Abstract
Microscopic findings in Alzheimer’s disease (AD) at autopsy include a wide cortical distribution of beta amyloid (Aβ)-containing plaques and diminished numbers of pyramidal neurons in CA1 of hippocampus and tyrosine hydroxylase-positive (TH+) neurons in the locus coeruleus (LC). To better understand the neuropathology underlying cognitive decline in AD, we analyzed the AD-type neuropathology in brains of triple transgenic (3×Tg) mice harboring mutations for APPswe, PS1M146V, and tauP301L. Histochemical and immunohistochemical staining and computerized stereology were carried out in age-matched young, early middle age, and late middle age 3×Tg mice. The 3×Tg mice showed an intracellular Aβ deposition in subiculum and CA1 pyramidal neurons and an extracellular distribution of amyloid plaques specifically in the subiculum of hippocampal formation and in neocortical layer V. The 3×Tg mice also showed an age-related loss of TH+ neurons in LC, with a loss of 37% of these neurons at 15 months of age. There was no loss of CA1 neurons at any age examined. Reduced AD-type neuropathology in CA1 of 3×Tg mice suggests a possible neuroprotective role for high intracellular-to-extracellular ratios of insoluble Aβ deposits. Understanding the neurobiology of this apparent neuroprotection could lead to an improved understanding of age-related cognitive function in general, and the development of novel strategies for the therapeutic management of AD patients.
Keywords: Locus coeruleus, Hippocampus, Alzheimer’s disease, Triple transgenic mice, Double transgenic mice
Introduction
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder with insidious onset of dementia. Diagnosis of probable AD typically occurs in the fifth or sixth decade of life, followed by relentless progression to severe dementia and death with 7 to 10 years. Currently, there are no effective treatments to slow the inexorable process of the disease.
The neuropathological diagnosis of AD is confirmed on the basis of semi-quantitative evidence of amyloid plaques and neurofibrillary tangles in cortical brain regions (Price et al. 1991; Berg et al. 1998). Among the brain regions associated with neuron loss in AD are marked reductions in pyramidal neurons in the CA1 subregion of hippocampus (West 1993; Ridley et al. 1995; West et al. 2000) and tyrosine hydroxylase-immunopositive (TH+) neurons in the locus coeruleus (LC) (Chan-Palay and Asan 1989; German et al. 1992; Manaye et al. 1995; Busch et al. 1997; Berridge and Waterhouse 2003; Yu and Dayan 2005; Doya 2008). Since neuron numbers in LC and CA1 are not diminished during normal (non-demented) brain aging (West 1993; Mouton et al. 1994; Ohm et al. 1997), the loss of these neurons appears to play a critical role in the progressive cognitive decline in AD (Price et al. 1991; Manaye et al. 1995; Berg et al. 1998; Mattson 2004; Grudzien et al. 2007). According to the amyloid hypothesis, the conversion of beta-amyloid (Aβ) from soluble to insoluble forms triggers the formation of amyloid plaques and neurofibrillary tangles. To date, however, the connection between these neuropathological markers and neuron loss in CA1 and LC is poorly understood vis-à-vis the progressive, age-related cognitive impairment in AD.
The development of transgenic techniques and cloned mutations associated with familial AD led to the development of heuristically useful transgenic rodent phenotypes with one or more of the neuropathological changes associated with AD. A common feature in these transgenic rodents is the over-expression of AD-type mutations for amyloid precursor protein (APP), a transmembrane protein that cleaves to form mutant beta-amyloid (Aβ) peptides, leading to the deposition of mutant Aβ-containing amyloid plaques in cortical tissue. The over-expression of a single APP mutation in mice leads to deposits of mutant Aβ peptides and progressive deposition of cortical amyloid plaques starting in middle age, but fails to achieve sufficient levels of extracellular mutant Aβ necessary for AD-type neuron loss (Calhoun et al. 1998; Duyckaerts et al. 2008; van Dooren et al. 2005). The double transgenic (dtg) over-expression of APP mutations with a human mutation for presenilin 1 (PS1) causes greater deposition of Aβ-containing amyloid plaques starting at younger ages (Duff et al. 1996; Borchelt et al. 1997; Holcomb et al. 1998), leading to AD-type neuron loss by 12 to 18 months, and cognitive impairment in late middle age (18 months). Over-expression of the Swedish APP mutation with the delta E9 mutation of presenilin 1 (dtg APPswe/PS1ΔE9) leads to heavy deposition of amyloidogenic Aβ1-42 in cortical tissue (Borchelt et al. 1997) and significant age-related loss of TH+ neurons in the LC by 12 months of age (O’Neil et al. 2007; Liu et al. 2008). By 15 to 18 months of age, these dtg APPswe/PS1ΔE9 mice show a strong correlation between total Aβ-containing plaque volume (amyloid load) and loss in total numbers of pyramidal neurons in the CA1 region and noradrenergic neurons in LC (O’Neil et al. 2007; Liu et al. 2008; Manaye et al. 2010), together with alterations in the total numbers of synapses in the striatum radiatum (West et al. 2009). By the late middle age (18 months), these dtg APPswe/PS1ΔE9 mice show impaired performance on spatial memory tasks that is strongly correlated to Aβ load values (Savonenko et al. 2005). As a model for understanding the neuropathological substrates underlying AD-type progressive cognitive decline, the dtg APPswe/PS1ΔE9 mice recapitulates many of the histopathological and cognitive changes found in AD; however, two neuropathological markers of AD that do not occur in dtg APPswe/PS1ΔE9 mice are neurofibrillary tangles and intracellular Aβ deposits in cortical tissue. Microinjection of APPswe and tauP301L mutations into single cells derived from monozygous PS1M146V knockin mice results in AD-type phenopathology that includes deposition of intracellular Aβ; extracellular Aβ-containing amyloid plaques; and phosphorylated tau (phospho-tau) pathology, a precursor to neurofibrillary tangles (Oddo et al. 2003a, b; Rohn et al. 2008; Mastrangelo and Bowers 2008; Overk et al. 2009). Furthermore, the distribution of phospho-tau pathology in 3×Tg mice closely recapitulates the spatial distribution of tangles found in AD (Oddo et al. 2003a, b).
