Abstract
Cortico-muscular coherence (CMC) at beta frequency (13–30 Hz) occurs particularly during weak to moderate isometric contraction. It is a well-established measure of communication between the primary motor cortex (M1) and corresponding muscles revealing information about the integrity of the pyramidal system. Although the slowing of brain and muscle dynamics during healthy aging has been evidenced, functional communication as determined by CMC has not been investigated so far. Since decline of motor functions at higher age is likely to be associated with CMC changes, the present study aims at shedding light on the functionality of the motor system from a functional interaction perspective. To this end, CMC was investigated in 27 healthy subjects aging between 22 and 77 years during isometric contraction of their right forearm. Neuromagnetic activity was measured using whole-head magnetoencephalography (MEG). Muscle activity was measured by means of surface electromyography (EMG) of the right extensor digitorum communis (EDC) muscle. Additionally, MEG-EMG phase lags were calculated in order to estimate conducting time. The analysis revealed CMC and M1 power amplitudes to be increased with age accompanied by slowing of M1, EMG, and CMC. Frequency changes were particularly found in subjects aged above 40 years suggesting that at this middle age, neurophysiological changes occur, possibly reflecting an early neurophysiological marker of seniority. Since MEG–EMG phase lags did not vary with age, changes cannot be explained by alterations of nerve conduction. We argue that the M1 power amplitude increase and the shift towards lower frequencies might represent a neurophysiological marker of healthy aging which is possibly compensated by increased CMC amplitude.
Keywords: Aging, Oscillations, Cortico-muscular coherence, Motor control, Magnetoencephalography
Introduction
Motor function declines with age characterized by increased movement variability, reduced coordination abilities, and slowing of movement speed (for reviews, see Krampe 2002; Seidler et al. 2010). Musculo-skeletal alterations, changes of peripheral and central nerve conduction, decreased levels of neurotransmitters, and loss of gray as well as white matter have been accounted for impaired motor functions in the elderly (for reviews, see Krampe 2002; Raz and Rodrigue 2006; Seidler et al. 2010; Ward 2006). The understanding of structural as well as functional age-related changes represents a fundamental prerequisite for shifting these changes into later decades of life span and thus preventing subjects from early onset of motor disturbances.
Cortico-muscular coherence (CMC) represents a binding mechanism between M1 and corresponding muscles (Conway et al. 1995; Gross et al. 2000; Halliday et al. 1998; Salenius and Hari 2003); reviewed in Brown 2000; Mima and Hallett 1999). It is caused by oscillatory drive in cortico-spinal pathways most likely maintaining stable motor output while the required computational effort is kept low (Brown 2000). CMC at beta frequency occurs during weak to moderate isometric contraction of the forearm and drops with movement initiation (Kilner et al. 1999). It develops during childhood and early adolescence and has been related to the maturation of intra-cortical and cortico-muscular connectivity (James et al. 2008). CMC most likely reflects conduction of fast pyramidal pathways (Mima et al. 2000; Salenius et al. 1997) representing a marker of the integrity of the pyramidal system. Accordingly, CMC plays a crucial role for sensorimotor integration representing a key mechanism for appropriate motor control (Classen et al. 1998; Kilner et al. 1999; Kristeva et al. 2007; Mackay 1997; Witte et al. 2007).
Alterations of movement-related brain processes are well known during healthy aging. In particular, increased activation and involvement of a widespread motor network in elderly subjects have been suggested by means of electroencephalography (EEG; Babiloni et al. 2006; Derambure et al. 1993; Sailer et al. 2000) and functional magnetic resonance imaging (fMRI) studies (Heuninckx et al. 2005; Noble et al. 2011; Ward and Frackowiak 2003). Additionally, slowing of brain dynamics has been evidenced with increasing age (Krampe 2002; Seidler et al. 2010). Accordingly, Semmler et al. (2003) found motor-unit coherence during isometric contraction of the hand shifted towards lower frequencies (i.e., 5–9 and 12–13 Hz) in older subjects. These data imply that the oscillatory input to motor neurons changes during healthy aging even during apparently simple motor tasks. Such changes have been related to reduced steadiness during sustained contraction observed in older subjects (Semmler et al. 2003). A recent study suggests that CMC and motor-unit coherence represent distinct neural processes (Boonstra et al. 2009). While CMC is a marker of direct communication between M1 and muscle, a cortical source of motor-unit coherence between both hands is less likely (Boonstra et al. 2009). Although the relevance of CMC for motor control is well established and slowing of electromyographic (EMG) activity as well as brain dynamics has been evidenced in the elderly, it is still a moot issue how EMG slowing is mediated by central processes. The present study aims at bridging the gap between local activation changes of M1 and the EMG by investigating functional interaction by means of CMC during healthy aging. Since CMC changes are likely to contribute to decline of motor functions in the elderly, results reveal an improved understanding of physiological changes of motor control processes which are potentially useful for prevention of age-related movement disturbances.
