Abstract
Background and Purpose
Leukoaraiosis (LA) and male sex have been associated with decreased cerebrovascular reactivity, which potentially adversely affects tissue viability in acute stroke. Therefore, we aimed to elucidate the contribution of LA-severity and sex to the extent of the hyperacute ischemic core volume following intracranial large artery occlusion (ILAO).
Methods
We analyzed data from 87 patients with acute ILAO who had acute multimodal CT-imaging. LA-severity was assessed using the van Swieten scale on non-contrast CT. CT-perfusion (CTP) data were analyzed using automatic calculation of the mean transit time (MTT) and hyperacute cerebral blood volume (CBV) defects. Multivariate linear and logistic regression analyses were used to identify independent predictors of the hyperacute infarct-volume.
Results
Severe LA (VSS 3–4; OR 43.22, 95%-CI 6.26–298.42, p<0.001) and male sex (OR 7.52, 95%-CI 1.38–40.86, p=0.020) were independently associated with a hyperacute CBV-lesion >25 mL on multivariate logistic regression analysis. Multivariate linear regression analysis confirmed the association between severe LA (p<0.001) and male sex (p=0.01) with larger CBV lesions. There was no significant difference in the absolute or relative MTT-lesion volumes when stratified by LA severity or sex. Women had significantly smaller CBV-lesion volumes compared to men (p=0.036).
Conclusions
Severe LA and male sex are associated with larger infarct cores, which adds to the notion that sex and LA alter the brain’s intrinsic susceptibility to acute cerebral ischemia. Future, larger studies are needed to confirm our observation that women have smaller core volumes and its significance.
Keywords: Acute Stroke, CT, focal ischemia, leukoaraiosis, Women & Minorities
Introduction
The infarct core is a critical predictor for outcome and response to thrombolysis.1–5 Identifying factors contributing to core expansion is important to understand how these affect outcome, develop novel therapies, and possibly aid in risk stratification. Key factors associated with acute ischemic core expansion include the time from symptom onset,4 collateral status,6 and site of arterial occlusion. There is mounting evidence that sex and extent of leukoaraiosis (LA) may significantly alter cerebrovascular reserve,7–10 which translates to greater infarct growth in animal stroke models.11,12 Only two respective MRI-based volumetric analyses reported that sex7 and LA9 were associated with the expansion of the acute ischemic lesion. However, the potential contribution of collaterals and site of arterial occlusion location was not reported. Lastly, compared to MRI perfusion CT (CTP) is more readily available and increasingly utilized in stroke management and studying factors associated with CTP-defined ischemic core is of increasing clinical relevance.
To test the hypothesis that the severity of preexisting LA and sex are independently associated with the CTP-defined ischemic core volume we examined the association between these factors while accounting for collateral status and site of arterial occlusion in a previously characterized patient population with intracranial large artery occlusion (ILAO).13
Methods
Patient Selection and Clinical Data
We reviewed data from 1,153 consecutive patients with imaging-confirmed diagnosis of ischemic stroke evaluated at a single academic emergency room between January 2007 and October 2010. Methods of the patient selection, collection of clinical data, and patient exclusion have previously been described in detail.13 In brief, we included patients that had an admission NCCT, immediately followed by CT-angiogram (CTA) and CTP, all performed within 24 hours after the time of last known well. Patients without evidence of anterior circulation intracranial arterial occlusion on CTA or with non-diagnostic CTP images were excluded. All 87 included patients had a follow-up NCCT performed 24 h after presentation. Patient demographics, admission vital signs, laboratory data, co-morbidities, pre-admission medications, and stroke etiology (Trial of Org 10172 in Acute Stroke Treatment [TOAST] classification)14 after completion of diagnostic work-up, were collected on all patients. Time-to-CTP was defined as the time (min) between last known well and completion of the CTP. National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS) were assessed at the time of presentation and at 90 days. This study was reviewed and approved by our Institutional Review Board.
