Abstract
Magnetic-based systems utilizing superparamagnetic nanoparticles and a magnetic field gradient to exert a force on these particles have been used in a wide range of biomedical applications. This review is focused on drug targeting applications that require penetration of a cellular barrier as well as strategies to improve the efficacy of targeting in these biomedical applications. Another focus of this review is regenerative applications utilizing tissue engineered scaffolds prepared with the aid of magnetic particles, the use of remote actuation for release of bioactive molecules and magneto–mechanical cell stimulation, cell seeding and cell patterning.
Keywords: cell therapy, drug delivery, forced transport, magnetic cell seeding, magnetic cell stimulation, magnetic targeting, patterned cell assemblies, superparamagnetic nanoparticles, time-varied magnetic field, tissue engineering
Recent progress in the use of magnetic nano- and micro-particles for biomedical applications has significantly exceeded expectations. The versatile intrinsic properties of magnetic particles enable their use in numerous medical applications, such as: localization of therapy, where magnetic carriers, associated with drugs, nucleic acids or loaded within cells can be directed or guided by means of a magnetic field gradient towards certain biological targets; magnetic fluid hyperthermia, where selective thermal ablation of tumors is achieved through heating of tumor-localized magnetic particles exposed to a high frequency magnetic field; tissue engineering, where particles can be used in remote actuation for control of cellular behavior enabling development of functional tissue or to provide means for a patterned cell assembly and facilitated seeding of tissue engineered scaffold with functional cells; and MRI, where magnetic particles are used as contrast agents. Recent reviews have covered the biological applications of magnetic colloidal particles discussing their magnetic properties, synthesis and surface functionalization strategies, biocompatibility, and toxicity and in vitro and in vivo applications [1–8]. In this review, we discuss drug targeting applications which require penetration of a cellular barrier as well as strategies to improve the efficacy of targeting in these applications. This review also addresses recent work in the use of engineered scaffolds prepared with the aid of magnetic particles for regenerative applications, the use of an applied magnetic field to release bioactive molecules and provide mechanical-cell stimulation within these scaffolds, and cell seeding and patterning.
Principles & components of magnetic targeting
Magnetic targeting is based on two main components: a magnetically responsive carrier of therapeutics and a source of a magnetic field gradient (i.e., a magnetic force) responsible for the attraction or positioning of magnetically responsive carriers in organs or tissues. A highly desirable property for a magnetic carrier is superparamagnetism, the ability to strongly magnetize (i.e., align all magnetic moments of atoms parallel along the direction of a magnetic field) when the material is exposed to a magnetic field and have no remnant or residual magnetization (remanence) when the magnetic field is removed. Superparamagnetic particles are typically chosen as a compromise between the desire to achieve strong magnetization and the need to avoid particle aggregation. The force on a magnetic carrier with magnetic moment m⃗ is governed by the equation:
To maximize the force, the magnet system should, on the one hand, generate field B⃗ that is sufficiently strong at the location of the carrier to maximize the induced carrier magnetization m⃗. On the other hand, the magnet system should generate strong field gradients at the carrier’s location. No single source of a magnetic field can meet both requirements [7].
Magnet systems for magnetic targeting that have been proposed or employed so far fall into two main classes. In one class, magnets external to the body provide both the field to magnetize the carrier and field gradients for targeting [9,10]. However, the use of external magnets imposes serious limitations in targeting deep tissues as their field strength and field gradient decrease exponentially with the distance from the surface. The other class is based on a combination of external magnets and magnets (or magnetizable devices) implanted local to the target region. In the second class of systems, the external magnet would typically provide the magnetizing field for the carrier, while the local magnet (or magnetizable implant) will provide the largest possible field gradients for targeting [11–15]. The second type of magnet system can be of potential use for targeting deep tissues, including blood vessels where magnetizable implants can be placed.
Magnetic drug targeting approaches in vivo
Drug delivery to the inner ear
Delivery of medications to the inner ear has been an area of considerable growth in both the research and clinical realms over the past several decades. The systemic route for delivery of medication is accompanied by some troubling drawbacks, including variable penetration into the inner ear due to the presence of a blood–cochlea barrier and the potential for undesirable systemic side effects [16]. Thus, investigators and clinicians have begun developing and utilizing techniques to deliver therapeutic agents locally. Most of the local delivery strategies to the inner ear have used the round window membrane (RWM) approach due to the RWM’s structure, function and permeability characteristics [17]. One of the strategies to locally deliver drugs to the inner ear is based on pulling superparamagnetic nanoparticles through the RWM to deliver therapeutic molecules for protection and possibly restoration of sensorineural hearing loss. A RWM model was designed to investigate the magnetically assisted transport of superparamagnetic nanoparticles through the membrane. The model consisted of a three-cell-layer assembly with epithelial cells cultured on both sides of a small intestinal submucosal matrix with fibroblasts seeded within the small intestinal submucosal matrix membrane, which was structurally and physically similar to the human RWM [18,19]. In the most recent study, poly(lactic-co-glycolic) acid (PLGA; 100 nm) superparamagnetic nanoparticles were pulled through the RWM model using permanent magnets with a flux density of 0.410 T at the pole face. Independent variables such as external magnetic force and exposure time, composition of hyaluronic acid hydrogel suspending media and particle characteristics, including magnetic susceptibility, were studied. The authors found that magnetically assisted transport of PLGA nanoparticles through the RWM inserts increased 2.1-fold in 1 h compared with the controls. Hyaluronic acid hydrogel did prevent particle accumulation on the surface of the RWM in a magnetic field but also impaired the mobility of these particles. Neither greater particle susceptibility nor stronger external magnetic fields significantly improved the transmembrane transport [18].
A recent in vivo study reported that magnetic force has been used to pull therapeutic payloads on nanoparticles into the inner ear of guinea pigs through the RWM [20]. These authors showed that magnetic nanoparticles (MNPs) were pulled into the cochlea by magnetic forces about 3.3-times above controls. However, scaling up this methodology to humans will require placement of an extremely strong pulling magnet on the opposite side of the head at a long working distance. Based on the guinea pig studies above, the necessary magnetic strength to create the same forces at the required working distance of 30–50 cm for humans would exceed current US FDA safety limits (8 T for adults, 4 T for children) [21–23]. To overcome this difficulty, Shapiro et al. have suggested pushing particles magnetically from the same side as the target ear, over a much shorter 5–10 cm working distance, using magnetic fields within the FDA safety limits [23]. The authors demonstrated, both in simulations and with two experiments, that a simple arrangement of just two magnets can be used to push away or ‘magnetically inject’ nanoparticles. This approach might be useful for a variety of clinical needs where pulling the particles in towards external or internally implanted magnets is either not desirable or is not possible. In particular, it would allow therapeutic MNPs to be pushed in through the RWM into the inner ear, thus bypassing the blood–cochlea barrier.
Drug delivery to the brain
The major problem in delivering drugs to the brain is related to the presence of the blood–brain barrier (BBB). The capillary endothelial cells that line the cerebral microvessels and surrounding perivascular elements (basal lamina, pericyte, astrocyte end-feet and interneurons) make up the BBB, separating the brain from the rest of the body with tight junctions. The glial cells, such as astrocytes, surround 85% of the surface of the capillaries. They are responsible for making the endothelial cells ‘tighten up’, producing a transendothelial electrical resistance (1500–2000 Ωcm2), which is much higher than that of the systemic endothelia (3–33 Ωcm2) [24,25]. The BBB is a dynamic interface through which substances pass using different mechanisms (i.e., passive transport, active transport, receptor-mediated transport, or more complex systems such as endocytosis or transcytosis).
The permeability of the BBB can be significantly affected by neurological disorders [26]. Brain tumors cause several BBB abnormalities, such as hyperplasia of the endothelial cells, the opening of the tight junctions and an increase in the fenestrations, and the presence of pinocitic vacuoles [27,28]. In the case of pathologies such as the cerebrovascular diseases, which compromise the BBB integrity, crossing the BBB is obviously facilitated. This condition can potentially enable drug delivery utilizing nanocarriers including physically facilitated transport of magnetic carriers loaded with drugs to the diseased part of the brain tissue. In early studies, magnetic drug delivery has been employed to target cytotoxic drugs to brain tumors. Hassan and Gallo proposed the use of a magnetic field in order to target iron oxide microspheres of cationic chitosan loaded with oxantrazole into the brain [29]. After intra-arterial administration, a higher brain concentration of the encapsulated drug was achieved when a magnetic field of 6000 Gauss was applied for 30 min. The increased localization of the carrier could be attributed both to the magnetic forces and to the possible interaction of cationic carriers with the anionic BBB. This approach was also tested by Pulfer and Gallo to direct microspheres to a brain glioma after an intercarotid injection in rats [30].
In order to understand the effect of particle size on tissue distribution in vivo, the authors studied the targeting of small 10–20 nm magnetic particles coated with neutral polymer administered to rats with brain tumors (glioma) [31]. The experiments showed that small neutral nanoparticles localized mainly into the brain tumor tissue after the activation of the external magnetic field. In comparison with larger (1 µm) diameter magnetic particles, small magnetic particles concentrated in brain tumor at significantly higher levels than the magnetic neutral dextran and cationic aminodextran microspheres previously studied.
More recent studies on magnetic targeting of brain tumors evaluating a number of factors were conducted by Yang’s group [32]. One study examined whether, with magnetic targeting, pathological alteration in brain tumor flow dynamics could be of value in discriminating the diseased site from healthy brain. The authors found that the decreased blood flow rate in glioma, reflecting tumor vascular abnormalities, is an important contributor to glioma-selective nanoparticle accumulation with magnetic targeting [32]. Chertok and coworkers also found that even in the absence of a magnetic field, systemically injected nanoparticles passively reach the brain tumor vasculature [33]. However, a magnetic field gradient generated near the brain tumor enabled prolonged retention of the magnetic carriers within glioma lesions, resulting in a fivefold higher nanoparticle concentration in the magnetically targeted tumor compared with animals not exposed to magnetic field gradients [33]. This group also studied whether the magnetic field topography along with carrier administration method would impact the efficacy of brain tumor targeting. The magnetic field configuration was designed to avoid aggregation of magnetic carriers in the afferent vasculature. Using a magnetic field of 350 mT, administration of magnetic carriers via carotid artery resulted in a 1.8-fold higher nanoparticle accumulation in glioma compared with intravenous administration.
Drug & cell delivery to stented blood vessels
The vascular system is another target where magnetically mediated drug delivery can be potentially applicable. This target is of particular interest for implementation of magnetically localized therapy because stents are widely used in current clinical practice to alleviate blockages in coronary and peripheral arteries. Although the implantation of either bare-metal stents or drug-eluting stents improves clinical outcomes of angioplasty procedures, they often lead to an injury-triggered reobstruction of stented blood vessels called in-stent restenosis. A recent review provides a wide-angle perspective for application of magnetic targeting for localized vascular delivery of therapy [34]. Drug-eluting stents have been developed as a means of preventing intimal hyperplasia and appear to have reduced the early risk of coronary restenosis, although this still occurs in over 10% of stented vessels at 12 months [35]. There are also concerns regarding increased incidence of stent thrombosis and myocardial infarction in patients who have had a drug-eluting stent inserted [35]. The primary limitations of this approach are the fixed amount of drug applied to the stent and its predetermined release profile. If redosing is required, the only solution is to implant another stent sandwiched on top of the initial one. Magnetically targeted drug delivery to stents can overcome these limitations and enable renewable and modulatable local levels of the therapeutic agents at the site of arterial lesion.