Method
Subjects
Twenty-seven healthy right-handed volunteers participated in the present study. Mean age was 47.9 ± 3.1 years (mean ± standard error of mean, SEM) and overall age ranged between 22 and 77 years. All subjects were free from chronic diseases and medical treatment. Only one subject was a smoker. None of the participants reported inconvenient intake of caffeine, ruling out the possibility that nicotine or caffeine might have affected the present results. Handedness was determined by means of the Edinburgh Handedness Inventory (Oldfield 1971). All subjects gave their written informed consent prior to the study, which was performed in accordance with the Declaration of Helsinki and approved by the local ethics committee.
MEG recordings
Subjects were comfortably seated in a magnetically shielded room during measurement and were asked to keep their heads stationary during the magnetoencephalography (MEG) recording. They were asked to perform a weak to moderate isometric contraction of their right forearm. To this end, they kept the forearm elevated at about 30°, with the hand outstretched and fingers slightly abducted. Subjects were asked to keep the contraction constant at about 20% of the maximal contraction force during MEG recordings. The EMG was recorded from the right extensor digitorum communis (EDC) muscle using bipolar surface electrodes. Signal strength was visually controlled during the entire measurement. In 12 subjects, neuromagnetic activity was recorded noninvasively with a helmet-shaped 122-channel whole-head NeuromagTM system (Ahonen et al. 1993). Due to a change of the MEG system (Elekta Oy, Helsinki, Finland), 15 further subjects were measured by means of a 306-channel system. Signals were continuously measured for 5 min with a 30-s break after each minute of isometric contraction to avoid physical fatigue. To control for eyeblinks, a vertical electrooculogram was simultaneously recorded. Subjects were additionally instructed to fixate a stationary dot on a projection screen in order to minimize eye movements. All signals were measured with a band-pass filter of 0.03 to 330 Hz, digitized at 1,000 Hz, and stored digitally for offline analysis. The EMG signal was high-pass filtered at 20 Hz and rectified offline. For MEG source localization, T1-weighted MRIs were acquired for those 12 participants who were investigated with the 122-channel MEG system using a 1.5-T Magnetom (SiemensTM, Germany). For subjects measured with the 306-channel system, MRIs were obtained by means of a 3.0-T Siemens-Magnetom. The exact position of the head with respect to the MEG sensor array was determined by measuring magnetic signals from four indicator coils placed on the scalp. Coil localizations with respect to three anatomical landmarks (nasion, left and right preauricular points) were identified using a 3D digitizer (Polhemus, Colchester, VT) and used for the alignment of MEG and MRI data.
Data analysis
After artifact rejection, time periods of isometric contraction were selected for spectral analysis. In order to exclude periods of arm movements preceding isometric contraction, the first 5 s after movement onset were discarded from the analysis.
Coherence spectra between MEG and EMG signals were calculated with a frequency resolution of 1 Hz by averaging the Fourier transforms across periods of isometric contraction. After applying a Hanning window, all MEG and EMG signals were converted in 1-s-long data segments. Hereafter, cross-spectral density was computed for all MEG sensors and the EMG signal. Coherence was determined as a measure of linear correlation in the frequency domain which is defined as the absolute value of the cross spectrum normalized by the square root of the auto spectra of two signals. Coherence equals one whenever two signals are in perfectly linear relationship and equals zero in case of absolute linear independence (Schnitzler and Gross 2005). For source localization, the analysis tool Dynamic Imaging of Coherent Sources (DICS) was used. DICS allows tomographic mapping of functional interaction in the entire brain (Gross et al. 2001). The localization of each source was determined in 3D space by using a spatial filter algorithm and a realistic head model of each individual. For visualization localization, maps of individual cortical sources were displayed using SPM99 in case of MEG recordings with the 122-channel system and with SPM8 in the remaining cases (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, http://www.fil.ion.ucl.ac.uk/spm). Additionally, the spectral power of M1 and EDC was calculated as a measure of local activity.
CMC and power were determined individually at alpha (8–12 Hz), beta (13–30 Hz), low gamma (30–60 Hz), and high gamma (60–90 Hz) frequencies, respectively. Data from the 122-channel system were analyzed by means of custom-made analysis tools, while data from the 306-channel system were analyzed using the fieldtrip toolbox (http://www.fieldtrip.fcdonders.nl) which are both based on Matlab®. Additionally, phase differences between M1 and EDC were calculated at the frequency of individual maximum CMC in order to determine conducting time. It represents a combined measure of pure neuronal conducting as well as peripheral synaptic computing (Gross et al. 2000). In order to determine phase lags, instantaneous phase and amplitude were separated by means of the Hilbert transform after applying a narrow band-pass filter around the individual peak frequency of CMC to both signals (for a detailed description of the procedure, please refer to Gross et al. 2000).
Statistics
All statistics were calculated with IBM® SPSS Statistics 19 using analysis of variance (ANOVA) with factor group and post hoc Scheffé test. To this end, subjects were divided into three groups with respect to their age: one group consisted of young subjects (30.0 ± 0.6 years; range, 22–39), a second group comprising subjects at middle age (47.3 ± 0.5 years; range, 41–55), and a third group of elderly subjects (66.4 ± 0.7 years; range, 58–77). Please note that in each group, MEG data of four subjects were acquired using the 122-channel system while five volunteers were measured by means of the 306-channel system. Correlation between age and neurophysiological measures (i.e., coherence amplitude and frequency, M1 power and frequency, EDC power and frequency) was calculated by means of Pearson's correlation.
