Abstract
Resveratrol, a polyphenolic compound, has been shown to extend lifespan in different organisms. Emerging evidence suggests that the prolongevity effect of resveratrol depends on dietary composition. However, the mechanisms underlying the interaction of resveratrol and dietary nutrients in modulating lifespan remain elusive. Here, we investigated the effect of resveratrol on lifespan of Drosophila melanogaster fed diets differing in the concentrations of sugar, yeast extract, and palmitic acid representing carbohydrate, protein, and fat, respectively. Resveratrol at up to 200 μM in diets did not affect lifespan of wild-type female flies fed a standard, restricted or high sugar–low protein diet, but extended lifespan of females fed a low sugar–high protein diet. Resveratrol at 400 μM extended lifespan of females fed a high-fat diet. Lifespan extension by resveratrol was associated with downregulation of genes in aging-related pathways, including antioxidant peroxiredoxins, insulin-like peptides involved in insulin-like signaling and several downstream genes in Jun-kinase signaling involved in oxidative stress response. Furthermore, resveratrol increased lifespan of superoxide dismutase 1 (sod1) knockdown mutant females fed a standard or high-fat diet. No lifespan extension by resveratrol was observed in wild-type and sod1 knockdown males under the culture conditions in this study. Our results suggest that the gender-specific prolongevity effect of resveratrol is influenced by dietary composition and resveratrol promotes the survival of flies by modulating genetic pathways that can reduce cellular damage. This study reveals the context-dependent effect of resveratrol on lifespan and suggests the importance of dietary nutrients in implementation of effective aging interventions using dietary supplements.
Keywords: Resveratrol, Lifespan, Dietary composition, Aging intervention, Superoxide dismutase 1, Oxidative stress
Introduction
Aging is a process that leads to physiologic decline and an increased vulnerability to disease and death (Fontana et al. 2010). While the process of aging is complex, advances in aging research have shown that age-related declines in lifespan and healthspan can often be delayed through genetic and environmental manipulation, e.g., diet or pharmacological agents (Fontana et al. 2010). One of the most widely used experimental paradigms that extend lifespan in model organisms is calorie or dietary restriction (CR or DR), a condition in which calories or dietary nutrients are modestly reduced while maintaining adequate nutrition (Masoro 2009; Fontana et al. 2010; Piper et al. 2011). DR has been reported to extend lifespan and attenuate age-related diseases in a wide variety of organisms including yeast, worms, flies, mice, rats, and probably nonhuman primates, rhesus monkeys (Colman et al. 2009; Fontana et al. 2010). However, recent reports in several insect species and mice have challenged the effectiveness of this intervention to increase lifespan. Several labs have conducted nutrition geometric studies, which have measured lifespan in several fly species, including Drosophila melanogaster, Queensland fruit fly (Bactrocera tryoni), and the Mexican fruit fly (Mexfly; Anastrepha ludens), under dietary conditions with various amount of sugar and protein (Carey et al. 2008; Lee et al. 2008; Skorupa et al. 2008; Fanson et al. 2009; Piper et al. 2011). These studies suggest that dietary composition or the ratio of carbohydrate relative to protein is more important than calorie intake in modulating lifespan. In mice, a study of lifespan in approximately 40 recombinant inbred lines under DR condition indicates that DR only extends lifespan in a subset of these mouse lines and even shortens lifespan in some (Liao et al. 2010). This suggests that the prolongevity effect of DR depends on genetic background of the organism. Further, it is questionable whether DR would be widely utilized as a health or longevity intervention in human populations where the abundance of food has led to an epidemic of obesity (Ingram et al. 2006). Obesity raises challenges to successful, healthy aging due its link to many diseases, including type 2 diabetes, stroke, and neurodegenerative disorders including Alzheimer’s disease (Desai et al. 2010). It is, therefore, important to assess the effect of interactions among various environmental and genetic factors in modulating lifespan.
Other research on the potential for nutritional modulation of lifespan has suggested the possibility that polyphenolic compounds from fruits and vegetables added to diets modulates healthy aging and lifespan (Lithgow et al. 2005; Obrenovich et al. 2010). For example, resveratrol, a phytoallexin that is a member of the stilbene family, has been shown to extend lifespan in yeast, worms, flies, and a short-lived fish (Howitz et al. 2003; Wood et al. 2004; Valenzano et al. 2006). Resveratrol affects activities of a number of proteins involved in aging, such as sirtuins and PGC-1α, to exert beneficial effects on health and lifespan (Pirola and Frojdo 2008). Numerous studies have revealed health benefits of resveratrol supplementation, including delayed decline of age-related cognitive function, improved pancreatic β-cell function, and reduced cardiac hypertrophy in rodent models (Pearson et al. 2008; Lee et al. 2011; Chan et al. 2008; Baur 2010). However, the effect of resveratrol on lifespan and the underlying mechanisms remain controversial. Emerging evidence indicates that the prolongevity effect of resveratrol depends on dietary conditions of the organism. Supplementation of resveratrol can extend the lifespan of mice placed on a high-fat diet when compared to their age- and diet-matched controls (Baur et al. 2006). Middle-aged mice fed a normal ad libitum diet supplemented with resveratrol were healthier but did not live longer than their age- and diet-matched controls (Pearson et al. 2008). In D. melanogaster, when resveratrol was added to a normal diet, lifespan extension was observed in one study (Wood et al. 2004) but not in another (Bass et al. 2007b).
Here, we evaluate the effect of resveratrol supplemented to diets varying the concentrations of sugar, yeast extract, and palmitic acid representing carbohydrate, protein, and fat, respectively, on lifespan in D. melanogaster. Our results reveal the interaction of resveratrol with dietary nutrients to modulate lifespan through stress-related pathways.
