Abstract
Aging and physiological androgen decay leads to structural changes in corpus cavernosum (CC) that associate with erectile function impairment. There is evidence that such changes relate to nitric oxide (NO) bioavailability, an endothelial compound produced by the action of endothelial NO synthase (eNOS), and is regulated by sirtuin-1 (Sirt1), a NAD+-dependent protein deacetylase. Taking into account the reduced NO synthesis observed in aging and erectile dysfunction, we aimed to characterize human CC of androgen-deprived, young, and aged individuals postulating that androgen deprivation induces modifications similar to those observed in aging. Human penile fragments were collected from young individuals submitted to male-to-female sex reassignment procedure, who undergone an androgen deprivation chemical regimen, from young organ donors and from aged patients submitted to penile deviation surgery. They were processed for histomorphometric analysis of smooth muscle (SM) and connective tissues (CT), and dual-immunofluorescence of alpha-actin/vWf or Sirt1, and endothelin-1/eNOS. Estrogen receptors were analyzed by immunohistochemistry and semiquantification of Sirt1, eNOS, and phospho-Akt was assayed by Western blotting. Androgen withdrawal, similarly to aging, leads to a noteworthy reduction of SM-to-CT ratio in CC. However, in contrast to young and aged, a significant increase in penile Sirt1 expression accompanied by an increase in total eNOS expression was observed in androgen-depleted individuals. No changes were evidenced in phospho-Akt system and estrogen receptors were undetectable. These findings indicate that Sirt1 regulates the expression of eNOS in human CC employing mechanisms influenced by androgen depletion.
Keywords: Hypogonadism, Human cavernous tissue, Endothelial dysfunction, Sirtuin-1, Endothelial nitric oxide synthase (eNOS)
Introduction
Androgens play an important role in the development of male secondary sexual characteristics, and their deficiency may result in structural abnormalities of the penis (Mills et al. 1996; Shabsigh 1997). In addition, androgen depletion after surgical or pharmacological castration of the adult male generally results in loss of libido and decline in erectile function (Shabsigh 1997). Despite these evidences, the mechanism by which androgens modify erectile function is still controversial.
During erection, the penis acts as a capacitor, accumulating blood under pressure by a veno-occlusive mechanism. Briefly, dilation of the resistance arterial bed of the penis favors blood flow and entrapment in the corpus cavernosum (CC), a combined effect of expansion of the lacunar spaces by relaxation of the trabecular smooth muscle (SM) and compression of the draining venules. The efficiency of this process strongly depends on the integrity of the trabecular SM as corpora expansion is related directly to its content and inversely to that of connective tissue (CT) (Luo et al. 2007; Nehra et al. 1996; Wespes et al. 1991). In fact, cavernous SM cell loss or functional impairment is the etiological basis of erectile dysfunction (ED) in many patients, since muscular tonus regulates the stages of erection, which cannot be achieved by vascular mechanisms alone (Wespes 2002). Any condition that results in structural changes in the cavernous tissue, such as aging or androgen depletion, is a potential contributor to ED.
Clinical and animal studies provided evidence that androgens protect cavernous SM, promoting cell proliferation and maintaining functional integrity (Andersson and Wagner 1995; Aversa et al. 2000, 2003; Garban et al. 1995; Huang et al. 2008; Lue and Dahiya 1997; Mills et al. 1992; Shabsigh 1997). It was also demonstrated that androgen deficiency contributes to endothelial damage in a castrated rat model (Lu et al. 2007). Furthermore, investigations showed that testosterone and dihydrotestosterone upregulate nitric oxide synthase (NOS) expression and activity in male genital tissues, particularly in cavernous tissue (Lugg et al. 1995; Marin et al. 1999; Penson et al. 1996; Zvara et al. 1995). The NOS/cyclic guanosine monophosphate pathway is critical for erectile function, and two constitutive isoenzymes, endothelial and neuronal NOS (eNOS and nNOS, respectively), are thought to play different roles in nitric oxide (NO) production during erection. NO derived from nNOS of the nerves supplying the penis initiates the erectile response, whereas its sustained production from eNOS is responsible for full erection and its maintenance (Musicki et al. 2009). Thus, most of the NO that mediates relaxation of the cavernous tissue SM results from eNOS activity (Traish 2009), emphasizing the role of the endothelium in the erection process.
Beyond androgen deprivation due to physiological decline in serum testosterone, aging itself alters vascular function (Yassin and Saad 2008), being considered the most important predisposing factor for ED (Ghalayini et al. 2010). The underlying cellular and molecular mechanisms that associate with endothelial dysfunction have not yet been elucidated, but increased breakdown of NO due to an augmented production of superoxide anions and changes in expression and/or activity of eNOS could favor this condition. The contribution of NO pathway for vascular aging is far from being consensual, as it was reported a reduction on eNOS mRNA levels (Challah et al. 1997) and contrariwise, an increase of eNOS enzyme expression combined with reduced activity (Cernadas et al. 1998) in aged animals. Nevertheless, eNOS could be overactivated, mainly through Akt-mediated phosphorylation (Lovren et al. 2009), as a compensatory mechanism to counterbalance endothelial dysfunction induced by age-dependent oxidative stress (van der Loo et al. 2000).
Vascular aging associates with transcriptional changes in gene expression (Zahn et al. 2007) that in part are regulated by sirtuin-1 (Sirt1) (Wood et al. 2004). Sirt1 belongs to the family of the mammalian homologues of the Sir2 (silent information regulator-2) of the yeast Saccharomyces cerevisiae. This is a NAD+-dependent protein deacetylase, present in cell cytoplasm and nucleus, that modulates the cell cycle, senescence, apoptosis, and metabolism by distinct mechanisms that include regulation of expression of individual genes (Picard et al. 2004), condensation of chromatin (Vaquero et al. 2007), or deacetylation of specific transcription factors (Brunet et al. 2004). Milne and Denu (2008) described protective action of Sirt1 in aged endothelium, heart, liver, and adipose tissue, while others reported pro-aging roles of sirtuins (Longo 2009), which may be due to specific time and tissue properties of Sirt1 activity. However, several evidences suggest that Sirt1 acts as a longevity factor in vascular tissue, particularly because of its critical role in endothelial homeostasis by regulating eNOS activity (Ota et al. 2010).
Sirt1 promotes endothelial-dependent vasodilatation by targeting eNOS for deacetylation, leading to an enhanced NO production. Likewise, blocking Sirt1 function results in decreased NO bioavailability and inhibited endothelium-dependent vasorelaxation (Mattagajasingh et al. 2007). Interestingly, eNOS has also been implicated in the regulation of the expression of Sirt1 (Nisoli et al. 2005), and NO itself could act as an inducer of Sirt1 activity in endothelium (Ota et al. 2010).
In the present study, we hypothesize that androgen deprivation induces modifications in the CC comparable to those observed in physiological aging and strongly contribute to ED onset. Hence, emphasizing structural features and Sirt1–eNOS axis regulation, we aim to characterize human cavernous tissue in young androgen-deprived, young, and healthy aged individuals.
Materials and methods
Tissue collection and processing
Penile fragments removed from the CC of Caucasian individuals submitted to male-to-female sex reassignment surgery (n = 3, 19–28 years), previously treated with 200 mg/day spironolactone and 50 mg/day cyproterone acetate, for 24 months, followed by 4 mg/day estradiol for 12 months, were included in the group of young androgen deprived. The young control CC fragments were removed from healthy potential organ donors (n = 6, 17–34 years), dissected simultaneously with the organ harvesting for transplant program. The cause of death was cranioencephalic trauma and all individuals were presumed to be potent considering the absence of ED risk factors. The healthy-aged CC samples were excised from the opposite side of Peyronie’s plaque of patients submitted to penile corporoplasty with Yachia technique (n = 7, 61–74 years), after preoperative detailed clinical examination, which included International Index of Erectile Function-5 scoring and penile Doppler ultrasound. No ED or risk factors for ED were found in any patient. The study design was authorized and approved by the local hospital and university ethics committees.
Each cavernous tissue sample was divided in two fragments: one was immediately stored at −80°C for molecular analysis, and the other was fixed in 10% buffered formaldehyde and paraffin embedded for Masson’s trichrome staining and immunohistochemical studies.
