Abstract
Background
Excessive chronic drinking is accompanied by a broad spectrum of emotional changes ranging from apathy and emotional flatness to deficits in comprehending emotional information, but their neural bases are poorly understood.
Methods
Emotional abnormalities associated with alcoholism were examined with functional magnetic resonance imaging in abstinent long-term alcoholic men in comparison to healthy demographically matched controls. Participants were presented with emotionally valenced words and photographs of faces during deep (semantic) and shallow (perceptual) encoding tasks followed by recognition.
Results
Overall, faces evoked stronger activation than words, with the expected material-specific laterality (left hemisphere for words, and right for faces) and depth of processing effects. However, whereas control participants showed stronger activation in the amygdala and hippocampus when viewing faces with emotional (relative to neutral) expressions, the alcoholics responded in an undifferentiated manner to all facial expressions. In the alcoholic participants, amygdala activity was inversely correlated with an increase in lateral prefrontal activity as a function of their behavioral deficits. Prefrontal modulation of emotional function as a compensation for the blunted amygdala activity during a socially relevant face appraisal task is in agreement with a distributed network engagement during emotional face processing.
Conclusions
Deficient activation of amygdala and hippocampus may underlie impaired processing of emotional faces associated with long-term alcoholism and may be a part of the wide array of behavioral problems including disinhibition, concurring with previously documented interpersonal difficulties in this population. Furthermore, the results suggest that alcoholics may rely on prefrontal rather than temporal limbic areas in order to compensate for reduced limbic responsivity and to maintain behavioral adequacy when faced with emotionally or socially challenging situations.
Keywords: Alcoholism, Emotion, Faces, Amygdala, Functional Magnetic Resonance Imaging
Emotions engage strong mental and affective states and rely on a seamless coordination among multiple neurophysiological systems spanning different levels of the neuraxis (Damasio, 1998; Halgren and Marinkovic, 1995; Panksepp, 1998). The emotional changes accompanying long-term chronic alcoholism cover a broad spectrum. Some of these changes, e.g., apathy and emotional flatness, are reminiscent of those seen in patients with bilateral frontal lobe damage (Lezak, 1995; Moselhy et al., 2001), or in patients with right-hemisphere damage (Kaplan, 1988; Oscar-Berman and Schendan, 2000). Other abnormalities are subtle. For example, alcoholics may make atypical judgments regarding the nature of facial emotional expressions (Clark et al., 2007; Foisy et al., 2005, 2007a,b; Kornreich et al., 2001; Maurage et al., 2008; Oscar-Berman et al., 1990; Philippot et al., 1999; Townshend and Duka, 2003) or intonations of emotional utterances (Monnot et al., 2001, 2002; Uekermann et al., 2005), suggesting that alcoholism may involve an underlying neurocognitive deficit in the capacity to comprehend emotional information. Furthermore, an individual’s genetic history can impact both a tendency toward alcoholism and the development of anomalies in areas of the brain involved in emotional processing (Dick and Bierut, 2006; Dick and Foroud, 2003; Oscar-Berman and Bowirrat, 2005).
Taken together, there is considerable uncertainty about the nature of emotional changes in alcoholism. However, it is clear that excessive chronic drinking can damage the brain, and emotional abnormalities in alcoholics can interfere with healthy interpersonal relationships. In this study, we used functional magnetic resonance imaging (fMRI) to examine the effects of long-term alcoholism on brain systems involved in emotional perception and memory. We were especially interested in the effects of alcoholism on prefrontal neocortical and mesial temporal limbic structures because (i) they are critically involved in emotional functioning (Aggleton, 2000; Ghashghaei et al., 2007; Pandya and Yeterian, 2001), (ii) they are susceptible to damage from long-term alcohol abuse (Crews, 2000; Harris et al., 2008; Kril and Halliday, 1999; Makris et al., 2008; Sullivan and Pfefferbaum, 2005), and (iii) they have common neuroanatomical connections, thereby underlining their functional associations (Goldman-Rakic et al., 1984; Koob, 2003).
Studies indicate that the frontal lobes are particularly susceptible to alcohol-related brain damage in terms of the loss of gray and white matter volume and compromised tract integrity (Crews, 2000; Harris et al., 2008; Sullivan and Pfefferbaum, 2005), reflected in impaired executive functions and personality aberrations (Oscar-Berman and Hutner, 1993). Volume reduction also has been observed in mesial temporal structures such as hippocampus (Agartz et al., 1999) and amygdala (Makris et al., 2008), with the amygdala volume decrease showing correlations with craving and increased drinking (Wrase et al., 2008). Together with the temporal limbic structures, prefrontal cortex is interactively involved in judgment, decision making, and social conduct relying on both cognitive and affective functions. For instance, whereas the amygdala is crucial in emotional perception and expression (Aggleton, 2000), the hippocampus is involved in memory formation (Squire and Zola-Morgan, 1991). Prefrontal regions play a role in cognitive evaluation and regulatory control of emotion-related behavior (Hariri et al., 2000), and their involvement is inversely related to amygdala activity during emotional regulation (Quirk and Beer, 2006). It is therefore plausible that the emotional impairments observed in chronic alcoholics are due to the dysfunctional cortico-limbic circuitry. It has been hypothesized that the right hemisphere is particularly vulnerable to chronic alcohol abuse based on the similarities between cognitive dysfunctions in chronic alcoholics and patients with right hemisphere lesions (Oscar-Berman, 1992). This hypothesis has received equivocal support by neuroimaging studies, which paint a more complex picture of the neural basis of the observed dysfunctions (Harris et al., 2008;Makris et al., 2008; Sullivan and Pfefferbaum, 2005; Volkow et al., 1992). However, material-specific lateralization of brain activity has been demonstrated in numerous studies with word and face-encoding tasks producing left- and right-lateralized activation, respectively (Braver et al., 2001;Kelley et al., 1998).
In this study, we employed both verbal (word) and nonverbal (face) materials in an effort to contrast relative hemispheric sensitivities to the cumulative effects of alcohol abuse. Both words and photographs of faces had different emotional valances (positive, negative, neutral) and were presented under “deep” (semantic) and “shallow” (perceptual) encoding conditions. Contrasting the 2 levels of depth at which information was encoded allowed us to evaluate subsequent recognition as affected by the level-of-processing (Craik and Lockhart, 1972), and potentially differential involvement of prefrontal regions which show sensitivity to encoding strategy (Demb et al., 1995; Kapur et al., 1994).
Based on previous evidence of the effects of long-term alcoholism on neurocircuitry (Oscar-Berman and Marinkovic, 2004; Sullivan and Pfefferbaum, 2005) and emotional function (Oscar-Berman, 2000), we hypothesized that mesial temporal and prefrontal contributions to encoding and retrieval of emotional faces would be especially compromised in long-term alcoholics as compared to matched controls.
METHODS
Research Participants
Participants in the study included 15 abstinent long-term alcoholics (ALC) (age, mean ± SD, 50.2 ± 12.8 years) and 15 nonalcoholic controls (NC) (54.1 ± 12.5 years), with comparable socioeconomic backgrounds, matched for age, education, and IQ (see Table 1). All subjects were right-handed, male, native English speakers, and were carefully screened. Potential participants were recruited through flyers placed in the Boston Veterans Affairs Healthcare System, Boston University School of Medicine, and after-care programs in the Boston area, and through advertisements placed with local newspapers and web sites. Subject selection procedures for both groups included an initial prescreening telephone interview to determine identifying information such as age, level-of-education, health history, and history of alcohol and drug use. Those eligible were invited to the laboratory for further screening and neuropsychological evaluations usually requiring between 5 and 7 hours of testing over a minimum of 1 to 2 days. Prior to screening, informed consent was obtained. Participants were reimbursed for time and travel expenses.
Table 1.
Subject Characteristics, Mean ± SD (range)
| NC (n = 15) | ALC (n = 15) | t(28)a | p-value (2-tailed) | |
|---|---|---|---|---|
| Age | 54.1 ± 12.5 (25–77) | 50.2 ± 12.8 (34–76) | −0.85 | 0.40 |
| Education | 14.7 ± 1.8 (12–18) | 13.8 ± 1.7 (12–18) | −1.28 | 0.21 |
| Full scale IQ | 109.1 ± 9.8 (88–123) | 105.5 ± 7.8 (90–116) | −1.12 | 0.27 |
| Verbal IQ | 109.1 ± 10.1 (92–128) | 106.6 ± 8.7 (92–121) | −0.71 | 0.48 |
| Performance IQ | 107.7 ± 11.1 (83–125) | 103.1 ± 9.6 (83–113) | −1.19 | 0.24 |
| Working memory | 108.4 ± 15.3 (83–136) | 113.3 ± 19.4 (83–155) | 0.74 | 0.46 |
| Verbal fluency | 44.3 ± 19.5 (10–80) | 43.1 ± 27.2 (10–80) | −0.24 | 0.81 |
| POMS – Depress. | 37.8 ± 5.0 (35–50) | 39.5 ± 8.2 (30–66) | 0.7 | 0.49 |
| POMS – Anx. | 40.3 ± 4.7 (37–53) | 42.0 ± 4.7 (32–53) | 0.4 | 0.69 |
| MAACL – Depr. | 46 ± 3.3 (40–53) | 46 ± 8.1 (40–71) | 0.0 | 1.0 |
| MAACL – Anx. | 43.7 ± 2.3 (37–45) | 43.5 ± 5.8 (37–58) | −0.15 | 0.88 |
| Hamilton Depr. | 0.93 ± 1.14 (0–3) | 1.6 ± 2.2 (0–8) | 1.08 | 0.29 |
| QFI | 0.4 ± 0.6 (0–2.0) | 11.9 ± 10.5 (1.0 –38.0) | 3.89 | 0.0006 |
| Years of ≥21 drinks/week | N/A | 16.4 ± 8.7 (5–35) | N/A | N/A |
| Years sober | N/A | 7.3 ± 12.0 (0.2–38) | N/A | N/A |
| FH+ first degree relatives | 21% | 73% | Fisher’s ET | <0.01 |
| FH+ fathers | 21% | 60% | Fisher’s ET | <0.06 |
| FH+ mothers | 0% | 20% | Fisher’s ET | 0.25 |
| ASPD symptoms | 14% | 67% | Fisher’s ET | <0.01 |
QFI, Quantity-Frequency Index was calculated based on the amount, type, and frequency of use of alcoholic beverages either over the last 6 months (for the nonalcoholics), or over the 6 months preceding cessation of drinking (for the alcoholics). FH+ denotes group percentages reporting Family History of alcoholism depending on the affected relative. Fisher’s ET (Exact Test) is used to calculate statistical significance of categorical data obtained from small samples.