A recent study published by Chorny and coworkers demonstrates the feasibility of site-specific drug delivery to implanted magnetizable stents by uniform field controlled targeting of MNPs with efficacy for in-stent restenosis [11]. These authors showed that local administration of MNPs applied to stented rat carotid arteries in the presence of a uniform magnetic field resulted in fourfold higher initial number of MNPs associated with the stented region compared with non-magnetic control conditions. After removal of the uniform field, the stent and MNPs lose their magnetic moments and targeted MNPs are redistributed from the stented arterial segment over time. Interestingly, the number of MNPs measured in the arteries of magnetically treated animals remained 5.5- to 9.5-fold higher than in the non-magnetic control group up to 5 days post-treatment. Despite the redistribution of a sizable fraction of initially captured MNP, a significant reduction in the neointima-to-media ratio was revealed 14 days postsurgery in the animals treated with paclitaxel-loaded particles in the presence of the uniform field, but not in control animals [11].
Another complication of the intravascular stent implantation is related to injury of the endothelial monolayer, which exposes the underlying media and induces a cascade of cellular and biological events, resulting in abnormal vascular wall function. Strategies that enhance the number of endothelial cells in the vessel wall following injury may limit complications such as thrombosis, vasospasm and neointimal formation through reconstitution of a luminal barrier and cellular secretion of paracrine factors [36]. The use of magnetic forces for localization of endothelial cells to stented segments of blood vessels is a relatively new concept that has been explored by several groups. The magnetic force in these animal studies was generated using either externally applied permanent magnets [37–39] or permanently magnetized ferromagnetic stents [13].
A proof-of-concept study published by Polyak and coworkers demonstrated the feasibility of targeted delivery of magnetically responsive endothelial cells to stented arteries in the presence of a uniform magnetic field [14]. Endothelial cell loading with MNPs was achieved with high efficiency rendering cells with high magnetic responsiveness and accompanied with a marginal reduction in cell viability. Targeting of cells was successfully carried out in the rat carotid stenting model either under interrupted or uninterrupted blood flow conditions. Notably, in both settings the bioluminescent signal (proportional to the number of viable cells) measured at the stented area was an order of magnitude higher in the magnetic delivery animals compared with the non-magnetic controls (Figure 1) [14]. Although the delivery of unmodified endothelial cells by itself seems to be a clinically worthwhile therapeutic strategy, the therapeutic potential of cell targeting can be enhanced by the expression of pharmacologically relevant levels of therapeutic gene, thus combining the benefits of cell and gene therapy. Further studies involving investigations to quantitatively determine the efficacy of the cell targeting, the fate of the off-targeted cells, re-endothelialization efficiency and therapeutic outcomes are necessary to reach any conclusions about the future clinical implementation of this method.
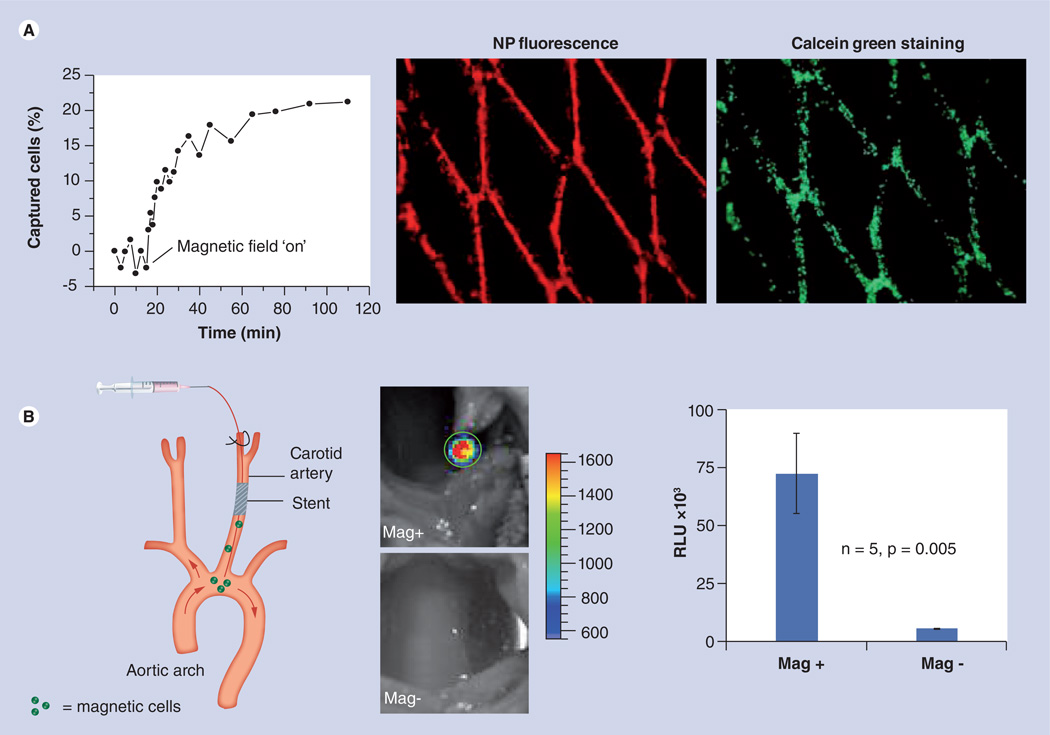
Figure 1. Magnetic targeting of magnetic nanoparticle/preloaded bovine aortic endothelial cells to stents under flow conditions.
(A) In vitro capture kinetics of magnetically responsive bovine aortic endothelial cells (BAECs) onto a 304-grade stainless steel stent in the presence of a uniform field of 1000 Gauss and a nonpulsatile flow rate of 30 ml/min. The data were obtained by measuring the fluorescence of magnetic nanoparticles (MNPs). Magnetically responsive BAECs captured in vitro onto a 304-grade stainless-steel stent as evidenced by the red fluorescence of MNPs and Calcein green staining of live cells. (B) In vivo cell delivery to stents deployed in the rat carotid artery under uninterrupted blood flow conditions. A catheter was introduced via the external carotid into the common carotid and advanced beyond the stent to the aortic arch. In the Mag+ group, the injection was carried out with animals placed in a magnetic field of 1000 Gauss, and the field was maintained for a total of 5 min after delivery. In Mag− rats, no magnetic field was applied. To track targeted cells, BAECs were first transduced in culture with replication-defective adenoviral vector encoding firefly luciferase and then loaded with MNPs. The animals were imaged 48 h after delivery by local perivascular administration of luciferin admixed in a pluronic gel. The signal emitted from the stented arterial segment due to the luciferase transgene expression was significantly higher in the animals that received cells in the presence of a magnetic field (Mag+).
Mag+: Magnetic group; Mag−: Control; NP: Nanoparticle; RLU: Relative luminescent units.
Adapted with permission from [14]. © (2008) National Academy of Sciences USA.
The need to transport magnetic drug carriers through soft tissues
Although the targeting strategies discussed above appear to be promising, MNPs often need not only be localized, but also transported through the cellular layers or barriers such as walls of blood vessels, the BBB, RWM or across other soft tissues to reach the target cells and enable retention of carriers in order to provide pharmacologically relevant drug levels in the treated tissue. Force-mediated localization of magnetic carriers in soft tissues still remains poorly understood [40–45]. Following the original investigations by Crick, rheological and magnetic actuation studies of cells have been conducted using magnetic beads; however, these studies did not address the aspects of active (external force controlled) transport of particles in tissues and cells [46,47].
In the context of MNP localization, soft tissues are significantly more complex than biological fluids. In viscous biological fluids, a magnetic force will move MNPs at a constant rate, proportional to the fluid viscosity. However, soft tissues have a complex material structure composed of liquids and solids. Such complex structures may exhibit a nonzero yield stress (resulting in practically no movement of MNPs if the external magnetic force does not exceed a certain threshold). The yield stress and viscous drag depend strongly on the rate of variation of the magnetic forces and on the characteristics of the MNPs, such as size, shape, surface charge and magnetic responsiveness. These magnetic force and MNP characteristics will govern the mechanisms of MNP transport and distribution in soft tissues. Thus, it is very important to determine and apply these parameters in each targeting application, in order to efficiently transport and localize MNPs in soft tissues, and achieve the most therapeutically desirable MNP distribution in the target area.
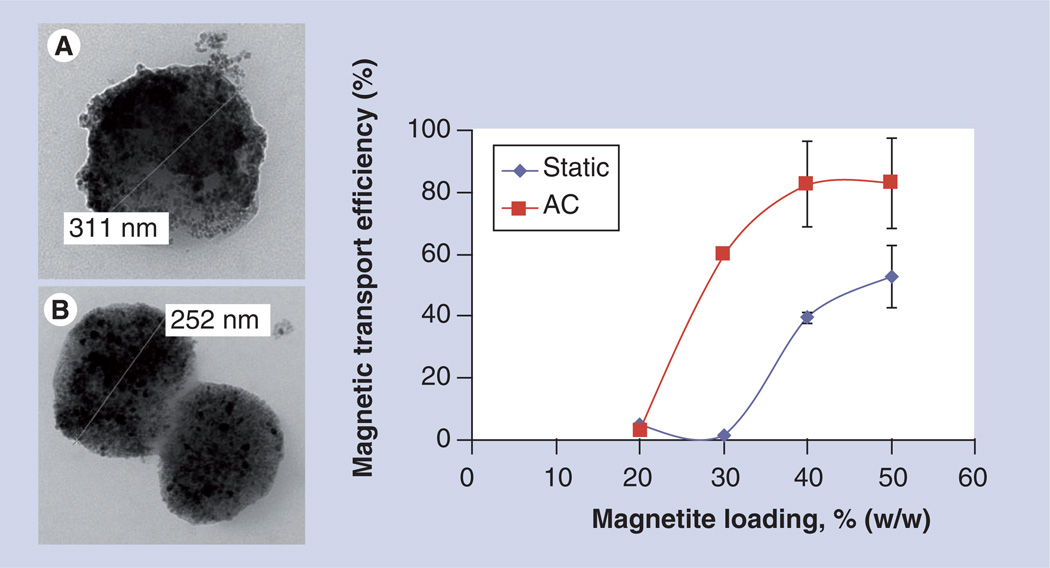
We have suggested recently that a time-varying magnetic field applied simultaneously with the static field gradient on MNPs can impact the mobility of particles in viscous medium by changing the balance between forces acting on particles (Figure 2). Our study shows that a time-varying magnetic field decreases effective viscous drag in a soft medium by generation of an oscillating force on the magnetic carriers, which dramatically improves transport of carriers through a soft medium [48].
Figure 2. Morphology and mobility of magnetic nanoparticles under various conditions.
Transmission electron microscopy of (A) 20% and (B) 50% (w/w) magnetite-loaded polylactic-based magnetic nanoparticles prepared by the emulsification–solvent evaporation method. The graph represents magnetic nanoparticle transport efficiency in gel as a function of different magnetic field settings (static vs static superimposed with an alternating, AC field) and magnetite loadings (n = 3).
AC: Alternating current; w/w: Weight to weight.
Adapted with permission from [48].