Materials and methods
Fly strains and culture conditions
Fly stains wild-type Canton S, da-Gal4 (w1118; P{w[+mW.hs] = GAL4-da.G32},3) and UAS-sod1IR (w1; P{UAS-Sod1.IR}F103/SM5) were obtained from the Bloomington Drosophila Stock Center (Bloomington, IN, USA). Fly stocks were routinely maintained on standard fly cornmeal agar medium at 25 ± 1°C, 60 ± 5% humidity, and a 12-h light/dark cycle (Ashburner et al. 2005). Five types of sugar-, yeast extract-, and palmitic acid-based diets as control diets were prepared as previously described (Boyd et al. 2011). The standard base diet contained 10% sugar and 10% yeast extract in weight/volume; the DR diet had 2.5% sugar and 2.5% yeast extract; the high sugar–low protein diet consisted of 18% sugar and 2% yeast extract; the low sugar–high protein diet contained 2% sugar and 18% yeast extract; and the high-fat diet had 10% sugar, 10% yeast extract, and 2% palmitic acid. For resveratrol-supplemented diets, resveratrol was dissolved in 100% ethanol and added to each of the above five diets to a final concentration of 100, 200, or 400 μM. All diets contained 1.5% agar and equal amount of ethanol.
Lifespan assay
For lifespan assays, progeny flies were collected within 24 h after eclosion from the standard cornmeal food and allowed to mate for 24 h on the standard base diet. Under light CO2 anesthesia, flies were sorted by sex into groups of 20 individuals and placed in 30 mL polypropylene vials that contained 5 mL of the base diet. After another 24 h, flies were transferred to a specified experimental diet as described above to start lifespan measurement. Flies were transferred to fresh food once every 2–3 days and the number of dead flies was recorded at each transfer in a Microsoft Excel® spreadsheet (Microsoft, Redmond, WA, USA) until all flies died. Flies were kept at 25 ± 1°C, 60 ± 5% humidity and a 12-h light/dark cycle at all times.
Food intake assay
Food intake was measured using the capillary feeder method (CAFE) with minor modifications previously described (Ja et al. 2007; Boyd et al. 2011). Female flies were fed the high-fat diet with or without 400 μM resveratrol until they were 14 days old. Randomly selected flies (n = 16) from each treatment were housed in the capillary feeding chambers with two flies per chamber. Thus, there were eight replicates per treatment. The feeding capillary was filled with liquid and agar-free high-fat diet with or without 400 μM resveratrol. To account for evaporation of food, two feeding chambers were set up with feeding capillaries but without flies. The volume of food intake was recorded once every 24 h for three consecutive days. Average daily food intake per fly was then calculated.
Quantitative polymerase chain reaction
Female flies exposed to the low sugar–high protein diet or high-fat diet, supplemented with or without 400 μM resveratrol were collected when they were 2 weeks old. Total RNA was isolated from the heads of approximately 20 flies per sample using the Trizol Reagent (Cat. No. 15596–026, Invitrogen Inc., Carlsbad, CA, USA) according to the manufacturer’s protocol. The quality and quantity of total RNA was assessed using the Nanodrop 1000 from Thermo Scientific (Wilmington, DE, USA). cDNA synthesis from total RNA was performed using Superscript® reverse transcriptase (Cat. No. 18064–022, Invitrogen Inc.). Quantitative polymerase chain reaction (qPCR) was performed using the StepOnePlus real-time PCR system from Applied Biosystems Inc. (Foster City, CA, USA) according to the manufacturer’s protocol. qPCR measurement for each gene was repeated with four to six independent biological samples. The transcript level of each gene was normalized to the internal control gene ribosomal protein L32 (Rpl32). The primer sequences for all genes in this study were described in a previous publication (Boyd et al. 2011).
Statistical analysis
Statistical analyses were performed using Statview 5.0 software (SAS, Cary, NC, USA). Data were expressed as means ± standard error. Lifespan difference was assessed by the log-rank test. Student’s t tests were performed for qPCR and food intake data; p < 0.05 was considered statistically significant.
Results
The effect of resveratrol on lifespan of flies fed the standard base and DR diets
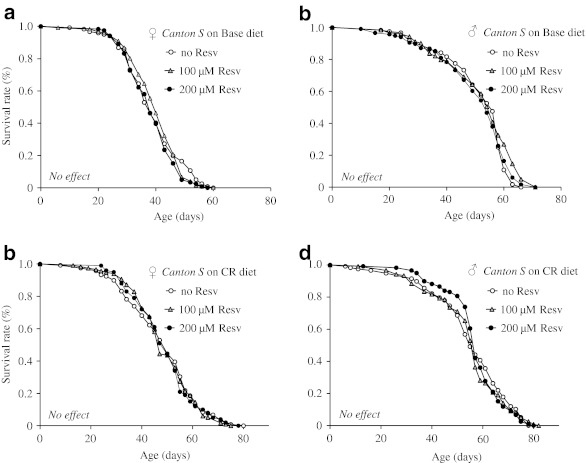
To assess the role of calorie content on the effect of resveratrol on lifespan, we measured the lifespan of wild type Canton S male and female flies on the standard base or DR diet supplemented with or without a final concentration of 100 and 200 μM resveratrol. Consistent with previous studies, the DR diet extended mean lifespan of both males and females when compared to the base diet (Fig. 1a–d and Table 1). However, no extension or shortening of mean lifespan was observed at either dose of resveratrol for either males or females (Fig. 1a–d). The base and DR diets used here are routinely employed in aging intervention and DR studies in D. melanogaster (Bross et al. 2005; Bass et al. 2007a; Zou et al. 2007). Our findings suggest that supplementation of resveratrol at up to 200 μM is not sufficient to promote longevity of flies under standard or restricted dietary conditions.
