Abstract
Mitochondrial dysfunction in heart failure includes greater susceptibility to mitochondrial permeability transition (MPT), which may worsen cardiac function and decrease survival. Treatment with a mixture of the n3 polyunsaturated fatty acids (n3 PUFAs) docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) is beneficial in heart failure patients and increases resistance to MPT in animal models. We assessed whether DHA and EPA have similar effects when given individually, and whether they prolong survival in heart failure. Male δ-sarcoglycan null cardiomyopathic hamsters were untreated or given either DHA, EPA, or a 1:1 mixture of DHA + EPA at 2.1% of energy intake. Treatment did not prolong survival: mean survival was 298 ± 15 days in untreated hamsters and 335 ± 17, 328 ± 14, and 311 ± 15 days with DHA, EPA, and DHA + EPA, respectively (n = 27–32/group). A subgroup of cardiomyopathic hamsters treated for 26 wk had impaired left ventricular function and increased cardiomyocyte apoptosis compared with normal hamsters, which was unaffected by n3 PUFA treatment. Evaluation of oxidative phosphorylation in isolated subsarcolemmal and interfibrillar mitochondria with substrates for complex I or II showed no effect of n3 PUFA treatment. On the other hand, interfibrillar mitochondria from cardiomyopathic hamsters were significantly more sensitive to Ca2+-induced MPT, which was completely normalized by treatment with DHA and partially corrected by EPA. In conclusion, treatment with DHA or EPA normalizes Ca2+-induced MPT in cardiomyopathic hamsters but does not prolong survival or improve cardiac function. This suggest that greater susceptibility to MPT is not a contributor to cardiac pathology and poor survival in heart failure.
Keywords: cardiomyopathy, diet, fish oil, metabolism, mitochondria
recent clinical studies suggest that a mixture of the marine n3 polyunsaturated fatty acids (n3 PUFAs) docosahexaenoic acid (DHA; 22:6n3) and eicosapentaenoic acid (EPA; 20:5n3) improves left ventricular (LV) function and clinical outcome in heart failure patients (13, 14, 33, 34). The Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico-Heart failure (GISSI-HF) trial assessed the effect of a low dose of DHA + EPA (0.84 g/day at a 45:55 ratio) in heart failure patients and found a small but significant improvement in survival compared with placebo after 3.9 yr (14). A recent dose-response study with short-term treatment with DHA + EPA in heart failure patients (33) and our work in a pressure overload model in rats (8) suggest that a higher dose (3–5 g/day) is optimal for preventing deterioration in LV function. Furthermore, the underlying mechanisms responsible for clinical improvement are not understood, nor is it clear whether clinical benefit requires treatment with both DHA and EPA.
We recently found major differences between treatment with DHA and EPA on myocardial phospholipid fatty acyl side-chain composition and mitochondrial function in rats (26, 27). There is growing evidence to support a causal role for mitochondrial dysfunction in the progression of heart failure. Heart failure induced by infarction, chronic hypertension, or genetic δ-sarcoglycan deficiency results in mitochondrial pathology as evidenced by impaired oxidative phosphorylation and ATP production and enhanced susceptibility to stress-induced mitochondrial permeability transition (MPT) (1, 11, 21, 23). MPT occurs with the formation and opening of the MPT pore and is generally considered to be a catastrophic event that collapses the proton motive force and causes mitochondrial swelling, cessation of ATP production, and efflux of matrix proteins that can trigger apoptotic pathways (16, 40, 41). We observed that treatment with either DHA or DHA + EPA, but not EPA alone, delays Ca2+-induced MPT in cardiac mitochondria from normal rats or rats with pathological cardiac hypertrophy or heart failure induced by myocardial infarction (26, 27, 36). Treatment with DHA increases both EPA and DHA in cardiac membrane phospholipids, but treatment with EPA does not increase DHA (26, 27), as there is low enzymatic capacity for EPA elongation to DHA in heart (22). Together, this suggests that the optimal formulation of marine n3 PUFAs for treatment of heart failure may be DHA, or a mixture of DHA + EPA but not EPA alone. To date, there have been no studies in animal models of heart failure to assess the effects of DHA and/or EPA on survival.
The goals of the present investigation were to determine the long-term effects of DHA and EPA given alone or as a 1:1 mixture on mitochondrial phospholipid fatty acid composition, Ca2+-induced MPT, and survival in heart failure. To avoid the confounding variables encountered in pressure overload and myocardial infarction models of heart failure, we used the δ-sarcoglycan null cardiomyopathic Bio TO2 hamster. This is a well-established rodent model of dilated cardiomyopathy in which the myocardium develops focal necrosis early on with hypertrophy of the remaining cells and clear mitochondrial dysfunction (2, 6, 21, 25, 32). This model recapitulates key aspects of human heart failure, specifically LV contractile dysfunction and chamber enlargement, neurohormonal activation, increased cardiomyocytes apoptosis, and early death (20, 29, 30, 45–47). Survival in this model is prolonged by treatment with pharmacological inhibitors of neurohormones, as observed in heart failure patients (29, 43, 45). Myocardial homogenates from cardiomyopathic Syrian hamster have a decreased capacity for mitochondrial oxidative metabolism (2, 25), which is due to a defect in interfibrillar mitochondria (IFM) (21). Furthermore, these animals have a greater susceptibility to stress-induced MPT and respond to nutritional and metabolic therapies (4, 10, 11, 21). We administered DHA and EPA at a clinically relevant dose (2.1% of energy intake; equivalent to ∼4.7 g/day in humans). Our first experimental series was a prevention protocol that assessed the ability of treatment with DHA, EPA, or DHA + EPA initiated at 6 wk of age to attenuate the development of mitochondrial and LV dysfunction and prolong survival. In a follow-up treatment protocol, we evaluated the ability of DHA to slow or reverse established heart failure by initiating treatment at 30 wk of age.
METHODS
All animal protocols were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee and conducted according to the Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health, publication no. 85-23). The animals were maintained on a 12-h:12-h light-dark cycle, and all procedures were performed between 2 and 5 h from the start of the light phase. Male δ-sarcoglycan null cardiomyopathic hamsters (Bio TO2 strain) were acquired at 5 wk of age from Bio Breeder (Watertown, MA). Healthy age-matched male F1B hamsters were used as a reference group and fed the standard diet. Animals were housed six per cage and given food and water ad libitum. Investigators were blinded to treatment when measurements were performed. Data from the F1B and T02 hamsters on the standard diet in the prevention protocol were previously reported in separate publications that evaluated the effect of high-fat and high-sugar diets (11, 19). In the present investigation, we compared data from the control groups with the n3 PUFA-treated groups. All experimental groups were contemporary and housed in the same room.
Diets
Cardiomyopathic hamsters were assigned to one of four diets (Table 1): a standard diet or diets supplemented with DHA, EPA, or combined DHA + EPA at 2.1% of energy intake. This dose of n3 PUFA is equivalent to ∼4.7 g/day in humans (assuming an energy intake of 2,000 kcal and 9 kcal/g n3 PUFA). The diets were custom manufactured using purified ingredients (Research Diets, New Brunswick, NJ), and they all contained 20% of energy from protein (casein + l-cystine), 68% of energy from carbohydrate sources (maltodextrin, 12% of total energy and corn starch, 55%), and 12% of energy from fat. The fat source for the standard diet was a mixture of lard, cocoa butter, and soybean oil. Equal amounts of lard and cocoa butter were replaced in the n3 PUFA-supplemented diets. DHA and EPA were from ethylester oils (90% pure; KD Pharma, Bexbach, Germany). The diets were analyzed for fatty acid content using gas chromatography coupled with mass spectrometry as described below for the analysis of myocardial membrane lipid composition, and detailed composition is given in Table 1.
Table 1.
Fatty acid compositions of the diet expressed as the molar percent of total fatty acids in the chow
| Fatty Acid | Standard Diet | DHA | EPA | DHA + EPA |
|---|---|---|---|---|
| C14:0 | 0.6 | 0.5 | 0.5 | 0.5 |
| C16:0 | 22.4 | 17.7 | 17.4 | 17.8 |
| C16:1 | 2.2 | 1.8 | 1.5 | 1.7 |
| C18:0 | 24.6 | 19.2 | 18.6 | 19.2 |
| C18:1n-9 | 28.4 | 22.5 | 21.3 | 22.3 |
| C18:1n7 | 2.4 | 1.9 | 1.7 | 1.9 |
| C18:2n-6 | 14.9 | 13.1 | 12.1 | 12.9 |
| C18:3n3 | 0.7 | 0.6 | 0.5 | 0.6 |
| C20:4n-6 | 3.7 | 3.1 | 2.6 | 2.8 |
| C20:5n3 | 1.4 | 20.5 | 10.7 | |
| C22:5n3 | 1.3 | 1.0 | ||
| C22:6n3 | 18.0 | 3.2 | 8.7 |
All diets derived 12% of total energy from fat.