Tissue engineering & regenerative medicine
Tissue engineering or regenerative medicine offers new possibilities for the functional and structural restoration of damaged or lost tissue. Tissue engineering involves either seeding cells into a 3D structure called a scaffold, to which the cells can attach and grow, or a bottom-up approach, which involves patterning cells according to a predefined organization that will guide the maturation of the tissue engineered construct [49]. Growth factors (GFs) are frequently added to enhance the proliferation and differentiation of the cells that have been seeded into the scaffold. Mechanical stimulation can also be applied for these purposes. MNPs and magnetic fields have been investigated for their applicability to all of these aspects of tissue engineering.
Scaffolds
The general requirements for scaffolds used in tissue engineering and the various techniques available for their manufacture are described elsewhere [49–52]. Magnetic scaffolds can provide unique capabilities not available with other methods and materials. Magnetic scaffolds can provide controlled release or redosing of GFs, mechanical stimulation of the seeded cells, improved cell seeding and the means of assembling a scaffold in the desired configuration.
The challenge of developing magnetic scaffolds extends beyond the technical problems of scaffold design and the development of an appropriate external magnetic system. Although the general requirements for scaffolds have been well described, the detailed requirements for many specific applications have not yet been determined. For example, mechanical stimulation, discussed in detail below, is known to increase cell differentiation. However, a detailed description of the optimum force required (amplitude, frequency, duration and direction) remains unknown for many applications. If the required forces were well characterized, the problem could be reduced to the still difficult task of designing a magnetic system to deliver this force to cells in a magnetically responsive scaffold.
GF delivery & release
In most tissue engineering approaches, GFs are preloaded into the scaffold prior to implantation. A general overview of GF delivery in tissue engineering, including an extensive list of commonly used GFs, is provided in [53]. Non-magnetic approaches for delivery or controlled release of GFs include the use of gelatin [54] and PLGA [55] microparticles incorporated within the scaffold pores, polymer microspheres or nanospheres produced from a number of degradable and nondegradable polymers of synthetic or natural origin [56,57], and surrounding the scaffold with a gelatin hydrogel loaded with GF [58]. Hydrogels have also been used in a more controlled approach through electrostatic binding of proteins and their subsequent sequential release at rates reflected by their equilibrium binding constants [59,60].
A review of the use of nanoparticles for GF delivery shows that although a large number of investigations of nanoparticle systems for GF delivery have been conducted, very few of these involved MNPs [61]. For some applications such as bone graft substitution, the optimum approach would be to continuously add GF to the engineered tissue in a way that mimics natural growth conditions. It might also be desirable in some situations to modify the release profile based on measurements of the growth rate. Although the non-magnetic approaches for delivery of GFs often provide controlled release, the release profile is preprogrammed prior to implantation. Magnetic approaches have the capability to modify the release profile as needed in vivo.
Possible methods for magnetic delivery and release of GFs include: attraction of MNP loaded with GFs to a magnetically responsive scaffold; magnetically mediated heating of a thermally responsive polymer through the application of an external magnetic field of high frequency; and exertion of a mechanical force to a magnetically responsive scaffold through the application of an external time-varying or continuous field.
Bock et al. have developed a magnetic scaffold to which GFs or other biologically active molecules bound to magnetic particles can be delivered on demand by means of an externally applied uniform magnetic field [62]. Magnetically responsive scaffold exposed to a uniform magnetic field alters the distribution of magnetic flux and leads to higher field gradients near/inside the scaffold in much the same manner as described for the magnetic stents. Commercial scaffolds made of hydroxyapatite and collagen are transformed into magnetic scaffolds by dip-coating the scaffolds in aqueous ferrofluids containing iron oxide nanoparticles stabilized by various macromolecules. Mesenchymal stem cells (MSCs) isolated from human bone marrow were seeded inside the scaffolds and cultured under static magnetic conditions. The continuous magnetization of scaffolds did not pose significant adverse effects on cell viability. Computer simulations performed for a spherical magnetic scaffold 1 cm in diameter, and 150-nm diameter MNPs showed the attractive force exerted by the scaffold on the MNP exceeded the weight of the particle when the field gradient reaches 13 Oe cm−1.
Another approach to making GFs available in an engineered tissue is to preload a magnetic scaffold with the appropriate GFs and utilize an external magnetic field to mechanically release the GFs to the cells as needed. It has been suggested that a ferroscaffold developed for drug release applications could be utilized as a scaffold for engineered tissues [63]. These scaffolds were fabricated using an in situ synthesis of iron oxide nanoparticles in the presence of various concentrations of biodegradable gelatin. Drug release was demonstrated using vitamin B12 and an external magnetic field of approximately 400 Oe that was switched on and off. No attempt was made to seed this scaffold with cells or to deliver GFs, but the authors state that the drug release properties demonstrated make this scaffold a good candidate for tissue engineering.
A macroporous ferrogel scaffold has been developed that can be remotely controlled by a magnetic field to deliver various biological agents on demand [64]. The active porous scaffold gives a large deformation and volume change of over 70% under a moderate magnetic field. Under applied magnetic fields, the macroporous ferrogel can give large and prompt deformation, causing water flow through the interconnected pores. The resulting deformation and water convection was shown to trigger and enhance the release of biological agents in a mouse model.
Considerable work has been done in the development of thermally responsive polymers that could release a drug or GF with the application of energy from external sources, such as ultrasound, near infrared, UV, visible wavelength light and magnetic fields [65]. Thermoresponsive materials have a sharp transition temperature at which they become either soluble or insoluble. When the transition is from a more soluble to a less soluble state, this temperature is known as the lower critical solution temperature. Conversely, if the transition is from a less soluble to a more soluble state, this temperature is known as the upper critical solution temperature [66]. The combination of MNPs and thermoresponsive polymers is unique because MNPs exposed to an alternating magnetic field of high frequency exhibit an increase in temperature due to magnetic hysteresis loss and Brownian relaxation [67,68]. This change in temperature can be conducted to a thermoresponsive polymer leading to a polymer phase transition and a consequent drug burst release.
Extensive research has been performed in using this technology for controlled drug release [69–72] but little work on extending this to controlled GF release has been published. Controlled GF release could be achieved either through direct heating of thermally responsive polymeric particles, which contain both MNPs, and the GF, or through secondary heating in which a thermally responsive particle containing the GF is surrounded by magnetic particles that are heated and the resulting increase in local temperature causes release of the GF from the thermally responsive polymeric particle.
Mechano–magnetic stimulation of cells within scaffolds
In tissue regeneration, it has been shown that in addition to molecular signals (e.g., GFs), physical cues, such as electrical signaling, mechanical stimulation of constructs and medium perfusion, may be essential for appropriate tissue formation [73–76]. These signals aim to either mimic signals found in vivo or induce beneficial cellular processes for tissue formation.
Mechanical stimulation of cells has been broadly investigated in the last couple decades. The most common examples for application of mechanical stimulation are bioreactors, developed to apply mechanical forces via piston/compression systems, substrate bending, hydrodynamic compression and fluid shear [77–80]. Although this approach was found to have a positive impact for many tissue types, it has its drawbacks. The forces are mainly applied to the scaffold rather than directly to the cell membrane or cytoskeleton where they are required, limiting their applicability to 3D cultured cells. In addition, its implementation is limited to in vitro application and cannot be extended to in vivo tissue engineering.
A major advantage of magnetically mediated stimulation is that nanomagnetic actuation allows ‘action at a distance’ (thus enabling actuation both in vitro and in vivo). The magnetic field can be coupled to the particle to actuate a process within a target cell regardless of whether there are intervening structures, such as tissue. In addition, stress parameters can also be varied dynamically, simply by changing the strength and frequency of the applied field. The ability to precisely target, manipulate and activate individual ion channels or targets within the cells is probably the biggest advantage of this approach.
Mechanotransduction, while being a very fundamental and important initiator of many biological processes, is still not fully understood in terms of mechanisms. Briefly, stretch-activated ion channels, responsible for the activation of the mechnotransduction pathway, are found within the cell membranes of almost every cell type. There is a large amount of evidence to suggest different kinetic activation patterns, including stretch [81–84], state of phosphorylation [85–87] and the presence of specific ligand [88]. Nonetheless, the majority of structural studies have shown that these channels can sense membrane tension directly. In order to test whether magnetically tunable particles could induce mechanical stimulation within cells, different application methods were used (broadly reviewed in [89]).
The feasibility of mechanical stimulation induced by magnetic forces has been shown in several previous works, mainly by binding magnetic particles to cellular targets. The idea to deform the cytoskeleton and test cell response through magnetically responsive particles bound to integrin receptor on the cell membrane led to numerous experiments already in the 1990s [90–92]. In this model, magnetic particles are specifically bound to cell surface integrins and actuated from outside by magnetic field application, leading to an overall membrane and cytoskeleton deformation, thus causing activation of the mechanosensitive channels and cell response.
Investigators in this field have aimed to increase binding specificity to ion channels only, thus enabling actuation of the targeted channel without interrupting normal cell function. This could be achieved by particle modification with ion channel-specific antibodies. Bound particles were later actuated by high gradient magnetic fields leading to channel opening and appropriate cellular response [93]. These and other examples proved the ability of externally applied magnetic fields to induce cellular response.
One of the existing drawbacks of the receptor stimulation technique is its inability to stimulate cells in the long-term due to internalization of the magnetic particles. Hence, the idea to stimulate cells when the particles attached on their surface was further developed by investigating the effect of internalized particles within the stimulated cells. Almost all studied cell types showed the ability to internalize nano- and submicron particles. The ability to internalize the particle is dependent on various factors such as cell type, particle size, the hydrophobicity and surface charge of the particle polymer, the nature of the particle surface coating and the proliferation rate of the cells, broadly reviewed by Hughes et al. [89]. These properties have recently been exploited for use in transfections and internal manipulations within the cell of interest [94–96]. Several studies investigated various conditions of magnetic cell loading for use in targeted cell delivery [97,98]. MacDonald et al. had shown that internalization of particles is an active (cytoskeleton reorganization-dependent) and magnetic force-dependent process [98].
