total FLICA FI per unit surface area of the vessel wall was 1,502 ± 116.8 AU/μm2 (Fig. 4C).
Fig. 4.
H2O2-induced vascular cell apoptosis in the absence or presence of l-NMMA, z-VAD-FMK, and LaCl3. A: representative confocal images of FLICA, Alexa488-Annexin-V, Calcein-AM, and Alexa488-Annexin-V with Calcein-AM double staining in each individual experiment. Vessels were perfused with 1% albumin-Ringer solution (first row), H2O2 at 10 (second row) and 100 μM (third row) for different time periods as shown on the lower left of each image. Arrows indicate the endothelial cells (ECs) and arrowheads indicate pericytes. *ECs not stained with Calcein-AM. B: inhibitory effect of l-NMMA , z-VAD-FMK, and LaCl3 on H2O2-induced vascular cell apoptosis (N = 6 per group). After preperfusion of l-NMMA, z-VAD-FMK, or LaCl3 for 30, 30, and 20 min, respectively, each vessel was perfused with H2O2 (10 μM) in the presence of each compound. The vessel was double-stained by FLICA (first column) or Annexin-V (second column) with Calcein-AM (third column). Each confocal image is the projection of the bottom half of the vessel. The scale bar represents 30 μm. The dotted line outlines the vascular wall. C: summary of the FLICA staining quantifications in each group. *P < 0.05, significant increase from control (BSA). †P < 0.05, significant decrease from the response to H2O2 treatment alone.
We also investigated the effect of a higher concentration of H2O2 (100 μM) on vascular cell apoptosis in 11 vessels. H2O2 at 100 μM induced a similar pattern of vascular cell apoptosis to that observed with 10 μM of H2O2 perfusion, but occurred in a shorter exposure time. After only 1-h perfusion of 100 μM of H2O2, the majority of pericytes and 30 ± 6.1% of the ECs showed positive staining of Annexin-V (Fig. 4A, bottom panel, N = 4 per group). Meanwhile, the vascular cells showed increases in FLICA staining with magnitude similar to that observed in vessels perfused with 10 μM of H2O2 for 2 h (Fig. 4, A and C).
H2O2-induced NO production contributes to vascular cell apoptosis, EC [Ca2+]i accumulation, and increases in Lp.
To elucidate the relationship between H2O2-induced NO production and cell apoptosis, we investigated the effect of l-NMMA on H2O2-induced vascular cell apoptosis. Blockade of H2O2-induced NO production (Fig. 2A) by l-NMMA prevented caspase 3/7 activation and PS externalization (Fig. 4B, top panel, and Fig. 4C; N = 3 per group). The causal relationship between H2O2-induced NO production and increases in EC [Ca2+]i and Lp was further examined in nine vessels. Without affecting the baseline EC [Ca2+]i and Lp, l-NMMA completely prevented H2O2-induced delayed increases in EC [Ca2+]i (N = 4) and Lp (N = 5). Figure 5, A and B, shows the results of two individual experiments. The summarized data are presented in Fig. 5C.
Fig. 5.
l-NMMA prevents H2O2-induced delayed increases in EC [Ca2+]i and Lp. A and B: H2O2-induced changes in Lp and EC [Ca2+]i in the presence of l-NMMA in 2 individual experiments. The application of l-NMMA prevented H2O2-induced increases in EC [Ca2+]i and Lp. C: summary of the Lp (N = 5) and EC [Ca2+]i (N = 4) results. †P < 0.05, significant decrease from the Lp and EC [Ca2+]i responses to H2O2 in the absence of l-NMMA.
Activation of caspase cascade downstream from H2O2-induced initial NO contributes to EC Ca2+ accumulation, cell apoptosis, and progressively increased Lp.
