Abstract
The time-course for aging-associated effects on vascular reactivity to U46619, a stable analogue of thromboxane A2 (TXA2), was studied in aorta from female senescence-accelerated mice-prone (SAMP8), a murine model of accelerated senescence. SAMP8 and senescence-accelerated mice-resistant (SAMR1) were divided into three groups: 3-, 6- and 10-month-old. Contractile curves to U46619 (10−9 to 10−6 M) were performed in aortic rings in the absence or in the presence of nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 10−4 M) and/or cyclooxygenase (COX) inhibitor indomethacin (10−5 M). Protein and gene expression for COX-1 and COX-2 were determined by immunofluorescence and real-time PCR, respectively. Maximal contraction to U46619 was markedly higher in SAMP8 at all ages. In SAMR1, increases were seen at 10 months, while SAMP8 displays augmented contraction at 6 months, which was further increased at 10 months. l-NAME enhanced U46619 contractions in both 6-month-old groups, although the increase was higher on vessels from SAMR1 at this age. Indomethacin equally increased U46619 contractions in both 3-month-old groups, suggesting the production of vasodilator prostaglandin in young animals. In contrast, at 6 and 10 months indomethacin decreased U46619 contractions in both groups, indicating an aging-associated swap to a release of contractile prostanoids in aorta. In conclusion, aging enhances contractile responses to TXA2 in aorta from female mice by a mechanism involving a decrease of NO production and increased action of contractile prostanoids. This process occurs earlier in SAMP8 mice, establishing these mice as good model to study cardiovascular aging in a convenient and standard time-course.
Keywords: U46619, NO bioavailability, Contractile prostanoids, Vascular reactivity
Introduction
Aging is a physiological process associated with an increase in cardiovascular morbidity and mortality, even in the absence of known cardiovascular risk factors (Lakatta and Levy 2003). Throughout aging, structural and functional alterations are produced in vasculature. Vascular aging is associated with endothelial dysfunction, arterial stiffening and remodeling, impaired angiogenesis, defective vascular repair, and with an increasing prevalence of atherosclerosis (Erusalimsky 2009; Lakatta and Levy 2003).
During their fertile life, women have a lower risk of cardiovascular disease, as compared to their male peers. However, this protection is lost after menopause, when besides aging, a decline of sex hormones production has been associated to increased risk of cardiovascular disease (Kannel 2002). Unfortunately, little information is available on the vascular effects of aging in females.
Changes in vascular pathophysiology have been extensively studied in mice models because their physiological and genetic parallel with human cardiovascular system (Paigen et al. 1994; Yutzey and Robbins 2007). Particularly, the senescence-accelerated mouse-prone 8 strain (SAMP8) has been described as one of the most appropriate models to study vascular aging and age-associated diseases compared with the senescence-accelerated mouse-resistant 1 (SAMR1) strain (Butterfield and Poon 2005; Miyamoto 1997). Vascular studies using these models are not abundant, but few studies in male (Llorens et al. 2007) and female (Novella et al. 2010) mice have shown morphological alterations, mechanical and endothelial dysfunction.
Thromboxane A2 (TXA2), a lipid mediator synthesized from arachidonic acid metabolism through the cyclooxygenase (COX) pathway, is a powerful constrictor of vascular smooth muscle (Narumiya et al. 1999). TXA2 is produced in far smaller quantities, primarily by the platelets, and to a lesser extent by some systemic blood vessels (Smith 1986). Recent studies reveal that the TXA2 pathway is modulated by estrogens, and therefore, may play an important role in the regulation of vascular tone and blood pressure in females, in both normal and in pathophysiological states (Sellers and Stallone 2008). Constrictor prostanoids, such as TXA2 are known to regulate vascular tone by increasing contractile response of the systemic vasculature. Studies have shown that endothelium-derived prostaglandins increase vessel tone in aged females (Stewart et al. 2000), although few studies have addressed to the specific role of TXA2 to vascular aging in females.
We have recently reported vascular alterations in middle-aged SAMP8 female mice. In this model, aging-related changes on vascular reactivity was associated to changes on NO and eicosanoids pathways (Novella et al. 2010). In this study, we aimed to identify the time points for functional and molecular changes in the vascular wall in response to TXA2 on SAMP8 and SAMR1 models. For that, female mice were studied at ages spanning from young adult to near the mean survival.
Material and methods
Experimental animals
Female SAMR1 (n = 42) and SAMP8 (n = 42) were obtained from the breeding stock at Parc Cientific de Barcelona and housed according to institutional guidelines (constant room temperature—22°C, 12 h light/dark cycle, 60% humidity, standard mice chow and water ad libitum). All protocols were approved by the Institutional Ethics Committee at the University of Valencia, conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Both SAMR1 and SAMP8 mice were euthanized under anesthesia at 3, 6, and 10 months old for tissue harvesting.
Determination of biochemical variables
Blood samples were withdrawn by cardiac puncture and centrifuged at 1,200 × g for 10 min. Plasma levels of glucose, creatinine, and 17β-estradiol were determined using an automatic analyzer (ADVIA Centaur® CP Immunoassay System, Siemens, Munich, Germany) at a commercial analytical service center. The levels of NO metabolites (NO−2/NO−3) were determined in the plasma by a commercial colorimetric assay kit (Cayman Chemical Company) following the supplier’s instructions.
