Abstract
Honeybees (Apis mellifera) are an attractive model system for studying aging. However, the aging level of worker honeybees from the field hive is in dispute. To eliminate the influence of task performance and confirm the relationship between chronological age and aging, we reared newly emerged workers in a thermostat at 34°C throughout their lives. A survivorship curve was obtained, indicating that workers can be reared away from the field hive, and the only difference between these workers is age. To confirm that these workers can be used for aging studies, we assayed age-related molecules in the trophocytes and fat cells of young and old workers. Old workers expressed more senescence-associated β-galactosidase, lipofuscin granules, lipid peroxidation, and protein oxidation than young workers. Furthermore, cellular energy metabolism molecules were also assayed. Old workers exhibited less ATP concentration, β-oxidation, and microtubule-associated protein light chain 3 (LC3) than young workers. These results demonstrate that honeybees reared in a thermostatic chamber can be used for aging studies and cellular energy metabolism in the trophocytes and fat cells of workers changes with advancing age.
Keywords: Trophocyte, Fat cell, Aging, Age-related molecules, Cellular energy metabolism, Honeybee
Introduction
Aging is a complex process involving a gradual decline in biological functions and an increased incidence of age-associated diseases, such as cardiovascular disease, cancer, arthritis, cataract, osteoporosis, type 2 diabetes, and Alzheimer’s disease. Understanding the biology of aging cannot only help to extend the life span of organisms but also improve their health.
Age-related molecules have been used as indices of aging in many organisms (Kishi et al. 2003; Genade et al. 2005; Hsu et al. 2008; Hsu and Chiu 2009; Hsieh and Hsu 2011). These age-related molecules include senescence-associated β-galactosidase (SA-β-Gal) (Dimri et al. 1995), lipofuscin granules (Nakano et al. 1995), lipid peroxidation (Draper and Hadley 1990; Almeida et al. 1998), and protein oxidation (Welis-Knecht et al. 1993). SA-β-Gal is a eukaryotic hydrolase that is localized in the lysosome (Kurz et al. 2000), and SA-β-Gal expression increases with age (Dimri et al. 1995; Kishi et al. 2003; Genade et al. 2005; Hsu et al. 2008; Hsieh and Hsu 2011). Lipofuscin is an intralysosomal polymeric material that originates from autophagocytosed cellular components oxidized outside or inside the lysosomal compartment (Terman and Brunk 2004). Lipofuscin cannot be degraded by lysosomal hydrolases or exocytosed. Accumulation of lipofuscin granules has been reported to increase with age (Nakano et al. 1995; Brunk and Terman 2002; Kishi et al. 2003; Genade et al. 2005; Hsu et al. 2008; Hsieh and Hsu 2011). Thus, accumulation of both SA-β-Gal and lipofuscin are considered reliable indices of advancing age. Lipids and proteins are damaged by reactive oxidative species, resulting in lipid peroxidation and protein oxidation. Lipid peroxidation and protein oxidation increase with age and are also indicators of aging (Sohal et al. 1993; Welis-Knecht et al. 1993; Almeida et al. 1998; Hsu et al. 2008; Hsieh and Hsu 2011). Accordingly, the fluctuation of age-related molecules can be used to evaluate the degree of aging in organisms (Mecocci et al. 1999; Hsu et al. 2008; Hsu and Chiu 2009; Hsieh and Hsu 2011).
Honeybees (Apis mellifera) are an attractive model system for studying aging because queens have a much longer life span than workers, even though they have the same genome. In addition, they live in large colonies, are easily manipulated, and their genome has been sequenced. Therefore, honeybees have been the model system for many aging studies (Remolina et al. 2007; Neukirch 1982; Rueppell et al. 2007a, b). A variety of honeybee specimens, including spermathecae, muscle, ventriculi, hemolymph plasma, semen, brain, trophocytes, and fat cells, have been used to study oxidative stress (Weirich et al. 2002; Seehuus et al. 2006a, b; Williams et al. 2008; Collins et al. 2004; Corona et al. 2005) and aging (Hsieh and Hsu 2011).
We recently assayed the expression of age-related molecules in the trophocytes and fat cells of newly emerged and old workers from the field hive (Hsieh and Hsu 2011). Trophocytes, which are large and irregularly shaped, and fat cells, which are small and spherical, attach to one another to form a single layer of cells around each segment of the honeybee’s abdomen. Trophocytes and fat cells do not divide during the adulthood of workers, are immersed in body fluid, and can be used to test anti-aging drugs by microinjecting them into the body fluid. In a sense, honeybees can be used as an animated biomicroincubator (Hsieh and Hsu 2011). Therefore, trophocytes and fat cells are good target cells for cellular senescence studies. This claim is supported by the assay of age-related molecules in the trophocytes and fat cells of workers from the field hive, showing that old workers exhibited more SA-β-Gal, lipofuscin granules, lipid peroxidation, and protein oxidation compared to newly emerged workers (Hsieh and Hsu 2011).
Although honeybees are a suitable model for aging studies, it is argued that the aging level of worker honeybees in the field hive is not certain because the aging level was claimed to depend on chronological age and task performance (Neukirch 1982; Rueppell et al. 2007a), or to be decoupled from chronological age (Seehuus et al. 2006a; Rueppell et al. 2007b). In order to eliminate the problem of task performance and confirm the relationship between chronological age and aging, in this study, we reared newly emerged workers in a thermostat at 34°C throughout their lives. The only difference between workers is age because they have no task performance as nurse bees or forager bees. We not only examined the age-related molecules such as SA-β-Gal, lipofuscin granules, lipid peroxidation, and protein oxidation, but also examined cellular energy metabolism molecules such as ATP concentration, β-oxidation, and LC3 in the trophocytes and fat cells of young and old workers.