Fig. 1.
The effect of resveratrol on the lifespan of wild type Canton S flies fed the standard base and calorie restriction (CR) diets. a–b Lifespan curves of females and males fed the standard base diet supplemented with 0, 100, or 200 μM resveratrol (Resv). c–d Lifespan curves of females and males fed the CR diet supplemented with 0, 100, or 200 μM resveratrol. The number of flies in each trial is more than 100. No effect supplementation of resveratrol does not extend lifespan under the diet condition specified
Table 1.
The effect of resveratrol on lifespan of flies on various diets
| Strain | Gender | Treatment | No. of flies | Mean lifespan (days) | % Lifespan difference (%)a | |
|---|---|---|---|---|---|---|
| diet | Resv conc. | |||||
| Canton S | Female | Base diet | No Resv | 121 | 38.9 ± 0.9 | |
| 100 μM | 121 | 39.6 ± 0.8 | 1.8 | |||
| 200 μM | 119 | 38.3 ± 0.8 | −1.5 | |||
| CR diet | No Resv | 119 | 47.9 ± 1.3 | |||
| 100 μM | 117 | 48.5 ± 1.1 | 1.2 | |||
| 200 μM | 100 | 48.7 ± 1.2 | 1.7 | |||
| HS–LP diet | No Resv | 120 | 50.3 ± 1.4 | |||
| 100 μM | 122 | 51.8 ± 1.0 | 3.0 | |||
| 200 μM | 117 | 48.8 ± 1.2 | −3.0 | |||
| LS–HP diet | No Resv | 117 | 20.6 ± 0.7 | |||
| 100 μM | 121 | 20.5 ± 0.8 | −0.5 | |||
| 200 μM | 124 | 23.6 ± 0.9 | 14.6*** | |||
| High-fat diet | No Resv | 157 | 30.3 ± 0.7 | |||
| 200 μM | 150 | 32.0 ± 0.6 | 5.6 | |||
| 400 μM | 149 | 33.2 ± 0.7 | 9.6** | |||
| Male | Base diet | No Resv | 121 | 51.2 ± 1.0 | ||
| 100 μM | 123 | 51.7 ± 1.2 | 1.0 | |||
| 200 μM | 116 | 50.1 ± 1.2 | −2.1 | |||
| CR diet | No Resv | 118 | 55.1 ± 1.4 | |||
| 100 μM | 121 | 54.5 ± 1.3 | −1.1 | |||
| 200 μM | 119 | 56.8 ± 1.0 | 3.1 | |||
| HS–LP diet | No Resv | 118 | 59.4 ± 0.9 | |||
| 100 μM | 120 | 59.4 ± 0.9 | 0.0 | |||
| 200 μM | 121 | 59.6 ± 0.8 | 0.3 | |||
| LS–HP diet | No Resv | 122 | 29.6 ± 0.8 | |||
| 100 μM | 114 | 31.9 ± 1.0 | 7.8*** | |||
| 200 μM | 111 | 30.5 ± 1.2 | 3** | |||
| High-fat diet | No Resv | 152 | 35.2 ± 1.1 | |||
| 200 μM | 143 | 35.6 ± 1.1 | 1.1 | |||
| 400 μM | 155 | 38.0 ± 1.0 | 8.0 | |||
| sod1RNAi | Female | Base diet | No Resv | 117 | 24.2 ± 0.4 | |
| 200 μM | 117 | 26.3 ± 0.4 | 8.7*** | |||
| 400 μM | 116 | 25.9 ± 0.4 | 7.0** | |||
| High-fat diet | No Resv | 123 | 20.2 ± 0.3 | |||
| 200 μM | 110 | 22.5 ± 0.3 | 11.4*** | |||
| 400 μM | 114 | 22.5 ± 0.3 | 11.4*** | |||
| Male | Base diet | No Resv | 153 | 16.6 ± 0.3 | ||
| 200 μM | 151 | 16.1 ± 0.3 | −3.0 | |||
| 400 μM | 139 | 15.0 ± 0.2 | −9.6*** | |||
| High-fat diet | No Resv | 130 | 13.3 ± 0.3 | |||
| 200 μM | 122 | 15.0 ± 0.4 | 12.8** | |||
| 400 μM | 115 | 14.2 ± 0.4 | 6.8 | |||
CR calorie restriction diet, HS–LP high sugar–low protein diet, LS–HP low sugar–high protein diet
aThe lifespan difference was derived from comparing resverarol fed flies to the nonsupplemented diet-matched controls. Positive number indicates lifespan increase, negative number indicates lifespan decrease
*p < 0.05, **p < 0.01, ***p < 0.001 by log-rank analysis
Influence of dietary composition on the effect of resveratrol on lifespan
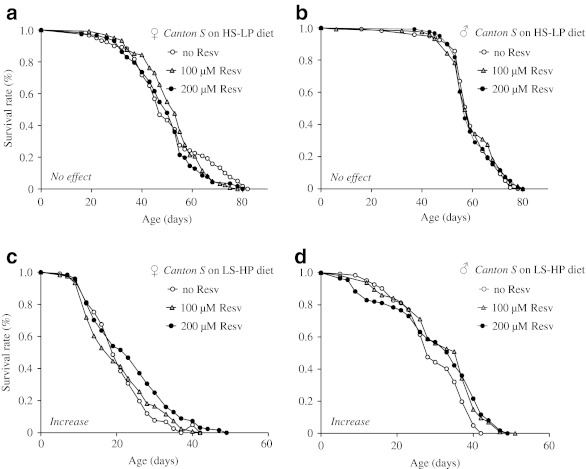
The ratio of carbohydrate to protein has been shown to be critical in modulating lifespan of flies (Lee et al. 2008; Skorupa et al. 2008). Here, we assessed the effect of resveratrol supplementation on lifespan of Canton S flies fed diets varying the amounts of sugar and yeast extract (SY). Yeast extract is the only protein source in the SY-based diet. Consistent with findings in the literature (Lee et al. 2008; Skorupa et al. 2008), we found that the high sugar–low protein diet increased mean lifespan, while the low sugar–high protein diet decreased mean lifespan in both males and females when compared to the standard base diet (Fig. 2a–d; Table 1). As in the case of the base diet, supplementation of resveratrol at up to 200 μM did not extend mean lifespan of either males or females fed the high sugar–low protein diet. However, supplementation of resveratrol at 200 μM but not 100 μM extended mean lifespan of females fed the low sugar–high protein diet by ∼15% (p < 0.001). Supplementation of resveratrol at these same concentrations did not extend the lifespan of males fed any diet. These findings suggest that composition of dietary nutrients influences the effect of resveratrol on lifespan in a gender-specific manner.