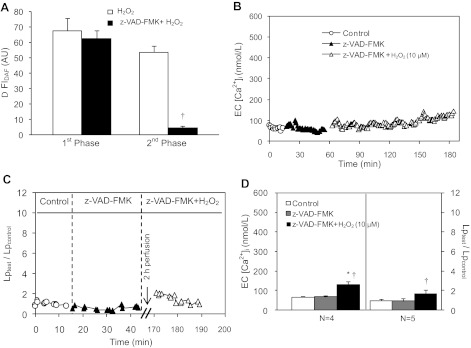
Our abovementioned results demonstrate that caspase 3/7 activation in H2O2-perfused vessels is dependent on H2O2-induced NO production. To elucidate the interrelationship between H2O2-induced NO production and caspase activation, we measured H2O2 (10 μM)-induced NO production in the presence of caspase inhibitor, z-VAD-FMK, in three vessels. Perfusion of z-VAD-FMK (20 μM) for 30 min did not affect the initial NO production (ΔFIDAF = 63 ± 5.2 vs. 68 ± 8.3 AU, P > 0.05), but prevented the NO production at the late phase (Fig. 6A). As the late-phase NO production is dependent on the delayed increases in EC [Ca2+]i (Fig. 2B), we further examined the direct effect of z-VAD-FMK on H2O2-induced increases in EC [Ca2+]i in four vessels. Without affecting the baseline EC [Ca2+]i, z-VAD-FMK completely blocked H2O2 (10 μM)-induced increases in EC [Ca2+]i during the first 90 min of H2O2 perfusion (Fig. 6B). At the end of 2-h H2O2 perfusion, EC [Ca2+]i was only 133 ± 18.4 nM, a significant reduction from 417 ± 45.0 nM that occurred in the absence of z-VAD-FMK (Fig. 6D).
Fig. 6.
Effects of Z-VAD-FMK on H2O2-induced NO production, EC Ca2+ accumulation, and increases in Lp. A: z-VAD-FMK has no effect on H2O2-induced initial NO production, but prevents the second phase of NO production (N = 3). †P < 0.05, significant decrease from responses to H2O2 treatment alone. B and C: individual experiments show that z-VAD-FMK prevents the H2O2-induced delayed increases in EC [Ca2+]i and Lp. D: summarized EC [Ca2+]i (N = 4) and Lp results (N = 5). *P < 0.05, significant increase from control. †P < 0.05, significant decrease from the responses to H2O2 treatment alone.
Under the same experimental conditions, the effect of z-VAD-FMK on H2O2 (10 μM)-induced cell apoptosis and Lp increase was investigated in 11 vessels. Perfusing vessels with z-VAD-FMK prevented H2O2-induced caspase 3/7 activation, PS externalization (Fig. 4B, middle panel, N = 3 per group), and increases in Lp (Lptest/Lpcontrol=1.7 ± 0.41, N = 5). Figure 6C shows the Lp result from one individual experiment, and Fig. 6D plots the summarized data. These results suggest that H2O2-induced initial NO production induces caspase 3/7 activation, which promotes delayed Ca2+ influx and initiates Ca2+-dependent second phase of NO production, resulting in vascular cell apoptosis and delayed increases in microvessel permeability.
H2O2-induced EC Ca2+ accumulation is the direct cause of cell apoptosis and increases in microvessel Lp.