Histomorphometric evaluation
Tissue sections 4–6 μm thick were cut with a microtome (RM2145, Leica Microsystems GmbH, Germany) and placed in 0.1% poly-l-lysine-covered microscopy slides for Masson’s trichrome staining by laboratory routine method. All the slides stained were observed and the images were captured in an optical microscope connected to a digital camera (Carl Zeiss MicroImaging GmbH, Göttingen, Germany). Then, the images were subjected to computer-assisted color histomorphometric analysis employing ImageJ® software (NIH, Maryland, USA). This allowed assessing the proportion of the trabecular SM and CT content to total erectile tissue area by pixel classification (red for SM and blue for CT). Areas belonging to tunica albuginea were excluded from the analysis. The ratio of SM to CT areas was also calculated in 20 fields per slide (two random slides per case, in a total of three to four cases per group).
Immunohistochemistry
The immunohistochemical study of estrogen receptors (ER) in human cavernous tissue sections was performed on a Ventana automated slide stainer (Ventana Medical System®, Tucson, Arizona, USA), with adequate detection kits and ancillary reagents. Rabbit monoclonal primary antibody anti-ER (SP1) (Ventana Medical System®) has been optimally diluted for use with Ventana iVIEW DAB Detection kit. The negative control consisted of non-immune mouse immunoglobulin G instead of the primary ER antibody. All the slides were observed and the images were captured in an optical microscope connected to a digital camera (Carl Zeiss MicroImaging GmbH).
Immunofluorescence
Immunofluorescence (IF) detection of von Willebrand factor (vWf)/alpha-actin, Sirt1/alpha-actin, and ET1/eNOS were performed. Briefly, sections were deparaffinized, rehydrated, exposed to 1 M HCl for epitope retrieval, and neutralized with 0.1 M borax, followed by incubation for 1 h with blocking solution (1% bovine serum albumin in phosphate-buffered saline). The sections were incubated overnight at 4°C with a mixture of primary antibodies: mouse anti-alpha-actin (Chemicon International, Temecula, CA, USA) with rabbit anti-vWf (Chemicon International) or with rabbit anti-Sirt1 (Santa Cruz Biotechnology, CA, USA) and goat anti-ET1 (Santa Cruz Biotechnology) with rabbit anti-eNOS (Santa Cruz Biotechnology). Negative controls were performed by primary antibody omission. After washing twice with PBS, the sections were incubated at room temperature, with a suitable mix of secondary antibodies, anti-mouse conjugated with AlexaTM 568 (red) and anti-rabbit conjugated with AlexaTM 488 (green), or anti-goat conjugated with AlexaTM 568 (red), and anti-mouse or anti-rabbit conjugated with AlexaTM 488 (green) (Molecular Probes, Leiden, Netherlands). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue) (Molecular Probes). Sections were mounted in buffered solution of glycerol and observed in an Apotome fluorescence microscope (Imager.Z1, Carl Zeiss MicroImaging GmbH, Göttingen, Germany). Images were acquired with AxionVision® software (Carl Zeiss MicroImaging GmbH).
Western blotting
For protein analysis, CC fragments were mechanically homogenized in 50 mM Tris–HCl pH 7.2, 0.1 M NaCl, 5 mM EDTA, and 0.5% TritonX-100 and supplemented with 2% Protease Inhibitor Cocktail P8340 (Sigma-Aldrich Co, Dorset, UK) and 0.2% Phosphatase Inhibitor Cocktails 1 and 2 (P2850 and P5726, respectively; Sigma-Aldrich Co). After total protein level determination by Bradford protein assay (BioRad Laboratories, CA, USA), 40 μg/lane from each sample was loaded on 8% and 12% sodium dodecylsulfate-polyacrylamide gel, separated using the Laemmli (1970) discontinuous buffer system (SDS-PAGE), and transferred to nitrocellulose membrane with 0.45-μm pore (BioRad Laboratories) for 2 h. After incubation with a blocking solution (5% non-fat milk Molico® in 0.1% Tween-20 Tris buffer saline) for 1 h, the membranes were immunoreacted with the following primary antibodies: rabbit anti-Sirt1 (Santa Cruz Biotechnology), rabbit anti-eNOS (Santa Cruz Biotechnology), rabbit anti-phospho-Akt (Ser-473) (Cell Signaling Technology, MA, USA), and rabbit anti-Akt (Cell Signaling Technology), diluted in 5% bovine serum albumin 0.1% Tween-20 Tris buffer saline, overnight at 4°C with shaking. After extensive washing and incubation with appropriated secondary antibody coupled to horseradish peroxidase, labeled bands were evidenced using chemiluminescent substrate (SuperSignal West Pico Chemiluminescent Substrate, Pierce Biotechnology, IL, USA). Membranes used for phospho-Akt detection were then stripped and probed with antibody for the index protein, rabbit anti-Akt. Normalization of the total levels of Sirt1, eNOS, and Akt was performed using β-actin as loading control (rabbit anti-β-actin, Abcam, Cambridge, UK). Phosphorylated Akt levels were normalized with total levels of Akt protein. Each experiment was repeated three times and performed in all samples of each group. Results were quantified by densitometry using the ScionImage® software (Scion Corporation, NIH, MN, USA) and represent the mean value of each protein in human androgen-deprived and aged groups, indicated in percentage, relative to young controls, considered 100%.
Statistical analysis
Data obtained from histomorphometric evaluation and semiquantification of proteins were statistically analyzed using one-way analysis of variance, followed by Bonferroni multiple comparison post hoc test or by t test when appropriate. Statistical analysis was performed using GraphPad Prism® software. Probability values less than 5% (P < 0.05) were considered significant.
Results
Histomorphometric study of human cavernous tissue
According to previous reports (Tomada et al. 2010a, b), cavernous tissue from all studied samples presented a sponge-like texture, exhibiting a mesh of interconnected cavernous spaces, lined by endothelium and separated by trabeculae composed mainly of SM fibers and sparse CT (constituted mainly by collagen fibers and fibroblasts). No adipocytes were observed neither in androgen-deprived nor in aged individuals.
