Abstract
The reproductive-cell cycle theory of aging posits that reproductive hormone changes associated with menopause and andropause drive senescence via altered cell cycle signaling. Using data from the Wisconsin Longitudinal Study (n = 5,034), we analyzed the relationship between longevity and menopause, including other factors that impact “ovarian lifespan” such as births, oophorectomy, and hormone replacement therapy. We found that later onset of menopause was associated with lower mortality, with and without adjusting for additional factors (years of education, smoking status, body mass index, and marital status). Each year of delayed menopause resulted in a 2.9% reduction in mortality; after including a number of additional controls, the effect was attenuated modestly but remained statistically significant (2.6% reduction in mortality). We also found that no other reproductive parameters assessed added to the prediction of longevity, suggesting that reproductive factors shown to affect longevity elsewhere may be mediated by age of menopause. Thus, surgical and natural menopause at age 40, for example, resulted in identical survival probabilities. These results support the maintenance of the hypothalamic–pituitary–gonadal axis in homeostasis in prolonging human longevity, which provides a coherent framework for understanding the relationship between reproduction and longevity.
Keywords: Wisconsin Longitudinal Study, Menopause, Mortality, Longevity, Survival probabilities, Hypothalamic–pituitary–gonadal axis, Reproduction, Ovarian lifespan, Births, Oophorectomy, Hormone replacement therapy, Endocrine dyscrasia, Hormone, Life expectancy
Introduction
The reproductive-cell cycle theory of aging postulates that the hormones that regulate reproduction act in an antagonistic pleiotropic manner to control aging via cell cycle signaling; they promote growth and development early in life to achieve reproduction but later in life, in a futile attempt to maintain reproduction, become dysregulated and drive senescence (Bowen and Atwood 2004). Thus, the dysregulation of the hypothalamic–pituitary–gonadal (HPG) axis (endocrine dyscrasia) leads to altered (dyotic) signaling to somatic and reproductive tissues, driving re-entry of cells into the cell cycle. In post-mitotic, terminally differentiated cell types, such signaling leads to attempted, albeit incomplete, cell division resulting in cellular dysfunction and/or death. In tissues containing totipotent stem cells, cellular division is completed at an enhanced rate.
The theory predicts that the longer the HPG axis is maintained in equilibrium, the longer an organism will live. Support for the dysregulation of the HPG axis in regulating lifespan comes from both animal and human studies (Helle et al. 2005; Horiuchi 1997). The most frequently reported relationship between reproduction and post-reproductive survival in humans is between late reproduction and longevity, with advanced age at last reproduction associated with improved longevity (Helle et al. 2005, Appendix A). In this study, we examined a number of reproductive life traits to determine whether maintenance of the HPG axis predicts increased longevity. We find that later age of menopause is consistently associated with reduced mortality among women in the Wisconsin Longitudinal Study (WLS) and that no other reproductive parameter assessed significantly predicted mortality. These results suggest that reproductive and somatic senescence are tightly coupled in humans and that selection to reproduce later (or longer) would favor enhanced longevity. Our findings suggest that strategies designed to maintain the HPG axis in equilibrium will increase longevity.
Methods
Data
The WLS design and history have been discussed in detail elsewhere (Hauser 2005; Hauser and Willis 2005; Sewell et al. 2004). Briefly, the WLS sample was originally comprised of 10,317 men and women who graduated from Wisconsin high schools in 1957 (one third of all who graduated, the modal year of birth was 1939). These individuals were surveyed in 1964, 1975, 1992, 2004, and 2010 (current round).1 In addition to the focal respondents, the WLS began interviewing one randomly selected sibling of each graduate (when possible) starting in 1977 (were mainly born between 1929 and 1949). Since then the survey content has been broadly parallel for graduate and sibling participants.
The WLS provides a comprehensive record of social background, adolescent ability and aspirations, schooling, military service, family formation, labor market experiences, health, personality, and social and civic participation of the original respondents. Data for the current study come primarily from the 1993 and 2004 waves of the WLS when female reproductive health and menopause questions were added to the supplemental mail surveys. These data were augmented with National Death Index (NDI) and Social Security Death Master File (SSA) death records.
Sample and response rates
The WLS has and continues to enjoy exceptional response rates. The WLS sample includes 5,326 female graduates (original sample) and 4,341 randomly selected female siblings (supplemental sample, from graduates of either gender).2 Of these, 7,344 were able to be located, alive, and sent a mail survey in the 1993 interview round. We take this as the base sample for computing response rates. A total of 5,827 (72%) sample members completed the mail survey. Notably, 276 of these cases (5%) were still menstruating at last contact and excluded from analysis. Data suitable for analysis were obtained for 5,034 (91%) cases.3
Measures
Longevity
Death was the primary outcome measure for this study. Death records were collected from two sources: (1) the NDI, a national registry of deaths from local and state reporting agencies with near complete coverage of domestic deaths, and/or (2) SSA, a death registry compiled by the Social Security Administration of Social Security beneficiaries. Records were first matched with the NDI. If a match was found using NDI confirming a death, the date of death was recorded. At the time of this study, NDI death information was available up through December 2008. To ascertain deaths after December 2008 or for records not in the NDI, we matched records with the SSA. Though SSA records may have somewhat lower coverage than NDI records, they were both more current (available through June 2010) and less likely to contain reporting errors for crucial matching information (e.g., social security number, date of birth). If a death match was found in the SSA, the date of death was recorded. Records not matching NDI, SSA, or WLS internal mortality records were presumed alive as of June 2010.