Isolated mouse aorta preparation
Thoracic aorta was excised, placed immediately in ice-cold Krebs–Henseleit solution and cleaned of surrounding tissue. Arteries were dissected into 4-mm rings, mounted between two stainless steel holders (100 μm inner diameter), and placed in a 4 ml organ baths containing modified Krebs–Henseleit (in mM: NaCl 115; KCl 4.6; KH2PO4 1.2; MgCl2 1.2; CaCl2 2.5; NaHCO3 25; glucose 11.1; EDTA 0.01, pH 7.3–7.4) kept at 37°C and aerated with 95% O2/5% CO2 for isometric force measurements (Grass FT03, Grass Instruments Division Astromed, Inc., West Warwick, RI, U.S.A.). Changes in isometric force were recorded by use of Chart v. 3.4/s software and a MacLab/8e data acquisition system (ADInstruments, East Sussex, UK). Once the optimal resting tension was reached (1 g), aortic rings were allowed to attain a steady level of tension during a 1-h equilibration period before testing. Functional integrity of the endothelium was confirmed routinely by the presence of relaxation induced by acetylcholine (10−7–10−6 M) during contraction obtained with serotonin (10−5 M).
In vitro tension measurement
Following the equilibration period, arterial segments were exposed to receptor-independent depolarizing agent KCl (60 mM) until the contraction reached a stable plateau (10 to 20 min). After washout and return to stable baseline, contractile responses were determined by cumulative concentration–response curves to KCl (5–120 mM) or the TXA2 mimetic U46619 (10−9 to 3 × 10−7 M). In some experiments, rings were incubated for 15 min with NG-nitro-l-arginine methyl ester (l-NAME, 10−4 M), indomethacin (10−5 M) or the combination of l-NAME (10−4 M) plus indomethacin (10−5 M).
Immunofluorescence
Protein expression and localization of prostaglandin-associated enzymes COX-1 and COX-2 were determined in aortic sections from SAMR1 and SAMP8 mice by immunofluorescence as previously described (Novella et al. 2010). Aortic sections (4 μm) were thaw-mounted onto polylysine covered slides, fixed in acetone (15 min) and blocked for 30 min with horse serum. Sections were incubated overnight at 4°C with primary antibodies: 1:200 anti-COX-1 (Santa Cruz—sc19998) or 1:200 anti-COX-2 (Santa Cruz—sc19999). Following washes, sections were co-stained with 10 μM phalloidin (Sigma) and secondary antibodies 1:500 Alexa Fluor 488 conjugated goat anti-rabbit (Invitrogen). Coverslips were mounted on slides using ProLong Gold antifade reagent with DAPI (Invitrogen), and sections were visualized through a confocal microscope (Axiovert 2000, Carl Zeiss Inc) with a 40× objective lens (Zeiss). For each image, light was passed through a different excitation filter: (1) 350 nm (for DAPI); (2) 490 nm (for Alexa 488); and (3) 590 nm (for phalloidin). Each aorta was recorded in three different regions and results were expressed as an average of fluorescence elicited using Mac Biophotonic ImageJ Software.
Quantitative real-time PCR
Total RNA was isolated and reverse transcribed as previously described (Novensa et al. 2010). mRNAs encoding COX-1 and COX-2 were quantified by Quantitative real-time PCR (qRT-PCR) based on SYBR® Green fluorescence, using the GAPDH mRNA as internal control. The specific primer sequences for mice were: COX-1 (NM_008969.3) 5′-CCA GTG CTG GGG CAG TGC TG-3′, 5′-ACA CGG ACG CCT GTT CCA CG-3′; COX-2 (NM_011198.3) 5′-TCC GAG CTG TGC TGC TCT GC-3′, 5′-GCC CAG TCC TCG GGT GAA CC-3′; GAPDH (NM_008084.2) 5′-ACC CCA GCA AGG ACA CTG AGC AAG-3′, 5′-TGG GGG TCT GGG ATG GAA ATT GTG-3′. Real-time PCR reactions were set following the manufacture’s conditions (Applied Biosystems). Ct values obtained for each gene were normalized to Ct of housekeeping gene GAPDH (ΔCt) and converted to the linear form using the term 2-ΔCt, and expressed as 2−ΔCt.
Drugs
The following drugs were used: serotonin hydrochloride, acetylcholine chloride, NG-nitro-l-arginine methyl ester hydrochloride (l-NAME), indomethacin and 9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α (U46619) were obtained from Sigma (Sigma Chemical Co, St. Louis, MO, USA). All drugs for vascular reactivity studies were dissolved in Krebs solution except U46619, which was dissolved initially in ethanol, and indomethacin, which was dissolved in ethanol and sodium bicarbonate solution (150 mM) and readjusted to pH 7.4 with HCl prior to use further diluted in Krebs solution to the proper final concentration. Stock solutions of each drug were freshly prepared at the day of experiment, and kept on ice throughout the experiment.
Data analysis
Data are expressed as means ± SEM. Contractions to KCl and U46619 are shown as absolute tension (in milligrams). The pD2 (negative logarithm of the molar concentration at which half-maximum contraction occurs) was determined from individual concentration–response curves by non-linear regression analysis. Area under the concentration–response curve (AUC) was calculated from each individual concentration–response curve to U46619 and was expressed as arbitrary units. The contribution of NO and prostanoids to the vascular contraction induced by the TXA2 analogue was calculated by subtracting from the AUC for U46619, the AUC for U46619 in the presence of l-NAME and/or indomethacin. In each experimental group n indicates the number of animals. Differences between mouse strains (i.e., SAMR1 vs. SAMP8) and by experimental groups (i.e., 3- vs. 6- vs. 10-month-old groups) were analyzed by two-way ANOVA, followed by Bonferroni’s post-test to compare replicate means. Statistical significance was accepted at P < 0.05. The statistical analysis was carried out using the Prism 4 software (GraphPad Software Inc., San Diego, CA, USA).