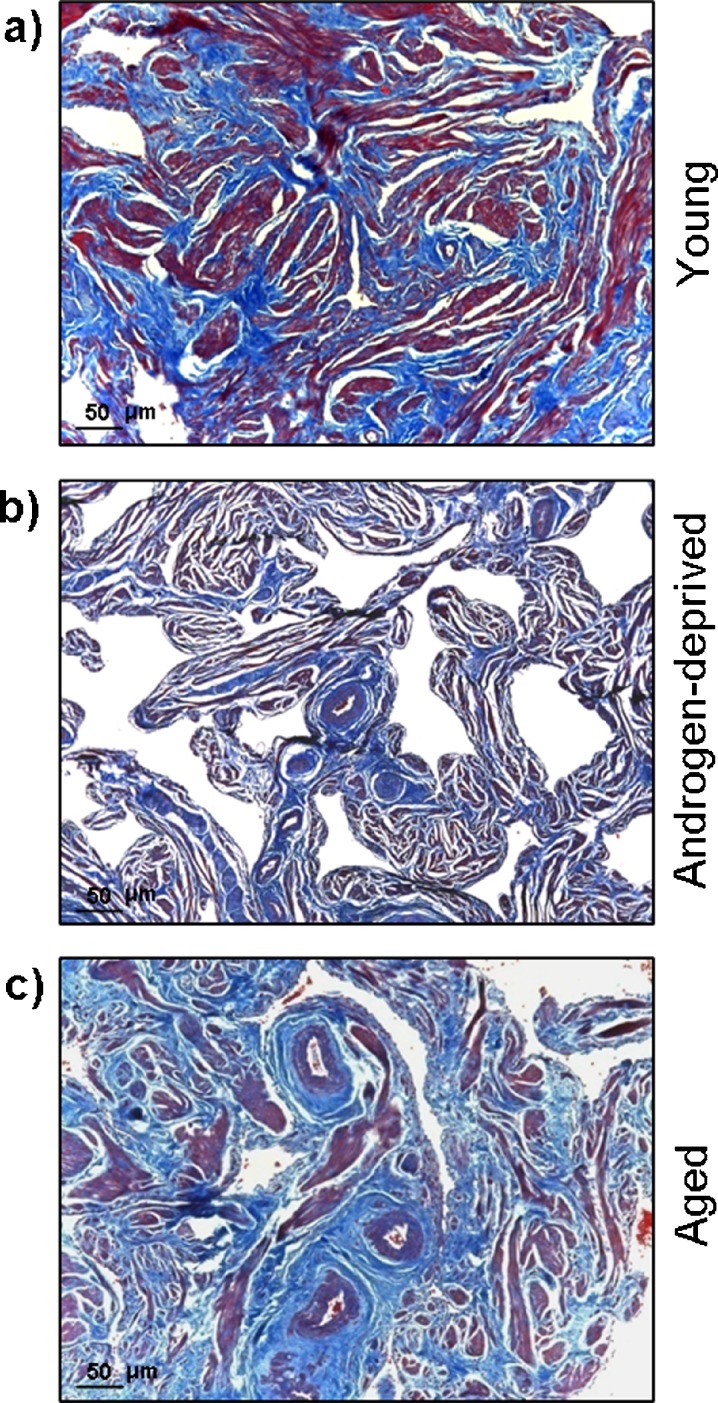
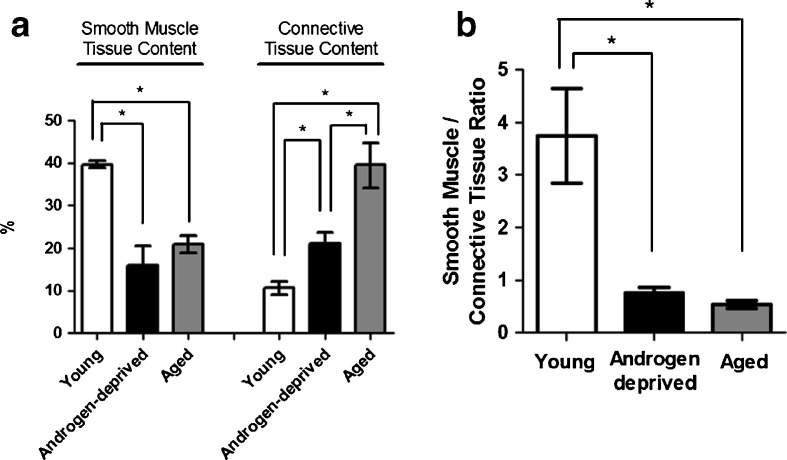
Masson’s trichrome staining (Fig. 1a–c) showed considerable differences in SM and CT contents (stained red and blue, respectively) in androgen-deprived cavernous tissue compared with young and aged, which was confirmed by quantitative computer-assisted histomorphometric analysis (Fig. 2a). In fact, both aging and androgen withdrawal led to a significant decrease in trabecular SM content compared with young cavernous tissue (39.7 ± 0.9% to 20.9 ± 2.0%, P = 0.000, and to 15.9 ± 4.7%, P = 0.002, for aged and androgen-deprived tissue, respectively), although no significant differences were detected in SM content between androgen deprived and aged CC (P = 0.108). Furthermore, CT percentage doubled in androgen deprived (21.2 ± 2.4%, P = 0.006) and near quadruplicated in aged individuals (39.5 ± 5.3%, P = 0.001) compared with young controls (10.6 ± 1.6%); in contrast, androgen deprived presented just about half of the aged CT content (P = 0.003). The SM-to-CT ratio was also calculated (Fig. 2b) and, as depicted within the graph, compared with young controls (3.75 ± 0.9); this ratio was significantly reduced both in androgen-deprived (0.75 ± 0.12, P = 0.000) and in aged cavernous tissue (0.53 ± 0.07, P = 0.000). Note that no differences were detected between androgen-deprived and aged CC (P = 0.625).
Fig. 1.
Masson’s trichrome staining of young (a), androgen-deprived (b), and aged (c) human cavernous tissue. Smooth muscle and connective tissues are stained in red and in blue, respectively
Fig. 2.
a Quantitative changes in smooth muscle and connective tissue contents, determined by computer-assisted histomorphometry, of young, androgen-deprived, and aged samples (n = 3–4 for each group). Significant differences between groups (P < 0.05) (asterisk). b Smooth muscle/connective tissue ratio, determined after computer-assisted histomorphometry of young, androgen-deprived, and aged samples (n = 3–4 for each group). Significant differences between groups (P < 0.05) (asterisk)
Estrogen receptors detection in human cavernous tissue
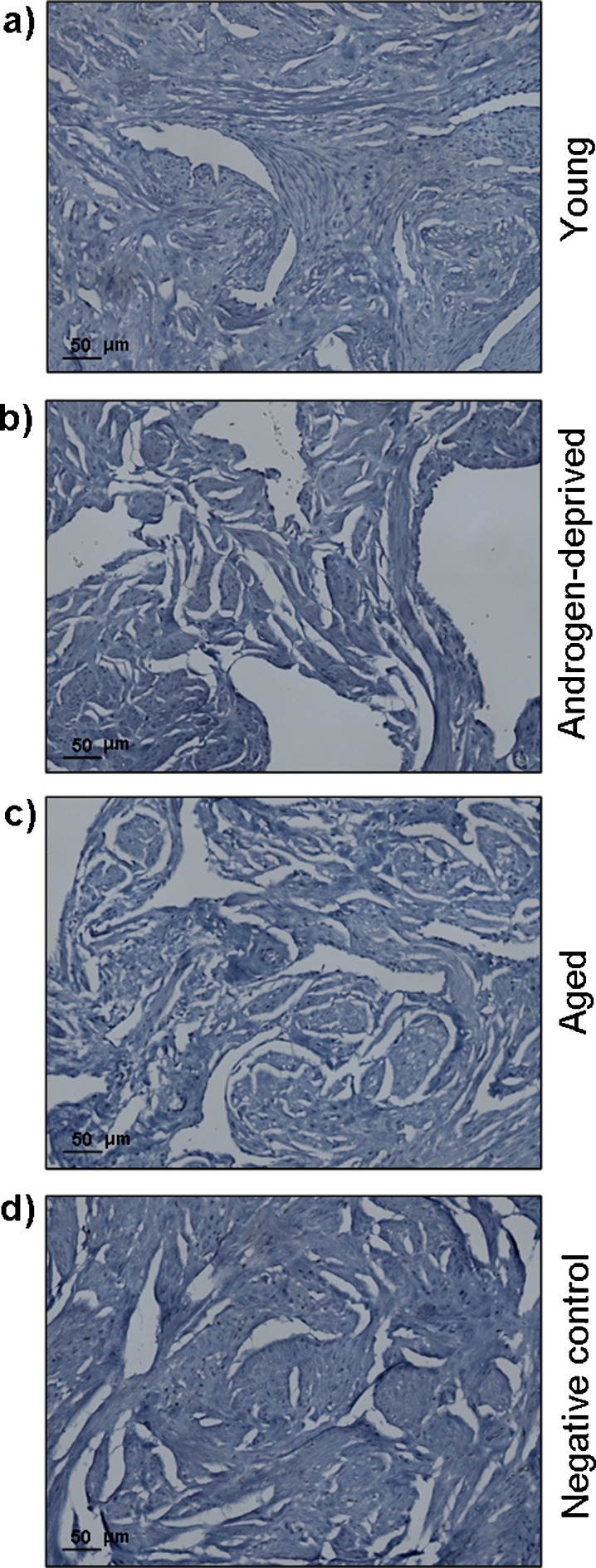
Androgen-deprived, young, and aged human cavernous tissue showed no positive staining for ER (Fig. 3a–c). The specificity of the primary antibody was validated by the absence of staining in the negative control of this tissue (Fig. 3d). This finding allowed us to exclude the direct influence of estradiol treatment on cavernous tissue morphological and molecular changes.
Fig. 3.
