Abstract
Neuroimaging studies have begun to uncover the neural substrates of cancer and treatment-related cognitive dysfunction, but the time course of these changes in the years following chemotherapy is unclear. This study analyzed multimodality 3T MRI scans to examine the structural and functional effects of chemotherapy and post-chemotherapy interval (PCI) in a cohort of breast cancer survivors (BCS; n=24; PCI mean 6, range 3–10 y) relative to age- and education-matched healthy controls (HC; n=23). Assessments included voxel-based morphometry (VBM) for gray matter density (GMD) and fMRI for activation profile during a 3-back working memory task. The relationships between brain regions associated with PCI and neuropsychological performance, self-reported cognition, and oxidative and direct DNA damage as measured in peripheral lymphocytes were assessed in secondary analyses. PCI was positively associated with GMD and activation on fMRI in the right anterior frontal region (Brodmann Areas 9 and 10) independent of participant age. GMD in this region was also positively correlated with global neuropsychological function. Memory dysfunction, cognitive complaints, and oxidative DNA damage were increased in BCS compared to HC. Imaging results indicated lower fMRI activation in several regions in the BCS group. BCS also had lower GMD than HC in several regions, and in these regions GMD was inversely related to oxidative DNA damage and learning and memory neuropsychological domain scores. This is the first study to show structural and functional effects of PCI and to relate oxidative DNA damage to brain alterations in BCS. The relationship between neuroimaging and cognitive function indicates the potential clinical relevance of these findings. The relationship with oxidative DNA damage provides a mechanistic clue warranting further investigation.
Keywords: breast cancer, voxel-based morphometry, functional MRI, chemotherapy, DNA damage
Introduction
Increasing evidence shows cognitive changes related to breast cancer and its treatments, as demonstrated by lower than expected performance on neuropsychological tests. Meta-analyses have shown changes in verbal and visuospatial domains [1], as well as executive function (including working memory) and processing speed [2–5]. Self-report measures show increased perceived cognitive difficulties in cancer patients [6].
Imaging studies have recently begun to reveal the neural substrates of such changes. Retrospective MRI studies have shown post-chemotherapy structural alterations in both gray [7–9] and white matter [10,11] in cohorts of breast cancer survivors (BCS) whose average post-chemotherapy intervals (PCIs) ranged from four months to 21 years. Retrospective functional MRI (fMRI) studies have shown hypoactivation during cognitive tasks, at an average of 4.7 years [12] or 10 years [13] post-chemotherapy, compared to BCS who never received chemotherapy. De Ruiter et al. demonstrated links between structural and functional results in a single cohort approximately 10 years post-chemotherapy (compared to non-chemotherapy treated BCS), with gray and white matter changes overlapping with regions of fMRI hypoactivation [7]. Recently, prospective structural, functional, and diffusion tensor MRI studies [14–16] have shown changes after treatment, particularly in the frontal cortex. The recent prospective data is particularly important given reports of cognitive [17] and functional [16,18,19] changes prior to treatment.
Many biological mechanisms for cancer- and treatment-associated cognitive dysfunction and brain changes have been proposed; prominent among them are oxidative stress, DNA damage, and compromised DNA repair, all of which may be shared risk factors for development of cancer and sensitivity to cancer- and treatment-related neurological side effects [20]. Several chemotherapeutic agents are known to produce oxidative DNA damage. Increased oxidative DNA damage in white blood cells has also been demonstrated in women with breast cancer pre-treatment, and these changes are exacerbated after chemotherapy [21].
Despite recent progress, the time course of changes during survivorship is poorly understood. The current study examines the association of PCI with gray matter density (GMD) and working memory-related functional MRI (fMRI) brain activation in BCS. The relationships among PCI, neuroimaging, cognitive changes and biological markers could help elucidate important mechanisms. We hypothesized that in BCS compared to healthy controls there would be increased cognitive dysfunction, changes on neuroimaging, and oxidative and direct DNA damage, and that these would be associated with shorter PCI.
Methods
Participants
BCS with a history of non-metastatic disease and chemotherapy treatment and age- and education-matched healthy controls (HC) were recruited as part of a larger study of the cognitive sequelae of cancer and its treatments [22,23]. Approximately 60% of the sample therefore had prior assessment with a subset of neuropsychological measures 2–4 years earlier. No practice effects were evident in the subset of the current sample enrolled in the previous study (data not shown). In the present study, 27 BCS and 24 HC underwent structural and functional MRI exams as well as a battery of neuropsychological tests and self-report measures. Blood was obtained for biomarker analysis, including Comet assays to assess DNA damage (see below). Four participants were excluded from all analyses: one HC for current use of antipsychotic medication, two BCS for history of stroke, and one BCS for extremely poor performance (>4 SD below the control mean) on neuropsychological tests that was thought to be due to a comorbid condition. Final group sizes in the current study were 24 BCS and 23 HC.