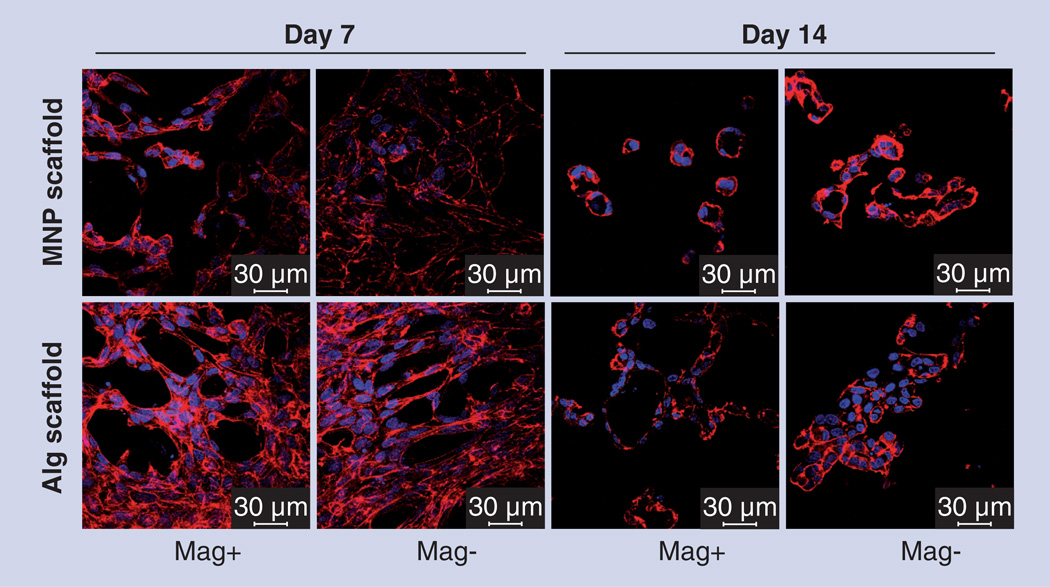
The authors of this review recently implemented a mechanical stimulation approach in 3D cultivation systems within polymeric scaffolds [99]. A new alginate-based composite biomaterial with tunable and externally controlled properties was explored in its ability to provide means of physical stimulation to endothelial cells (Figure 3). We created magnetite-impregnated alginate scaffolds proven to be magnetically responsive under exposure to an alternating magnetic field. These scaffolds were seeded with bovine aortic endothelial cells and stimulated by an alternating magnetic field (10–15 Gauss, 40 Hz) during the first 7 days of a 14-day experimental course. Cells within stimulated constructs showed significantly elevated metabolic activity during the stimulation period, implying a migration and reorganization processes within the cells. Immunostaining and confocal microscopy analyses further confirmed this observation showing that on day 14, in magnetically stimulated scaffolds without the addition of any GFs or other supplements, cellular vessel-like (loop) structures, known as indicators of vasculogenesis and angiogenesis were formed compared with cell sheets or aggregates observed in the nonstimulated (control) scaffolds (Figure 4). Accurate control of cellular organization to form tissue-engineered constructs together with additional molecular signals could lead to the creation of an efficient prevascularized tissue construct with potential applicability for transplantation.
Figure 3. Scaffold morphology.
Scanning electron microscopy images of the (A) 1.2% (weight/volume) magnetic nanoparticle–alginate and (B) non-magnetic alginate scaffolds. (C) Macroscopic view of the (i) dry, (ii) prewetted for 30 min and (iii) hydrated with culture medium for 24 h scaffolds.
Reproduced with permission from [99].
Figure 4. Immunostaining and confocal microscopy studies.
Endothelial cell organization in magnetic nanoparticle-impregnated alginate (MNP–alginate) and alginate constructs, on days 7 and 14 postcell seeding stimulated by an alternating magnetic field (10–15 Gauss, 40 Hz), Mag+ group. Mag− group is nonstimulated control. The cells are stained for F-actin (red) and nuclei (blue) (scale bar: 30 µm). By day 14, in the magnetically stimulated scaffolds without addition of any growth factors or other supplements, cellular vessel-like (loop) structures, known as indicators of vasculogenesis and angiogenesis were formed as compared with cell sheets or aggregates observed in the nonstimulated (control) scaffolds.
Alg: Alginate; Mag+: Magnetic group; Mag−: Control; MNP: Magnetic nanoparticle.
Reproduced with permission from [99].
The exact mechanism of cell stimulation within the magnetically responsive matrix is yet to be determined. Because MNPs and their aggregates are anisotropic in terms of their geometrical shape (i.e., not of ideally spherical geometry) they might generate local torque forces applied directly on cells adhered to the MNP-decorated scaffold wall surface and result in a local magneto–mechanical effect applied on cells. Alternatively, when the magnetic particle density within the scaffold is relatively high, enabling nanoparticles to experience magnetic attraction; a magnetostrictive mechanism could be employed.
Magnetostriction is a property of ferro- and ferri-magnetic materials that causes material to change its shape or dimensions during the process of magnetization. Individual magnetite crystals in the size range of 5–20 nm are superparamagnetic due to their small size. However in the alginate-composite material, they aggregate into larger structures of 776 ± 416 nm, displaying slight hysteresis, which is indicative of a slow magnetic relaxation process, resulting in a remnant magnetization or ferrimagnetism [99]. When the composite magnetic material is exposed to a magnetic field, magnetization on particles generates magnetostrictive strain due to particle attraction, leading to overall scaffold deformation and change in dimensions mimicking the behavior of domains in bulk ferro- or ferri-magnetic materials. For example, such alternating deformation can be scaffold contraction leading to a direct mechanical effect applied to cells. These hypotheses should be corroborated experimentally.
Scaffold production
Another application for MNPs in tissue engineering scaffolds is the use of MNPs and an applied magnetic field in the initial fabrication of the scaffold [100–103]. The ordered structure of the extracellular matrix of tissues in living organisms plays a key role in cellular response. Duplicating these structures at the nanoscale in the laboratory for tissue engineering applications has proved to be a difficult endeavor.
Alsberg et al. have developed a method to spatially control the self-assembly of fibrin lattices [100]. Fibrin has been investigated extensively as a biological scaffold for bone cartilage, neural, adipose and blood vessel regeneration. The structure and morphology of fibrin networks (i.e., fiber size, branching and fiber spacing) influence their physical properties as a scaffold, and the ability to control the structure is essential. In this technique superparamagnetic microbeads were first coated with thrombin and positioned by a controlled magnetic field into hexagonal arrays. When a fibrinogen solution was added to the magnetically aligned array, the fibrin nano-fibrils that subsequently polymerized from the beads preferentially oriented along the main bead–bead axes in a triangulated geodesic pattern. The authors demonstrated biocompatibility of human microvascular endothelial cells that were cultured on the resultant fibrin matrices.
Control of scaffold porosity in addition to layout is another important factor in developing scaffolds for tissue engineering. Hu et al.combined particulate leaching technology using sugar with magnetic microparticles and a magnetic field to fabricate 2D and 3D porous biodegradable scaffolds made of poly(l-lactide-co-ε-caprolactone) [104]. Ferrite micro-/nano-particles were encapsulated in sugar microspheres to enable their magnetization. A magnetic apparatus consisting of a block-type neodymium magnet underneath a grid of steel wires was magnetized by the magnet and was then used to form an assembled template for polymer. After polymer casting and removal of the sugar template, spherical pores were generated inside the scaffold. The authors demonstrated that this approach could be extended to 3D scaffolds, such as those needed for vascular tissue engineering by winding the 2D porous sheets on sacrificial molds. The biocompatibility of the developed scaffold was confirmed by viable cells after 4-day culture.
Hydrogels are widely used as tissue-engineering scaffolds. One of the challenges has been to use hydrogels in a ‘bottom-up’ assembly approaches that attempt to replicate nature’s use of repeating structures to build constructs by assembling well characterized building blocks. Yuet et al. have approached this problem through the microfluidic synthesis and field-driven self-assembly of monodisperse, multifunctional Janus hydrogel particles with anisotropic superparamagnetic susceptibility and chemical composition [103]. Janus particles have the property that surfaces of the two hemispheres exhibit different chemical properties. In this case, one hemisphere is superparamagnetic and the other is non-magnetic. This results in an anisotropic magnetic susceptibility and, under an applied field, permits one hemisphere to interact with satellite particles or nearby chains while preserving the chain’s symmetry in a lateral field. Under varying conditions the authors were able to demonstrate self-assembly into a stationary, semiregular array and mesh-like superstructures formed as parallel chains zippered together. MNPs have been shown to enhance osteoinduction even without the presence of a magnetic field [105]. This finding has been used as a basis for development of magnetic biodegradable fibrous materials with potential applications in bone regeneration [106]. Nanofibrous membranes were fabricated by electrospinning Fe3O4/chitosan/polyvinyl alcohol. MG63 human osteoblast-like cells were seeded on the membranes and showed good cell adhesion and proliferation based on scanning electron microscopy observation and MTT assay. Using tissue culture plates as controls, cells cultured on these membranes had increased proliferation on days 3, 5 and 7 and this improvement increased with higher Fe3O4 nanoparticle loading. The authors conclude that the results suggest that the magnetic biodegradable nanofibrous membranes can be a promising biomaterial for enhancement of osteogenesis. Because cell adhesion and proliferation correlated with the nanoparticle loading, the authors also suggested the possibility of further controlling cell function through regulation of the Fe3O4 nanoparticle loading content in the membranes.
Cell patterning
The use of scaffolds for tissue engineering presents some limitations that could limit their effectiveness in certain tissue engineering applications. Scaffolds can slow or delay the organization of cells and the establishment of cell–cell interactions [107]. Scaffolds could be poor substitutes for the extracellular matrix due to a number of factors including insufficient biological activity, immunogenicity and elevated inflammatory reactions, fluctuating degradation rate and uncontrollable cell–biomaterial interactions [108]. A detailed list of the advantages and disadvantages of scaffold and scaffold-free tissue engineering approaches for different applications is provided in [109]. A review of non-magnetic approaches for cell patterning including cell sheets, cell-laden hydrogels, 3D printing, inkjet printing and laser-assisted bioprinting is provided in [49]. MNPs and magnetic fields can be used to position cells in a pattern suitable for tissue engineering without the use of artificial scaffolds.
A technique named magnetic force-based tissue engineering (Mag-TE) has been developed [110] and applied to a number of applications including preparation of artificial skeletal muscles [111], bone tissue for repair of defects [112,113], small-diameter vascular tissue for graft survival [114] and retinal pigment epithelium for choroidal neovascularization [115]. Although there are variations in the details for the various applications, the basic concept involves using magnetite cationic liposomes containing magnetite nanoparticles that electrostatically interact with cell membranes, and can therefore be used for magnetically labeling live cells. These magnetically labeled cells are accumulated in a desired pattern through an applied magnetic field and steel structure under a cell culture surface. The authors report that both patterned lines of single cells and complex cell patterns (curved, parallel or crossing patterns) were successfully fabricated.
The Mag-TE technique has also been used to generate a MSC sheet to treat severe ischemic diseases [116]. Using the Mag-TE techniques, magnetized MSCs were formed into multilayered cell sheets. These sheets were placed into nude mice subjected to unilateral hind limb ischemia and compared with both saline and injected MSCs. The MSC sheet group had a greater angiogenesis in ischemic tissues compared with the control and MSC-injected groups as measured by capillary density and arteriole density in histological sections harvested from the ischemic adductor and gastrocnemius muscles.
A nonuniform applied magnetic field has been used to create a 3D cell assembly of magnetically labeled cells [117] based, in part, on earlier work by Wilhelm et al. [118]. Experiments were conducted using human endothelial progenitor cells and mouse macrophages magnetically labeled using anionic citrate-coated iron oxide nanoparticles. Magnetic field gradients were applied to suspensions of these cells using either a cylindrical tip or a truncated tip placed on a permanent magnet. For the cylindrical tip, the gradient was approximately 1000 T/m at 500 µm resulting in a magnetic force several orders of magnitude higher than other forces experienced by a cell in suspension, including Brownian motion or buoyancy. Cells progressively stacked near the tip to form a 3D aggregate. The authors were able to control the packing density of cells by tuning the magnetic field gradient geometry and intensity, the magnetic cellular load and the number of cells. From the packing density the authors made some structural inferences based on a comparison of packing density to that of a simple cubic crystal. The authors believe that this ability to control cell density and distribution is essential for the formation of tissues in vitro.
Magnetic levitation has been evaluated to address the challenge of 3D tissue culture [107]. The authors demonstrated that the structure of the tissue culture can be manipulated, and multicellular clustering of different cell types in co-culture can be accomplished through control of the field configuration. Using a variety of ring-shaped magnets, cells were levitated and the resultant structures evaluated. Using a large-radius magnet, it was observed that the shape of the cell pattern generated was ring-shaped and this pattern was maintained after the magnet was removed. The concept of magnetic levitation was also used in development of 3D tumor spheroids that mimic in vivo tumors for potential anticancer drug screening [119].