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Experimental Protocols
Two series of experiments were performed: a prevention protocol and a treatment protocol. The prevention protocol included two cohorts: 1) a survival group that was treated from 6 wk of age until death or a maximum of 78 wk and 2) a physiological assessment group treated from 6 to 30 wk of age, at which time LV function was evaluated by echocardiography, followed by euthanasia and assessment of mitochondria physiology. A second treatment protocol evaluated only the effects of DHA on survival, LV function, and mitochondrial physiology in older hamsters with established heart failure. Treatment was initiated at 30 wk of age, and survival was assessed over 30 wk of treatment, after which time (60 wk of age) LV function was assessed and the animals were euthanized and mitochondrial function was assessed.
Prevention protocol: survival group.
Six-week-old TO2 hamsters were assigned to either the standard diet (n = 32), DHA (n = 27), EPA (n = 29), or a combined DHA + EPA (n = 30). The healthy reference group of F1B hamsters were fed the standard diet (n = 17). Previous studies found that Bio TO-2 hamsters have a median survival between 37 to 46 wk of age, with 100% death by 55–75 wk (30, 42, 47); thus, we set the maximum length of treatment at 78 wk, after which any surviving animals were euthanized by exsanguination under deep general anesthesia (5% inhaled isoflurane). The hamsters were monitored daily for mortality, activity, and thriftiness and were weighed weekly but were otherwise not handled. The following criteria for euthanasia as an end-point alternative to natural death were established before the study: weight loss > 15%, dyspnea, lethargy lasting more than 4 h, or pulmonary congestion as identified by audible wheezing. No animals met these criteria, and none were euthanized.
Prevention protocol: physiological assessment group.
The effects of n3 PUFA on cardiac and mitochondrial function were evaluated following 24 wk of dietary treatment (from 6 to 30 wk of age). Six-week-old TO2 hamsters were assigned to either the standard diet (n = 12), DHA (n = 11), EPA (n = 11), or a combined DHA + EPA (n = 12). The healthy reference group of F1B hamsters were fed the standard diet (n = 12). After 23 wk of treatment, animals underwent an echocardiogram and were euthanized the following week. Under deep general anesthesia (isofluane by mask, 2–4% to effect), blood was collected by cardiac puncture, and the animal was euthanized by exsanguination. The organs and hearts were harvested for biochemical analysis and mitochondrial isolation. Sections of the LV free wall were taken for biochemical analysis and histology, frozen in liquid nitrogen, and the remainder used for mitochondrial isolation. Cardiac mitochondria were analyzed for respiration, Ca2+ retention capacity, and size as described in Mitochondrial measurements.
Treatment protocol.
An additional protocol was performed to evaluate the effects of DHA started at 30 wk. The rationale for the treatment protocol was that it would more realistically mimic treatment of clinical cardiomyopathies and heart failure where therapies are initiated after cardiac dysfunction is established, not prior as in the prevention protocol. TO2 hamsters were assigned to either the standard diet or DHA (n = 42/group and maintained for 30 wk to assess survival and mitochondrial respiration). After 29 wk of treatment, animals underwent an echocardiogram and were euthanized the following week, as described in Prevention protocol: physiological assessment group.
Experimental Methods
Echocardiography.
Cardiac function was assessed by echocardiography as previously described (11). Briefly, hamsters were anesthetized with inhaled isoflurane by mask (1.5–2.0% in 100% O2) and placed on a warming pad. The level of anesthesia was adjusted as needed to ensure the absence of reflex response to foot pinch. Two-dimensional cine loops and guided M-mode frames were recorded from the parasternal short axis using a Vevo 770 (Visual Sonics, Toronto, Canada) with a 15-MHz linear array transducer (model 716). Fractional shortening was calculated as (end-diastolic diameter − end-systolic diameter)/end-diastolic diameter.
Mitochondrial measurements.
Subsarcolemmal mitochondria (SSM) and IFM were isolated as described previously (11, 27). Briefly, LV tissue (∼250 mg) was minced and homogenized in 1:10 cold modified buffer consisting of 100 mM KCl, 50 mM MOPS, 5 mM MgSO4, 1 mM EGTA, 1 mM ATP, and 0.2 mg/ml BSA and centrifuged at 500 g. Subsequent centrifugations separated and purified SSM. Separation of the IFM was achieved by incubation on ice with 5-mg purified trypsin/g wet tissue for 10 min and subsequent purification by differential centrifugations.
Mitochondrial oxygen consumption was assessed as previously described using a Clarke-type electrode chamber at 37°C (11). Isolated mitochondria (0.5 mg mitochondrial protein/ml) were resuspended in buffer containing 100 mM KCl, 50 mM MOPS, 5 mM KH2PO4, 1 mM EGTA, and 1 mg/ml BSA. State 3 respiration was measured with glutamate + malate (10 and 5 mM, respectively) or succinate + rotenone (10 mM and 7.5 μM, respectively), and state 4 with oligomycin (4 μM), and the respiratory control ratio was calculated as state 3/state 4. Mitochondrial diameter of IFM and SSM was measured by flow cytometry, as previously described (5).
The ability of mitochondrial to retain Ca2+, an index of MPT pore opening, was assessed in isolated mitochondrial as described previously in detail (11, 27). Briefly, with the use of a 96-well fluorescence plate reader (FLUOstar Optima, BMG Labtech), 25 μg of mitochondrial protein was suspended in 200 μl of respiration buffer without BSA and supplemented with 5 μM EGTA and 1 mM MgCl2. Glutamate and malate were added as substrates (10 and 5 mM, respectively), and extramitochondrial Ca2+ was monitored using 1 μM calcium-green 5N. Fluorescence was measured every 17 s at excitation and emission wavelengths of 485 and 538 nm, respectively, at 37°C. Ca2+ was added in increments of 25 nmol/mg mitochondrial protein at 7-min intervals.
Metabolic and biochemical parameters.
Plasma glucose, triglycerides, free fatty acids, and insulin were measured using spectrophotometric enzymatic methods as previous described (11). Maximal activities of the mitochondrial oxidative enzymes isocitrate dehydrogenase, medium-chain acyl-CoA dehydrogenase, and citrate synthase were measured spectophotometrically at 37°C from homogenates made from frozen tissue, as previously described (11).
Mitochondrial membrane lipid composition.
The phospholipid fatty acid composition of IFM in the prevention protocol was measured using gas chromatography-mass spectrometry in homogenates made from frozen aliquots according to a modification of the transesterification method as previously described (12, 28).
Histology.
Myocardium from the LV lateral free wall was evaluated for myocyte cross-sectional area, interstitial fibrosis, and cardiomyocyte apoptosis as previously described (11).
Statistical Analysis
Data are presented as means ± SE. Survival was assessed with a Kaplan-Meier analysis. To establish the extent of pathology, physiological, biochemical parameters, and LV function were compared between the F1B and TO2 hamsters on the standard diet using an unpaired t-test. Differences among the various dietary treatments in the physiological substudy in the prevention protocol were determined using a one-way ANOVA and a Holm-Sidak post hoc test for multiple comparisons. In the treatment protocol, the DHA and standard diets were compared using an unpaired t-test. Differences in Ca2+ in the retention assay were evaluated using a two-way ANOVA for repeated measures with a Holm-Sidak post hoc test. A value of P < 0.05 was considered significant.