The present study used immunocytochemistry, histochemistry, and computerized stereology to investigate the spatiotemporal distribution of AD-type neuropathology, including age-related losses of pyramidal neurons in CA1 and TH+ neurons in LC in 3×Tg mice, in comparison to similar changes in dtg APPswe/PS1ΔE9 mice (O’Neil et al. 2007; Liu et al. 2008; Manaye et al. 2010).
Materials and methods
Mice
To assess age-related changes in AD-type neuropathology, n = 16 female 3×Tg mice were divided into three age groups: young (8 months, n = 6), middle age (12 months, n = 5), and late middle age (15 months, n = 5). Mice were obtained by Dr. Scott Turner at the Georgetown University Medical Center in Washington, D.C., USA. Brains were fixed in situ by intra-arterial perfusion and processed as detailed below at Dr. Kebreten Manaye’s laboratory at Howard University College of Medicine in Washington, DC. All protocols and procedures were in strict compliance with animal care and handling guidelines from the National Institutes of Health and the Animal Care and Use Committees at the Georgetown University Medical Center and the Howard University College of Medicine in Washington, D.C., USA
Tissue preparation
Tissue processing, sectioning, and staining of female 3×Tg mice in this study followed the same procedures used previously to prepare age-matched dtg APPswe/PS1ΔE9 female mice (Borchelt et al. 1997) for design-based stereology (O’Neil et al. 2007; Manaye et al. 2010). In this study, n = 16 female 3×Tg mice were sacrificed by perfusion with 4% paraformaldehyde fixative in 0.1 M phosphate-buffered saline, pH 7.4 (PBS). The brains were removed and stored at −80°C until sectioning. Each brain was serially sectioned in the coronal plane on a frozen sliding microtome at an instrument setting of 40 μm. Two entire regions of interest, LC and hippocampus, were sampled in a systematic-random manner for computerized stereology studies, as detailed elsewhere (O’Neil et al. 2007; Mouton et al. 2009; Manaye et al. 2010; for stereology review, see Mouton 2002).
Tissue processing
For quantification of total numbers of TH+ neurons, a set of systematic-random sections throughout the LC were collected in 12-well plates and washed in 0.1 M PBS, incubated in 1% hydrogen peroxide for 30 min at RT, washed again in 0.1 M PBS, and placed in 0.3% Triton X-100 for 10 min at RT. Sections were transferred into 5% normal goat serum in 0.1 M PBS for 30 min at RT to block non-specific binding, then incubated overnight in rabbit anti-tyrosine hydroxylase antibody (polyclonal, Chemicon International, Temecula, CA, USA) diluted to 1:1000 with 2% normal goat serum and 0.3% Triton X-100 in 0.1 M PBS at 4°C. After incubation, sections were washed in 0.1 M PBS and incubated in biotinylated secondary goat anti-rabbit antibody (1:400, Vector Laboratories, Burlingame, CA, USA) with 1% normal goat serum in 0.1 M PBS for 90 min at RT. Sections were washed in 0.1 M PBS and re-incubated for another 90 min in ABC solution (Vector Laboratories, Burlingame, CA, USA) at RT, rinsed in 0.1 M PBS, and colorized using DAB (Vectastain kit, Vector Laboratories, Burlingame, CA, USA) for 6 to 10 min. All sections for TH immunocytochemistry were lightly counterstained in a 0.1% solution of cresyl violet, rinsed, dehydrated through an ascending graded series of alcohol, cleared in xylene, and coverslipped. Adjacent sets of sections were processed using the same immunocytochemical approach for visualization of intracellular amyloid deposits and phospho tau. Systematic-random sections through the hippocampus were immunostained with mouse anti-6E10 antibody (monoclonal, Covance, Princeton, NJ, USA) and mouse anti-PHF-Tau (clone AT8, Thermo Scientific Pierce, Rockford, IL, USA) diluted to 1:1000 and 1:100, respectively. To visualize extracellular amyloid deposits, an adjacent set of systematic-random sections through the hippocampus was counterstained with Congo red for estimation of total amyloid volume (load), as reported previously (Lee et al. 2005; Mouton et al. 2009). Finally, for quantification of pyramidal neurons in CA1, an adjacent set of 8–12 sections through the hippocampus were sampled in a systematic-random manner and histochemically stained with 0.1% cresyl violet, dehydrated through an ascending alcohol series, cleared in xylene and coverslipped. The average section thickness after all tissue processing was 17 μm ± 2 SEM.