Materials and methods
Honeybees
The honeycomb frames containing pupae from the source colony were purchased from a single commercial breeder (Hsinchu, Taiwan) and transferred to an incubator (34°C, 95% relative humidity). One hundred newly emerged workers were collected in a cage (15 × 10 × 12 cm3) and put into a 34°C thermostat (NK system, Nippon, Japan). Workers were fed honey and fresh pollen grains mixed with honey every day. Their survivorship was recorded every day. This experiment replicated three times. The survivorship, mean life span, and maximum life span were analyzed by SPSS software (version 10, SPSS, Chicago, IL, USA). After establishing survivorship curve, 5-day-old and 30-day-old workers were selected to serve as young and old workers, respectively, for the following studies.
Assay of SA-β-Gal
SA-β-Gal expression in the trophocytes and fat cells of young and old workers was measured as described previously (Dimri et al. 1995). Briefly, workers were dissected. Trophocytes and fat cells from abdomen were detached from the cuticle of a young or old worker in honeybee saline (Hsu et al. 2007), immersed in SA-β-Gal staining solution at 37°C for 6 h, washed with PBS, mounted onto glass slides, and viewed under light microscope (Olympus BX-61, Tokyo, Japan). SA-β-Gal area was analyzed by QWin image processing and analysis software (version 2.5, Leica, Wetzlar, Germany). This experiment was replicated five times.
Assay of lipofuscin granules
Lipofuscin granules in trophocytes and fat cells were observed by confocal microscopy (Brunk and Terman 2002). Trophocytes and fat cells were isolated from a young or old worker in honeybee saline, mounted onto glass slides, and viewed under a confocal microscope (Leica TCS SP2; Leica, Wetzlar, Germany). Autofluorescence of lipofuscin granules was evaluated with blue (450–490 nm) excitation light, with 520 nm emission filters. Lipofuscin granules area in trophocytes and fat cells was analyzed by QWin image processing and analysis software (version 2.5, Leica, Wetzlar, Germany). This experiment was replicated 14 times.
Assay of lipid peroxidation
The trophocytes and fat cells from three young and old workers were homogenized in 1.0 ml 50 mM phosphate buffer (pH 7.5) containing protease inhibitors (leupeptin 10 μg/ml, pepstatin 1 μg/ml and phenylmethylsurfonyl fluoride 40 μg/ml) by using a polytron and sonicator, and centrifuged at 5,000×g for 10 min to obtain the resulting supernatant, which was used in following experiments. The protein content of supernatant was determined by protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA; 500–0.006). Lipid peroxidation was evaluated by the thiobarbituric acid reactive substances procedure (Draper and Hadley 1990). Briefly, 210 μl of the resulting supernatant were mixed with 420 μl of 28% trichloroacetic acid solutions in an Eppendorf tube. After boiling for 15 min, 630 μl of 1% thiobarbituric acid (TBA, pH < 2) solution were added in Eppendorf, which was then placed in a boiling water bath for 15 min. After cooling, the absorbance of solution was measured using a spectrophotometer at 532 nm. The concentration of malondialdehyde (MDA) was calculated by the absorbance coefficient of the MDA–TBA complex (absorbance coefficient = 1.56 × 105 cm−1 M−1) and expressed as nanomoles per gram of protein. This experiment was replicated 11 times.
Assay of protein oxidation
Protein carbonyls in trophocytes and fat cells were determined using a spectrometric 2,4-dinitrophenylhydrazones (DNPH) assay with minor modification. Briefly, 300 μl of the resulting supernatant (described in Assay of lipid peroxidation) were treated with 300 μl of 10 mM DNPH dissolved in 2 M HCl or with 2 M HCl in the controls. Samples were then incubated for 1 h at room temperature, stirred every 10 min, precipitated with 300 μl of 28% trichloroacetic acid, and centrifuged at 11,000×g for 3 min. The pellet was washed with 1 ml of ethanol/ethyl acetate, 1:1 (v/v) and redissolved in 1 ml of 6 M guanidine in 10 mM phosphate buffer/trifluoroacetic acid, pH 2.3. Any trace insoluble material was removed by centrifugation at 8,000×g for 3 min. The difference in absorbance between the DNPH-treated and the HCl-treated sample was determined at 366 nm, and the results were expressed as nanomoles of carbonyl groups per milligrams of protein, using the extinction coefficient of 22.0 mM−1 cm−1 for aliphatic hydrazones (Sohal et al. 1993). This experiment was replicated eight times.
Assay of ATP concentration
ATP in the trophocytes and fat cells in young and old workers was quantified by the ATP determination kit (Invitrogen, Carlsbad, CA, USA; A22066). Briefly, after background luminescence was recorded, 10 μl of diluted ATP standard solution (1, 10, 100, 1,000, 5,000, 10,000 nM) or 10 μl of the supernatant (described in Assay of lipid peroxidation) was added to the standard reaction solution, and measured spectrophotometrically at 560 nm at room temperature. Resultant luminescence, standardized to background, yielded a standard curve, which, in turn, was used to obtain the ATP value and expressed as picomole per milligram protein. This experiment was replicated seven times.
