Abstract
Insecticide resistance in the malaria vector An. gambiae s.l. threatens insecticide-based control efforts, necessitating regular monitoring. We assessed resistance in field-collected An. gambiae s.l. from Jinja, Uganda using WHO biosassays. Only An. gambiae s.s. and An. arabiensis (≈70%) were present. Female An. gambiae exhibited extremely high pyrethroid resistance (permethrin LT50 >2h; deltamethrin LT50 >5h). Female An. arabiensis were resistant to permethrin and exhibited reduced susceptibility to deltamethrin. However, whilst An. gambiae were DDT resistant, An. arabiensis were fully susceptible. Both species were fully susceptible to bendiocarb and fenitrothion. Kdr 1014S has increased rapidly in the Jinja population of An. gambiae s.s. and now approaches fixation (≈95%), consistent with insecticide-mediated selection, but is currently at low frequency in An. arabiensis (0.07%). Kdr 1014F was also at low frequency in An. gambiae. These frequencies preclude adequately-powered tests for association with phenotypic resistance. PBO synergist bioassays resulted in near complete recovery of pyrethroid susceptibility suggesting involvement of CYP450s in resistance. A small number (0.22%) of An. gambiae s.s. x An. arabiensis hybrids were found, suggesting the possibility of introgression of resistance alleles between species. The high levels of pyrethroid resistance encountered in Jinja threaten to reduce the efficacy of vector control programmes which rely on pyrethroid impregnated bednets or indoor spraying of pyrethroids.
Keywords: Anopheles arabiensis, kdr, metabolic resistance, malaria, permethrin, introgression, hybridisation
Background
In this era of malaria control and elimination, the World Health Organization (WHO) is advocating rapid scale-up of vector control interventions with major roles for insecticide treated nets (ITNs) and indoor residual spraying (IRS) (WHO, 2011). However, only four classes of mosquito adulticides (organochlorines, pyrethroids, carbamates and organophosphates) are available for vector control (Nauen, 2007; Ranson et al., 2011) and no new public-health insecticide has been licensed in the past two decades (Nauen, 2007; Kelly-Hope et al., 2008). The four classes share just two target sites (pyrethroids and DDT target the voltage-gated sodium channel, carbamates and organophosphates target acetylcholinesterase) and with this severe limitation the selection pressure induced by control strategies is likely to lead to the development of resistance.
Whilst rotational use of insecticides from different classes in order to mitigate against resistance is feasible for IRS application, ITNs are reliant solely on pyrethroids due to their low mammalian toxicity. Whilst conclusive proof is presently lacking, there is some empirical evidence that insecticide resistance may reduce the efficacy of malaria vector control efforts (N’Guessan et al., 2007; Sharp et al., 2007; Ranson et al., 2011; Asidi et al., 2012). Resistance to pyrethroids in the most important African malaria vector An. gambiae sensu stricto (della Torre et al., 2002) has been widely reported in Sub-Saharan Africa (Ranson et al., 2011) and recently in Uganda (Verhaeghen et al., 2006; Rubaihayo et al., 2008; Ramphul et al., 2009; Verhaeghen et al., 2010). If insecticide resistance does impact upon control then this is a major concern for malaria control efforts in Uganda (Yeka et al., 2012) and regular resistance monitoring will be pivotal in counteracting this threat.
Resistance to pyrethroid insecticides is associated predominantly with target site insensitivity and increased detoxification (Berge et al., 1998; Ranson et al., 2011). Two single base substitutions in the voltage-gated sodium channel commonly referred to as knockdown resistance (kdr) mutations that confer cross-resistance to DDT and pyrethroids have been described in An. gambiae sensu lato populations (Martinez-Torres et al., 1998; Ranson et al., 2000). A leucine-serine substitution at position 1014 (1014S) identified originally in Kenyan An. gambiae s.s. (Ranson et al., 2000) and referred to as kdr-east and a leucine-phenylalanine substitution at the same amino acid position (1014F) identified originally in West Africa (Martinez-Torres et al., 1998) and referred to as kdr-west have a strong association with resistance (Donnelly et al., 2009). The 1014S variant is now found in An. gambiae s.s. throughout much of East/Central Africa (Etang et al., 2006; Pinto et al., 2006; Ridl et al., 2008) and there has been a rapid rise in frequency of 1014S in Kenyan An. gambiae s.s. coinciding with the scaling up of ITN distribution (Mathias et al., 2011). Whilst the 1014F allele is at high frequency in West Africa it has been only rarely reported in East African populations including at very low frequency in Ugandan An. gambiae s.s. (Verhaeghen et al., 2006)
The An. gambiae s.l. species complex is regarded as the primary target for ITN and IRS vector control strategies in Uganda (Ugandan MOH, 2010) and previous studies have detected pyrethroid and DDT resistance in these vectors in Uganda (Ramphul et al., 2009; Verhaeghen et al., 2010). In recent work in Apac, northern Uganda, resistance of the local An. gambiae population to pyrethroid insecticides and DDT prompted a switch to a carbamate insecticide to which no resistance had been detected. Whilst IRS using DDT in the first round of spraying and the class II pyrethroid α-cypermethrin in the second round gave rise to a modest (but significant) decrease in malaria morbidity in the <5y age group (Odds Ratios of 0.76 and 0.83 respectively), when IRS switched to bendiocarb to which the mosquito population was fully susceptible, the decrease in malaria morbidity was much greater (OR = 0.16–0.34 over the three rounds of IRS conducted) (Kigozi et al., 2012). Thus, knowledge of resistance patterns can have important implications for effective control.