Reproductive traits
Data on menopause, number of live births, unilateral and bilateral oophorectomy, hysterectomy, and hormone replacement therapy (HRT) were collected. Age of menopause—approximated by age of last menstrual period—was constructed from a combination of several items, including age of last menstrual cycle, menstruation within the last 12 months, and age at time of survey. Menopause and reproductive health data (surgery, HRT, etc.) were primarily extracted from the 1993 survey and updated, if necessary, from information collected at follow-up in 2004. Number of live births was collected from rosters of children that were updated at each survey wave.
Other factors
Other covariates investigated were year of birth, adolescent IQ, years of education, marital status, family income, smoking status, and body mass index (BMI).4 These were collected at baseline (1993) or from administrative records. See Table 1 for sample descriptive statistics.
Table 1.
Sample descriptive statistics
| N/mean/% | Range | |
|---|---|---|
| Sample | ||
| N | 5,034 | |
| Age at 1993 interview (SD) | 54.1 (4.14) | 35.7–77.8 |
| Deaths | ||
| N (%) | 439 (8.7%) | |
| Age at death (SD) | 65.9 (6.89) | 51–91 |
| Reproductive parameters | ||
| Age of last period (SD) | 47.7 (7.08) | 21–65 |
| Biological children (SD) | 2.8 (1.70) | 0–18 |
| Any HRT (%) | 61.6% | |
| Oophorectomy, one or both (%) | 28.2% | |
| Baseline and additional controls | ||
| Adolescent IQ (SD) | 102.7 (14.6) | 61–145 |
| Years of education (SD) | 13.4 (2.12) | 2–21 |
| Current smoker | 17.5% | |
| Body mass index (SD) | 26.1 (5.16) | 10–83 |
| Currently married | 80.0% | |
| Median family income | $47,600 | −$12,000–$1,000,000 |
Analyses
We used a prospective analytic design. We estimated two sets of proportional hazards regressions predicting mortality risk, with and without covariates (Cox 1972; SAS Institute 2011).5 These types of models relate time-to-event data to a set of covariates and an underlying hazard function. These models included both right and left censoring; right censoring was used for post-survey survival, while left censoring was used to account for survival from age of last period to age at survey. We also used a robust standard error correction for sibling pairs.
Preliminary models included all the controls listed in other factors above. Non-significant controls were dropped from the final “adjusted” regressions presented below. The significant controls that were retained were (a) years of education, (b) smoking status, (c) BMI, and (d) marital status. Preliminary models also explored alternative specifications of age at last period (i.e., age groups) with similar findings.
Results
The proportional hazards regression models indicated that menopause significantly predicts mortality (see Table 2). Unadjusted data indicated a 2.9% reduction in mortality for every year of later menopause. Adjusting for years of education, smoking status, BMI, and marital status modestly attenuated the reduction in mortality to 2.6%.
Table 2.