Neuroimaging protocol
All CT sequences were obtained on a 64 row detector Philips scanner. NCCT was performed in a non-helical mode at 120 KvP and 200 mA with data reconstruction at 5 mm axial slices. CTA was performed using 64 × 0.625 mm detector configuration with a pitch of 0.673, from the arch of aorta to the vertex using 120 KvP, 300 mA, and 0.5 s rotation time. Patients received 60–80 mL of Isovue 370 (Bracco Diagnostics, Princeton, NJ) in the antecubital vein at a rate of 4 mL/s through a power injector followed by 40 mL saline. 3D orthogonal maximum intensity projection images were created in 3 planes. CTP was performed with detector configuration of 64 × 0.625 mm at 80 KvP and 100 mAs creating 4 slices of 10 mm thickness (total coverage 40 mm). The plane of imaging was parallel to the floor of the anterior cranial fossa starting just above the orbits. 30 cycles were obtained every 2 s with a total scan time of 60 s.
Image post-processing
CTP data were analyzed utilizing a stand-alone version of CTP software developed by Philips Medical Systems (Cleveland, Ohio), which automatically calculated mean transit time (MTT) and hyperacute CBV defects as previously described.15 The anterior cerebral artery was used for arterial input function.16 Hemispheric volume within the CTP-slices and ischemic lesions on follow-up NCCT were measured free-hand. The following perfused parameters were used to calculate lesion volumes (mL): MTT-lesion (more than 145% of contralateral normal), CBV-lesion (core below a threshold of 2.0 mL×100 g−1), and MTT-CBV mismatch (penumbra).15 To account for potential differences in brain volumes, the MTT-, CBV-, and MTT-CBV mismatch volumes were also expressed relative (%) to the corresponding hemispheric volume within the CTP slices. Finally, infarct volume on follow-up NCCT and lesion growth (infarct volume on follow-up NCCT minus CBV-lesion volume on admission CTP) were calculated.
Image review and analysis
Admission NCCT, CTA, and CTP were reviewed independently by two experienced readers blinded to both clinical data and any follow-up scans. Disagreements in readings were resolved by consensus. Variable window width and center-level settings were used for optimal ischemic hypoattenuation detection with NCCT and CTA images.17 Presence and extent of LA was assessed utilizing the van Swieten Scale (VSS) grading the white matter lesions for the regions anterior and posterior to the central sulcus on a 3-point scale from 0 (no LA) to 2 (confluent white matter involvement from the ventricles to the grey matter). Summing the score from the anterior and posterior region provided a total score ranging from 0–4.18 In the present study, LA was separately assessed in each hemisphere but only the score from the non-ischemic hemisphere was considered after un-blinding. LA was distinguished from lacunar infarcts, which were defined as a sharply marginated low density lesion on CT without mass effect and location within a recognized arterial territory as previously described.18 Location of occlusions within vessel segments was noted on CTA. CTA source images were used to assess for the presence of collateral vessels as previously described.17 Briefly, leptomeningeal collaterals were graded on a five-point scale (from 1 denoting absence to 5 denoting exuberant collaterals) compared to the unaffected side. To avoid classification bias and to minimize inter-rater variability, we also dichotomized the degree of LA (VSS 0–2 vs. 3–4) and collateral status (1–2 vs. 3–5) for statistical purposes.
Statistics
Weighted kappa statistics were used to determine the degree of agreement in 40 randomly chosen patients. Kappa values were interpreted to represent inter-observer agreement as slight (0.01–0.2), fair (0.21–0.4), moderate (0.41–0.6), substantial (0.61–0.8), or almost perfect (0.81–1.0).19
Continuous variables are reported as mean±SD or as median±interquartile range (IQR). Categorical variables are reported as proportions.
Between-group comparisons for continuous variables were made with unpaired t-test, Kruskal Wallis, and Mann-Whitney U-test, as appropriate. Categorical variables were compared using the χ2-test or Fisher exact test as appropriate. For the multivariate logistic analyses, initial CBV-lesion volume was dichotomized in <25 mL vs. >25 mL based on reports that the 25 mL threshold predicted the presence of tissue at risk, early neurological deterioration, response to thrombolysis, and excellent outcome.1,2 Recently, it has been suggested that a more stringent CBV-lesion volume of <16 mL may best predict a favorable outcome.5 We therefore repeated all analyses using the 16 mL CBV-lesion volume threshold (secondary analysis). Multivariate logistic regression with forward elimination (probability value for elimination of 0.1) was used to identify independent predictors for hyperacute CBV-volume >25 mL (or >16 mL).
Finally, to assess predictors of the hyperacute CBV-lesion independent of a predefined lesion volume threshold we also performed univariate and multivariate (probability value for elimination of 0.1) linear regression analyses.