Fig. 2.
The effect of resveratrol on the lifespan of wild type Canton S flies fed the high sugar–low protein (HS–LP) and low sugar–high protein (LS–HP) diets. a–b Lifespan curves of females and males fed the HS–LP diet supplemented with 0, 100, or 200 μm resveratrol (Resv). c–d Lifespan curves of females and males fed the LS-HP diet supplemented with 0, 100, or 200 μm resveratrol. The number of flies in each trial is more than 100. No effect supplementation of resveratrol does not extend lifespan under the diet condition specified; Increase supplementation of resveratrol extends lifespan under the specified diet condition
The effect of resveratrol on lifespan of flies fed a high-fat diet
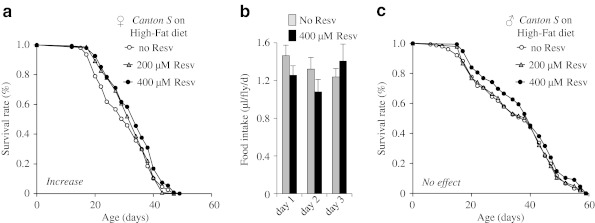
Resveratrol has been shown to promote the survival of mice fed a high-fat but not standard rodent chow diet (Baur et al. 2006; Pearson et al. 2008). Here, we measured lifespan of Canton S flies fed a high-fat diet with 2% palmitic acid supplemented with or without 200 or 400 μM resveratrol (Fig. 3a–c; Table 1). Supplementation of resveratrol at either concentration did not extend the lifespan of males fed the high-fat diet (Fig. 3c). However, supplementation of resveratrol at 400 μM increased mean lifespan of females fed the high-fat diet by ∼10% (p < 0.01), when compared to the nonsupplemented controls (Fig. 3a; Table 1). The lifespan of females on the high-fat diet supplemented with 200 μM resveratrol was higher than the nonsupplemented controls, but the difference was not statistically significant. We also measured daily food intake of female flies fed with the agar-free high-fat diet supplemented with or without 400 μM resveratrol for three consecutive days using the CAFE method (Ja et al. 2007). Resveratrol did not change the daily food intake in these flies, indicating that lifespan extension by resveratrol in female flies fed the high-fat diet is not due to the difference in food intake (Fig. 3b). Taken together, these findings suggest that resveratrol can alleviate the detrimental effect of fat on the lifespan of females.
Fig. 3.
The effect of resveratrol on the lifespan of wild type Canton S flies fed the high-fat diet. a Lifespan curves of females fed the high diet supplemented with 0, 200, or 400 μM resveratrol (Resv). b Food intake of females fed the high-fat diet supplemented with 0 or 400 μM resveratrol. The bar graph shows average daily food intake in volume per fly for females 2 weeks old in three consecutive days. The error bars indicate standard error. c Lifespan curves of males fed the high diet supplemented with 0, 200, or 400 μM resveratrol. The number of flies in each trial is more than 100. No effect supplementation of resveratrol does not extend lifespan under the specified diet condition; Increase supplementation of resveratrol extends lifespan under the specified diet condition
The effect of resveratrol on expression of aging-related genes
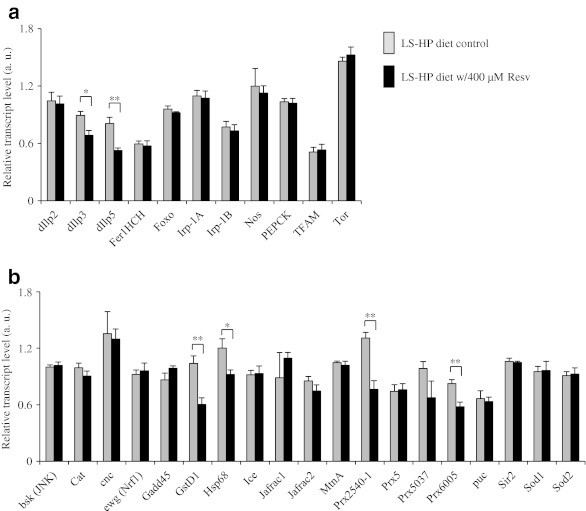
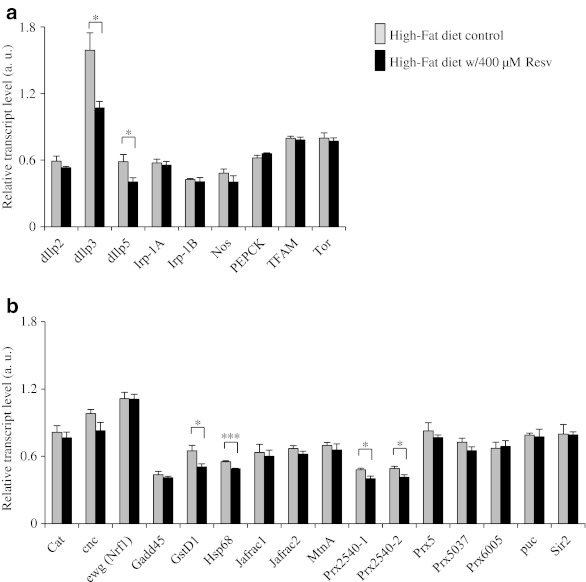
To investigate the mechanisms underlying the prosurvival effect of resveratrol, we measured transcript levels of approximately 30 genes involved in aging-related pathways, such as nutrient sensing and oxidative stress response pathways, in females fed the low sugar–high protein diet supplemented with or without 400 μM resveratrol. Supplementation of resveratrol at 400 μM increased mean lifespan of females fed the low sugar–high protein diet by 15.4% when compared to the nonsupplemented control (p < 0.01, lifespan curve not shown). Most of the genes surveyed here were not regulated by supplementation of resveratrol (Fig. 4). None of these genes were up-regulated by resveratrol, either. However, six genes were found down-regulated by resveratrol. Among them are Drosophila insulin-like peptide 3 and 5 (dIlp3 and dIlp5) involved in insulin-like signaling, which is a major pathway known to modulate lifespan in worms, flies, and rodents (Broughton et al. 