Results
Biochemical and morphological parameters
In both strains, body weight significantly increased with age, although SAMP8 weighed much less at 6 and 10 months than SAMR1 (Table 1). Uterine weights and estrogen levels did not differ among groups, indicating that female mice did not change ovarian function with aging. Besides, a trend to increase glucose was observed and creatinine plasmatic concentrations were not affected by aging.
Table 1.
Physiological and biochemical parameters in female SAMR1 and SAMP8
| SAMR1 | SAMP8 | |||||
|---|---|---|---|---|---|---|
| 3 months | 6 months | 10 months | 3 months | 6 months | 10 months | |
| Body weight (g) | 23.7 ± 0.2 | 26.7 ± 0.6* | 30.7 ± 1.0*,*** | 23.2 ± 0.3 | 24.6 ± 0.4*,**** | 27.4 ± 0.7*,***,**** |
| Uterus weight (mg) | 105 ± 8 | 108 ± 10 | 112 ± 7 | 100 ± 5 | 100 ± 7 | 109 ± 5 |
| Glucose (mg/dL) | 165 ± 31 | 170 ± 18 | 185 ± 17 | 159 ± 13 | 173 ± 15 | 197 ± 11 |
| Creatinine (mg/dL) | 0.29 ± 0.01 | 0.27 ± 0.01 | 0.26 ± 0.02 | 0.29 ± 0.02 | 0.28 ± 0.01 | 0.30 ± 0.02 |
| 17β-estradiol (pg/mL) | 286 ± 76 | 317 ± 66 | 283 ± 67 | 328 ± 59 | 286 ± 50 | 309 ± 36 |
| NO−2/NO−3 (μM) | 22.2 ± 1.3 | 16.8 ± 1.0 | 13.1 ± 1.1** | 19.1 ± 1.7 | 10.5 ± 1.6**,**** | 8.3 ± 0.4**,**** |
Values are means ± SEM. n = 10 animals per group
*P < 0.05 and **P < 0.01 versus 3-month-old group of the same strain, ***P < 0.05 versus 6-month-old group of the same strain, ****P < 0.05 versus SAMR1 with same age
Effects of age on contractile responses
Age-dependent increase of contraction in response to KCl
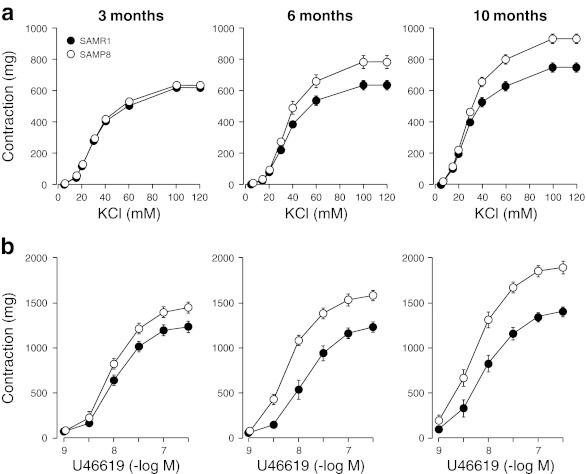
Figure 1a shows concentration-dependent responses induced by cumulative applications of KCl to aortic rings from SAMR1 and SAMP8 groups, and Table 2 shows the corresponding pD2 values and maximal contractions (Emax). In aorta from 3-month-old groups, pD2 values and maximal responses did not differ in both SAMR1 and SAMP8 mice. Differences between strains start to appear at 6 months, when maximal contractions in response to KCl were significantly enhanced in aorta from SAMP8 compared to SAMR1, and continue increasing when mice are 10 months old. Responses at 10-month-old SAMR1 were comparable to that obtained at 6-month-old SAMP8.
Fig. 1.
Concentration–response curves to a KCl (5–120 mM) and b the analogue of thromboxane A2 U46619 (10−9 to 3×10−7 M) in aortic rings from 3-, 6-, and 10-month-old SAMR1 and SAMP8 mice. Each data point show mean ± SEM from n = 10 mice for each group
Table 2.
pD2 values and maximal responses (Emax) to KCl in aortic rings from 3-, 6-, and 10-month-old groups of both SAMR1 and SAMP8 mice
| SAMR1 | SAMP8 | |||
|---|---|---|---|---|
| pD2 | Emax | pD2 | Emax | |
| 3 months | 1.48 ± 0.01 | 620 ±15 | 1.48 ± 0.01 | 637 ± 21 |
| 6 months | 1.44 ± 0.01 | 634 ± 25 | 1.45 ± 0.02 | 784 ± 38*,** |
| 10 months | 1.53 ± 0.02**,*** | 748 ± 24**,*** | 1.52 ± 0.01**,*** | 933 ± 23*,**,*** |
Values are means ± SEM. n = 10 animals per group
*P < 0.05 vs. SAMR1 group with same age, and **P < 0.05 vs. 3-month-old group of the same strain, and ***P < 0.05 vs. 6-month-old group of the same strain
Age-dependent increase of contraction in response to U46619
Contractile responses to the TXA2 analogue U46619 were increased in all groups of SAMP8 in comparison to SAMR1 (Fig. 1b and Table 3), although a marked difference on the time-course for hyper-reactivity to TXA2 was observed when SAMR1 and SAMP8 were compared. In SAMR1, increases on Emax and pD2 values by U46619 were only seen at 10-month-old mice (Fig. 1b and Table 3). Conversely in SAMP8, both Emax and pD2 values to U46619 starts to enhance at 6-month-old group, and is further increased at 10 months of age (P < 0.05; Fig. 1b and Table 3).