In this study, we assess the insecticide resistance status of field-collected An. gambiae s.l. from Jinja, a site of medium-level malaria transmission in which no wide-scale IRS or ITN distribution is currently undertaken.
Methods
Study site
The study was conducted between the months of July and October, 2011 in Jinja district-Walukuba sub-county (N 00°25.857′ E 033°13.739′) located in Eastern Uganda along the shoreline of Lake Victoria (Fig. 1). Jinja, is one of the largest urban centres in Uganda. The region is characterised by hilly grassland and has a bimodal rainfall pattern with a long rainy season between July and November and a short rainy season between February and May. The temperature is relatively constant throughout the year with an average of 25°C. In the most recent survey malaria endemicity was characterised as mesoendemic (Okello et al., 2006).
Figure 1.
Map of eastern Uganda depicting the location of Jinja.
Mosquito sample collections and morphological identification
Mosquitoes were collected as larvae by the dipping method (Service, 1993) from a variety of breeding sites including rice fields, temporary pools along roadsides, tyre tracks and cow hoof prints. Larvae were transferred to the insectary at Walukuba Health Centre, fed on finely ground Tetramin fish food, and reared to adulthood. Emerging adults were fed on 10% sugar solution and identified as belonging to the Anopheles gambiae species complex using morphological keys (Gillies & De Meillon, 1968; Gillies & Coetzee, 1987).
Insecticide susceptibility tests
Three to five day old adult male and non-blood-fed female mosquitoes were exposed to insecticide treated papers impregnated with WHO diagnostic concentrations (0.05% deltamethrin; 0.75% permethrin; 0.1% bendiocarb; 1% fenitrothion and 4% DDT). Bioassays were conducted in accordance with standard WHO insecticide susceptibility testing procedures (W.H.O., 1998). Batches of 20–25 mosquitoes were exposed to each insecticide for 1h to determine the baseline susceptibility in the population. Secondary investigations were performed to establish time response curves and to characterise the LT50 (exposure time required for 50% mortality) to deltamethrin and permethrin through exposure of batches of 20–25 mosquitoes to either deltamethrin or permethrin at different time intervals (1, 5, 15, 30, 45, 60, 90,120 and 150min for deltamethrin; 5, 15, 30, 45, 60, 90,120, 240 and 360min for permethrin). Apart from the differing time of exposure, these tests were in all other ways compliant with the WHO protocol. Temperature and humidity were recorded during both exposure and holding periods, and mortality scored 24h post exposure. With the exception of mosquitoes that survived exposure beyond 60min, samples were stored individually over silica gel for further molecular analysis. Mosquitoes that survived exposure time ≥ 60min were killed by aspiration into 90% ethanol, blotted dry, and stored immediately in RNALater (Ambion) for future microarray analysis. Legs from these specimens were stored separately for species identification PCR and kdr taqman assays (see below).
For comparison, and to permit generation of resistance ratios (RRs), LT curves were also generated from the pyrethroid susceptible An, gambiae Kisumu strain, with exposure times of 0.25, 0.5, 1, 2, 3, 5, 10 minutes (deltamethrin) and 0.5, 1, 3, 5, 8, 10, 15 minutes (permethrin).