2005; Fontana et al. 2010). Supplementation of resveratrol also downregulated glutathione S transferase D1 (GstD1) and heat shock protein 68, two downstream targets of Jun kinase (JNK) signaling pathway, which is a major oxidative stress response pathway and is known to modulate lifespan in several model organisms (Wang et al. 2005; Karpac and Jasper 2009). In addition, two peroxiredoxin (Prx) genes, Prx2540-1 and Prx6005, were downregulated by supplementation of resveratrol. Peroxiredoxins are antioxidant enzymes critical for maintaining redox status in the cell (Radyuk et al. 2003; Wood et al. 2003). Taken together, these findings suggest that supplementation of resveratrol promotes the survival of flies by reducing oxidative damage.
Fig. 4.
The effect of resveratrol on gene expression in Canton S female flies fed the low sugar–high protein (LS–HP) diet as determined by quantitative PCR (qPCR). a Expression patterns of metabolism-related genes in flies fed the LS–HP diet with or without 400 μM resveratrol. b Expression patterns of oxidative stress-related genes in flies fed the LS–HP diet with or without 400 μm resveratrol. The relative transcript levels of all genes are in arbitrary units (a. u.) after being normalized to the transcript level of rpL32. The values are expressed as means ± standard error. Four to six biologically independent samples were used in qPCR for each diet. *p < 0.05, **p < 0.01, ***p < 0.001 based on Student’s t test
We also measured transcript levels for most of the genes described above for females fed the high-fat diet supplemented with or without 400 μM resveratrol. Similar to the case for the low sugar–high protein diet, supplementation of resveratrol did not change transcript levels of most of the genes surveyed here (Fig. 5). Five of the six genes, dIlp3, dIlp5, gstD1, hsp68, and Prx2540-1, downregulated by resveratrol in flies fed the low sugar–high protein diet, were also downregulated by resveratrol in flies fed the high-fat diet. Supplementation of resveratrol also reduced the transcript level of Prx2540-2 instead of Prx6005. These findings are consistent with what we observed in the case of the low sugar–high protein diet and further support the notion that supplementation of resveratrol can reduce oxidative damage in flies.
Fig. 5.
The effect of resveratrol on gene expression and food intake in Canton S female flies fed the high-fat diet as determined by qPCR. a Expression patterns of metabolism-related genes in flies fed the high-fat diet with or without 400 μM resveratrol. b Expression patterns of oxidative stress-related genes in flies fed the high-fat diet with or without 400 μM resveratrol. The relative transcript levels of all genes are in arbitrary units (a.u.) after being normalized to the transcript level of rpL32. Four to six biological independent samples were used in qPCR for each diet. *p < 0.05, **p < 0.01, ***p < 0.001 based on Student’s t test
The effect of resveratrol on lifespan of superoxide dismutase 1 knockdown flies
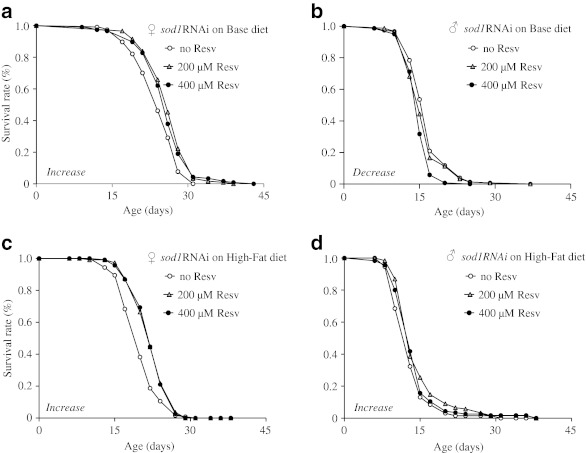
To further investigate the prosurvival effect of resveratrol, we measured the lifespan of superoxide dismutase 1 (sod1) knockdown flies fed the base and high-fat diets supplemented with or without 200 and 400 μM resveratrol. Reduction of sod1 expression in flies was achieved by the RNA interference (RNAi) method. Expression of double-stranded RNA was induced from the UAS-sod1IR (inverted repeat) transgene by a ubiquitously expressed GAL4, daughterless (da)-Gal4, as previously described (Boyd et al. 2011; Martin et al. 2009). The sod1 knockdown flies were called sod1RNAi flies here. Supplementation of resveratrol at either 200 or 400 μM slightly extended mean lifespan of sod1RNAi females but not males fed the standard base diet or the high-fat diet by up to 8.7% and 11.4%, respectively (p < 0.01; Fig. 6a–d). These findings further support the role of resveratrol in modulating oxidative damage in flies.