Table 3.
pD2 values and maximal responses (Emax) to U46619 in aortic rings in the absence (Control) and in the presence of l-NAME (10−4 M), indomethacin (10−5 M) or l-NAME plus indomethacin from 3-, 6-, and 10-month-old of both SAMR1 and SAMP8 mice groups
| SAMR1 | SAMP8 | |||
|---|---|---|---|---|
| pD2 | Emax | pD2 | Emax | |
| Control | ||||
| 3 months | 7.93 ± 0.04 | 1,224 ± 50 | 8.07 ± 0.04 | 1,435 ± 60* |
| 6 months | 7.92 ± 0.09 | 1,230 ± 60 | 8.24 ± 0.03*,** | 1,581 ± 51*,** |
| 10 months | 8.13 ± 0.08**,*** | 1,400 ± 44**,*** | 8.30 ± 0.08*,** | 1,893 ± 67*,**,*** |
| l-NAME 10−4 M | ||||
| 3 months | 8.27 ± 0.06**** | 1,496 ± 83**** | 8.30 ± 0.05**** | 1,702 ± 99**** |
| 6 months | 8.21 ± 0.08**** | 1,553 ± 81**** | 8.19 ± 0.05 | 1,851 ± 49**** |
| 10 months | 8.16 ± 0.06 | 1,484 ± 54 | 8.26 ± 0.05 | 1,876 ± 80 |
| Indomethacin 10−5 M | ||||
| 3 months | 7.90 ± 0.05 | 1,435 ± 49**** | 8.01 ± 0.04 | 1,651 ± 81**** |
| 6 months | 7.58 ± 0.07**,**** | 1,146 ± 77** | 7.73 ± 0.05**,**** | 1,544 ± 83 |
| 10 months | 7.60 ± 0.06**,**** | 1,177 ± 70**,**** | 7.78 ± 0.04***,**** | 1,582 ± 61**** |
| l-NAME 10−4 M + indomethacin 10−5 M | ||||
| 3 months | 8.02 ± 0.02 | 1,578 ± 60**** | 8.12 ± 0.05 | 1,839 ± 87*,****,***** |
| 6 months | 7.67 ± 0.03** | 1,326 ± 70 | 7.88 ± 0.05***** | 1,733 ± 58* |
| 10 months | 7.76 ± 0.04**,***** | 1,493 ± 77 | 8.00 ± 0.04***** | 1,888 ± 64*,***** |
Values are means ± SEM. n = 10 animals per group
*P < 0.05 vs. SAMR1 group with same age, **P < 0.05 vs. 3-month-old group of the same strain and treated group, ***P < 0.05 vs. 6-month-old group of the same strain and treated group, ****P < 0.05 vs. same age and strain of control group and *****P < 0.05 vs. same age and same strain indomethacin group
Effects of aging on NO contribution to vascular function
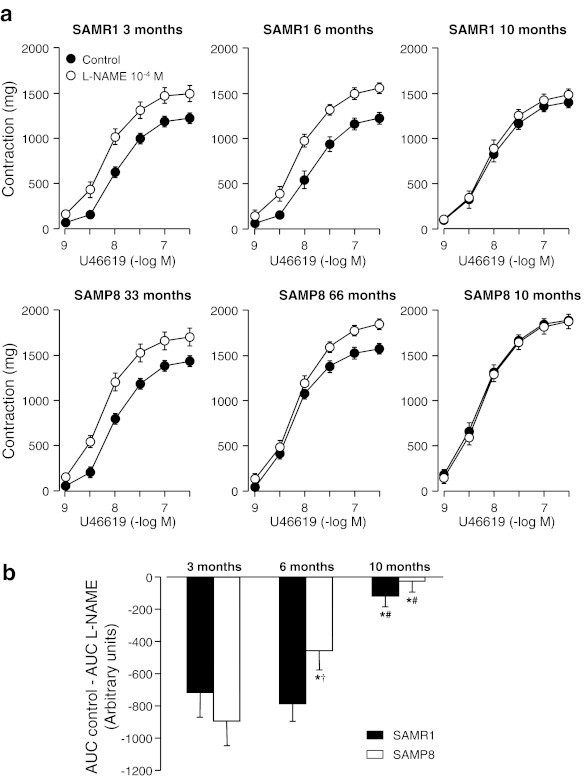
At 3 months of age, treatment with the NOS inhibitor l-NAME (10−4 M) similarly increased Emax and pD2 values to the TXA2 analogue U46619 in both SAMR1 (ΔAUC, 717 ± 125) and SAMP8 (ΔAUC, 895 ± 156). Differences on NO contribution to TXA2-induced contractions were noticed at 6 months of age. Although l-NAME enhanced U46619 contractions in both 6-month-old SAMR1 and SAMP8, the increase observed on vessels from SAMR1 was much higher compared to SAMP8 (ΔAUC, 790 ± 129 and 520 ± 122, respectively). At the age of 10 months, l-NAME treatment induced no effects on contractions by U46619 in both SAMR1 and SAMP8 (Fig. 2 and Table 3). In support to the data on vascular reactivity, biochemical studies reveal an earlier and more pronounced aging-associated decrease of NO production in SAMP8 when compared to SAMR1. Measurement of NO metabolites NO−2/NO−3 in plasma samples of SAMR1 and SAMP8 mice reveals a progressive decrease on NO production in SAMP8, while SAMR1 mice shows a significant decrease on NO production only at age of 10 months (Table 1).
