Abstract
Aging has profound yet unpredictable effects on pain perception and incidence of anxiety disorders. However, the mechanisms underlying age-related pathologies are confounded by contradictory observations in rodent models. Therefore, the goal of our study was to test the hypothesis that genetic variability contributes to age-related pain behaviors and susceptibility to anxiety. To address this hypothesis, we examined pain and anxiety-like behavior in young or old Brown Norway (BN), Fisher 344, and BN/F344 (F1), three rat strains used in studies to evaluate the effect of aging. Mechanosensitive thresholds were assessed using the Von Frey assay, and visceral pain sensitivity was measured via the visceromotor response to colorectal distension. Anxiety-like behavior and exploration was quantified in the elevated plus maze. In the BN strain, old rats exhibited increased mechanosensitive thresholds compared to young rats; however, age did not affect visceral sensitivity in this strain. In F344-BN rats, the number of abdominal contractions induced by the highest colonic distension pressure was significantly lower in old rats. However, following colonic sensitization, a difference was no longer apparent. In the F-344 strain, visceral hypersensitivity following afferent sensitization was evident in young rats at all distension pressures but was not observed in older animals at 20 mmHg. Aging significantly reduced maze exploration across all strains. Our data demonstrate that age- and strain-related alterations exist in pain behavior and highlight the effects of aging on exploratory behavior. These findings suggest that strain differences contribute to the controversial data on the effects of aging on pain perception.
Keywords: Genetic diversity, Strain differences, Visceral pain, Mechanosensitive thresholds, Anxiety, Aging
Introduction
Abdominal pain due to visceral hypersensitivity, the primary symptom of the irritable bowel syndrome (IBS), is most often diagnosed in patients in their mid-twenties and, as a result, has largely been considered a disorder present in younger patients (Bennett and Talley 2002). However, epidemiological studies have shown that 10–20% of geriatric patients within the USA report IBS-like symptomatology, which is consistent with the national average for IBS within the general population (Friedel and Krevsky 2000; Ehrenpreis 2005; Talley et al. 1991). In response to rectal distension, elderly individuals have elevated sensory thresholds for first sensation, urge to defecate, and first painful sensation as compared to young control subjects (Lagier et al. 1999). Additionally, the elderly exhibit a higher threshold to painful sensation in response to intraesophageal balloon distension (Lasch et al. 1997). In addition, while some clinical studies show little or no difference in somatic pain sensitivity, many have demonstrated that elderly patients have altered sensitivity to thermonociception, ischemic pain, and pressure pain (Harkins et al. 1986; Edwards and Fillingim 2001; Lautenbacher et al. 2005). Taken together, these studies suggest an overall reduction in global pain sensitivity with advancing age.
A strong connection between altered pain perception and heightened anxiety has been observed with over 60% of IBS patients with a diagnosed anxiety disorder (Whitehead et al. 2002). These patients have altered hypothalamic–pituitary–adrenal (HPA) axis activity that results in enhanced release of stress hormones at baseline and following stressful stimuli (Dinan et al. 2006; Chang et al. 2009). Altered anxiety has also been reported within the geriatric population where the rate of anxiety symptom reporting is at least three times that of the general population (Scrable et al. 2009).
The mechanisms responsible for abnormal pain perception and increased anxiety in the elderly are currently unknown, and previous mechanistic investigations using rodents have produced inconsistent results which do not allow for a definitive conclusion on the effects of aging on pain and behavior (Gagliese and Melzack 2000). There are numerous sources of variability within these data, but the most obvious inconsistencies likely arise as a result of genetic diversity between rodent strains and methodological variations between studies. Therefore, the aims of the present study were designed specifically to (1) determine potential effects of rodent strain on changes in mechanosensitive thresholds and visceral pain behavior with age and (2) to examine the effect of aging on anxiety-related behavior in three rodent strains using uniform methodological assessments within a single laboratory.
To investigate these aims, we selected three rodent strains which are currently available from the National Institute of Aging (NIA): the Brown Norway (BN), Brown Norway–Fischer 344 (BN/F344 (F1)) (F1), and the F344 rats. In these animals, we measured mechanosensitive thresholds and visceral sensitivity. Since IBS is a disorder characterized by visceral hypersensitivity (Whitehead et al. 1990), visceral sensitivity assessment was performed at baseline and following induction of visceral hypersensitivity. In addition, rats were evaluated on the elevated plus maze (EPM) to assess anxiety-like behavior. The data from this study elucidate the differential effects of aging on pain in three rat strains which may translate to the heterogeneous results observed in human studies concerning pain perception in the elderly.
Materials and methods
Animals
Young (6 month) and old (26 month) Brown Norway, Brown Norway–F344, and F344 rats were obtained from the NIA colonies at Harlan Sprague–Dawley Inc. (Indianapolis, IN, USA). All rats in the current study were male with an experimental number of seven to nine animals per group. Rats were given food and water ad libitum at 23°C in a 12:12-h light-dark-controlled room. Due to the large size of the aged rats, animals were single housed upon arrival to prevent cage-mate aggression. To reduce the stress associated with shipping and the novel laboratory environment, all rats were acclimated to the animal facility for 1 week followed by an additional week of acclimatization to the experimenter and the laboratory environment. During the second week of acclimation, rats were brought into the laboratory between the hours of 9:00 AM and 2:00 PM, weighed, and handled by the investigator. All experiments were approved by the Institutional Animal Care and Use Committee (protocol number 08–065).