Given their reported association with lesion size, the following additional variables were forced into the respective multivariate regression models: admission mean arterial blood pressure (aMAP) and admission blood glucose. A P<0.05 was considered statistically significant. All statistical analyses were performed using IBM® SPSS® Statistics 19.0.0.1 (IBM®-Armonk, NY).
Results
Baseline Characteristics
Briefly, of 1153 patients included in the database, 87 patients had acute anterior circulation ILAO with adequate image quality for analysis. Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics (unadjusted) of the studied patient population as stratified by initial CBV-lesion volume
| Characteristics | All patients (n=87) | CBV <25 mL (n=63) | CBV >25 mL (n=24) | P-value |
|---|---|---|---|---|
| Age, years | 67 (±16) | 65 (±17) | 71 (±14) | 0.130 |
| Male sex | 48 (55%) | 30 (48%) | 18 (75%) | 0.030 |
| Baseline NIHSS, median (IQR) | 15 (9–21) | 12 (8–20) | 19 (15–24) | 0.001 |
| Baseline mRS, median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.896 |
| Mean arterial blood pressure, mmHg | 102 (±22) | 101 (±18) | 107 (±30) | 0.543 |
| Admission glucose, mg/dL | 126 (±35) | 123 (±33) | 133 (±40) | 0.220 |
| Admission creatinine, mg/dL | 1.10 (±0.86) | 1.08 (±0.98) | 1.14 (±0.44) | 0.032 |
| Admission white blood cells, th/mm | 9.5 (±4.1) | 9.4 (±4.1) | 10.0 (±4.1) | 0.464 |
| LDL-C within 24 hours of admission, mg/dL | 82 (±35) | 84 (±38) | 79 (±26) | 0.591 |
| Preadmission medications | ||||
| Antiplatelets | 31 (36%) | 22 (35%) | 9 (38%) | 0.822 |
| Warfarin | 6 (7%) | 3 (5%) | 3 (13%) | 0.203 |
| Statin | 34 (39%) | 23 (37%) | 11 (46%) | 0.467 |
| Hypoglycemic agents | 10 (11%) | 8 (13%) | 2 (8%) | 0.568 |
| ACEi/ARB | 26 (30%) | 18 (29%) | 8 (33%) | 0.794 |
| Preexisting risk factors | ||||
| Hypertension | 62 (71%) | 41 (65%) | 21 (88%) | 0.062 |
| Atrial fibrillation | 27 (31%) | 20 (32%) | 7 (29%) | 0.816 |
| Dyslipidemia | 44 (51%) | 32 (51%) | 12 (50%) | 0.947 |
| Diabetes | 13 (15%) | 11 (17%) | 2 (8%) | 0.501 |
| Prior stroke or transient ischemic attack | 14 (16%) | 9 (14%) | 5 (21%) | 0.458 |
| Coronary artery disease | 29 (33%) | 18 (29%) | 11 (46%) | 0.127 |
| Congestive heart failure | 17 (20%) | 12 (19%) | 5 (21%) | 0.851 |
| Peripheral vascular disease | 5 (6%) | 2 (3%) | 3 (13%) | 0.126 |
| Dementia | 4 (5%) | 3 (5%) | 1 (4%) | 1.000 |
| Smoking | 25 (29%) | 19 (30%) | 6 (25%) | 0.793 |
| Alcohol abuse | 9 (10%) | 6 (10%) | 3 (13%) | 0.702 |
| Onset-to-CTP Time, min | 339±269 | 332 (±217) | 360 (±378) | 0.301 |
| Acute intervention | 0.327 | |||
| Conservative management | 30 (34%) | 24 (38%) | 6 (25%) | |
| i.v rtPA | 15 (17%) | 9 (14%) | 6 (25%) | |
| Endovascular intervention without i.v. rtPA | 27 (31%) | 21 (33%) | 6 (25%) | |
| Endovascular intervention with i.v. rtPA | 15 (17%) | 9 (14%) | 6 (25%) | |