One of the challenges in engineering 3D cell-dense tissues is the conflicting objectives of increasing porosity within fibrous scaffolds to improve cellular infiltration and the negative effect that this has on fiber alignment [120]. To address these issues an electrospinning technique was used to fabricate fibrous bundles consisting of composite fibers of poly(l-lactic-co-glycolic) acid and MNPs. C2C12 myoblasts were seeded on the bundles and were grown along the direction of the underlying fibers. When treated with the differentiating medium heat-inactivated horse serum, the myoblasts fused together and formed multinucleated myotubes. After exposure to an external magnetic field the resultant cell rods responded by self-assembling into 3D tissues with a highly ordered architecture. After 3 days, 3D cell-dense tissue architecture was retained when the magnetic field was removed.
Microscale cell-laden hydrogels fabricated using the photopatterning method can be useful building blocks for tissue engineering applications. However, 3D assembly of these microgels to form larger 3D complex constructs is still a challenge. To address this challenge an approach has been developed in which MNP-loaded cell-encapsulating microscale hydrogels were fabricated and assembled into 3D multilayer constructs using magnetic fields [121]. By spatially controlling the magnetic field, the authors demonstrated that 3D construct geometry can be manipulated, and multilayer assembly of multiple microgel layers can be achieved.
A low-cost method termed magnetic hydrogel-based cell patterning has been developed in an attempt to perform cell patterning in a way that is not dependent on cell labeling [122]. Using hydrogel blocks and a simple magnet, the authors were able to produce complicated cell patterns. The blocks were fabricated by mixing magnetic particles with the hydrogel and then generating the desired patterns through photolithography. Cells were seeded into a culture plate into which the hydrogel blocks had been placed. The hydrogel blocks prevented cell adhesion in those areas onto which they had been inserted. The blocks were subsequently removed using a magnet and the cell pattern maintained. Heterotypic cell patterning can be achieved by seeding a second type of cell, which preferentially adheres to areas not already seeded.
Cell seeding
Cell seeding into scaffolds for tissue engineering presents several challenges that can be addressed through the use MNPs and applied magnetic fields. Proper distribution of the cells within the scaffold is often difficult to achieve due to the hydrophobic nature of most scaffold materials. In addition, with 3D scaffolds it is relatively easy to seed cells onto the surface layers but much more difficult to seed cells at the proper density into the interior of the scaffold. Non-magnetic approaches to solve these problems include various dynamic seeding methods and bioreactors [123–125]. These methods may have limitations in that they may either destroy the porous scaffold architecture or fail to produce tissue of adequate thickness [126]. None of the studies cited below directly compared magnetic cell seeding effectiveness with these other approaches. One potential advantage of magnetic seeding is that it could possibly control the distribution of cells, perhaps in a nonhomogenous configuration, within the scaffold through appropriate design of the magnetic field. Several of the papers below mention this possibility, but it has not been demonstrated experimentally.
A magnetic tweezer system has been developed to apply controlled forces to a large number of magnetic beads or magnetically labeled cells inside a scaffold [127]. Although the primary purpose of this research is to use the magnetic force acting on magnetic objects of various sizes to determine local physical parameters of the scaffold, the technology can also allow optimization of cell seeding in the construct and induce a defined 3D cellular organization.
The combined use of MNP-seeded cells and magnetic force was shown to increase the infiltration and distribution of cells into PLGA salt-leached scaffolds compared with controls [126]. 3T3 fibroblast cells containing magnetic iron/platinum nanoparticles were seeded along one side of the scaffold. A cylindrical neodymium magnet (magnetic field, 4000 Gauss) was placed underneath the well plate containing the seeded scaffold. After 3 days of incubation, measurements were taken of cell penetration into the scaffold. Magnetic seeding resulted in a more than tenfold increase in density in the center of the scaffold compared with non-magnetic controls.
The Mag-TE technology described above has been applied to the problem of cell seeding into a scaffold using a technique termed ‘mag-seeding’ [128]. NIH/3T3 fibroblasts, magnetically labeled with magnetite cationic liposomes (described above), were seeded onto six types of commercially available scaffolds. A magnet (4000 Gauss) was placed under the scaffold. The cell-seeding efficiency for all scaffolds was enhanced by Mag-seeding relative to static seeding (58.9 vs 10.8%).
The potential of magnetically mediated multilayered cell seeding in a tubular architecture for generation of vascular grafts was demonstrated by Perea and coworkers [129]. In this work, a radial magnetic force generated around the collagen-based tubular scaffold guided the sequential seeding of five layers of magnetically labeled human smooth muscle cells followed by the deposition of one layer of human umbilical vein endothelial cells. Co-cultured tubular graft incubated over a 5-day period demonstrated densely packed multilayers of smooth muscle cells coated with one layer of endothelial cells resembling the natural blood vessel architecture. Magnetically mediated cell seeding in tubular geometry enables rapid cell deposition, avoiding cell settling effects due to gravity, leading to accelerated cell–substrate adhesion. Creation of tubular constructs using this methodology is immediate as compared with the dynamic rotational seeding, which occurs at a much longer timescale.
Conclusion & future perspective
Magnetic-based systems utilizing superparamagnetic nanoparticles and various configurations of magnetic fields and field gradients provide a range of new opportunities for a number of clinical applications. For certain medical conditions, localized therapy is highly desirable as it can enable administration of a significantly lower drug dose, thus minimizing systemic drug-induced toxicity. Magnetically mediated localization of therapeutic agents (e.g., drugs, genes and cells) is a promising approach to improve the efficacy and safety of the administered therapy resulting in improved clinical outcomes. Furthermore, magnetic targeting can enable redosing or administration of an additional drug at the diseased site, which for example, is highly desirable for treatment of vascular lesions where metallic stents are currently used to alleviate re-obstruction of the blood vessels.
Although promising results have been achieved demonstrating the potential of magnetic targeting, many challenges still exist in order to successfully translate this technology into clinical settings. Substantial improvements in both the magnetic carriers and the targeting magnet systems are likely to be necessary to make the magnetic targeting a viable clinical treatment modality. A number of applications, such as drug delivery to brain tumors, the inner ear and stented blood vessels, which require penetration of a cellular barrier (e.g., BBB, RWM and endothelial luminal layer underlying the walls of blood vessels). The overall objectives of drug targeting consist not only in physical guidance, but also in the retention of the therapeutic agent at the target site. The retention of therapy could be achieved by the magnetically facilitated transport of magnetic carriers through the tissue, overcoming the cellular barriers mentioned above. Efficient tissue penetration of therapeutic carriers could also result in a more pharmacologically optimal drug distribution within target tissue improving the therapeutic outcome. Use of a time-varying uniform magnetic field overlaid with the static gradient field has been shown to radically improve transport of magnetic carriers in gels [48]. This approach can be implemented in real tissues to retain and distribute magnetic drug carriers. Additional strategies that can change the effective resistance of biological tissues and improve drug carrier tissue penetration include use of magnetic carriers with a proteolytic surface to increase the carrier’s mobility through the extracellular matrix [43] or utilization of ultrasound to generate inertial cavitation to improve transdermal drug delivery [130,131]. A better understanding of these mechanisms will enable the development of means by which distribution of MNPs in soft tissues can be controlled to a significant extent in a number of clinical strategies, radically improving therapeutic outcomes.
Scaling up the magnetic drug delivery technology from animals and laboratory models to humans would probably require the design of magnet systems enabling the pushing of a magnetic carrier inward in contrast to a conventional pulling of carriers, usually achieved using permanent magnets. The need to develop such carrier pushing-magnet systems has been recognized and work is ongoing in this direction [23].
Besides the aspects of magnet system design, it is important to realize that there are many challenges related to the nanoparticle–biomolecule interface when magnetic carriers are administered systemically. This phenomenon is termed as the ‘protein (biomolecule) corona’ and is related to the formation of a protein layer on the surface of nanoparticles, the so called ‘bio–nano’ interface that the cell actually ‘sees’ when it interacts with particles [132]. This biomolecule corona can mask targeting ligands anchored at the particle surface, influence biodistribution in vivo, and affect cell internalization and intracellular trafficking. Considering the implications of the biomolecule corona effect for MNP targeting, there are two possible approaches that can be taken regarding nanoparticle design. One approach is related to surface engineering and would suggest designing an MNP interface that will experience minimal interactions with the surrounding biological environment except for displaying the specific affinity/targeting desired (i.e., a type of adsorption-proof nanoparticle). A second approach would involve exploitation of the ‘corona’ layer itself for targeting, through understanding which biomolecules promote the delivery of particles to which location [133].
The manipulation and control of cells and cellular structures through MNP-based actuation is a relatively new strategy that has shown great potential for tissue engineering and regenerative medicine applications. Magnetically responsive composite scaffolds are very promising materials for these fields as they can enable control of the spatial distribution and temporal kinetics of GF release. They also can enable local control over the induction of tissue regeneration, as opposed to the effect of a diffusible GF, and will provide the first capability to control precisely where proliferation, maturation or differentiation occurs in an engineered tissue. In the future, this positional control of cellular behavior may facilitate the production of tissues composed of multiple cell lineages derived from a single stem cell type.
In summary, we believe that the use of MNPs has great potential for clinical applications. It is also clear that more animal and clinical studies are necessary to fully realize the clinical potential of these magnetic-based systems. Successful translation of this technology to the clinic will require the joint efforts of researchers from multiple disciplinary backgrounds.
Executive summary.
Physical considerations for magnetic targeting
-
▪
A magnetic carrier should be superparamagnetic (to prevent agglomeration, avoiding embolism) and capable of acquiring a sufficient magnetic moment to experience a magnetic force as large as possible to overcome drag and yield forces.
-
▪
To maximize the force acting on a magnetic carrier a magnet system should generate a sufficiently strong uniform magnetizing field (to maximize the induced carrier magnetization) and generate strong gradients at the targeting region.
Therapy delivery/targeting
-
▪
Scaling up the magnetic targeting technique to deliver therapy to the inner ear would require design of magnet systems enabling the pushing of therapeutic carriers through the round window membrane, because a pulling magnet system would not satisfy US FDA magnetic safety limits.
-
▪
Pathologies associated with certain neurological disorders compromise the integrity of the blood–brain barrier making it more susceptible to physically facilitated transport of magnetic carriers loaded with drugs. Conditions including carrier size and surface charge, brain tumor flow dynamics, carrier administration route and topography of a magnetic field should be carefully considered to enable efficient brain targeting.
-
▪
A combination of external uniform magnetic field with magnetizable implants can enable the generation of local high field gradients for targeting deep tissue, including blood vessels where magnetizable implants can be placed.
-
▪
A time-varying uniform magnetic field overlaid with a permanent gradient field offers a promising approach to enhance transport and localization of magnetic carriers in soft tissues. The ‘oscillating’ effect of a time-varying magnetic field significantly improves displacement of (magnetic nanoparticles [MNPs]) within a soft medium, possibly by reducing viscous drag.
Tissue engineering & regenerative medicine
-
▪
Composite biomaterials consisting of magnetic particles and thermoresponsive polymers can enable remote control of the release profiles of growth factors within tissue engineering scaffolds in vivo.