Assay of β-hydroxylacyl-coenzyme A dehydrogenase activity and citrate synthase activity
β-Hydroxylacyl-coenzyme A dehydrogenase (HOAD) activity assay was performed as previously described (Thibeault et al. 1997). Briefly, 50 μl of supernatant (described in Assay of lipid peroxidation) mixed with 930 μl of solution (containing 100 mM triethanolamine–HCl, 5 mM EDTA, and 1 mM KCN), 10 μl of 11.5 mM NADH, and 10 μl of 0.05 mM acetoacetyl-coenzyme A (CoA) (pH 7.0) (omitted for the control). Enzyme activity was measured from the oxidation of NADH at 340 nm by using a UV/VIS spectrophotometer (Beckman/DU 70, Brea, CA, USA) at 37°C and expressed as nanomoles per minute per milligram protein. Extinction coefficient of NADH at 340 nm is 6.22 ml μmol−1 cm−1. This experiment was replicated seven times.
Citrate synthase (CS) activity was determined by citrate synthase assay kit (Sigma, Saint Louis, MO, USA; CS0720). Briefly, 50 μl of supernatant (described in Assay of lipid peroxidation) mixed with 900 μl of assay solution (including acetyl CoA solution and 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB)) and 50 μl of 100 mM Tris buffer for background luminescence measurement. Fifty microliter of 10 mM oxaloacetic acid (OAA) solution replaced with 50 μl of 100 mM Tris buffer to initiate the reaction and measured spectrophotometrically at 412 nm at room temperature. CS activity was expressed as nanomoles per minute per milligram protein. Extinction coefficient of DTNB at 412 nm is 13.6 ml μmol−1 cm−1. This experiment was replicated seven times.
Assay of LC3 expression
Proteins (30 μg) from the supernatant (described in Assay of lipid peroxidation) mixed with sampling buffer were electrophoresed in a 10–15% acrylamide SDS/PAGE, and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking 1 h at 25°C, PVDF membranes were incubated with antibodies of LC3 (1:1,000) (Abgent, San Diego, CA, USA; ap1801b) or tubulin (1:10,000) (Abcam, Cambridge, MA, USA; ab6046), respectively. Then, membranes were probed with their respective secondary antibody labeled with horseradish peroxidase (1:10,000). Immunolabeled proteins were detected by using a chemiluminiscence method (PerkinElmer, Covina, CA, USA) and analyzed with Image J software (NIH, Bethesda, MA, USA). This experiment was replicated five times.
Statistical analysis
Differences in mean values between the two age groups were examined using two-sample t test. A p value of less than 0.05 was considered statistically significant.
Results
Survivorship
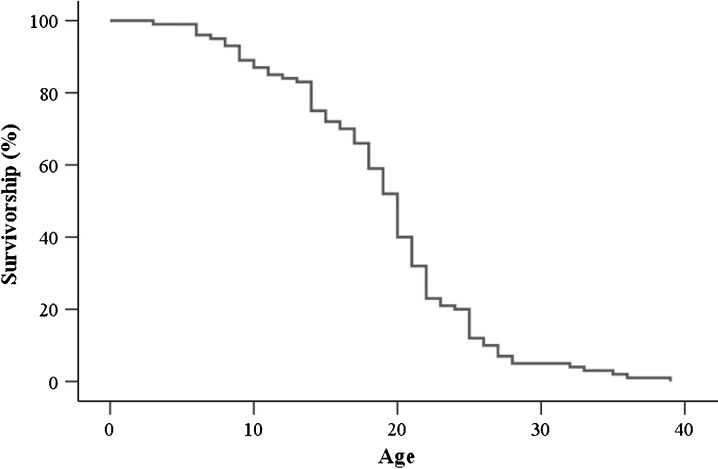
A survivorship curve was obtained from newly emerged workers which reared in a thermostatic chamber at 34°C (Fig. 1). The mean life span is 18.86 ± 0.47 days. The maximum life span is 31.57 ± 2.54 days. In subsequent studies, 5-day workers were selected and served as young workers, 30-day workers were selected and served as old workers, upon which SA-β-Gal, lipofuscin granules, lipid peroxidation, protein oxidation, ATP concentration, β-oxidation, and LC3 were assayed.
Fig. 1.
Survivorship curve of workers reared in a thermostatic chamber at 34°C
SA-β-Gal and lipofuscin granules
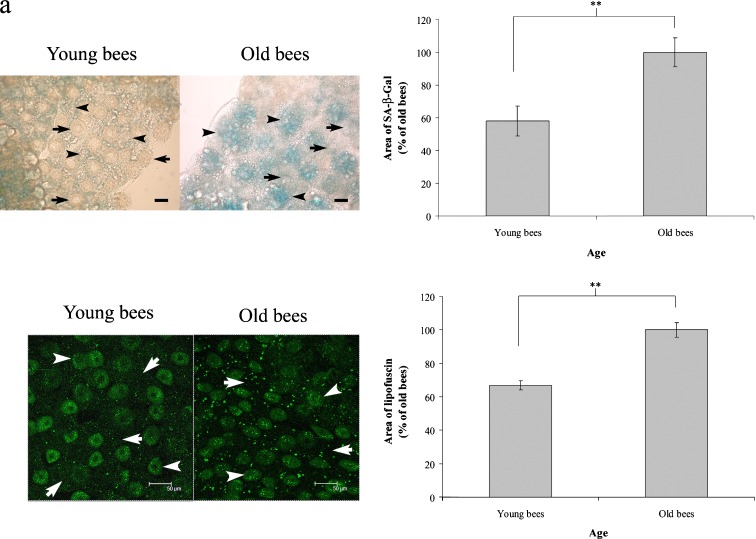
In order to confirm that workers reared in a thermostatic chamber at 34°C can be used for aging studies, we assayed SA-β-Gal activity and lipofuscin granules in trophocytes and fat cells of young and old workers. Cellular SA-β-Gal activity in trophocytes and fat cells, observed by the intensity of blue staining, was low in young workers and high in old workers (Fig. 2a). Statistical analysis revealed that the area of trophocytes and fat cells staining positive for SA-β-Gal activity increased with age (t = −4.888, n = 5, P < 0.01) (Fig. 2b). These results show that SA-β-Gal activity increases with age in workers reared in a thermostatic chamber at 34°C.