Reference spaces and stereology methods
On sets of systematically sampled sections, two reference spaces (CA1, LC) were outlined under low power magnification (×4), with borders of the reference spaces defined according to the stereotaxic mouse brain atlas of Franklin and Paxinos (2008). Because TH immunoreactivity is not confined to specific or readily definable LC subnuclei, we quantified the TH+ neurons in the LC region of the brainstem, including the nucleus subcoeruleus. For both LC and CA1, a total of about 100 to 200 neurons were counted within using a high-resolution oil immersion objective (×60, 1.4 NA). Mean total neuron numbers in CA1 of the hippocampus and TH+ neurons in LC were estimated using the optical fractionator (West et al. 1991), and the total volume of Congo red-stained amyloid (amyloid load) in the subiculum region of the hippocampus was estimated using the area fraction probe, as previously detailed (Long et al. 1998; Mouton 2002; Mouton et al. 2009; O’Neil et al. 2007). Neurons were distinguished from other cell types on the basis of neuronal phenotype, i.e., nucleolus, a well-formed nuclear membrane and, in the case of noradrenergic neurons in the LC, TH immunoreactivity. To avoid artifacts at the sectioning surface, e.g., lost caps, a guard volume where no cells were counted was observed a minimum of 2 μm above and below the disector. Sampling of each reference space was continued to a mean coefficient of error of 0.05 to 0.10, according to Gundersen et al. (1999). Amyloid plaque distribution in 3×Tg mice was examined by three histological methods, one histochemical stain (Congo red), and two immunohistochemical stains (6E10 and 4 G8) to determine its distribution pattern and detect age-related changes in density or distribution of AD-type Aβ plaques in hippocampus and neocortex regions. A trained operator blind to treatment carried out these stereology studies with assistance from a computerized stereology system (Stereo Investigator, MBF Bioscience, Williston, VT, USA).