Proportional hazards regression results
| Hazard ratio | p | 95% CI | |
|---|---|---|---|
| Age of menopause (years) | |||
| Unadjusted | 0.971 | <0.001 | 0.959–0.983 |
| Adjusteda | 0.974 | <0.001 | 0.962–0.986 |
aAdjusted for baseline education, smoking, BMI, and marital status
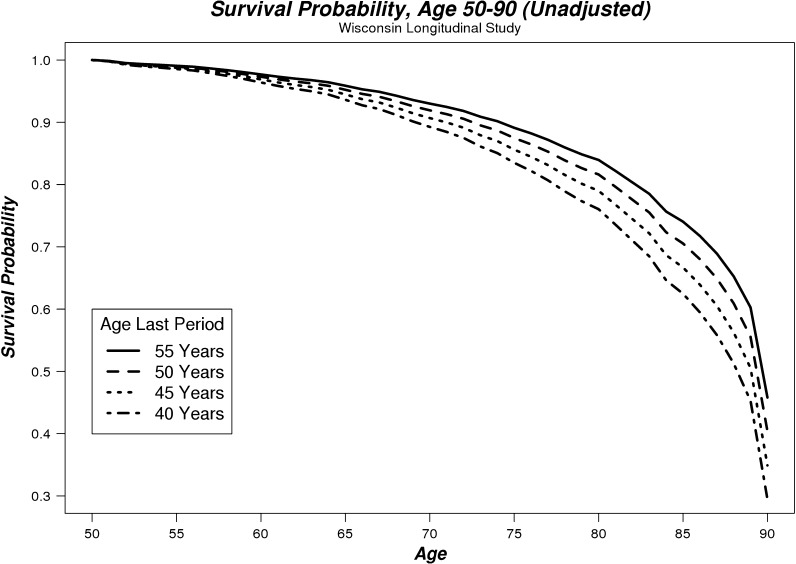
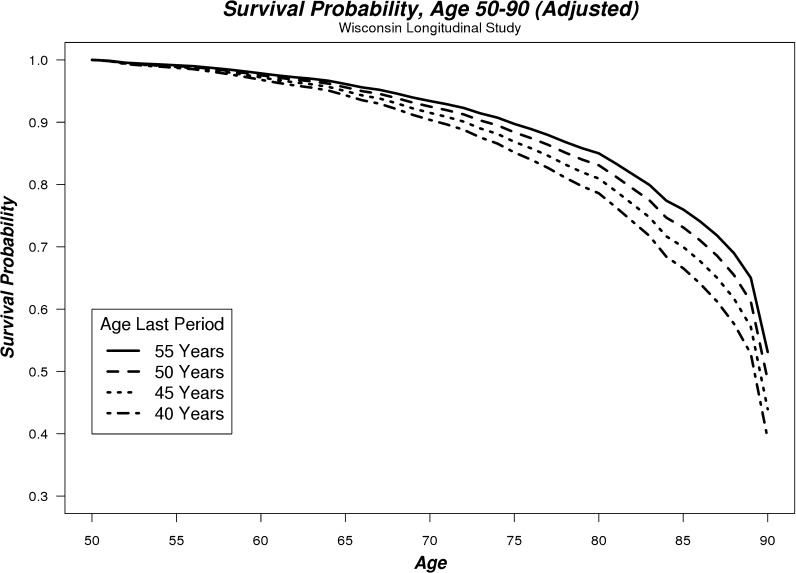
Estimated survival probabilities from these regressions are shown in Table 3 and plotted in Figs. 1 and 2 between the ages of 50 and 90. The data were modeled to represent the majority of menopause ages in our sample (40–55 years), with other controls evaluated at their mean. These illustrate how increasing age at menopause is associated with increased probability of survival for both unadjusted and adjusted regression models. Women in our sample who reached menopause at 40 years of age had a 39% chance of surviving to age 90, while women who reached menopause at 55 years of age had a 53% chance of surviving to age 90 (based on adjusted regressions). For example, this reduction in mortality (between menopause at age 55 vs 40) translated into a life expectancy advantage over the 50-90 age range of 1.58 years (4.6%).
Table 3.
Estimated survival probabilities from age 50–90 by age of last period
| Age last period | Estimated survival to agea | |||
|---|---|---|---|---|
| 60 (%) | 70 (%) | 80 (%) | 90 (%) | |
| 40 years | 97 | 90 | 79 | 39 |
| 45 years | 97 | 92 | 81 | 44 |
| 50 years | 98 | 93 | 83 | 49 |
| 55 years | 98 | 93 | 85 | 53 |
aAdjusted for baseline years of education, smoking status, BMI, and marital status
Fig. 1.
Unadjusted survival probability curves for women aged between 50 and 90 whose last menstrual period occurred at 40, 45, 50, or 55 years of age
Fig. 2.
Adjusted survival probability curves for women aged between 50 and 90 whose last menstrual period occurred at 40, 45, 50, or 55 years of age
However, other reproductive traits that we explored in preliminary work (unilateral or bilateral oophorectomy, number of biological children, and HRT) did not significantly contribute to predicting mortality and were not included in our final regressions. In other words, no other reproductive parameters assessed added to the prediction of longevity, with or without considering age of menopause. Thus, surgical and natural menopause at age 40, for example, resulted in identical survival probabilities. It is important to note, however, that number of biological children and HRT were significantly associated with age of menopause. That is, number of biological children was positively associated with age of menopause, while use of HRT was negatively associated with age of menopause. It is also important to note that preliminary simulations did not suggest that loss to follow-up or missing data would significantly alter our main findings.
Discussion
Our findings suggest that maintenance of HPG axis homeostasis is a predictor of mortality. Later age of last period consistently predicted reduced mortality (Figs. 1 and 2; Table 2); even when adjusted for a number of additional covariates, the effect remained largely unchanged. We also found in our preliminary regressions that surgical and natural menopause resulted in identical survival probabilities, as might be expected given that both result in the dysregulation of the HPG axis.