Fig. 2.
SA-β-Gal activity and lipofuscin granules in the trophocytes and fat cells of young and old workers. a Blue staining indicates SA-β-Gal activity. Arrows point to trophocytes. Arrowheads point to fat cells. Scale bar, 25 μm. b Quantification of SA-β-Gal in young and old workers. Bars represent mean ± SEM (n = 5). c Brightly stained green dots indicate lipofuscin granules. Arrows point to trophocytes. Arrowheads point to fat cells. Scale bar, 50 μm. d Quantification of lipofuscin granules in young and old workers. Bars represent mean ± SEM (n = 14). Asterisks indicate statistical significance as determined by two-sample t test (**P < 0.01)
Lipofuscin granules appeared as bright-green-colored dots in the cells. The trophocytes and fat cells of young workers exhibited few lipofuscin granules, whereas those of old workers expressed numerous lipofuscin granules (Fig. 2c). Statistical analysis indicated that the trophocytes and fat cells of old workers exhibited more lipofuscin granules than those of young workers (t = −6.378, n = 14, P < 0.01) (Fig. 2d). These results showed that the expression of lipofuscin granules increases with age in workers reared in a thermostatic chamber at 34°C.
Lipid peroxidation and protein oxidation
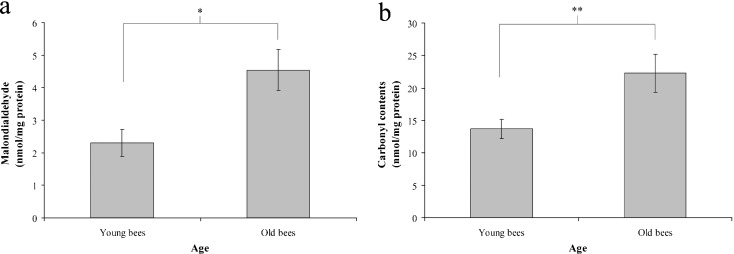
In order to confirm that workers reared in a thermostatic chamber at 34°C can be used for aging studies, we further assayed lipid peroxidation and protein oxidation in trophocytes and fat cells of young and old workers. Lipid peroxidation was assessed by determining the levels of MDA, a metabolite of lipid peroxidation. The mean values obtained for MDA were 2.14 ± 0.23 and 5.11 ± 1.11 nmol mg−1 protein in young and old workers, respectively (t = −2.444, n = 11, P < 0.05) (Fig. 3a). These data showed that the level of MDA, and therefore, lipid peroxidation increases with age in workers reared in a thermostatic chamber at 34°C.
Fig. 3.
Lipid peroxidation (a) and protein oxidation (b) in the trophocytes and fat cells of young and old workers. Bars represent mean ± SEM (n = 33 in (a), n = 24 in (b)). Asterisks indicate statistical significance as determined by two-sample t test (*P < 0.05; **P < 0.01)
Protein oxidation in trophocytes and fat cells was assessed by determining the carbonyl contents of amino acids using DNPH. The mean values obtained for carbonyl-group content were 1.27 ± 0.62 and 8.17 ± 1.66 nmol mg−1 protein in young and old workers, respectively (t = −3.879, n = 8, P < 0.01) (Fig. 3b). These data reveal that the level of carbonyl group and, therefore, protein oxidation increases with age in workers reared in a thermostatic chamber at 34°C.