Image Acquisition, Processing, and Analysis
All images were acquired on the same Siemens MAGNETOM Tim Trio 3T scanner using a 12-channel head coil as previously described [16,24]. Structural scans were a sagittal T1-weighted MP-RAGE (magnetization prepared rapid gradient echo) sequence with the parameters: 160 contiguous 1.2 mm slices, TR: 2300 ms, TE: 2.91 ms, TI: 900 ms, flip angle: 9, NEX: 1, BW/Pixel: 240, FOV: 256 mm, matrix 256×256, in-plane resolution: 1.0×1.0 mm. Blood oxygenation level-dependent (BOLD) fMRI images were acquired axially with a T2*-weighted single shot echo-planar imaging (EPI) pulse sequence with the parameters: TR: 2250 ms, TE: 29 ms, flip angle: 79, FOV: 220 mm, matrix: 88×88, slice thickness: 3.5 mm, NEX: 1, yielding 39 contiguous axial slices and a voxel dimension of 2.5×2.5×3.5 mm.
As in our prior fMRI studies of breast cancer patients [16,25], a block design verbal “N-back” task was used. During scanning, participants saw a series of consonant letters (except L, W, and Y) presented one every three seconds. Task conditions were 0-, 1-, 2-, and 3-back. Each condition was presented in 27-second epochs preceded by three seconds of instruction (e.g., “the match is one back”). For each letter, participants responded via button press to indicate whether the current letter was a match (i.e., was the same as the designated target or the letter presented 1, 2, or 3 back in the sequence, depending on the condition) or a non-match. The four experimental conditions were each presented three times in pseudorandom order for a total of 12 task blocks (total duration = 6:54). Participants practiced a version of the task prior to scanning to ensure comprehension of the task. Presentation software (Neurobehavioral Systems, Inc., Albany, CA) was used to program the task and record response accuracy and reaction times.
Image processing for VBM used in-house MATLAB (Version 7.9 (R2009b), Mathworks, Inc., Natick, MA) scripts to implement optimized VBM methods [26–28] using Statistical Parametric Mapping software (SPM; Version 8, Wellcome Department of Imaging Neuroscience, London, UK), similar to our prior studies [29–31,15,16]. Briefly, after reconstruction, scans were registered to the Montreal Neurological Institute T1-weighted template and segmented into gray matter, white matter, and cerebrospinal fluid compartments using the MNI T1-weighted template and corresponding tissue probability maps. Gray matter maps were then spatially normalized to MNI space, resampled to 1 mm isotropic voxels, and smoothed using an isotropic Gaussian spatial filter (FHWM = 10 mm). The smoothed, normalized gray matter maps were used for second-level multi-subject voxelwise analyses. The SPM8 prior probability gray matter template was used to restrict statistical comparisons to the gray matter compartment.
fMRI image processing also utilized SPM8. Spatial realignment using a six parameter model was performed on raw scan data to remove minor motion-related signal change. Realignment parameters were entered as covariates at the subject level, and all volumes were normalized into standardized atlas space, resampled to 2 mm3 isotropic voxels, and smoothed to a FWHM of 8 mm. Contrast images comparing pairs of working memory load conditions (e.g., 3-back > 0-back) were created for each participant. These contrast images were then used in second-level multi-subject voxelwise analyses. Mixed model analyses accounted for both random effects (scan) and fixed effects (task condition).
Voxelwise random effects analyses for both VBM and fMRI were conducted using linear regression (for PCI) or t-tests (between-group analysis) as implemented in SPM8 to construct maps of voxels in which local GMD or activation differed as a function of PCI or between BCS and HC groups. Age was included as a covariate in all group analyses. Statistical significance was set at an uncorrected voxel-level pcrit of 0.001 and a minimum cluster size (k) of 10 voxels. Voxel-level significance values represent the chance (under the null hypothesis) of finding a voxel with as great or greater a height threshold (Z). Cluster-level significance can be interpreted as the probability (under the null hypothesis) of finding a cluster with as great or greater a number of voxels, with family-wise error correction for the whole-brain search volume (i.e., correction for multiple comparisons). In fMRI comparisons, a mask of the main effect of the 3-back > 0-back comparison (pcrit = 0.05) in all participants was applied in order to search in only regions of task-related activation.
In order to correlate imaging data with behavioral and Comet assay data, MarsBaR v0.42 (http://marsbar.sourceforge.net/) was used to extract mean GMD or activation values from each statistically significant cluster. To reduce dimensionality of imaging data from each analysis, these mean cluster values were averaged to create an overall mean value of significant clusters for each analysis.
Neurocognitive Testing and Self-Report Measures
Raw neurocognitive test scores were normalized using the mean and standard deviation of the HC group scores. Domain scores were created for each participant by averaging the z-scores of the included tests. Domain scores were then adjusted for age. The learning domain consisted of the Rey Auditory-Verbal Learning Test (AVLT) total learning score [32,33], story recall (immediate) [34], and the sum of recalled items for initial learning trials on the Brown Learning Test (BLT) [35]. The memory domain comprised Rey AVLT delayed recall, story recall (10 minute delay) [34], and BLT long delay score. The attention domain included Wechsler Adult Intelligence Scale (WAIS-III) Digit Span forward total score [36] and the Rao Paced Auditory Serial Addition Test (PASAT) 2 and 3 minute trial total scores [37]. The language domain included the Wide Range Achievement Test (WRAT-4) Word Reading test [38] and the Wechsler Abbreviated Scale of Intelligence (WASI) Vocabulary test [39]. The visuospatial domain was the WASI Block Design raw score. The executive domain consisted of the Digit Span backward total score, the Controlled Oral Word Association (COWA) Test total score [32,33], Delis-Kaplan Executive Function System (D-KEFS) Color-Word Interference Test inhibition and inhibition/switching trials times [40], D-KEFS Sorting Test number of correct sorts, and D-KEFS Trail Making Test number-letter switching trial time. The psychomotor domain consisted of D-KEFS Color-Word Interference Test color naming trial time, D-KEFS Trail Making Test number sequencing trial time, Symbol Digit Modalities Test oral total score [41], and Grooved Pegboard total time [42].