Fig. 6.
The effect of resveratrol on the lifespan of sod1RNAi flies fed the standard base and high-fat diets. a–b Lifespan curves of sod1RNAi females and males fed the standard base diet supplemented with 0, 200, or 400 μm resveratrol. c–d Lifespan curves of sod1RNAi females and males fed the high-fat diet supplemented with 0, 200, or 400 μm resveratrol. The number of flies in each trial is more than 100. Increase and decrease marked in the panel indicate that supplementation of resveratrol extends and reduces lifespan, respectively under the specified diet condition
Discussion
Resveratrol has been shown to increase lifespan and promote health span in model organisms ranging from yeast, worms, flies, and mice (Allard et al. 2009; Baur and Sinclair 2006; Pirola and Frojdo 2008). However, the prolongevity effect of resveratrol and its underlying mechanisms have been challenged by studies from several labs and remain controversial (Bass et al. 2007b; Kaeberlein et al. 2005). In this study, we demonstrated the context-dependent effect of resveratrol on lifespan in D. melanogaster. We found that resveratrol at up to 200 μM did not extend the lifespan of female flies fed a standard, DR, or high sugar–low protein diet. However, resveratrol at 200 μM increased lifespan of females of wild-type Canton S strain fed a low sugar–high protein. Resveratrol at 400 μM extended the lifespan of wild-type females fed a high-fat diet, as well as the lifespan of sod1RNAi females on the standard or high-fat diet. We did not observe lifespan extension by resveratrol in males under any of the dietary conditions tested here except that resveratrol at 400 μM slightly decreased lifespan of sod1RNAi males fed the standard base diet and resveratrol at 200 μM slightly increased lifespan of sod1RNAi males fed the high-fat diet. Although we have not measured the lifespan of flies fed a standard, DR or high sugar–low protein diet supplemented with higher than 200 μM resveratrol, our results suggest that female flies have a heightened responsiveness to supplementation of resveratrol than males and dietary composition modulates the prolongevity effect of resveratrol. Flies fed the low sugar–high protein or high-fat diet had shorter lifespan than those on the standard base diet. sod1RNAi flies were also short-lived than their diet-matched controls. The lifespan extension by resveratrol was found to be associated with downregulation of insulin-like peptides and oxidative stress response genes. Taken together, our findings suggest that resveratrol is more effective to promote the survival of flies under stress.
Resveratrol was originally discovered as an activator of sirtuins to promote longevity (Howitz et al. 2003). Sirtuins are a family of deacetylases found in species ranging from yeast to humans (Wood et al. 2004; Baur and Sinclair 2006; Guarente 2007; Baur 2010). Sirtuins are generally thought to mediate lifespan by DR, although this view along with the prolongevity effect of resveratrol has been challenged (Baur 2010). A number of follow-up studies have revealed that resveratrol modulates activities of many targets besides sirtuins, including AMP-activated protein kinase (AMPK) and PGC-1α, known to be regulated by DR in mammals (Pirola and Frojdo 2008). Through these targets, resveratrol exerts numerous beneficial effects in mammals ranging from increased insulin sensitivity, reduced plasma glucose and triglyceride levels, and attenuated oxidative damage. In this study, we have found that resveratrol reduces expression of two insulin-like peptide genes, dIlp3 and dIlp5, under the low sugar–high protein or high-fat diet condition when resveratrol promotes the survival of flies. This finding is consistent with results from mammalian studies, which have shown reduction of insulin-like grow factor-1 (IGF-1) by supplementation of resveratrol in diets (Baur et al. 2006; Baur 2010). In Drosophila, reducing the levels of dIlp2, dIlp3, and dIlp5 by partial ablation of insulin-like peptide-producing median neurosecretory cells in the brain resulted in longer lifespan and increased resistance to oxidative stress (Broughton et al. 2005). In the present study, we have also shown that under the low sugar–high protein and high-fat diet conditions, supplementation of resveratrol leads to lower transcript levels of several oxidative stress response genes, including gstD1, hsp68, and Prx. GstD1 and hsp68 are downstream targets of JNK signaling, a major oxidative stress response pathway known to modulate lifespan in worms and flies (Wang et al. 2005; Karpac and Jasper 2009). Prxs are a family of anti-oxidants that protect cells from metabolically produced intracellular reactive oxygen species (Radyuk et al. 2003; Wood et al. 2003). Resveratrol also increased the survival of sod1RNAi but not wild type Canton S females fed the standard base diet. These findings suggest that one of the mechanisms by which resveratrol promotes the survival in flies is to reduce insulin-like signaling and oxidative damage in the cell. Further, our results suggest that resveratrol indirectly modulates JNK signaling by altering oxidative stress levels in the cell.