Synergist bioassays
To investigate the possible role of insecticide detoxification by P450 monooxygenases in the resistance phenotype, mosquitoes were exposed for 1h to papers treated with the synergist piperonyl butoxide (PBO; 4%) prior to exposure to either DDT, deltamethrin or permethrin insecticide treated papers for a further 60min. Mortality was scored after a 24h recovery period. Controls were exposed to PBO treated papers then control papers.
Molecular analysis
The species identification PCR of Scott et al. (1993) was applied to all samples. An. gambiae s.s. molecular form identification (M and S molecular forms) of a subset of samples was completed using the SINE PCR of Santolamazza et al. (2008). Kdr (1014S and 1014F) genotype at codon 1014 was determined using the Taqman PCR of Bass et al. (2007). Exon 21 of the VGSC was sequenced from representative samples using the primers of Lynd et al. (2010) to address the issue of introgression of kdr alleles from An. gambiae to An. arabiensis.
Data analysis
Binomial confidence intervals around mortality estimates (Newcombe, 1998) were calculated using Vassar Stats (http://faculty.vassar.edu/lowry/VassarStats.html). LT curves were calculated using a custom R script (R Development Core Team, 2011). Allelic associations with phenotype were investigated using Fisher Exact tests calculated using Vassar Stats.
Results
Insecticide susceptibility tests and synergist bioassays
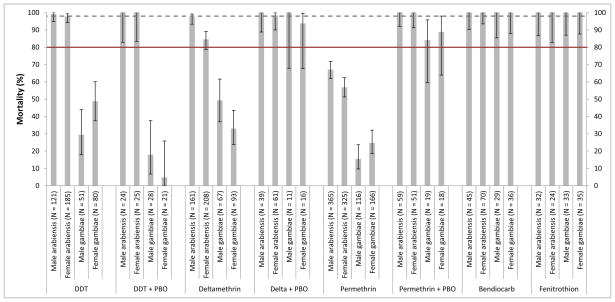
A total of 7,202 non-blood-fed An. gambiae s.l. aged 3–5 days, were assayed for resistance using WHO standard 60min exposures, or for determination of LT curves for permethrin and deltamethrin. In total, 707 An. gambiae s.s. and 1,536 An. arabiensis were exposed to diagnostic doses of deltamethrin (0.05%), permethrin (0.75%), bendiocarb (0.1%), fenitrothion (1%) and DDT (4%) for 1h. Complete susceptibility to bendiocarb (carbamate) and fenitrothion (organophosphate) was observed in both species and to DDT in An. arabiensis. In An. gambiae s.s. resistance was detected to DDT (♀ mortality following 1h exposure = 48.8 %; ♂ = 29.4%), deltamethrin (♀ 33% and ♂ 49.3%) and permethrin (♀ 24.7% and ♂ 15.5%), and in An. arabiensis to permethrin (♀ 56.9% and ♂ 67.1%) with reduced susceptibility to deltamethrin in females only (♀ 84.6 and ♂ 97.5%). Data with 95% confidence intervals are shown in Figure 2. The possible involvement of cytochrome P450s in the resistance phenotype was investigated using the synergist PBO. Exposure to PBO prior to bioassays was found to fully recover the susceptibility of An. gambiae s.s. to deltamethrin, and produce near full recovery of susceptibility to permethrin, suggesting that P450s are involved in the resistance phenotype. PBO exposure did not alter susceptibility to DDT (Figure 2). In An. arabiensis both permethrin and deltamethrin susceptibility was recovered through prior exposure to PBO.
Figure 2.
Mortality levels for An. gambiae s.s. and An. arabiensis males and females exposed for 1h to insecticides and the synergist Piperonyl Butoxide (PBO) plus insecticide. The straight line depicts the level of 80% mortality and the dashed line 98% mortality. Mortalities <80% are indicative of resistance under WHO terminology and mortality of 80–98% indicates incipient resistance.
LT curves for permethrin and deltamethrin are shown in Figs 3 and 4 respectively. We found the LT50s for permethrin in An. gambiae s.s. from Jinja to be 207min (♂) and 132min (♀). For comparison, the LT50s for the Kisumu strain were 7.8min (♀) and 5.3 min (♂) giving female and male resistance ratios of 16.9 and 39 respectively. In An. arabiensis the LT50 for permethrin was 49min (females) and 33min (males) with resistance ratios relative to Kisumu of 6.2 for both sexes. The LT50s for deltamethrin in An. gambiae were higher than those for permethrin (♀ = 319min; ♂ = 458min) whilst mortalities for An. arabiensis were comparable to the levels for permethrin in this species (♀ = 49min and ♂ = 42min) (Figure 3). For Kisumu, the LT50s for deltamethrin were 1.09min for females and 0.09 min (95% CIs 0.04–0.24) for males. Because of the extreme susceptibility of males to deltamethrin, the LT50 is not reliable and we have utilised the upper confidence limit of the estimate in RR calculations, producing RRs for An, gambiae of 292 and 1908 in females and males respectively, and for An. arabiensis RRs of 45 (females) and 175 (males).