Results
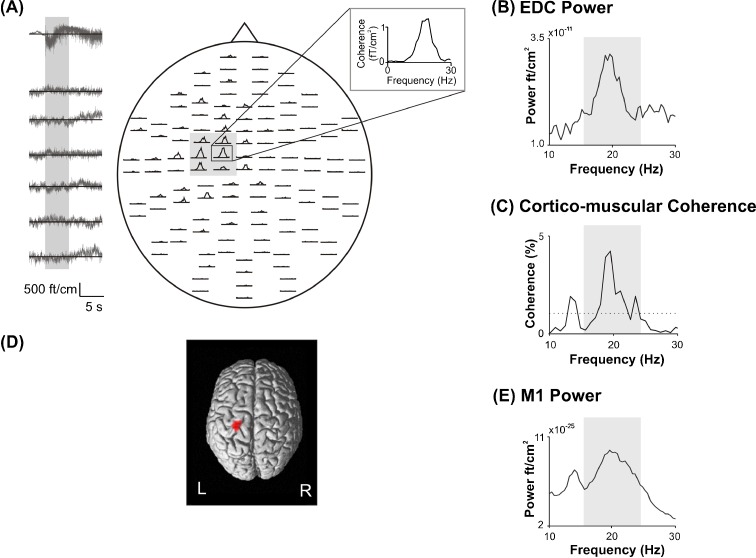
The Edinburgh Handedness Inventory revealed individual laterality quotients above 90 indicating that all subjects were strictly right handed. Groups differed significantly with respect to age (F(2,24) = 94.92, p < 0.00). Figure 1 indicates that the main CMC peak was detectable between 13 and 30 Hz corresponding to the beta frequency range (Fig. 1a, c). The cortical origin of CMC was localized within the contralateral handknob corresponding to M1 in each individual (Fig. 1d).
Fig. 1.
Spectral characteristics of one representative subject. a Cerebro-muscular coherence between all MEG sensors and right EDC muscle. The insert indicates one sensor covering the contralateral fronto-parietal cortex corresponding to the primary sensorimotor (M1) cortex. The left panel indicates a 15-s-long segment of the EDC muscle raw signal (upper row) and of the six MEG channels covering contralateral M1 shaded in gray. The gray bar indicates the time period of 5 s at the beginning of the contraction period, which has been discarded from the analysis in order to exclude movement-related oscillatory activity. b EDC power spectrum. c CMC between M1 and contralateral EDC. The dotted line indicates the 95% level of significance. d CMC source localization, e power spectrum of contralateral M1
EMG
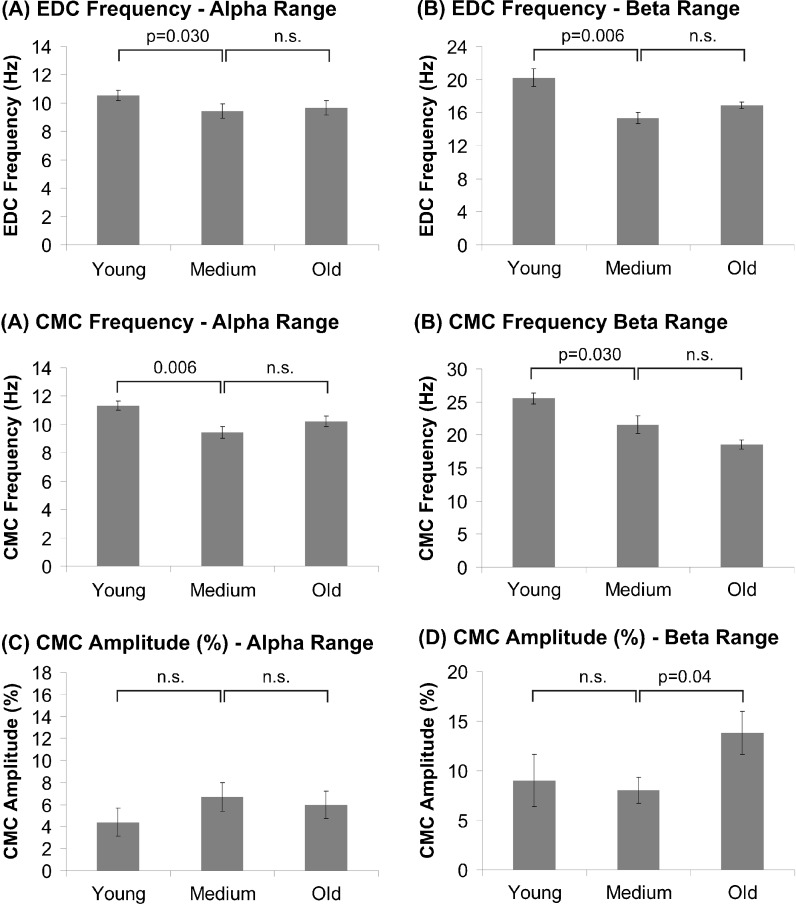
The EMG power maximum was determined at 20.1 ± 1.0 Hz in young subjects, at 15.3 ± 0.7 Hz in middle-aged subjects, and at 17.9 ± 0.4 Hz in elderly subjects (F(2,24) = 12.53; p < 0.00). Scheffé test revealed higher EMG frequencies in younger as compared to middle-aged subjects (p = 0.006), while middle-aged and elderly subjects did not differ significantly from each other (p = 0.43). The amplitude was on average 164.7 ± 31.4 μV (young), 215.6 ± 41.5 μV (middle-aged), and 202.0 ± 45.8 μV (old; F(2,24) = 1.03, p = 0.37). Slightly higher amplitudes in middle-aged and elderly subjects were particularly due to one subject in each group and did not represent a characteristic feature of these groups. Additional peaks were found at alpha, low, and high gamma frequencies but, statistical analysis did not reveal significant differences between groups, neither of frequency nor of amplitude.