These data fit within the biological and evolutionary framework of the reproductive-cell cycle theory of aging that posits the endocrine dyscrasia resulting from the loss of negative feedback by sex steroids and inhibins on hypothalamic and pituitary hormonal production is driving our senescent phenotype and ultimately mortality. Thus, factors that regulate the commencement (puberty), end (menopause, andropause, oophorectomy), or modulation of HPG axis homeostasis (pregnancy, lactation, amenorrhea, and HRT) might be predicted to regulate senescence (age-related diseases) and mortality. Below we discuss the evidence for each of these factors with respect to age-related disease incidence and longevity.
Age at menopause
Previous studies, together with our current study, indicate that higher age at menopause is associated with prolonged female post-reproductive lifespan (Helle et al. 2005; Cooper et al. 1998; Jacobsen et al. 1997, 2003; Johnston 2001, 2003; Ossewaarde et al. 2005; Snowdon et al. 1989; van der Schouw et al. 1996). The most frequently reported relationship between reproduction and post-reproductive survival in humans is between late reproduction and longevity, with advanced age at last reproduction being associated with improved longevity (Helle et al. 2005, Appendix A). In addition, delaying of an entire reproductive effort to later age covaries with the enhanced post-reproductive survival of mothers (Helle et al. 2002). Conversely, early menopause induced by bilateral oophorectomy at the time of hysterectomy for benign disease is associated with an increased risk of morbidity and mortality (Parker and Manson 2009; Parker et al. 2009; Rivera et al. 2009a, b; Rocca et al. 2006). Oophorectomy is not associated with increased survival at any age (Parker et al. 2009). In this connection, it has been shown that experimental manipulation of germ cell number affects longevity. Transplantation of reproductively viable ovaries from young mice into senescent mice significantly extended lifespan (Cargill et al. 2003).
Further evidence that reproductive endocrine dyscrasia lies at the heart of mortality is indicated by the relationship between the age at menopause and age-related diseases. In women with later menopause, there is a reduced risk for developing cardiovascular disease (de Kleijn et al. 2002; van der Schouw et al. 1996; Jacobsen et al. 1997; Hu et al. 1999; Ossewaarde et al. 2005), calcifications in the aorta (Witteman et al. 1989), atherosclerosis (Joakimsen et al. 2000), cognitive decline (McLay et al. 2003), and bone fractures (van Der Voort et al. 2003). The risk of colorectal cancer also is decreased (van Wayenburg et al. 2000), and despite an increase in death from uterine and ovarian cancer with increasing age at menopause, the net effect of later menopause is an increased lifespan (Ossewaarde et al. 2005).
As might be expected, early reproductive endocrine dyscrasia, occurring naturally or induced by unilateral or bilateral oophorectomy in premenopausal women, is associated with increased risk of developing dementia, cognitive decline, stroke, fatal and non-fatal coronary heart disease, Parkinsonism, osteoporosis, hip fracture, lung cancer, depression, and anxiety (Parker and Manson 2009; Parker et al. 2009; Rivera et al. 2009a, b; Rocca et al. 2006; Nappi et al. 1999; Rocca et al. 2007; Shuster et al. 2010; Gleason et al. 2005; Rocca et al. 2008a, b, c, 2009; Lisabeth et al. 2009; Baba et al. 2010; Koushik et al. 2009). Indeed, the increased prevalence of cognitive disease in women correlates with the abrupt earlier loss of gonadal function (Jorm et al. 1987; McGonigal et al. 1993; Brookmeyer et al. 1998; Gao et al. 1998; Andersen et al. 1999; Hy and Keller 2000). Prospective cohort studies indicate that E2 replacement therapy reduces the incidence (Henderson et al. 1994) and delays the onset of cognitive decline in women and men (Tang et al. 1996; Kawas et al. 1997; Paganini-Hill and Henderson 1996; Baldereschi et al. 1998). It should be noted that similar protection is not always afforded by the use of non-physiological estrogens such as conjugated equine estrogens (CEE) and non-physiological progestagens such as medroxyprogesterone (MPA; Shumaker et al, 2003). This might explain the null finding in our study for HRT and longevity, since most subjects taking HRT in the US were prescribed either Premarin (CEE) or Prempro (CEE + MPA). Similarly, suppression of circulating gonadotropins (and sex steroids) with GnRH agonist therapy halves the risk of death from AD (Bowen et al. 2004; D’Amico et al. 2010), suggesting elevations in gonadotropins drive cognitive decline (Bryan et al. 2010; Casadesus et al. 2006, 2007). Indeed, it has been demonstrated that down-regulation of serum gonadotropins is as effective as estrogen replacement at improving menopause-associated cognitive deficits in mice (Bryan et al. 2010).
Breast and ovarian cancer are the only age-related diseases where risk decreases with early reproductive endocrine dyscrasia, i.e., following oophorectomy (Parker et al. 2009). Conversely, early menarche, higher numbers of ovulatory cycles, nulliparity, and late menopause are associated with increased risk for female reproductive cancers (Gladwell 2000; Parsa and Parsa 2009).