RESULTS
Prevention Protocol
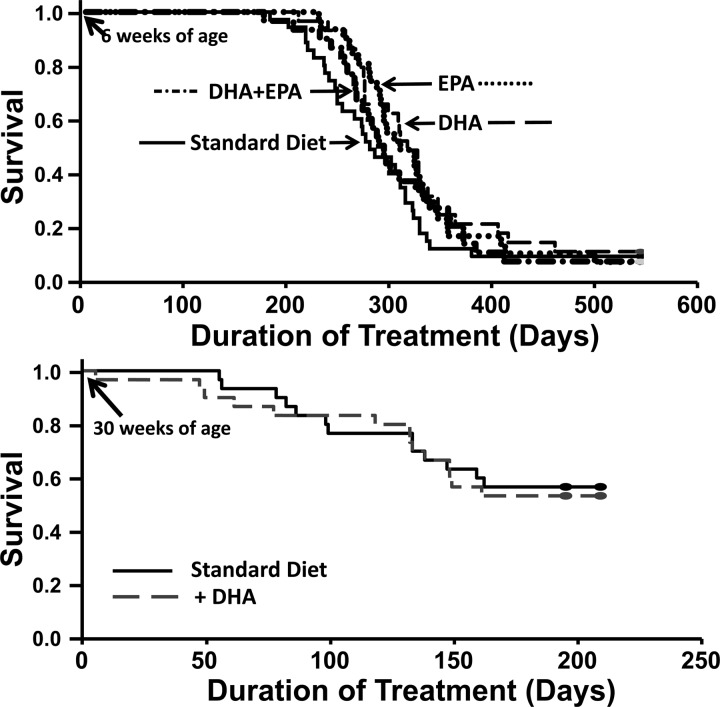
The survival study showed that cardiomyopathic hamsters on the standard diet had a mean survival of 298 ± 15 days on treatment, which was not different from groups treated with the n3 PUFA diets (335 ± 17, 328 ± 14, and 311 ± 15 days for DHA, EPA, and DHA + EPA, respectively) (Fig. 1).
Fig. 1.
Animal survival plotted as a function of duration of dietary treatment. Top: prevention study wherein hamsters initiated dietary treatment at 6 wk of age [standard diet (n = 32), docosahexaenoic acid (DHA; n = 27), eicosapentaenoic acid (EPA; n = 29), or a combined DHA + EPA (n = 30)]. Bottom: treatment study wherein DHA was started at 30 wk of age (n = 42/group).
The results from the physiological assessment group found that the TO2 hamsters had a significantly lower body and LV mass at 30 wk of age compared with healthy F1B hamsters (Table 2), which was unaffected by the n3 PUFA diets. Cardiomyocyte apoptosis was elevated in the TO2 hamsters compared with the F1B hamsters and was not significantly altered by n3 PUFA (Table 2). TO2 hamsters had higher circulating glucose levels (F1B vs. TO2 control, P < 0.05), which were significantly reduced by treatment with DHA, but not EPA or DHA + EPA (Table 2). There were no differences among groups in circulating insulin, free fatty acids, or triglycerides (Table 2).
Table 2.
Morphometric and metabolic parameters: prevention groups
| TO2 |
|||||
|---|---|---|---|---|---|
| F1B Standard Diet | Standard diet | DHA | EPA | DHA + EPA | |
| Body mass, g | 166 ± 4 | 131 ± 3* | 136 ± 3 | 131 ± 3 | 127 ± 2 |
| LV mass/tibia length, mg/mm | 15.1 ± 0.3 | 12.3 ± 0.2* | 11.8 ± 0.3 | 11.6 ± 0.3 | 11.6 ± 0.2 |
| RV mass/tibia length, mg/mm | 3.61 ± 0.22 | 2.92 ± 0.28 | 3.23 ± 0.18 | 2.77 ± 0.27 | 3.09 ± 0.32 |
| Metabolic parameters | |||||
| Glucose, mg/dl | 113 ± 4 | 143 ± 7* | 114 ± 8# | 149 ± 10 | 141 ± 7 |
| Insulin, ng/ml | 1.6 ±0.4 | 1.1 ± 0.2 | 0.7 ± 0.1 | 1.3 ± 0.4 | 1.3 ± 0.2 |
| Free fatty acids, mM | 0.61 ± 0.13 | 0.78 ± 0.13 | 0.60 ± 0.06 | 0.84 ± 0.20 | 0.58 ± 0.07 |
| Triglycerides, mg/dl | 333 ± 119 | 505 ± 84 | 346 ± 100 | 598 ± 153 | 389 ± 82 |
| Histology | |||||
| TUNEL-positive cardiomyocyte nuclei, per 10,000 nuclei | 2.3 ± 0.8 | 5.2 ± 1.3* | 4.4 ± 1.0 | 4.5 ± 1.2 | 4.9 ± 0.8 |
| Cardiomyocyte cross-sectional area, μm2 | 603 ± 26 | 665 ± 27* | 615 ± 35 | 630 ± 25 | 657 ± 24 |
| Interstitial fibrosis, %total area | 10.0 ± 1.1 | 10.6 ± 0.6 | 9.9 ± 0.6 | 10.5 ± 0.7 | 10.1 ± 0.8 |
Values are means ± SE; standard diet, n = 12; DHA, n = 11; EPA, n = 11; and DHA + EPA, n = 12.
LV, left ventricular; RV, right ventricular; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
P < 0.05 vs. F1B;
P < 0.05 vs. TO2 control (CTRL), one-way ANOVA; †P < 0.05 vs. TO2 DHA.
Cardiac Function
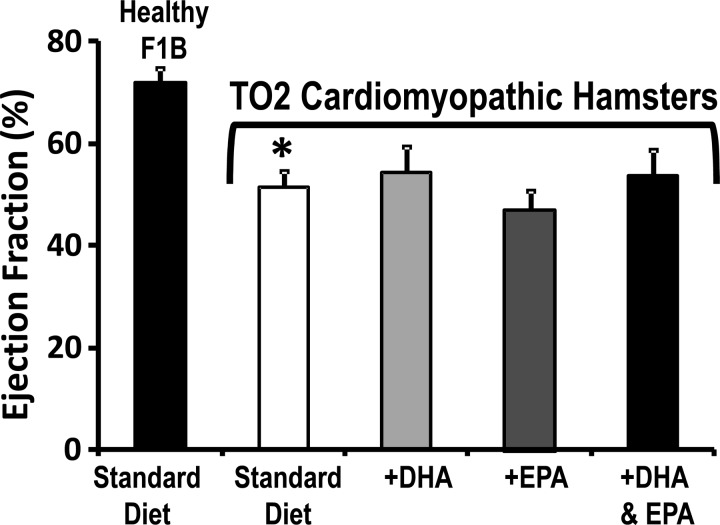
When compared to F1B hamsters, the TO2 hamsters displayed systolic dysfunction and dilation, as evidenced by an increased end-systolic diameter and decreased end-diastolic wall thickness and ejection fraction, which were unaffected by supplementation with the various n3 PUFA diets (Fig. 2, and Table 3).
Fig. 2.
Myocardial ejection fraction after 30 wk of treatment in the prevention study. *P < 0.05 vs. F1B. (standard diet, n = 12; DHA, n = 11; EPA, n = 11; and DHA + EPA, n = 12).
Table 3.
Echocardiographic measurements: prevention groups
| TO2 |
|||||
|---|---|---|---|---|---|
| F1B Standard Diet | Standard diet | DHA | EPA | DHA + EPA | |
| LV end-diastolic diameter, mm | 4.8 ± 0.2 | 5.1 ± 0.2 | 5.1 ± 0.2 | 5.3 ± 0.2 | 4.7 ± 0.3 |
| LV end-systolic diameter, mm | 3.1 ± 0.2 | 4.0 ± 0.2* | 3.9 ± 0.3 | 4.3 ± 0.3 | 3.6 ± 0.3 |
| End-diastolic anterior wall thickness, mm | 1.9 ± 0.1 | 1.5 ± 0.1* | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 |
| End-diastolic posterior wall thickness, mm | 1.8 ± 0.1 | 1.4 ± 0.1* | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.6 ± 0.1 |
| LV area of fractional shortening | 0.37 ± 0.02 | 0.23 ± 0.02* | 0.25 ± 0.03 | 0.20 ± 0.02 | 0.24 ± 0.03 |
| Ejection fraction, % | 71.9 ± 2.8 | 51.5 ± 3.0* | 54.2 ± 5.3 | 46.8 ± 4.1 | 53.5 ± 5.2 |
Values are means ± SE; standard diet, n = 12; DHA, n = 11; EPA, n = 11; and DHA + EPA, n = 12.
P < 0.05 vs. F1B.