M1
The analysis of M1 spectral power revealed discernible peaks at each of the mentioned frequency ranges, but ANOVA did not result in significant group differences regarding frequency or amplitude in any of the frequency ranges.
Cerebro-muscular coherence
CMC was prominent at 25.5 ± 0.8 Hz (young), at 21.5 ± 1.3 Hz (middle-aged), and at 18.6 ± 0.6 Hz (elderly; F(2,24) = 12.5, p < 0.00). Post hoc analysis showed that CMC was significantly faster in young subjects as compared to middle-aged and elderly subjects (p = 0.03), while the latter groups did not differ significantly from each other (p = 0.12). At alpha frequency, discernible peaks occurred at 11.3 ± 0.3 Hz (young), 9.4 ± 0.4 Hz (middle-aged), and 10.2 ± 0.4 Hz (elderly; F(2,24) = 6.5, p = 0.005). Scheffé test again revealed a significant decrease of CMC frequency from young to middle-aged subjects (p = 0.006), while the latter did not differ from the elderly (p = 0.35). Neither at low nor at high gamma frequencies were significant group effects evident.
Analysis of CMC amplitude at alpha frequency did not reveal significant group effects (F(2,24) = 0.85, p = 0.44). At beta frequency, CMC amplitude was 9.0 ± 2.6% (young), 8.0 ± 1.3 Hz (middle-aged), and 13.8 ± 2.2% (elderly). ANOVA revealed a trend towards group differences (F(2,24) = 2.8, p = 0.08) indicating that CMC amplitude was higher in the elderly as compared to middle-aged subjects (p = 0.04), while no significant difference between young and middle-aged subjects was found (p = 0.70). Figure 2 illustrates group comparisons at alpha and beta frequencies. Neither at low nor at high gamma frequencies was significant amplitude or frequency differences between groups found (p > 0.5).
Fig. 2.
Mean EDC power frequency, CMC frequency, and CMC amplitude at alpha (left column) and beta frequency (right column). Error bars indicate SEM
Phase lags between M1 and EDC were 12.0 ± 1.9 ms (young), 14.6 ± 1.9 ms (middle-aged), and 14.8 ± 2.2 ms (elderly). Groups did not differ significantly from each other (F(2,24) = 0.58, p = 0.57).
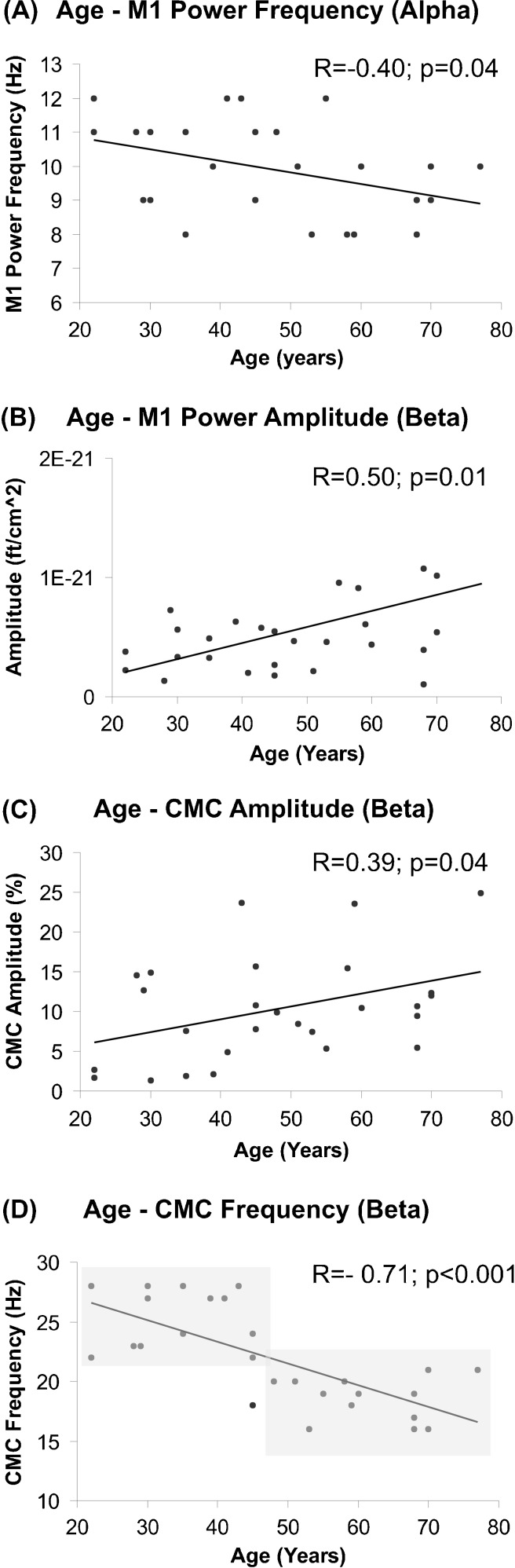
Age was significantly correlated with M1 power frequency at the alpha range (R = −0.40, p = 0.04; Fig. 3a). At beta frequency, CMC amplitude (R = 0.39, p = 0.04; Fig. 3c), CMC frequency (R = −0.71, p < 0.00; Fig. 3d), and M1 power amplitude (R = 0.50, p = 0.01; Fig. 3b) were significantly correlated with age.