| Stroke mechanism | 0.598 | |||
| Large-artery atherosclerosis | 20 (23%) | 15 (24%) | 5 (21%) | |
| Cardioembolic | 38 (44%) | 26 (41%) | 12 (50%) | |
| Other | 11 (13%) | 6 (10%) | 5 (21%) | |
| Undetermined | 18 (21%) | 16 (25%) | 2 (8%) | |
| Site of intracranial occlusion | 0.592 | |||
| ICA | 6 (7%) | 5 (8%) | 1 (4%) | |
| ICA+MCA | 22 (25%) | 16 (25%) | 6 (25%) | |
| MCA M1 | 40 (46%) | 26 (41%) | 14 (58%) | |
| MCA M2 | 16 (18%) | 13 (21%) | 3 (13%) | |
| MCA M3 | 3 (3%) | 3 (5%) | 0 (0%) | |
| Leukoaraiosis grade (Van Swieten Score) | <0.001 | |||
| 0 | 30 (34%) | 29 (46%) | 1 (4%) | |
| 1 | 17 (20%) | 15 (24%) | 2 (8%) | |
| 2 | 19 (22%) | 14 (22%) | 5 (21%) | |
| 3 | 10 (11%) | 3 (5%) | 7 (29%) | |
| 4 | 11 (13%) | 2 (3%) | 9 (38%) | |
| Collateral grade | <0.001 | |||
| 1 (absent) | 24 (28%) | 7 (11%) | 17 (71%) | |
| 2 (less than contralateral hemisphere) | 44 (51%) | 39 (62%) | 5 (21%) | |
| 3 (equal to contralateral hemisphere) | 15 (17%) | 14 (22%) | 1 (4%) | |
| 4 (greater than contralateral hemisphere) | 4 (5%) | 3 (5%) | 1 (4%) | |
| 5 (exuberant) | 0 (0%) | 0 (0%) | 0 (0%) | |
ACEi=angiotensin converting enzyme inhibitor; ARB=angiotensin receptor blocker; ICA=internal carotid artery; i.v. rtPA= intravenous recombinant tissue plasminogen activator; IQR=interquartile range; MCA=middle cerebral artery
Inter-observer Agreement
Free marginal kappa values for inter-observer agreement were as follows: graded LA (VSS; κ=0.63; percentage of agreement=70%); dichotomized LA-severity (κ=0.85; percentage of agreement=93%); collateral status (κ=0.69; percentage of agreement=75%); dichotomized collateral status (κ=0.85; percentage of agreement=93%).
Univariate Binary Analyses
Twenty-four (28%) patients had a CBV-lesion (ischemic core) >25 mL. Compared to patients with a CBV-lesion <25 mL, they were more frequently male (p=0.03), had a higher admission NIHSS (p=0.001), had a higher serum creatinine (p=0.032), more severe LA (p<0.001), and worse collateral status (p<0.001).
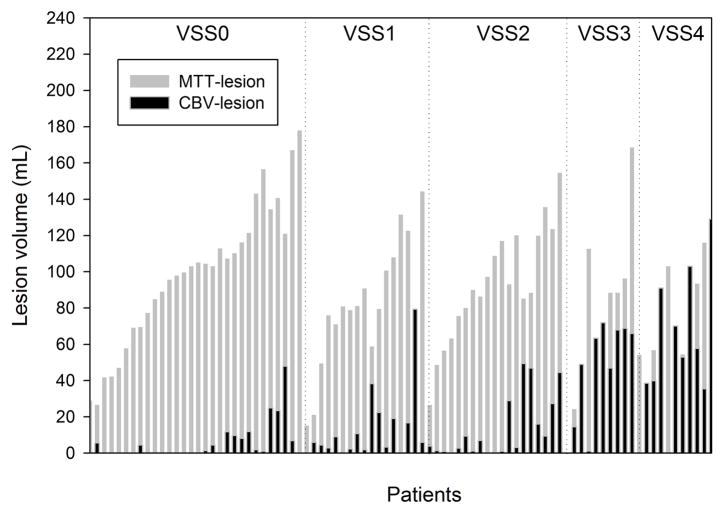
Compared to patients with absent-to-moderate LA (VSS grades 0 to 2), patients with severe LA (VSS grades 3 and 4) were more likely to have hypertension (p=0.028), coronary artery disease (p=0.015), higher admission NIHSS (p=0.003), worse 90-day mRS (p<0.001), and older age (p=0.002). There was a significant inverse association between LA severity and robustness of collateral status as well as absolute CBV-lesion volumes, respectively (p<0.001). Figure 1 depicts the MTT- and CBV-lesion volumes of all patients stratified by LA-severity. There was no significant difference in the absolute or relative MTT-lesion volumes when stratified by leukoaraiosis severity (Figure 2A&B). Subjects with a VSS of 2 to 4 had significantly greater lesion growth (p<0.05) and significantly larger final infarct volumes (p<0.05) compared to subjects without LA (Figure 3).