Self-reported cognition was assessed using the Multiple Ability Self-Report Questionnaire (MASQ) [43] and the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-COG) [44]. It should be noted that, unlike other self-report data presented, higher scores on the FACT-COG are indicative of decreased cognitive complaints. The Center for Epidemiologic Studies-Depression Scale (CES-D) [45] and the State-Trait Anxiety Inventory-State Scale (STAI-S) [46] were used to assess depression and anxiety symptoms.
Assessment of Direct and Oxidative DNA Damage: Comet Assay
Whole blood (10μl) was mixed with 0.5 ml RPMI 1640 containing 10% FBS, 10% DMSO, 1 mM deferoxamine, step-frozen and stored at −80 °C until analysis. The Comet assay was performed as described previously [47]. Briefly, 6 μl of blood was mixed with 70 μl 1% low melting point agarose and applied onto Trevigen CometSlides. Cells were lysed, placed in alkali buffer, and then electrophoresed. Slides were stained with ethidium bromide and 100 randomly selected nuclei/treatment were evaluated (Komet 4.0; Kinetic Imaging Ltd., Liverpool, UK). For the assessment of oxidative DNA damage, a modified alkaline Comet assay was performed that included enzymatic digestion with formamidopyrimidine DNA glycosylase (fpg) prior to electrophoresis. Both direct and oxidative DNA damage were expressed as Comet (Olive) tail moment [(tail mean – head mean)*tail%DNA/100]. Increases in tail moment indicate increased DNA damage.
Statistical Analyses
Linear regression within BCS only was used to assess the effect of PCI on self-reported cognition, neuropsychological test scores, and DNA damage. T-tests were used to assess significance of between-group effects with these same variables. To examine the possible pattern of relationships between imaging results and other key variables, we tested the association between mean GMD and activation values with the above measures separately in each group using regression analyses. For these secondary analyses, a liberal significance threshold of p ≤0.05 was employed due to the relatively small sample size and likely non-independence among cognitive variables.
Results
Demographic information, self-report, Comet assay, age-adjusted neuropsychological domain, and cancer- and treatment-related information are shown in Table 1. Groups did not differ in age, education, handedness, APOE status, estimated IQ, STAI-S, or CES-D scores. Patients were an average of 6.4 years post-chemotherapy (range: 3.2–10.2). PCI was not significantly correlated with age (r = −0.086, p = 0.688).
Table 1.
Demographic, self-reported cognition, DNA damage, age-adjusted neuropsychological domain, and breast cancer treatment information. Values shown are mean ± SD.
| Healthy Controls | Breast Cancer Survivors | |
|---|---|---|
| N | 23 | 24 |
| Age (years) | 61.2±9.9 (range 46–79) | 57.8±9.6 (range 41–78) |
| Education (years) | 16.0±2.3 | 15.7±2.1 |
| Handedness (R:L) | 19:4 | 22:2 |
| APOE ε4 allele positive | 6 (27%)^ | 5 (24%)^ |
| Full Scale IQ Estimate (Barona Index [56]) | 113.4±3.9 | 113.3±4.3 |
| CES-D raw score | 8.7±6.9 | 7.5±5.8 |
| STAI-S raw score | 31.9±9.1 | 30.2±7.9 |
| 3-back score (corrected for guessing) | 54.1±21.9 | 53.7±21.2 |
| 3-back reaction time (seconds) | 0.772±0.186 | 0.825±0.200 |
| FACT-COG total raw score* | 135.3±13.0 | 116.2±28.8 |
| MASQ total raw score* | 79.9±17.7 | 97.1±20.3 |
| Direct DNA damage (alkaline Comet assay tail moment) | 1.0±0.3 | 0.8±0.3 |
| Oxidative DNA damage (fpg Comet assay tail moment)* | 1.0±0.6 | 1.5±1.2 |
| Age-adjusted neuropsychological domain z-scores: | ||
| Learning | 0.2±0.7 | −0.2±0.7 |
| Memory* | 0.03±0.5 | −0.3±0.6 |
| Attention | 0.1±0.7 | 0.4±0.6 |
| Language | −0.03±0.9 | 0.3±0.8 |
| Visuospatial | 0.1±0.9 | −0.5±1.0 |
| Executive | 0.04±0.6 | −0.04±0.7 |
| Psychomotor | 0.04±0.4 | −0.1±0.4 |
| Average | 0.1±0.4 | −0.1±0.5 |
| Age at diagnosis | 51.2±10.1 | |
| Received radiation | 19 | |
| Received tamoxifen | 13 | |
| Received aromatase inhibitor | 17 | |
| Average post-chemotherapy to MRI interval (years) | 6.4±2.1 (range 3.2–10.2) | |
| Stage I number of patients (percent) | 7 (29) | |
| Stage IIa | 8 (33) | |
| Stage IIb | 6 (25) | |
| Stage IIIa | 2 (8) | |
| Stage IIIb | 1 (4) | |
| Chemotherapy regimens: | ||
| Doxorubicin, cyclophosphamide (AC) | 7 | |
| Doxorubicin, cyclophosphamide, taxane (AC-T) | 5 | |
| Doxorubicin, cyclophosphamide, 5-fluorouracil (CAF) | 2 | |
| Doxorubicin, taxane (A-T) | 3 | |
| Cyclophosphamide, methotrexate, 5-fluorouracil (CMF) | 2 | |
| CMF and CAF | 1 | |
| Taxane only | 1 | |
| AC-T and capecitabine | 2 | |
| Taxane and capecitabine | 1 |
APOE genotypes were only available for 22 HC and 21 BCS participants
Between groups p ≤ 0.05.