-
▪
Magnetic composite scaffolds also can provide means of remote physical stimulation to cultivated cells within scaffolds in vitro and in vivo. This methodology, combined with controlled release of chemical signals (i.e., growth factors), can potentially lead to the creation of an efficient prevascularized tissue construct in vitro, which can be transplanted and efficiently integrated in vivo with the host tissue.
-
▪
MNPs and magnetic fields can be used in the initial fabrication of the tissue engineered scaffolds enabling spatial control in the scaffold self-assembly process.
-
▪
In applications where scaffolds present limitations for production of tissue, MNPs and magnetic fields offer an alternative to position or assemble cells in a pattern suitable for tissue engineering without the use of scaffolds.
-
▪
Magnetically facilitated seeding of cells within scaffolds can allow possible control over the distribution of cells, perhaps in a nonhomogeneous configuration, within the scaffold through appropriate design of the magnetic field.
Acknowledgments
This work was partially supported by the Louis and Bessie Stein Family foundation through the Drexel University College of Medicine, American Associates of the Ben-Gurion University of the Negev, Israel (S Cohen and B Polyak), USA Award Number 5R01HL107771-02 from the National Heart, Lung and Blood Institute (B Polyak) and the European Union FWP7 (INELPY; S Cohen). Y Sapir gratefully acknowledges the generous fellowship from the late Daniel Falkner and his daughter Ann Berger and thanks the Azrieli Foundation for the award of an Azrieli Fellowship supporting her PhD program.
Footnotes
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the NIH.
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1. Colombo M, Carregal-Romero S, Casula MF, et al. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012;41(11):4306–4334. doi: 10.1039/c2cs15337h. ▪▪ Excellent overview of the biological applications of magnetic colloidal nanoparticles with particular focus on synthesis, characterization and discussion of the challenges for an extended application of magnetic nanoparticles in medicine.
- 2. Krishnan KM. Biomedical nanomagnetics: a spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans. Magn. 2010;46(7):2523–2558. doi: 10.1109/TMAG.2010.2046907. ▪▪ Comprehensive review focusing on targeted drug delivery and triggered release, novel contrast agents for MRI magnetic fluid hyperthermia for cancer therapy in vitro diagnostics and the emerging magnetic particle imaging technique.
- 3.Laurent S, Dutz S, Hafeli UO, Mahmoudi M. Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011;166(1–2):8–23. doi: 10.1016/j.cis.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, Forge D, Port M, et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008;108(6):2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011;63(1–2):24–46. doi: 10.1016/j.addr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Pankhurst QA, Thanh NTK, Jones SK, Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D. Appl. Phys. 2009;42:224001. [Google Scholar]
- 7.Polyak B, Friedman G. Magnetic targeting for site-specific drug delivery: applications and clinical potential. Expert Opin. Drug Deliv. 2009;6(1):53–70. doi: 10.1517/17425240802662795. [DOI] [PubMed] [Google Scholar]
- 8.Wankhede M, Bouras A, Kaluzova M, Hadjipanayis CG. Magnetic nanoparticles: an emerging technology for malignant brain tumor imaging and therapy. Expert Rev. Clin. Pharm. 2012;5(2):173–186. doi: 10.1586/ecp.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexiou C, Schmid RJ, Jurgons R, et al. Targeting cancer cells: magnetic nanoparticles as drug carriers. Eur. Biophys. J. 2006;35(5):446–450. doi: 10.1007/s00249-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 10.Lubbe AS, Alexiou C, Bergemann C. Clinical applications of magnetic drug targeting. J. Surg. Res. 2001;95(2):200–206. doi: 10.1006/jsre.2000.6030. [DOI] [PubMed] [Google Scholar]
- 11. Chorny M, Fishbein I, Yellen BB, et al. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc. Natl Acad. Sci. USA. 2010;107(18):8346–8351. doi: 10.1073/pnas.0909506107. ▪▪ High-quality study that demonstrates the feasibility of targeted drug delivery to intravascular stents mediated by a uniform magnetic field for the treatment of in-stent restenosis.
- 12.Pislaru SV, Harbuzariu A, Agarwal G, et al. Magnetic forces enable rapid endothelialization of synthetic vascular grafts. Circulation. 2006;114(Suppl. 1):I314–I318. doi: 10.1161/CIRCULATIONAHA.105.001446. [DOI] [PubMed] [Google Scholar]
- 13.Pislaru SV, Harbuzariu A, Gulati R, et al. Magnetically targeted endothelial cell localization in stented vessels. J. Coll. Cardiol. 2006;48(9):1839–1845. doi: 10.1016/j.jacc.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 14. Polyak B, Fishbein I, Chorny M, et al. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc. Natl Acad. Sci. USA. 2008;105(2):698–703. doi: 10.1073/pnas.0708338105. ▪▪ Thorough investigation demonstrating the feasibility of magnetically mediated endothelial cell targeting to magnetizable intravascular stents in vitro and in vivo.
- 15.Yellen BB, Forbes ZG, Halverson DS, et al. Targeted drug delivery to magnetic implants for therapeutic applications. J. Magn. Magn. Mater. 2005;293(1):647–654. [Google Scholar]
- 16.Mccall AA, Swan EE, Borenstein JT, Sewell WF, Kujawa SG, Mckenna MJ. Drug delivery for treatment of inner ear disease: current state of knowledge. Ear Hearing. 2010;31(2):156–165. doi: 10.1097/AUD.0b013e3181c351f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goycoolea MV, Lundman L. Round window membrane. Structure function and permeability: a review. Microsc. Res. Tech. 1997;36(3):201–211. doi: 10.1002/(SICI)1097-0029(19970201)36:3<201::AID-JEMT8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Wang Y, Chen K, Grady BP, Dormer KJ, Kopke RD. Magnetic assisted transport of PLGA nanoparticles through a human round window membrane model. J. Nanotechnol. Eng. Med. 2010;1(3):031010–031016. [Google Scholar]
- 19.Mondalek Fadee G, Zhang Yuan Y, Kropp B, et al. The permeability of SPION over an artificial three-layer membrane is enhanced by external magnetic field. J. Nanobiotechnol. 2006;4(4) doi: 10.1186/1477-3155-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dormer KJ, Awasthi V, Galbraith W, Kopke RD, Chen K, Wassel R. Magnetically-targeted, technetium 99m-labeled nanoparticles to the inner ear. J. Biomed. Nanotechnol. 2008;4(2):174–184. [Google Scholar]
- 21.Chakeres DW, De Vocht F. Static magnetic field effects on human subjects related to magnetic resonance imaging systems. Prog. Biophys. Mol. Biol. 2005;87(2–3):255–265. doi: 10.1016/j.pbiomolbio.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Schenck JF. Physical interactions of static magnetic fields with living tissues. Prog. Biophys. Mol. Biol. 2005;87(2–3):185–204. doi: 10.1016/j.pbiomolbio.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 23. Shapiro B, Dormer K, Rutel IB. A two-magnet system to push therapeutic nanoparticles. AIP Conf. Proc. 2010;1311(1):77–88. doi: 10.1063/1.3530064. ▪ Interesting initial work on the design of a magnet system to push magnetic carriers.
- 24.Brightman M. Ultrastructure of brain endothelium. In: Bradbury MWB, editor. Physiology and Pharmacology of the Blood–Brain Barrier. Berlin, Germany: Springer-Verlag; 1992. pp. 1–22. [Google Scholar]
- 25.Lo EH, Singhal AB, Torchilin VP, Abbott NJ. Drug delivery to damaged brain. Brain Res. Brain Res. Rev. 2001;38(1–2):140–148. doi: 10.1016/s0165-0173(01)00083-2. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Garcia E, Andrieux K, Gil S, Couvreur P. Colloidal carriers and blood-brain barrier (BBB) translocation: a way to deliver drugs to the brain? Int. J. Pharm. 2005;298(2):274–292. doi: 10.1016/j.ijpharm.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc. Res. 1999;58(3):312–328. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- 28.Vajkoczy P, Menger MD. Vascular microenvironment in gliomas. Cancer Treat. Res. 2004;117:249–262. doi: 10.1007/978-1-4419-8871-3_15. [DOI] [PubMed] [Google Scholar]
- 29.Hassan EE, Gallo JM. Targeting anticancer drugs to the brain. I: Enhanced brain delivery of oxantrazole following administration in magnetic cationic microspheres. J. Drug Target. 1993;1(1):7–14. doi: 10.3109/10611869308998759. [DOI] [PubMed] [Google Scholar]
- 30.Pulfer SK, Gallo JM. Enhanced brain tumor selectivity of cationic magnetic polysaccharide microspheres. J. Drug Target. 1998;6(3):215–227. doi: 10.3109/10611869808997896. [DOI] [PubMed] [Google Scholar]
- 31.Pulfer SK, Ciccotto SL, Gallo JM. Distribution of small magnetic particles in brain tumor-bearing rats. J. Neurooncol. 1999;41(2):99–105. doi: 10.1023/a:1006137523591. [DOI] [PubMed] [Google Scholar]
- 32.Chertok B, David AE, Huang Y, Yang VC. Glioma selectivity of magnetically targeted nanoparticles: a role of abnormal tumor hydrodynamics. J. Control. Release. 2007;122(3):315–323. doi: 10.1016/j.jconrel.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chertok B, Moffat BA, David AE, et al. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29(4):487–496. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chorny M, Fishbein I, Forbes S, Alferiev I. Magnetic nanoparticles for targeted vascular delivery. IUBMB Life. 2011;63(8):613–620. doi: 10.1002/iub.479. ▪ Good recent review focusing on the aspects of targeted drug, cell and gene delivery for vascular applications.
- 35.Roiron C, Sanchez P, Bouzamondo A, Lechat P, Montalescot G. Drug eluting stents: an updated meta-analysis of randomised controlled trials. Heart. 2006;92(5):641–649. doi: 10.1136/hrt.2005.061622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gulati R, Jevremovic D, Witt TA, et al. Modulation of the vascular response to injury by autologous blood-derived outgrowth endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2004;287(2):H512–H517. doi: 10.1152/ajpheart.00063.2004. [DOI] [PubMed] [Google Scholar]
- 37.Hofmann A, Wenzel D, Becher UM, et al. Combined targeting of lentiviral vectors and positioning of transduced cells by magnetic nanoparticles. Proc. Natl Acad. Sci. USA. 2009;106(1):44–49. doi: 10.1073/pnas.0803746106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyrtatos PG, Lehtolainen P, Junemann-Ramirez M, et al. Magnetic tagging increases delivery of circulating progenitors in vascular injury. JACC. Cardiovasc. Interv. 2009;2(8):794–802. doi: 10.1016/j.jcin.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Consigny PM, Silverberg DA, Vitali NJ. Use of endothelial cells containing superparamagnetic microspheres to improve endothelial cell delivery to arterial surfaces after angioplasty. J. Vasc. Interv. Radiol. 1999;10(2 Pt 1):155–163. doi: 10.1016/s1051-0443(99)70458-6. [DOI] [PubMed] [Google Scholar]
- 40.Barnes AL, Wassel RA, Mondalek F, Che K, Dormer KJ, Kopke RD. Magnetic characterization of superparamagnetic nanoparticles pulled through model membranes. BioMag. Res. Technol. 2007;5(1):1–10. doi: 10.1186/1477-044X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalambur VS, Han B, Hammer BE, Shield TW, Bischof JC. In vitro characterization of movement, heating and visualization of magnetic nanoparticles for biomedical applications. Nanotechnology. 2005;16:1221–1233. [Google Scholar]
- 42.Kuhn SJ, Hallahan DE, Giorgio TD. Characterization of superparamagnetic nanoparticle interactions with extracellular matrix in an in vitro system. Ann. Biomed. Eng. 2006;34(1):51–58. doi: 10.1007/s10439-005-9004-5. [DOI] [PubMed] [Google Scholar]
- 43.Kuhn SJ, Finch SK, Hallahan DE, Giorgio TD. Proteolytic surface functionalization enhances in vitro magnetic nanoparticle mobility through extracellular matrix. Nano Lett. 2006;6(2):306–312. doi: 10.1021/nl052241g. [DOI] [PubMed] [Google Scholar]
- 44.Mondalek FG, Zhang YY, Kropp B, et al. The permeability of SPION over an artificial three-layer membrane is enhanced by external magnetic field. J. Nanobiotechnol. 2006;4(4):1–9. doi: 10.1186/1477-3155-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rotariu O, Udrea LE, Strachan NJC, Badescu V. The guidance of magnetic colloids in simulated tissues for targeted drug delivery. J. Optoelect. Adv. Mat. 2007;9(4):942–945. [Google Scholar]
- 46.Crick FHC, Hughes AFW. The physical properties of cytoplasm: a study by means of the magnetic particle method. Exp. Cell. Res. 1950;1:37–80. [Google Scholar]
- 47. Dobson J. Remote control of cellular behaviour with magnetic nanoparticles. Nat. Nanotechnol. 2008;3(3):139–143. doi: 10.1038/nnano.2008.39. ▪▪ Highly interesting review discussing magnetic actuation to remotely manipulate and control cell function with an external magnetic field and magnetic nanoparticles linked to cells, focusing in particular on applications for tissue engineering and regenerative medicine.