Figure 3.
Species- and sex-specific LT curves for exposure of adult mosquitoes to 0.75% permethrin in a WHO tube bioassay (females: solid lines, black circles; males: dashed lines, crosses). The LT50 calculated from these figures are An. arabiensis ♀ 48.19min, An. arabiensis ♂ 33.21min, An. gambiae ♀ 135.7min, An. gambiae ♂ 213.3min.
Figure 4.
Species- and sex-specific LT curves for exposure of adult mosquitoes to 0.05% deltamethrin in a WHO tube bioassay. The LT50 calculated from these figures are An. arabiensis ♀ 48.72min, An. arabiensis ♂ 41.46min, An. gambiae ♀ 319min, An. gambiae ♂ 458min.
Molecular analysis
Only An. arabiensis and An. gambiae s.s. were detected in Jinja with An. arabiensis the more common (70.4%) with An. gambiae x An. arabiensis hybrids detected at low frequency (0.22%). Only An. gambiae S form were found when a subset of An. gambiae (N = 145) was analysed with the SINE PCR diagnostic. The kdr 1014S allele approached fixation in An. gambiae s.s. (95.04%; C.I. 94.11–95.83%) whereas in An. arabiensis only 4 out of 2,988 specimens were L/S heterozygotes (frequency of 1014S 0.07%; C.I. 0.03–0.18%) – see Table 1. We sequenced An. arabiensis heterozygotes (L/S) and L/L homozygotes, and An. gambiae homozygous for S/S to determine whether 1014S might have introgressed from An. gambiae to An. arabiensis. Sequencing confirmed the presence of the 1014S allele in An. arabiensis but there is insufficient variation in this fragment to discriminate between de novo mutation and introgression as the cause. No significant associations between 1014S and resistance were found for either species phenotyped with any insecticide (alive following exposure to ≥ 60 mins, dead following exposure to ≤ 60mins). Although consistent with an hypothesis that 1014S does not show very strong resistance associations within the population, without genotyping considerably very large sample sizes, the low minor allele frequencies (i.e. commonness of the serine allele in An. gambiae and rarity in An. arabiensis) curtail the power of the association tests to detect weak-moderate association with resistance phenotypes.
Table 1.
Genotype counts at codon 1014 of the voltage gated sodium channel in An. gambiae and An. arabiensis from Walukuba, Jinja in samples phenotyped for permethrin, deltamethrin and DDT. P values are from 2-tailed exact tests.
| Insecticide | Sex | Species |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alive ≥ 60 min
|
Dead ≤ 60 min
|
|
|||||||||
| LL | LS | SS | FS | LL | LS | SS | FS | P | |||
| Permethrin | Female | An. gambiae | 1 | 14 | 152 | 1 | 0 | 10 | 38 | 0 | 0.051 |
| Permethrin | Male | An. gambiae | 3 | 12 | 132 | 0 | 0 | 3 | 22 | 0 | 1 |
| DDT | Female | An. gambiae | 0 | 4 | 37 | 0 | 0 | 4 | 35 | 0 | 1 |
| DDT | Male | An. gambiae | 0 | 2 | 34 | 0 | 0 | 1 | 14 | 0 | 1 |
| Deltamethrin | Female | An. gambiae | 3 | 5 | 64 | 0 | 0 | 1 | 41 | 0 | 0.06 |
| Deltamethrin | Male | An. gambiae | 1 | 3 | 36 | 0 | 1 | 3 | 32 | 0 | 1 |
| Permethrin | Female | An. arabiensis | 177 | 1 | 0 | 0 | 259 | 0 | 0 | 0 | 0.41 |
| Permethrin | Male | An. arabiensis | 116 | 1 | 0 | 0 | 295 | 0 | 0 | 0 | 0.28 |
| DDT | Female | An. arabiensis | 4 | 0 | 0 | 0 | 166 | 0 | 0 | 0 | 1 |
| DDT | Male | An. arabiensis | 1 | 0 | 0 | 0 | 110 | 1 | 0 | 0 | 0.4 |
| Deltamethrin | Female | An. arabiensis | 33 | 0 | 0 | 0 | 186 | 0 | 0 | 0 | 1 |
| Deltamethrin | Male | An. arabiensis | 8 | 0 | 0 | 0 | 198 | 0 | 0 | 0 | 1 |
Through screening 126 An. arabiensis and 150 An. gambiae for 1014F, only 1 An. gambiae S/F heterozygote was detected and this was confirmed through sequencing (frequency of 1014F = 0.33% in An. gambiae; C.I. 0.06–1.86%).