Cognitive and affective scores and QFI were not recorded for 1 control subject; therefore, t(27) is listed.
At the laboratory, a medical history interview and a vision test were administered, along with a handedness questionnaire (Briggs and Nebes, 1975). All participants were given a computerized version of the NIMH Diagnostic Interview Schedule (Robins et al., 1989) to provide lifetime psychiatric diagnoses according to DSM-IV (APA, 1994) criteria. The groups differed on symptoms of antisocial personality disorder (ASPD) at some point in their lives, but none of the participants in either group reported experiencing such symptoms currently. Family history of alcoholism was probed using a diagrammatic family tree on which subject indicated first- and second-degree relatives known to be alcoholics. ALC participants reported a high incidence of family history of alcoholism compared to the NC group (Table 1). Additional tests were given to measure affective state: the Hamilton Depression Scale (Hamilton, 1960), the Profile of Mood States (POMS; McNair et al., 1981), and the Multiple Affect Adjective Check List–Revised (Zuckerman and Lubin, 1965). The groups did not differ on measures of affective state (Table 1). Participants also were given a structured interview (Cahalan et al., 1969; MacVane et al., 1982) in which they were questioned about drinking patterns, the number of years of heavy drinking (>21 drinks/wk), and length of abstinence. This permitted a Quantity-Frequency Index (QFI) to be calculated for each participant. The QFI takes into consideration the amount, type, and frequency of use of alcoholic beverages either over the last 6 months (for the NC subjects), or over the 6 months preceding cessation of drinking (for the ALC subjects). The ALC subjects met DSM-IV criteria for alcohol abuse or dependence for at least 5 years (mean 16.4 years; range 5–35 years), and they had abstained from alcohol use for at least 4 weeks prior to testing (mean 7.3 years; range: 0.2–38 years). This information was validated by the following: DIS scores; medical records; and interviews with staff of collaborating medical facilities and family members when possible. The participants also were given the Mini Mental Status Exam (Folstein et al., 1975), the Wechsler Adult Intelligence Scale–III (Wechsler, 1997a), the Wechsler Memory Scale–III (Wechsler, 1997b), and a Verbal Fluency Test (FAS, Borkowski et al., 1967) to ensure consistency of intellectual assessment procedures of ALC and NC groups.
Participants were excluded if any source (i.e., DIS scores, hospital records, or personal interviews) indicated that they had 1 of the following: neurological dysfunction (e.g., major head injury with loss of consciousness greater than 30 minutes, stroke, epilepsy, or seizures unrelated to alcohol withdrawal); electroconvulsive therapy; major psychiatric disease (e.g., schizophrenic disorders, current symptoms of ASPD, or current major depression); current polydrug abuse or use of psychotropic medications; HIV; severe hepatic disease; history of serious learning disability or dyslexia; uncorrected vision or hearing problems. Additionally, individuals for whom comprehension of the experimental conditions was in doubt were excluded, as well as individuals with pacemakers, surgical metal clips, or implants, and those who had suffered injuries involving shrapnel or other metal.
Image Acquisition and Analysis
Imaging data were acquired using a 3.0T Siemens (Erlangen, Germany) Trio whole-body high-speed magnetic resonance scanner. Exposure to scanner noise was reduced with 29 dB earplugs, and head movements were minimized with foam padding. Following automated shimming and scout image acquisition, two 8-minute high-resolution 3DMP-RAGE (magnetization-prepared rapid gradient echo) sequences that optimize contrast for a range of tissue properties were obtained with: repetition time (TR) = 2530 ms, echo time (TE) = 3.25 ms, flip angle = 7°, field of view (FOV) = 256 mm, 128 sagittal slices with in-plane resolution 1 × 1 mm, slice thickness = 1.33 mm. These 2 high-resolution structural images were used for slice prescription, spatial normalization, and cortical surface reconstruction. Functional whole-brain blood oxygen level-dependent (BOLD) images were obtained with a gradient echo T2*-weighted sequence (TR = 3 s, TE = 25 ms, FOV = 200 mm, flip angle = 90°). Twenty-eight contiguous axial-oblique slices aligned to the anterior / posterior commissure line (voxel size: 3.1 × 3.1 × 5 mm) were acquired interleaved and with no gap.
The imaging data were analyzed using FreeSurfer and FS-FAST (FreeSurfer – Functional Analysis Stream) analysis packages (Burock and Dale, 2000; Dale et al., 1999; Fischl et al., 1999a). Based on the averaged structural scans, individual cortical surfaces were reconstructed using an automatic gray / white segmentation and tessellation and inflation of the folded surface tessellation patterns (http://surfer.nmr.mgh.harvard.edu/). Furthermore, these surfaces were registered with a canonical brain surface created from an average of brains in each subject group based on the sulcal / gyral pattern (Fischl et al., 1999b) allowing for high-resolution averaging based on matching of homologous cortical locations across subjects, while minimizing metric distortion. Using the atlas-based segmentation algorithm and volumetric labeling within the same FreeSurfer analysis stream (Fischl et al., 2002), volumetric measures were obtained for the amygdala and hippocampus, as well as cortical mantle for each hemisphere and for each subject. Overall, the ALC and NC groups did not differ with respect to the volumes of amygdala, hippocampus, or cortical mantle, all p > 0.34.
Data from each functional imaging session were motion corrected using the analysis of functional neuroimages (AFNI) algorithm (Cox and Jesmanowicz, 1999). There were no group differences in the amount of head motion; this did not exceed the maximum of 3.5 mm in any subject. After spatial smoothing with a 3D 8 mm full width at half maximum Gaussian kernel and intensity normalization, condition-specific effects were estimated by fitting the amplitudes of boxcar functions convolved with a gamma function to the BOLD signal across all runs (Burock and Dale, 2000). The estimated hemodynamic response was defined by a gamma function of 2.25 seconds hemodynamic delay and 1.25 seconds dispersion. Statistical activation maps were constructed from averaged responses for each contrast / stimulus condition for each subject and were resampled onto the common cortical surface space (for the prefrontal surface-based analyses) and Talairach space (for the analyses of the mesial temporal lobe activations). The group average analyses were based on a random-effects model which takes into account the inter-subject variance, allowing for inferences to the population (Friston et al., 1999).
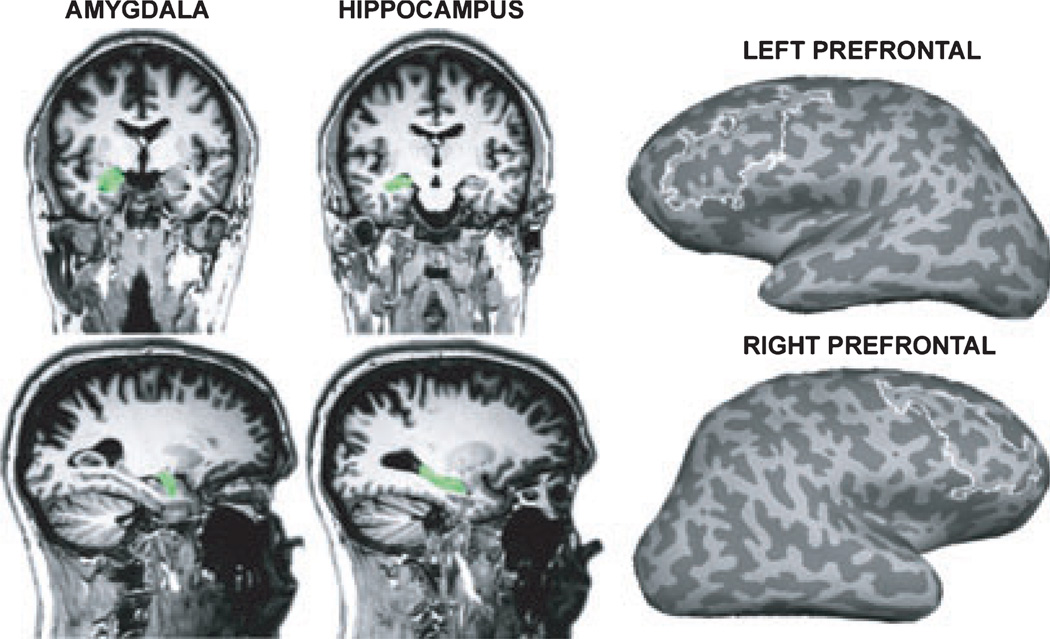
Region-of-interest (ROI) analyses were conducted for the mesial temporal and prefrontal regions based on our a priori hypotheses concerning their role in emotional and mnemonic functions. The ROIs were defined as amygdala and hippocampus volumes bilaterally based on each individual’s anatomy. Furthermore, left and right prefrontal ROIs were anatomically defined to include the inferior and middle prefrontal gyri and sulci (Fig. 1). All the ROIs were defined based on automatic parcellation (Fischl et al., 2004). Within these anatomical boundaries, functional constraint for the prefrontal ROI analysis in each subject was based on the unbiased orthogonal contrast (i.e., all conditions vs. fixation) and included the voxels within each anatomical label that were active at a threshold of p < 0.0001. Percent signal changes from baseline were computed for each ROI and each subject and submitted to ANOVAs comparing activity levels across groups and conditions. Statistical analyses were performed on activity levels (percent signal change from baseline) for each of these ROIs within the general linear model with the between-group factor of Group (ALC, NC) and within-subject factors of Material (faces, words), Level of Processing (deep, shallow), Emotion (negative, positive, neutral), and Hemispheric Laterality (left, right). Statistical analyses were performed with SPSS and GANOVA programs (SPSS for Windows; Woodward et al., 1990).