Age at menarche
Just as earlier endocrine dyscrasia results in increased mortality, so too early age at menarche (before age 12 years), is correlated with increased risk of cardiovascular morbidity and mortality and overall mortality in women (Lakshman et al. 2009). Conversely, two recent studies have found a 4.5% and 2.4% reduced risk of overall mortality for each year later menarche occurs (Jacobsen et al. 2007, 2009). However, it is not clear if later age at menarche decreases risk of cardiovascular morbidity and mortality, per se, or if later age of menarche operates through later age of menopause as suggested by our results. Since earlier menarche might be associated with earlier menopause, with later menarche associating with later menopause, these data indicate that maintaining the HPG axis in equilibrium longer, for example, will decrease the risk of morbidity and mortality.
Partial re-establishment of the HPG axis with HRT
The most compelling evidence in humans for reproductive endocrine dyscrasia regulating longevity comes from epidemiological studies of estrogen replacement therapy use after menopause. Partial balancing of the HPG axis with estrogen therapy (decreasing gonadotropin/GnRH production) extends longevity. Over 15 studies have demonstrated a reduction in the risk of mortality in those taking estrogen replacement therapies (reviewed in Paganini-Hill et al. 2006). These studies consistently show a 20% to 50% decrease in mortality among users of estrogens. Recently, Paganini-Hill et al. (2006) reported increased longevity even in older users of post-menopausal estrogen therapy. There is considerable evidence that physiological hormone replacement therapies also delay the onset, halt the progression of, and even reverse the course of age-related diseases including heart disease, cerebrovascular disease (stroke), Alzheimer’s disease, cancer, and osteoporosis (Atwood and Bowen 2011). Thus, partial rebalancing of the HPG axis following menopause and during andropause via exposure to sex steroids increases longevity. Although there is mounting evidence that the elevations in gonadotropins promotes age-related diseases (Bowen et al. 2004; Wilson et al. 2008; Bryan et al. 2010; Atwood and Bowen 2011; Sun et al. 2006), gonadotropins are of course downregulated by sex steroids (and GnRH agonists/antagonists). Thus, either the maintenance of sex steroid concentrations or the suppression of gonadotropin concentrations using sex steroids and/or GnRH agonists/antagonists would be expected to delay age-related diseases and extend longevity.
In animals, re-establishment of the negative feedback loops in the HPG axis of post-reproductive mice (22 months of age) following transplantation of reproductively viable ovaries from young mice (3 months of age) has been demonstrated to extend lifespan by up to 40% (Cargill et al. 2003; Mason et al. 2009). Gonad manipulation in model organisms provides strong evidence for a direct link between reproduction and longevity (Arantes-Oliveira et al. 2002; Muller et al. 2001). In the hermaphroditic worm Caenorhabditis elegans, neonatal ablation of the gonadal germ line cells while leaving the somatic gonad intact results in increased life span, but removal of the entire gonad yielded no change in life span (Hsin and Kenyon 1999). Ablation of the germ line must be prior to germ line stem cell proliferation for lifespan extension to occur (Arantes-Oliveira et al. 2002). However, no extension of lifespan was found following ablation of the germ line in Drosophila melanogaster (Barnes et al. 2006). Characterization and quantitation of the endocrine signals in these animal models might help explain the discrepant results regarding longevity. In this respect, our own data indicate that suppression of GnRHR signaling in C. elegans significantly decreases reproduction 46% and prolongs lifespan 15% (23% at lower temperature) compared with wild-type worms (Vadakkadath Meethal et al. 2006; Vadakkadath Meethal, and Atwood, unpublished data).
Limitations
Our study is limited in a few ways. First, WLS participants are almost exclusively non-Hispanic white and grew up and have lived largely in Wisconsin. Second, while our preliminary work indicated that no other reproductive parameters were significantly associated with mortality, we cannot definitely rule out some other possible explanations for our findings, like pre-existing health problems. Third, our analyses relied exclusively on self-reported data for all predictors. Fourth, because of relatively low mortality in our sample and certain data limitations, we were not able to disaggregate our analyses by cause of death.
Acknowledgments
This research uses data from the Wisconsin Longitudinal Study (WLS) of the University of Wisconsin—Madison. Since 1991, the WLS has been supported principally by the National Institute on Aging (AG-9775, AG-21079, and AG-033285), with additional support from the Vilas Estate Trust, the National Science Foundation, the Spencer Foundation, and the Graduate School of the University of Wisconsin—Madison. A public use file of data from the Wisconsin Longitudinal Study is available from the Wisconsin Longitudinal Study, University of Wisconsin—Madison, 1180 Observatory Drive, Madison, Wisconsin 53706 and at http://www.ssc.wisc.edu/wlsresearch/data/. The opinions expressed herein are those of the authors. This material is the result of work supported with the use of facilities at the William S. Middleton Memorial Veterans Hospital, Madison, WI. The contents do not represent the views of the Department of Veterans Affairs or the US Government. This is Geriatrics Research, Education and Clinical Center VA paper # 2011–21.