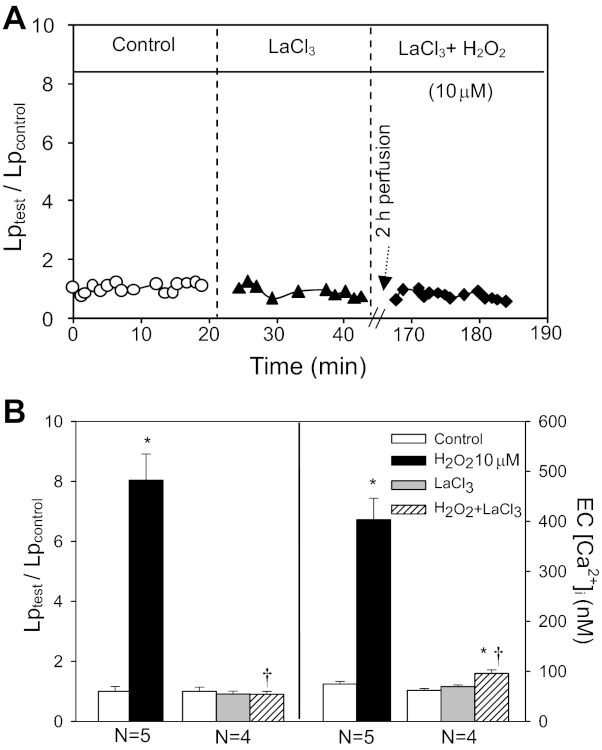
Loss of intracellular Ca2+ homeostasis plays important roles in cell apoptosis. We examined the role of H2O2-induced progressively increased EC [Ca2+]i in cell apoptosis and Lp increase in 10 vessels using LaCl3. Blocking Ca2+ influx by LaCl3 significantly attenuated H2O2 (10 μM)-induced caspase 3/7 activation. The FI of FLICA per unit surface area of vessel wall decreased from 1,502 ± 116.8 to 351 ± 62.2 AU/μm2 after 2-h perfusion of H2O2 (Fig. 4, B and C, N = 3). LaCl3 also blocked H2O2-induced PS exposure (Fig. 4B, N = 3) and the delayed increases in Lp (mean Lptest/Lpcontrol=0.9 ± 0.09, N = 4). Figure 7A shows a representative Lp response to H2O2 in the presence of LaCl3 from one individual experiment. Figure 7B summarizes the Lp and EC [Ca2+]i data.
Fig. 7.
LaCl3 abolishes H2O2-induced endothelial Ca2+ accumulation and Lp increases. A: an individual experiment of Lp measurements in the presence of LaCl3 (50 μM). B: summarized EC [Ca2+]i and Lp results (N = 4 per group). *P < 0.05, significant increase from control. †P < 0.05, significant decrease from H2O2-induced response.
DISCUSSION
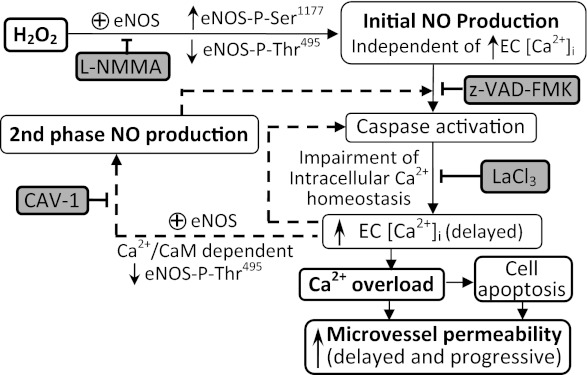
Our study, using combined quantitative permeability measurements with fluorescence imaging and confocal microscopy, demonstrated cellular and molecular mechanisms of H2O2-induced increases in microvessel permeability in intact venules. H2O2 at 10 μM, a concentration close to the plasma level under human disease conditions (21), induces an immediate and large-magnitude NO production in ECs of intact venules in the absence of an initial increase in EC [Ca2+]i. This large amount of NO, instead of causing an immediate increase in microvessel permeability that occurred in PAF-stimulated microvessels (39, 43), results in caspase activation and intracellular Ca2+ accumulation, thus leading to vascular cell apoptosis and progressively increased microvessel permeability. Our results provide the first in vivo evidence that H2O2-induced eNOS activation in intact venules involves sequential Ca-independent and Ca-dependent mechanisms and delineate the interrelationship between increased EC [Ca2+]i, CaM binding with eNOS, and eNOS phosphorylation at Ser1177 and Thr495 in H2O2-induced NO production in intact microvessels. Most importantly, we provide a time-dependent correlation of the involved signaling pathways with eNOS activity and changes in microvessel permeability. To our knowledge, this information has not previously been reported in either in vitro or in vivo studies. To translate the remarkable knowledge gained from in vitro studies to clinical therapeutic applications, it is essential to evaluate the cellular and molecular mechanisms in their native state. Multidimensional protein identification revealed that forty-one percent of proteins expressed in vivo are not detected in vitro, indicating that distinct protein expression is apparently regulated by the tissue microenvironment that cannot yet be duplicated in standard cell culture (8). Therefore, investigating the cellular and molecular mechanisms in intact microvessels within an in vivo environment is necessary and important. The mechanisms revealed from this study may provide new insights into the pathogenesis of elevated H2O2-involved cardiovascular diseases. A diagram in Fig. 8 summarizes details of the study.