Figure 1. Hyperacute CBV- and MTT-lesion volumes of analyzed patients.
Absolute CBV- and MTT-lesion volumes of the analyzed patients show increasing CBV-to-MTT volume ratios with worsening LA-status.
Figure 2. Hyperacute CBV- and MTT-lesion volumes.
Absolute (A) and relative (B) CBV-lesion volumes increased with LA-severity (*p<0.05 vs. VSS of zero). There was a significant absolute (A) and relative (B) MTT-CBV lesion volume mismatch at all LA-grades (‡p<0.01 vs. MTT-lesion volume). Women had significantly smaller absolute (C) and relative (D) CBV-lesion volumes compared to men. Both sexes demonstrated significant absolute (C, p<0.001) and relative (D, p<0.001) MTT-CBV mismatch volumes, respectively. There was no significant difference in the respective absolute and relative MTT-lesion volumes when stratified by leukoaraiosis severity or sex (p>0.05).
Figure 3. Infarct volumes and lesion growth.
Compared to patients with no leukoaraiosis (VSS=0), subjects with a VSS of 2 to 4 had significantly greater lesion growth (p<0.05) and significantly larger final infarct volumes (p<0.05).
Compared to men, women were more likely to have atrial fibrillation (17% vs. 49%, p=0.001). Women had smaller hemispheric volumes (307±35 vs. 274±38 mL, p<0.001) as well as significantly smaller absolute (25±30 vs. 13±23 mL, p=0.036, Figure 2C) and relative (adjusted for hemispheric volume; p=0.044, Figure 2D) CBV-lesion volumes compared to men, respectively. There was no significant difference in the absolute or relative MTT-lesion volumes when stratified by sex (Fig 2C&D). Both sexes had significant CTP-derived absolute (p<0.001) and relative (p<0.001) MTT-CBV mismatch volumes. There were no differences in all other assessed variables (supplemental Table 1). In particular, there was no inter-sex difference in age (66±15 vs. 68±17 years, p=0.585), admission NIHSS (p=0.910), VSS (p=0.858), collateral status (p=0.699), and time-to-CTP (344±329 vs. 333±173 min, p=0.840).
Multivariate Logistic Regression Analysis
Severe LA (OR 43.22, 95%-CI 6.26–298.42, p<0.001) and male sex (OR 7.52, 95%-CI 1.38–40.86, p=0.020) were independently associated with a hyperacute CBV-lesion >25 mL (Table 2). Repeating the analysis using the 16 mL-threshold yielded similar results, showing an association between severe LA (OR 10.81, 95%-CI 2.50–46.78, p<0.001), male sex (OR 5.90, 95%-CI 1.60–21.74, p=0.008), and admission NIHSS (for each 1-point increase OR 1.12, 95%-CI 1.02–1.23, p=0.022) with a hyperacute CBV-lesion >16 mL.
Table 2.
Multivariate logistic regression analysis of factors independently associated with a CBV-lesion volume (>25 mL)
| Independent variable | OR (95% CI) | P-value |
|---|---|---|
| Age | 0.96 (0.90–1.03) | 0.242 |
| Male sex | 7.52 (1.38–40.86) | 0.020 |
| Admission NIHSS | 1.12 (0.98–1.27) | 0.087 |
| LA grade (VSS 3–4) | 43.22 (6.26–298.42) | <0.001 |
| Collateral status (Grade 1–2) | 1.93 (0.19–19.96) | 0.582 |
| Hypertension | 2.25 (0.29–17.39) | 0.439 |
| Mean arterial blood pressure, mmHg | 1.01 (0.97–1.04) | 0.745 |
| Admission glucose, mg/dL | 1.01 (0.99–1.03) | 0.287 |
| Admission creatinine, mg/dL | 1.12 (0.56–2.40) | 0.689 |
Multivariate Linear Regression Analysis
After adjusting for confounders, severe LA (VSS 3–4; unstandardized β-coefficient 39.64, 95%-CI 28.55–50.73, p<0.001) and male sex (unstandardized β-coefficient 11.55, 95%-CI 2.89–20.21, p=0.01) were significantly associated with greater CBV-lesion volumes.