Mitochondrial Enzymes and Function
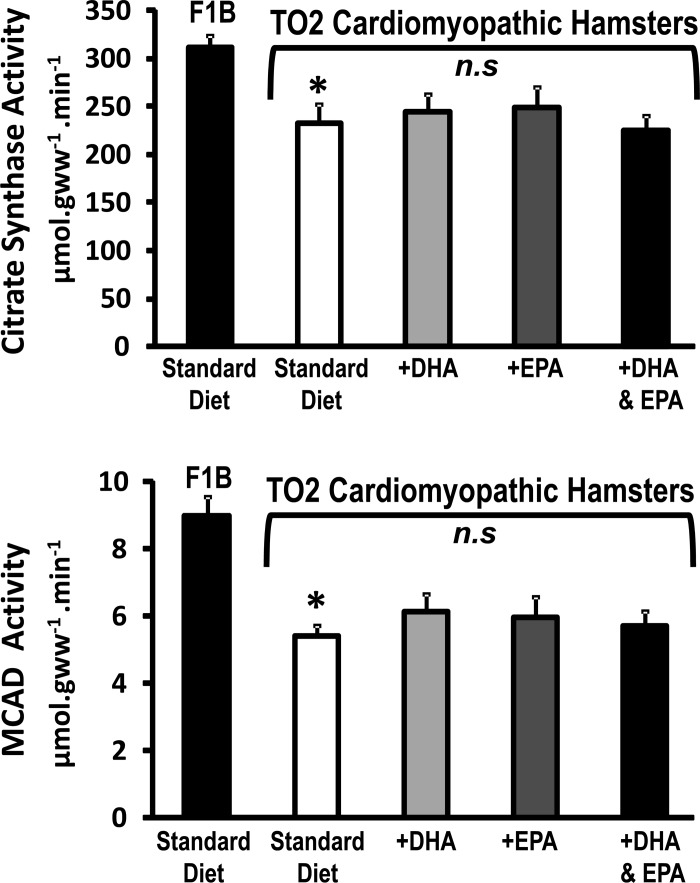
TO2 hamsters displayed a significant decrease in mitochondrial yield and myocardial tissue oxidative capacity, as reflected in lower activity of citrate synthase, medium-chain acyl-CoA dehydrogenase, and isocitrate dehydrogenase (Fig. 3, Table 4). These parameters were not significantly altered by the n3 PUFA diets. The diameter of SSM and IFM were not different between normal and cardiomyopathic hamster and was not affected by treatment. Assessment of isolated mitochondria revealed a decrease in respiratory capacity (decreased state 3 and state 4 succinate supported respiration) in both SSM and IFM compared with mitochondria from F1B hamsters, with no change in respiratory control ratio (Table 4).
Fig. 3.
Myocardial activity of citrate synthase, a citric acid cycle enzyme, and of medium-chain acyl-CoA dehydrogenase (MCAD), a fatty acid β-oxidation enzyme. *P < 0.05 vs. F1B. NS, not significant; gww, gram wet weight.
Table 4.
Mitochondrial parameters and the activity of mitochondrial enzymes in cardiac tissue from the prevention study
| TO2 |
|||||
|---|---|---|---|---|---|
| F1B Standard Diet | Standard diet | DHA | EPA | DHA + EPA | |
| SSM | |||||
| State 3, nA O·mg−1·min−1 | |||||
| Malate + glutamate | 148 ± 10 | 148 ± 17 | 148 ± 17 | 127 ± 10 | 143 ± 16 |
| Succinate | 296 ± 16 | 258 ± 15* | 271 ± 9 | 281 ± 22 | 267 ± 20 |
| State 4, nA O·mg−1·min−1 | |||||
| Malate + glutamate | 28.8 ± 5.4 | 21.6 ± 5.9 | 20.1 ± 3.6 | 14.5 ± 3.8 | 25.4 ± 6.1 |
| Succinate | 79.0 ± 7.1 | 55.2 ± 6.4* | 66.1 ± 6.4 | 67.0 ± 3.6 | 68.1 ± 6.5 |
| RCR | |||||
| Malate + glutamate | 7.4 ± 1.5 | 10.4 ± 2.8 | 8.7 ± 1.6 | 9.8 ± 2.4 | 7.7 ± 1.3 |
| Succinate | 4.1 ± 0.4 | 5.1 ± 0.5 | 4.4 ± 0.5 | 4.3 ± 0.3 | 3.9 ± 0.3 |
| IFM | |||||
| State 3, nA O·mg−1·min−1 | |||||
| Malate + glutamate | 201 ± 26 | 154 ± 23 | 197 ± 21 | 149 ± 13 | 198 ± 24 |
| Succinate | 357 ± 28 | 282 ± 20* | 373 ± 39 | 296 ± 26 | 323 ± 29 |
| State 4, nA O·mg−1·min−1 | |||||
| Malate + glutamate | 39.4 ± 10.1 | 29.5 ± 3.7 | 31.2 ± 6.5 | 24.5 ± 6.9 | 47.8 ± 13.6 |
| Succinate | 101.0 ± 11.6 | 72.6 ± 6.5* | 94.7 ± 17.8 | 81.9 ± 8.0 | 83.9 ± 12.5 |
| RCR | |||||
| Malate + glutamate | 10.3 ± 4.4 | 5.5 ± 0.7 | 7.8 ± 1.0 | 10.6 ± 2.4 | 5.4 ± 0.7 |
| Succinate | 3.8 ± 0.4 | 4.0 ± 0.3 | 4.5 ± 0.5 | 3.9 ± 0.4 | 3.5 ± 0.3 |
| Mitochondrial parameters | |||||
| SSM yield, mg protein/g wet wt | 6.0 ± 0.6 | 4.1 ± 0.3* | 5.9 ± 0.8 | 5.2 ± 0.7 | 5.4 ± 0.6 |
| SSM diameter, μm | 0.56 ± 0.03 | 0.55 ± 0.02 | 0.56 ± 0.02 | 0.58 ± 0.01 | 0.57 ± 0.02 |
| IFM yield, mg protein/g wet wt | 9.0 ± 0.6 | 4.1 ± 0.6* | 5.1 ± 0.9 | 5.5 ± 1.0 | 5.4 ± 0.7 |
| IFM diameter, μm | 0.60 ± 0.03 | 0.55 ± 0.01 | 0.56 ± 0.01 | 0.56 ± 0.01 | 0.54 ± 0.02 |
| Mitochondrial enzyme activities | |||||
| Isocitrate dehydrogenase, mmol·min−1·g wet wt−1 | 87.6 ± 3.5 | 76.2 ± 4.9* | 77.1 ± 6.1 | 75.8 ± 5.1 | 72.4 ± 3.9 |
| Citrate synthase, μmol·min−1·g wet wt−1 | 311 ± 13 | 233 ± 20* | 244 ± 18 | 249 ± 22 | 225 ± 17 |
| Medium-chain acyl-CoA dehydrogenase, μmol·min−1·g wet wt−1 | 9.0 ± 0.6 | 5.4 ± 0.3* | 6.1 ± 0.5 | 6.0 ± 0.6 | 5.7 ± 0.5 |
Values are means ± SE; standard diet, n = 12; DHA, n = 11; EPA, n = 11; and DHA + EPA, n = 12.
SSM, subsarcolemmal mitochondria; RCR, respiratory control ratio; IFM, interfibrillar mitochondria.
P < 0.05 vs. F1B.
Mitochondrial Phospholipid Fatty Acid Composition
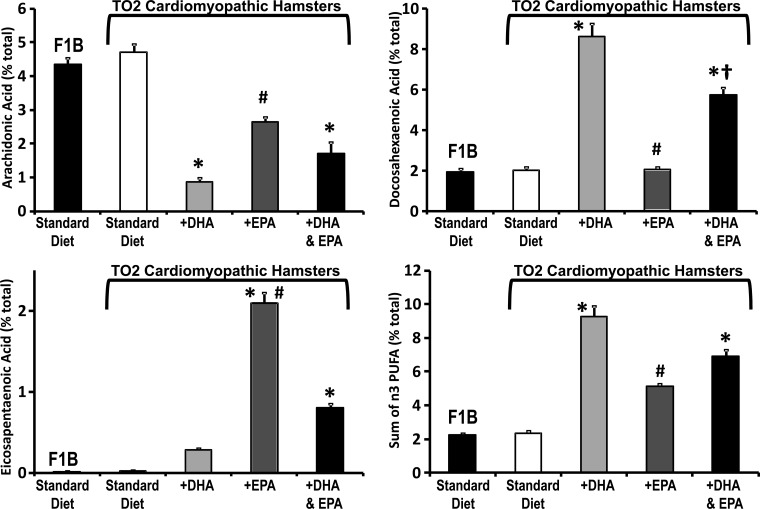
Mitochondrial phospholipid fatty acyl side-chain composition was not different between F1B and TO2 hamsters but was profoundly altered by the three n3 PUFA diets (Fig. 4, and Table 5). Supplementation with DHA increased membrane DHA and decreased membrane arachidonic acid (Fig. 4). Supplementation with EPA increased membrane EPA and also decreased arachindonic acid but did not change DHA compared with the standard diet, consistent with previous studies in rats showing lack of EPA elongation and desaturation in the heart (26, 27, 37). The decrease in arachidonic acid was greater with DHA than with EPA treatment (Fig. 4). Supplementation with DHA + EPA increased both membrane DHA and EPA and decreased arachidonic acid (Fig. 4). Overall, supplementation with DHA increased total n3 PUFA and depleted arachidonic acid more than did EPA supplementation (Fig. 4, and Table 5). Supplementation with EPA did not change total membrane n3 PUFA significantly, though a strong positive trend was present (Fig. 4, and Table 5). Total saturated fatty acid content was not different among the three n3 PUFA treatment groups; however, they were all significantly lower (∼15–30%) compared with the standard diet (Table 5).