Fig. 8.
Schematic diagram demonstrating the underlying mechanisms by which H2O2 induces eNOS activation and increases in microvessel permeability. H2O2, at near pathological concentration, induces two phases of eNOS activation in ECs of intact venules. The initial eNOS activation is independent of increased EC [Ca2+]i but is associated with increased eNOS phosphorylation at Ser1177 and decreased phosphorylation at Thr495. This initial NO production leads to the activation of caspase cascade, which in turn disrupts the intracellular Ca2+ homeostasis, causing delayed Ca2+ accumulation, vascular cell apoptosis, and increases in microvessel permeability. Blockade of NO production by l-NMMA inhibits all the downstream events. Of note, the delayed increase in EC [Ca2+]i also triggers a second pulse of elevated EC [Ca2+]i-dependent eNOS activation which is associated with a decrease in eNOS Thr495 phosphorylation and an increase in CaM binding. CAV-1, a CaM binding antagonist, abolishes the second NO production. Caspase activation is further amplified by the delayed increases in EC [Ca2+]i and the second NO production through positive feedback, leading to Ca2+ overload and cell apoptosis. Dashed lines indicate the positive-feedback loop. Each inhibitor listed in the shaded box can effectively block the downstream events.
Although H2O2 has been reported to induce NO production in large arteries and play a role in vasomotion, whether it induces NO production in intact venules had not been investigated previously. Our previous studies have demonstrated that inflammatory mediator-induced NO depends on increased EC [Ca2+]i and the magnitude of NO is directly correlated with the peak of transiently increased microvessel permeability (13, 39, 41, 43). In the present study, we demonstrated a different role of EC NO in the regulation of microvessel permeability in intact venules. We found that, even in the absence of an initial increase in EC [Ca2+]i, the magnitude of H2O2-induced initial NO production (ΔFIDAF-2 = 68 ± 8.3 AU) was 5 times more than that produced in PAF-perfused vessels (ΔFIDAF-2 = 12.4 ± 0.71 AU) (40). Most interestingly, this large amount of NO did not cause an immediate and transient permeability increase as that observed in PAF-perfused vessels (39). Instead, it caused a delayed and progressively increased microvessel permeability. These results suggest that the role of NO in mediating permeability increases might depend on the concurrent activations of other signaling pathways, and therefore, the involved cellular mechanisms and the patterns of increased permeability can be stimulus specific. The H2O2-induced time- and dose-dependent permeability increases suggest that the effect of H2O2 on endothelial cells is cumulative.
Recent studies demonstrate that eNOS activation under oxidative stress produces superoxide rather than NO due to S-glutathionylation of eNOS (5), or the deficiency of l-arginine and tetrahydrobioterin (H4B) (4, 35). This phenomenon has been referred to as “eNOS uncoupling” and contributes to decreased NO bioavailability in various cardiovascular diseases (4, 15, 35). Our NO measurements clearly demonstrated that an excessive amount of NO was produced by eNOS when the intact venule was exposed to H2O2, which do not support ROS-mediated eNOS uncoupling, at least, during a short-term exposure and before vascular cells undergo apoptosis.