Cellular energy metabolism: ATP concentration, β-oxidation, and LC3 expression
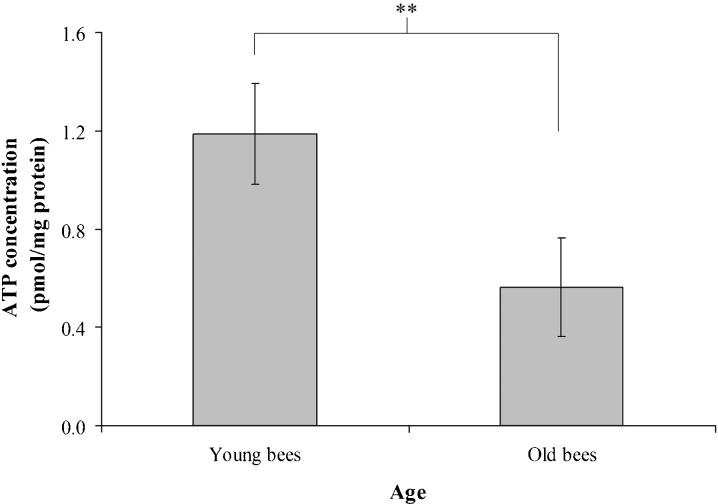
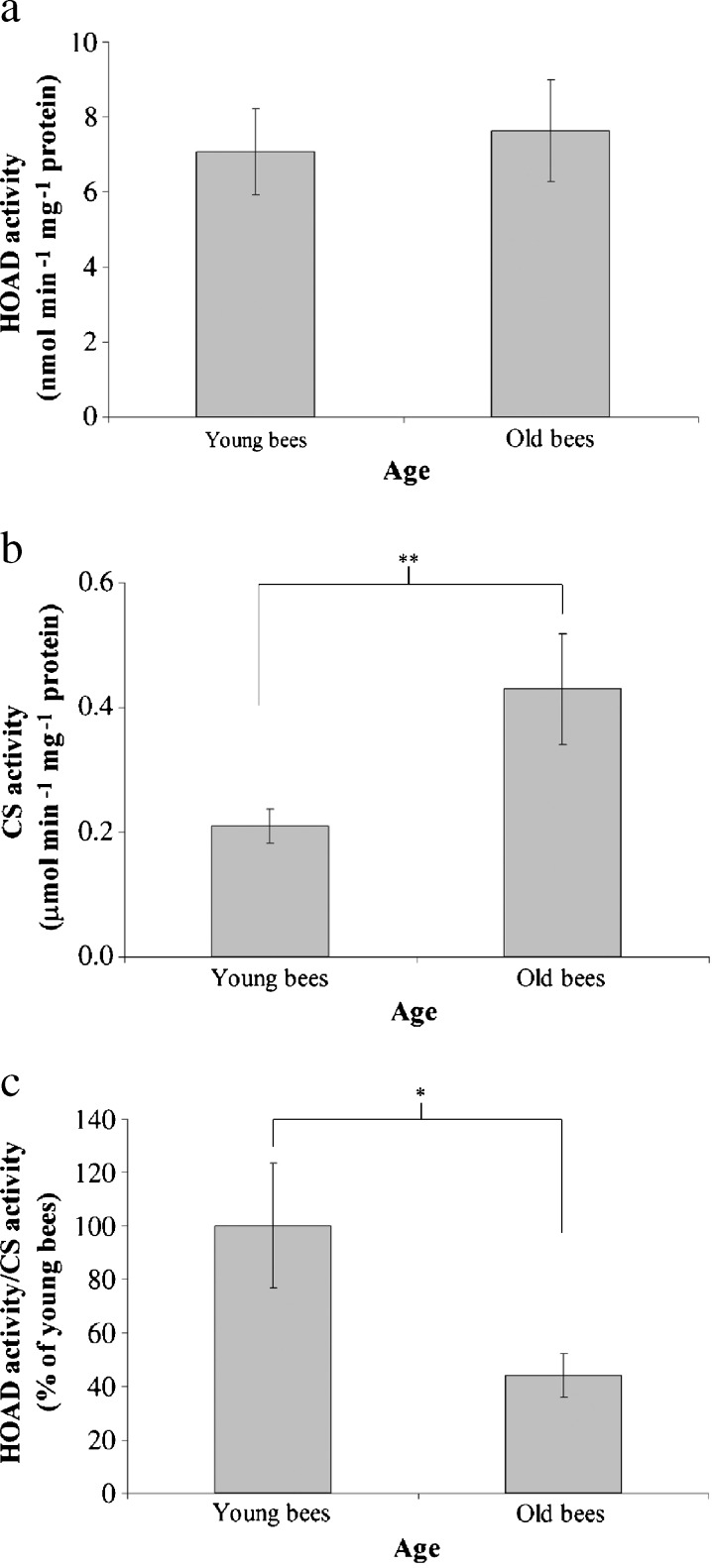
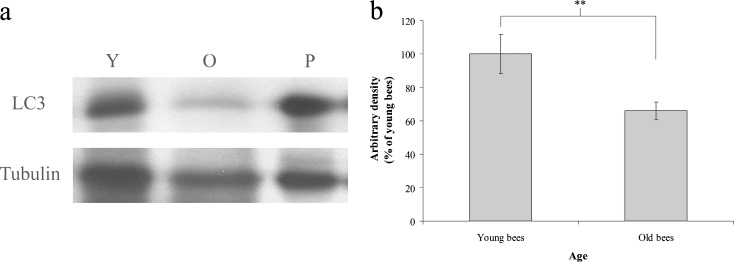
To understand whether cellular energy metabolism changes with advancing age in workers reared in a thermostatic chamber at 34°C, we assayed ATP concentration, β-oxidation, and LC3 in the trophocytes and fat cells of young and old workers. The mean values of ATP concentration were 1.19 ± 0.20 and 0.56 ± 0.20 pmol mg−1 protein in young and old workers, respectively (t = 4.114, n = 7, P < 0.05) (Fig. 4), indicating that young workers have higher ATP concentration than old workers. The ratio of HOAD activity/CS activity shows the relative importance of β-oxidation for aerobic metabolism (Cordiner and Egginton 1997). There were no significant difference between the HOAD activity of young and old workers in this study; means of 7.07 ± 1.14 and 7.63 ± 1.36 nmol min−1 mg−1 protein were observed for young and old workers, respectively (t = −0.267, n = 7, P > 0.05) (Fig. 5a). CS activity was positively correlated with advancing age with means of 0.21 ± 0.03 and 0.43 ± 0.09 nmol min−1 mg−1 protein in young and old workers, respectively (t = −5.263, n = 7, P < 0.01) (Fig. 5b). The ratio of HOAD/CS activity was higher in young workers than in old workers (t = 2.497, n = 7, P < 0.05) (Fig. 5c), indicating that young workers had higher β-oxidation activity than old workers. LC3 is a biomarker of macroautophage (Kadowaki et al. 2006). The trophocytes and fat cells of young workers expressed more LC3 than those of old workers (Fig. 6a). Statistical analysis showed that LC3 expression was significantly higher in young workers than in old workers (t = 5.081, n = 5, P < 0.01) (Fig. 6b), indicating that young workers have higher macroautophage degradation activity. These results demonstrate that ATP concentration, β-oxidation, and LC3 expression, and, therefore, cellular energy metabolism decreased with age in workers reared in a thermostatic chamber at 34°C.
Fig. 4.
ATP concentration in the trophocytes and fat cells of young and old workers. Bars represent mean ± SEM (n = 21). Asterisks indicate statistical significance as determined by two-sample t test (**P < 0.01)
Fig. 5.