Footnotes
Other than a nominal $10 payment included in the 2004 mail survey, WLS respondents have never been reimbursed for participation.
Siblings were randomly selected from rosters of siblings ascertained during the 1975 graduate telephone interviews. However, some graduates declined to provide contact information for selected siblings. This number represents the number of female selected siblings regardless of whether contact information was obtained and should be interpreted as the maximum possible female sibling sample.
The primary reasons for incomplete data were individual item refusal and/or unclassifiable responses.
Global self-reported health was excluded as a predictor as menopause induces changes in global health.
We explored other analytic approaches, including predicting 5-year survival using logistic regression, with similar results.
References
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, et al. Gender differences in the incidence of AD and vascular dementia: the EURODEM Studies. EURODEM Incidence Research Group. Neurology. 1999;53(9):1992–1997. doi: 10.1212/WNL.53.9.1992. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295(5554):502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Bowen RL. The reproductive-cell cycle theory of aging: an update. Exp Gerontol. 2011;46(2–3):100–107. doi: 10.1016/j.exger.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Baba Y, Ishikawa S, Amagi Y, Kayaba K, Gotoh T, Kajii E. Premature menopause is associated with increased risk of cerebral infarction in Japanese women. Menopause. 2010;17(3):506–510. doi: 10.1097/gme.0b013e3181c7dd41. [DOI] [PubMed] [Google Scholar]
- Baldereschi M, Di Carlo A, Lepore V, Bracco L, Maggi S, Grigoletto F, Scarlato G, Amaducci L. Estrogen-replacement therapy and Alzheimer’s disease in the Italian Longitudinal Study on Aging. Neurology. 1998;50(4):996–1002. doi: 10.1212/WNL.50.4.996. [DOI] [PubMed] [Google Scholar]
- Barnes AI, Boone JM, Jacobson J, Partridge L, Chapman T. No extension of lifespan by ablation of germ line in Drosophila. Proc Biol Sci. 2006;273(1589):939–947. doi: 10.1098/rspb.2005.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen RL, Atwood CS. Living and dying for sex—a theory of aging based on the modulation of cell cycle signaling by reproductive hormones. Gerontol. 2004;50(5):265–290. doi: 10.1159/000079125. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Beaird H, Atwood CS, Smith MA, Rimm AA (2004) Men treated for prostate cancer have a decreased incidence of dementia. In: 9th International Congress on AD
- Bowen RL, Verdile G, Liu T, Perry G, Smith MA, Martins RN, Atwood CS. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-ß protein precursor and amyloid-ß deposition. Journal of Biological Chemistry. 2004;279(19):20539–45. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337–1342. doi: 10.2105/AJPH.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan KJ, Mudd JC, Richardson SL, Chang J, Lee HG, Zhu XW, Smith MA, Casadesus G. Down-regulation of serum gonadotropins is as effective as estrogen replacement at improving menopause-associated cognitive deficits. J Neurochem. 2010;112(4):870–881. doi: 10.1111/j.1471-4159.2009.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill SL, Carey JR, Muller HG, Anderson G. Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell. 2003;2(3):185–190. doi: 10.1046/j.1474-9728.2003.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA. Luteinizing hormone modulates cognition and amyloid-deposition in Alzheimer APP transgenic mice. Bba-Mol Basis Dis. 2006;1762(4):447–452. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao CV, Perry G, Keri RA, Smith MA. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol. 2007;269(1–2):107–111. doi: 10.1016/j.mce.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Ephross SA, Weinberg CR, Baird DD, Whelan EA, Sandler DP. Menstrual and reproductive risk factors for ischemic heart disease. Am J Epidemiol. 1998;147(11):S50. [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. J Roy Stat Soc B. 1972;34(2):187. [Google Scholar]
- D’Amico AV, Braccioforte MH, Moran BJ, Chen MH. Luteinizing-hormone releasing hormone therapy and the risk of death from Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24(1):85–89. doi: 10.1097/WAD.0b013e31819cb8f4. [DOI] [PubMed] [Google Scholar]
- de Kleijn MJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga JD, van der Graaf Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155(4):339–345. doi: 10.1093/aje/155.4.339. [DOI] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55(9):809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Gladwell M. John rock’s error. The New Yorker. 2000;13:52–58. [Google Scholar]
- Gleason CE, Cholerton B, Carlsson CM, Johnson SC, Asthana S. Neuroprotective effects of female sex steroids in humans: current controversies and future directions. Cell Mol Life Sci. 2005;62(3):299–312. doi: 10.1007/s00018-004-4385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RM. Survey response in the long run: the Wisconsin Longitudinal Study. Field Methods. 2005;17:3–29. doi: 10.1177/1525822X04272452. [DOI] [Google Scholar]