Calmodulin (CaM) binding to eNOS, and the changes in eNOS phosphorylation at different residues, particularly at Ser1177 and Thr495 (human and rat sequence), have all been demonstrated to enhance eNOS activity (9). However, their interrelationship in the regulation of eNOS activity has not been well understood, especially in intact microvessels. Studies on purified proteins reported that phosphorylation of eNOS at Ser1177 enhances eNOS activity by reducing CaM dissociation from activated eNOS when calcium levels are low (23). On the other hand, CaM binding has been proposed to induce a conformational change in the eNOS, which increases Ser1177 phosphorylation (24). Studies on cultured ECs reported that eNOS-Ser1177 phosphorylation can occur in the absence or presence of increased EC [Ca2+]i (10), but the dephosphorylation of Thr495 and CaM binding to eNOS are dependent on elevated [Ca2+]i (11). Short-term exposure of cultured ECs to H2O2 (within one h at 50–500 μM) has been shown to cause eNOS activation and increased NO production (3, 18, 33), but the underlying mechanisms, especially with regard to the interplay between Ca2+/CaM binding to eNOS and eNOS phosphorylation at Ser1177 and Thr495 in H2O2-induced eNOS activation, has not been clearly demonstrated. Our study provided the first evidence in intact microvessels that H2O2 (10 μM) induces two phases of NO production, and that each phase of increased NO involves different mechanisms of eNOS activation. The large magnitude of H2O2-induced initial NO production, that was associated with increased eNOS Ser1177 phosphorylation without elevated EC [Ca2+]i, demonstrates that Ser1177 phosphorylation in the absence of increased EC [Ca2+]i is able to stimulate a large increase in eNOS activity. In contrast, the H2O2-induced second phase of NO production was found to be associated with elevated EC [Ca2+]i-dependent Thr495 dephosphorylation and Ca2+/CaM binding to eNOS without increases in Ser1177 phosphorylation. Dephosphorylation of Thr495 has been reported to enhance the CaM binding to eNOS and to completely depend on elevated Ca2+ in agonist-stimulated NO production in cultured ECs (11). Caveolin-1 has been demonstrated to specifically interact with eNOS to inhibit CaM binding (12, 19). The use of AP-CAV, a fusion peptide that contains caveolin-1 scaffolding domain, in this study allows us to further elucidate the role of Ca2+/CaM in H2O2-induced eNOS activation and NO production. Our previous study demonstrated that this peptide competes with increased EC [Ca2+]i, inhibiting PAF-induced Ca2+/CaM-dependent eNOS activation and microvessel permeability in intact venules (39). Both the decreased phosphorylation of Thr495 at the peak rate of the second phase of NO production, and a complete inhibition of the second phase of NO by AP-CAV, support that Ca2+/CaM-mediated eNOS activation is responsible for the second phase of H2O2-induced NO. These observations also indicate that the increased EC [Ca2+]i-initiated CaM binding to eNOS with Thr495 dephosphorylation is sufficient to increase eNOS activity in the absence of elevated eNOS phosphorylation at Ser1177. Although Thr495 dephosphorylation and CaM binding to eNOS have been recognized as increased EC Ca2+ dependent, a modest decrease in Thr495 phosphorylation concomitant with the increased eNOS Ser1177 phosphorylation was found at H2O2-induced initial NO production that occurred in the absence of increased EC [Ca2+]i. Moreover, the application of AP-CAV that promotes the CaM dissociation from eNOS attenuated H2O2-induced eNOS Ser1177 phosphorylation and decreased the initial NO production. Taken together, these results indicate that Thr495 dephosphorylation and CaM/eNOS binding can occur at basal levels of EC [Ca2+]i and play an important role in eNOS Ser1177 phosphorylation and increased eNOS activity. Another potential mechanism is that Ser1177 phosphorylation may prevent the CaM dissociation from activated eNOS at lower Ca2+ conditions as that proposed in purified protein studies (23).
In vitro studies suggest that high levels of NO may cause endothelial cell apoptosis (29, 34). Currently, cell apoptosis has been mainly detected in cultured cells, cross-sections of embedded tissue, and homogenized cell lysate. In the present study, we combined fluorescent confocal imaging with single vessel perfusion techniques, enabling us to detect cell apoptosis with both temporal and spatial resolution in the vascular wall, and thus allowed cell signaling and structural changes to be directly linked to the changes in vascular functions. Using this approach, we found that H2O2 perfusion induced caspase 3/7 activation and PS translocation in both pericytes and ECs, and that these changes were prevented by blocking H2O2-induced NO production by l-NMMA (Fig. 4B). These data provided in vivo evidence that H2O2-induced excessive NO production can lead to vascular cell apoptosis. Furthermore, we identified that more pericytes underwent apoptosis than ECs at the same H2O2 exposure time (Fig. 4A). Our identification of pericytes was based on cell morphology. Pericytes on the venules have multiple cellular processes that wrap around the vascular wall (Fig. 4A and Supplemental video, available with the online version of this article), which are distinct from ECs and those tightly wrapped smooth muscle cells in the arterioles (26). As endothelium is the first layer exposed to H2O2 through perfusion, the EC accessibility to H2O2 should not be a problem. Therefore, our results suggest that pericytes are more susceptible to H2O2-mediated cell apoptosis than ECs. This may explain the early loss of microvascular pericytes and aneurism formation in diabetic retinopathy, in which the increased H2O2 level has been proposed to play a role (31).