Statistical analysis
Age-related differences were assessed using ANOVA and correlations between parameters by the Pearson product moment, respectively, with statistical significance at p ≤ 0.05.
Results
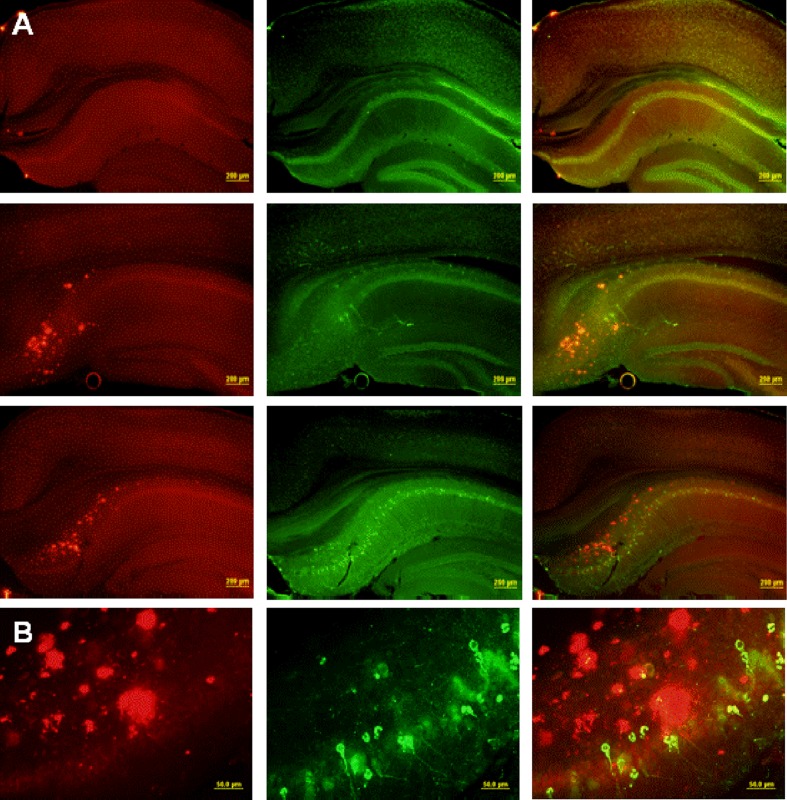
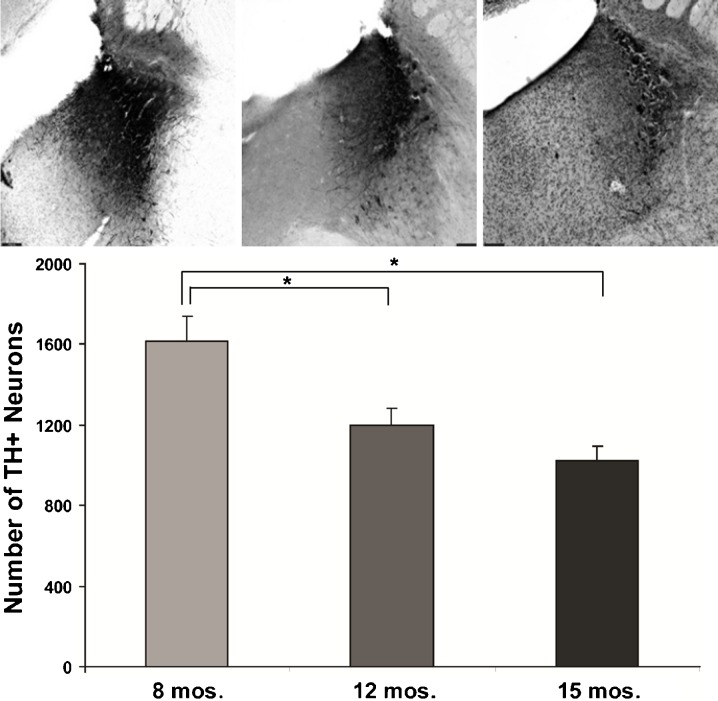
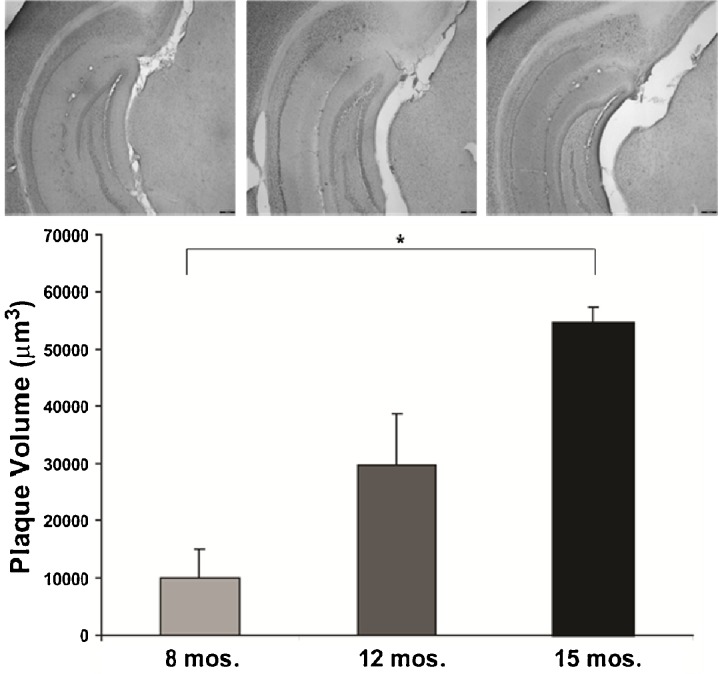
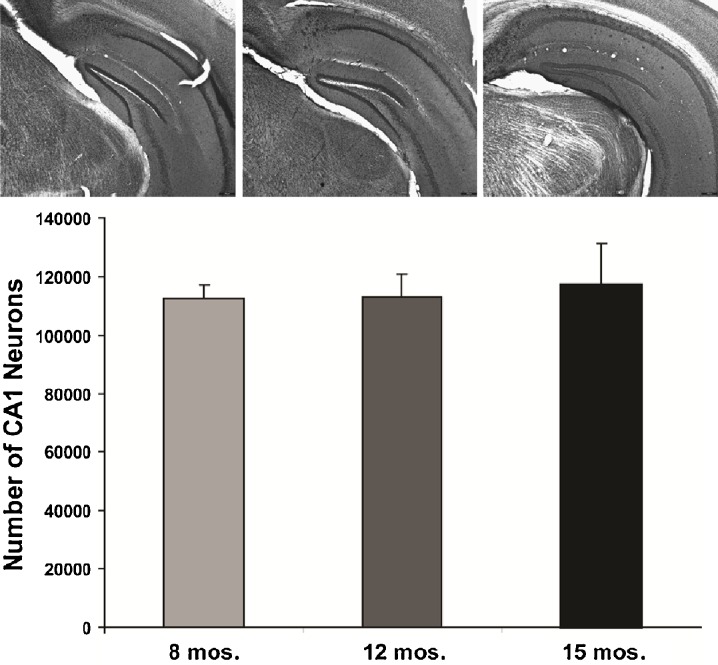
All three staining methods confirmed age-related increases in Aβ peptide deposits, amyloid plaques, and phospho tau in hippocampal formation and neocortex from young adult to late middle age in 3×Tg mice (Fig. 1). Congo red staining revealed extracellular amyloid deposits primarily restricted to the subiculum region of the hippocampal formation and layer V of neocortex (Figs. 1 and 2). Immunostaining with 6E10 and 4G8 confirmed intracellular Aβ-immunopositive deposits in the pyramidal neuron layer of CA1, a region without extracellular plaques stained by Congo red. Computerized stereology was used to assess total number (total N) of neurons in two regions, the LC and CA1 subregion of the hippocampus, that are associated with neuron loss in AD. As shown in Fig. 3, there was a significant 37% reduction in the mean total number of TH+ neurons in LC of late middle-aged mice compared to that in young adult mice (p < 0.05). In early middle-aged 3×Tg mice, there was a 26% reduction from total number of TH+ mice in LC of young adult mice (p < 0.05), and a non-significant reduction in late middle-aged mice compared to early middle-aged mice (p = 0.2). There was also an age-dependent increase in plaque volume in the hippocampal area of 3×Tg mice (p < 0.05; Fig. 4). In contrast to these significant age-related changes, there was no relationship between age and number of CA1 pyramidal neurons (Fig. 5).