Fig. 3.
Correlation analysis between age and M1 power frequency at the alpha range (a), age and M1 power amplitude (b), age and CMC amplitude (c), and age and CMC frequency (d) at beta frequency, respectively. Shaded areas indicate that subjects below 45 years generally tend to show CMC at higher frequencies, while subjects above 45 years seem to be characterized by CMC at lower frequencies suggesting a fundamental change of functional communication between motor cortex and muscles in middle-aged subjects
To exclude the possibility that employment of the two different MEG systems used here might have affected the present results, t tests comparing data from the 122-channel and 306-channel systems were performed without revealing any significant differences between systems (p > 0.5).
Discussion
Age-related decrements of motor performance have been evidenced in numerous studies leading to the hypothesis that brain processes are slowed in elderly subjects (reviewed in Krampe 2002; Seidler et al. 2010). In line with this hypothesis, the present results revealed significant slowing of CMC, EDC, and M1 while CMC and M1 power amplitudes were shown to linearly increase with age.
EMG power
The analysis did not reveal evidence for differences of EMG power strength between groups suggesting that muscle force expended was comparable in all groups. EMG power frequency at alpha and beta frequencies decreased with age suggesting that lower frequencies predominate in the elderly supporting previous findings (Esposito et al. 1996). Additionally, a shift of motor-unit coherence towards lower frequencies with increasing age has been evidenced (Semmler et al. 2003). In general, slowing of EMG and motor-unit activity has been related to progressive motor-unit loss associated with normal aging (Brown et al. 1988; Doherty et al. 1993; Wang et al. 1999).
M1 power
All subjects showed significant CMC at beta frequency which originated in contralateral M1 replicating previous findings (e.g., Gross et al. 2000). At the alpha range, M1 power frequency was inversely correlated with age supporting the hypothesis that brain dynamics shift towards lower frequencies with increasing age. At beta frequency, power amplitude was shown to systematically increase with age. Power represents an established measure of local brain activation in the frequency domain. Spontaneous oscillations within sensorimotor areas at alpha and beta frequencies have been regarded as idling rhythm (Pfurtscheller 1977; Pfurtscheller and Aranibar 1977) which is decreased by movements. Thus, the observed power increase most likely indicates lower neuronal activation within primary sensorimotor areas (Hari and Salmelin 1997). The present data suggest a decrease of M1 activation (as indicated by higher power) with increasing age. This result might reflect (1) reduction of gray matter shown in elderly subjects (for review, see Raz and Rodrigue 2006) or (2) the reduced ability of motor cortical neurons to desynchronize their oscillatory discharge. The latter interpretation goes along with previous data showing reduced efficacy of motor neuron recruitment during isometric contraction in older subjects (Erim et al. 1999). Nevertheless, we would like to stress that the observation of activation decrease associated with aging might be seen to be in conflict with previous fMRI (D'Esposito et al. 1999) and EEG studies (Sailer et al. 2000; Ziegler et al. 2010) showing increased motor cortical activation in older subjects. Although not entirely clear at the moment, this missing analogy might be due to different motor task demands. Since in the study of Sailer et al. (2000), a finger-tapping task was investigated, one might speculate that controlling each finger tap requires higher level motor control strategies than isometric contraction. Accordingly, increased M1 activation in elderly subjects has been particularly shown in more complex motor tasks (Kim et al. 2010; Noble et al. 2011).