Composition of dietary nutrients has a significant impact on lifespan (Fontana et al. 2010; Piper et al. 2011). A major finding of our study is that the prosurvival effect of resveratrol depends on dietary context in Drosophila. The effect of resveratrol on lifespan extension is not likely due to its effect on food intake. Wood et al. showed that resveratrol did not decrease food intake in female flies fed a diet with 15% sugar and 15% yeast extract (Wood et al. 2004). In this study, we found that resveratrol did not change food intake in females fed the high-fat diet. These data suggest that resveratrol does not affect palatability of the food or induce food aversion. In addition, the context-dependent effect of resveratrol is unlikely due to the difference in resveratrol uptake by flies under different dietary conditions. Our lab and others observed that food intake among females on the high sugar–low protein, low sugar–high protein and standard base diets differed by no more than 20% (Mair et al. 2005; our unpublished observation). Thus, resveratrol uptake should not be much different among these three diets. On the other hand, we previously demonstrated that female flies on the DR and high-fat diets increased food intake in volume by about twofold, when compared to those on the standard base diet (Zeng et al. 2011; Boyd et al. 2011). Thus, flies ingested about twofold more resveratrol on the DR or high-fat diet, when compared to the base diet with the same concentration of resveratrol. However, two doses of resveratrol differing in twofold were tested for each diet in our study. The amount of resveratrol intake for flies on the DR or high-fat diet with the lower dose of resveratrol should be comparable to that for flies on the base diet with the higher dose of resveratrol. Therefore, the actual biological doses of resveratrol among diets are unlikely the main cause of the diet-dependent prolongevity effect of resveratrol.
The context-dependent effect of resveratrol on lifespan apparently is not a unique feature of resveratrol. Phytochemicals found in fruits and vegetables have been shown to be beneficial to health, promote healthy aging, and possibly longevity (Lithgow et al. 2005; Obrenovich et al. 2010). Emerging evidence indicates that prolongevity effects of phytochemicals depend on dietary conditions of the organism. To name a few, we have previously demonstrated that a freeze-dried extract from açai, a berry found in the Amazon region, can increase the survival in female Drosophila under a high-fat but not standard diet condition (Sun et al. 2010). This lifespan extension is associated with transcriptional changes of genes involved in gluconeogenesis and JNK signaling. A mixture of cranberry and oregano extracts can extend the lifespan of females but not male Mexflies under dietary conditions with relative high sugar to protein ratios without decreasing reproduction (Zou et al. 2010; Zou et al. 2011). Resveratrol itself has been shown to promote the longevity of female Mexflies under a narrow dietary condition (Zou et al. 2009). The gender-specific response to phytochemicals is intriguing but poorly understood. Some phytochemicals including resveratrol are also phytoestrogens (Szkudelska and Szkudelski 2010), which could potentially act synergistically with other hormones in female flies. This may result in a heightened responsiveness of females to supplementation of phytochemicals than males. Resveratrol can increase the lifespan of middle-aged male mice under a high-fat but not standard diet, which is association with reduction of IGF-1 and activation of AMPK by resveratrol (Baur et al. 2006; Pearson et al. 2008). The effect of resveratrol in female mice has not been investigated yet. Taken together, our study along with a number of published works suggests that an efficient aging intervention using phytochemicals including resveratrol depends on diet composition. Furthermore, dietary conditions under which a phytochemical is effective in promoting longevity vary among different types of phytochemicals.
Over the last decade, an increasing body of evidence has accumulated to indicate that lifespan modulation by oxidative stress is context dependent (Salmon et al. 2010). High fat and high protein diets are pronounced in modern societies producing increasing numbers of obese individuals including the aged (Szkudelska and Szkudelski 2010). These individuals are more susceptible to type 2 diabetes, cardiovascular disease, stroke, and a host of other age-related diseases. Studies in mice and humans have shown that resveratrol can lower glucose levels and may be an effective intervention for the treatment of type 2 diabetes, a disease that is prevalent in both aging and obesity (Baur et al. 2006; Milne et al. 2007; Szkudelska and Szkudelski 2010). Resveratrol has also been shown to reduce oxidative damage in human cells and animal models (Szkudelska and Szkudelski 2010; Baur 2010; Frankel et al. 2011). These studies along with the current findings support the hypothesis that resveratrol can promote health span partly through modulating oxidative stress pathways. However, our findings along with results published by other laboratories indicate that the prolongevity effect of resveratrol depends on metabolic conditions. Future studies directed to understand the mechanisms underlying the context dependent effect of resveratrol should provide not only insight into the oxidative stress theory of aging but also valuable guidance for implementing efficient aging interventions.
Acknowledgements
This study was supported by funding from the Intramural Research Program of the National Institute on Aging, NIH to SZ. CXW was supported by a scholarship sponsored by the Chinese Scholarship Council.
Abbreviations
- sod1
Superoxide dismutase 1
- CR
Calorie restriction
- DR
Dietary restriction
- IR
Inverted repeat
- Mexfly
Mexican fruit fly
- qPCR
Quantitative polymerase chain reaction
- SY
Sugar and yeast extract
- dIlp3
Drosophila insulin-like peptide 3
- dIlp5
Drosophila insulin-like peptide 5
- gstD1
Glutathione S transferase D1
- hsp68
Heat shock protein 68
- Prx
Peroxiredoxin
- JNK
Jun kinase
- RNAi
RNA interference
- AMPK
AMP-activated protein kinase
- IGF-1
Insulin-like growth factor 1
Footnotes
Chunxu Wang and Charles T. Wheeler contributed equally to this study.