Fig. 1.
Distribution of Aβ and phospho-tau at the hippocampal formation in the 3×Tg mouse model. a Low-magnification (×4) images of Aβ-40 (left column), PHF-tau (middle column), and combined staining (right column) of 3×Tg mice at 8 months (top row), 12 months (middle row), and 15 months of age (bottom row). b High-magnification (×60) images from a 15-month-old mouse
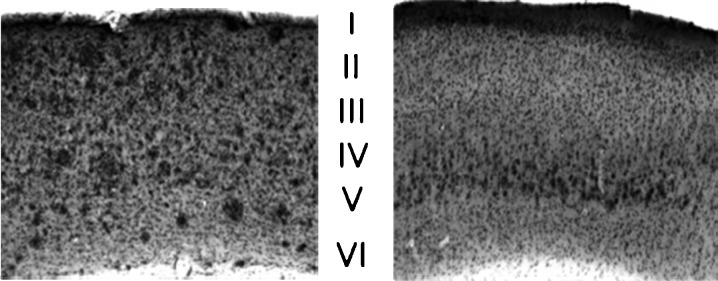
Fig. 2.
Preferential accumulation of extracellular Aβ in cortical layer V. A low-magnification (×4) comparison of Aβ deposition in cortex of 13-month-old dtg (left) and 12-month-old 3×Tg (right) mice shows that while Aβ deposits are found in all layers of cortex of dtg mice, they are found only in layer V of 3×Tg mice
Fig. 3.
Age-dependent loss of norepinephrine neurons in the 3×Tg mice. Low-magnification (×4) image of TH staining in LC region of 3×Tg mice at 8 (a), 12 (b), and 15 months (c). d Stereological analysis carried out at high magnification (×60) revealed an age-dependent loss of TH-ir neurons in LC of 3×Tg mice (*p < 0.05)
Fig. 4.
Accumulation of extracellular Aβ with age in the hippocampus. Low-magnification (×4) image of Congo red staining in hippocampus of 3×Tg mice at 8 (a), 12 (b), and 15 months (c). d Stereological analysis revealed an age-dependent accumulation of extracellular deposits in 3×Tg mice (*p < 0.05)
Fig. 5.
Sparing of CA1 neurons in 3×Tg mice. Low-magnification (×4) image of Nissl staining in CA1 subregion of 3×Tg mice at 8 (a), 12 (b), and 15 months (c). d Stereological analysis revealed no relationship between age and the number of CA1 pyramidal neurons
Discussion
The availability of transgenic mouse models with AD-type neuropathology provides the opportunity to better understand the interaction between the deposition of these markers and age-related neuron loss that correlates strongly with the progression of dementia in AD. Although amyloid plaques and neurofibrillary tangles in cortical brain regions have historically been the microscopic neuropathology markers of AD, these structures occur in normal (non-demented) brain aging and fail to correlate with the severity of cognitive decline (Price et al. 1991; Berg et al. 1998; Mattson 2004; van Dooren et al. 2005; Duyckaerts et al. 2008). In contrast, the finding of neuron loss in circuits associated with cognitive function is thought to play a central role in the progressive dementia in AD (Price et al. 1991; Manaye et al. 1995; Berg et al. 1998; Mattson 2004; Grudzien et al. 2007).