Estrogen receptors detection by immunohistochemistry in human cavernous tissue from young (a), androgen-deprived (b), and aged (c) individuals. Note the absence of staining in the negative control (d)
Dual immunolabeling of vWf/alpha-actin, Sirt1/alpha-actin, and ET1/eNOS
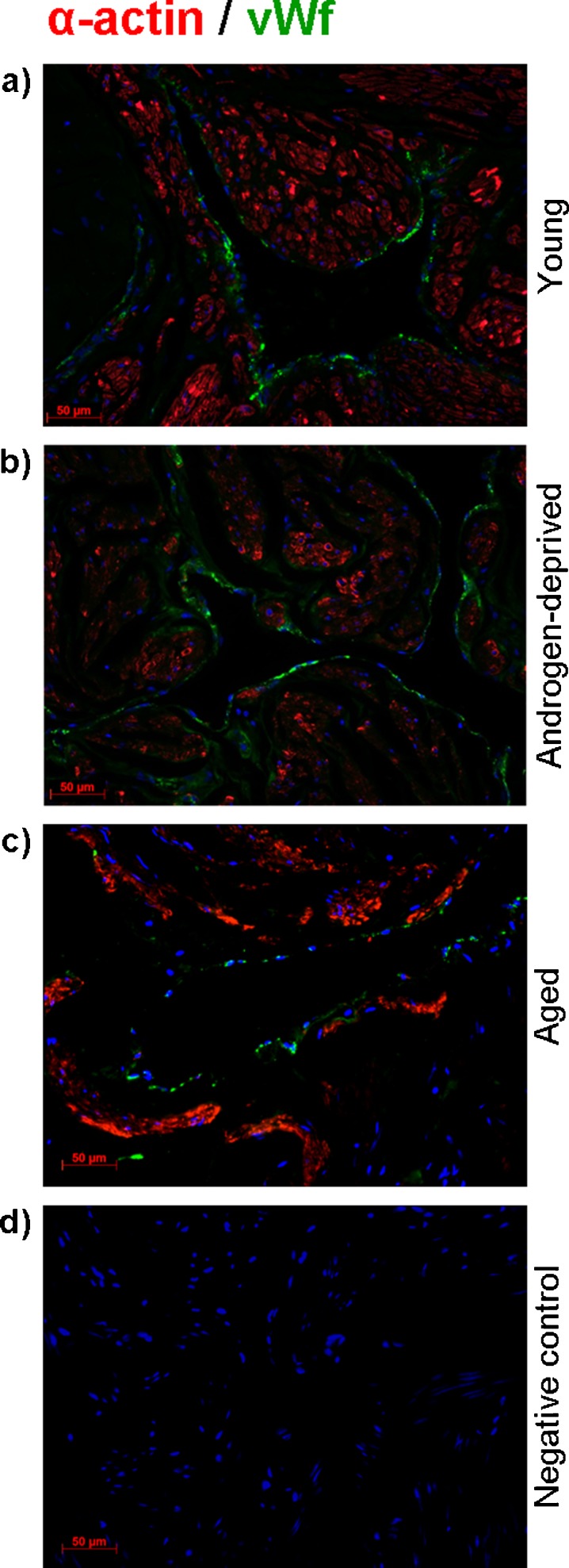
Characterization of SM and endothelial cells (EC) distribution in cavernous tissue in the studied conditions was done by IF detection of specific cell markers, respectively, alpha-actin (red) for SM cells and vWf (green) for EC (Fig. 4a–c). In all groups, IF staining of vWf was confined to EC that outlined the sinusoidal trabeculae, whereas alpha-actin was detected exclusively in fiber-organized SM cells. Corroborating histomorphometric evaluation, we found that androgen-deprived and aged samples presented a sparse intensity of alpha-actin staining compared with young specimens.
Fig. 4.
Dual-immunolabelling by immunofluorescence of alpha-actin (red) and von Willebrand factor (vWf) (green) in young (a), androgen-deprived (b), and aged (c) human cavernous tissue. The nucleus is stained blue (DAPI). Negative control was performed by primary antibody omission (d)
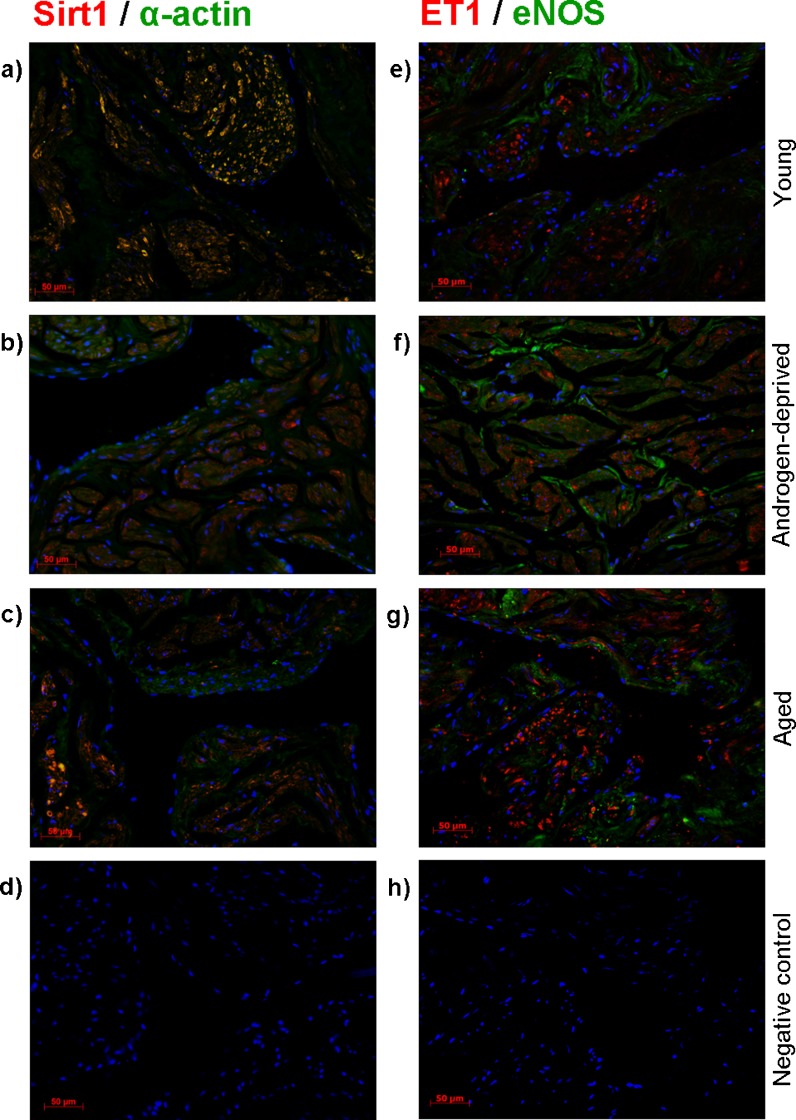
Sirt1 (red) and alpha-actin (green) IF detection (Fig. 5a–c) demonstrated that the former is clearly expressed in cavernous SM cells in all groups studied. However, co-localization (yellow) of both proteins is markedly higher in CC of young and aged individuals when compared with the androgen-deprived tissue.
Fig. 5.
Dual-immunofluorescence detection of sirtuin-1 (Sirt1) (red) and alpha-actin (green) (a–d) and of endothelin-1 (ET1) (red) and endothelial nitric oxide synthase (eNOS) (green) (e–h) in young (a, e), androgen-deprived (b, f), and aged (c, g) human cavernous tissue. The nucleus is stained blue (DAPI). Negative controls were performed by primary antibody omission (d, h)
Dual-immunolabelling of ET1 (red) and eNOS (green) (Fig. 5e–g) revealed that ET1 is exclusively expressed by SM cells, while eNOS was detected in both SM and EC cells, without co-localization. Androgen-deprived human samples evidenced a higher intensity of ET1 expression compared with young samples, but apparently less than in the aged specimens. The endothelial expression of eNOS was hardly observed in young cavernous tissue.
The specificity of the primary antibodies was confirmed by absence of staining in the negative controls (Figs. 4d and 5d, h).