Vascular cell apoptosis has been implicated in the pathogenesis of many cardiovascular diseases (32). However, the relationship between vascular cell apoptosis and microvessel barrier functions remains undefined. In this study, we found that H2O2 (10 or 100 μM)-induced apoptosis in ECs and pericytes occurred at the same time when we observed significant increases in microvessel Lp. Moreover, preventing cell apoptosis by inhibition of caspase activation or blockade of Ca2+ influx blocked H2O2-induced increases in microvessel Lp. These results strongly suggest that H2O2-induced vascular cell apoptosis is responsible for the delayed and progressive increases in microvessel permeability. ECs serve as the main barrier for transport functions of the microvessels. There is no doubt that EC apoptosis contributes significantly to the impairment of vascular barrier function, whereas the role of pericytes in the regulation of microvessel permeability remains to be determined. Our recent study conducted in inflammation-induced remodeled microvessels demonstrated that the extended pericyte processes provide a complete coverage of endothelial gaps at PAF-induced peak increase in permeability, serving as an additional barrier that lessens the degree of vascular leakage and protects the structural integrity of the vascular wall (37). Based on these findings, we predict that microvessels with apoptotic pericytes may lose the protective function of pericytes when the endothelial barrier is impaired, making them susceptible to augmented permeability increases in response to stimuli.
Given that the blockade of H2O2-induced NO production by l-NMMA prevented caspase 3/7 activation, EC Ca2+ accumulation, and cell apoptosis, the results support that H2O2-induced initial NO production is responsible for the activation of caspase cascade and the subsequent events. The formation of peroxynitrite by NO and superoxide may play a role in caspase activation through opening of the mitochondrial permeability transition pore (34). The inhibition of caspase that did not affect the H2O2-induced initial NO production, but blocked Ca2+ accumulation and cell apoptosis, indicates a causal relationship between caspase activation and the impairment of Ca2+ homeostasis and Ca2+ overload-induced cell apoptosis. As for how the caspase activation resulted in elevation of EC [Ca2+]i, different mechanisms have been proposed under different experimental conditions (17, 25, 27, 28). Caspase activation may selectively cleave a restricted set of target proteins (16). Cleavage of the plasma membrane calcium pump, which impairs Ca2+ extrusion, was reported to be associated with neuronal death (28). On the other hand, the activation of cation channels, which increases Ca2+ influx, was found to be associated with oxidative stress-induced intracellular Ca2+ overload and cell death (17, 25, 27). Though detailed mechanisms remain to be elucidated, our results that caspase inhibition prevented H2O2-induced cell apoptosis and the increases in microvessel permeability indicate that inhibition of caspases might serve as a therapeutic target in oxidative stress-involved vascular dysfunction in cardiovascular diseases.
Our results also showed that not only caspase activation results in increases in EC [Ca2+]i, but that the increased [Ca2+] can also further activate caspases, because blocking Ca2+ influx by LaCl3 significantly attenuated H2O2-induced caspase activation. These observations suggest that the H2O2-induced, NO-mediated intracellular Ca2+ overload and cell apoptosis can be augmented through a positive feedback. The attenuated caspase activation by LaCl3 can also be attributed to the inhibition of Ca2+-dependent late-phase NO production through the same positive-feedback loop. Although both caspase activation and intracellular Ca2+ accumulation are essential events in H2O2-induced vascular cell apoptosis, our results suggest that the intracellular Ca2+ overload is the key step, because inhibition of Ca2+ influx by LaCl3 sufficiently prevents H2O2-induced cell apoptosis and permeability increase, even in the presence of activated caspases.