Additional sensitivity analyses
To reduce heterogeneity we reanalyzed our dataset excluding (a) patients with distal (M2 or M3) middle cerebral artery occlusion (n=19); (b) subjects presenting beyond 8 hours of last known well time-to-CTP (“wake up stroke,” n=14 or “found down,” n=3); (c) patients with M2 or M3-occlusion and/or a time-to-CTP >8 hours (n=31). The respective multivariate logistic (supplemental Tables 2–4) and linear (not shown) regression analyses from these analyses confirmed significant associations between LA and sex with the acute CBV-lesion (not shown).
Discussion
The most significant findings of this study are absent to moderate LA and female sex independently predict a small acute ischemic core (CBV)-lesion volumes. These results are important because larger acute core volumes are associated with poor response to treatment and adverse outcome.13,20 Recent data indicates that a cut off value of <25 mL may indicate better response to thrombolysis, less frequent early neurological deterioration, and more frequent excellent outcome.1,2
We have previously shown that preexisting LA is associated with worse clinical outcome in patients with ILAO.13 The mechanisms by which LA affects post-stroke outcome are not entirely clear but reduced cerebral blood flow and vascular reserve have been shown to be reduced in regions affected by LA, which is expected to reduce tissue survival in acute stroke.8,21 Together with experimental data,11 our data supports the theory that severe LA is associated with decreased penumbral survival, which may translate to a worse outcome. However, further study is required to establish a definite link between LA, vascular reserve, core expansion, and outcome.
LA severity did not affect the overall extent of tissue hypoperfusion (MTT-lesion) and the relationship between CBV- and MTT-lesion volumes did not change when adjusted for the hemispheric volumes. This finding is important because it indicates more rapid expansion of the core rather than of the tissue at risk in affected patients. This can be explained by the observation that LA preferentially affects deep vs. superficial brain areas and largely spares the cerebral cortex.21 Hence, in patients with similar occlusion location, collateral supply, and hemodynamics (such as in our study) the MTT-lesion is expected to remain restricted to the vascular territory of an occluded intracranial artery even in severe LA.
It has been hypothesized that LA-related chronic hypoperfusion may promote the development of the cerebral collateral circulation; however, no such association was previously noted.22 Our conflicting observation of a significant inverse relation between LA severity and robustness of collaterals may be related to differing methodology in ascertaining collaterals and LA as well as the fact that the former study included patients with posterior circulation strokes, who may have a different underlying pathology.
Sex differences regarding etiology, presentation, treatment, and stroke outcome are increasingly recognized, which are thought to be in part related to endogenous sex steroid hormones, particularly estrogens.23 Both young and old women have been noted to have better cerebral autoregulation compared to men.10 In the setting of an acute ischemic stroke this may result in longer survival of the ischemic penumbra. Indeed, similar to experimental data we found that women had significantly smaller core volumes compared to men.12 This contrasts with a prior report showing similar ischemic lesion volumes in men and women following ILAO as assessed on CTA source images.24 Since this approach cannot inform on different perfusion states a direct comparison to our results is not possible.24 More importantly, when the same group investigated the association of gender with MRI-derived lesion volumes, they noted significantly less ischemic tissue progressing towards infarction in women ≤70 years compared to males in the similar age strata.7 In light of generally observed poorer clinical outcome of women23 our observation is intriguing because it offers a possible explanation as to why women over-proportionally benefit from recanalization strategies, which may abate the usual sex differences in stroke outcome.25