HOAD activity (a), CS activity (b), and the ratio of HOAD activity/CS activity (c) in the trophocytes and fat cells of young and old workers. The ratio of HOAD activity/CS activity (β-oxidation) was normalized to the expression in young workers; the results are shown as percentages and represent the mean ± SEM (n = 21). Asterisks indicate statistical significance as determined by two-sample t test (*P < 0.05; **P < 0.01)
Fig. 6.
LC3 expression in the trophocytes and fat cells of young and old workers. a LC3 was analyzed by western blot. Tubulin served as the loading control. Y young workers, O old workers, P positive control; the muscle of rats. b LC3 was normalized to the expression in young workers; the results are shown as percentages and represent the mean ± SEM (n = 15). Asterisks indicate statistical significance as determined by two-sample t test (**P < 0.01)
Discussion
In this study, we determined the age-related molecules and cellular energy metabolism molecules in the trophocytes and fat cells of young and old workers reared in a thermostatic chamber at 34°C. The only difference between these workers is age because they have no task performance as nurse bees or forager bees. Age-related molecules and cellular energy metabolism molecules assays showed that old workers expressed more SA-β-Gal, lipofuscin granules, lipid peroxidation, and protein oxidation than young workers, whereas old workers expressed less ATP concentration, β-oxidation, and LC3 than young workers. These results validate that honeybees reared in a thermostatic chamber can be used for aging studies and that cellular energy metabolism in the trophocytes and fat cells of workers changes with age.
Survivorship
Survival curves are divided into aging survival curves and stochastic survival curves (Guarente 1997). The survival curve of workers reared in a thermostatic chamber at 34°C is similar to the aging survival curve of zebrafish, Nothobranchius rachovii, Cynolebias nigripinnis (Herrera and Jagadeeswaran 2004), Nothobranchius furzeri (Genade et al. 2005), and mice (Baur et al. 2006). Therefore, the survival curve of workers reared in a thermostatic chamber at 34°C is an aging survival curve. Herrera and Jagadeeswaran explained that the survivorship curves express the natural aging of the fish and is not attributable to infectious disease or other non-age-dependent variables (Herrera and Jagadeeswaran 2004). Genade et al. (2005) also reported that this curve is associated with aging. The death of workers in our study is most probably associated with aging.
SA-β-Gal and lipofuscin granules
The expression of SA-β-Gal has been broadly used to examine the cellular senescence in many cell types such as the dermal fibroblasts and epidermal keratinocytes of human (Dimri et al. 1995), the endothelial and smooth muscle cells of human and rabbit blood vessels (van der Loo et al. 1998; Kurz et al. 2000), the skin and dermis of zebrafish (Danio rerio) (Kishi et al. 2003), the skin and dermis of the annual fish N. furzeri (Genade et al. 2005), and the gills of the annual fish N. rachovii (Hsu et al. 2008). The accumulation of lipofuscin granules has also been extensively used to evaluate cellular senescence in many cell types such as the skin, dermis, and muscle of zebrafish (D. rerio) (Kishi et al. 2003), the brain and heart of rats (Nakano et al. 1995), the neonatal cardiac myocytes of rats (Brunk and Terman 2002), the liver and caudal peduncle of N. furzeri (Genade et al. 2005), and the gills of N. rachovii (Hsu et al. 2008). SA-β-Gal and lipofuscin granules have been also used to determine the aging of trophocytes and fat cells in workers reared in the field hive (Hsieh and Hsu 2011). The expression of SA-β-Gal and the accumulation of lipofuscin granules increased with the aging of workers from the field hive. In this study, the expression of SA-β-Gal and the accumulation of lipofuscin granules increased with the age of workers reared in a thermostatic chamber at 34°C. In other words, their expression and accumulation increased with the aging of workers. The results of SA-β-Gal and lipofuscin granules from workers reared in a thermostatic chamber at 34°C is similar to those from workers reared in the field hive (Hsieh and Hsu 2011).
Protein oxidation and lipid peroxidation
Lipid peroxidation has been used to assess cellular senescence in many cell types such as the adrenal gland of rats (Almeida et al. 1998) and the muscle of N. rachovii (Hsu et al. 2008). Protein oxidation has been used to estimate cellular senescence in many cell types such as the muscle of Drosophila melanogaster (Sohal et al. 1993), the lens cells of human (Welis-Knecht et al. 1993), and the muscle of N. rachovii (Hsu et al. 2008). Lipid peroxidation and protein oxidation have also been used to determine the aging of trophocytes and fat cells in workers reared in the field hive (Hsieh and Hsu 2011). Lipid peroxidation and protein oxidation increased with the aging of workers from the field hive. In this study, lipid peroxidation and protein oxidation increased with the age of workers reared in a thermostatic chamber at 34°C. On the other hand, lipid peroxidation and protein oxidation increased with the aging of workers. The results of lipid peroxidation and protein oxidation from workers reared in a thermostatic chamber at 34°C is similar to those from workers reared in the field hive (Hsieh and Hsu 2011).
Workers with advancing age from the field hive can also be used for aging studies
The expression of age-related molecules in the trophocytes and fat cells of young and old workers reared in a thermostatic chamber at 34°C is similar to that of workers reared in the field hive (Hsieh and Hsu 2011). This fact indicates that the aging level of workers increased with advancing age, regardless of their source. Therefore, workers with advancing age from the field hive can also be used for aging studies. The aging level of workers from the field hive derives from the accumulated effects of chronological age and task performance. Each worker goes through similar tasks with advancing age under normal conditions. The surviving workers can represent the aging workers. In addition, the results of this study are consistent with previous studies of worker honeybees (Remolina et al. 2007; Jemielity and Keller 2007; Hsieh and Hsu 2011) and with that of Drosophila (Grotewiel et al. 2005).