The cause(s) of age-related cognitive loss is currently unknown in AD or for transgenic mouse models that form AD-type amyloid plaques containing mutant Aβ peptides. Studies in humans and experimental animals support the view that short-term memory loss in AD is closely related to disruptions in hippocampal circuitry, particularly pyramidal neurons in the CA1 subregion (Squire 1992). Furthermore, disruptions in the noradrenergic LC are thought to account for a wide spectrum of behavioral abnormalities in AD, including apathy, agitation, anxiety, irritability, dysphoria and aberrant motor behavior, delusion, disinhibition, anxiety, and hallucinations (Mega et al. 1996; Berridge and Waterhouse 2003; Weinshenker 2008; Sara 2009). The majority of studies show strong correlations between severity of dementia in AD and loss of noradrenergic neurons in the LC and loss of pyramidal neurons in hippocampal CA1 (Chan-Palay and Asan 1989; German et al. 1992; Busch et al. 1997; West et al. 2000; Grudzien et al. 2007; Weinshenker 2008). Because neuron losses in LC and CA1 do not occur during normal brain aging (West 1993; Mouton et al. 1994; Ohm et al. 1997), understanding the process underlying the degeneration of these brain regions could provide important clues to the underlying cause of cognitive decline in AD.
This study investigated neuron loss in LC and CA1 of two mouse models of AD-type neuropathology with variable intracellular/extracellular ratios of Aβ immunocytochemistry in hippocampus. We report several remarkable similarities and differences in the 3×Tg mouse line (Oddo et al. 2003a, b) compared to the dtg APPswe/PS1ΔE9 (Borchelt et al. 1997). The 3×Tg mice showed significant age-related loss of TH+ neurons in LC that was significantly correlated to increasing accumulation of extracellular amyloid plaques, with stability in the total number of pyramidal neurons in CA1. The lack of age-related loss of CA1 neurons in 3×Tg mice distinguishes this line from dtg APPswe/PS1ΔE9 (Manaye et al. 2010) and the APPSLPS1KI model (Casas et al. 2004), both of which report significant loss of pyramidal neurons in CA1 of the hippocampus at 13 and 17 months of age, respectively. Since the oldest 3×Tg mice in this study were 18 months of age, the possibility remains that CA1 neuronal loss occurs at later ages.
The spatiotemporal pattern of amyloid plaques and intracellular/extracellular Aβ deposition in cortical tissue of 3×Tg differs markedly from that in dtg APPswe/PS1ΔE9 mice. As reported in previous studies of 3×Tg mice (Carroll et al. 2007; Mastrangelo and Bowers 2008; Oh et al. 2010) and 5×Tg mice (Oakley et al. 2006), our studies of 3×Tg mice show age-related increases in intraneuronal Aβ-immunoreactive deposits in the subiculum and, to a lesser extent, in the neocortex and hippocampal CA1 region. In contrast, there was little to no evidence of intraneuronal Aβ immunoreactivity in the subiculum, hippocampus, or neocortex of the dtg APPswe/PS1ΔE9 mouse. In addition to age-related intraneuronal accumulation of Aβ peptides in the subiculum, there is progressive deposition of extracellular Aβ-immunoreactivity in the subiculum and occasionally in hippocampus and entorhinal cortex starting around 8 months of age in the 3×Tg mice. In the dtg APPswe/PS1ΔE9 mice, the progressive increase in the deposition of amyloid plaques begins about 4 months earlier (Borchelt et al. 1997; Manaye et al. 2007; Mouton et al. 2009; Manaye et al. 2010) than in the 3×Tg mice. Compared to age-matched dtg APPswe/PS1ΔE9 mice, Congo red histochemistry and immunocytochemistry for 6E10 and 4G83xTg mice showed noticeably diminished levels of peak amyloid staining and Aβ deposition in hippocampus. In the dtg APPswe/PS1ΔE9 mice, there was early deposition of Aβ immunoreactive deposits in hippocampus, with spread to subiculum and neocortex regions, whereas Aβ immunoreactive deposits in 3×Tg mice were primarily limited to the subiculum. Thus, the two lines of mice showed distinct spatial and temporal patterns of deposition of intracellular Aβ and extracellular Aβ, as well as different rates of amyloid plaque formation in the hippocampus and neocortex.
Our computerized stereology analysis indicates stability in the number of pyramidal neurons in CA1 of 3×Tg mice, together with high ratios of intracellular/extracellular deposition of Aβ peptides in CA1 region. This high ratio of intracellular/extracellular Aβ in the CA1 region of the 3×Tg mouse supports an attractive hypothesis for the lack of pyramidal neuron loss in the CA1 region: diminished inflammatory reactivity coincident with reduced extracellular deposition of mutant human Aβ peptides. In the dtg APPswe/PS1ΔE9 mice, which have relatively heavy deposition of extracellular Aβ peptides and no detectable levels of intracellular Aβ in the CA1 region, neuron loss occurs in both the LC and CA1 (O’Neil et al. 2007; Liu et al. 2008; Manaye et al. 2010). Thus, relatively low concentrations of extracellular Aβ peptides in the CA1 region of the 3×Tg mice may protect pyramidal neurons in CA1 against inflammatory damage and death secondary to reactive neurogliosis. In support of this idea, the 3×Tg mice show markedly reduced levels of astrocytic activation, as evidenced by reduced GFAP immunoreactivity in the molecular layers of the CA1 region compared to that in dtg APPswe/PS1ΔE9 mice.