- Hauser RM, Willis RJ. Survey design and methodology in the health and retirement study and the Wisconsin Longitudinal Study. In: Waite LJ, editor. Aging, health, and public policy: demographic and economic perspectives. New York: Population Council; 2005. pp. 209–235. [Google Scholar]
- Helle SI, Ekse D, Holly JMP, Lonning PE. The IGF-system in healthy pre- and postmenopausal women: relations to demographic variables and sex-steroids. J Steroid Biochem. 2002;81(1):95–102. doi: 10.1016/S0960-0760(02)00052-3. [DOI] [PubMed] [Google Scholar]
- Helle S, Lummaa V, Jokela J. Are reproductive and somatic senescence coupled in humans? Late, but not early, reproduction correlated with longevity in historical Sami women. P Roy Soc Lond B Bio. 2005;272(1558):29–37. doi: 10.1098/rspb.2004.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Paganini-Hill A, Emanuel CK, Dunn ME, Buckwalter JG. Estrogen replacement therapy in older women. Comparisons between Alzheimer’s disease cases and nondemented control subjects. Arch Neurol. 1994;51(9):896–900. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- Horiuchi S. Postmenopausal acceleration of age-related mortality increase. J Gerontol Ser Biol Sci Med Sci. 1997;52(1):B78–B92. doi: 10.1093/gerona/52A.1.B78. [DOI] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399(6734):362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, Rosner B, Stampfer MJ. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159(10):1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- Hy LX, Keller DM. Prevalence of AD among whites: a summary by levels of severity. Neurology. 2000;55(2):198–204. doi: 10.1212/WNL.55.2.198. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Nilssen S, Heuch I, Kvale G. Does age at natural menopause affect mortality from ischemic heart disease. J Clin Epidemiol. 1997;50(4):475–479. doi: 10.1016/S0895-4356(96)00425-8. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Heuch I, Kvale G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol. 2003;157(10):923–929. doi: 10.1093/aje/kwg066. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Heuch I, Kvale G. Association of low age at menarche with increased all-cause mortality: a 37-year follow-up of 61,319 Norwegian women. Am J Epidemiol. 2007;166(12):1431–1437. doi: 10.1093/aje/kwm237. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Oda K, Knutsen SF, Fraser GE. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: the adventist health study, 1976–88. Int J Epidemiol. 2009;38(1):245–252. doi: 10.1093/ije/dyn251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joakimsen O, Bonaa KH, Stensland-Bugge E, Jacobsen BK. Population-based study of age at menopause and ultrasound assessed carotid atherosclerosis: the Tromso Study. J Clin Epidemiol. 2000;53(5):525–530. doi: 10.1016/S0895-4356(99)00197-3. [DOI] [PubMed] [Google Scholar]
- Johnston SL. Associations with age at natural menopause in Blackfeet women. Am J Hum Biol. 2001;13(4):512–520. doi: 10.1002/ajhb.1083. [DOI] [PubMed] [Google Scholar]
- Johnston SL. Menopause in Blackfeet women—a life span perspective. Coll Antropol. 2003;27(1):57–66. [PubMed] [Google Scholar]
- Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand. 1987;76(5):465–479. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48(6):1517–1521. doi: 10.1212/WNL.48.6.1517. [DOI] [PubMed] [Google Scholar]
- Koushik A, Parent ME, Siemiatycki J. Characteristics of menstruation and pregnancy and the risk of lung cancer in women. Int J Cancer. 2009;125(10):2428–2433. doi: 10.1002/ijc.24560. [DOI] [PubMed] [Google Scholar]
- Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, Wareham NJ, Ong KK. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94(12):4953–4960. doi: 10.1210/jc.2009-1789. [DOI] [PubMed] [Google Scholar]
- Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham Heart Study. Stroke. 2009;40(4):1044–1049. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB, Cargill SL, Anderson GB, Carey JR. Transplantation of young ovaries to old mice increased life span in transplant recipients. J Gerontol A Biol Sci Med Sci. 2009;64(12):1207–1211. doi: 10.1093/gerona/glp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigal G, Thomas B, McQuade C, Starr JM, MacLennan WJ, Whalley LJ. Epidemiology of Alzheimer’s presenile dementia in Scotland, 1974–88. BMJ. 1993;306(6879):680–683. doi: 10.1136/bmj.306.6879.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay RN, Maki PM, Lyketsos CG. Nulliparity and late menopause are associated with decreased cognitive decline. J Neuropsychiatr Clin Neurosci. 2003;15(2):161–167. doi: 10.1176/appi.neuropsych.15.2.161. [DOI] [PubMed] [Google Scholar]
- Muller HG, Carey JR, Wu D, Liedo P, Vaupel JW. Reproductive potential predicts longevity of female Mediterranean fruitflies. Proc Biol Sci. 2001;268(1466):445–450. doi: 10.1098/rspb.2000.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappi G. Memory functioning at menopause: impact of age in ovariectomized women. Gynecol Obstet Invest. 1999;47(1):29–36. doi: 10.1159/000010058. [DOI] [PubMed] [Google Scholar]