Post-chemotherapy interval
Imaging
In voxelwise regressions with PCI (BCS group only), structural and functional effects converged in the right anterior frontal lobe (Figure 1): GMD was positively correlated with PCI in the right superior and middle frontal gyri (Brodmann Areas (BA) 9, 10) and activation was negatively correlated with PCI in the right middle frontal gyrus (BA 10). Increased GMD was associated with longer PCI in 10 clusters within the bilateral frontal and parietal lobes and basal ganglia, as well as the right temporal lobe (Table 2). No regions were evident in which decreased GMD was associated with longer PCI or in which activation was positively correlated with PCI.
Figure 1.
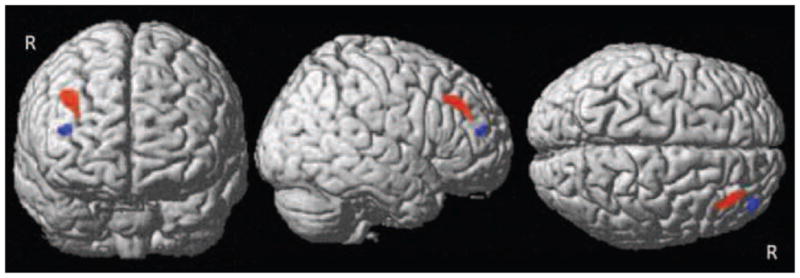
Functional and structural overlap in the right anterior middle frontal gyrus, in which gray matter density (red) was positively correlated with post-chemotherapy interval. Working memory-related activation (blue) was negatively correlated with post-chemotherapy interval (voxelwise p uncorrected = 0.001, cluster size=10 for both modalities)
Table 2.
Statistically significant clusters from imaging analyses (voxel-level p uncorrected = 0.001, cluster size = 10 voxels for all analyses).
| Peak MNI coordinates (x y z) | Cluster extent (k) | Cluster-level pcorrected (FWE) | Cluster-level puncorrected | Z | Region description of local maxima within cluster | Brodmann Area (BA) |
|---|---|---|---|---|---|---|
| VBM: Positive correlation with post-chemotherapy interval | ||||||
| 9 9 42 | 79 | 0.996 | 0.465 | 4.15 | Right cingulate gyrus | 32 |
| 64 8 8 | 160 | 0.970 | 0.294 | 4.10 | Right precentral gyrus | 44 |
| 37 37 33 | 608 | 0.453 | 0.051 | 4.09 | Right superior and middle frontal gyri | 9, 10 |
| 20 21 5 | 73 | 0.997 | 0.484 | 3.63 | Right caudate | N/A |
| −8 16 47 | 48 | 0.999 | 0.577 | 3.50 | Left cingulate gyrus | 32 |
| −21 11 −23 | 105 | 0.991 | 0.396 | 3.44 | Left inferior frontal gyrus | 47 |
| −21 20 2 | 11 | >0.999 | 0.814 | 3.44 | Left putamen | N/A |
| 15 −80 33 | 26 | >0.999 | 0.694 | 3.42 | Right precuneus | 7 |
| −9 83 14 | 37 | 0.999 | 0.630 | 3.38 | Left cuneus | 18 |
| 64 −20 −2 | 40 | 0.998 | 0.615 | 3.35 | Right superior temporal gyrus | 21 |
| 3-back > 0-back: Negative correlation with post-chemotherapy interval | ||||||
| 40 52 16 | 16 | 0.922 | 0.095 | 3.84 | Right middle frontal gyrus | 10 |
| VBM: Healthy Control > Breast Cancer Survivor | ||||||
| −43 −67 8 | 143 | 0.971 | 0.411 | 3.67 | Left middle temporal gyrus | 37 |
| 16 −26 −4 | 188 | 0.948 | 0.344 | 3.60 | Right midbrain | N/A |
| −14 −23 6 | 408 | 0.762 | 0.167 | 3.58 | Left thalamus | N/A |
| 2 −23 −17 | 184 | 0.950 | 0.349 | 3.53 | Right midbrain | N/A |
| 38 −66 −39 | 461 | 0.709 | 0.143 | 3.48 | Right cerebellum | N/A |
| 49 −21 23 | 59 | 0.995 | 0.611 | 3.37 | Right insula | 13 |
| 3-back > 0-back: Healthy Control > Breast Cancer Survivor | ||||||
| 10 −68 42 | 23 | 0.808 | 0.080 | 3.56 | Right precuneus | 7 |
| −28 −66 22 | 15 | 0.954 | 0.150 | 3.47 | Left middle temporal gyrus | 39 |
3-back task performance and reaction times, cognitive testing, self-reports, and Comet assay
PCI was not significantly correlated with 3-back task performance or reaction time, any neuropsychological domain or self-report measure, or DNA damage.