Fig. 4.
Effect of diet on mitochondrial membrane phospholipid composition. Myocardial mitochondria phospholipid fatty acid composition expressed as a percentage of total fatty acids (top, bottom left) and the sum of n3 polyunsaturated fatty acids (n3 PUFA; bottom right) after 24 wk of dietary treatment. *P < 0.05 vs. TO2 control; #P < 0.05 vs. TO2 DHA; †P < 0.05 vs. TO2 EPA.
Table 5.
Fatty acid compositions of mitochondrial phospholipids from the prevention study
| TO2 |
|||||
|---|---|---|---|---|---|
| F1B Standard Diet | Standard diet | DHA | EPA | DHA + EPA | |
| C14:0 | 0.25 ± 0.02 | 0.27 ± 0.02 | 0.26 ± 0.03 | 0.41 ± 0.08 | 0.92 ± 0.53 |
| C16:0 | 30.4 ± 0.9 | 30.4 ± 1.2 | 35.0 ± 1.0 | 33.4 ± 1.9 | 34.6 ± 1.1 |
| C16:1 | 0.48 ± 0.04 | 0.44 ± 0.03 | 0.42 ± 0.05 | 0.58 ± 0.04 | 0.52 ± 0.04 |
| C18:0 | 20.4 ± 0.60 | 22.8 ± 0.8 | 22.4 ± 0.9 | 23.8 ± 1.0 | 22.6 ± 0.4 |
| C18:1n-9 | 15.0 ± 0.3 | 13.8 ± 0.4 | 9.6 ± 0.5# | 11.6 ± 0.5#† | 10.8 ± 0.4# |
| C18:1n7 | 1.24 ± 0.06 | 1.34 ± 0.10 | 1.46 ± 0.07 | 1.46 ± 0.05 | 1.50 ± 0.07 |
| C18:2n-6 | 25.5 ± 0.7 | 23.6 ± 0.9 | 20.6 ± 0.8 | 20.9 ± 0.7 | 20.3 ± 0.6# |
| C18:3n-3 | 0.20 ± 0.01 | 0.23 ± 0.01 | 0.25 ± 0.01 | 0.28 ± 0.01# | 0.22 ± 0.01‡ |
| C20:3n-6 | 0.12 ± 0.00 | 0.12 ± 0.01 | 0.07 ± 0.00 | 0.06 ± 0.00# | 0.06 ± 0.00# |
| C20:4n-6 | 4.34 ± 0.21 | 4.70 ± 0.26 | 0.87 ± 0.13# | 2.64 ± 0.14† | 1.71 ± 0.34# |
| C20:5n-3 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.29 ± 0.02 | 2.09 ± 0.13#† | 0.80 ± 0.06† |
| C22:5n-3 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.69 ± 0.04#† | 0.13 ± 0.01# |
| C22:6n-3 | 1.96 ± 0.13 | 2.04 ± 0.13 | 8.64 ± 0.62# | 2.06 ± 0.15† | 5.74 ± 0.38# |
| Σ n3 PUFA | 2.24 ± 0.12 | 2.35 ± 0.13 | 9.25 ± 0.65# | 5.11 ± 0.22† | 6.89 ± 0.45# |
| Σ n6 PUFA | 30.0 ± 0.9 | 28.5 ± 1.1 | 21.6 ± 0.9# | 23.7 ± 0.8 | 22.1 ± 0.7# |
| Σ Sat fat | 51.1 ± 1.2 | 53.5 ± 1.3 | 57.7 ± 1.5 | 57.6 ± 1.3 | 58.2 ± 1.0 |
| Σ MUFA | 16.7 ± 0.4 | 15.6 ± 0.4 | 11.5 ± 0.4# | 13.6 ± 0.6#† | 12.8 ± 0.4# |
| Σ PUFA | 32.2 ± 1.0 | 30.8 ± 1.2 | 30.8 ± 1.5 | 28.8 ± 1.0 | 29.0 ± 1.1 |
Values are means ± SE.
PUFA, polyunsaturated fatty acid; MUFA, monounsaturated fatty acid; Sat fat, saturated fat.
* P < 0.05 vs. F1B;
P < 0.05 vs. TO2 CTRL;
P < 0.05 vs. TO2 DHA;
P < 0.05 vs. TO2 EPA.
Mitochondrial Permeability Transition
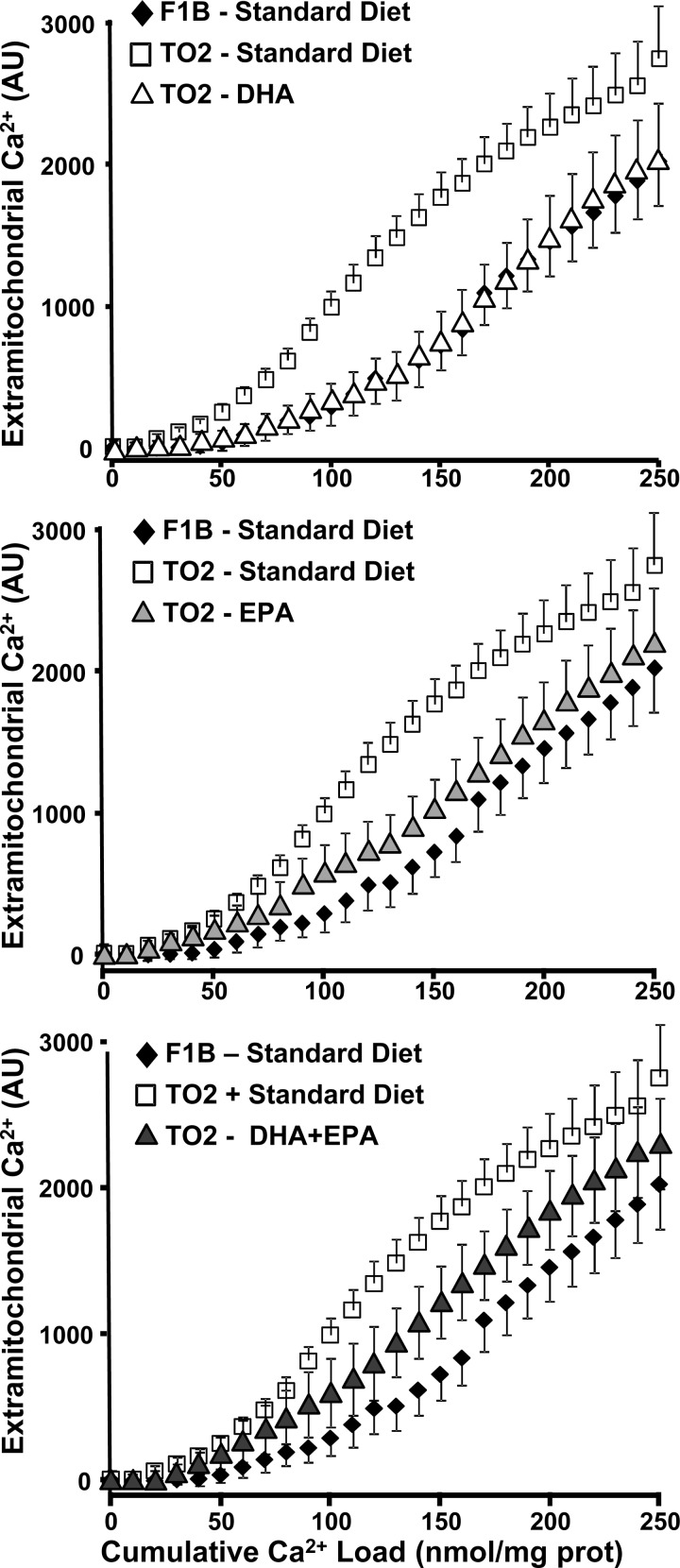
Ca2+-induce MPT was assessed in both SSM and IFM of the prevention groups. Consistent with previous studies, there were no differences between F1B and TO2 SSM or between TO2 control and n3 PUFA-supplemented SSM (Fig. 5). On the other hand, in IFM there was a significant decrease in the cumulative Ca2+ load needed to induce MPT in TO2 hamsters fed the standard diet compared with F1B (Fig. 5). Supplementation with DHA, EPA, or DHA + EPA significantly prevented Ca2+-induced MPT, as seen in the rightward shift in the relationship between extramitochondrial [Ca2+] (Fig. 5) and cumulative Ca2+ load. There was no difference among DHA, EPA, or DHA + EPA in this relationship.