Western blot semiquantification of Sirt1, total eNOS, and total and phospho-Akt
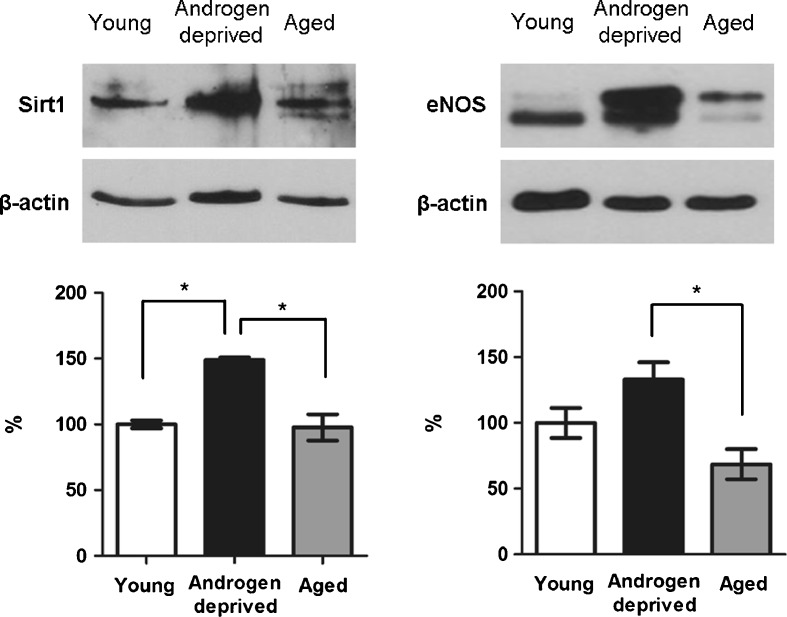
WB representative bands of Sirt1 and total eNOS expression in androgen-deprived, young, and aged human cavernous tissue is presented in Fig. 6 (semiquantitative analysis is detailed in the graphs). Values relative to expression of studied proteins are indicated in percentage, always considering young controls 100%.
Fig. 6.
Representative bands obtained by Western immunoblotting analysis of sirtuin-1 (Sirt1) and endothelial nitric oxide synthase (eNOS) in young, androgen-deprived, and aged human cavernous tissue. The graphs below represent the semiquantitative analysis of the aforementioned proteins (performed in all samples of each group), calculated by the fraction of pixels presented by each band relative to β-actin band used as loading control. Values represent the mean value of each protein in human cavernous tissue from androgen-deprived and aged groups, indicated in percentage, relative to young controls, considered 100%. Error bars represent standard deviation of the mean. Significant differences between groups (P < 0.05) (asterisk)
A significant increase of Sirt1 protein levels of androgen-deprived individuals (148.9%) compared with young (P = 0.000) and aged (P = 0.000) was observed. No significant differences were observed between young and aged individuals (97.7%, P = 0.975).
Concerning total eNOS expression, the semiquantitative analysis demonstrated a significant increase in cavernous tissue of androgen-deprived samples compared with aged ones (133.1% versus 68.6%, P = 0.016). However, no differences were observed between these groups and young controls (P = 0.298 and P = 0.223 for androgen deprived and aged, respectively).
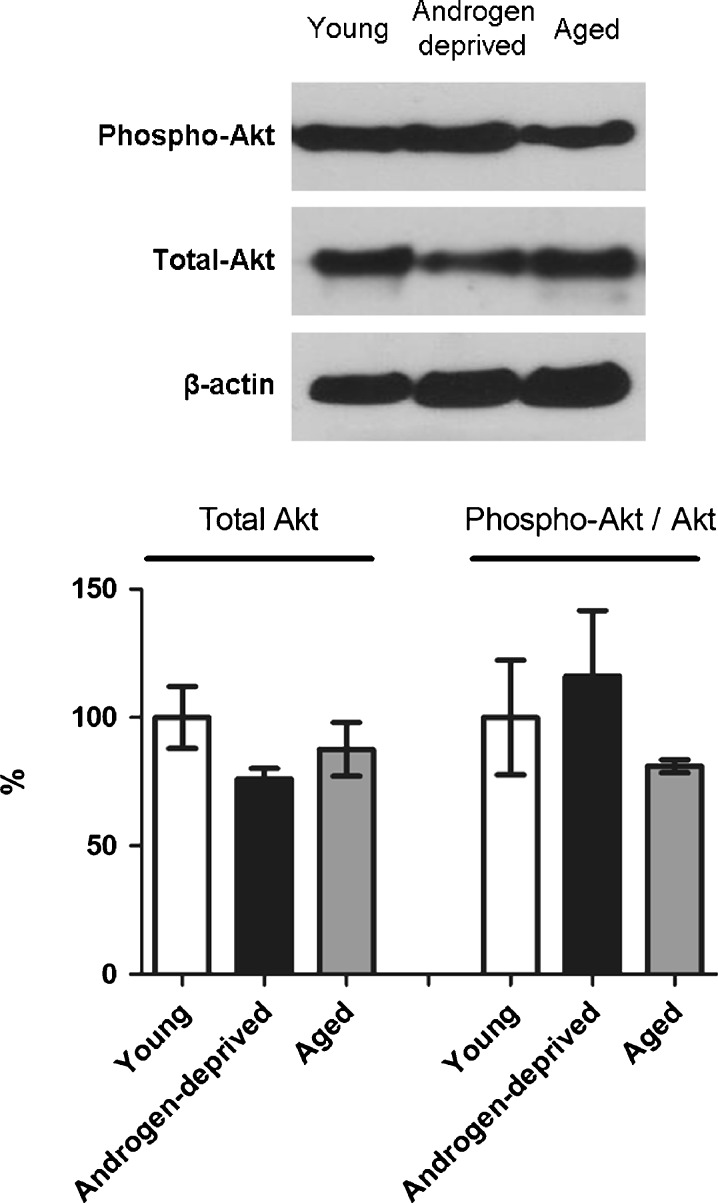
Figure 7 presents the representative bands of phospho-Akt and total Akt detection in all studied human groups. Despite the slight increase observed in androgen-deprived samples and the decrease in aged individuals compared with young cavernous tissue, phospho-Akt did not present significant differences (P = 0.547). Similarly, total Akt expression did not exhibit statistical differences between groups (P = 0.333), although a decrease in both androgen-deprived and aged groups was observed.
Fig. 7.
Representative bands obtained by Western immunoblotting analysis of total and phosphorylated Akt in young, androgen-deprived, and aged human cavernous tissue. The graph below represents the semiquantitative analysis of total Akt and phospho-Akt (performed in all samples of each group). Total Akt and phospho-Akt levels were normalized with total levels of β-actin and total Akt proteins, respectively. Values represent the mean value of each protein in human cavernous tissue from androgen-deprived and aged groups, indicated in percentage, relative to young controls, considered 100%. Error bars represent standard deviation of the mean. Significant differences between groups (P < 0.05) (asterisk)
Discussion
Penile erection is modulated by a complex crosstalk of neurotransmitters, vasoactive agents, and endocrine factors (Adams et al. 1997; Andersson and Wagner 1995) and involves the close coordination of three hemodynamic events: increased arterial inflow, sinusoidal SM relaxation, and decreased venous outflow. Thus, the mechanical properties of erectile tissue are strictly dependent on the integrity of its structure (Moreland 2000; Nehra et al. 1996).
The effect of androgens on erectile function is complex, considering their influence upon distinct features such as integration of stimulatory impulses from central and peripheral nervous systems and penile morphofunctional homeostasis (Mills et al. 1992; Mirone et al. 2009). Previous studies in animal models suggest that androgen deprivation reversibly alters the structure and function of the cavernous tissue (Traish and Kim 2005) and results in critical increase in the number of apoptotic trabecular SM cells, which compromise the veno-occlusive mechanism and erectile function (Shabsigh 1997; Traish et al. 1999, 2007). Nevertheless, the exact mode of androgen action in the regulation of erectile function in human remains unknown.
Aging also leads to change of the cavernous tissue structure (Ferrer et al. 2010; Luo et al. 2007; Tomada et al. 2008, 2010b; Wespes et al. 1991) and, despite the controversy of reports concerning SM and CT content, such changes seem to be one of multiple contributing factors for the age-dependent erectile dysfunction (El-Sakka and Yassin 2010; Yassin and Saad 2008).