Relationship of imaging to other measures
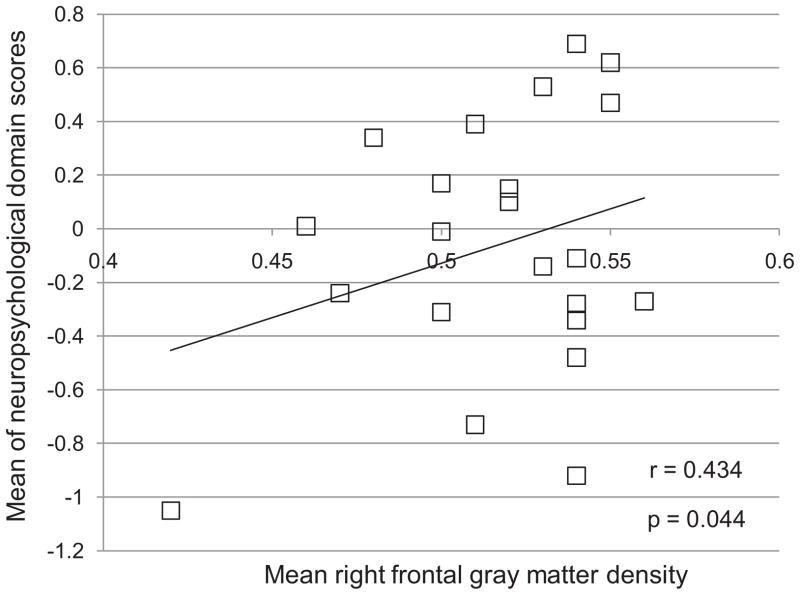
In secondary analyses, the mean GMD value of the right frontal cluster depicted in Figure 1 (red) was positively correlated with the overall neuropsychological performance (mean of domain scores, r = 0.434, p = 0.044) (Figure 2). The mean activation of the 3-back cluster depicted in Figure 1 (blue) was not associated with any other measures at p ≤ 0.05. The average GMD of all 10 significant clusters derived from a positive correlation with PCI (Table 2) was not associated with any other measures at p ≤ 0.05.
Figure 2.
In breast cancer survivors (BCS), the average of neuropsychological domain z-scores vs. mean gray matter density in right middle and superior frontal gyri (Brodmann Areas 9, 10)
Between-group analyses
Imaging
Imaging analyses revealed differences between the BCS and HC groups. BCS showed decreased GMD relative to HC in multiple regions including the left temporal lobe, right midbrain, left thalamus, right cerebellum, and right insula (Table 2). BCS did not show any regions of increased GMD relative to HC. Functional imaging revealed relatively decreased working memory-related brain activation in BCS compared to HC in the right precuneus and left middle temporal gyrus. No areas were associated with relatively greater activation in BCS.
3-back task performance and reaction times, cognitive testing, self-reports, and Comet assay
Performance accuracy and reaction times for the 3-back task did not differ between groups. BCS scored lower than HC on the memory domain on neuropsychological testing (F (1, 45) = 4.20, p = 0.046). The BCS and HC groups also differed on the FACT-COG (F (1, 45) = 8.45, p = 0.006) and MASQ (F (1, 45) = 9.61, p = 0.003) self-report measures, with BCS reporting more cognitive complaints on both measures. Oxidative DNA damage was increased in BCS (F (1, 45) = 4.00, p = 0.052) (Table 1).
Relationship of imaging to other measures
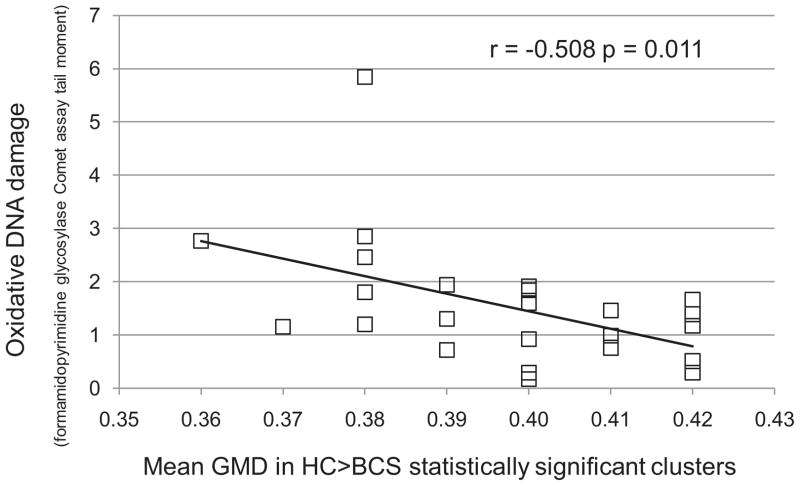
Within the BCS group, overall neuropsychological function and all but two domains were uncorrelated with the mean GMD of the regions showing between-group differences. The learning and memory domain scores, however, were negatively correlated with GMD (r = −0.639, p = 0.001 and r = −0.647, p = 0.001, respectively). Increased oxidative damage was associated with lower GMD in BCS (r = −0.508, p = 0.011, Figure 3). Activation did not correlate with any other measure. Within the HC group, no significant correlations were found.