Cellular energy metabolism molecules: ATP concentration, β-oxidation, and LC3 expression
The rate of ATP synthesis is principally governed by substrate availability, namely ADP and Pi. In addition, mitochondrial membrane potential formed by electron transport and proton leakage controls ATP synthesis. Therefore, ATP concentration can be an index of cellular energy metabolism. In this study, ATP concentrations in trophocytes and fat cells decreased with advancing age in workers reared in a thermostatic chamber at 34°C. This finding is consistent with previous studies showing that ATP production decrease with age due to the decline of mitochondrial membrane potential (Petersen et al. 2004; Kelley et al. 2002).
HOAD is located in the mitochondrial matrix and catalyzes the reaction between 3-hydroxylacyl- CoA and NAD+ to form 3-ketoacyl-CoA and NADH. CS is the initial enzyme in the tricarboxylic acid cycle and catalyzes the reaction between acetyl CoA and OAA to form citric acid. The HOAD/CS ratio can represent the β-oxidation activity (Cordiner and Egginton 1997). In this study, CS activity in trophocytes and fat cells increased with advancing age, which is consistent with previous studies showing that CS activity was found to increase with the foraging experience in the thorax flight muscle of workers (Schippers et al. 2006, 2010). The HOAD/CS ratio in trophocytes and fat cells was reduced with advancing age, indicating that β-oxidation activity was higher in young workers. These results coincide with previous studies, which showed that children have higher fat oxidation than adults (Kostyak et al. 2007) and elderly adults (Levadoux et al. 2001).
Autophagy is responsible for the degradation of most long-lived proteins and some organelles (Cuervo et al. 2005). The amino acids, fatty acids, and carbohydrates produced by autophagy can be reused for biosynthesis or energy production (Lum et al. 2005). Therefore, molecules of autophage can be used to appraise cellular energy metabolism. In this study, LC3, a regulatory molecule of macroautophagy, was selected to serve as an index of cellular energy metabolism (Kadowaki et al. 2006). LC3 expression decreased with advancing age in the trophocytes and fat cells of workers reared in thermostatic chamber at 34°C, indicating that old workers have lower macroautophagy activity and young workers have higher macroautophagy activity. This result corresponds to a previous study showing that LC3 expression was lower in extraocular muscles and hearts of aged rats (McMullen et al. 2009; Taneike et al. 2010). The results of ATP concentration, β-oxidation, and LC3 expression demonstrate that cellular energy metabolism in the trophocytes and fat cells of workers change with advancing age.
Honeybees have many important merits for studying aging as described above. Now honeybees can be reared in a thermostatic chamber, which provides controlled, isolated, and clean environment for studying aging. This system can be used to study the mechanism of lifespan extension by ambient temperature reduction and the screening of anti-aging drugs.
Acknowledgments
This work was supported by CMRPD 170361 grant from Chang Gung Memorial Hospital, Taiwan. We thank Scott C. Schuyler for critically reading the manuscript.
References
- Almeida H, Magalhães MC, Magalhães MM. Age-related changes in lipid peroxidation products in rat adrenal gland. Age. 1998;21:119–121. doi: 10.1007/s11357-998-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Couteur DL, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell functions. Free Radic Biol Med. 2002;33:611–619. doi: 10.1016/S0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- Collins AM, Williams V, Evans JD. Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera. Insect Mol Biol. 2004;13:141–146. doi: 10.1111/j.0962-1075.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- Cordiner S, Egginton S. Effects of seasonal temperature acclimatization on muscle metabolism in rainbow trout, Oncorhynchus mykiss. Fish Physiol Biochem. 1997;16:333–343. doi: 10.1023/A:1007732003452. [DOI] [Google Scholar]
- Corona M, Hughes KA, Weaver DB, Robinson GE. Gene expression patterns associated with queen honey bee longevity. Mech Ageing Dev. 2005;6:1230–1238. doi: 10.1016/j.mad.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Bergamini E, Brunk UT, Drӧge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;86:421–431. doi: 10.1016/0076-6879(90)86135-I. [DOI] [PubMed] [Google Scholar]
- Genade T, Benedetti M, Terzibasi E, Roncaglia P, Valenzano DR, Cattaneo A, Cellerino A. Annual fishes of the genus Nothobranchius as a model system for aging research. Aging Cell. 2005;4:223–233. doi: 10.1111/j.1474-9726.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Martin I, Bhandari P, Cook-Wiens E. Functional senescence in Drosophila melanogaster. Ageing Res Rev. 2005;4:372–397. doi: 10.1016/j.arr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Guarente L. Link between aging and the nucleolus. Genes Dev. 1997;11:2449–2455. doi: 10.1101/gad.11.19.2449. [DOI] [PubMed] [Google Scholar]
- Herrera M, Jagadeeswaran P. Annual fish as a genetic model for aging. J Gerontol A Biol Sci Med Sci. 2004;59:101–107. doi: 10.1093/gerona/59.2.B101. [DOI] [PubMed] [Google Scholar]