To our knowledge, the present study is the first reporting morphological changes in human penile tissue, consequent to pharmacological androgen deprivation treatment in the setting of preparation for surgical sex change. The treatment includes a 24-month administration of antiandrogen drugs, to which estrogen is added to further enhance the female phenotype. Attenuation of male gender characteristics decreased spontaneous erections and reduced testicular volume (Moore et al. 2003).
Comparing healthy young subjects with androgen-deprived individuals, morphometric and immunohistochemical data demonstrate a significant increase in cavernous CT content, coupled to a significant decrease in trabecular SM content. Similar findings were observed in aged individuals of this study and agree with previous observations in aged humans (Tomada et al. 2008, 2010b) and ED patients (Shabsigh et al. 1998). They provide substantial evidence that long-standing androgen deprivation, either physiologically, as it occurs in aging, or following pharmacological treatment, leads to cavernous tissue remodeling that results in a replacement of SM cells by fibroblasts, increase in collagen deposition, and reduction of the SM-to-CT ratio. The consequent change in fibroelastic properties of penile tissue compromises the compliance and hemodynamics, results in corporeal veno-occlusive dysfunction (Nehra et al. 1996) and decreased capacity of sinuses to fill with blood, and produces an effective erection (Ferrer et al. 2010).
In parallel to structural findings, the immunofluorescence data suggest that enhanced expression of the vasoconstrictor peptide ET1 in androgen-deprived and aged individuals here observed adds further to the local age-related impairment of CC relaxation (Saenz de Tejada et al. 1991). Interestingly, a previous report showed increased circulating levels of ET1 in hypogonadic men, which were normalized with testosterone replacement (Kumanov et al. 2007). ET1 is in fact considered the most potent stimulator of penile SM cell contractility (Filippi et al. 2003; Granchi et al. 2002; Mills et al. 2001; Saenz de Tejada et al. 1991). Mainly originated in EC, ET1 was also evidenced in SM cells of the human penis (Granchi et al. 2002; Saenz de Tejada et al. 1991; present investigation) and is thus involved in paracrine and autocrine effects (Granchi et al. 2002).
The loss of SM fibers of male cavernous tissue leads to reduced oxygen tension in the penis. This condition compromises the oxygenation of the organ and appears to downregulate local NO synthesis, a major neurotransmitter in erection (Wespes 2002).
Most of NO synthesis result from eNOS activity in SM cells, which make them important intervenients in vascular NO synthesis (Buchwalow et al. 2002; Komori et al. 2008). This point is of major importance because when eNOS-derived NO bioactivity is reduced, there is an impairment of endothelial-dependent relaxation and endothelial dysfunction ensues (Mattagajasingh et al. 2007).
Several studies point to Sirt1 as a chief regulator of NO synthesis in endothelium (Milne et al. 2007; Pillarisetti 2008; Potente et al. 2007) employing an Akt-mediated pathway of eNOS phosphorylation (Lovren et al. 2009) or promoting eNOS catalytic activity through deacetylation of lysines 496 and 506 (Mattagajasingh et al. 2007). As NO itself appears to reciprocally activate Sirt1 expression and activity (Ota et al. 2007), then Sirt1–eNOS axis emerges as a decisive regulatory mechanism in CC endothelium.
Albeit the similarity of structural features observed in aging and androgen deprivation here described, the underlying mechanisms involving NO synthesis are probably different as the molecular studies indicate.
In fact, comparing young and aged subjects, the variation in the Sirt1–eNOS axis and its additional regulator Akt is minimal. These findings agree with previous observations (Figueiredo et al. 2011) and suggest that a stable condition was achieved, notwithstanding the structural differences. However, in contrast with the unaltered effects involving Sirt1–eNOS axis in the young and the aged individuals, there is a significant enhancement of Sirt1 and eNOS expression and a slight increase in phospho-Akt expression in androgen-deprived subjects. This does not seem to result from estradiol administration. Although estrogens are able to rapidly induce NO production in ECs without altering the expression of eNOS gene or protein (Caulin-Glasser et al. 1997; Haynes et al. 2000; Hisamoto et al. 2001; Russel et al. 2000; Lantin-Hermoso et al. 1997), by a mechanism that involves PI3-kinase-dependent activation of Akt, neither estrogen receptors were expressed nor phospho-Akt expression was significantly changed, although Akt activation occurring in Sirt1 overexpression appears to be limited to insulin-resistant conditions (Sun et al. 2007).
The administration of anti-androgen does not seem to be a direct reason too because there is evidence that the upregulation of Sirt1–eNOS axis is actually due to androgens. In fact, penile nNOS and eNOS activities of castrated rats were reduced by half and later restored following androgen administration (Bilavacqua et al. 2004; Lugg et al. 1995; Marin et al. 1999; Penson et al. 1996; Zvara et al. 1995).
Therefore, the data strongly support the point that in the young penis CC, there is a local compensatory activation of the axis that is maintained by the continued anti-androgen drug administration. Additional studies would be necessary to unravel its fine details and mechanism.
The data also emphasize the importance of androgens and SM cells in the local regulation of the erection process, which make of them important therapeutic targets for ED. In fact, the use of phosphodiesterase-5 inhibitors (iPDE5) for the treatment of ED revealed efficacy and enhanced awareness for the condition, but some men still failed to respond to iPDE5 alone. In these, testosterone replacement therapy with subphysiological doses improves erectile function and it is now accepted that full therapeutic potential of iPDE5 will only manifest in a eugonadal state (Gooren 2006). Because these drugs also need a critical amount of NO, which is a limitation in some patients, therapeutic strategies that promote NO synthesis present a double benefit considering that they improve erectile function and enhance the efficacy of iPDE5 too (Fukuhara et al. 2011). The study suggests that CC is normally endowed with similar mechanisms.
Conclusions
Our results cumulatively suggest that androgens play an important role in the overall structure of human CC, and that androgen deprivation induces changes in cavernous tissue equivalent to those observed in chronological aging, in particular, significant decrease in SM cell content associated to an increase in CT. In what concerns Sirt1–eNOS axis, the present data strongly suggest that Sirt1 regulates the expression of eNOS, and that androgen deprivation induces upregulation of this system, an effect that was not observed in the aged tissue. Nevertheless, further studies are required to fully elucidate the roles of Sirt1 in age-related changes of CC and its implication for endothelial dysfunction progression.
Therefore, in contrast with long-term physiological involution that occurs in aging, where ET1 effects may be more relevant, anti-androgenic pharmacological intervention triggers a different mechanism in penis CC, which includes a compensatory enhancement of the Sirt1–eNOS axis.
Acknowledgments
The authors thank Dra. Bárbara Gomes and Mrs. Helena Pereira from the Pathology Department of Centro Hospitalar S. João for estrogen receptor immunodetection in human cavernous tissue. This study was supported by Fundação para a Ciência e Tecnologia (FCT), Portugal—Pluriannual funding and SFRH/BD/46009/2008.