Figure 3.
Oxidative DNA damage in breast cancer survivors (BCS) as measured by Comet assay of peripheral lymphocytes vs. mean gray matter density (GMD) of clusters in which BCS showed relatively decreased GMD compared to healthy controls (HC)
Discussion
This multimodal MRI study of long-term BCS found PCI associated with both structural and functional changes on brain MRI. Regional changes on imaging were in turn related to neuropsychological performance. Furthermore, a plasma biomarker showed increased oxidative DNA damage in BCS compared to controls which was also inversely associated with brain structural changes.
GMD was positively correlated with PCI in distributed brain regions with the largest clusters in the frontal lobes. This suggests that frontal gray matter recovers over time after an initial chemotherapy insult, as indicated by two prior prospective studies [15,24]. Working memory-related brain activation was inversely associated with PCI in a region proximal to the largest structural finding. The convergence of structural and functional changes in the frontal region is consistent with prior reports of changes in executive cognitive functions after chemotherapy [2–5,48] as well as retrospective [12,13] and prospective [16] functional neuroimaging studies. Results of previous functional imaging studies in breast cancer patients have been interpreted as showing compensatory hyperactivation in similar frontal regions [25,18,19,16]. The present results suggest that frontal hyperactivation diminishes over time, becoming less necessary as structural recovery proceeds. Alternatively, decreased activation with a longer PCI may reflect a failure of compensatory brain activation over time. Although neuropsychological performance could potentially help address this issue, the current study cannot differentiate between these mechanisms in the absence of substantial cognitive deficits after 6 years.
Comparisons between BCS and controls indicated decreases in GMD and activation in left middle temporal gyrus. Taken together, the structural and functional findings indicate an incomplete recovery after an average of 6.4 years post-treatment. This is generally consistent with previous GMD findings at a range of PCIs [7–9] as well as fMRI reports of hypoactivation 5–10 years after chemotherapy in BCS [12,13]. Of note, there is also evidence for increased blood flow on O15-PET during a memory task an average of 5–10 years after chemotherapy [49].
Relationships among imaging and cognitive variables are a particularly interesting aspect of this study that increases the potential clinical significance of our findings. Greater GMD in a right frontal cluster derived from a voxelwise regression with PCI was associated with better global neuropsychological function. GMD clusters derived from between-group imaging analyses showed an unexpected negative correlation between GMD and learning and memory scores.
DNA damage in cancer patients is attributable to multiple factors, including cancer-related inflammation and oxidative stress, deficits in DNA repair mechanisms, and treatment-related insult [20]. Chemotherapeutic regimens can damage DNA in several ways [20,50]. Direct DNA damage may occur as a result of the mechanism of action of a drug; for example, doxorubicin intercalates with DNA, causing strand breaks. Introduction of chemotherapeutic agent into a cell can also lead to generation of free radicals, resulting in oxidative damage. Substantial increases in both oxidative and direct DNA damage measured in peripheral lymphocytes by the Comet assay have been reported from before to shortly after chemotherapy (1–2 weeks), and both oxidative and direct DNA damage were increased in BC patients relative to HC before treatment initiation [21]. In the present study, the increased oxidative but not direct DNA damage in BCS an average of 6 years post-chemotherapy relative to HC may suggest that oxidative damage is longer-lasting in this population. However, lack of correlation between oxidative DNA damage and PCI suggests a variable time course. Increased oxidative DNA damage in BCS was associated with decreased GMD in clusters derived from between-group VBM analyses. This is an interesting finding with implications for the mechanism of chemotherapy-related brain changes, and warrants further investigation. Notably, increases in oxidative and direct DNA damage measured via peripheral lymphocyte Comet assay have also been reported in Parkinson’s disease [51] as well as both mild cognitive impairment and Alzheimer’s disease [52], suggesting that a link between oxidative DNA damage and neurodegeneration may be a common phenomenon.
Limitations of the present study include a retrospective, cross-sectional design that does not permit firm conclusions with regard to change over time. Our patient cohort had a history of several different chemotherapy regimens, although many agents were common across patients. Sample size does not allow for differentiation of effects of individual regimens. Further, our BCS experienced a range of time courses and types of endocrine treatments, which have been shown to affect cognition differentially from chemotherapy [53–55], and a majority of this cohort was using these therapies at the time of the study. Cohort size does not permit conclusions about these effects of these therapies. Finally, the current study performed a number of statistical analyses, and multiple comparison corrections were not employed due to limited sample size, leaving the possibility that some of our findings are due to chance.
To our knowledge this is the first imaging study of the effects of PCI on brain structure and function. We employed multi-modal MRI in concert with neuropsychological testing, cognitive self-report, and biomarkers to examine the relationship among these variables, and found points of convergence. The potential clinical significance of imaging findings was underscored by association with cognitive measures. The link between neuroimaging variables and oxidative DNA damage in peripheral lymphocytes may provide a direction for further mechanistic analysis. Prospective multi-modal investigations of treatment and survivorship will continue to be important as will recruitment of larger cohorts to enhance statistical power and generalizability. Multi-center collaborations may be required to achieve sufficient recruitment, which has proven challenging in this population. Although challenges remain to fully understand the time course of cognitive and biological changes after chemotherapy, the present study demonstrates the important role that PCI may play in neural outcomes after chemotherapy.