References
- Allard JS, Perez E, Zou S, de Cabo R. Dietary activators of Sirt1. Mol Cell Endocrinol. 2009;299(1):58–63. doi: 10.1016/j.mce.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Golic KG, Hawley RS, editors. Drosophila: a laboratory handbook. 2. Woodbury, NY: Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- Bass TM, Grandison RC, Wong R, Martinez P, Partridge L, Piper MD. Optimization of dietary restriction protocols in Drosophila. J Gerontol A Biol Sci Med Sci. 2007;62(10):1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128(10):546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baur JA. Resveratrol, sirtuins, and the promise of a DR mimetic. Mech Ageing Dev. 2010;131(4):261–269. doi: 10.1016/j.mad.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd O, Weng P, Sun X, Alberico T, Laslo M, Obenland DM, Kern B, Zou S. Nectarine promotes longevity in Drosophila melanogaster. Free Radic Biol Med. 2011;50(11):1669–1678. doi: 10.1016/j.freeradbiomed.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bross TG, Rogina B, Helfand SL. Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell. 2005;4(6):309–317. doi: 10.1111/j.1474-9726.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA. 2005;102(8):3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Harshman LG, Liedo P, Muller HG, Wang JL, Zhang Z. Longevity–fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell. 2008;7(4):470–477. doi: 10.1111/j.1474-9726.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, Dyck JR. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem. 2008;283(35):24194–24201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1–16. doi: 10.1016/j.cger.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Fanson BG, Weldon CW, Perez-Staples D, Simpson SJ, Taylor PW. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni) Aging Cell. 2009;8(5):514–523. doi: 10.1111/j.1474-9726.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel S, Ziafazeli T, Rogina B. dSir2 and longevity in Drosophila. Exp Gerontol. 2011;46(5):391–396. doi: 10.1016/j.exger.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol. 2007;72:483–488. doi: 10.1101/sqb.2007.72.024. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Roth GS, Lane MA, Ottinger MA, Zou S, de Cabo R, Mattison JA. The potential for dietary restriction to increase longevity in humans: extrapolation from monkey studies. Biogerontology. 2006;7(3):143–148. doi: 10.1007/s10522-006-9013-2. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fan AY, Liong AY, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci USA. 2007;104(20):8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280(17):17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Karpac J, Jasper H. Insulin and JNK: optimizing metabolic homeostasis and lifespan. Trends Endocrinol Metab. 2009;20(3):100–106. doi: 10.1016/j.tem.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci USA. 2008;105(7):2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Yang H, Tartar DM, Gao B, Luo X, Ye SQ, Zaghouani H, Fang D. Prevention and treatment of diabetes with resveratrol in a non-obese mouse model of type 1 diabetes. Diabetologia. 2011;54(5):1136–1146. doi: 10.1007/s00125-011-2064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9(1):92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow GJ, Gill MS, Olsen A, Sampayo JN. Pharmacological intervention in invertebrate aging. Age. 2005;27(3):213–223. doi: 10.1007/s11357-005-3625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3(7):e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Jones MA, Grotewiel M. Manipulation of Sod1 expression ubiquitously, but not in the nervous system or muscle, impacts age-related parameters in Drosophila. FEBS Lett. 2009;583(13):2308–2314. doi: 10.1016/j.febslet.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction-induced life extension of rats and mice: a critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790(10):1040–1048. doi: 10.1016/j.bbagen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrenovich ME, Nair NG, Beyaz A, Aliev G, Reddy VP. The role of polyphenolic antioxidants in health, disease, and aging. Rejuvenation Res. 2010;13(6):631–643. doi: 10.1089/rej.2010.1043. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8(2):157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MD, Partridge L, Raubenheimer D, Simpson SJ. Dietary restriction and aging: a unifying perspective. Cell Metab. 2011;14(2):154–160. doi: 10.1016/j.cmet.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirola L, Frojdo S. Resveratrol: one molecule, many targets. IUBMB Life. 2008;60(5):323–332. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- Radyuk SN, Sohal RS, Orr WC. Thioredoxin peroxidases can foster cytoprotection or cell death in response to different stressors: over- and under-expression of thioredoxin peroxidase in Drosophila cells. Biochem J. 2003;371(Pt 3):743–752. doi: 10.1042/BJ20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon AB, Richardson A, Perez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010;48(5):642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7(4):478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Seeberger J, Alberico T, Wang C, Wheeler CT, Schauss AG, Zou S. Acai palm fruit (Euterpe oleracea Mart.) pulp improves survival of flies on a high fat diet. Exp Gerontol. 2010;45(3):243–251. doi: 10.1016/j.exger.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 2010;635(1–3):1–8. doi: 10.1016/j.ejphar.2010.02.054. [DOI] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16(3):296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121(1):115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300(5619):650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Zeng C, Du Y, Alberico T, Seeberger J, Sun X, Zou S. Gender-specific prandial response to dietary restriction and oxidative stress in Drosophila melanogaster. Fly. 2011;5(3):174–180. doi: 10.4161/fly.5.3.15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Sinclair J, Wilson MA, Carey JR, Liedo P, Oropeza A, Kalra A, de Cabo R, Ingram DK, Longo DL, Wolkow CA. Comparative approaches to facilitate the discovery of prolongevity interventions: effects of tocopherols on lifespan of three invertebrate species. Mech Ageing Dev. 2007;128(2):222–226. doi: 10.1016/j.mad.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Carey JR, Liedo P, Ingram DK, Muller HG, Wang JL, Yao F, Yu B, Zhou A. The prolongevity effect of resveratrol depends on dietary composition and calorie intake in a tephritid fruit fly. Exp Gerontol. 2009;44(6–7):472–476. doi: 10.1016/j.exger.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Carey JR, Liedo P, Ingram DK, Yu B, Ghaedian R. Prolongevity effects of an oregano and cranberry extract are diet dependent in the Mexican fruit fly (Anastrepha ludens) J Gerontol A Biol Sci. 2010;65(1):41–50. doi: 10.1093/gerona/glp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Carey JR, Liedo P, Ingram DK, Yu B (2011) Prolongevity effects of a botanical with oregano and cranberry extracts in Mexican fruit flies: examining interactions of diet restriction and age. Age (in press) [DOI] [PMC free article] [PubMed]