Discussion
In this study we have demonstrated resistance to pyrethroid insecticides in An. gambiae s.l. from Jinja, one of the largest population centres in Uganda. For An. gambiae s.s., the resistance levels are particularly high with an LT50 for females of over 2h for permethrin exposure and over 5h for deltamethrin exposure. Such high levels of resistance are a major concern for malaria control efforts owing to reliance on pyrethroid-impregnated bednets. Pyrethroid and DDT resistance in Ugandan An. gambiae s.l. has been documented previously (Rubaihayo et al., 2008; Ramphul et al., 2009; Verhaeghen et al., 2010). Prevalence of phenotypic resistance appears to have risen sharply in recent years in Jinja. Using standard 1h WHO diagnostic tests Verhaeghen et al. (2010) observed 74–99% mortality in permethrin-exposed female An. gambiae collected between 2004 and 2006. We now observe 25% mortality. No deltamethrin resistance was detected by Verhaeghen et al. though we now detect only 33% mortality to deltamethrin. Over the same period, DDT mortality was 67–87% and is now 49% (this study). Whilst there are no previous data on resistance for An. arabiensis in Jinja, data from Tororo and Busolwe (≈ 100km east of Jinja) from 2008 indicate full susceptibility to permethrin and deltamethrin and incipient resistance to DDT in the Busolwe An. arabiensis population (Ramphul et al., 2009). Here, we report resistance to permethrin and incipient resistance to deltamethrin in female An. arabiensis, suggesting that resistance might be increasing Uganda. Whilst there is evidence that An. arabiensis and An. gambiae do not contribute equally to malaria transmission e.g. Okello et al. (2006) reported 45% abundance of An. arabiensis in Jinja in 2001–2 but these gave only a 23% contribution to malaria transmission, nevertheless the apparently higher frequency of An. arabiensis in this study (70%) than in the 2001–2002 collections of Okello et al. (2006) suggests a potentially increased role in overall malaria transmission. Recently, Bayoh et al. (2010) documented a species shift in Western Kenya with gradual replacement of An. gambiae s.s. by An. arabiensis which was postulated to be in response to increased ITN use. However, ITNs are not used widely in Jinja and hence this is unlikely to explain the apparent rise in An. arabiensis frequency in the Jinja population.
In the present study we failed to detect significant associations between the kdr 1014S mutation and any resistance phenotype. However, given the power constraints resulting from low minor allele frequencies in both species (see Weetman et al. 2010) it would be unwise to reject involvement of 1014S in resistance, especially given previous findings of major involvement of 1014S in DDT and permethrin resistance in eastern Uganda (Ramphul et al., 2009). Moreover, allele frequency dynamics strongly suggest a strong recent role in resistance. Verhaeghen, et al. (2010) showed a significant increase of kdr 1014S frequency in An. gambiae s.s from Jinja, from 13% in 2001/2002 to 34% in 2004/2006. Our data show that the increase in kdr 1014S frequency has continued, with the mutation now close to fixation in An. gambiae s.s. (≈ 95%). Such a rapid rate of increase is unlikely to have arisen in the absence of strong DDT or pyrethroid-mediated selection, as implicated elsewhere in cases of kdr increase (Protopopoff et al., 2008; Lynd et al., 2010; Mathias et al., 2011). However, 1014S remains at a very low frequency in An. arabiensis (0.07%). We also confirm the finding of Verhaeghen et al. (2006) that 1014F is present in An. gambiae in Uganda, and should now be monitored routinely alongside 1014S.