References
- Adams MA, Banting JD, Maurice DH, Morales A, Heaton JP. Vascular control mechanisms in penile erection: phylogeny and the inevitability of multiple and overlapping systems. Int J Impot Res. 1997;9:85–91. doi: 10.1038/sj.ijir.3900275. [DOI] [PubMed] [Google Scholar]
- Andersson KE, Wagner G. The physiology of penile erection. Physiol Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- Aversa A, Isidori AM, De Martino MU, Caprio M, Fabbrini E, Rocchietti-March M, Frajese G, Fabbri A. Androgens and penile erection: evidence for a direct relationship between free testosterone and cavernous dilatation in men with erectile dysfunction. Clin Endocrinol. 2000;53:517–522. doi: 10.1046/j.1365-2265.2000.01118.x. [DOI] [PubMed] [Google Scholar]
- Aversa A, Isidori AM, Spera G, Lenzi A, Fabbri A. Androgens improve cavernous dilatation and response to sildenafil in patients with erectile dysfunction. Clin Endocrinol. 2003;58:632–638. doi: 10.1046/j.1365-2265.2003.01764.x. [DOI] [PubMed] [Google Scholar]
- Bilavacqua TJ, Deng W, Champion HC, Helltrom WJ, Kadowitz PJ. Gene therapy techniques for the delivery of endothelial nitric oxide synthase to the corpora cavernosa for erectile dysfunction. Methods Mol Biol. 2004;279:173–185. doi: 10.1385/1-59259-807-2:173. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Buchwalow IB, Podzuweit T, Bocker W, Samoilova VE, Thomas S, Wellner M, Baba HA, Robenek H, Schnekenburger J, Lerch MM. Vascular smooth muscle and nitric oxide synthase. Faseb. 2002;16:500–508. doi: 10.1096/fj.01-0842com. [DOI] [PubMed] [Google Scholar]
- Caulin-Glasser T, Garcia-Cardena G, Sarrel P, Sessa WC, Bender JR. 17β-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res. 1997;81:885–892. doi: 10.1161/01.RES.81.5.885. [DOI] [PubMed] [Google Scholar]
- Cernadas MR, Miguel LS, García-Durán M, González-Fernández F, Millás I, Montón M, Rodrigo J, Rico L, Fernández P, Frutos T, Rodríguez-Feo JA, Guerra J, Caramelo C, Casado S, López-Farré A. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.RES.83.3.279. [DOI] [PubMed] [Google Scholar]
- Challah M, Nadaud ST, Philippe M, Battle T, Soubrier S, Corman B, Michel JB. Circulating and cellular markers of endothelial dysfunction with aging in rats. Am J Physiol. 1997;273:1941–1948. doi: 10.1152/ajpheart.1997.273.4.H1941. [DOI] [PubMed] [Google Scholar]
- El-Sakka AI, Yassin AA. Amelioration of penile fibrosis: myth or reality. J Androl. 2010;31:324–335. doi: 10.2164/jandrol.109.008730. [DOI] [PubMed] [Google Scholar]
- Ferrer JE, Velez JD, Herrera AM. Age-related morphological changes in smooth muscle and collagen content in human corpus cavernosum. J Sex Med. 2010;7:2723–2728. doi: 10.1111/j.1743-6109.2009.01508.x. [DOI] [PubMed] [Google Scholar]
- Figueiredo A, Cordeiro AL, Tomada N, Tomada I, Rodrigues A, Almeida H, Gouveia A, Neves D. Real-time PCR studt of Ang1, Ang2, Tie2, VEGF and KDR expression in human erectile tissue during aging. J Sex Med. 2011;8:1341–1351. doi: 10.1111/j.1743-6109.2010.02116.x. [DOI] [PubMed] [Google Scholar]
- Filippi S, Marini M, Vannelli GB, Crescioli C, Granchi S, Vignozzi L, Luconi M, Ferruzzi P, Morelli A, Forti G, Ledda F, Maggi M. Effects of hypoxia on endothelin-1 sensitivity in the corpus cavernosum. Mol Hum Reprod. 2003;9:765–774. doi: 10.1093/molehr/gag096. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Tsujimura A, Okuda H, Yamamoto K, Takao T, Miyagawa Y, Nonomura N, Okuyama A. Vardenafil and resveratrol synergistically enhance the nitric oxide/cyclic guanosine monophosphate pathway in corpus cavernosal smooth muscle cells and its therapeutic potential for erectile dysfunction in the streptozotocin-induced diabetic rat: preliminary findings. J Sex Med. 2011;8:1061–1071. doi: 10.1111/j.1743-6109.2010.02193.x. [DOI] [PubMed] [Google Scholar]
- Garban H, Marquez D, Cai L, Rafner J, Gonzalez-Cadavid NF. Restoration of normal adult penile erectile response in aged rats by long-term treatment with androgens. Biol Reprod. 1995;53:1365–1372. doi: 10.1095/biolreprod53.6.1365. [DOI] [PubMed] [Google Scholar]
- Ghalayini IF, Al-Ghazo MA, Al-Azab R, Bani-Hani I, Matani YS, Barham AE, Harfeil MN, Haddad Y. Erectile dysfunction in a Mediterranean country: results of an epidemiological survey of a representative sample of men. Int J Impot Res. 2010;22:196–203. doi: 10.1038/ijir.2009.65. [DOI] [PubMed] [Google Scholar]
- Gooren L. The role of testosterone in erectile function and dysfunction. J Men’s Health Gender. 2006;3:292–298. doi: 10.1016/j.jmhg.2006.08.001. [DOI] [Google Scholar]
- Granchi S, Vannelli GB, Vignozzi L, Crescioli C, Ferruzzi P, Mancina R, Vinci MC, Forti G, Filippi S, Luconi M, Ledda F, Maggi M. Expression and regulation of endothelin-1 and its receptors in human penile smooth muscle cells. Mol Hum Reprod. 2002;8:1053–1064. doi: 10.1093/molehr/8.12.1053. [DOI] [PubMed] [Google Scholar]
- Haynes MP, Sinha D, Russel KS, Collinge M, Fulton D, Moralez-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87:677–682. doi: 10.1161/01.RES.87.8.677. [DOI] [PubMed] [Google Scholar]
- Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, Taniguchi N, Murata Y. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2001;276:3459–3467. doi: 10.1074/jbc.M005036200. [DOI] [PubMed] [Google Scholar]
- Huang Y, Pan L, Xia X, Feng Y, Jiang C, Cui Y. Long term effects of phytoestrogen daidzein on penile cavernosal structures in adult rats. Urology. 2008;72:220–224. doi: 10.1016/j.urology.2007.11.064. [DOI] [PubMed] [Google Scholar]
- Komori K, Tsujimura A, Takao T, Matsuoka Y, Miyagawa Y, Takada S, Nonomura N, Okuyama A. Nitric oxide synthesis leads to vascular endothelial growth factor synthesis via the NO/cyclic guanosine 3′,5′-monophosphate (cGMP) pathway in human corpus cavernosal smooth muscle cells. J Sex Med. 2008;5:1623–1635. doi: 10.1111/j.1743-6109.2008.00772.x. [DOI] [PubMed] [Google Scholar]
- Kumanov P, Tomova A, Kirilov G. Testosterone replacement therapy in male hypogonadism is not associated with increase of endothelin-1 levels. Int J Androl. 2007;30:41–47. doi: 10.1111/j.1365-2605.2006.00706.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriphage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lantin-Hermoso RL, Rosenfeld CR, Yuhanna IS, Germian Z, Chen Z, Shaul PW. Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am J Physiol Lung Cell Mol Physiol. 1997;273:L119–L126. doi: 10.1152/ajplung.1997.273.1.L119. [DOI] [PubMed] [Google Scholar]
- Longo VD. Linking sirtuins, IGF-I signalling, and starvation. Exp Gerontol. 2009;44:70–74. doi: 10.1016/j.exger.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Lovren F, Pan Y, Shukla PC, Quan A, Teoh H, Szmitko PE, Peterson MD, Gupta M, Al-Omran M, Verma S. Visfatin activates eNOS via Akt and MAP kinases and improves endothelial cell function and angiogenesis in vitro and in vivo: translational implications for atherosclerosis. Am J Physiol Endocrinol Metab. 2009;296:E1440–E1449. doi: 10.1152/ajpendo.90780.2008. [DOI] [PubMed] [Google Scholar]
- Lu Y, Kuang L, Zhu H, Wu H, Wang XF, Pang YP, Wang NJ, Yu DL. Changes in aortic endothelium ultrastructure in male rats following castration, replacement with testosterone and administration of 5alpha-reductase inhibitor. Asian J Androl. 2007;9:843–847. doi: 10.1111/j.1745-7262.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- Lue T, Dahiya M. Molecular biology of erectile function and dysfunction. Mol Urol. 1997;1:35–48. [Google Scholar]
- Lugg JA, Rajfer J, Gonzalez-Cadavid NF. Dihydrotestosterone is the active androgen in maintenance of nitric oxide-mediated penile erection in the rat. Endocrinology. 1995;136:1495–1501. doi: 10.1210/en.136.4.1495. [DOI] [PubMed] [Google Scholar]