Fig. 1.
Regions of interest (ROIs) were defined in the amygdala and hippocampus bilaterally based on each individual’s anatomy (Fischl et al., 2004). The amygdala ROI was centered at Talairach coordinates: ±20, −6 to −17; hippocampus : ±32, −11 to −20, and prefrontal ROIs included the inferior and middle prefrontal gyri and sulci.
Behavioral Tasks
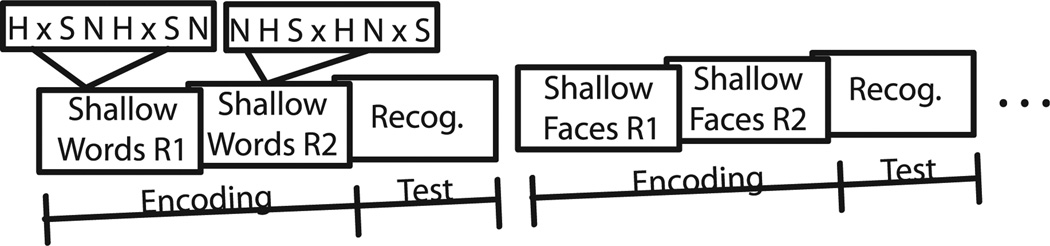
Subjects were asked to perform 4 different encoding tasks that varied both Type of Material (faces or words) and Depth of Encoding (deep or shallow). Each task (e.g., Shallow Word Encoding) consisted of 2 encoding runs immediately followed by a recognition run. Therefore, each subject performed 8 encoding run (2 for each encoding task) and 4 corresponding recognition runs that immediately followed encoding runs. A partial example of the task suite is presented in Fig. 2. The order of the tasks was counterbalanced across subjects. Each encoding run lasted 2 minute 48 seconds and consisted of 8 blocks. Each block consisted of 7 stimuli of either 1 emotional condition (positive, negative, or neutral), or fixation trials, resulting in 14 stimuli per emotion per run, totaling 42 stimuli per run. The 2 encoding runs comprised the same stimuli, but the stimulus and block order was differently randomized within each of the 2 runs. Randomization order was matched for the 2 material types within each encoding task and 2 different randomization lists were counterbalanced across subjects. Different stimulus sets were used for deep and shallow encoding tasks within each material type. The factors of Material Type and Depth of Encoding, along with the factor of Emotional Valence (positive, negative, neutral), were combined into a 2 × 2 × 3 factorial design. During encoding, participants were provided with 2 sets of instructions that varied the level of semantic processing. “Deep” encoding requires semantic elaboration (e.g., whether a word is abstract of concrete), resulting in a stronger memory trace when compared to “shallow” encoding, which is limited to perceptual stimulus characteristics (e.g., color) (Craik and Lockhart, 1972). The entire fMRI experiment lasted approximately 45 minutes, including between-run instructions and practice within the bore of the magnet. Prior to scanning, the participants practiced the tasks outside the scanner and with additional stimuli that were not used during the actual experiment.
Fig. 2.
A partial example of 1 version of a task suite. In this case, a Shallow Words Encoding task (“Is the word written in color or in white print?”) was presented in 2 runs (R1 and R2). Each run consisted of 8 blocks and each block consisted of 7 different words with an emotional valence (H, happy; S, sad; N, neutral) or fixation trials (x). The encoding set was followed by a recognition task (“Seen before?”) with 50% novel words and subsequently by other encoding tasks in a manner counterbalanced across subjects.
Encoding of Words
In the shallow encoding condition, the subjects were asked to press the right button if a word was printed in color and to press the left button if a word was in white print. The font color was randomized across stimuli. In the deep encoding condition, the subjects were instructed to press the right button in response to abstract words and to press the left button for concrete words. In this setting, abstract was defined as “something that is theoretical – not a specific thing or instance, for example, ‘concentration’.” Concrete was defined as “something that is real – a specific thing or instance, for example, ‘chair’.”
Encoding of Faces
In the shallow encoding condition, the subjects’ task was to press the right button to faces appearing in their natural color and to press the left button if the faces appeared in grayscale. The deep encoding task instructed the subjects to judge whether a face seemed intelligent (right button) or not (left button). Even though the stimuli were blocked by emotional valence, stimulus color was randomized across stimuli.
Recognition Tasks
Immediately after each encoding set, subjects completed a recognition task. They were asked to press the right button to those faces or words they had seen during the preceding encoding set and to press the left button to those faces or words they had not seen before. Each recognition run consisted of 8 blocks of 14 stimuli for each emotion (50% repeated), and 2 blocks of fixation. Stimuli were blocked by emotion (positive, negative, or neutral), but the repeated and novel stimuli were randomly intermixed.
Using the Presentation® software package (Neurobehavioral Systems, Albany, CA), the stimuli were shown in the center of a rear-projection presentation screen in a manner synchronized with the scanner. Each stimulus was presented for 2 seconds, and was followed by 1 seconds of fixation. Subjects indicated their responses by pressing buttons on a magnet-compatible response box.
Words were equated for length (means: 6.13 letters for negative, 6.27 for positive, and 6.27 for neutral words), and balanced for emotional valence (mean ratings of 5.9 for negative, 5.7 for positive, and 2.3 for neutral) and imagery (mean ratings of 4.7 for negative, 4.8 for positive, and 4.5 for neutral words) (Paivio et al., 1968; Rubin and Friendly, 1986). Words were printed in capital letters in white or in color (red, blue, green, or yellow) against a black background. The face stimuli were photographs of unfamiliar young adults without facial hair, glasses, or jewelry. A large number of volunteers posed in each photograph as happy, sad, or neutral, according to instructions and after practice. The stimulus set used here was selected from a much larger set based on 95% consistency of emotional expression evaluations performed by 25 independent judges (Marinkovic and Halgren, 1998). The faces were shown either in color or grayscale against a black background.
Behavioral data from both the encoding and recognition portions of the experiment were analyzed with respect to accuracy/ratings and reaction time (RT). Mixed design ANOVA with the factors of Group (ALC, NC), Material (faces, words), Color (black and white), and Emotion (negative, positive, neutral) were applied to the data. Due to dissimilar nature of the deep and shallow encoding tasks, they were analyzed separately. However, the factor of Level of Processing (deep, shallow) was included in the analyses of the recognition data.
RESULTS
Behavioral Measures
In the shallow encoding task, i.e., as participants decided whether each word or face was presented in color or not, the overall accuracy was very high, although significantly higher for the color (97.8%) than for the black- and white-stimuli (95.7%) (F1,28 = 5.8, p < 0.05). The NC group was more accurate than the ALC group for the face stimuli (F1,28 = 5.0, p < 0.05), means = 98.6% and 95.7%, respectively. RT were faster overall for words (824 ms) than for faces (881 ms) (F1,28 = 4.8, p < 0.05). Emotional valence influenced the assessments since RT was faster for the neutral and positive, as compared to negative stimuli (F1,28 = 13.2, p < 0.001). Significant Group × Material × Color × Emotion interaction (F2,27 = 6.8, p < 0.01) was due to the ALC’s slower RTs to emotionally valenced as compared to neutral faces when presented in black and white. This emotional Stroop-like effect was significant for the ALC (F1,28 = 8.0, p < 0.001), but not for NC (F1,28 = 0.05, p > 0.5).