Cerebro-muscular coherence
CMC is a well-established measure of the pyramidal pathway integrity representing an index of motor cortical involvement to a specific motor output (Mima and Hallett 1999). Its reproducibility particularly of coherence frequency has been evidenced previously at least on the group level (Pohja et al. 2005). In line with previous data, CMC was particularly found at beta frequencies which has been established as a characteristic feature of static isometric contraction (Andrykiewicz et al. 2007; Kilner et al. 2000; Kristeva et al. 2007; Salenius and Hari 2003), while CMC at gamma frequencies has been established as a feature of dynamic force contraction (Andrykiewicz et al. 2007; Omlor et al. 2007).
Data analysis revealed a linear increase of CMC amplitude at beta frequency during healthy aging suggesting stronger motor cortical participation with increasing age. This result is in line with EEG (Derambure et al. 1993) and fMRI studies (Heuninckx et al. 2005; Noble et al. 2011; Ward and Frackowiak 2003), suggesting the involvement of an extended network subserving motor control and increased task-related activation, particularly in subcortical brain areas (Noble et al. 2011) in older subjects. It has been argued that with increasing age, motor control might shift from an automatic towards a cognitive mode (Heuninckx et al. 2005). Accordingly, brain activation patterns differing between young and older subjects were particularly found when motor control demands were increased (Kim et al. 2010; Krampe 2002; Noble et al. 2011; Seidler et al. 2010), indicating that additional neural resources are required by the elderly in order to achieve the same performance as younger subjects. Thus, one might speculate that the observed changes might compensate for structural and biochemical decline occurring during healthy aging.
The increase of CMC strength in older subjects indicates a stronger motor cortical involvement to isometric contraction. Thus, decrease of motor cortical activation—as suggested by the present data—might require stronger functional interaction between M1 and muscles in order to stabilize steady-state contraction. This interpretation is in line with the notion that CMC facilitates effective cortico-spinal interaction (Kristeva et al. 2007). Taken together, the observed age-related increase of CMC strength might represent a compensatory mechanism. But, two alternative interpretations can be considered for this result as well. Firstly, one might speculate that the increase of CMC might be simply due to M1 power increase in the elderly yielding an improved signal-to-noise ratio. But, two pharmacological intervention studies suggest that CMC and M1 oscillations represent separate phenomena and strengthen the hypothesis that CMC reflects a significant mechanism rather than a simple transfer of an essential cortical phenomenon (Baker and Baker 2003; Riddle et al. 2004). Secondly, CMC strength and frequency vary with force (Chakarov et al. 2009; Witte et al. 2007). At very low forces, (i.e., 4% of maximum voluntary contraction, MVC) CMC frequency is higher while CMC strength is lower as compared to weak forces (i.e., 16% of MVC; Witte et al. 2007). But, it seems less likely that particularly younger subjects should have systematically adopted lower forces than the elderly. Moreover, force differences should affect the EMG power amplitude as well (Yang et al. 2009), a result that was not found in the present data. All in all, the present finding of increased CMC strength in the elderly is more likely to reflect a functionally relevant mechanism rather than an epiphenomenon.
Increased motor-unit coherence particularly at lower frequencies (i.e., 5–9 Hz) has been shown in older subjects (Semmler et al. 2003) suggesting enhanced common oscillatory input to motor neurons at this frequency. It has been argued that such age-related alterations might contribute to reduced steadiness during sustained contraction in elderly subjects (Semmler et al. 2003) and increased variability of muscle force (Olafsdottir et al. 2007), possibly indicating a neurophysiological marker of physiological aging. Along this line, reduced efficacy of motor neuron recruitment during isometric contraction in older subjects has been suggested (Erim et al. 1999). Since it is likely that CMC represents a neurophysiological marker of conduction in fast pyramidal tracts, the present data imply slowing of cortico-spinal communication associated with aging which might be simply due to alterations of central and peripheral conduction. But, analysis of phase lag did not reveal differences between groups weakening this assumption.
All in all, previous data suggest that slowing of oscillatory activity might represent a general phenomenon of the aging human brain possibly yielding decrements of motor and cognitive performance (Krampe 2002). Interestingly, the observed frequency shift was particularly found between young and middle-aged subjects. This finding implies that in these middle-agers, neurophysiological changes occur possibly reflecting an early neurophysiological marker of seniority. Interestingly enough, changes of CMC amplitude occur as recently as higher age suggesting that compensatory mechanisms take place not in parallel to age-related frequency shifts but when such changes persist.
Conclusion
The present data suggest changes of M1 activity and CMC during healthy aging. While a decline of M1 activity and slowing of M1, EDC, and CMC might represent a general marker of aging, increased coherence amplitude might denote a compensatory mechanism to maintain isometric contraction. Interestingly, frequency shifts were particularly observed in middle-aged subjects suggesting that such changes might represent an early marker of healthy aging.
Acknowledgments
Bettina Pollok is grateful for support from the Deutsche Forschungsgemeinschaft (PO 806/3-1) and from the Research Commission of the Medical Faculty of the Heinrich-Heine University (9772440). Vanessa Krause is grateful for financial support by a grant from Heinrich-Heine-University (9772467). We would like to thank Erika Rädisch for her technical support during MRI scans.