- 48. Macdonald C, Friedman G, Alamia J, Barbee K, Polyak B. Time-varied magnetic field enhances transport of magnetic nanoparticles in viscous gel. Nanomedicine (Lond.) 2010;5(1):65–76. doi: 10.2217/nnm.09.97. ▪ Initial work representing a strategy to improve transport of magnetic carriers in soft tissues.
- 49.Guillotin B, Guillemot F. Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol. 2011;29(4):183–190. doi: 10.1016/j.tibtech.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cell. Mater. 2003;5:29–39. doi: 10.22203/ecm.v005a03. discussion 39–40. [DOI] [PubMed] [Google Scholar]
- 51.Ikada Y. Challenges in tissue engineering. J. R. Soc. Interface. 2006;3(10):589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howard D, Buttery LD, Shakesheff KM, Roberts SJ. Tissue engineering: strategies, stem cells and scaffolds. J. Anat. 2008;213(1):66–72. doi: 10.1111/j.1469-7580.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc. Interface. 2011;8(55):153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel ZS, Young S, Tabata Y, Jansen JA, Wong MEK, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J. Biomed. Mat. Res. Part A. 2003;65(4):489–497. doi: 10.1002/jbm.a.10542. [DOI] [PubMed] [Google Scholar]
- 56.Tayalia P, Mooney DJ. Controlled growth factor delivery for tissue engineering. Adv. Mater. 2009;21(32–33):3269–3285. doi: 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- 57.Wei GB, Jin QM, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J. Control. Release. 2006;112(1):103–110. doi: 10.1016/j.jconrel.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kempen DHR, Lu LC, Heijink A, et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30(14):2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 59.Freeman I, Cohen S. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials. 2009;30(11):2122–2131. doi: 10.1016/j.biomaterials.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 60.Freeman I, Kedem A, Cohen S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 2008;29(22):3260–3268. doi: 10.1016/j.biomaterials.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 61.Zhang SF, Uludag H. Nanoparticulate systems for growth factor delivery. Pharm. Res. 2009;26(7):1561–1580. doi: 10.1007/s11095-009-9897-z. [DOI] [PubMed] [Google Scholar]
- 62.Bock N, Riminucci A, Dionigi C, et al. A novel route in bone tissue engineering: magnetic biomimetic scaffolds. Acta Biomater. 2010;6(3):786–796. doi: 10.1016/j.actbio.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 63.Hu SH, Liu TY, Tsai CH, Chen SY. Preparation and characterization of magnetic ferroscaffolds for tissue engineering. J. Magn. Magn. Mater. 2007;310(2):2871–2873. [Google Scholar]
- 64.Zhao XH, Kim J, Cezar CA, et al. Active scaffolds for on-demand drug and cell delivery. Proc. Natl Acad. Sci. USA. 2011;108(1):67–72. doi: 10.1073/pnas.1007862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Timko BP, Dvir T, Kohane DS. Remotely triggerable drug delivery systems. Adv. Mat. 2010;22(44):4925–4943. doi: 10.1002/adma.201002072. [DOI] [PubMed] [Google Scholar]
- 66.Ward MA, Georgiou TK. Thermoresponsive polymers for biomedical applications. Polymers. 2011;3(3):1215–1242. [Google Scholar]
- 67.Hiergeist R, Andra W, Buske N, et al. Application of magnetite ferrofluids for hyperthermia. J. Magn. Magn. Mater. 1999;201:420–422. [Google Scholar]
- 68.Mornet S, Vasseur S, Grasset F, et al. Magnetic nanoparticle design for medical applications. Prog. Solid State Chem. 2006;34(2–4):237–247. [Google Scholar]
- 69.Brule S, Levy M, Wilhelm C, et al. Doxorubicin release triggered by alginate embedded magnetic nanoheaters: a combined therapy. Adv. Mater. 2011;23(6):787–790. doi: 10.1002/adma.201003763. [DOI] [PubMed] [Google Scholar]
- 70.Liu TY, Hu SH, Liu DM, Chen SY, Chen IW. Biomedical nanoparticle carriers with combined thermal and magnetic responses. Nano Today. 2009;4(1):52–65. [Google Scholar]
- 71.Derfus AM, Von Maltzahn G, Harris TJ, et al. Remotely triggered release from magnetic nanoparticles. Adv. Mat. 2007;19(22):3932–3936. [Google Scholar]
- 72.Hoare T, Santamaria J, Goya GF, et al. A magnetically triggered composite membrane for on-demand drug delivery. Nano Lett. 2009;9(10):3651–3657. doi: 10.1021/nl9018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akhyari P, Fedak PW, Weisel RD, et al. Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation. 2002;106(12 Suppl. 1):I137–I142. [PubMed] [Google Scholar]
- 74.Zimmermann WH, Schneiderbanger K, Schubert P, et al. Tissue engineering of a differentiated cardiac muscle construct. Circ. Res. 2002;90(2):223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 75.Radisic M, Park H, Shing H, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc. Natl Acad. Sci. USA. 2004;101(52):18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Radisic M, Yang L, Boublik J, et al. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am. J. Physiol. Heart Circ. Physiol. 2004;286(2):H507–H516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 77.Dvir T, Levy O, Shachar M, Granot Y, Cohen S. Activation of the ERK1/2 cascade via pulsatile interstitial fluid flow promotes cardiac tissue assembly. Tissue Eng. 2007;13(9):2185–2193. doi: 10.1089/ten.2006.0364. [DOI] [PubMed] [Google Scholar]
- 78.Carver SE, Heath CA. Semi-continuous perfusion system for delivering intermittent physiological pressure to regenerating cartilage. Tissue Eng. 1999;5(1):1–11. doi: 10.1089/ten.1999.5.1. [DOI] [PubMed] [Google Scholar]
- 79.Guldberg RE. Consideration of mechanical factors. Ann. NY Acad. Sci. 2002;961:312–314. doi: 10.1111/j.1749-6632.2002.tb03111.x. [DOI] [PubMed] [Google Scholar]
- 80.Roberts SR, Knight MM, Lee DA, Bader DL. Mechanical compression influences intracellular Ca2+ signaling in chondrocytes seeded in agarose constructs. J. Appl. Physiol. 2001;90(4):1385–1391. doi: 10.1152/jappl.2001.90.4.1385. [DOI] [PubMed] [Google Scholar]
- 81.Wu X, Davis MJ. Characterization of stretch-activated cation current in coronary smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2001;280(4):H1751–H1761. doi: 10.1152/ajpheart.2001.280.4.H1751. [DOI] [PubMed] [Google Scholar]
- 82.Naruse K, Yamada T, Sokabe M. Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am. J. Physiol. 1998;274(5 Pt 2):H1532–H1538. doi: 10.1152/ajpheart.1998.274.5.H1532. [DOI] [PubMed] [Google Scholar]
- 83.Kamkin A, Kiseleva I, Wagner KD, et al. Characterization of stretch-activated ion currents in isolated atrial myocytes from human hearts. Pflugers. Arch. Eur J. Physiol. 2003;446(3):339–346. doi: 10.1007/s00424-002-0948-0. [DOI] [PubMed] [Google Scholar]
- 84.Frank D, Kuhn C, Brors B, et al. Gene expression pattern in biomechanically stretched cardiomyocytes: evidence for a stretch-specific gene program. Hypertension. 2008;51(2):309–318. doi: 10.1161/HYPERTENSIONAHA.107.098046. [DOI] [PubMed] [Google Scholar]
- 85.Park SM, Liu G, Kubal A, Fury M, Cao L, Marx SO. Direct interaction between BKCa potassium channel and microtubule-associated protein 1A. FEBS Lett. 2004;570(1–3):143–148. doi: 10.1016/j.febslet.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 86.Bockenhauer D, Zilberberg N, Goldstein SA. KCNK2: reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat. Neurosci. 2001;4(5):486–491. doi: 10.1038/87434. [DOI] [PubMed] [Google Scholar]
- 87.Lee HS, Millward-Sadler SJ, Wright MO, Nuki G, Salter DM. Integrin and mechanosensitive ion channel-dependent tyrosine phosphorylation of focal adhesion proteins and β-catenin in human articular chondrocytes after mechanical stimulation. J. Bone Miner. Res. 2000;15(8):1501–1509. doi: 10.1359/jbmr.2000.15.8.1501. [DOI] [PubMed] [Google Scholar]
- 88.Paoletti P, Ascher P. Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron. 1994;13(3):645–655. doi: 10.1016/0896-6273(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 89.Hughes S, El Haj AJ, Dobson J. Magnetic micro- and nanoparticle mediated activation of mechanosensitive ion channels. Med. Eng. Phys. 2005;27(9):754–762. doi: 10.1016/j.medengphy.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 90.Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J. Cell. Sci. 2006;119(Pt 3):508–518. doi: 10.1242/jcs.02760. [DOI] [PubMed] [Google Scholar]
- 91.Hughes S, Dobson J, El Haj AJ. Magnetic targeting of mechanosensors in bone cells for tissue engineering applications. J. Biomech. 2007;40(Suppl. 1):S96–S104. doi: 10.1016/j.jbiomech.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 92.Kanczler JM, Sura HS, Magnay J, et al. Controlled differentiation of human bone marrow stromal cells using magnetic nanoparticle technology. Tissue Eng. Part A. 16(10):3241–3250. doi: 10.1089/ten.TEA.2009.0638. [DOI] [PubMed] [Google Scholar]
- 93.Hughes S, McBain S, Dobson J, El Haj AJ. Selective activation of mechanosensitive ion channels using magnetic particles. J. R. Soc. Interface. 2008;5(25):855–863. doi: 10.1098/rsif.2007.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pickard M, Chari D. Enhancement of magnetic nanoparticle-mediated gene transfer to astrocytes by ‘magnetofection’: effects of static and oscillating fields. Nanomedicine (Lond.) 2010;5(2):217–232. doi: 10.2217/nnm.09.109. [DOI] [PubMed] [Google Scholar]
- 95.Mcbain SC, Griesenbach U, Xenariou S, et al. Magnetic nanoparticles as gene delivery agents: enhanced transfection in the presence of oscillating magnet arrays. Nanotechnology. 2008;19(40):405102. doi: 10.1088/0957-4484/19/40/405102. [DOI] [PubMed] [Google Scholar]
- 96.Chorny M, Polyak B, Alferiev IS, Walsh K, Friedman G, Levy RJ. Magnetically driven plasmid DNA delivery with biodegradable polymeric nanoparticles. FASEB J. 2007;21(10):2510–2519. doi: 10.1096/fj.06-8070com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chorny M, Alferiev IS, Fishbein I, et al. Formulation and in vitro characterization of composite biodegradable magnetic nanoparticles for magnetically guided cell delivery. Pharm. Res. 2012;29(5):1232–1241. doi: 10.1007/s11095-012-0675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Macdonald C, Barbee K, Polyak B. Force dependent internalization of magnetic nanoparticles results in highly loaded endothelial cells for use as potential therapy delivery vectors. Pharm. Res. 2012;29(5):1270–1281. doi: 10.1007/s11095-011-0663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sapir Y, Cohen S, Friedman G, Polyak B. The promotion of in vitro vessel-like organization of endothelial cells in magnetically responsive alginate scaffolds. Biomaterials. 2012;33(16):4100–4109. doi: 10.1016/j.biomaterials.2012.02.037. ▪ Describes initial steps in remote stimulation of cells within magnetically responsive alginate scaffolds for the generation of vascularized tissue constructs.