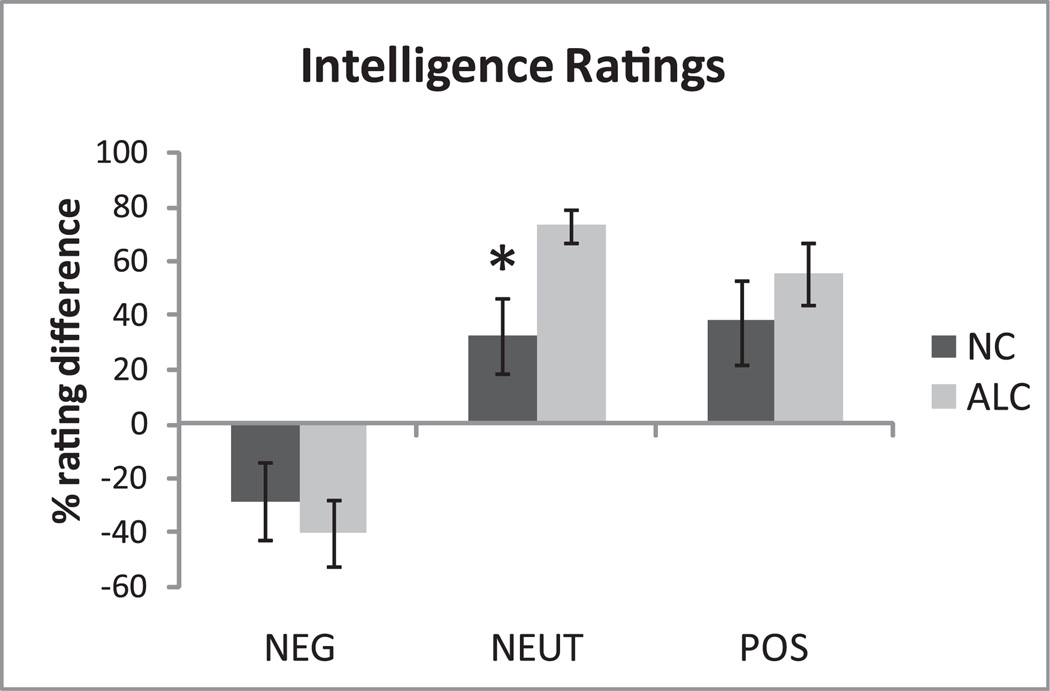
During deep encoding, faces with neutral and positive expressions were rated as more intelligent, whereas the negatively valenced faces were perceived as less intelligent overall (F1,27 = 36.8, p < 0.0001). A significant interaction among the factors of Group, Emotional Valence, and Rating (F2,56 = 3.6, p < 0.05) indicated that the ALC group was particularly inclined to this effect. In comparison to the NC group, the ALC group rated the neutral faces as more intelligent (F1,28 = 7.4, p < 0.01); see Fig. 3. They also hesitated more (i.e., had slower RT) than the NC group when rating a face as unintelligent, as indicated by a significant Group × Rating interaction (F1,28 = 7.1, p < 0.05). Correlations between this behavioral effect and other measures are presented in Table 2. Prolonged RTs observed in the ALC group correlated negatively with Working Memory (r = −0.54, p < 0.05) and Verbal IQ (r = −0.53, p < 0.05), suggesting that intelligence judgment was verbally mediated. Positive correlation between RTs and the length of heavy drinking (r = 0.52, p < 0.05) indicates that chronic alcohol use affects speed of reactions during socially relevant assessment tasks.
Fig. 3.
During “deep” face encoding, participants judged whether a face seemed intelligent or not. Shown are relative (“intelligent” minus “unintelligent”) ratings for both groups across the 3 emotional valences (mean ± SEM). Abstinent alcoholics (ALC) rated neutral faces as more intelligent than the nonalcoholic control (NC) group.
Table 2.
Correlation Coefficients for Measures Obtained During Deep Face-Encoding Task
| Correlations | BOLD in right PFC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RTs to negative faces rated as “unintelligent” | Negative | Neutral | Positive | ||||||||||
| Amyg LH | Amyg RH | Hip LH | Hip RH | Work Mem | VIQ | Heavy drink | Amyg LH− | Amyg LH neutral | Amyg LH+ | ||||
| ALC | −0.12 | 0.42 | 0.47* | 0.3 | −0.54** | −0.53** | 0.52** | −0.47* | −0.48* | 0.0 | |||
| >0.6 | <0.12 | <0.08 | >0.27 | <0.05 | <0.05 | <0.05 | <0.08 | <0.07 | >0.99 | ||||
| NC | 0.52** | 0.07 | −0.15 | −0.11 | 0.01 | 0.19 | n/a | 0.09 | 0.24 | 0.28 | |||
| <0.05 | >0.8 | >0.58 | >0.68 | >0.9 | >0.19 | n/a | >0.76 | >0.4 | >0.3 | ||||
The left panel contains correlations between the RTs to negative faces rated as “unintelligent” and BOLD activity in the amygdala (Amyg) and hippocampus (Hip), Working Memory, Verbal IQ (VIQ), and duration of heavy drinking for both ALC and NC groups. The right panel contains correlations between BOLD activity measured in the right prefrontal cortex (PFC) during Deep Face Encoding of negative, neutral, and positive faces, and the left amygdala activity to those same stimuli.
LH, left hemisphere; RH, right hemisphere.
denotes trend toward significance, p < 0.1;
denotes significance at the p < 0.5 level.
The ALC group was significantly slower than the NC group during the deep word encoding task, i.e., when rating the words as abstract or concrete (F1,28 = 4.8, p < 0.05; means: 1257 ms and 1396 ms for the NC and ALC groups, respectively). For ALC participants, RTs showed significant negative correlation with Working Memory (r = −0.53, p < 0.05) and marginal negative correlation with Verbal Fluency (r = −0.45, p < 0.11), suggesting that participants with worse working memory and verbal skills found this task more difficult. The RTs also showed a tendency to correlate with the length of heavy drinking (r = 0.44, p < 0.1), indicating that long-term heavy alcohol intake affects speed on verbal tasks. Behavioral data for the recognition portion of the experiment were analyzed with 14 subjects per group, as data for 2 subjects were not available. There were no group differences in accuracy or RT during recognition. Overall, stimuli encoded under deep conditions were recognized with greater accuracy (F1,26 = 96.4, p < 0.0001; means: 82% vs. 70%) and speed (F1,24 = 32, p < 0.0001; means: 1153 ms vs. 1226 ms), than those encoded under shallow instructions, confirming the level-of-processing effect (Craik and Lockhart, 1972). Furthermore, words were recognized better than faces (F1,26 = 35.4, p < 0.0001; means: 82% vs. 70%), and also faster (F1,24 = 19.3, p < 0.0001, means: 1140 ms vs. 1240 ms).
Neuroimaging Results: Limbic Structures in the Temporal Lobes
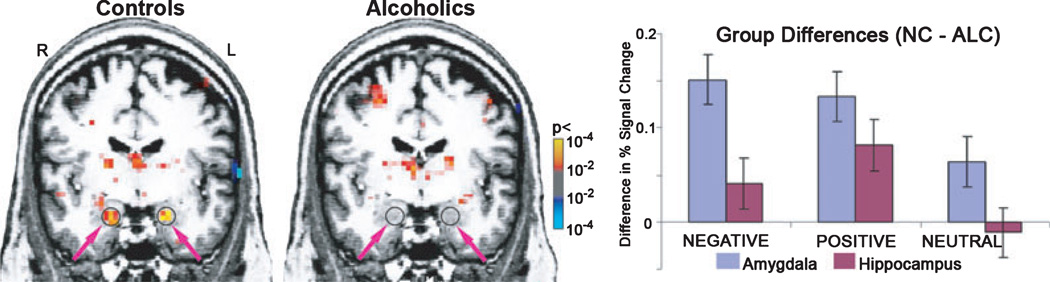
Voxel-wise analyses were performed using random-effects analysis model of the group data and indicated a group-related difference in activity during the deep face-encoding task, as illustrated in Fig. 5. Statistical analyses were performed on activity levels (percent signal change from baseline) for the ROIs defined in amygdala and hippocampus within the general linear model. Significant effects described in this section are also listed in Table 3.
Fig. 5.
Deep encoding of faces evoked amygdala activity in the nonalcoholic control (NC) group, but not in abstinent alcoholic (ALC) group. Left panel: Circles and arrows point to group averaged activations in right and left amygdalae, Talairach coordinates: −20.2, −5.9, −17.5; 20.2, −5.9, −17.5. The color-bar denotes p-values obtained with random effects group analyses of the Deep Face Encoding (averaged across all 3 emotions) vs. Fixation contrast. Right panel: Group differences (NC minus ALC) in brain activity (mean percent signal change over baseline ± SEM) observed in the amygdala and hippocampus to negative, positive, and neutral faces during deep encoding. The activity to emotionally expressive faces in these temporal limbic structures is significantly blunted in abstinent chronic alcoholics.
Table 3.