Fig. 5.
Effect of diet on Ca2+-induced mitochondrial permeability transition. Extramitochondrial [Ca2+] plotted as a function of the cumulative Ca2+ added to subsarcolemmal mitochondria (top) and interfibrillar mitochondria (bottom). AU, arbitrary unit; Prot, protein.
Treatment Protocol
There was no difference in survival between the standard diet and DHA treatment (Fig. 1, bottom). DHA had no effect on body or organ mass or metabolic parameters with the exception of a 25% reduction in plasma triglyceride (Table 6). Echocardiographic assessment revealed a modest but significant increase in LV fractional area of shortening and ejection fraction with DHA treatment (Table 7). There were no significant effects on wall thickness, LV dimensions, or heart rate (Table 7). DHA treatment did not affect mitochondrial yield or respiration (Table 8).
Table 6.
Morphometric and metabolic parameters in TO2 hamsters in the treatment study
| Standard Diet | DHA | |
|---|---|---|
| Body weight, g | 113 ± 2* | 113 ± 3 |
| LV weight/tibia length, mg/mm | 11.0 ± 0.3* | 11.3 ± 0.3 |
| RV weight/tibia length, mg/mm | 2.74 ± 0.15 | 3.01 ± 0.18 |
| Metabolic parameters | ||
| Glucose, mg/dl | 221 ± 8* | 227 ± 14 |
| Free fatty acids, mM | 0.46 ± 0.05 | 0.44 ± 0.05 |
| Triglycerides, mg/dl | 440 ± 56* | 307 ± 30# |
Values are means ± SE; n = 12/group.
P < 0.05 vs. standard diet.
Table 7.
Echocardiographic measurements from the treatment study
| Standard Diet | DHA | |
|---|---|---|
| Heart rate, beats/min | 326 ± 8 | 312 ± 5 |
| LV end-diastolic diameter, mm | 5.4 ± 0.2 | 5.4 ± 0.3 |
| LV end-systolic diameter, mm | 4.4 ± 0.2 | 4.2 ± 0.2 |
| End-diastolic anterior wall thickness, mm | 2.1 ± 0.1 | 2.0 ± 0.1 |
| End-diastolic posterior wall thickness, mm | 1.9 ± 0.1 | 1.9 ± 0.1 |
| LV area of fractional shortening | 0.19 ± 0.01 | 0.23 ± 0.02# |
| Ejection fraction, % | 45.3 ± 2.1 | 53.0 ± 2.6# |
Values are means ± SE; n = 12/group.
P < 0.05 vs. TO2 CTRL.
Table 8.
Mitochondrial parameters: treatment groups
| TO2 |
||
|---|---|---|
| Standard diet | DHA | |
| SSM | ||
| State 3, nA O·mg−1·min−1 | ||
| Malate + glutamate | 118 ± 12 | 104 ± 10 |
| Succinate | 192 ± 19 | 169 ± 15 |
| Palm CoA + M + C | 79 ± 8 | 78 ± 9 |
| State 4, nA O·mg−1·min−1 | ||
| Malate + glutamate | 29.9 ± 3.5 | 31.3 ± 4.0 |
| Succinate | 66.5 ± 3.1 | 60.6 ± 5.7 |
| Palm CoA + M + C | 24.5 ± 2.2 | 22.7 ± 2.0 |
| RCR | ||
| Malate + glutamate | 8.8 ± 1.2 | 8.5 ± 2.2 |
| Succinate | 4.0 ± 0.4 | 3.6 ± 0.3 |
| Palm CoA + M + C | 7.7 ± 0.5 | 7.7 ± 0.9 |
| IFM | ||
| State 3, nA O·mg−1·min−1 | ||
| Malate + glutamate | 122 ± 20 | 139 ± 20 |
| Succinate | 201 ± 24 | 172 ± 16 |
| Palm CoA + M + C | 84.3 ± 14.3 | 93.1 ± 12.5 |
| State 4, nA O·mg−1·min−1 | ||
| Malate + glutamate | 34.5 ± 2.8 | 32.3 ± 2.8 |
| Succinate | 68.3 ± 8.1 | 69.7 ± 6.1 |
| Palm CoA + M + C | 28.6 ± 3.3 | 31.2 ± 3.9 |
| RCR | ||
| Malate + glutamate | 3.5 ± 0.4 | 4.3 ± 0.5 |
| Succinate | 3.0 ± 0.4 | 2.5 ± 0.1 |
| Palm CoA + M + C | 5.4 ± 0.7 | 5.8 ± 0.6 |
| Mitochondrial yield, mg/g wet wt | ||
| SSM | 6.2 ± 0.9 | 6.2 ± 0.9 |
| IFM | 5.9 ± 0.5 | 5.9 ± 0.5 |
Values are means ± SE; n = 12/group.
M, malate; C, carnitine.
DISCUSSION
Mitochondrial dysfunction in heart failure includes greater susceptibility to MPT, which has been proposed to worsen ATP production, impair cardiac function, trigger cardiomyocyte apoptosis, and decrease survival (15, 17, 24, 40, 41). Thus prevention of permeability transition in cardiac mitochondria has been a putative therapeutic target in heart failure, with the concept being that interventions that prevent MPT will increase the capacity for oxidative phosphorylation, prevent cardiomyocyte death, and improve LV function and survival. The results of the present study show that despite an increased resistance to Ca2+-induced MPT, n3 PUFA supplementation did not significantly prolong survival or improve LV function in cardiomyopathic hamsters. Thus our results do not support the concept that delayed MPT pore opening will prevent cardiac dysfunction and prolong survival in heart failure and suggests that greater susceptibility to MPT is not a major contributor to cardiac pathology and poor survival in heart failure.
Whereas our findings suggest that marine n3 PUFAs are not effective for treatment of cardiomyopathies due to defects in δ-sarcoglycan, a positive effect of marine n3 PUFA on survival and/or LV function has been previously observed in clinical and animal studies. A beneficial effect was observed in patients with heart failure associated with ischemic heart disease or hypertension (14, 33, 34) and in most (3, 7, 8, 31, 35, 38), but not all (26, 36), studies in rats and mice with infarct-induced heart failure or pathological LV hypertrophy. The absence of a beneficial effect in the present investigation could be due to species differences in the response to n3 PUFAs and/or differences in the composition of membrane phospholipid fatty acids. Analysis of cardiac mitochondrial phospholipid fatty acid composition demonstrates a dramatic difference between rats and hamsters when fed a standard diet. Mitochondria from cardiomyopathic hamsters had lower DHA and arachidonic acid (2 and 4% of total fatty acid side chains) than rats (10 and 15%), but greater total long-chain saturated fatty acids (palmitate + stearate of ∼51 vs. ∼35% in rats) (Fig. 4, Table 5) (26, 27). We could find no information in the literature regarding the fatty acid composition of cardiac mitochondria from humans or mice. Whole tissue analysis of ventricular myocardium from humans, hamsters, rats, and mice shows that these species all have low cardiac membrane phospholipid EPA (<1%) and that humans have lower DHA than rodents (∼3 vs. ∼7% in hamsters, 10% in rats, and ∼30% in mice) (8, 11, 18, 35). On the other hand, human and hamster myocardium have similar phospholipid saturated fatty acid content (palmitate + stearate of ∼41%), which is higher than rats and mice (∼32%) (8, 11, 18, 35). Furthermore, arachidonic acid content in myocardial phospholipids is similar in humans and hamsters (∼11%), which is much lower than in rats (23%) but similar to mice (8%) (8, 11, 18, 35). Taken together, hamster myocardium appears to have a more human-like phospholipid fatty acid composition than mice and rats, particularly for DHA, arachidonic acid, and long-chain saturated fatty acids. Thus the lack of benefit in the present investigation may be due to species differences in cardiac membrane phospholipid fatty acid metabolism and composition. Furthermore, there may be import species differences among the phospholipid subclasses that impact mitochondrial structure and function in heart failure; however, this has not been addressed in the literature or in the current investigation.