Mechanosensitive threshold assessment
The Von Frey assay is a standard technique to record mechanosensitivie thresholds in rodents. Following a 30-min acclimation to the experimental environment, rats were placed individually in a clear, plexiglass cage on an elevated wire mesh floor and allowed to acclimate for an additional 30 min to the apparatus. After the acclimation period, an IITC 2390 series Electronic Von Frey Anesthesiometer (IITC Life 164 Science Inc, Woodland Hills CA, USA) was used to digitally record the threshold to elicit hindpaw withdrawal in response to mechanical stimulation. In this procedure, the Von Frey probe was applied with constant pressure to the plantar surface of the hind paw and the minimal force required to elicit hind paw withdrawal was recorded from the digital display of the anesthesiometer. The procedure was performed in triplicate on the same hind paw of each animal with 5 min between each test and somatic mechanosensitive thresholds were quantified as the average withdrawal threshold (Myers et al. 2007).
Visceral sensitivity assessment
Following an overnight fast (16–18 h), all rats were briefly anesthetized by isoflurane inhalation (2–5%). A strain gauge force transducer was surgically attached to the external abdominal oblique musculature to quantify the visceromotor response (VMR) to colorectal distension (CRD). The strain gauge lead wires were externally looped around to the dorsal surface of the animal and attached to the back using a single stitch. A 5-cm colorectal balloon was inserted 9–11 cm into the distal colon and secured by tape at the base of the tail. Rats were allowed to recover for 30 min before assessment of visceral sensitivity. The VMR to colorectal distension CRD was quantified by observing the number of abdominal contractions recorded by the strain gauge sutured onto the abdominal musculature. Following a 10-min control period where the balloon was inserted but not distended, three isobaric distensions were performed at 20, 40, and 60 mmHg. The distension pressures were maintained for a period of 10 min while the number of abdominal contractions was recorded to indicate the level of visceral sensitivity at each distension pressure. A 10-min resting period was allowed between distensions. During this procedure, rats were fully conscious and ambulatory (Ness and Gebhart 1988; Myers and Greenwood-Van Meerveld 2007; Tyler et al. 2007). Following an initial series of CRD, rats were given a colonic infusion of dilute acetic acid (1.5 ml of 0.6% COOH). The acetic acid solution was used to cause acute sensitization of colonic afferent fibers, thereby inducing visceral hypersensitivity within 60 min (Langlois et al. 1994). One hour after acetic acid infusion, a second series of CRD was performed using the same balloon distention protocol as previously described (baseline, 20-, 40-, and 60-mmHg distensions) and the VMR was recorded. Immediately following the last distension, rats were anesthetized using isoflurane inhalation (5%) and euthanized.
Assessment of anxiety-like behavior: elevated plus maze
One day following the Von Frey assay, anxiety-like behavior was determined using a standard EPM (elevated 50 cm from the ground with each arm 50 cm in length). The maze was cleaned with a 20% ethanol solution and allowed to dry before experimental testing. Following a 30-min acclimation period to the experimental room, animals were placed on the maze facing an open arm and allowed to explore for a period of 5 min. The percentage of time spent in the open arms was recorded as an indicator of anxiety, and maze exploration was recorded as the total number of arm entries, number of head dips, number of rears, and end explorations during the 5-min testing period (Myers and Greenwood-Van Meerveld 2007). Behavior of the animal on the maze was recorded by a digital video camera which was mounted directly above the maze. The digital video was reviewed by a blinded observer for behavioral scoring.
Data analysis
Data are represented as the mean ± SEM. A two-way ANOVA was used to analyze potential differences in the percent time in open arms (anxiety-like behavior), total number of arm entries (locomotor activity) on the EPM, and mechanosensitive thresholds with age and strain as factors and Bonferroni post tests. Changes in the VMR due to CRD were analyzed with a 2 × 3 × 4 × 2 repeated measures ANOVA with afferent sensitization (normosensitive vs. hypersensitive), strain, distension pressure, and age as factors followed by Tukey–Kramer post hoc tests. Within-strain comparisons of VMR to CRD were analyzed with a repeated measures two-way ANOVA with distension pressure and age or afferent sensitization as factors followed by Bonferroni post tests. Animal numbers were seven to nine per group for each assay.
Results
Mechanosensitive thresholds
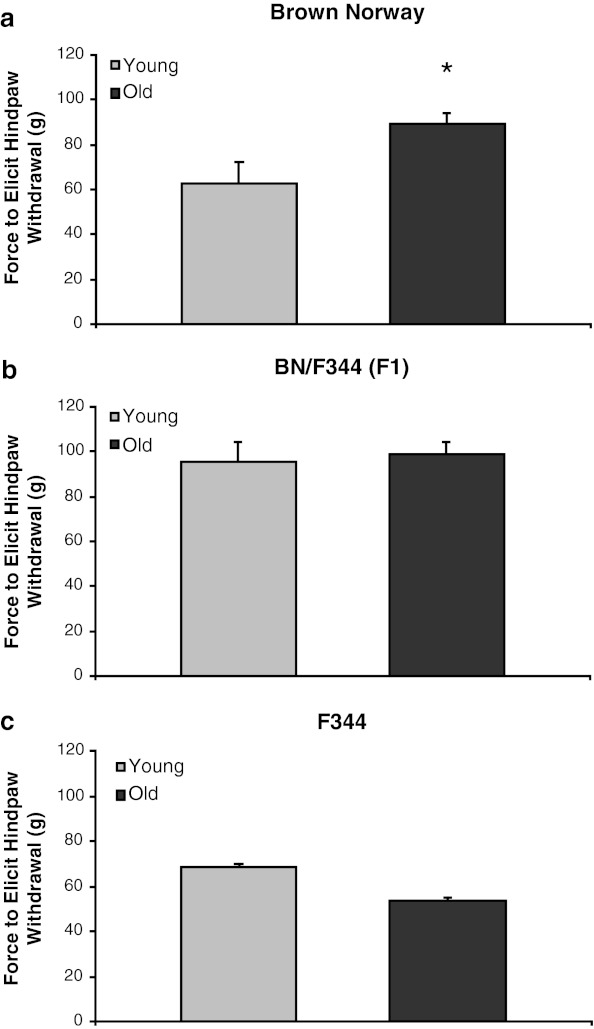
Assessment of mechanosensitive thresholds revealed a significant main effect of strain [F(2,41) = 16.2, p < 0.0001]. Although there was no main effect of age, there was a significant strain × age interaction [F(2,41) = 6.48, p < 0.01], which is explained by the impact of age on somatic threshold to mechanical stimulation in the BN strain (Fig. 1a). Old BN rats had reduced mechanical sensitivity quantified as an increased amount of force required to elicit a nociceptive hindpaw withdrawal reflex compared to their younger counterparts. However, no difference was observed between old and young rats in the nociceptive withdrawal reflex of BN/F344 (F1) (Fig. 1b) or F344 rats (Fig. 1c).