Although 3×Tg mice deposit phospho-tau in hippocampal regions in an age-related manner, this neuropathology does not appear to be associated with cognitive decline in these mice. A line of 3×Tg mice with a repressible human tau variant were developed (Santacruz et al. 2005) that over-express mutant tau by 7–13 fold, resulting in mice that develop a tauopathy associated with neurodegeneration and impaired cognitive function. Prior to the extracellular deposition of AD-like distributions of Aβ and tau pathologies, the 3×Tg mice develop long-term potentiation in association with intracellular Aβ immunoreactivity. After suppression of transgenic tau, memory function recovered in these mice despite continued accumulation of NFTs. This finding indicates that NFTs alone likely cannot account for the cognitive decline in this 3×Tg model of tauopathy. Although no morphometric studies were carried out in that study, the observed cognitive impairment could result from loss of noradrenergic innervation to cortical brain regions, which, according to the present study, begins to increase in an age-dependent manner by early middle age (12 months) in the 3×Tg line. In support of the view that loss of TH+ neurons in LC may be responsible for the cognitive impairment in 3×Tg mice, dtg APPswe/PS1ΔE9 mice show cognitive impairment at age 18 months (Savonenko et al. 2005). Since both the 3×Tg and dtg APPswe/PS1ΔE9 lines have significant loss of TH+ neurons in LC by 18 months of age, but the total number of pyramidal neurons in CA1 is stable in the 3×Tg mice, the loss of noradrenergic innervation to cortical regions may be responsible for the cognitive impairments in both the 3×Tg mice and the dtg APPswe/PS1ΔE9 mice.
Acknowledgments
This research was supported by grants from the US Public Health Service NINDS/NIH, SNRP 2U54NS039409-10 (KFM), R01 AG0245478 (RST), R44MH076541-04 (PRM), and GU Pilot Grant (KFM).
References
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55(3):326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42(1):33–84. doi: 10.1016/S0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19(4):939–945. doi: 10.1016/S0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- Busch C, Bohl J, Ohm TG. Spatial, temporal and numeric analysis of Alzheimer changes in the nucleus coeruleus. Neurobiol Aging. 1997;18(4):401–406. doi: 10.1016/S0197-4580(97)00035-3. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Wiederhold KH, Abramowski D, Phinney AL, Probst A, Sturchler-Pierrat C, Staufenbiel M, Sommer B, Jucker M. Neuron loss in APP transgenic mice. Nature. 1998;395(6704):755–756. doi: 10.1038/27351. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27(48):13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas C, Sergeant N, Itier JM, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol. 2004;165:1289–1300. doi: 10.1016/S0002-9440(10)63388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V, Asan E. Quantitation of catecholamine neurons in the locus coeruleus in human brains of normal young and older adults and in depression. J Comp Neurol. 1989;287(3):357–372. doi: 10.1002/cne.902870307. [DOI] [PubMed] [Google Scholar]
- Doya K. Modulators of decision making. Nat Neurosci. 2008;11(4):410–416. doi: 10.1038/nn2077. [DOI] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383(6602):710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C, Potier MC, Delatour B. Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115(1):5–38. doi: 10.1007/s00401-007-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3. Boston: Elsevier/Academic Press, Amsterdam; 2008. [Google Scholar]
- German DC, Manaye KF, White CL, 3rd, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32(5):667–676. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- Grudzien A, Shaw P, Weintraub S, Bigio E, Mash DC, Mesulam MM. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging. 2007;28(3):327–335. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology—reconsidered. J Microsc. 1999;193(Pt 3):199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Holcomb I, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzaen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada C, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Lee GD, Aruna JH, Barrett PM, Lei DL, Ingram DK, Mouton PR. Stereological analysis of microvascular parameters in a double transgenic model of Alzheimer’s disease. Brain Res Bull. 2005;65(4):317–322. doi: 10.1016/j.brainresbull.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yoo MJ, Savonenko A, Stirling W, Price DL, Borchelt DR, Mamounas L, Lyons WE, Blue ME, Lee MK. Amyloid pathology is associated with progressive monoaminergic neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2008;28(51):13805–13814. doi: 10.1523/JNEUROSCI.4218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, Kalehua AN, Muth NJ, Calhoun ME, Jucker M, Hengemihle JM, Ingram DK, Mouton PR. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19(5):497–503. doi: 10.1016/S0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Manaye KF, McIntire DD, Mann DM, German DC. Locus coeruleus cell loss in the aging human brain: a non-random process. J Comp Neurol. 1995;358(1):79–87. doi: 10.1002/cne.903580105. [DOI] [PubMed] [Google Scholar]