Fig. 2.
a Concentration–response curves to U46619 in aortic rings from 3-, 6-, and 10-month-old SAMR1 and SAMP8 mice in the absence (Control) and in the presence of NOS synthase inhibitor l-NAME (10−4 M). b Area under curves (AUC) from concentration–response curves in absence (control) minus AUC in the presence of l-NAME (10−4 M). Each data point show mean ± SEM from n = 10 mice for each group. *P < 0.05 vs. 3-month-old group of the same strain and treated group, #P < 0.05 vs. 6-month-old group of the same strain, and †P < 0.05 vs. SAMR1 group with same age
Aging-associated effects on COX pathway
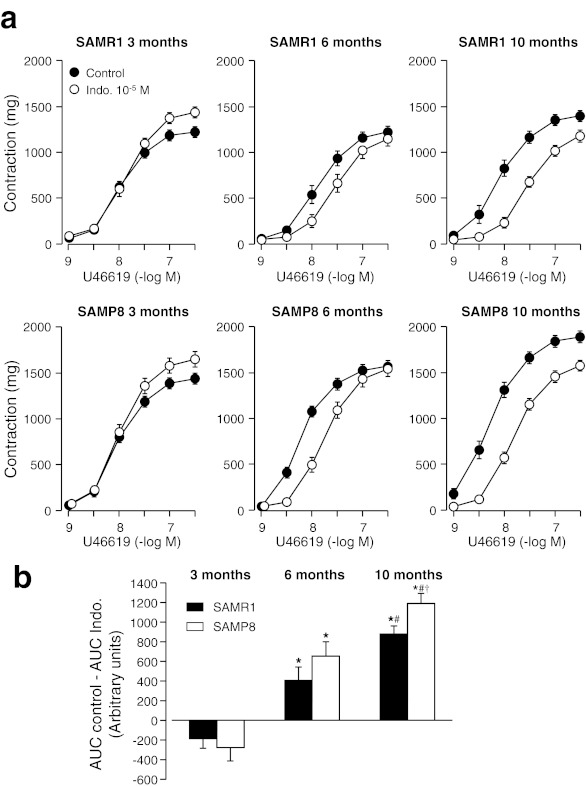
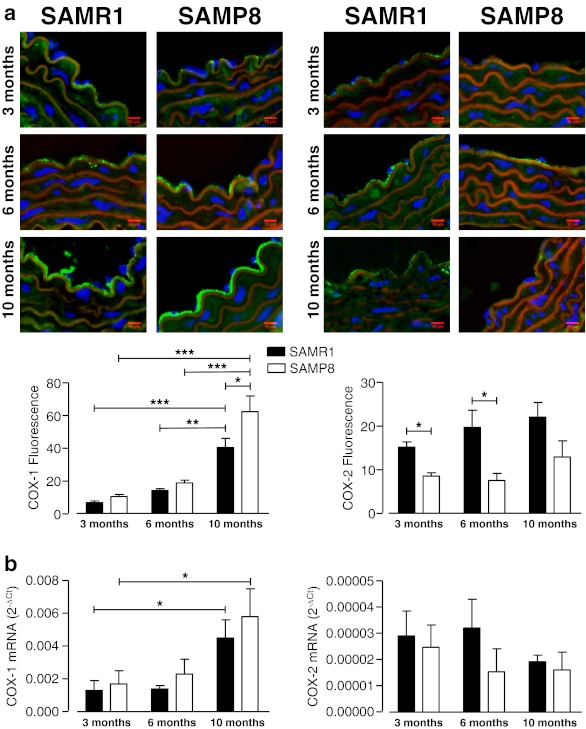
The COX inhibitor indomethacin (10−5 M) did not change the pD2 values but significantly increased the maximal response to U46619 in aortic rings of both SAMR1 and SAMP8 mice at 3 months of age, indicating the involvement of relaxing prostanoids as modulators of the U46619 response at this age (Fig. 3 and Table 3). Conversely, indomethacin decreased the sensitivity to U46619 at 6 months, and the sensitivity and Emax to U46619 at 10 months (Fig. 3 and Table 3), suggesting a swap from the contribution of relaxing prostaglandins to a higher involvement of vasoconstrictor prostaglandins to the modulation of vascular contraction. Analysis of gene and endothelial protein expression of the two subtypes of COX (COX-1 and COX-2) show an aging-associated increase of COX-1 expression that was evident at 10 months of age (Fig. 4a and b). Conversely, aging did not affect COX-2 expression, even though vessels from SAMR1 express a higher level of COX-2 protein than do SAMP8 mice (Fig. 4a and b).
Fig. 3.