References
- Ahonen AI, Hamalainen MS, Ilmoniemi RJ, Kajola MJ, Knuutila JE, Simola JT, Vilkman VA. Sampling theory for neuromagnetic detector arrays. IEEE Trans Biomed Eng. 1993;40:859–869. doi: 10.1109/10.245606. [DOI] [PubMed] [Google Scholar]
- Andrykiewicz A, Patino L, Naranjo JR, Witte M, Hepp-Reymond MC, Kristeva R. Corticomuscular synchronization with small and large dynamic force output. BMC Neurosci. 2007;8:101. doi: 10.1186/1471-2202-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Binetti G, Cassarino A, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Galderisi S, Hirata K, Lanuzza B, Miniussi C, Mucci A, Nobili F, Rodriguez G, Luca Romani G, Rossini PM. Sources of cortical rhythms in adults during physiological aging: a multicentric EEG study. Hum Brain Mapp. 2006;27:162–172. doi: 10.1002/hbm.20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MR, Baker SN. The effect of diazepam on motor cortical oscillations and corticomuscular coherence studied in man. J Physiol. 2003;546:931–942. doi: 10.1113/jphysiol.2002.029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra TW, van Wijk BC, Praamstra P, Daffertshofer A. Corticomuscular and bilateral EMG coherence reflect distinct aspects of neural synchronization. Neurosci Lett. 2009;463:17–21. doi: 10.1016/j.neulet.2009.07.043. [DOI] [PubMed] [Google Scholar]
- Brown P. Cortical drives to human muscle: the Piper and related rhythms. Prog Neurobiol. 2000;60:97–108. doi: 10.1016/S0301-0082(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Brown WF, Strong MJ, Snow R. Methods for estimating numbers of motor-units in biceps-brachialis muscles and losses of motor-units with aging. Muscle Nerve. 1988;11:423–432. doi: 10.1002/mus.880110503. [DOI] [PubMed] [Google Scholar]
- Chakarov V, Naranjo JR, Schulte-Monting J, Omlor W, Huethe F, Kristeva R. Betarange EEG-EMG coherence with isometric compensation for increasing modulated low-level forces. J Neurophysiol. 2009;102:1115–1120. doi: 10.1152/jn.91095.2008. [DOI] [PubMed] [Google Scholar]
- Classen J, Gerloff C, Honda M, Hallett M. Integrative visuomotor behavior is associated with interregionally coherent oscillations in the human brain. J Neurophysiol. 1998;79:1567–1573. doi: 10.1152/jn.1998.79.3.1567. [DOI] [PubMed] [Google Scholar]
- Conway B, Halliday D, Farmer S, Shahani U, Maas P, Weir A, Rosenberg J. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task. J Physiol. 1995;489:917–924. doi: 10.1113/jphysiol.1995.sp021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derambure P, Defebvre L, Dujardin K, Bourriez JL, Jacquesson JM, Destee A, Guieu JD. Effect of aging on the spatio-temporal pattern of event-related desynchronization during a voluntary movement. Electroencephalogr Clin Neurophysiol. 1993;89:197–203. doi: 10.1016/0168-5597(93)90133-A. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor-unit losses on strength in older men and women. J Appl Physiol. 1993;74:868–874. doi: 10.1063/1.354879. [DOI] [PubMed] [Google Scholar]
- Erim Z, Beg MF, Burke DT, de Luca CJ. Effects of aging on motor-unit control properties. J Neurophysiol. 1999;82:2081–2091. doi: 10.1152/jn.1999.82.5.2081. [DOI] [PubMed] [Google Scholar]
- Esposito F, Malgrati D, Veicsteinas A, Orizio C. Time and frequency domain analysis of electromyogram and sound myogram in the elderly. Eur J Appl Physiol Occup Physiol. 1996;73:503–510. doi: 10.1007/BF00357671. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci USA. 2001;98:694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Tass PA, Salenius S, Hari R, Freund HJ, Schnitzler A. Cortico-muscular synchronization during isometric muscle contraction in humans as revealed by magnetoencephalography. J Physiol. 2000;527(Pt 3):623–631. doi: 10.1111/j.1469-7793.2000.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett. 1998;241:5–8. doi: 10.1016/S0304-3940(97)00964-6. [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. TINS. 1997;20:44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. J Neurosci. 2005;25:6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LM, Halliday DM, Stephens JA, Farmer SF. On the development of human corticospinal oscillations: age-related changes in EEG-EMG coherence and cumulant. Eur J Neurosci. 2008;27:3369–3379. doi: 10.1111/j.1460-9568.2008.06277.x. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci. 2000;20:8838–8845. doi: 10.1523/JNEUROSCI.20-23-08838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Jousmaki V, Hari R, Lemon RN. Task-dependent modulation of 15–30 Hz coherence between rectified EMGs from human hand and forearm muscles. J Physiol. 1999;516(Pt 2):559–570. doi: 10.1111/j.1469-7793.1999.0559v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee YS, Lee JJ, Song HJ, Yoo DS, Lee HJ, Kim HJ, Chang Y. Functional magnetic resonance imaging reveals age-related alterations to motor networks in weighted elbow flexion-extension movement. Neurol Res. 2010;32:995–1001. doi: 10.1179/016164110X12670144737693. [DOI] [PubMed] [Google Scholar]