Fig. 1.
Effects of aging on somatic sensitivity in three rodent strains. a In the BN strain, old rats required an increased force to elicit a nociceptive withdrawal reflex of the hindpaw as compared to young animals. Aging had no effect on somatic sensitivity in b F344-BN or c F344 rats. *p < 0.05 vs. young animals of the same strain
Visceral sensitivity assessment
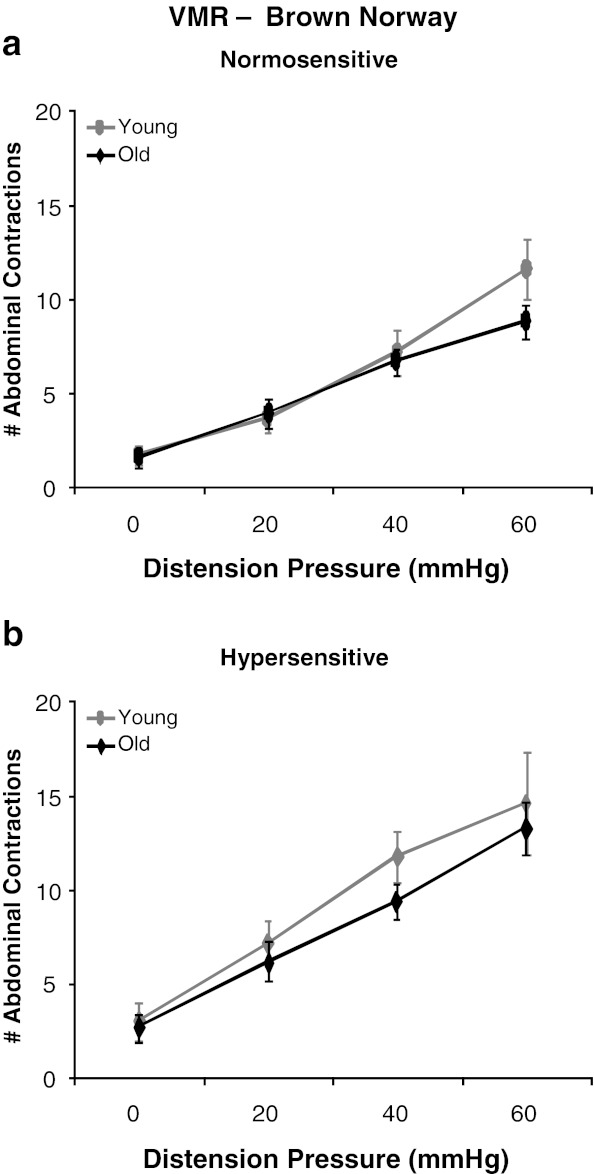
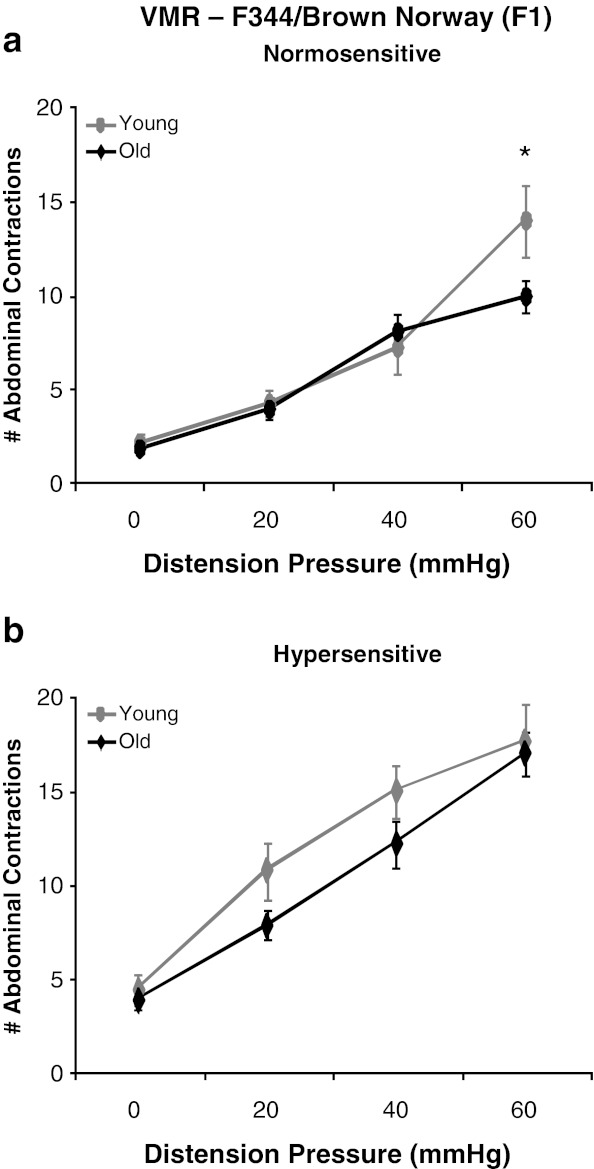
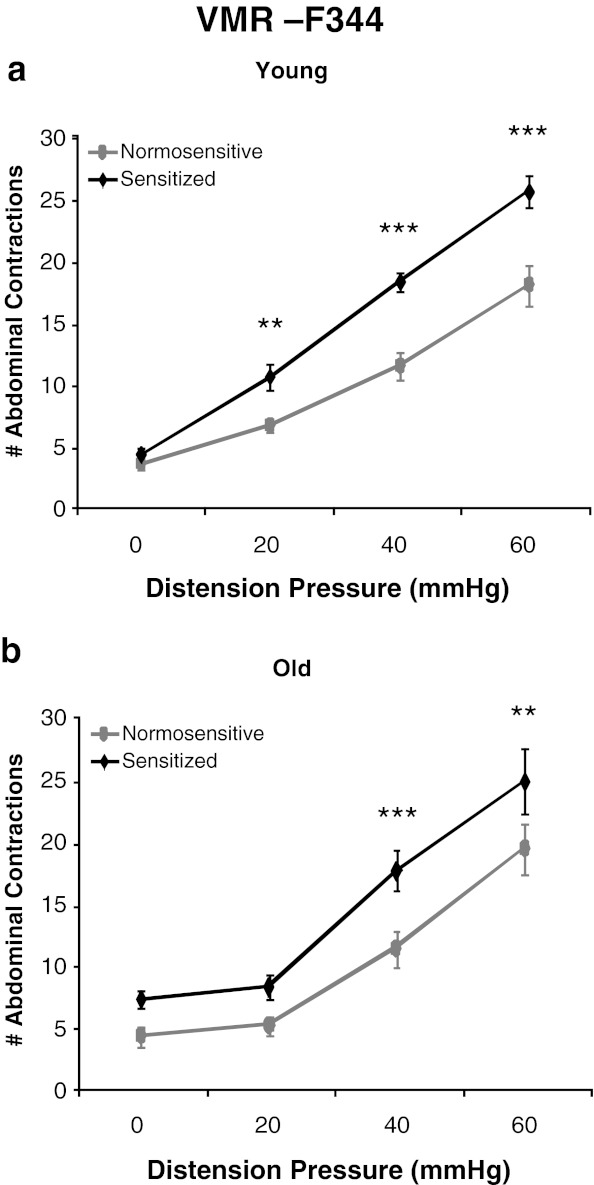
Visceral sensitivity was assessed by measurement of a nociceptive behavioral reflex to luminal distension of the colorectum. A main effect of pressure was observed as a graded increase in the VMR to CRD in both young and old rats [F(3,120) = 208.61, p < 0.0001]. Consistent with previous results, the ANOVA revealed a main effect of colonic sensitization where the VMR to CRD was increased following intracolonic acetic acid administration (sensitized) in both young and old rats of all rodent strains (Figs. 2, 3, and 4) (Langlois et al. 1994). A main effect of strain was also observed [F(2,40) = 32.45, p < 0.0001] where post hoc tests revealed that BN rats are least responsive to CRD, F344 rats exhibited the highest VMR to CRD, and the BN/F344 (F1) strain was an intermediate between BN and F344 animals. Although there was no main effect of aging across all rodent strains, within-strain comparison indicated differential effects of age on visceral hypersensitivity. In the BN strain, there was no main effect of age and both young and old rats exhibit similar levels of visceral sensitivity across all distension pressures at baseline and following intracolonic infusion of acetic acid (Fig. 2a and b). In BN/F344 (F1) rats, there