Summary of Statistical Results for Each ROI Including Talairach Coordinates, Volumes, and Group Comparisons
| Amygdala | ||
| Talairach coord.: ±20, −6, −17 | ||
| Vol (cm3): NC = 1.76, ALC = 1.66 | t28 = 0.74, | >0.47 |
| Amygdala – Encoding | ||
| Group × Material × Depth | F1,28 = 7.2 | <0.05 |
| Material × Depth | F1,28 = 6.9 | <0.05 |
| Material (faces elicit more activity) | F1,28 = 21.1 | <0.0001 |
| Material × Emotion × Laterality | F2,56 = 3.2 | <0.05 |
| Emotion × Laterality | F2,56 = 3.7 | <0.05 |
| Group × Emotional faces on the left | F1,28 = 9.9 | <0.005 |
| Group × Emotional faces on the right | F1,28 = 6.2 | <0.05 |
| Amygdala – Recognition | ||
| Material (faces elicit more activity) | F1,28 = 5.9 | <0.05 |
| Face Emotion effect in NC | F2,56 = 6.3 | <0.005 |
| Face Emotion effect in ALC | F2,56 = 0.08 | <0.5 |
| Emotion (neg. elicit most activity) | F2,56 = 3.4 | <0.05 |
| Emotion × Material × Laterality | F2,56 = 3.7 | <0.05 |
| Hippocampus | ||
| Talairach coord. ±32, −11, −20 | ||
| Vol (cm3): NC = 3.33, ALC = 3.37 | t28 = −0.25 | >0.8 |
| Hippocampus – Encoding | ||
| Laterality × Material | F1,28 = 6.5 | <0.05 |
| Faces > Words on the right in NC | F1,28 = 9.8 | <0.005 |
| Faces = Words on the right in ALC | F1,28 = 0.2= Words on the right in ALC | >0.5 |
| Group × Emotional faces on the left | F1,28 = 9.2 | <0.01 |
| Group × Emotional faces on the right | F1,28 = 9.3 | <0.005 |
| Hippocampus – Recognition | ||
| Group × Emotion | F2,56 = 4.0 | <0.05 |
| Material × Laterality | F1,28 = 8.8 | <0.01 |
| Faces > Words on the right | F1,28 = 4.8 | <0.05 |
| Material × Emotion × Laterality | F2,56 = 4.7 | <0.05 |
| Emotion × Depth | F2,56 = 4.2 | <0.05 |
| Lateral prefrontal cx. | ||
| Talairach coord. Left: −39, 31, 28 | ||
| Right: 37, 43, 13; ROI vols (cm3) | ||
| Anatom.: NC = 23.55, ALC = 23.56 | t28 = 0.02 | >0.9 |
| Masked: NC = 5.68, ALC = 5.34 | t28 = 1.4 | >0.16 |
| Prefrontal – Encoding | ||
| Depth (deep > shallow) | F1,28 = 13.9 | <0.001 |
| Material × Laterality | F1,28 = 19.0 | <0.0005 |
| Right > Left for faces | F1,28 = 18.2 | <0.0005 |
| Left > Right for words | F1,28 = 5.9 | <0.05 |
| Group × Material for Deep | F1,28 = 5.0 | <0.05 |
| Material × Laterality for Deep | F1,28 = 19.5 | <0.0001 |
| Faces > Words on the right in ALC | F1,28 = 22.0 | <0.0001 |
| Right > Left for faces in NC | F1,28 = 5.0 | <0.05 |
| Left > Right for words in NC | F1,28 = 8.8 | <0.01 |
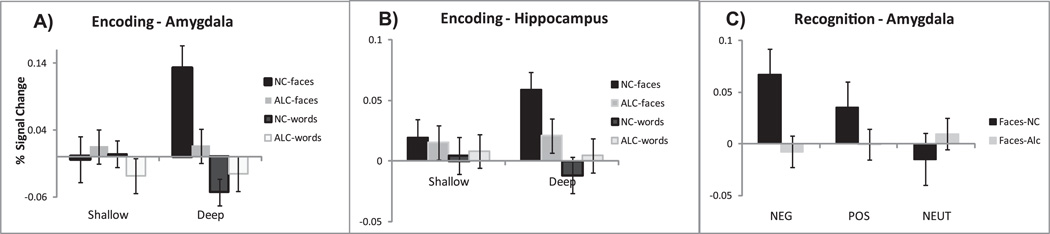
Amygdala Activity During Encoding Tasks
A significant 3-way interaction was observed in the amygdala bilaterally among the factors of Group × Material × Depth of Processing (F1,28 = 7.2, p = 0.05), as well as a significant interaction of Material and Depth of Processing (F1,28 = 6.9, p < 0.05); see Fig. 4A. The face-encoding task activated amygdala under the deep processing condition in the NC group. The main effect of Material indicated that the amygdala was selectively activated by faces (F1,28 = 21.1, p < 0.0001). Significant interactions of Material × Emotion × Laterality (F2,56 = 3.2, p < 0.05), and Emotion × Laterality (F2,56 = 3.7, p < 0.05), were due to a higher sensitivity of the right amygdala to faces, as well as its higher sensitivity to the emotional valence of the stimuli. Negatively valenced faces in particular tended to evoke a stronger activation in the right as compared to the left amygdala (F1,28 = 3.9, p < 0.06).
Fig. 4.
Bilateral amygdala (A) and hippocampus (B) responses (average percent signal change from baseline ± SEM) to faces and words during shallow and deep encoding conditions. The strongest activity in both structures was elicited by the Deep Face-Encoding task, particularly in the NC group. (C) Bilateral amygdala activity during recognition of deeply encoding faces. It was responsive to faces with emotional valence in the NC but not in the ALC group.
Group differences were further modulated by the factor of emotional valence in the context of the deep face-encoding task (Fig. 5). Whereas neutral faces yielded no significant group differences in amygdala activation, faces with negative and positive emotional expressions evoked stronger activity in the NC group than in the ALC group both in the left (F1,28 = 9.9, p < 0.005) and in the right amygdala (F1,28 = 6.2, p < 0.05). Thus, the NC group responded more strongly to the faces with positive and negative emotional expressions than to the neutral faces. In contrast, the amygdala in the ALC group responded to the emotionally valenced face stimuli in an undifferentiated manner.
Volumetric measures of amygdala and hippocampus did not correlate with the BOLD functional activations during deep face encoding, (both p > 0.27, covaried for age), suggesting that the reduced activation to emotional expressions in the ALC group was unrelated to volumetric changes in these limbic structures.
Amygdala Activity During Recognition Tasks
The same analyses were applied to data from the recognition tasks. A significant main effect of Material was observed (F1,28 = 5.9, p < 0.05), with faces eliciting stronger activity than words, replicating results seen during the encoding tasks. Furthermore, the group difference in activity evoked by Emotion during encoding was replicated during the recognition task as well. Whereas a significant differentiation among emotions was observed in the NC group (F2,56 = 6.3, p < 0.005), amygdala activity in the ALC group did not differentiate among the stimuli with emotional valence (F2,56 = 0.08, p > 0.5), Fig. 4C. A significant main effect of Emotion (F2,56 = 3.4, p < 0.05) indicated that the negative stimuli elicited the strongest activity, followed by the stimuli with positive and neutral valence. Emotional valence interacted significantly with the factors of Material and Laterality (F2,56 = 3.7, p < 0.05) so that the faces with emotional expressions evoked stronger activity in the right amygdala than faces with neutral expressions (F1,28 = 5.0, p < 0.05).
Hippocampus Activity During Encoding Tasks
During encoding, a significant Laterality × Material interaction (F1,28 = 6.5, p < 0.05) was reflected in a stronger activation of the right hippocampus by faces as compared to words (F1,28 = 4.9, p < 0.05). This effect was significant for the NC group (F1,28 = 9.8, p < 0.005), but not for the ALC group (F1,28 = 0.2, p > 0.5). Whereas stronger hippocampal activation to the emotionally expressive faces during deep encoding was observed in the NC group, the activation in the ALC group did not differentiate among the emotions. This group difference was significant for the left (F1,28 = 9.2, p < 0.01) and for the right hippocampus (F1,28 = 9.3, p < 0.005), similar to the effects observed in the amygdala during the face-encoding task (Fig. 5).
Hippocampus Activity During Recognition Tasks
A significant interaction of the factors of Group and Emotion (F2,56 = 4.0, p < 0.05) resulted from higher hippocampal activation to negative stimuli in the NC group, as compared to ALC group (F1,28 = 4.5, p < 0.05). A significant Material × Laterality interaction (F1,28 = 8.8, p < 0.01) was due to stronger activity of the right hippocampus to face stimuli in comparison to words (F1,28 = 4.8, p < 0.05). A significant Material × Emotion × Laterality interaction (F2,56 = 4.7, p < 0.05) resulted from stronger activation of the right hippocampus to emotionally expressive faces as compared to the neutral faces. The hippocampus was more strongly activated by deeply encoded stimuli, as indicated by a significant Emotion × Level of Processing interaction (F2,56 = 4.2, p < 0.05).
Neuroimaging Results: Prefrontal ROIs
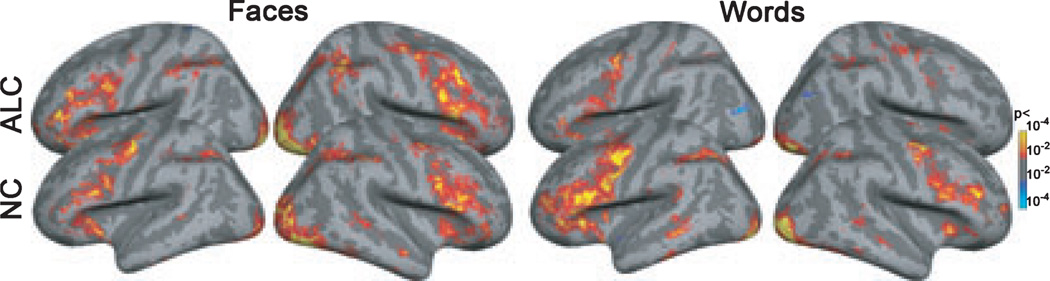
Voxel-wise analysis of the activity in the cortical mantle was performed using random-effects analysis model of the group data. Figure 6 shows the overall activity during deep encoding tasks for both groups, material types, and hemispheres. Direct voxel-wise intergroup comparison did not yield reliable overall group differences, but interactions of group, laterality, and material type in terms of activity levels (percent signal change) were explored within the prefrontal ROIs (Fig. 1) with the same set of analyses as described for amygdala and hippocampus.
Fig. 6.
Voxel-wise statistical activity maps are displayed on the inflated lateral cortical surfaces for the left and right hemispheres for both groups. The color-bar denotes p-values obtained with the random effects group analyses of the Deep Face (left) and Deep Word (right) encoding vs. Fixation contrasts.