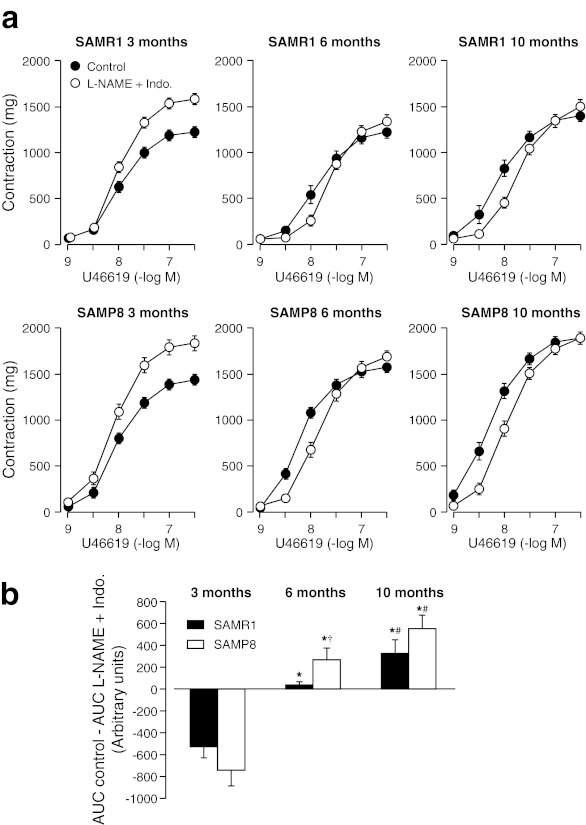
a Concentration–response curves to U46619 in aortic rings from 3-, 6-, and 10-month-old SAMR1 and SAMP8 mice in the absence (Control) and in the presence of the nonselective COX inhibitor indomethacin (Indo. 10−5 M). b Area under curves (AUC) from concentration–response curves in absence (control) minus AUC in the presence of Indo. (10−5 M). Each data point show mean ± SEM from n = 10 mice for each group. *P < 0.05 vs. 3-month-old group of the same strain, #P < 0.05 vs. 6-month-old group of the same strain, and †P < 0.05 vs. SAMR1 group with same age
Fig. 4.
Cyclooxygenase (COX) expression in thoracic aorta from SAMR1 and SAMP8 female mice at different ages: atop: representative immunofluorescent merged images of COX-1 (left) and COX-2 (right). Staining shows nucleus (blue, DAPI), actin fibers (red, phalloidin), COX (green). Bar graphs (bottom) show the results of densitometric analyses from pooled data of endothelial expression of COX-1 and COX-2. b COX-1 (left) and COX-2 (right) mRNA expression in mice aorta normalized to the expression of GAPDH, which was used as an endogenous reference gene. Data are plotted as the mean ± SEM derived from four to eight independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001
Effects of both NOS- and COX- inhibition on contractile responses to U46619
Concomitant treatment with l-NAME (10−4 M) and indomethacin (10−5 M) did not change pD2 values, but increased significantly the maximal response to U46619 in aortic rings of both SAMR1 and SAMP8 mice at the age of 3 months. In the 6- and 10-month-old groups maximal responses did not change, but significantly decreased the pD2 values. Interestingly, COX inhibition partially restored the increasing effects of l-NAME on the contractile responses to U46619 (Fig. 5 and Table 3).
Fig. 5.
a Concentration–response curves to U46619 in aortic rings from 3-, 6-, and 10-month-old SAMR1 and SAMP8 mice in the absence (Control) and in the presence of l-NAME (10−4 M) plus indomethacin (Indo. 10−5 M). b Area under curves (AUC) from concentration–response curves in absence (control) minus AUC in the presence of l-NAME (10−4 M) plus Indo. (10−5 M). Each data point show mean ± SEM from n = 10 mice for each group. *P < 0.05 vs. 3-month-old group of the same strain and treated group, #P < 0.05 vs. 6-month-old group of the same strain, and †P < 0.05 vs. SAMR1 group with same age. Each data point show mean ± SEM from n = 10 mice for each group
Discussion
The present study brings new insights in the comprehension of the physiological role of aging on TXA2 responses in aorta from female mice with accelerated senescence. Our results indicate that aging (1) induces an increment in contractile responses to KCl and TXA2 analogue U46619, (2) evokes a decrease of NO bioavailability in response to U46619, and (3) modifies the functional prostanoids involved in the regulation of TXA2 contraction. Although aging similarly affects vascular function in both SAMR1 and SAMP8, those responses were accelerated in SAMP8, establishing this strain as an appropriate model to study vascular effects of aging in females.
At 3 months of age, both strains exhibited a similar, receptor-independent contractile response induced by high extracellular concentrations of KCl, thus discarding differences due to strain-specific changes. Nevertheless, at 6 months old, and even further at 10 months old, contractile responses in SAMP8 mice were higher than those of SAMR1. The increased contractions to KCl in SAMP8 indicate that intrinsic mechanisms contribute to the altered vasoconstrictor responses. KCl-induced constriction is mediated primarily via depolarization-induced opening of voltage-gated Ca2+ channels and influx of extracellular Ca2+ (Hall et al. 2006). Accordingly, the ability of aging in SAMP8 mice to increase constrictor responses to KCl is suggestive of an action on voltage-gated Ca2+ channels or distal mechanisms that respond to this influx of Ca2+.
Examination of plasma glucose and creatinine level, demonstrated that there were no differences between SAMR1 and SAMP8 mice, which rules out a possible contribution of age-induced metabolic changes or impaired renal function to vasomotor dysfunction. Furthermore, measurements of 17β-estradiol levels did not differ between SAMR1 and SAMP8 animals at different ages, which exclude hormonal status as an explanation for the observed differences in vascular responses. Like other rodents, SAM mice do not reach reproductive senescence until later in life (Yuan et al. 2005). According to uterine weight data and 17β-estradiol plasma levels in SAM animals, we can suggest that, at least until 10 months, SAMR1 and SAMP8 exhibit similar hormonal status, confirming this hypothesis. For this reason, the SAM model still could not be considered, as such, as a good model to study natural menopause, despite being a good model to study progressive aging. In this regard, in a previous study we have described middle-aged SAMP8 ovariectomized mice as a new model to concomitantly study the effects of aging and menopause in female mice (Novella et al. 2010).