was a significant age × pressure interaction where old animals had a significant reduction in the VMR to CRD at 60 mmHg as compared to young adult rats [F(3,39) = 3.91, p = 0.0156] which suggests that aging decreases visceral sensitivity to high-intensity nociceptive stimuli within this strain (Fig. 3a). However, this decreased visceral sensitivity with age is abolished following direct sensitization of colonic afferents where no difference is observed in the VMR to CRD between young and old BN/F344 (F1) rats across all distension pressures following intracolonic acetic acid (Fig. 3b). Young F344 animals were highly susceptible to the effects of acetic acid on colonic afferent sensitization and show visceral hypersensitivity following intracolonic infusion of acetic acid at all distention pressures (Fig. 4a). However, in the F344 strain, colonic afferents become less vulnerable to acetic acid sensitization at the lowest distension pressure of 20 mmHg where old rats exhibit an increase in the VMR to CRD at only 40 and 60 mmHg following intracolonic acetic acid (Fig. 4b).
Fig. 2.
Effect of age on visceral sensitivity in BN rats. a The VMR to CRD was similar at all distension pressures in both young and old rats with normosensitive colons. b Visceral sensitivity was not affected by age following acute sensitization with dilute acetic acid. p > 0.05 at all distension pressures
Fig. 3.
Effect of age on visceral sensitivity in F344-BN (F1) rats. a Old F344-BN (F1) rats exhibited a reduced VMR to CRD at 60 mmHg as compared to young animals. b Following colonic sensitization, young and old F344-BN (F1) rats had a similar VMR to CRD at all distension pressures. *p < 0.05 vs. old animals at 60 mmHg
Fig. 4.