In conclusion, our study has demonstrated that H2O2 induces a large magnitude of NO production in the ECs of intact venules through coordinated actions of CaM binding to eNOS and the changes in eNOS phosphorylation at residues Ser1177 and Thr495 in the absence and presence of increased EC [Ca2+]i. Instead of causing immediate permeability increases, this large amount of NO activates caspase cascades, resulting in the impairment of intracellular Ca2+ homeostasis, vascular cell apoptosis, and delayed and progressive increases in microvessel permeability. The signaling cascade and the sequential events found in H2O2-perfused intact microvessels as illustrated in Fig. 8 might contribute to a better understanding of the pathogenesis of microvascular dysfunction and benefit the development of targeted therapeutics in ROS-related cardiovascular diseases.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-56237 and HL-084338 to P. He, and by a predoctoral fellowship from the American Heart Association [10PRE3050008 to X. P. Zhou].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.Z. and P.H. conception and design of research; X.Z., D.Y., and M.W. performed experiments; X.Z., D.Y., M.W., and P.H. analyzed data; X.Z., D.Y., and P.H. interpreted results of experiments; X.Z., D.Y., M.W., and P.H. prepared figures; X.Z. and P.H. drafted manuscript; X.Z. and P.H. edited and revised manuscript; X.Z. and P.H. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Banerjee D, Madhusoodanan UK, Nayak S, Jacob J. Urinary hydrogen peroxide: a probable marker of oxidative stress in malignancy. Clin Chim Acta 334: 205–209, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Cai H, Li Z, Davis ME, Kanner W, Harrison DG, Dudley SC., Jr Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol Pharmacol 63: 325–331, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci USA 102: 9056–9061, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468: 1115–1118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curry FE, Huxley VH, Sarelius IH. Techniques in microcirculation: measurement of permeability, pressure and flow. In: Cardiovascular Physiology. Techniques in the Life Sciences. New York:Elsevier, 1983, p. 1–34 [Google Scholar]
- 7.Del Maestro RF, Bjork J, Arfors KE. Increase in microvascular permeability induced by enzymatically generated free radicals. II. Role of superoxide anion radical, hydrogen peroxide, and hydroxyl radical. Microvasc Res 22: 255–270, 1981 [DOI] [PubMed] [Google Scholar]
- 8.Durr E, Yu J, Krasinska KM, Carver LA, Yates JR, Testa JE, Oh P, Schnitzer JE. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol 22: 985–992, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 284: R1–R12, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Fleming I, Busse R. Signal transduction of eNOS activation. Cardiovasc Res 43: 532–541, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: E68–E75, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, Gachhui R, Crooks C, Wu C, Lisanti MP, Stuehr DJ. Interaction between caveolin-1 and the reductase domain of endothelial nitric-oxide synthase. Consequences for catalysis. J Biol Chem 273: 22267–22271, 1998 [DOI] [PubMed] [Google Scholar]
- 13.He P, Liu B, Curry FE. Effect of nitric oxide synthase inhibitors on endothelial [Ca2+]i and microvessel permeability. Am J Physiol Heart Circ Physiol 272: H176–H185, 1997 [DOI] [PubMed] [Google Scholar]
- 14.He P, Zhang X, Curry FE. Ca2+ entry through conductive pathway modulates receptor-mediated increase in microvessel permeability. Am J Physiol Heart Circ Physiol 271: H2377–H2387, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Heinzel B, John M, Klatt P, Bohme E, Mayer B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J 281: 627–630, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hengartner MO. The biochemistry of apoptosis. Nature 407: 770–776, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Herson PS, Lee K, Pinnock RD, Hughes J, Ashford ML. Hydrogen peroxide induces intracellular calcium overload by activation of a non-selective cation channel in an insulin-secreting cell line. J Biol Chem 274: 833–841, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Hu Z, Chen J, Wei Q, Xia Y. Bidirectional actions of hydrogen peroxide on endothelial nitric-oxide synthase phosphorylation and function: co-commitment and interplay of Akt and AMPK. J Biol Chem 283: 25256–25263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem 272: 18522–18525, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Kendall S, Michel CC. The measurement of permeability in single rat venules using the red cell microperfusion technique. Exp Physiol 80: 359–372, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Lacy F, Kailasam MT, O'Connor DT, Schmid-Schonbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension 36: 878–884, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Mayhan WG. Role of nitric oxide in modulating permeability of hamster cheek pouch in response to adenosine 5′-diphosphate and bradykinin. Inflammation 16: 295–305, 1992 [DOI] [PubMed] [Google Scholar]