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute (R01CA101318, PI:AJS; R25CA117865, PI: VLC), the American Cancer Society (ACS RSGBP-04-089-01-PBP, PI: VLC), The Indiana University Melvin and Bren Simon Cancer Center Translational Research Acceleration Collaboration (PI: FWU), The National Institutes of Health, National Institute on Aging (F30 AG039959, PI: SKC), and the Indiana University Medical Scientist Training Program (National Institute of General Medical Sciences GM077229-02).
Footnotes
Disclosures: None
References
- 1.Jim HSL, Phillips KM, Chait S, Faul LA, Popa MA, Lee Y-H, Hussin MG, Jacobsen PB, Small BJ. Meta-Analysis of Cognitive Functioning in Breast Cancer Survivors Previously Treated With Standard-Dose Chemotherapy. Journal of Clinical Oncology. 2012;30(29):3578–3587. doi: 10.1200/jco.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc. 2003;9 (7):967–982. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- 3.Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. Cancer J. 2008;14 (6):396–400. doi: 10.1097/PPO.0b013e31818d8769. [DOI] [PubMed] [Google Scholar]
- 4.Stewart A, Bielajew C, Collins B, Parkinson M, Tomiak E. A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin Neuropsychol. 2006;20 (1):76–89. doi: 10.1080/138540491005875. [DOI] [PubMed] [Google Scholar]
- 5.Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J, Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer. 2005;104 (10):2222–2233. doi: 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- 6.Pullens MJ, De Vries J, Roukema JA. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psychooncology. 2010;19(11):1127–1138. doi: 10.1002/pon.1673. [DOI] [PubMed] [Google Scholar]
- 7.de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, Lavini C, Linn SC, Boven E, van Dam FSAM, Schagen SB. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: Converging results from multimodal magnetic resonance imaging. Human Brain Mapping. 2011 doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koppelmans V, de Ruiter MB, van der Lijn F, Boogerd W, Seynaeve C, van der Lugt A, Vrooman H, Niessen WJ, Breteler MM, Schagen SB. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, Wada N, Imoto S, Murakami K, Uchitomi Y The Breast Cancer Survivors’ Brain MRIDG. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109 (1):146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 10.Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Stock JVd, Smeets A, Christiaens M-R, Leemans A, Hecke WV, Vandenberghe J, Vandenbulcke M, Sunaert S. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Human Brain Mapping. 2011;32(3):480–493. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abraham J, Haut MW, Moran MT, Filburn S, Lemiuex S, Kuwabara H. Adjuvant chemotherapy for breast cancer: effects on cerebral white matter seen in diffusion tensor imaging. Clinical Breast Cancer. 2008;8 (1):88–91. doi: 10.3816/CBC.2008.n.007. [DOI] [PubMed] [Google Scholar]
- 12.Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011;68(11):1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FS, Nederveen AJ, Boven E, Schagen SB. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Human Brain Mapping. 2011;32(8):1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W, Verhoeven JS, Christiaens M-R, Vandenberghe J, Vandenbulcke M, Sunaert S. Longitudinal Assessment of Chemotherapy-Induced Structural Changes in Cerebral White Matter and Its Correlation With Impaired Cognitive Functioning. Journal of Clinical Oncology. 2012;30(3):274–281. doi: 10.1200/jco.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 15.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123(3):819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in Brain Activation during Working Memory Processing Associated with Breast Cancer and Treatment: A Prospective Functional MRI Study. Journal of Clincal Oncology. 2012;30(20):2500–8. doi: 10.1200/JCO.2011.38.5674. JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole B, Hanscom BS, Mulrooney TJ, Schwartz G, Kaufman PA. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cimprich B, Reuter-Lorenz P, Nelson J, Clark PM, Therrien B, Normolle D, Berman MG, Hayes DF, Noll DC, Peltier S, Welsh RC. Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol. 2010;32(3):324–331. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- 19.Scherling C, Collins B, Mackenzie J, Bielajew C, Smith A. Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: an FMRI study. Frontiers in human neuroscience. 2011;5:122. doi: 10.3389/fnhum.2011.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nature Reviews Cancer. 2007;7 (3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blasiak J, Arabski M, Krupa R, Wozniak K, Rykala J, Kolacinska A, Morawiec Z, Drzewoski J, Zadrozny M. Basal, oxidative and alkylative DNA damage, DNA repair efficacy and mutagen sensitivity in breast cancer. Mutation research. 2004;554(1–2):139–148. doi: 10.1016/j.mrfmmm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Unverzagt FW, Monahan PO, Moser LR, Zhao Q, Carpenter JS, Sledge GW, Jr, Champion VL. The Indiana University telephone-based assessment of neuropsychological status: a new method for large scale neuropsychological assessment. J Int Neuropsychol Soc. 2007;13(5):799–806. doi: 10.1017/s1355617707071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Von Ah D, Harvison KW, Monahan PO, Moser LR, Zhao Q, Carpenter JS, Sledge GW, Jr, Champion VL, Unverzagt FW. Cognitive function in breast cancer survivors compared to healthy age- and education-matched women. Clin Neuropsychol. 2009;23(4):661–674. doi: 10.1080/13854040802541439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal Gray Matter Reduction after Breast Cancer Chemotherapy and Association with Executive Symptoms: A Replication and Extension Study. Brain, Behavior, and Immunity. 