Effects of primary afferent sensitization in young and old F344 rats. a Young rats have an increased number of abdominal contractions at 20, 40, and 60 mmHg following acetic acid infusion. b An increase in the VMR to CRD is observed at 40 and 60 mmHg following intracolonic acetic acid in old F344 rats
Anxiety-like behavior and maze exploration
Although aging did not appear to affect anxiety-like behavior as measured by percent time in the open arms [F(1,38) = 3.24, p = 0.08], there was a main effect of age on exploration of the elevated plus maze [F(1,38) = 12.14, p < 0.01], observed as a significant decrease in total arm entries in old as compared to young rats Additionally, no differences were noted in number of head dips or end exploration between young and old rats. However, the was a significant strain × age interaction [F(2,27) = 4.57, p < 0.05], explained by an increased number of rears in young vs. old F344 rats (Table 1).
Table 1.
Effect of age on behavior in the elevated plus maze
| % Time in open arms | Total number of arm entries | Number of rears | Number of head dips | Number of end explorations | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Young | Old | Young* | Old | Young | Old | Young | Old | Young | Old | |
| Brown Norway | 15.8 ± 6.0 | 33.7 ± 11.5 | 7.4 ± 1.0 | 5.7 ± 0.6 | 12.5 ± 1.3 | 10.2 ± 0.8 | 8.0 ± 2.9 | 4.4 ± 1.6 | 0.4 ± 0.4 | 0.2 ± 0.2 |
| BN/F344 (F1) | 23.8 ± 8.3 | 14.3 ± 4.2 | 7.3 ± 1.0 | 4.4 ± 1.1 | 14.2 ± 1.1 | 14.3 ± 1.3 | 7.5 ± 2.6 | 7.5 ± 1.7 | 1.0 ± 0.6 | 0.8 ± 0.5 |
| F344 | 13.7 ± 3.2 | 31.8 ± 7.7 | 7.1 ± 1.1 | 3.4 ± 0.6 | 13.3 ± 3.2* | 5.1 ± 1.3 | 10.7 ± 3.2 | 8.3 ± 1.3 | 1.3 ± 0.3 | 1.4 ± 0.6 |
Aging had no significant effect on the percent time spent in the open arms of the EPM. A significant main effect of aging on maze exploration was observed where old rats had a decreased total number of arm entries. Additionally, young F344 rats exhibited increased rearing as compared to old rats of the same strain. Data are represented as the mean ± SEM.
*p < 0.01 young vs. old animals
Discussion
In the current study, age was found to have an effect on mechanosensitive thresholds and visceral pain behavior which varies with rodent strain; whereas exploration of the EPM decreases with age across all strains. Our data suggest that inconsistent results reported previously on the effects of aging on pain likely arise, in part, due to strain differences between studies, suggesting that genetic background can contribute to differences in the effect of age on pain behavior. Furthermore, the data indicate that each strain utilized within these experiments is uniquely suited to investigate the underlying mechanisms of changes in pain perception with age which occur within the clinical population.
Although controversial, the majority of clinical observations have shown an increase in somatic pain thresholds in old patients as compared to young adults (Gibson and Helme 2001; Horch et al. 1992; Meliala et al. 1999; Harkins et al. 1986; Lautenbacher and Strian 1991; Jensen et al. 1992). Specifically, geriatric patients have a higher pain threshold with exposure to sharpened Von Frey filaments or stimulation with a hypodermic needle, which indicates a decrease in mechanosensitive nociceptive thresholds with age (Horch et al. 1992; Meliala et al. 1999). This phenomenon is also observed in the present study where old BN rats exhibit an increased threshold to elicit nociceptive withdrawal of the hindpaw in response to mechanical stimulation, indicating a decrease in tactile sensitivity. Therefore, the finding that mechanosensitive thresholds were increased with age in BN rats suggests that the BN strain represents an appropriate model for studying the mechanisms responsible for elevated mechanical sensory thresholds, which has been observed within a specific aged cohort in the clinical setting. Previous reports indicate that somatic pain assessments may be confounded by age-related deficits in complex behavioral endpoints, such as reduced hindpaw licking following the hot plate test (Berry et al. 2007). However, these age-related behavioral deficits are unlikely to be a confounding factor in the current study as the Von Frey method relies on simple, reflexive behavior, which has been shown to remain intact with age (Gagliese and Melzack 2000).
Although mechanosensitive thresholds were altered with age in the BN rat strain, the BN/F344 (F1) strain did not exhibit age-dependent changes in tactile mechanical sensitivity. However, we observed a decrease in the number of abdominal muscle contractions in response to luminal distension of the colon at the high-intensity distention pressure of 60 mmHg in aged BN/F344 (F1) rats. These results suggest that aged BN/F344 (F1) rats have reduced sensitivity to high-intensity, nociceptive visceral stimuli. Similar results have also been observed in Sprague Dawley rats where decreased nociceptive sensitivity is only observed in aged rats with the application of a high-intensity stimulus (Goettl et al. 2000). Furthermore, these animal data compliment a clinical study where older adults rate a noxious stimulus as less intense than their younger counterparts (Gibson et al. 1991). Additional clinical studies have reported that elderly patients have a higher threshold to first painful sensation in response to rectal and esophageal distension (Lasch et al. 1997; Lagier et al. 1999). Therefore, BN/F344 rats may represent a model for investigating the mechanisms responsible for decreased sensitivity to visceral nociceptive stimuli with age, which has been observed in the elderly population. Our data show that the decreased visceral sensitivity to high-intensity stimuli with age is lost following colonic infusion of dilute acetic acid. Intracolonic acetic acid has been shown to induce visceral hypersensitivity within 1 h and may involve a subset of neurons called “silent nociceptors” which are polymodal nerves that are normally quiescent but can be activated by chemical irritants (Sengupta and Gebhart 1994; Robinson and Gebhart 2008; Langlois et al. 1994). These silent nociceptors may activate in response to acetic acid stimulation and compensate for the decreased visceral sensitivity to nociceptive distension observed in the non-sensitized, aged cohort.