- Hsieh YS, Hsu CY. Honeybee trophocytes and fat cells as target cells for cellular senescence studies. Exp Gerontol. 2011;46:233–240. doi: 10.1016/j.exger.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Chiu YC. Ambient temperature influences aging in an annual fish (Nothobranchius rachovii) Aging Cell. 2009;8:726–737. doi: 10.1111/j.1474-9726.2009.00525.x. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Ko FY, Li CW, Fann K, Lue JT. Magnetoreception system in honeybees (Apis mellifera) PLoS One. 2007;2(4):e395. doi: 10.1371/journal.pone.0000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CY, Chiu YC, Hsu WL, Chan YP. Age-related markers assayed at different developmental stages of the annual fish Nothobranchius rachovii. J Gerontol A Biol Sci Med Sci. 2008;63A:1267–1276. doi: 10.1093/gerona/63.12.1267. [DOI] [PubMed] [Google Scholar]
- Jemielity S, Keller L. Aging: a young mind in old bees. Curr Biol. 2007;17:R294–R295. doi: 10.1016/j.cub.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Kadowaki M, Karim MR, Carpi A, Miotto G. Nutrient control of macroautophagy in mammalian cells. Mol Aspects Med. 2006;27:426–443. doi: 10.1016/j.mam.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Kelley D, He J, Menshikova E, Ritov V. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Kishi S, Uchiyama J, Baughman A, Goto T, Lin M, Tsai S. The zebrafish as a vertebrate model of functional aging and very gradual senescence. Exp Gerontol. 2003;38:777–786. doi: 10.1016/S0531-5565(03)00108-6. [DOI] [PubMed] [Google Scholar]
- Kostyak JC, Kris-Etherton P, Bagshaw D, DeLany JP, Farrell PA. Relative fat oxidation is higher in children than adults. Nutr J. 2007;6:19. doi: 10.1186/1475-2891-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated β-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci. 2000;113:3613–3622. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- Levadoux E, Morio B, Montaurier C, Puissant V, Boirie Y, Fellmann N, Picard B, Rousset P, Beaufrere B, Ritz P. Reduced whole-body fat oxidation in women and in the elderly. Int J Obes Relat Metab Disord. 2001;25:39–44. doi: 10.1038/sj.ijo.0801530. [DOI] [PubMed] [Google Scholar]
- Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- McMullen CA, Ferry AL, Gamboa JL, Andrade FH, Dupont-Versteegden EE. Age-related changes of cell death pathways in rat extraocular muscle. Exp Gerontol. 2009;44:420–425. doi: 10.1016/j.exger.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, Fano G, Fulle S, MacGarvey U, Shinobu L, Polidori MC, Cherubini A, Vecchirt J, Senin U, Beal MF. Age-dependent increase in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999;26:303–308. doi: 10.1016/S0891-5849(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Nakano M, Oenzil F, Mizuno T, Gotoh S. Age-related changes in the lipofuscin accumulation of brain and heart. Gerontology. 1995;41:69–79. doi: 10.1159/000213726. [DOI] [PubMed] [Google Scholar]
- Neukirch A. Dependence of the life span of the honeybee (Apis mellifera) upon flight performance and energy consumption. J Comp Physiol. 1982;146:35–40. [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remolina SC, Hafez DM, Robinson GE, Hughes KA. Senescence in the worker honey bee Apis mellifera. J Insect Physiol. 2007;53:1027–1033. doi: 10.1016/j.jinsphys.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Bachelier C, Fondrk MK, Page RE. Regulation of life history determines lifespan of worker honey bees (Apis mellifera L.) Exp Gerontol. 2007;42:1020–1032. doi: 10.1016/j.exger.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Christine S, Mulcrone C, Groves L. Aging without functional senescence in honey bee workers. Curr Biol. 2007;17:R274–R275. doi: 10.1016/j.cub.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers MP, Dukas R, Smith RW, Wang J, Smolen K, McClelland GB. Lifetime performance in foraging honeybees: behaviour and physiology. J Exp Biol. 2006;209:3828–3836. doi: 10.1242/jeb.02450. [DOI] [PubMed] [Google Scholar]
- Schippers MP, Dukas R, McClelland GB. Lifetime- and caste-specific changes in flight metabolic rate and muscle biochemistry of honeybees, Apis mellifera. J Comp Physiol B. 2010;180:45–55. doi: 10.1007/s00360-009-0386-9. [DOI] [PubMed] [Google Scholar]
- Seehuus SC, Krekling T, Amdam GV. Cellular senescence in honey bee brain is largely independent of chronological age. Exp Gerontol. 2006;41:1117–1125. doi: 10.1016/j.exger.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc Natl Acad Sci U S A. 2006;103:962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Dubey A, Orr WC. Protein oxidative damage is associated with life expectancy of houseflies. Proc Natl Acad Sci U S A. 1993;90:7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Shirasawa T, Mizushima N, Otsu K. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. Lipofuscin. Int J Biochem Cell Biol. 2004;36:1400–1404. doi: 10.1016/j.biocel.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Thibeault M, Blier PU, Guderley H. Seasonal variation of muscle metabolism organization in rainbow trout (Oncorhynchus mykiss) Fish Physiol Biochem. 1997;16:139–155. doi: 10.1007/BF00004671. [DOI] [Google Scholar]
- van der Loo B, Fenton MJ, Erusalimsky JD. Cytochemical detection of a senescence-associated b-galactosidase in endothelial and smooth muscle cells from human and rabbit blood vessels. Exp Cell Res. 1998;241:309–315. doi: 10.1006/excr.1998.4035. [DOI] [PubMed] [Google Scholar]
- Weirich GF, Collins AM, Williams VP. Antioxidant enzymes in the honey bee, Apis mellifera. Apidologie. 2002;33:3–14. doi: 10.1051/apido:2001001. [DOI] [Google Scholar]
- Welis-Knecht MC, Huggins TG, Dyer G, Thorpe SR, Baynes JW. Oxidized amino acids in lens protein with age. J Biol Chem. 1993;268:12348–12352. [PubMed] [Google Scholar]
- Williams JB, Roberts SP, Elekonich MM. Age and natural metabolically intensive behavior affect oxidative stress and antioxidant mechanisms. Exp Gerontol. 2008;43:538–549. doi: 10.1016/j.exger.2008.02.001. [DOI] [PubMed] [Google Scholar]