- 23.McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem 275: 6123–6128, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PR, Kemp BE, Pearson RB. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol 9: 845–848, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Miller BA. The role of TRP channels in oxidative stress-induced cell death. J Membr Biol 209: 31–41, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Murfee WL, Skalak TC, Peirce SM. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation 12: 151–160, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Schieven GL, Ledbetter JA. Activation of tyrosine kinase signal pathways by radiation and oxidative stress. Trends Endocrinol Metab 5: 383–388, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Schwab BL, Guerini D, Didszun C, Bano D, Ferrando-May E, Fava E, Tam J, Xu D, Xanthoudakis S, Nicholson DW, Carafoli E, Nicotera P. Cleavage of plasma membrane calcium pumps by caspases: a link between apoptosis and necrosis. Cell Death Differ 9: 818–831, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Shen YH, Wang XL, Wilcken DE. Nitric oxide induces and inhibits apoptosis through different pathways. FEBS Lett 433: 125–131, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Shimizu S, Nomoto M, Naito S, Yamamoto T, Momose K. Stimulation of nitric oxide synthase during oxidative endothelial cell injury. Biochem Pharmacol 55: 77–83, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Shojaee N, Patton WF, Hechtman HB, Shepro D. Myosin translocation in retinal pericytes during free-radical induced apoptosis. J Cell Biochem 75: 118–129, 1999 [PubMed] [Google Scholar]
- 32.Stefanec T. Endothelial apoptosis: could it have a role in the pathogenesis and treatment of disease? Chest 117: 841–854, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Thomas SR, Chen K, Keaney JF., Jr Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem 277: 6017–6024, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Walford GA, Moussignac RL, Scribner AW, Loscalzo J, Leopold JA. Hypoxia potentiates nitric oxide-mediated apoptosis in endothelial cells via peroxynitrite-induced activation of mitochondria-dependent and -independent pathways. J Biol Chem 279: 4425–4432, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem 273: 25804–25808, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Yang Z, Zhang A, Altura BT, Altura BM. Hydrogen peroxide-induced endothelium-dependent relaxation of rat aorta involvement of Ca2+ and other cellular metabolites. Gen Pharmacol 33: 325–336, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Yuan D, He P. Vascular remodeling alters adhesion protein and cytoskeleton reactions to inflammatory stimuli resulting in enhanced permeability increases in rat venules. J Appl Physiol 113: 1110–1120, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan Y, Granger HJ, Zawieja DC, DeFily DV, Chilian WM. Histamine increases venular permeability via a phospholipase C-NO synthase-guanylate cyclase cascade. Am J Physiol Heart Circ Physiol 264: H1734–H1739, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Zhou X, He P. Endothelial [Ca2+]i and caveolin-1 antagonistically regulate eNOS activity and microvessel permeability in rat venules. Cardiovasc Res 87: 340–347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X, He P. Improved measurements of intracellular nitric oxide in intact microvessels using 4,5-diaminofluorescein diacetate. Am J Physiol Heart Circ Physiol 301: H108–H114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X, He P. Temporal and spatial correlation of platelet activating factor-induced increases in endothelial [Ca2+]i, nitric oxide, and gap formation in intact venules. Am J Physiol Heart Circ Physiol 301: H1788–H1797, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X, Wen K, Yuan D, Ai L, He P. Calcium influx-dependent differential actions of superoxide and hydrogen peroxide on microvessel permeability. Am J Physiol Heart Circ Physiol 296: H1096–H1107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu L, He P. Platelet-activating factor increases endothelial [Ca2+]i and NO production in individually perfused intact microvessels. Am J Physiol Heart Circ Physiol 288: H2869–H2877, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.