The strengths of our study are the analysis of a homogenous patient population undergoing multimodal CT-imaging and the collection of extensive clinical information. Finally, we investigated various variables that have been associated with the ischemic lesion volume in the setting of large arterial stroke. Our data expands on prior observations10,13 by considering potential important confounders such as occlusion location, collateral status, and detailed comorbid conditions. Further, we also included patients having a mismatch of <20%, receiving endovascular therapy, subsequently developing hemorrhagic conversion, brain edema, or requiring hemicraniectomy allowing for better generalization of our results. Finally, previously used sophisticated approaches to LA volume quantification with MRI are not readily available to the majority of clinical centers and require dedicated image post-processing, which is not feasible in the acute setting.9
Our study has important limitations attributed to its retrospective design and to the relatively small number of included patients in our cohort. Second, CTA and CTP were obtained at the treating stroke physician’s discretion, which may have biased towards the selection of patients having a “favorable” appearing NCCT. This may explain the lack of significant association between the time to imaging, site of vascular occlusion, stroke etiology, and treatment modality with the initial core volume in the univariate analysis. Nevertheless, the impact of this would not be clinically significant because this approach represents relevant clinical practice. Third, we did not perform a volumetric analysis of LA-burden and LA-misclassification is possible given the CT-based visual grading system. However, as noted the LA classification used in our study is easy to perform, had a high inter-observer agreement, and does not require extensive post-processing.18 Forth, the optimal parameters for identifying the acute infarct on CTP remain debated.26 However, the widespread availability and clinical use of CTP and possibility to perform automated, rater-independent, validated volumetric measures of core and penumbra remain major advantages of this technique.15,16,26 Using threshold independent analyses such as the use of a CTP-ASPECTS may allow for obtaining additional semiquantitative data to increase the predictive power for lesion extent assessment in future studies.26 Finally, no information regarding menopause status/hormonal therapy was available, which precludes deriving conclusions regarding the biological contribution of sex hormones to the observed findings.
In conclusion, our results add to the mounting evidence that sex and LA alter the brain’s intrinsic susceptibility to acute cerebral ischemia. If confirmed in larger prospective studies, the observation that women had smaller core volumes may provide novel insight as to why women may over-proportionally benefit from thrombolysis. Our data need to be interpreted cautiously due to the retrospective design and small cohort size and should be considered hypothesis generating for further studies elucidating the pathophysiology underlying the interaction between sex, LA, and tissue outcome.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported by institutional funds.
Dr. Selim receives research support from the NIH/NINDS (U01 NS074425).
Dr. Moonis receives research support through a Indo-US Science & Technology Forum grant (<$10,000).
Footnotes
Author contributions:
Nils Henninger: Study concept or design; Acquisition of data
Eugene Lin: Analysis or interpretation of data; Acquisition of data
Diogo C. Haussen: Drafting/revising the manuscript for content, including medical writing for content; Statistical analysis
Laura L. Lehman: Drafting/revising the manuscript for content, including medical writing for content
Deepak Takhtani: Analysis or interpretation of data
Magdy Selim: Drafting/revising the manuscript for content, including medical writing for content
Majaz Moonis: Drafting/revising the manuscript for content, including medical writing for content
Disclosures
The authors report no conflicts of interest.
Literature
- 1.Parsons MW, Christensen S, McElduff P, Levi CR, Butcher KS, De Silva DA, et al. Pretreatment diffusion- and perfusion-mr lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab. 2010;30:1214–1225. doi: 10.1038/jcbfm.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davalos A, Blanco M, Pedraza S, Leira R, Castellanos M, Pumar JM, et al. The clinical-dwi mismatch: A new diagnostic approach to the brain tissue at risk of infarction. Neurology. 2004;62:2187–2192. doi: 10.1212/01.wnl.0000130570.41127.ea. [DOI] [PubMed] [Google Scholar]
- 3.Gasparotti R, Grassi M, Mardighian D, Frigerio M, Pavia M, Liserre R, et al. Perfusion ct in patients with acute ischemic stroke treated with intra-arterial thrombolysis: Predictive value of infarct core size on clinical outcome. AJNR Am J Neuroradiol. 2009;30:722–727. doi: 10.3174/ajnr.A1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demchuk AM, Menon B, Goyal M. Imaging-based selection in acute ischemic stroke trials - a quest for imaging sweet spots. Ann N Y Acad Sci. 2012;1268:63–71. doi: 10.1111/j.1749-6632.2012.06732.x. [DOI] [PubMed] [Google Scholar]