In the F344 strain, we observed that, while young rats exhibited visceral hypersensitivity at all distension pressures, colonic sensitization was not effective at inducing visceral hypersensitivity at the 20-mmHg distension in old rats, which has been shown to be a non-noxious distension pressure (Ness and Gebhart 1988). These data indicate that visceral afferents of aged rats are less vulnerable to the effects of acetic acid sensitization which suggests that F344 rats have decreased sensitivity to a visceral insult with age. Our data have a high degree of clinical relevance given that the physiological role of pain is to alert the central nervous system to insults which may result in tissue damage, and a decreased awareness of visceral injury with age have severe health-related consequences resulting from a delayed diagnosis of specific disease states (Robinson and Gebhart 2008; Moore and Clinch 2004; Tresch 1998; Liston et al. 1994; Hilton et al. 2001). Indeed, our results are consistent with clinical literature where geriatric patients with organic disorders such as peptic ulcer disease may not present with abdominal pain until the occurrence of severe gastric damage, resulting in a poor prognosis and increased mortality from diseases-related complications (Vreeburg et al. 1997). Therefore, these experiments suggest that the F344 strain represents an appropriate animal model for investigating the underlying mechanisms responsible for diminished perception of visceral injury with age. Future translational research in the F344 strain may allow for significant prevention of disease-related complications in elderly patients.
Clinical researchers have shown that anxiety-related disorders are significantly more prominent in elderly and heightened anxiety often develops as elderly individuals become aware of the cognitive and physical decline associated with age (Himmelfarb and Murrell 1984; Jorm et al. 1997; Preville et al. 2002; Sinoff and Werner 2003; Jonas et al. 1997). Additionally, a recent study has shown that aging is associated with increased variability in the HPA response to stress in BN/F344 and F344 rats (Segar et al. 2009). Therefore, changes in anxiety-like behavior may be expected to occur with age in these rodent strains. In the current study, the elevated plus maze was utilized to assess anxiety-like behavior which is quantified as the amount of time spent in the open vs. the closed arms and requires that each group exhibit similar maze exploration for accurate data interpretation (Hogg 1996). However, our findings indicate that there is a decrease in maze exploration quantified as a reduced number of total arm entries in aged animals and reduced rearing, which is significant in old F344 rats. These data are consistent with previous results found in 129 Sv/Ev mice, where older mice were less exploratory than young animals (Berry et al. 2007). In the current study, having shown that young and old animals do not participate in the plus maze assay to the same degree, the time spent in open arms cannot be used to accurately compare anxiety-like behavior. Furthermore, the use of alternate assays (i.e., light–dark emergence test and acoustic startle) to compare anxiety-like behavior between young and old rats may also be problematic due to age-related sensory degradation including a decline in visual acuity and hearing (Katz and Robison 1986; Gratton et al. 2008; Syka 2010; Caspary et al. 1999). Such age-related confounding factors may be overcome by additional behavioral tests to assess locomotive activity and/or visual acuity.
A previous study reported that survivorship curves vary with rodent strain (Turturro et al. 1999). Therefore, a potential confounding factor in interpreting the current data is that the old group of animals was defined according to a chronological age of 26 months rather than based upon the survival curves of the respective strains. In the current study, the ages of the rats were selected in an effort to remain consistent with previous literature on the effects of age on pain perception. In such studies, 3–6 months of age is commonly used to represent adulthood and “aged” cohorts typically range from 24 to 28 months, regardless of strain (Gagliese and Melzack 2000; Novak et al. 1999; Crosby et al. 2006; Crisp et al. 1994a, b; Akunne and Soliman 1994; Knisely and Hamm 1989).
In summary, given the recent substantial increase in the proportion of elderly in the population and the large influence of pain on the quality of life in older patients, the study of altered visceral and somatic pain perception with age is increasingly important. Translational research using rodent models will help identify specific mechanisms responsible for altered pain perception in healthy aging adults. Our study is the first to investigate the influence of age on pain behavior and anxiety in three separate rodent models and highlights the importance of rodent genetic background on nociception while reducing inter-laboratory differences. These results highlight the importance of strain selection in conducting research on the effects of age on pain with rodent models. The important finding of these strain-specific effects of aging may aid in the investigation of specific mechanisms involved in altered pain perception with age and potentially lead to the development of novel therapeutic targets for treatment of pain specifically in the elderly.
Acknowledgments
We would like to thank William Sonntag, Ph.D. for his thoughts and comments on the final drafts of the manuscript and Anthony Johnson, M.S. and Michael Anderson, Ph.D. for their input on statistical analysis. This work was supported by the Reynolds Oklahoma Center on Aging and a Department of Veterans of Affairs Merit Grant to BGVM.