In addition to the changes on intrinsic mechanisms to evoke vasoconstriction by aging, SAMP8 model reveled to be a great model to study aging-associated modifications on signaling pathways involved in the control of vasomotion. In our studies, we have observed a significant aging-associated increase on vasoconstriction induced by the TXA2 analogue U46619, which was more pronounced in females with accelerated senescence (SAMP8) than those with a normal life span (SAMR1). The enhanced contractile responses observed in our study to U46619 were associated to a decrease of vasodilators and/or an increase of vasoconstrictors. Our data suggest that increase in the release of NO and vasodilator prostaglandins in response to TXA2 receptor activation may contribute to a lower contractile response in young females, since both NOS inhibitor (l-NAME) and the COX inhibitor (indomethacin) augmented contractile responses in aorta from mice at 3 month of age. The increased contractions induced by l-NAME were reduced with aging in both SAMR1 and SAMP8 mice, suggesting a decrease of NO bioavailability by aging. Besides, our measurement of plasmatic concentration of NO metabolites NO−2/NO−3 revealed an aging-associated decrease on NO concentration. These findings are consistent with previous observations of aging-related decline in NO bioavailability (Collins and Tzima 2011; Taddei et al. 1995; 1997). Nevertheless these effects were observed before in SAMP8 (at 6 months) than in SAMR1 (only at 10 months), suggesting that a decrease of NO bioavailability by aging starts earlier in our model of accelerated senescence. Contrary to our data, an age-related increase of NOS activity without changes in NO levels has been described in cerebral cortex of SAMP8 mice (Inada et al. 1996). The increased NOS activity observed in the brain may be associated to increased mRNA expression of the neuronal isoform of NOS (nNOS) (Colas et al. 2006). In aortas from 6-month-old male mice, where endothelial NOS (eNOS) is the predominant isoform, both SAMR1 and SAMP8 showed similar eNOS immunoreactivity, suggesting that decreased NO bioavailability might not be related to changes in eNOS protein expression in SAMP8 (Llorens et al. 2007).
Regarding to prostaglandin production by U46619, contractions induced by this agonist were interestingly attenuated by the nonselective COX inhibitor (indomethacin) at 6 and 10 months, suggesting a higher production of vasoconstrictor prostanoids. With aging, along with a further impairment of l-arginine–NO pathway, COX-dependent vasoconstrictors production becomes evident (Rodriguez-Manas et al. 2009; Taddei et al. 1997). This is supported to the fact that aspirin (a well-known COX inhibitor) is routinely prescribed to patients with vascular disease. Furthermore, Aspirin use has been associated with significantly lower risk of all-cause mortality, and more specifically, cardiovascular mortality, among women with stable cardiovascular disease (Berger et al. 2009). Moreover, older women were noted to have the greatest benefit with aspirin therapy, a finding consistent with the Women’s Health Initiative study (Ridker et al. 2005). Since stimulation or upregulation of COX is able to produce vasodilator and vasoconstrictor products, our results in aorta from SAM mice interestingly demonstrate that aging is associated with a shift towards vasoconstrictor products.
Agonist-induced activation of the arachidonic acid cascade leads to the production of both vasodilator and vasoconstrictor prostaglandins by the endothelium, and how distinct pathophysiological conditions affect the equilibrium of prostaglandin release in either direction is largely unknown. In our studies, we attempt to determine if changes on COX-1/COX-2 expression could account to the swap on prostaglandin release during aging. Our data show a significant increase of both gene and protein expression of COX-1, although this increase does not explain the shift on prostaglandin-mediated responses since it was seen only when mice were 10 months old. In previous studies, we have demonstrated an increased contraction to U46619 in association with TXA2 receptor expression in 6-month-old SAMP8 female aorta compared with age-matched SAMR1 (Novella et al. 2010). In corroboration with our studies is an observation that augmented prostanoid-dependent vasoconstriction in other pathophysiological condition (i.e., obesity) is associated to increased TXA2 receptor gene expression rather than change of prostaglandin metabolism (Traupe et al. 2002).
One of the trickiest parts in the field concerns to the selection of the most appropriate age to investigate mechanisms involved in vascular aging when we use rodent models. Most studies have been done in mice on the verge of senescence and thus were unable to correlate vascular alterations that may occur during the aging process or at early stages of aging, when cardiovascular damage starts. Our functional and molecular analyses in SAMR1 and SAMP8 mice indicate that contractile responses are similar or slightly enhanced between young mice at 3 months of age, but become statistically different by 6 months and continue to change thereafter. This should be considered when selecting the most appropriate time points for investigating the mechanisms mediating vascular aging.
Our results demonstrated that contractile response to TXA2 is increased with aging by a mechanism involving the decreased bioactivity of NO and increased production of contractile prostanoids. The inhibition of COX induces an increase of NO bioavailability. These effects occur earlier in aorta from female SAMP8 mice and are manifest at 6-month-old mice, establishing SAMP8 mice as a good model to study cardiovascular aging in a convenient and standard time-course.
Acknowledgments
We are indebted to Drs. Inmaculada Noguera and Ana Diaz for their excellent veterinary assistance, to Nadia Castillo for her exceptional technical assistance and to Maria Calvo and her group on Confocal Facility at IDIBAPS for great assistance with experiments using confocal imaging. This work was supported by the Spanish Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III - FEDER-ERDF (grants FIS PI10/00518, FIS PI080176 and Red HERACLES RD06/0009), Consellería de Sanidad, Generalitat Valenciana (grants AP 097/2011, AP 104/2011 and GE 027/2011), and Spanish Society of Cardiology (DN040480).