- 5.Kruetzelmann A, Kohrmann M, Sobesky J, Cheng B, Rosenkranz M, Rother J, et al. Pretreatment diffusion-weighted imaging lesion volume predicts favorable outcome after intravenous thrombolysis with tissue-type plasminogen activator in acute ischemic stroke. Stroke. 2011;42:1251–1254. doi: 10.1161/STROKEAHA.110.600148. [DOI] [PubMed] [Google Scholar]
- 6.Souza LC, Yoo AJ, Chaudhry ZA, Payabvash S, Kemmling A, Schaefer PW, et al. Malignant cta collateral profile is highly specific for large admission dwi infarct core and poor outcome in acute stroke. AJNR Am J Neuroradiol. 2012;33:1331–1336. doi: 10.3174/ajnr.A2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gokcay F, Arsava EM, Baykaner T, Vangel M, Garg P, Wu O, et al. Age-dependent susceptibility to infarct growth in women. Stroke. 2011;42:947–951. doi: 10.1161/STROKEAHA.110.603902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uh J, Yezhuvath U, Cheng Y, Lu H. In vivo vascular hallmarks of diffuse leukoaraiosis. J Magn Reson Imaging. 2010;32:184–190. doi: 10.1002/jmri.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–1413. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]
- 10.Deegan BM, Sorond FA, Lipsitz LA, Olaighin G, Serrador JM. Gender related differences in cerebral autoregulation in older healthy subjects. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2859–2862. doi: 10.1109/IEMBS.2009.5333604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCabe C, Gallagher L, Gsell W, Graham D, Dominiczak AF, Macrae IM. Differences in the evolution of the ischemic penumbra in stroke-prone spontaneously hypertensive and wistar-kyoto rats. Stroke. 2009;40:3864–3868. doi: 10.1161/STROKEAHA.109.559021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- 13.Henninger N, Lin E, Baker SP, Wakhloo AK, Takhtani D, Moonis M. Leukoaraiosis predicts poor 90-day outcome after acute large cerebral artery occlusion. Cerebrovasc Dis. 2012;33:525–531. doi: 10.1159/000337335. [DOI] [PubMed] [Google Scholar]
- 14.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D, et al. Perfusion-ct assessment of infarct core and penumbra: Receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–985. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 16.Wintermark M, Lau BC, Chien J, Arora S. The anterior cerebral artery is an appropriate arterial input function for perfusion-ct processing in patients with acute stroke. Neuroradiology. 2008;50:227–236. doi: 10.1007/s00234-007-0336-8. [DOI] [PubMed] [Google Scholar]
- 17.Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, et al. The pattern of leptomeningeal collaterals on ct angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010;41:2316–2322. doi: 10.1161/STROKEAHA.110.592303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Swieten JC, Hijdra A, Koudstaal PJ, van Gijn J. Grading white matter lesions on ct and mri: A simple scale. J Neurol Neurosurg Psychiatry. 1990;53:1080–1083. doi: 10.1136/jnnp.53.12.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 20.Shi ZS, Loh Y, Liebeskind DS, Saver JL, Gonzalez NR, Tateshima S, et al. Leukoaraiosis predicts parenchymal hematoma after mechanical thrombectomy in acute ischemic stroke. Stroke. 2012;43:1806–1811. doi: 10.1161/STROKEAHA.111.649152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC, et al. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321–326. doi: 10.1212/wnl.59.3.321. [DOI] [PubMed] [Google Scholar]
- 22.Sanossian N, Ovbiagele B, Saver JL, Alger JR, Starkman S, Kim D, et al. Leukoaraiosis and collaterals in acute ischemic stroke. J Neuroimaging. 2011;21:232–235. doi: 10.1111/j.1552-6569.2010.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva GS, Lima FO, Camargo EC, Smith WS, Lev MH, Harris GJ, et al. Gender differences in outcomes after ischemic stroke: Role of ischemic lesion volume and intracranial large-artery occlusion. Cerebrovasc Dis. 2010;30:470–475. doi: 10.1159/000317088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kent DM, Price LL, Ringleb P, Hill MD, Selker HP. Sex-based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: A pooled analysis of randomized clinical trials. Stroke. 2005;36:62–65. doi: 10.1161/01.STR.0000150515.15576.29. [DOI] [PubMed] [Google Scholar]
- 26.Lin K, Rapalino O, Lee B, Do KG, Sussmann AR, Law M, et al. Correlation of volumetric mismatch and mismatch of alberta stroke program early ct scores on ct perfusion maps. Neuroradiology. 2009;51:17–23. doi: 10.1007/s00234-008-0454-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.