The low frequency of 1014S in An. arabiensis suggests that current levels of phenotypic resistance in this species are not related to target site mechanisms and implicate involvement of other factors such as metabolic resistance, cuticular changes or behavioural avoidance. Metabolic resistance to pyrethroids in An. gambiae has often been shown to be mediated by cytochrome P450 enzymes (e.g. Müller et al., 2008; Stevenson et al., 2011). The near complete restoration of susceptibility of both species to permethrin and deltamethrin by prior exposure to PBO suggests that P450s are also involved with resistance in the present study. However, this effect was not observed when PBO was used in combination with DDT. Whilst there is evidence that DDT can be metabolised by cytochrome P450s (Chiu et al., 2008; Mitchell et al., 2012) DDT resistance is more commonly associated with metabolism by GSTs rather than P450s (Chiu et al., 2008) and GSTs are inhibited by DEM (diethyl maleate), which was not employed as a synergist in the present study. Future microarray experiments will aid in understanding the mechanisms underlying the resistance patterns.
We detected a low frequency (0.22%) of hybrids in this population. The presence of hybrids is indicative of the potential for some level of gene flow between the two species (Diabate et al., 2004; Donnelly et al., 2004). An. gambiae s.s. x An. arabiensis hybrids have also been found in Kenya (Petrarca et al., 1991; Stump et al., 2004) at comparable frequencies (0.2%) and also elsewhere in East Africa (White et al., 1972; Stump et al., 2004). Hybridisation between species offers the opportunity for introgression of resistance alleles between species. We detected the kdr 1014S allele at a low frequency in An. arabiensis and this allele could have arisen de novo in An. arabiensis (Diabate et al., 2004) or through genetic introgression from An. gambiae s.s. (Stump et al., 2004). Unfortunately in the sequence we analysed around the 1014 codon there is insufficient variation to distinguish between these two hypotheses. The presence of kdr in An. gambiae s.s. populations is believed to threaten malaria control efforts (Vulule et al., 1994; N’Guessan et al., 2007; Rubaihayo et al., 2008; Mathias et al., 2011) hence, the recent appearance of this mutation in An. arabiensis indicates that we should assess its impact on malaria control; the very low kdr frequency in An. arabiensis in this population, presents a valuable opportunity to develop a time series on the frequency of 1014S in this species for modelling studies (Barbosa et al., 2011).
Whilst resistance to pyrethroids is extremely high in An. gambiae and significant in An. arabiensis, complete susceptibility to bendiocarb and fenitrothrion was found in both species suggesting promise for carbamates or organophosphates in future control efforts. A switch from DDT to bendiocarb use in the IRS strategy employed in Apac, northern Uganda, following identification of resistance to DDT and pyrethroids resulted in a significant drop in malaria cases (Kigozi et al., 2012). The complete susceptibility of the Jinja mosquito population to these insecticides suggests that they are currently likely to be efficacious against both An. gambiae and An. arabiensis.
Acknowledgments
The project described was supported by Award Number U19AI089674 from the National Institute of Allergy and Infectious Diseases (NIAID). HDM was supported by the Uganda Malaria Clinical Operational and Health Services (COHRE) Training Program at Makerere University, Grant #D43-TW00807701A1, from the Fogarty International Center (FIC) at the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, FIC or NIH. We thank the staff of Walukuba Health Centre for provision of insectary facilities and the staff of the molecular laboratory (MOLAB) at Makerere University for facilitating access to molecular laboratory facilities.
References
- Asidi A, N’Guessan R, Akogbeto M, Curtis C, Rowland M. Loss of household protection from use of insecticide-treated nets against pyrethroid-resistant mosquitoes, Benin, Emerging Infectious. Diseases. 2012;18:1101–1106. doi: 10.3201/eid1807.120218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa S, Black WC, IV, Hastings I. Challenges in estimating insecticide selection pressures from mosquito field data. PLoS Neglected Tropical Diseases. 2011;5:e1387. doi: 10.1371/journal.pntd.0001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malaria Journal. 2007;6:111. doi: 10.1186/1475-2875-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malaria Journal. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge JB, Feyereisen R, Amichot M. Cytochrome P450 monooxygenases and insecticide resistance in insects. Philosophical Transactions of the Royal Society of London B Biological Science. 1998;353:1701–1705. doi: 10.1098/rstb.1998.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu TL, Wen Z, Rupasinghe SG, Schuler MA. Comparative molecular modeling of Anopheles gambiae CYP6Z1, a mosquito P450 capable of metabolizing DDT. Proceedings of the National Academy of Sciences USA. 2008;105:8855–8860. doi: 10.1073/pnas.0709249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- della Torre A, Costantini C, Besansky NJ, Caccone A, Petrarca V, Powell JR, et al. Speciation within Anopheles gambiae - the glass is half full. Science. 2002;298:115–117. doi: 10.1126/science.1078170. [DOI] [PubMed] [Google Scholar]