- Luo H, Goldstein I, Udelson D. A three-dimensional theoretical model of the relationship between cavernosal expandability and percent cavernosal smooth muscle. J Sex Med. 2007;4:644–645. doi: 10.1111/j.1743-6109.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- Marin R, Escrig A, Abreu P, Mas M. Androgen-dependent nitric oxide release in rat penis correlates with levels of constitutive nitric oxide synthase isoenzymes. Biol Reprod. 1999;61:1012–1016. doi: 10.1095/biolreprod61.4.1012. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno J, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. PNAS. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills TM, Wiedmeir VT, Stopper VS. Androgen maintenance of erectile function in the rat penis. Biol Reprod. 1992;46:342–348. doi: 10.1095/biolreprod46.3.342. [DOI] [PubMed] [Google Scholar]
- Mills TM, Reilly CM, Lewis RW. Androgens and penile erection: a review. J Androl. 1996;17:633–638. [PubMed] [Google Scholar]
- Mills TM, Chitaley K, Lewis RW. Vasoconstrictors in erectile physiology. Int J Impot Res. 2001;13:S29–S34. doi: 10.1038/sj.ijir.3900774. [DOI] [PubMed] [Google Scholar]
- Milne JC, Denu JM. The sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol. 2008;12:11–17. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirone V, Imbimbo C, Fusco F, Verze P, Creta M, Tajana G. Androgens and morphologic remodeling at penile and cardiovascular levels: a common piece in complicated puzzles? Eur Urol. 2009;56:309–316. doi: 10.1016/j.eururo.2008.12.037. [DOI] [PubMed] [Google Scholar]
- Moore E, Wisniewski A, Dobs A. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrinol Metab. 2003;88:3467–3473. doi: 10.1210/jc.2002-021967. [DOI] [PubMed] [Google Scholar]
- Moreland RB. Pathophysiology of erectile dysfunction: the contributions of trabecular structure to function and role of function antagonism. Int J Impot Res. 2000;12(Suppl 4):S39–S46. doi: 10.1038/sj.ijir.3900576. [DOI] [PubMed] [Google Scholar]
- Musicki B, Ross AE, Champion HC, Burnett AL, Bivalacqua TJ. Posttranslational modification of constitutive nitric oxide synthase in the penis. J Androl. 2009;30:352–362. doi: 10.2164/jandrol.108.006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehra A, Goldstein I, Pabby A, Nugent M, Huang YH, de Las Morenas A, Krane RJ, Udelson D, Saenz de Tejada I, Moreland RB. Mechanisms of venous leakage: a prospective clinicopathological correlation of corporeal function and structure. J Urol. 1996;156:1320–1329. doi: 10.1016/S0022-5347(01)65578-2. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Moll Cell Cardiol. 2007;43:571–579. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Ota H, Eto M, Ogawa S, Iijima K, Akishita M, Ouchi Y. SIRT1/eNOS axis as a potential target against vascular senescence, dysfunction and atherosclerosis. J Atheroscler Thromb. 2010;17:431–435. doi: 10.5551/jat.3525. [DOI] [PubMed] [Google Scholar]
- Penson DF, Ng C, Cai L, Rajfer J, Gonzalez-Cadavid NF. Androgen and pituitary control of penile nitric oxide synthase and erectile function in the rat. Biol Reprod. 1996;55:567–674. doi: 10.1095/biolreprod55.3.567. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado de Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillarisetti S. A review of Sirt1 and sirt1 modulators in cardiovascular and metabolic diseases. Recent Pat Cardiovasc Drug Discov. 2008;3:156–164. doi: 10.2174/157489008786263989. [DOI] [PubMed] [Google Scholar]
- Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. Sirt1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel KS, Haynes MP, Caulin-Glasser T, Rosneck J, Sessa WC, Bender JR. Estrogen stimulates heat shock protein 90 binding to endothelial nitric oxide synthase in human vascular endothelial cells. J Biol Chem. 2000;275:5026–5030. doi: 10.1074/jbc.275.7.5026. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I, Carson MP, de las Morenas A, Goldstein I, Traish AM. Endothelin: localization, synthesis, activity, and receptor types in human corpus cavernosum. Am J Physiol. 1991;261:H1078–H1085. doi: 10.1152/ajpheart.1991.261.4.H1078. [DOI] [PubMed] [Google Scholar]
- Shabsigh R. The effects of testosterone on the cavernous tissue and erection. World J Urol. 1997;15:21–26. doi: 10.1007/BF01275152. [DOI] [PubMed] [Google Scholar]
- Shabsigh R, Raymond JF, Olsson CA, O’Toole K, Buttyan R. Androgen induction of DNA synthesis in the rat penis. Urology. 1998;52:723–728. doi: 10.1016/S0090-4295(98)00233-7. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:306–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Tomada N, Oliveira R, Tomada I, Vendeira P, Neves D. Comparative ultrastructural study of human corpus cavernosum during ageing. Microsc Microanal. 2008;14(suppl3):152–155. doi: 10.1017/S1431927608089733. [DOI] [Google Scholar]
- Tomada N, Tomada I, Vendeira P, Neves D. Expression of vascular endothelial growth factor and angiopoietins in human corpus cavernosum. BJU Int. 2010;105(2):269–273. doi: 10.1111/j.1464-410X.2009.08663.x. [DOI] [PubMed] [Google Scholar]
- Tomada N, Tomada I, Cruz F, Vendeira P, Neves D. Characterization of VEGF and angiopoietins in human corpus cavernosum during aging. J Sex Med. 2010;7:1410–1418. doi: 10.1111/j.1743-6109.2009.01648.x. [DOI] [PubMed] [Google Scholar]
- Traish A. Androgens play a pivotal role in maintaining penile tissue architecture and erection: a review. J Androl. 2009;30:363–369. doi: 10.2164/jandrol.108.006007. [DOI] [PubMed] [Google Scholar]
- Traish AM, Kim N. Weapons of penile smooth muscle destruction: androgen deficiency promotes accumulation of adipocytes in the corpus cavernosum. Aging Male. 2005;8:141–146. doi: 10.1080/13685530500328183. [DOI] [PubMed] [Google Scholar]
- Traish AM, Park K, Dhir V, Kim NN, Moreland RB, Goldstein I. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology. 1999;140:1861–1868. doi: 10.1210/en.140.4.1861. [DOI] [PubMed] [Google Scholar]
- Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgens insufficiency and erectile dysfunction. Eur Urol. 2007;52:54–70. doi: 10.1016/j.eururo.2007.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachsmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1743. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero A, Scher M, Erdjument-Bromage H, Tempst P, Serrano L, Reinberg D. SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature. 2007;450:440–444. doi: 10.1038/nature06268. [DOI] [PubMed] [Google Scholar]
- Wespes E. Smooth muscle pathology and erectile dysfunction. Int J Impot Res. 2002;14(Suppl 2):S17–S21. doi: 10.1038/sj.ijir.3900792. [DOI] [PubMed] [Google Scholar]
- Wespes E, Goes PM, Schiffman S, Depierreux M, Vaderhaeghen JJ, Schulmann CC. Computerized analysis of smooth muscle fibers in potent and impotent patients. J Urol. 1991;146:1015–1017. doi: 10.1016/s0022-5347(17)37990-9. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Yassin AA, Saad F. Testosterone and erectile dysfunction. J Androl. 2008;29:593–604. doi: 10.2164/jandrol.107.004630. [DOI] [PubMed] [Google Scholar]
- Zahn JM, Poosala S, Owen AB, Ingram DK, Lustig A, Carter A, Weeraratna AT, Taub DD, Gorospe M, Mazan-Mamczarz K, Lakatta EG, Boheler KR, Xu X, Mattson MP, Falco G, Ko MS, Schlessinger D, Firman J, Kummerfeld SK, Wood WH, 3rd, Zonderman AB, Kim SK, Becker KG. AGEMAP: a gene expression database for aging in mice. PLoS Genet. 2007;3:e201. doi: 10.1371/journal.pgen.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvara P, Sioufi R, Schipper HM, Begin LR, Brock GB. Nitric oxide mediated erectile activity is a testosterone dependent event: a rat erection model. Int J Impot Res. 1995;7:209–219. [PubMed] [Google Scholar]