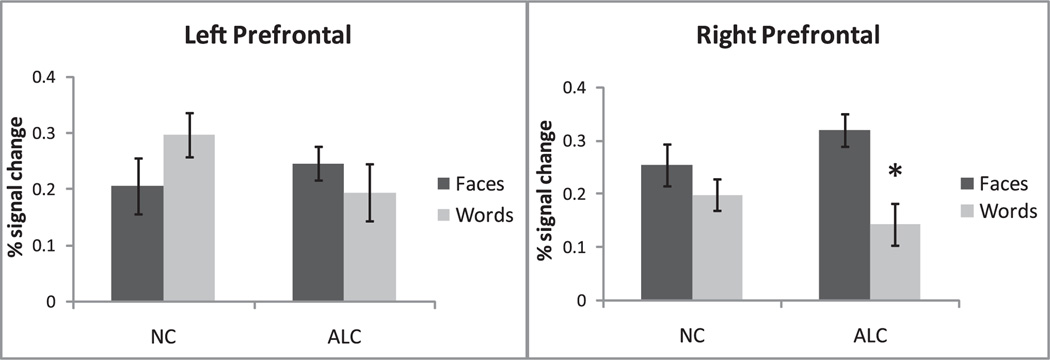
During encoding, deep processing evoked a significantly stronger activation overall as compared to the shallow tasks (F1,28 = 13.9, p < 0.001). Prefrontal regions showed an expected material-related sensitivity as indicated by a significant Material × Laterality interaction (F1,28 = 19.0, p < 0.0005). Laterality differences were particularly pronounced for faces, as they activated the prefrontal area more on the right than on the left (F1,28 = 18.2, p < 0.0005). Conversely, prefrontal activation to words was stronger on the left than on the right (F1,28 = 5.9, p < 0.05). Interactions between the factors of Group and Material (F1,28 = 5.0, p < 0.05) and Material and Laterality (F1,28 = 19.5, p < 0.0001) during deep encoding tasks resulted from different patterns of activation to faces and words exhibited by the ALC and NC groups (Figs. 6 and 7). Even though the groups did not differ in overall activity levels, their material-specific laterality patterns were different. Whereas for the ALC group, faces elicited stronger activation than words in the right hemisphere (F1,28 = 22.0, p < 0.0001) with no difference on the left, the NC group exhibited effects of laterality both for faces (F1,28 = 5.0, p < 0.05) and words (F1,28 = 8.8, p < 0.01).
Fig. 7.
Left and right prefrontal ROI analyses for the NC and ALC groups during the face and the word deep encoding tasks. Presented are mean percent signal change ± SEM for each group/condition. For the ALC group faces elicited significantly stronger activation than words, particularly in the right hemisphere.
Behavioral impairments observed in the ALC group to negative faces (prolonged RTs) and neutral faces (ALC rated them as more intelligent than the NC) indicated that the ALC group found this task to be challenging. As shown in Table 2, BOLD activity measured in the amygdala during negative and neutral conditions correlated negatively with the right prefrontal activity during the corresponding tasks, its decrease corresponding to the relative increase in the right prefrontal activity in the ALC group to face stimuli. This finding is consistent with the possibility that the prefrontal regions were engaged to compensate for the amygdala’s failure to contribute fully to the task performance in the ALC group, as discussed further below. Recognition task analyses yielded no significant effects.
DISCUSSION
Results of this study suggest that deficient activation of amygdala and hippocampus may underlie impaired processing of emotional faces in ALCs. Whereas the NC group showed stronger activation in the amygdala and hippocampus when viewing photographs of faces with emotional, relative to neutral expressions, for the ALC group, temporal limbic activation was undifferentiated to the emotionally valenced and neutral faces. This effect was observed both during deep encoding (i.e., judging “intelligence”) and face recognition tasks, providing internal validity to the findings.
Amygdala, Hippocampus, and Emotional Functions
Numerous studies have shown that the amygdala is critical for emotional functions (Aggleton, 2000) and that it is activated by faces with positive or negative emotional expressions (Breiter et al., 1996; Fitzgerald et al., 2006; Winston et al., 2003). In agreement with other studies, our results suggest a stronger sensitivity of the right amygdala to emotional expressions (Anderson et al., 2000), with the hemispheric difference particularly evident for negative face stimuli (Silberman and Weingartner, 1986). Furthermore, our observation of similar, albeit weaker activation of the hippocampus is in accord with other studies suggesting hippocampal involvement in face processing in general (Gur et al., 2002), and amygdala-hippocampus interactions during tasks of emotional memory in particular (Phelps, 2004).
The amygdala’s sensitivity to emotional face stimuli is crucial for recognizing a range of social emotions (Adolphs et al., 2002), and it has been probed in a variety of clinical populations. Overall, its activity corresponds to the emotional symptomatology of the underlying disorder or syndrome. More specifically, increased amygdala activity to emotional face expressions has been observed in social phobia (Birbaumer et al., 1998), anxiety (Stein et al., 2007), depression (Drevets, 2000), and in post-traumatic stress disorder (Rauch et al., 2000). In contrast, amygdala hypoactivity has been observed in psychopathy (Kiehl et al., 2001) and in youths with callous-unemotional traits (Marsh et al., 2008).
In a study comparing young social drinkers with (FH+) or without (FH−) family history of alcoholism, only the FH− individuals showed amygdala activation to faces (Glahn et al., 2007). Across both groups, the degree of activation correlated with the measures of behavioral disinhibition. These results are in line with this study suggesting that amygdala hypoactivity may underlie the emotional dysfunction in chronic alcoholics. Furthermore, they suggest that the emotional dysfunction may precede alcohol abuse and may be a part of the wide array of behavioral problems including impulsivity, disinhibition, and disregard for social norms.
Alcoholism, Emotions, and Compensatory Hypothesis
Alcoholism-related impairments in the perception of emotional face expressions have been reported in numerous studies (Clark et al., 2007; Foisy et al., 2005, 2007a,b; Kornreich et al., 2001; Maurage et al., 2008; Oscar-Berman et al., 1990; Philippot et al., 1999; Townshend and Duka, 2003). This study has confirmed and extended these findings in 2 ways. We observed that the NC participants were differentially reactive to the emotional face expressions as compared to ALC participants in an appraisal task in which the emotional valence of the stimuli was incidental to the task. More importantly, however, this study indicates that these emotional deficits may be due to impaired temporal limbic contributions to processing emotionally expressive faces.
In this study, the ALC’s hypoactivity of amygdala and hippocampus to emotional faces was accompanied by behavioral deficits, as the ALC participants took longer to judge a face as being unintelligent. Their RTs correlated negatively with their verbal IQ and working memory, suggesting that ALC individuals with better-preserved cognitive functions were able to furnish these judgments more efficiently. The length of heavy drinking prior to their abstinence correlated positively with the RTs, indicating effects of long-term alcohol abuse on this socially relevant assessment task. Furthermore, the ALC group rated neutral faces as more intelligent than the NC group. Similar findings have been reported for individuals with selective amygdala damage who judged negative faces as more trustworthy and approachable than control subjects (Adolphs et al., 1998).
In this study, the ALC participants were impaired on the intelligence-appraisal task possibly due to their dampened amygdala activity. Synchronous increase in prefrontal activity may have made it possible to performthe task, though at the cost of behavioral impairment. This possibly compensatory engagement of the prefrontal areas is suggested by the negative correlations between left amygdala activity and the right prefrontal region for the negative and neutral conditions, on which the ALC showed behavioral deficits (Table 2). Dampened amygdala activity (Fig. 5) in the ALC group was accompanied by a relative increase in right prefrontal activity to faces (Figs. 6 and 7). This pattern of activity is consistent with reduced amygdala activity and concurrent compensatory prefrontal engagement observed in healthy participants in studies using emotional reappraisal or suppression paradigms (Ochsner et al., 2002; Quirk and Beer, 2006).
Hypoactivation in the amygdala may partially account for the known deficits in alcoholism-related emotional functioning, and it could contribute to the interpersonal difficulties observed in this population (Kornreich et al., 2002). However, the amygdala functions within a multifocal interactive brain system that is flexibly engaged depending on the characteristics of the eliciting situation. Intracranial recordings in humans and neuroimaging studies using face stimuli have shown that emotional face processing relies on a distributed network including the amygdala and lateral prefrontal regions (Halgren and Marinkovic, 1995; Ishai et al., 2005). In fact, resection of a right ventrolateral prefrontal region where face-selective responses were recorded produced a profound deficit in recognizing the facial expression of fear (Marinkovic et al., 2000).
The compensatory hypothesis has been advanced in the alcoholism field as a way of interpreting alcoholics’ unimpaired performance that was accompanied by increased fronto-cerebellar activity in demanding cognitive tasks such as working memory (Desmond et al., 2003; Sullivan and Pfefferbaum, 2005). Results of this study are strongly suggestive of a similar mechanism whereby prefrontal regions modulate emotional functioning to compensate for the blunted amygdala activity during a socially relevant face appraisal task. This compensatory prefrontal engagement may underlie a coping strategy that the ALCs assume when faced with emotionally or socially challenging situations.
Other emotion-evoked limbic deficits in chronic alcoholics have been observed as well. Salloum and colleagues (2007) reported deficient activation of the ventral anterior cingulate region to negative facial emotions. Studies using diffusion tensor imaging have reported microstructure deficits in frontal white matter tracts connecting prefrontal and limbic areas (Harris et al., 2008), as well as disruptions in callosal connections (Schulte et al., 2005). Furthermore, significant volume reductions in dorsolateral prefrontal cortex, amygdala, and nucleus accumbens were observed in ALCs (Makris et al., 2008), confirming previous reports of alcoholism-related damage in the frontal and limbic structures (Agartz et al., 1999; Chanraud et al., 2007; De Bellis et al., 2005; Mukamal, 2004; Pfefferbaum et al., 1998; Sullivan et al., 1995). Taken together, this evidence suggests that alcoholism-related impairments in emotional functions may be observed when the cortico-limbic circuitry is unable to compensate for blunted amygdala contributions during a task challenging emotional or social functions.
Diathesis of Amygdala Hypoactivity in Chronic Alcoholics
Family History of Alcoholism and Disinhibited or Antisocial Traits
Development of alcohol dependence is influenced both by genetic factors and family environment. Most of the ALC participants in our study (73%) reported incidence of alcoholism in their first-degree relatives, as compared to 21% in the NC group and the majority was linked to paternal alcoholism. Moreover, a majority (67%) of the ALC participants reported experiencing symptoms of ASPD at some point in their lives, compared to 14% of NC, although none of the participants reported current symptomatology. The observed blunted amygdala responsiveness to emotional faces in our ALC group resonates with evidence of amygdala hypoactivity to faces in young individuals with positive family history of alcoholism, particularly in those with more disinhibited traits (Glahn et al., 2007). Reduced amygdala responses to emotional faces have also been observed in individuals with psychopathic tendencies (Blair, 2008; Kiehl et al., 2001). Thus, amygdala hypoactivity may underlie the emotional dysfunction in chronic alcoholics which may precede alcohol abuse and may be a part of the wide array of behavioral problems including impulsivity, disinhibition, and disregard for social norms (Goldstein et al., 2007), partially reflecting genetic vulnerabilities to alcohol abuse (Schuckit, 2009).