- Diabate A, Brengues C, Baldet T, Dabiré KR, Hougard JM, Akogbeto M, et al. The spread of the Leu-Phe kdr mutation through Anopheles gambiae complex in Burkina Faso: genetic introgression and de novo phenomena. Tropical Medicine and International Health. 2004;9:1267–1273. doi: 10.1111/j.1365-3156.2004.01336.x. [DOI] [PubMed] [Google Scholar]
- Donnelly MJ, Corbel V, Weetman D, Wilding CS, Williamson MS, Black WC., IV Does kdr genotype predict insecticide-resistance phenotype in mosquitoes? Trends in Parasitology. 2009;25:213–219. doi: 10.1016/j.pt.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Donnelly MJ, Pinto J, Girod R, Besansky NJ, Lehmann T. Revisiting the role of introgression vs shared ancestral polymorphisms as key processes shaping genetic diversity in the recently separated sibling species of the Anopheles gambiae complex. Heredity. 2004;92:61–68. doi: 10.1038/sj.hdy.6800377. [DOI] [PubMed] [Google Scholar]
- Etang J, Fondjo E, Chandre F, Morlais I, Brengues C, Nwane P, et al. First report of knockdown mutations in the malaria vector Anopheles gambiae from Cameroon. American Journal of Tropical Medicine and Hygiene. 2006;74:795–797. [PubMed] [Google Scholar]
- Gillies M, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical region) South African Institute for Medical Research; 1987. [Google Scholar]
- Gillies M, De Meillon B. The Anophelinae of Africa south of the Sahara: (Ethiopian Zoogeographical Region) South African Institute for Medical Research; 1968. [Google Scholar]
- Kelly-Hope L, Ranson H, Hemingway J. Lessons from the past: managing insecticide resistance in malaria control and eradication programmes. Lancet Infectious Diseases. 2008;8:387–389. doi: 10.1016/S1473-3099(08)70045-8. [DOI] [PubMed] [Google Scholar]
- Kigozi R, Baxi S, Gasasira A, Sserwanga A, Kakeeto S, Nasr S, et al. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoSOne. 2012 doi: 10.1371/journal.pone.0042857. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd A, Weetman D, Barbosa S, Egyir Yawson A, Mitchell S, Pinto J, et al. Field, genetic, and modeling approaches show strong positive selection acting upon an insecticide resistance mutation in Anopheles gambiae s.s. Molecular Biology and Evolution. 2010;27:1117–1125. doi: 10.1093/molbev/msq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Molecular Biology. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- Mathias DK, Ochomo E, Atieli F, Ombok M, Bayoh MN, Olang G, et al. Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in Western Kenya. Malaria Journal. 2011;10:10. doi: 10.1186/1475-2875-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SN, Stevenson BJ, Müller P, Wilding CS, Yawson AE, Field SG, et al. Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proceedings of the National Academy of Sciences USA. 2012;109:6147–6152. doi: 10.1073/pnas.1203452109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P, Warr E, Stevenson BJ, Pignatelli PM, Morgan JC, Steven A, et al. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genetics. 2008;4:e1000286. doi: 10.1371/journal.pgen.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Guessan R, Corbel V, Akogbeto M, Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerging Infectious Diseases. 2007;13:199–206. doi: 10.3201/eid1302.060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Management Science. 2007;63:628–633. doi: 10.1002/ps.1406. [DOI] [PubMed] [Google Scholar]
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Statistics in Medicine. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. American Journal of Tropical Medicine and Hygiene. 2006;75:219–225. [PubMed] [Google Scholar]
- Petrarca V, Beier JC, Onyango F, Koros J, Asiago C, Koech DK, et al. Species composition of the Anopheles gambiae complex (diptera: Culicidae) at two sites in western Kenya. Journal of Medical Entomology. 1991;28:307–313. doi: 10.1093/jmedent/28.3.307. [DOI] [PubMed] [Google Scholar]
- Pinto J, Lynd A, Elissa N, Donnelly MJ, Costa C, Gentile G, et al. Co-occurrence of East and West African kdr mutations suggests high levels of resistance to pyrethroid insecticides in Anopheles gambiae from Libreville, Gabon. Medical and Veterinary Entomology. 2006;20:27–32. doi: 10.1111/j.1365-2915.2006.00611.x. [DOI] [PubMed] [Google Scholar]