References
- Akunne HC, Soliman KF. Serotonin modulation of pain responsiveness in the aged rat. Pharmacol Biochem Behav. 1994;48(2):411–416. doi: 10.1016/0091-3057(94)90545-2. [DOI] [PubMed] [Google Scholar]
- Bennett G, Talley NJ. Irritable bowel syndrome in the elderly. Best Pract Res Clin Gastroenterol. 2002;16(1):63–76. doi: 10.1053/bega.2001.0266. [DOI] [PubMed] [Google Scholar]
- Berry A, Capone F, Giorgio M, Pelicci PG, de Kloet ER, Alleva E, Minghetti L, Cirulli F. Deletion of the life span determinant p66Shc prevents age-dependent increases in emotionality and pain sensitivity in mice. Exp Gerontol. 2007;42:37–45. doi: 10.1016/j.exger.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age-related changes in GABA(A) receptor subunit composition and function in rat auditory system. Neuroscience. 1999;93(1):307–312. doi: 10.1016/S0306-4522(99)00121-9. [DOI] [PubMed] [Google Scholar]
- Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, Ameen VZ, Mayer EA. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21(2):149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp T, Stafinsky JL, Hoskins DL, Dayal B, Chinrock KM, Uram M. Effects of aging on spinal opioid-induced antinociception. Neurobiol Aging. 1994;15(2):169–174. doi: 10.1016/0197-4580(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Crisp T, Stafinsky JL, Hoskins DL, Perni VC, Uram M, Gordon TL. Age-related changes in the spinal antinociceptive effects of DAGO, DPDPE and beta-endorphin in the rat. Brain Res. 1994;643(1–2):282–286. doi: 10.1016/0006-8993(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Crosby SJ, Knapp CM, Kornetsky C. Nociceptive threshold and analgesic response to morphine in aged and young adult rats as determined by thermal radiation and intracerebral electrical stimulation. Pharmacol Biochem Behav. 2006;84(1):148–157. doi: 10.1016/j.pbb.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Quigley EM, Ahmed SM, Scully P, O'Brien S, O'Mahony L, O'Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130(2):304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB. Age-associated differences in responses to noxious stimuli. J Gerontol A Biol Sci Med Sci. 2001;56(3):M180–M185. doi: 10.1093/gerona/56.3.M180. [DOI] [PubMed] [Google Scholar]
- Ehrenpreis ED. Irritable bowel syndrome: 10% to 20% of older adults have symptoms consistent with diagnosis. Gastroenterology. 2005;60(1):25–28. [PubMed] [Google Scholar]
- Friedel D, Krevsky B. Irritable bowel syndrome in the elderly. Clin Geriatr. 2000;8(9):36–47. [Google Scholar]
- Gagliese L, Melzack R. Age differences in nociception and pain behaviours in the rat. Neurosci Biobehav Rev. 2000;24(8):843–854. doi: 10.1016/S0149-7634(00)00041-5. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Helme RD. Age-related differences in pain perception and report. Clin Geriatr Med. 2001;17(3):433–456. doi: 10.1016/S0749-0690(05)70079-3. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Gorman MM, Helme RD (1991) Assessment of pain in the elderly using event related cerebral potentials. In: Bond MR, Charlton JE, Woolf CJ (eds) Proceedings of the VIth World Congress on Pain. Elsevier, Amsterdam, p. 527
- Goettl VM, Lindsey AE, Neff NH, Hadjiconstantinou M. GM1 ganglioside restores abnormal responses to acute thermal and mechanical stimuli in aged rats. Brain Res. 2000;858(2):380–385. doi: 10.1016/S0006-8993(00)01960-0. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Bateman K, Cannuscio JF, Saunders JC. Outer- and middle-ear contributions to presbycusis in the Brown Norway rat. Audiol Neurootol. 2008;13(1):37–52. doi: 10.1159/000107551. [DOI] [PubMed] [Google Scholar]
- Harkins SW, Price DD, Martelli M. Effects of age on pain perception—thermonociception. J Gerontol. 1986;41(1):58–63. doi: 10.1093/geronj/41.1.58. [DOI] [PubMed] [Google Scholar]
- Hilton D, Iman N, Burke GJ, Moore A, O'Mara G, Signorini D, Lyons D, Banerjee AK, Clinch D. Absence of abdominal pain in older persons with endoscopic ulcers: a prospective study. Am J Gastroenterol. 2001;96(2):380–384. doi: 10.1111/j.1572-0241.2001.03455.x. [DOI] [PubMed] [Google Scholar]
- Himmelfarb S, Murrell SA. The prevalence and correlates of anxiety symptoms in older adults. J Psychol. 1984;116(2d Half):159–167. doi: 10.1080/00223980.1984.9923632. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54(1):21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Horch K, Hardy M, Jimenez S, Jabaley M. An automated tactile tester for evaluation of cutaneous sensibility. J Hand Surg Am. 1992;17(5):829–837. doi: 10.1016/0363-5023(92)90452-U. [DOI] [PubMed] [Google Scholar]
- Jensen R, Rasmussen BK, Pedersen B, Lous I, Olesen J. Cephalic muscle tenderness and pressure pain threshold in a general population. Pain. 1992;48(2):197–203. doi: 10.1016/0304-3959(92)90059-K. [DOI] [PubMed] [Google Scholar]
- Jonas BS, Franks P, Ingram DD. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Fam Med. 1997;6(1):43–49. doi: 10.1001/archfami.6.1.43. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Korten AE, Henderson AS, Jacomb PA, Mackinnon A. Do cognitive complaints either predict future cognitive decline or reflect past cognitive decline? A longitudinal study of an elderly community sample. Psychol Med. 1997;27(1):91–98. doi: 10.1017/S0033291796003923. [DOI] [PubMed] [Google Scholar]
- Katz ML, Robison WG., Jr Evidence of cell loss from the rat retina during senescence. Exp Eye Res. 1986;42(4):293–304. doi: 10.1016/0014-4835(86)90022-9. [DOI] [PubMed] [Google Scholar]