Amygdala and Dopaminergic Deficits in Chronic Alcoholism
Since dopamine function was not manipulated in this study, any related interpretation is necessarily speculative. However, several converging lines of evidence suggest a possibility that dopaminergic deficits may contribute to the observed amygdala hypoactivity to emotionally expressive faces. Pharmacological agents modulate amygdala activity as the dopaminergic agonists increase (Hariri et al., 2002), and antagonists decrease activity to emotional stimuli in healthy subjects (Takahashi et al., 2005). Dopaminergic receptor density in amygdala is severely decreased in chronic alcoholics (Tupala et al., 2001), which might contribute to its decreased sensitivity to emotional stimuli observed in this study. Dopaminergic abnormalities have been known to mediate alcohol and drug addiction (Bowirrat and Oscar-Berman, 2005; Everitt et al., 1999; Koob, 2003; Volkow et al., 2002) and predict the risk of relapse (Heinz et al., 2005). Moreover, dopaminergic deficits are a part of a wider array of interrelated abnormalities affecting the brain reward circuitry in which the amygdala plays an essential role (Koob and LeMoal, 2005).
Prefrontal Cortex, Material Specificity, and Depth of Processing
Another focus of interest in this study was the lateral prefrontal region because of its susceptibility to alcohol-induced damage (Makris et al., 2008; Moselhy et al., 2001; Oscar-Berman and Hutner, 1993), as well as its contribution to encoding of emotionally expressive faces (Sergerie et al., 2005). In contrast to temporal limbic structures, activity in prefrontal regions was not sensitive to emotional valence in this study during encoding tasks. Instead, they appeared to be involved in “cognitive” aspects of the task such as depth of processing and material type. Faces and words evoked partially different prefrontal activation patterns in ALC and NC groups during encoding tasks. The NC group showed expected right-dominant activity to faces and left-dominant to words, in agreement with other studies showing materially specific laterality effects during encoding tasks (Braver et al., 2001; Kelley et al., 1998). In contrast, stronger activation to faces overall in the ALC group, particularly in the right prefrontal area, correlated negatively with amygdala activity, possibly compensating for its diminished activity to emotional faces.
Depth of processing was manipulated in this study by means of the shallow (judging color) and deep encoding conditions (judging whether words were abstract or concrete and whether faces were intelligent or not). Behavioral results indicated that those words and faces that were encoded in the deep condition were remembered with greater speed and accuracy than with the shallow encoding condition, confirming the level-of-processing effect (Craik and Lockhart, 1972). Furthermore, stimuli that were encoded under deep encoding conditions evoked significantly stronger activation in mesial temporal limbic regions, and even more strongly in prefrontal regions, than those processed under shallow encoding conditions. This finding agrees with other studies showing that deeper, semantic processing is associated with increased activity in prefrontal and mesial temporal regions (Grady et al., 1998; Kapur et al., 1994), possibly reflecting their interaction in binding information into episodic memory traces (Buckner et al., 2000; Makris et al., 2008). In addition, although we found an overall stronger activation by faces as compared to words in the amygdala, the laterality of the examined structures was materially specific, with right dominance for faces and left for words.
Limitations of the Study
Results of this study should be interpreted with due consideration of their limitations. The sample size was small and it did not include women, which necessarily limits the generalizability of the findings. Voxel-wise comparison of the cortical activity did not yield reliable differences in the overall activity between the 2 groups. However, hypothesis-based analysis of the prefrontal ROIs revealed material-dependent group differences in the activity patterns. A large number of conditions included in our design decreased the power to observe potential overall group-wise differences necessitating follow-up studies that can investigate more specific aspects of alcoholism-related deficits in emotional function.
CONCLUSIONS
Results of this study confirmed and extended observations of impaired emotional functioning in ALCs. Neuroimaging evidence showed deficient activation of the amygdala and hippocampus during cognitive tasks using emotional face expressions. Whereas in NC subjects, stronger activation was observed to faces with positive and negative, as compared to neutral emotional expressions, the activation to emotional faces was significantly blunted in the ALC subjects. The emotion-induced deficiency in limbic activation in alcoholics is consistent with clinical evidence of their interpersonal difficulties and could be a contributing factor to adverse repercussions in social interactions for this population. This finding is in agreement with studies showing amygdala hypoactivity in psychopathy (Blair, 2008) and also in individuals with family history of alcoholism, particularly those with more disinhibited traits (Glahn et al., 2007). Thus, amygdala hypoactivity may underlie the emotional dysfunction in chronic alcoholics, which may precede alcohol abuse and may be a part of the wide array of behavioral problems including disinhibition and disregard for social norms (Goldstein et al., 2007).
The ALC participants were impaired on the intelligence-appraisal task, possibly due to their dampened amygdala activity. However, amygdala hypoactivity was correlated with a synchronous increase in prefrontal activity on the conditions on which the ALC group showed behavioral deficits, suggesting compensatory engagement of the prefrontal regions. This pattern of inversely related activity in the amygdala and prefrontal cortex is consistent with the evidence obtained from healthy participants in studies using emotional reappraisal or suppression paradigms. This compensatory prefrontal engagement may underlie a coping strategy that alcoholic individuals assume when faced with emotionally or socially challenging situations.
ACKNOWLEDGMENTS
This research was supported by funds from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), grants R01-AA07112 and K05-AA00219, and Medical Research Service of the US Department of Veterans Affairs to Dr. Marlene Oscar-Berman; NIAAA K01-AA13402, R01-AA016624, and Alcoholic Beverage Medical Research Foundation to Dr. Ksenija Marinkovic, by P41RR14075, and MIND Institute. We thank Diane Merritt for recruitment assistance and Sheeva Azma and Susan Mosher for help with data collection and analysis.
REFERENCES
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14:1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. The Amygdala: A Functional Analysis. 2nd ed. Oxford, New York: Oxford University Press; 2000. [Google Scholar]
- Anderson AK, Spencer DD, Fulbright RK, Phelps EA. Contribution of the anteromedial temporal lobes to the evaluation of facial emotion. Neuropsychology. 2000;14:526–536. doi: 10.1037//0894-4105.14.4.526. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Schneider F, Weiss U, Flor H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9:1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philos Trans R Soc Lond B Biol Sci. 2008;363:2557–2565. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski J, Benton A, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. Am J Med Genet B Neuropsychiatry Genet. 2005;132:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, Snyder AZ, Ollinger JM, Akbudak E, Conturo TE, Petersen SE. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage. 2001;14(1 Pt 1):48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975;11:230–238. doi: 10.1016/s0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Logan J, Donaldson DI, Wheeler ME. Cognitive neuroscience of episodic memory encoding. Acta Psychol (Amst) 2000;105:127–139. doi: 10.1016/s0001-6918(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Burock MA, Dale AM. Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Hum Brain Mapp. 2000;11:249–260. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. Rutgers Center of Alcohol Studies. New Brunswick, NJ: 1969. American Drinking Practices: A National Study of Drinking Behavior and Attitudes. Monograph #6. [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Clark US, Oscar-Berman M, Shagrin B, Pencina M. Alcoholism and judgments of affective stimuli. Neuropsychology. 2007;21:346–362. doi: 10.1037/0894-4105.21.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Craik FI, Lockhart RS. Levels of processing: a framework for memory research. J Verb Learn Verb Behav. 1972;11:671–684. [Google Scholar]
- Crews FT. Research Monograph. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism (NIAAA); 2000. Neurotoxicity of Alcohol: Excitotoxicity, Oxidative Stress, Neurotrophic Factors, Apoptosis, and Cell Adhesion Molecules, Vol. 34. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Emotion in the perspective of an integrated nervous system. Brain Res Brain Res Rev. 1998;26:83–86. doi: 10.1016/s0165-0173(97)00064-7. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Dick DM, Bierut LJ. The genetics of alcohol dependence. Curr Psychiatry Rep. 2006;8:151–157. doi: 10.1007/s11920-006-0015-1. [DOI] [PubMed] [Google Scholar]