- Manaye KF, Wang PC, O’Neil JN, Huang SY, Xu T, Lei DL, Tizabi Y, Ottinger MA, Ingram DK, Mouton PR. Neuropathological quantification of dtg APP/PS1: neuroimaging, stereology, and biochemistry. Age (Dordr) 2007;29(2–3):87–96. doi: 10.1007/s11357-007-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaye KF, Allard JS, Kalifa S, Drew AC, Xu G, Ingram DK, Cabo RD, Mouton PR (2010) 17alpha-estradiol attenuates neuron lossin ovariectomized Dtg AbetaPP/PS1 mice. J Alzheimers Dis. doi:10.3233/JAD-2010-100993 [DOI] [PMC free article] [PubMed]
- Mastrangelo MA, Bowers WJ. Detailed immunohistochemical characterization of temporal and spatial progression of Alzheimer’s disease-related pathologies in male triple-transgenic mice. BMC Neurosci. 2008;9:81. doi: 10.1186/1471-2202-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430(7000):631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46(1):130–135. doi: 10.1212/WNL.46.1.130. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principles and practices of unbiased stereology: an introduction for bioscientists. Baltimore: The Johns Hopkins University Press; 2002. [Google Scholar]
- Mouton PR, Pakkenberg B, Gundersen HJ, Price DL. Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. J Chem Neuroanat. 1994;7(3):185–190. doi: 10.1016/0891-0618(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Chachich ME, Quigley C, Spangler E, Ingram DK. Caloric restriction attenuates cortical amyloidosis in a double transgenic mouse model of Alzheimer’s disease. Neurosci Lett. 2009;464:184–187. doi: 10.1016/j.neulet.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil JN, Mouton PR, Tizabi Y, Ottinger MA, Lei DL, Ingram DK, Manaye KF. Catecholaminergic neuronal loss in locus coeruleus of aged female dtg APP/PS1 mice. J Chem Neuroanat. 2007;34(3–4):102–107. doi: 10.1016/j.jchemneu.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003;24(8):1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oh KJ, Perez SE, Lagalwar S, Vana L, Binder L, Mufson EJ (2010) Staging of Alzheimer’s pathology in triple transgenic mice: a light and electron microscopic analysis. Int J Alzheimers Dis 2010. doi:10.4061/2010/780102 [DOI] [PMC free article] [PubMed]
- Ohm TG, Busch C, Bohl J. Unbiased estimation of neuronal numbers in the human nucleus coeruleus during aging. Neurobiol Aging. 1997;18(4):393–399. doi: 10.1016/S0197-4580(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Overk CR, Kelley CM, Mufson EJ. Brainstem Alzheimer’s-like pathology in the triple transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;35(3):415–425. doi: 10.1016/j.nbd.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12(4):295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Timothy CJ, Maclean CJ, Baker HF. Conditional learning and memory impairments following neurotoxic lesion of the CA1 field of the hippocampus. Neuroscience. 1995;67(2):263–275. doi: 10.1016/0306-4522(95)00063-O. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Vyas V, Hernandez-Estrada T, Nichol KE, Christie LA, Head E. Lack of pathology in a triple transgenic mouse model of Alzheimer’s disease after overexpression of the anti-apoptotic protein Bcl-2. J Neurosci. 2008;28(12):3051–3059. doi: 10.1523/JNEUROSCI.5620-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10(3):211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Savonenko A, Xu GM, Melnikova T, Morton JL, Gonzales V, Wong MP, Price DL, Tang F, Markowska AL, Borchelt DR. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer’s disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005;18(3):602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037/0033-295X.99.2.195. [DOI] [PubMed] [Google Scholar]
- van Dooren T, Dewachter I, Borghgraef P, van Leuven F. Transgenic mouse models for APP processing and Alzheimer’s disease: early and late defects. Subcell Biochem. 2005;38:45–63. doi: 10.1007/0-387-23226-5_2. [DOI] [PubMed] [Google Scholar]
- Weinshenker D. Functional consequences of locus coeruleus degeneration in Alzheimer’s disease. Curr Alzheimer Res. 2008;5(3):342–345. doi: 10.2174/156720508784533286. [DOI] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993;14(4):287–293. doi: 10.1016/0197-4580(93)90113-P. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- West MJ, Kawas CH, Martin LJ, Troncoso JC. The CA1 region of the human hippocampus is a hot spot in Alzheimer’s disease. Ann N Y Acad Sci. 2000;908:255–259. doi: 10.1111/j.1749-6632.2000.tb06652.x. [DOI] [PubMed] [Google Scholar]
- West MJ, Bach G, Soderman A, Jensen JL. Synaptic contact number and size in stratum radiatum CA1 of APP/PS1DeltaE9 transgenic mice. Neurobiol Aging. 2009;30(11):1756–1776. doi: 10.1016/j.neurobiolaging.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46(4):681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]