- Krampe RT. Aging, expertise and fine motor movement. Neurosci Biobehav Rev. 2002;26:769–776. doi: 10.1016/S0149-7634(02)00064-7. [DOI] [PubMed] [Google Scholar]
- Kristeva R, Patino L, Omlor W. Betarange cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output. Neuroimage. 2007;36:785–792. doi: 10.1016/j.neuroimage.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Mackay WA. Synchronized neuronal oscillations and their role in motor processes. Trends Cogn Sci. 1997;1:176–183. doi: 10.1016/S1364-6613(97)01059-0. [DOI] [PubMed] [Google Scholar]
- Mima T, Hallett M. Corticomuscular coherence: a review. J Clin Neurophysiol. 1999;16:501–511. doi: 10.1097/00004691-199911000-00002. [DOI] [PubMed] [Google Scholar]
- Mima T, Steger J, Schulman AE, Gerloff C, Hallett M. Electroencephalographic measurement of motor cortex control of muscle activity in humans. Clin Neurophysiol. 2000;111:326–337. doi: 10.1016/S1388-2457(99)00229-1. [DOI] [PubMed] [Google Scholar]
- Noble JW, Eng JJ, Kokotilo KJ, Boyd LA. Aging effects on the control of grip force magnitude: an fMRI study. Exp Gerontol. 2011;46:453–461. doi: 10.1016/j.exger.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsdottir H, Zhang W, Zatsiorsky VM, Latash ML. Age-related changes in multifinger synergies in accurate moment of force production tasks. J Appl Physiol. 2007;102:1490–1501. doi: 10.1152/japplphysiol.00966.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Omlor W, Patino L, Hepp-Reymond MC, Kristeva R. Gammarange corticomuscular coherence during dynamic force output. Neuroimage. 2007;34:1191–1198. doi: 10.1016/j.neuroimage.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Graphical display and statistical evaluation of event-related desynchronization (ERD) Electroencephalogr Clin Neurophysiol. 1977;43:757–760. doi: 10.1016/0013-4694(77)90092-X. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol. 1977;42:817–826. doi: 10.1016/0013-4694(77)90235-8. [DOI] [PubMed] [Google Scholar]
- Pohja M, Salenius S, Hari R. Reproducibility of cortex-muscle coherence. Neuroimage. 2005;26:764–770. doi: 10.1016/j.neuroimage.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Baker MR, Baker SN. The effect of carbamazepine on human corticomuscular coherence. Neuroimage. 2004;22:333–340. doi: 10.1016/j.neuroimage.2003.12.040. [DOI] [PubMed] [Google Scholar]
- Sailer A, Dichgans J, Gerloff C. The influence of normal aging on the cortical processing of a simple motor task. Neurology. 2000;55:979–985. doi: 10.1212/WNL.55.7.979. [DOI] [PubMed] [Google Scholar]
- Salenius S, Hari R. Synchronous cortical oscillatory activity during motor action. Curr Opin Neurobiol. 2003;13:678–684. doi: 10.1016/j.conb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol. 1997;77:3401–3405. doi: 10.1152/jn.1997.77.6.3401. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Neurosci Rev. 2005;6:285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler JG, Kornatz KW, Enoka RM. Motor-unit coherence during isometric contractions is greater in a hand muscle of older adults. J Neurophysiol. 2003;90:1346–1349. doi: 10.1152/jn.00941.2002. [DOI] [PubMed] [Google Scholar]
- Wang FC, de Pasqua V, Delwaide PJ. Age-related changes in fastest and slowest conducting axons of thenar motor-units. Muscle Nerve. 1999;22:1022–1029. doi: 10.1002/(SICI)1097-4598(199908)22:8<1022::AID-MUS3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Ward NS. Compensatory mechanisms in the aging motor system. Ageing Res Rev. 2006;5:239–254. doi: 10.1016/j.arr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte M, Patino L, Andrykiewicz A, Hepp-Reymond MC, Kristeva R. Modulation of human corticomuscular betarange coherence with low-level static forces. Eur J Neurosci. 2007;26:3564–3570. doi: 10.1111/j.1460-9568.2007.05942.x. [DOI] [PubMed] [Google Scholar]
- Yang Q, Fang Y, Sun CK, Siemionow V, Ranganathan VK, Khoshknabi D, Davis MP, Walsh D, Sahgal V, Yue GH. Weakening of functional corticomuscular coupling during muscle fatigue. Brain Res. 2009;1250:101–112. doi: 10.1016/j.brainres.2008.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Pritchett DL, Hosseini-Varnamkhasti P, Corkin S, Hamalainen M, Moore CI, Jones SR. Transformations in oscillatory activity and evoked responses in primary somatosensory cortex in middle age: a combined computational neural modeling and MEG study. Neuroimage. 2010;52:897–912. doi: 10.1016/j.neuroimage.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]