Footnotes
Susana Novella and Ana Paula Dantas contributed equally to this work.
References
- Berger JS, Brown DL, Burke GL, Oberman A, Kostis JB, Langer RD, Wong ND, Wassertheil-Smoller S. Aspirin use, dose, and clinical outcomes in postmenopausal women with stable cardiovascular disease: the Women’s Health Initiative Observational Study. Circ Cardiovasc Qual Outcomes. 2009;2:78–87. doi: 10.1161/CIRCOUTCOMES.108.791269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF. The senescence-accelerated prone mouse (SAMP8): a model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp Gerontol. 2005;40:774–783. doi: 10.1016/j.exger.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Colas D, Gharib A, Bezin L, Morales A, Guidon G, Cespuglio R, Sarda N. Regional age-related changes in neuronal nitric oxide synthase (nNOS), messenger RNA levels and activity in SAMP8 brain. BMC Neurosci. 2006;7:81. doi: 10.1186/1471-2202-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C, Tzima E. Hemodynamic forces in endothelial dysfunction and vascular aging. Exp Gerontol. 2011;46:185–188. doi: 10.1016/j.exger.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol. 2009;106:326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Jones TH, Channer KS, Jones RD. Mechanisms of agonist-induced constriction in isolated human mesenteric arteries. Vascul Pharmacol. 2006;44:427–433. doi: 10.1016/j.vph.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Inada K, Yokoi I, Kabuto H, Habu H, Mori A, Ogawa N. Age-related increase in nitric oxide synthase activity in senescence accelerated mouse brain and the effect of long-term administration of superoxide radical scavenger. Mech Ageing Dev. 1996;89:95–102. doi: 10.1016/0047-6374(96)01743-5. [DOI] [PubMed] [Google Scholar]
- Kannel WB. The Framingham Study: historical insight on the impact of cardiovascular risk factors in men versus women. J Gend Specif Med. 2002;5:27–37. [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Llorens S, de Mera RM, Pascual A, Prieto-Martin A, Mendizabal Y, de Cabo C, Nava E, Jordan J. The senescence-accelerated mouse (SAM-P8) as a model for the study of vascular functional alterations during aging. Biogerontology. 2007;8:663–672. doi: 10.1007/s10522-007-9108-4. [DOI] [PubMed] [Google Scholar]
- Miyamoto M. Characteristics of age-related behavioral changes in senescence-accelerated mouse SAMP8 and SAMP10. Exp Gerontol. 1997;32:139–148. doi: 10.1016/S0531-5565(96)00061-7. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Novella S, Dantas AP, Segarra G, Novensa L, Bueno C, Heras M, Hermenegildo C, Medina P. Gathering of aging and estrogen withdrawal in vascular dysfunction of senescent accelerated mice. Exp Gerontol. 2010;45:868–874. doi: 10.1016/j.exger.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Novensa L, Selent J, Pastor M, Sandberg K, Heras M, Dantas AP. Equine estrogens impair nitric oxide production and endothelial nitric oxide synthase transcription in human endothelial cells compared with the natural 17b-estradiol. Hypertension. 2010;56:405–411. doi: 10.1161/HYPERTENSIONAHA.110.151969. [DOI] [PubMed] [Google Scholar]
- Paigen B, Plump AS, Rubin EM. The mouse as a model for human cardiovascular disease and hyperlipidemia. Curr Opin Lipidol. 1994;5:258–264. doi: 10.1097/00041433-199408000-00003. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manas L, El-Assar M, Vallejo S, Lopez-Doriga P, Solis J, Petidier R, Montes M, Nevado J, Castro M, Gomez-Guerrero C, Peiro C, Sanchez-Ferrer CF. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8:226–238. doi: 10.1111/j.1474-9726.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- Sellers MM, Stallone JN. Sympathy for the devil: the role of thromboxane in the regulation of vascular tone and blood pressure. Am J Physiol Heart Circ Physiol. 2008;294:H1978–H1986. doi: 10.1152/ajpheart.01318.2007. [DOI] [PubMed] [Google Scholar]
- Smith WL. Prostaglandin biosynthesis and its compartmentation in vascular smooth muscle and endothelial cells. Annu Rev Physiol. 1986;48:251–262. doi: 10.1146/annurev.ph.48.030186.001343. [DOI] [PubMed] [Google Scholar]
- Stewart KG, Zhang Y, Davidge ST. Aging increases PGHS-2-dependent vasoconstriction in rat mesenteric arteries. Hypertension. 2000;35:1242–1247. doi: 10.1161/01.HYP.35.6.1242. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.CIR.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997;29:736–743. doi: 10.1161/01.HYP.29.3.736. [DOI] [PubMed] [Google Scholar]
- Traupe T, Lang M, Goettsch W, Munter K, Morawietz H, Vetter W, Barton M. Obesity increases prostanoid-mediated vasoconstriction and vascular thromboxane receptor gene expression. J Hypertens. 2002;20:2239–2245. doi: 10.1097/00004872-200211000-00024. [DOI] [PubMed] [Google Scholar]
- Yuan M, Wen-Xia Z, Jun-Ping C, Yong-Xiang Z. Age-related changes in the oestrous cycle and reproductive hormones in senescence-accelerated mouse. Reprod Fertil Dev. 2005;17:507–512. doi: 10.1071/RD04099. [DOI] [PubMed] [Google Scholar]
- Yutzey KE, Robbins J. Principles of genetic murine models for cardiac disease. Circulation. 2007;115:792–799. doi: 10.1161/CIRCULATIONAHA.106.682534. [DOI] [PubMed] [Google Scholar]