The lack of effect with dietary n3 PUFAs raises the possibility that this model of heart failure will not respond to manipulations in dietary lipid intake. This is clearly not the case, as two previous studies in δ-sarcoglycan null cardiomyopathic hamsters found clear improvement in survival with dietary interventions. Fiaccavento et al. (10) found that a diet comprised of flaxseeds, apples, and carrots that was high in α-linolenic acid (18:3n3) prolonged survival compared with a standard commercial laboratory chow (10); however, because of the complex composition of the diet, it is not possible to determine the extent that α-linolenic acid was responsible for the improved outcome. We observed in a parallel study that shared a common control group with the present investigation that cardiomyopathic hamsters live significantly longer on a high-fat diet (45% of energy from fat) comprised of palmitate (11%), stearate (12%), and oleate (18%) compared with a standard low-fat diet (12% energy from fat) (11). Mean survival on treatment increased from 278 days with the low-fat diet to 361 days with high-fat diet (P < 0.01). This beneficial effect was not associated with correction of myocardial mitochondrial oxidative capacity, changes in Ca2+-induced MPT, or improved cardiac pump function. In any case, these findings show that this model is responsive to dietary interventions, albeit any beneficial effects appear to be independent of mitochondrial mechanisms.
Systemic metabolic dysfunction consistent with the metabolic syndrome and early diabetes characterized by elevated circulating glucose, free fatty acids and triglycerides, and low insulin is an important feature of the δ-sarcoglycan null cardiomyopathic hamster model (Table 2) (11, 19, 32). In the present study, treatment with DHA resulted in a significant reduction in plasma glucose concentration without an increase in insulin levels, suggesting better glucose tolerance. While n3 PUFA intake can prevent the development of insulin resistance in obese rats (44), this is not a consistent finding, particularly in humans (9). There may be greater systemic metabolic abnormalities in the cardiomyopathic hamster than in rodent heart failure models using infarction or pressure overload. Future studies on the effects of n3 PUFA should assess more sensitive measures of insulin sensitivity and glucose tolerance, as these parameters may partially mediate the effects of n3 PUFA.
The dissociation between the susceptibility to MPT, cardiac function, and survival suggests that this aspect of mitochondrial dysfunction in heart failure does not contribute to poor cardiac function or mortality. The precise role of MPT in the heart is not clear. Cardiomyocytes from dogs with heart failure secondary to irreversible ischemic injury have accelerated MPT pore opening and impaired ADP-stimulated mitochondrial respiration compared with healthy normal dogs (39). Interestingly, the addition of cyclosporin A, an effective inhibitor of MPT, prevented MPT pore opening and partially restored maximal mitochondrial oxygen consumption toward normal values (39). On the other hand, cyclosporin had no effect in cardiomyocytes from healthy dogs. This suggests that greater MPT contributes to impaired oxidative phosphorylation in the failing heart. However, in the present study the improvement in resistance to Ca2+-induced MPT in IFM with n3 PUFA was not associated with a greater capacity for oxidative phosphorylation. Clearly, further work is needed to unravel the role of MPT in the pathophysiology of heart failure.
The recent GISSI-HF clinical trial found small (∼6%) but significant (P < 0.045) survival effects with a low dose of DHA + EPA compared with placebo after 4 yr of treatment in a large cohort of heart failure patients (∼3,490 patients per treatment group) (14). We cannot exclude the possibility that marine n3 PUFA would also have a small but significant survival benefit in the cardiomyopathic hamster model if more animals were assessed. We used the typical number of animals employed for this type of study (∼30/group) and observed that median survival was ∼10% longer with either DHA or EPA. While this was not significantly different, however, we cannot exclude the possibility that a small significant improvement in survival might be detected with a larger sample size.
In summary, we show that supplementation with marine n3 PUFA does not improve survival in a cardiomyopathic hamster model of heart failure. Both DHA and EPA significantly altered mitochondrial membrane phospholipid composition and delayed Ca2+-induced MPT opening without a beneficial effect on mitochondrial function or cardiomyocyte apoptosis. Thus the results from the present study do not support the notion that prevention of MPT improves cardiac dysfunction and prolongs survival in a genetic model of heart failure. That said, it is important to consider that this genetic model of cardiomyopathy does not recapitulate typical clinical heart failure, which presents later in life and is associate with diabetes, hypertension, and ischemic heart disease. Further studies need to assess whether the marine n3 PUFA would be more effective in more commonly acquired, nongenetic cardiac failure found with advance age.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-074237, HL-101434, and HL-072751.
DISCLOSURES
William Stanley is the inventor on a pending US patent by the University of Maryland for the use of docosahexaenoic acid for the treatment of heart failure.
AUTHOR CONTRIBUTIONS
T.F.G., E.R.D., B.H.B., P.A.H., K.A.O., and K.M.O. performed experiments; T.F.G., R.J.K., E.R.D., B.H.B., P.A.H., K.A.O., K.M.O., S.R., C.D., C.D.R., and W.C.S. analyzed data; T.F.G., R.J.K., E.R.D., P.A.H., K.A.O., H.N.S., S.R., C.D., C.D.R., and W.C.S. interpreted results of experiments; T.F.G., R.J.K., E.R.D., K.A.O., H.N.S., C.D.R., and W.C.S. edited and revised manuscript; T.F.G., R.J.K., E.R.D., B.H.B., P.A.H., K.A.O., H.N.S., S.R., C.D.R., and W.C.S. approved final version of manuscript; R.J.K. and W.C.S. prepared figures; R.J.K., E.R.D., and W.C.S. drafted manuscript; E.R.D. and W.C.S. conception and design of research.
ACKNOWLEDGMENTS
We thank Rogerio F. Ribeiro, Jr., for assistance with biochemical analysis.