2012 doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. Journal of Clinical Oncology. 2007;25 (25):3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashburner J, Friston KF. Voxel-based morphometry—the methods. Neuroimage. 2000;11 (6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 27.Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage. 2001;14 (6):1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- 28.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14 (1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 29.Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC Alzheimer’s Disease Neuroimaging I. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res. 2009;6 (4):347–361. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wishart HA, Saykin AJ, McAllister TW, Rabin LA, McDonald BC, Flashman LA, Roth RM, Mamourian AC, Tsongalis GJ, Rhodes CH. Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology. 2006;67(7):1221–1224. doi: 10.1212/01.wnl.0000238079.00472.3a. [DOI] [PubMed] [Google Scholar]
- 32.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. Oxford University Press; New York: 2004. [Google Scholar]
- 33.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; New York: 2006. [Google Scholar]
- 34.Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging. 1996;17 (1):123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- 35.Brown FC, Roth RM, Saykin AJ, Beverly-Gibson G. A new measure of visual location learning and memory: development and psychometric properties for the Brown Location Test (BLT) Clin Neuropsychol. 2007;21(5):811–825. doi: 10.1080/13854040600878777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Psychological Corporation. WMS Wechsler memory scale 3rd ed., Updated technical manual. 3. The Psychological Corporation; San Antonio, TX: 1997. WAIS-III Wechsler memory scale. [Google Scholar]
- 37.Fischer JS, Jak AJ, Kniker JE, Rudick RA. Administration and Scoring Manual for the Multiple Sclerosis Functional Composite Measure (MSFC) National Multiple Sclerosis Society; 2001. [Google Scholar]
- 38.Wilkinson GS, Robertson GJ. WRAT4 Wide Range Achievement Test Professional Manual. Psychological Assessment Resources, Inc; Lutz, FL: 2006. [Google Scholar]
- 39.The Psychological Corporation. Wechsler abbreviated scale of intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 40.Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan executive function system. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 41.Smith A. Symbol Digit Modalities Test. Western Psychological Services; Los Angeles: 1982. [Google Scholar]
- 42.Lafayette Instrument. Grooved pegboard: instruction/ owner’s manual. Lafayette Instrument; Lafayette, IN: 1989. [Google Scholar]
- 43.Seidenberg M, Haltiner A, Taylor MA, Hermann BB, Wyler A. Development and validation of a Multiple Ability Self-Report Questionnaire. J Clin Exp Neuropsychol. 1994;16(1):93–104. doi: 10.1080/01688639408402620. [DOI] [PubMed] [Google Scholar]
- 44.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11 (3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 45.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1 (3):385–401. [Google Scholar]
- 46.Spielberger CD. State-Trait Anxiety Inventory. Consulting Psychologists Press, Inc; Palo Alto, CA: 1983. [Google Scholar]
- 47.Pu X, Kamendulis LM, Klaunig JE. Acrylonitrile-induced oxidative stress and oxidative DNA damage in male Sprague-Dawley rats. Toxicological sciences : an official journal of the Society of Toxicology. 2009;111(1):64–71. doi: 10.1093/toxsci/kfp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Longitudinal Assessment of Cognitive Changes Associated With Adjuvant Treatment for Breast Cancer: Impact of Age and Cognitive Reserve. Journal of Clinical Oncology. 2010;28(29):4434–4440. doi: 10.1200/jco.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103 (3):303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Jungsuwadee P, Vore M, Butterfield DA, St Clair DK. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Molecular Interventions. 2007;7 (3):147–156. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- 51.Migliore L, Petrozzi L, Lucetti C, Gambaccini G, Bernardini S, Scarpato R, Trippi F, Barale R, Frenzilli G, Rodilla V, Bonuccelli U. Oxidative damage and cytogenetic analysis in leukocytes of Parkinson’s disease patients. Neurology. 2002;58 (12):1809–1815. doi: 10.1212/wnl.58.12.1809. [DOI] [PubMed] [Google Scholar]
- 52.Migliore L, Fontana I, Trippi F, Colognato R, Coppede F, Tognoni G, Nucciarone B, Siciliano G. Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiol Aging. 2005;26(5):567–573. doi: 10.1016/j.neurobiolaging.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 53.Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA, Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. Journal of Clinical & Experimental Neuropsychology: Official Journal of the International Neuropsychological Society. 2004;26 (7):955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 54.Shilling V, Jenkins V, Fallowfield L, Howell T, Shilling V, Jenkins V, Fallowfield L, Howell T. The effects of hormone therapy on cognition in breast cancer.[erratum appears in J Steroid Biochem Mol Biol. 2005 Jun;96(1):93] Journal of Steroid Biochemistry & Molecular Biology. 2003;86 (3–5):405–412. doi: 10.1016/j.jsbmb.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Jenkins V, Shilling V, Fallowfield L, Howell A, Hutton S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psychooncology. 2004;13(1):61–66. doi: 10.1002/pon.709. [DOI] [PubMed] [Google Scholar]
- 56.Barona A, Reynolds CR, Chastain R. A demographically based index of pre-morbid intelligence for the WAIS-R. Journal of Consulting and Clinical Psychology. 1984;52 (5):885–887. [Google Scholar]