- Protopopoff N, Verhaeghen K, Van Bortel W, Roelants P, Marcotty T, Baza D, et al. A significant increase in kdr in Anopheles gambiae is associated with an intensive vector control intervention in Burundi highlands. Tropical Medicine and International Health. 2008;13:1479–1487. doi: 10.1111/j.1365-3156.2008.02164.x. [DOI] [PubMed] [Google Scholar]
- R Devlopment Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. [Google Scholar]
- Ramphul U, Boase T, Bass C, Okedi LM, Donnelly MJ, Müller P. Insecticide resistance and its association with target-site mutations in natural populations of Anopheles gambiae from eastern Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103:1121–1126. doi: 10.1016/j.trstmh.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Molecular Biology. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- Ranson H, N Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends in Parasitology. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Ridl FC, Bass C, Torrez M, Govender D, Ramdeen V, Yellot L, et al. A pre-intervention study of malaria vector abundance in Rio Muni, Equatorial Guinea: their role in malaria transmission and the incidence of insecticide resistance alleles. Malaria Journal. 2008;7:194. doi: 10.1186/1475-2875-7-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubaihayo R, Tukesiga E, Abaasa A. Reduced susceptibility to pyrethroid insecticide treated nets by the malaria vector Anopheles gambiae s.l. in western Uganda. Malaria Journal. 2008;7:92. doi: 10.1186/1475-2875-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, della Torre A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malaria Journal. 2008;7:163. doi: 10.1186/1475-2875-7-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. American Journal of Tropical Medicine and Hygiene. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Service M. Mosquito Ecology: Field Sampling Methods. Chapman and Hall; London: 1993. [Google Scholar]
- Stevenson BJ, Bibby J, Pignatelli P, Muangnoicharoen S, O’Neill PM, Lian LY, et al. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: sequential metabolism of deltamethrin revealed. Insect Biochemistry and Molecular Biology. 2011;41:492–502. doi: 10.1016/j.ibmb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Sharp BL, Ridl FC, Govender D, Kuklinski J, Kleinschmidt I. Malaria vector control by indoor residual insecticide spraying on the tropical island of Bioko, Equatorial Guinea. Malaria Journal. 2007;6:52. doi: 10.1186/1475-2875-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump AD, Atieli FK, Vulule JM, Besansky NJ. Dynamics of the pyrethroid knockdown resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bed net trials. American Journal of Tropical Medicine and Hygiene. 2004;70:591–596. [PubMed] [Google Scholar]
- Ugandan Ministry of Health. Uganda Malaria Control Strategic Plan 2005/6 – 2009/10. Malaria Control Programme Ministry of Health; 2010. [Google Scholar]
- Verhaeghen K, Bortel WV, Roelants P, Okello PE, Talisuna A, Coosemans M. Spatio-temporal patterns in kdr frequency in permethrin and DDT resistant Anopheles gambiae s.s. from Uganda. American Journal of Tropical Medicine and Hygiene. 2010;82:566–573. doi: 10.4269/ajtmh.2010.08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen K, Van Bortel W, Roelants P, Backeljau T, Coosemans M. Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malaria Journal. 2006;5:16. doi: 10.1186/1475-2875-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulule JM, Beach RF, Atieli FK, Roberts JM, Mount DL, Mwangi RW. Reduced susceptibility of Anopheles gambiae to permethrin associated with the use of permethrin-impregnated bednets and curtains in Kenya. Medical Veterinary Entomology. 1994;8:71–75. doi: 10.1111/j.1365-2915.1994.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Weetman D, Wilding CS, Steen K, Morgan JC, Simard F, Donnelly MJ. Association mapping of insecticide resistance in wild Anopheles gambiae populations: major variants identified in a low-linkage disequilbrium genome. PLoS One. 2010;5(10):e13140. doi: 10.1371/journal.pone.0013140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- W.H.O. Test procedures for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticide on treated surfaces. Geneva: 1998. WHO/CDS/CPC/MAL/98.12. [Google Scholar]
- W.H.O. World Malaria Report. World Health Organisation; Geneva: 2011. [Google Scholar]
- White G, Magayuka S, Boreham P. Comparative studies on sibling species of the Anopheles gambiae Giles complex (Dipt., Culicidae): bionomics and vectorial activity of species A and species B at Segera, Tanzania. Bull of Entomological Research. 1972;62:295–317. [Google Scholar]
- Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, et al. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Tropica. 2012;121:184–195. doi: 10.1016/j.actatropica.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]