- 100.Alsberg E, Feinstein E, Joy MP, Prentiss M, Ingber DE. Magnetically-guided self-assembly of fibrin matrices with ordered nano-scale structure for tissue engineering. Tissue Eng. 2006;12:3247–3256. doi: 10.1089/ten.2006.12.3247. [DOI] [PubMed] [Google Scholar]
- 101.Takamatsu R, Oura H, Uchida T, et al. Development of biodegradable scaffolds by leaching self-assembled magnetic sugar particles. Presented at: 20th Anniversary MHS 2009 and Micro-Nano Global COE – 2009 International Symposium on Micro-NanoMechatronics and Human Science; 8–11 November 2009; Nagoya, Japan. [Google Scholar]
- 102.Uchida T, Oura H, Ikeda S, Arai F, Negoro M, Fukuda T. Fabrication of biodegradable scaffolds by use of self-assembled magnetic sugar particles as a casting template. Presented at: 2008 IEEE International Conference on Robotics and Automation, ICRA 2008; 19–23 May 2008; Pasadena, CA, USA. [Google Scholar]
- 103.Yuet KP, Hwang DK, Haghgooie R, Doyle PS. Multifunctional superparamagnetic janus particles. Langmuir. 2010;26(6):4281–4287. doi: 10.1021/la903348s. [DOI] [PubMed] [Google Scholar]
- 104.Hu C, Uchida T, Tercero C, et al. Development of biodegradable scaffolds based on magnetically guided assembly of magnetic sugar particles. J. Biotechnol. 2012;159(1–2):90–98. doi: 10.1016/j.jbiotec.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 105.Wu Y, Jiang W, Wen XT, et al. A novel calcium phosphate ceramic-magnetic nanoparticle composite as a potential bone substitute. Biomed. Mater. 2010;5(1):15001. doi: 10.1088/1748-6041/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 106.Wei Y, Zhang XH, Song Y, et al. Magnetic biodegradable Fe(3)O(4)/CS/PVA nanofibrous membranes for bone regeneration. Biomed. Mater. 2011;6(5):055008. doi: 10.1088/1748-6041/6/5/055008. [DOI] [PubMed] [Google Scholar]
- 107.Souza GR, Molina JR, Raphael RM, et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 2010;5(4):291–296. doi: 10.1038/nnano.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu X, Kaminski MD, Riffle JS, et al. Preparation and characterization of biodegradable magnetic carriers by single emulsion-solvent evaporation. J. Magn. Magn. Mater. 2007;311(1):84–87. [Google Scholar]
- 109.Demirbag B, Huri P, Kose G, Buyuksungur A, Hasirci V. Tissue engineering – to scaffold or not to scaffold. Bioessays. 2012;34(2):A3–A3. [Google Scholar]
- 110.Ito A, Takizawa Y, Honda H, et al. Tissue engineering using magnetite nanoparticles and magnetic force: heterotypic layers of cocultured hepatocytes and endothelial cells. Tissue Eng. 2004;10(5–6):833–840. doi: 10.1089/1076327041348301. [DOI] [PubMed] [Google Scholar]
- 111.Yamamoto Y, Ito A, Kato M, et al. Preparation of artificial skeletal muscle tissues by a magnetic force-based tissue engineering technique. J. Biosci. Bioeng. 2009;108(6):538–543. doi: 10.1016/j.jbiosc.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 112.Shimizu K, Ito A, Yoshida T, Yamada Y, Ueda M, Honda H. Bone tissue engineering with human mesenchymal stem cell sheets constructed using magnetite nanoparticles and magnetic force. J. Biomed. Mater. Res. B. Appl. Biomater. 2007;82(2):471–480. doi: 10.1002/jbm.b.30752. [DOI] [PubMed] [Google Scholar]
- 113.Shimizu K, Ito A, Honda H. Mag-seeding of rat bone marrow stromal cells into porous hydroxyapatite scaffolds for bone tissue engineering. J. Biosci. Bioeng. 2007;104(3):171–177. doi: 10.1263/jbb.104.171. [DOI] [PubMed] [Google Scholar]
- 114.Ito A, Ino K, Shimizu K, Honda H, Kamihira M. Fabrication of 3D tissue-like structure using magnetite nanoparticles and magnetic force. Presented at: 2006 IEEE International Symposium on Micro-Nano Mechanical and Human Science, MHS; 5–8 November 2006; Nagoya, Japan. [Google Scholar]
- 115.Ito A, Hibino E, Kobayashi C, et al. Construction and delivery of tissue-engineered human retinal pigment epithelial cell sheets, using magnetite nanoparticles and magnetic force. Tissue Eng. 2005;11(3–4):489–496. doi: 10.1089/ten.2005.11.489. [DOI] [PubMed] [Google Scholar]
- 116.Ishii M, Shibata R, Numaguchi Y, et al. Enhanced angiogenesis by transplantation of mesenchymal stem cell sheet created by a novel magnetic tissue engineering method. Arterioscler. Thromb. Vasc. Biol. 2011;31(10):2210–2215. doi: 10.1161/ATVBAHA.111.231100. [DOI] [PubMed] [Google Scholar]
- 117.Frasca G, Gazeau F, Wilhelm C. Formation of a three-dimensional multicellular assembly using magnetic patterning. Langmuir. 2009;25(4):2348–2354. doi: 10.1021/la8030792. [DOI] [PubMed] [Google Scholar]
- 118.Wilhelm C, Riviere C, Biais N. Magnetic control of dictyostelium aggregation. Phys. Rev. E. 2007;75(4):041906. doi: 10.1103/PhysRevE.75.041906. [DOI] [PubMed] [Google Scholar]
- 119.Lee WR, Oh KT, Park SY, et al. Magnetic levitating polymeric nano/microparticular substrates for three-dimensional tumor cell culture. Colloid Surf. B-Biointerfaces. 2011;85(2):379–384. doi: 10.1016/j.colsurfb.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 120.Lee WY, Cheng WY, Yeh YC, et al. Magnetically directed self-assembly of electrospun superparamagnetic fibrous bundles to form three-dimensional tissues with a highly ordered architecture. Tissue Eng. Part C Methods. 2011;17(6):651–661. doi: 10.1089/ten.TEC.2010.0621. [DOI] [PubMed] [Google Scholar]
- 121.Xu F, Wu CA, Rengarajan V, et al. Three-dimensional magnetic assembly of microscale hydrogels. Adv. Mater. 2011;23(37):4254–4260. doi: 10.1002/adma.201101962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fu CY, Lin CY, Chu WC, Chang HY. A simple cell patterning method using magnetic particle-containing photosensitive poly (ethylene glycol) hydrogel blocks: a technical note. Tissue Eng. Part C Methods. 2011;17(8):871–877. doi: 10.1089/ten.TEC.2010.0690. [DOI] [PubMed] [Google Scholar]
- 123.Weinand C, Xu JW, Peretti GM, Bonassar LJ, Gill TJ. Conditions affecting cell seeding onto three-dimensional scaffolds for cellular-based biodegradable implants. J. Biomed. Mater. Res. Part B. 2009;91B(1):80–87. doi: 10.1002/jbm.b.31376. [DOI] [PubMed] [Google Scholar]
- 124.Zhao F, Ma T. Perfusion bioreactor system for human mesenchymal stem cell tissue engineering: dynamic cell seeding and construct development. Biotech. Bioeng. 2005;91(4):482–493. doi: 10.1002/bit.20532. [DOI] [PubMed] [Google Scholar]
- 125.Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotech. 2004;22(2):80–86. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 126.Thevenot P, Sohaebuddin S, Poudyal N, Ping Liu J, Tang L. Magnetic nanoparticles to enhance cell seeding and distribution in tissue engineering scaffolds. Presented at: 2008 8th IEEE Conference on Nanotechnology, IEEE-NANO; 18–21 August 2008; Arlington, TX, USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Robert D, Fayol D, Le Visage C, et al. Magnetic micro-manipulations to probe the local physical properties of porous scaffolds and to confine stem cells. Biomaterials. 2010;31(7):1586–1595. doi: 10.1016/j.biomaterials.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 128.Shimizu K, Ito A, Honda H. Enhanced cell-seeding into 3D porous scaffolds by use of magnetite nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006;77:265–272. doi: 10.1002/jbm.b.30443. [DOI] [PubMed] [Google Scholar]
- 129.Perea H, Aigner J, Hopfner U, Wintermantel E. Direct magnetic tubular cell seeding: a novel approach for vascular tissue engineering. Cells Tissues Organs. 2006;183(3):156–165. doi: 10.1159/000095989. [DOI] [PubMed] [Google Scholar]
- 130.Mitragotri S. Transdermal drug delivery using low-frequency sonophoresis. BioMEMS Biomed. Nanotechnol. 2006;3:223–236. [Google Scholar]
- 131.Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269(5225):850–853. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- 132.Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA. What the cell ‘sees’ in bionanoscience. J. Chem. Soc. 2010;132(16):5761–5768. doi: 10.1021/ja910675v. [DOI] [PubMed] [Google Scholar]
- 133. Mahon E, Salvati A, Baldelli Bombelli F, Lynch I, Dawson KA. Designing the nanoparticle-biomolecule interface for ‘targeting and therapeutic delivery’. J. Control. Release. 2012;161(2):164–174. doi: 10.1016/j.jconrel.2012.04.009. ▪▪ Comprehensive review describing the phenomenon of the ‘protein (biomolecule) corona’ formed at the surface of the nanocarriers in a biological environment and highlighting key aspects essential for the successful realization of therapeutic targeting.