- Knisely JS, Hamm RJ. Physostigmine-induced analgesia in young, middle-aged, and senescent rats. Exp Aging Res. 1989;15(1–2):3–11. doi: 10.1080/03610738908259752. [DOI] [PubMed] [Google Scholar]
- Lagier E, Delvaux M, Vellas B, Fioramonti J, Bueno L, Albarede JL, Frexinos J. Influence of age on rectal tone and sensitivity to distension in healthy subjects. Neurogastroenterol Motil. 1999;11(2):101–107. doi: 10.1046/j.1365-2982.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- Langlois A, Diop L, Riviere PJ, Pascaud X, Junien JL. Effect of fedotozine on the cardiovascular pain reflex induced by distension of the irritated colon in the anesthetized rat. Eur J Pharmacol. 1994;271(2–3):245–251. doi: 10.1016/0014-2999(94)90780-3. [DOI] [PubMed] [Google Scholar]
- Lasch H, Castell DO, Castell JA. Evidence for diminished visceral pain with aging: studies using graded intraesophageal balloon distension. Am J Physiol. 1997;272(1 Pt 1):G1–G3. doi: 10.1152/ajpgi.1997.272.1.G1. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Strian F. Similarities in age differences in heat pain perception and thermal sensitivity. Funct Neurol. 1991;6(2):129–135. [PubMed] [Google Scholar]
- Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115(3):410–418. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Liston R, McLoughlin R, Clinch D. Acute pneumothorax: a comparison of elderly with younger patients. Age Ageing. 1994;23(5):393–395. doi: 10.1093/ageing/23.5.393. [DOI] [PubMed] [Google Scholar]
- Meliala A, Gibson SJ, Helme RD. The effect of stimulation site on detection and pain thresholds in young and older adults. Abstracts: IXth World Congress on Pain. Seattle: IASP Press; 1999. p. 559. [Google Scholar]
- Moore AR, Clinch D. Underlying mechanisms of impaired visceral pain perception in older people. J Am Geriatr Soc. 2004;52(1):132–136. doi: 10.1111/j.1532-5415.2004.52023.x. [DOI] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B. Corticosteroid receptor-mediated mechanisms in the amygdala regulate anxiety and colonic sensitivity. Am J Physiol Gastrointest Liver Physiol. 2007;292(6):G1622–G1629. doi: 10.1152/ajpgi.00080.2007. [DOI] [PubMed] [Google Scholar]
- Myers B, Dittmeyer K, Greenwood-Van Meerveld B. Involvement of amygdaloid corticosterone in altered visceral and somatic sensation. Behav Brain Res. 2007;181(1):163–167. doi: 10.1016/j.bbr.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450(1–2):153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Novak JC, Lovell JA, Stuesse SL, Cruce WL, McBurney DL, Crisp T. Aging and neuropathic pain. Brain Res. 1999;833(2):308–310. doi: 10.1016/S0006-8993(99)01522-X. [DOI] [PubMed] [Google Scholar]
- Preville M, Hebert R, Bravo G, Boyer R. Predisposing and facilitating factors of severe psychological distress among frail elderly adults. Can J Aging. 2002;21(2):195–204. doi: 10.1017/S071498080000146X. [DOI] [Google Scholar]
- Robinson DR, Gebhart GF. Inside information: the unique features of visceral sensation. Mol Interv. 2008;8(5):242–253. doi: 10.1124/mi.8.5.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrable H, Burns-Cusato M, Medrano S. Anxiety and the aging brain: stressed out over p53? Biochim Biophys Acta. 2009;1790(12):1587–1591. doi: 10.1016/j.bbagen.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segar TM, Kaxkow JW, Welge JA, Herman JP. Heterogeneity of neuroendocrine stress responses in aging rat strains. Physiol Behav. 2009;96:6–11. doi: 10.1016/j.physbeh.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol. 1994;71(6):2046–2060. doi: 10.1152/jn.1994.71.6.2046. [DOI] [PubMed] [Google Scholar]
- Sinoff G, Werner P. Anxiety disorder and accompanying subjective memory loss in the elderly as a predictor of future cognitive decline. Int J Geriatr Psychiatry. 2003;18(10):951–959. doi: 10.1002/gps.1004. [DOI] [PubMed] [Google Scholar]
- Syka J. The Fischer 344 rat as a model of prebycusis. Hear Res. 2010;264(1–2):70–78. doi: 10.1016/j.heares.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Talley NJ, Zinsmeister AR, Van Dyke C, Melton LJ., 3rd Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991;101(4):927–934. doi: 10.1016/0016-5085(91)90717-y. [DOI] [PubMed] [Google Scholar]
- Tresch DD. Management of the older patient with acute myocardial infarction: difference in clinical presentations between older and younger patients. J Am Geriatr Soc. 1998;46(9):1157–1162. [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–B501. doi: 10.1093/gerona/54.11.B492. [DOI] [PubMed] [Google Scholar]
- Tyler K, Moriceau S, Sullivan RM, Greenwood-van Meerveld B. Long-term colonic hypersensitivity in adult rats induced by neonatal unpredictable vs predictable shock. Neurogastroenterol Motil. 2007;19(9):761–768. doi: 10.1111/j.1365-2982.2007.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeburg EM, Snel P, de Bruijne JW, Bartelsman JF, Rauws EA, Tytgat GN. Acute upper gastrointestinal bleeding in the Amsterdam area: incidence, diagnosis, and clinical outcome. Am J Gastroenterol. 1997;92(2):236–243. [PubMed] [Google Scholar]
- Whitehead WE, Holtkotter B, Enck P, Hoelzl R, Holmes KD, Anthony J, Shabsin HS, Schuster MM. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98(5 Pt 1):1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122(4):1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]