- Ossewaarde ME, Bots ML, Verbeek ALM, Peeters PHM, van der Graaf Y, Grobbee DE, van der Schouw YT. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16(4):556–562. doi: 10.1097/01.ede.0000165392.35273.d4. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med. 1996;156(19):2213–2217. doi: 10.1001/archinte.1996.00440180075009. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Corrada MM, Kawas CH. Increased longevity in older users of postmenopausal estrogen therapy: the Leisure World Cohort Study. Menopause. 2006;13(1):12–18. doi: 10.1097/01.gme.0000172880.40831.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WH, Manson JE. Oophorectomy and cardiovascular mortality: is there a link? Menopause. 2009;16(1):1–2. doi: 10.1097/gme.0b013e31818d64d6. [DOI] [PubMed] [Google Scholar]
- Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, Shoupe D, Berek JS, Hankinson S, Manson JE. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009;113(5):1027–1037. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa P, Parsa B. Effects of reproductive factors on risk of breast cancer: a literature review. Asian Pac J Cancer Prev. 2009;10(4):545–550. [PubMed] [Google Scholar]
- Rivera CM, Grossardt BR, Rhodes DJ, Brown RD, Jr, Roger VL, Melton LJ, 3rd, Rocca WA. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16(1):15–23. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera CM, Grossardt BR, Rhodes DJ, Rocca WA. Increased mortality for neurological and mental diseases following early bilateral oophorectomy. Neuroepidemiology. 2009;33(1):32–40. doi: 10.1159/000211951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ., 3rd Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7(10):821–828. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Geda YE, Gostout BS, Bower JH, Maraganore DM, de Andrade M, Melton LJ., 3rd Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15(6):1050–1059. doi: 10.1097/gme.0b013e318174f155. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Maraganore DM. The long-term effects of oophorectomy on cognitive and motor aging are age dependent. Neurodegener Dis. 2008;5(3–4):257–260. doi: 10.1159/000113718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70(3):200–209. doi: 10.1212/01.wnl.0000280573.30975.6a. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, Melton LJ., 3rd Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond Engl) 2009;5(1):39–48. doi: 10.2217/17455057.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute SAS. Base Sas 9.3 procedures guide. Cary: SAS Institute; 2011. [Google Scholar]
- Sewell WH, Hauser RM, Springer KW, Hauser TS. As we age: the Wisconsin Longitudinal Study, 1957–2001. In: Leicht K, editor. In: research in social stratification and mobility. London: Elsevier; 2004. pp. 3–111. [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J, Investigators WHIMS. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65(2):161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon DA, Kane RL, Beeson WL, Burke GL, Sprafka JM, Potter J, Iso H, Jacobs DR, Phillips RL. Is early natural menopause a biologic marker of health and aging. Am J Public Health. 1989;79(6):709–714. doi: 10.2105/AJPH.79.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell. 2006;125(2):247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348(9025):429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- Vadakkadath Meethal S, Gallego MJ, Haasl RJ, Petras SJ, 3rd, Sgro JY, Atwood CS. Identification of a gonadotropin-releasing hormone receptor orthologue in Caenorhabditis elegans. BMC Evol Biol. 2006;6:103. doi: 10.1186/1471-2148-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans MJC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347(9003):714–718. doi: 10.1016/S0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- van Der Voort DJ, van Der Weijer PH, Barentsen R. Early menopause: increased fracture risk at older age. Osteoporos Int. 2003;14(6):525–530. doi: 10.1007/s00198-003-1408-1. [DOI] [PubMed] [Google Scholar]
- van Wayenburg CA, van der Schouw YT, van Noord PA, Peeters PH. Age at menopause, body mass index, and the risk of colorectal cancer mortality in the Dutch Diagnostisch Onderzoek Mammacarcinoom (DOM) cohort. Epidemiology. 2000;11(3):304–308. doi: 10.1097/00001648-200005000-00013. [DOI] [PubMed] [Google Scholar]
- Wilson AC, Clemente L, Liu T, Bowen RL, Vadakkadath Meethal S, Atwood CS. Reproductive hormones regulate the selective permeability of the blood-brain barrier. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2008;1782(6):401–407. doi: 10.1016/j.bbadis.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Witteman JC, Grobbee DE, Kok FJ, Hofman A, Valkenburg HA. Increased risk of atherosclerosis in women after the menopause. BMJ. 1989;298(6674):642–644. doi: 10.1136/bmj.298.6674.642. [DOI] [PMC free article] [PubMed] [Google Scholar]