- Dick DM, Foroud T. Candidate genes for alcohol dependence: a review of genetic evidence from human studies. Alcohol Clin Exp Res. 2003;27:868–879. doi: 10.1097/01.ALC.0000065436.24221.63. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution inter-subject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Foisy ML, Kornreich C, Fobe A, D’Hondt L, Pelc I, Hanak C, Verbanck P, Philippot P. Impaired emotional facial expression recognition in alcohol dependence: do these deficits persist with midterm abstinence? Alcohol Clin Exp Res. 2007a;31:404–410. doi: 10.1111/j.1530-0277.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- Foisy ML, Kornreich C, Petiau C, Parez A, Hanak C, Verbanck P, Pelc I, Philippot P. Impaired emotional facial expression recognition in alcoholics: are these deficits specific to emotional cues? Psychiatry Res. 2007b;150:33–41. doi: 10.1016/j.psychres.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Foisy ML, Philippot P, Verbanck P, Pelc I, van der Straten G, Kornreich C. Emotional facial expression decoding impairment in persons dependent on multiple substances: impact of a history of alcohol dependence. J Stud Alcohol. 2005;66:673–681. doi: 10.15288/jsa.2005.66.673. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Goldstein RB, Dawson DA, Saha TD, Ruan WJ, Compton WM, Grant BF. Antisocial behavioral syndromes and DSM-IV alcohol use disorders: results from the National Epidemiologic Survey on alcohol and related conditions. Alcohol Clin Exp Res. 2007;31:814–828. doi: 10.1111/j.1530-0277.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Craik FI. Neural correlates of the episodic encoding of pictures and words. Proc Natl Acad Sci U.S.A. 1998;95:2703–2708. doi: 10.1073/pnas.95.5.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, Alsop D, Maldjian J, Gur RE. Brain activation during facial emotion processing. Neuroimage. 2002;16(3 Pt 1):651–662. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K. Neurophysiological networks integrating human emotions. In: Gazzaniga M, editor. The Cognitive Neurosciences. Cambridge, MA: MIT Press; 1995. pp. 1137–1151. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27:1036–1040. doi: 10.1016/S0893-133X(02)00373-1. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, Papadimitriou GM, Makris N, Oscar-Berman M. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol Clin Exp Res. 2008;32:1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Res Bull. 2005;67:87–93. doi: 10.1016/j.brainresbull.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Kaplan E. A process approach to neuropsychological assessment. In: Boll T, Bryant BK, editors. Clinical Neuropsychology and Brain Function: Research, Measurement, and Practice. Washington, DC: American Psychological Association; 1988. pp. 125–167. [Google Scholar]
- Kapur S, Rose R, Liddle PF, Zipursky RB, Brown GM, Stuss D, Houle S, Tulving E. The role of the left prefrontal cortex in verbal processing: semantic processing or willed action? Neuroreport. 1994;5:2193–2196. doi: 10.1097/00001756-199410270-00051. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, Liddle PF. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of Addiction. San Diego, CA: Elsevier Academic Press; 2005. [Google Scholar]
- Kornreich C, Blairy S, Philippot P, Hess U, Noel X, Streel E, Le Bon O, Dan B, Pelc I, Verbanck P. Deficits in recognition of emotional facial expression are still present in alcoholics after mid- to long-term abstinence. J Stud Alcohol. 2001;62:533–542. doi: 10.15288/jsa.2001.62.533. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Philippot P, Foisy ML, Blairy S, Raynaud E, Dan B, Hess U, Noel X, Pelc I, Verbanck P. Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol Alcohol. 2002;37:394–400. doi: 10.1093/alcalc/37.4.394. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM. Brain shrinkage in alcoholics: a decade on and what have we learned? Prog Neurobiol. 1999;58:381–387. doi: 10.1016/s0301-0082(98)00091-4. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. New York: Oxford University Press; 1995. [Google Scholar]
- MacVane J, Butters N, Montgomery K, Farber J. Cognitive functioning in men social drinkers; a replication study. J Stud Alcohol. 1982;43:81–95. doi: 10.15288/jsa.1982.43.81. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Halgren E. Human brain potentials related to the emotional expression, repetition, and gender of faces. Psychobiology. 1998;26:348–356. [Google Scholar]
- Marinkovic K, Trebon P, Chauvel P, Halgren E. Localised face processing by the human prefrontal cortex: Face-selective intracerebral potentials and post-lesion deficits. Cogn Neuropsychol. 2000;17:187–199. doi: 10.1080/026432900380562. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJ. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, Martin S, de Timary P. Face processing in chronic alcoholism: a specific deficit for emotional features. Alcohol Clin Exp Res. 2008;32:600–606. doi: 10.1111/j.1530-0277.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States Manual. San Diego, CA: Educational Testing Service; 1981. [Google Scholar]
- Monnot M, Lovallo WR, Nixon SJ, Ross E. Neurological basis of deficits in affective prosody comprehension among alcoholics and fetal alcohol-exposed adults. J Neuropsychiatry Clin Neurosci. 2002;14:321–328. doi: 10.1176/jnp.14.3.321. [DOI] [PubMed] [Google Scholar]
- Monnot M, Nixon S, Lovallo W, Ross E. Altered emotional perception in alcoholics: deficits in affective prosody comprehension. Alcohol Clin Exp Res. 2001;25:362–369. [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ. Alcohol consumption and abnormalities of brain structure and vasculature. Am J Geriatr Cardiol. 2004;13:22–28. doi: 10.1111/j.1076-7460.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Alcoholism and asymmetries of brain function. Alcohol Health Res World. 1992;16:273–279. [Google Scholar]
- Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. Vol. 34. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2000. pp. 149–158. Research Monograph. [Google Scholar]
- Oscar-Berman M, Bowirrat A. Genetic influences in emotional dysfunction and alcoholism-related brain damage. Neuropsychiatric Dis Treat. 2005;1:211–229. [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Hancock M, Mildworf B, Hutner N, Weber DA. Emotional perception and memory in alcoholism and aging. Alcohol Clin Exp Res. 1990;14:383–393. doi: 10.1111/j.1530-0277.1990.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Hutner N. Frontal lobe changes after chronic alcohol ingestion. In: Hunt WA, Nixon SJ, editors. Alcohol-Induced Brain Damage. Vol. 22. Rockville, MD: Department of Health and Human Services; 1993. pp. 121–156. NIAAA Research Monograph. [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcoholism and the brain: an overview. Alcohol Res Health. 2004;27:125–133. [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Schendan HE. Asymmetries of brain function in alcoholism: relationship to aging. In: Obler L, Connor LT, editors. Neurobehavior of Language and Cognition: Studies of Normal Aging and Brain Damage. New York: Kluwer Academic Publishers; 2000. pp. 213–240. [Google Scholar]
- Paivio A, Yuille JC, Madigan SA. Concreteness, imagery, and meaningfulness values for 925 nouns. J Exp Psychol. 1968;76(Suppl. 1):1–25. doi: 10.1037/h0025327. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. The anatomical substrate of emotional behavior: the role of the cerebral cortex. In: Gainotte G, editor. Emotional Disorder and its Behavior. Vol. 5. Amsterdam: Elsevier; 2001. pp. 49–87. [Google Scholar]
- Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotions. Oxford: Oxford University Press; 1998. [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Philippot P, Kornreich C, Blairy S, Baert I, Den Dulk A, Le Bon O, Streel E, Hess U, Pelc I, Verbanck P. Alcoholics’ deficits in the decoding of emotional facial expression. Alcohol Clin Exp Res. 1999;23:1031–1038. [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Robins L, Helzer J, Cottler L, Goldring E. NIMH Diagnostic Interview Schedule: Version III Revised (DIS-III-R) St Louis, MO: Washington University; 1989. [Google Scholar]
- Rubin DC, Friendly M. Predicting which words get recalled: measures of free recall, availability, goodness, emotionality, and pronunciability for 925 nouns. Mem Cognit. 1986;14:79–94. doi: 10.3758/bf03209231. [DOI] [PubMed] [Google Scholar]
- Salloum JB, Ramchandani VA, Bodurka J, Rawlings R, Momenan R, George D, Hommer DW. Blunted rostral anterior cingulate response during a simplified decoding task of negative emotional facial expressions in alcoholic patients. Alcohol Clin Exp Res. 2007;31:1490–1504. doi: 10.1111/j.1530-0277.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–S14. [PubMed] [Google Scholar]
- Sergerie K, Lepage M, Armony JL. A face to remember: emotional expression modulates prefrontal activity during memory formation. Neuroimage. 2005;24:580–585. doi: 10.1016/j.neuroimage.2004.08.051. [DOI] [PubMed] [Google Scholar]
- Schulte T, Sullivan EV, Muller-Oehring EM, Adalsteinsson E, Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb Cortex. 2005;15:1384–1392. doi: 10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- Silberman EK, Weingartner H. Hemispheric lateralization of functions related to emotion. Brain Cogn. 1986;5:322–353. doi: 10.1016/0278-2626(86)90035-7. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Takano A, Asai K, Suhara T, Okubo Y. Effects of dopaminergic and serotonergic manipulation on emotional processing: a pharmacological fMRI study. Neuroimage. 2005;27:991–1001. doi: 10.1016/j.neuroimage.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Mixed emotions: alcoholics’ impairments in the recognition of specific emotional facial expressions. Neuropsychologia. 2003;41:773–782. doi: 10.1016/s0028-3932(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Tupala E, Hall H, Bergstrom K, Sarkioja T, Rasanen P, Mantere T, Callaway J, Hiltunen J, Tiihonen J. Dopamine D(2)/D(3)-receptor and transporter densities in nucleus accumbens and amygdala of type 1 and 2 alcoholics. Mol Psychiatry. 2001;6:261–267. doi: 10.1038/sj.mp.4000859. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Daum I, Schlebusch P, Trenckmann U. Processing of affective stimuli in alcoholism. Cortex. 2005;41:189–194. doi: 10.1016/s0010-9452(08)70893-1. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Burr G, Pascani K, Dewey SL, Wolf AP. Decreased brain metabolism in neurologically intact healthy alcoholics. Am J Psychiatry. 1992;149:1016–1022. doi: 10.1176/ajp.149.8.1016. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hitzemann R, Wong C, Pappas N. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res. 2002;116:163–172. doi: 10.1016/s0925-4927(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio, TX: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-III. San Antonio, TX: The Psychological Corporation; 1997b. [Google Scholar]
- Winston JS, O’Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage. 2003;20:84–97. doi: 10.1016/s1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]
- Woodward JA, Bonett DG, Brecht ML. Introduction to Linear Models and Experimental Design. San Diego, CA: Harcourt Brace Jovanovich; 1990. [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M, Ziegler DA, Davis OC, Kissling C, Schumann G, Breiter HC, Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Lubin B. Multiple Affect Adjective Check List. San Diego, CA: Educational and Industrial Testing Service; 1965. [Google Scholar]