REFERENCES
- 1.Abel ED, Doenst T. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res 90: 234–242, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barakat H, Brown W, Henry SD. Studies of fatty acid oxidation in homogenates of the cardiomyopathic hamster. Life Sci 23: 1835–1840, 1978 [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Shearer GC, Chen Q, Healy CL, Beyer AJ, Nareddy VB, Gerdes AM, Harris WS, O'Connell TD, Wang D. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation 123: 584–593, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Hahan N, Taouil K, Dassouli A, Morel JE. Long-term therapy with trimetazidine in cardiomyopathic Syrian hamster BIO 14:6. Eur J Pharmacol 328: 163–174, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Dabkowski ER, Williamson CL, Hollander JM. Mitochondria-specific transgenic overexpression of phospholipid hydroperoxide glutathione peroxidase (GPx4) attenuates ischemia/reperfusion-associated cardiac dysfunction. Free Radic Biol Med 45: 855–865, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Di Lisa F, Fan CZ, Gambassi G, Hogue BA, Kudryashova I, Hansford RG. Altered pyruvate dehydrogenase control and mitochondrial free Ca2+ in hearts of cardiomyopathic hamsters. Am J Physiol Heart Circ Physiol 264: H2188–H2197, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Duda MK, O'Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hoit BD, Kop WJ, Stanley WC. Dietary supplementation with omega-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res 76: 303–310, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duda MK, O'Shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, Chess DJ, Azimzadeh AM, Harris WS, Sharov VG, Sabbah HN, Stanley WC. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res 81: 319–327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care 12: 138–146, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Fiaccavento R, Carotenuto F, Minieri M, Masuelli L, Vecchini A, Bei R, Modesti A, Binaglia L, Fusco A, Bertoli A, Forte G, Carosella L, Di NP. Alpha-linolenic acid-enriched diet prevents myocardial damage and expands longevity in cardiomyopathic hamsters. Am J Pathol 169: 1913–1924, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galvao TF, Brown BH, Hecker PA, O'Connell KA, O'Shea KM, Sabbah HN, Rastogi S, Daneault C, Des Rosiers C, Stanley WC. High intake of saturated fat, but not polyunsaturated fat, improves survival in heart failure despite persistent mitochondrial defects. Cardiovasc Res 93: 24–32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelinas R, Thompson-Legault J, Bouchard B, Daneault C, Mansour A, Gillis MA, Charron G, Gavino V, Labarthe F, Des Rosiers C. Prolonged QT interval and lipid alterations beyond β-oxidation in very long-chain acyl-CoA dehydrogenase null mouse hearts. Am J Physiol Heart Circ Physiol 301: H813–H823, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghio S, Scelsi L, Latini R, Masson S, Eleuteri E, Palvarini M, Vriz O, Pasotti M, Gorini M, Marchioli R, Maggioni A, Tavazzi L. Effects of n-3 polyunsaturated fatty acids and of rosuvastatin on left ventricular function in chronic heart failure: a substudy of GISSI-HF trial. Eur J Heart Fail 12: 1345–1353, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Gissi-HFInvestigators, Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372: 1223–1230, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res 77: 334–343, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans 38: 841–860, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta 1787: 1402–1405, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation 110: 1645–1649, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Hecker PA, Galvao TF, O'Shea KM, Brown BH, Henderson R, Jr, Riggle H, Gupte SA, Stanley WC. High-sugar intake does not exacerbate metabolic abnormalities or cardiac dysfunction in genetic cardiomyopathy. Nutrition 28: 520–526, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hikoso S, Ikeda Y, Yamaguchi O, Takeda T, Higuchi Y, Hirotani S, Kashiwase K, Yamada M, Asahi M, Matsumura Y, Nishida K, Matsuzaki M, Hori M, Otsu K. Progression of heart failure was suppressed by inhibition of apoptosis signal-regulating kinase 1 via transcoronary gene transfer. J Am Coll Cardiol 50: 453–462, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Hoppel CL, Tandler B, Parland W, Turkaly JS, Albers LD. Hamster cardiomyopathy: a defect in oxidative phosphorylation in the cardiac interfibrillar mitochondria. J Biol Chem 257: 1540–1548, 1982 [PubMed] [Google Scholar]
- 22.Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Rat heart cannot synthesize docosahexaenoic acid from circulating alpha-linolenic acid because it lacks elongase-2. J Lipid Res 49: 1735–1745, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javadov S, Huang C, Kirshenbaum L, Karmazyn M. NHE-1 inhibition improves impaired mitochondrial permeability transition and respiratory function during postinfarction remodelling in the rat. J Mol Cell Cardiol 38: 135–143, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem 20: 1–22, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kako KJ, Thornton MJ, Heggtveit HA. Depressed fatty acid and acetate oxidation and other metabolic defects in homogenates from hearts of hamsters with hereditary cardiomyopathy. Circ Res 34: 570–580, 1974 [DOI] [PubMed] [Google Scholar]
- 26.Khairallah RJ, O'Shea KM, Brown BH, Khanna N, Des Rosiers C, Stanley WC. Treatment with docosahexaenoic acid, but not eicosapentaenoic acid, delays Ca2+-induced mitochondria permeability transition in normal and hypertrophied myocardium. J Pharmacol Exp Ther 335: 155–162, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khairallah RJ, Sparagna GC, Khanna N, O'Shea KM, Hecker PA, Kristian T, Fiskum G, Des Rosiers C, Polster BM, Stanley WC. Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition. Biochim Biophys Acta 1797: 1555–1562, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khairallah RJ, Kim J, O'Shea KM, O'Connell KA, Brown BH, Galvao T, Des Rosiers C, Polster BM, Hoppel CL, Stanley WC. Improved mitochondrial function with diet-induced increase in either docosahexaenoic acid or arachidonic acid in membrane phospholipids. PLoS One 7: e34402, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laviolle B, Pape D, Turlin B, Bellissant E. Direct effects of 3 combinations of enalapril, metoprolol, and spironolactone on cardiac remodeling in dilated cardiomyopathic hamsters. J Card Fail 12: 752–758, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Mattera GG, Lo GP, Loi FM, Vanoli E, Gagnol JP, Borsini F, Carminati P. Istaroxime: a new luso-inotropic agent for heart failure. Am J Cardiol 99: 33A–40A, 2007 [DOI] [PubMed] [Google Scholar]
- 31.McLennan PL, Abeywardena MY, Dallimore JA, Raederstorff D. Dietary fish oil preserves cardiac function in the hypertrophied rat heart. Br J Nutr 108: 645–654, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Missihoun C, Zisa D, Shabbir A, Lin H, Lee T. Myocardial oxidative stress, osteogenic phenotype, and energy metabolism are differentially involved in the initiation and early progression of delta-sarcoglycan-null cardiomyopathy. Mol Cell Biochem 321: 45–52, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moertl D, Hammer A, Steiner S, Hutuleac R, Vonbank K, Berger R. Dose-dependent effects of omega-3-polyunsaturated fatty acids on systolic left ventricular function, endothelial function, and markers of inflammation in chronic heart failure of nonischemic origin: a double-blind, placebo-controlled, 3-arm study. Am Heart J 161: 915–919, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, Gheorghiade M, Dei CL. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J Am Coll Cardiol 57: 870–879, 2011 [DOI] [PubMed] [Google Scholar]
- 35.O'Shea KM, Chess DJ, Khairallah RJ, Hecker PA, Lei B, Walsh K, Des Rosiers C, Stanley WC. ω-3 Polyunsaturated fatty acids prevent pressure overload-induced ventricular dilation and decrease in mitochondrial enzymes despite no change in adiponectin. Lipids Health Dis 9: 95, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Shea KM, Khairallah RJ, Sparagna GC, Xu W, Hecker PA, Robillard-Frayne I, Des Rosiers C, Kristian T, Murphy RC, Fiskum G, Stanley WC. Dietary omega-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J Mol Cell Cardiol 47: 819–827, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sergiel JP, Martine L, Raederstorff D, Grynberg A, Demaison L. Individual effects of dietary EPA and DHA on the functioning of the isolated working rat heart. Can J Physiol Pharmacol 76: 728–736, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Shah KB, Duda MK, O'Shea KM, Sparagna GC, Chess DJ, Khairallah RJ, Robillard-Frayne I, Xu W, Murphy RC, Des Rosiers C, Stanley WC. The cardioprotective effects of fish oil during pressure overload are blocked by high fat intake: role of cardiac phospholipid remodeling. Hypertension 54: 605–611, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharov VG, Sabbah HN, Shimoyama H, Goussev AV, Lesch M, Goldstein S. Evidence of cardiocyte apoptosis in myocardium of dogs with chronic heart failure. Am J Pathol 148: 141–149, 1996 [PMC free article] [PubMed] [Google Scholar]
- 40.Sharov VG, Todor A, Khanal S, Imai M, Sabbah HN. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. J Mol Cell Cardiol 42: 150–158, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharov VG, Todor AV, Imai M, Sabbah HN. Inhibition of mitochondrial permeability transition pores by cyclosporine A improves cytochrome C oxidase function and increases rate of ATP synthesis in failing cardiomyocytes. Heart Fail Rev 10: 305–310, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Shimizu T, Okamoto H, Chiba S, Matsui Y, Sugawara T, Akino M, Nan J, Kumamoto H, Onozuka H, Mikami T, Kitabatake A. VEGF-mediated angiogenesis is impaired by angiotensin type 1 receptor blockade in cardiomyopathic hamster hearts. Cardiovasc Res 58: 203–212, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Shimizu T, Okamoto H, Chiba S, Matsui Y, Sugawara T, Onozuka H, Mikami T, Kumamoto H, Kitabatake A. Long-term combined therapy with an angiotensin type I receptor blocker and an angiotensin converting enzyme inhibitor prolongs survival in dilated cardiomyopathy. Jpn Heart J 43: 531–543, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 237: 885–888, 1987 [DOI] [PubMed] [Google Scholar]
- 45.Trippodo NC, Fox M, Monticello TM, Panchal BC, Asaad MM. Vasopeptidase inhibition with omapatrilat improves cardiac geometry and survival in cardiomyopathic hamsters more than does ACE inhibition with captopril. J Cardiovasc Pharmacol 34: 782–790, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Witte K, Schnecko A, Hauth D, Wirzius S, Lemmer B. Effects of chronic application of propranolol on beta-adrenergic signal transduction in heart ventricles from myopathic BIO TO2 and control hamsters. Br J Pharmacol 125: 1033–1041, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu T, Zhou L, Mori S, Wang Z, McTiernan CF, Qiao C, Chen C, Wang DW, Li J, Xiao X. Sustained whole-body functional rescue in congestive heart failure and muscular dystrophy hamsters by systemic gene transfer. Circulation 112: 2650–2659, 2005 [DOI] [PubMed] [Google Scholar]