Abstract
Background
The Melanocortin (MC) peptides and opiod peptide β-endorphin are cleaved from the polypeptide precursor proopiomelanocortin (POMC). POMC-derived peptides are generated by extensive post-translational processing that involves several enzymes including prohormome convertase 1/3 and 2 (PC1/3 and PC2). Because ethanol decreases POMC mRNA levels, we determined if exposure to an ethanol-containing diet (ED) would significantly reduce central immunoreactivity of POMC, PC1/3, PC2 and β-endorphin.
Methods
Male Sprague-Dawley rats were given 18 days of access to a normal rodent chow or a control diet (CD), or short-term (4 days) or long term (18 days) access to an ED. At the end of the study, rats were perfused with 4% paraformaldehyde and their brains were sectioned into sets for processing with POMC, PC1/3, PC2 and β-endorphin immunoreactivity (IR).
Results
Rats exposed to an ethanol containing diet for 18 days (ED18) exhibited significant reductions of POMC and PC1/3 IR in the arcuate nucleus of the hypothalamus (Arc) relative to rats pair-fed a control diet (CD). On the other hand, rats exposed to an ethanol containing diet did not show any changes of central β-endorphin or PC2 IR relative to rats pair-fed a CD, regardless of length of exposure. Because there were no differences in body weights or caloric intake between the CD and ED groups, reductions of POMC and PC1/3 IR in ED-treated rats are best explain by ethanol exposure rather than altered energy balance.
Conclusions
The present study shows that ethanol site-specifically reduces POMC and PC1/3 IR in rat brain. These observations are consistent with ethanol-induced reductions of α-MSH and POMC IR that were previously reported. Since MC agonists have been shown to blunt ethanol intake in rodents, exogenous MC receptor agonists, as well as targets that may increase the synthesis of endogenous α-MSH (e.g. PC1/3) may have therapeutic value for treating alcohol abuse disorders and alcoholism.
Keywords: ethanol consumption, rats, melanocortin, POMC, PC1/3
Introduction
The polypeptide precursor pro-opiomelanocortin (POMC) gives rise to β-endorphin, an endogenous opioid peptide, and the melanocortin (MC) peptides adrenocorticotropic hormone (ACTH), α-melanocyte-stimulating hormone (α-MSH), β-MSH, and γ-MSH (Hadley and Haskell-Luevano, 1999). These peptides are produced by neurons within the hypothalamic arcuate nucleus (Arc), the nucleus of the solitary tract, and the medulla (Dores et al., 1986; Hadley and Haskell-Luevano, 1999; Jacobowitz and O’Donohue, 1978; O’Donohue and Dorsa, 1982).
The MC system has been implicated in the regulation of the neurobiological responses to drugs of abuse and drug-self-administration. For example, administration of α-MSH into the ventral tegmental area (VTA) and central infusion of the non-selective MC receptor agonist, melanotan II (MTII), alter dopamine signaling in the nucleus accumbens (NAc) and the VTA (Lindblom et al., 2001; 2002). Chronic treatment of a high dose of morphine decreases MC-4 receptor (MC4R) mRNA in the NAc, the periaqueductal gray, and neostriatum (Alvaro et al., 1996). Furthermore, chronic treatment with low doses of morphine or cocaine increases MC4R mRNA in the striatum and the NAc (Hsu et al., 2005). There are several observations which suggest that the MC system is a prime candidate for regulating neurobiological responses of ethanol. Central infusion of the MC receptor agonist, MTII, significantly reduces voluntary ethanol consumption in rats selectively bred for high ethanol drinking (Ploj et al., 2002). Additionally, intracerebroventricular (i.c.v.) infusion of MTII reduces ethanol consumption by high ethanol drinking C57BL/6J mice, and MTII-induced reduction of ethanol drinking is receptor-mediated (Navarro et al., 2003). I.c.v. infusion of a selective MC4R agonist significantly reduces ethanol intake, while i.c.v. infusion of the non-selective MCR antagonist AgRP(83–132) significantly increases ethanol drinking, by high ethanol drinking C57BL/6J mice (Navarro et al., 2005). Consistently, i.c.v. infusion of MTII fails to blunt ethanol drinking in mutant mice lacking the MC4R, indicating that MCs modulate ethanol intake via the MC4R (Navarro et al., 2011).
In addition to MC peptides, the POMC-derived opioid neuropeptide, β-endorphin, has been implicated in the modulation of the neurobiological responses to ethanol (Scanlon et al., 1992; Froehlich and Li, 1993; Zhou et al., 2000; Gianoulakis, 2001; Rasmussen et al., 2002). Non-selective opioid receptor antagonists, as well as those selective for the μ or δ opioid receptors, reduce ethanol consumption (Gianoulakis, 2001) and ethanol intake is reduced in μ opioid receptor knockout mice (Roberts et al, 2000; Hall et al., 2001). Local administration of an opioid antagonist into the NAc decreases ethanol consumption and operant responding for alcohol in rats (Heyser et al., 1999; Hyytiä and Kiianmaa, 2001; June et al., 2004).
POMC-derived neuropeptides are generated by extensive post-translational processing that involves several enzymes, including prohormone convertases 1/3 and 2 (PC1/3 and PC2). In the Arc, POMC is initially cleaved by PC1/3 to generate proACTH and β-lipotrophin. ProACTH is further cleaved by PC1/3 to N-terminal proopiocortin (N-POC), joining peptide, and ACTH. PC2 then cleaves ACTH 1–39 to generate ACTH 1–17 and corticotrophin-like intermediate lobe peptide. Further posttranslational modifications of ACTH 1–17 result in α-MSH (Benjannet et al., 1991; Thomas et al., 1991), and β-lipotrophin is further cleaved by PC2 to generate β-endorphin (Liotta et al., 1984). Interestingly, environmental factors, such as food restriction, can alter the expression of both POMC and PC1/3. Thus, fasting-induced alterations of α-MSH expression may result, in part, from alterations of post-translational processing of POMC (Perello et al., 2007).
Ethanol exposure produces direct effects on the expression of POMC (Rasmussen et al., 1998; 2002; Scanlon et al., 1992; Zhou et al., 2000), β-endorphin (Lam et al., 2010; 2011), and α-MSH (Navarro et al., 2008; Korare et al., 2008). Ethanol exposure has been shown to increase (Angelogianni and Gianoulakis, 1993) as well as decrease (Rasmussen et al., 1998; 2002) hypothalamic POMC expression in rats, and to increase (Schulz et al., 1980; Patel and Pohorecky, 1989), decrease (Przewlocka et al., 1990) or have no effect (Seizinger et al., 1983; Popp and Erickson, 1998) on β-endorphin levels in the hypothalamus of rats. We have shown that exposure to an ethanol containing diet leads to a significant reduction of central α-MSH immunoreactivity in regions of the hypothalamus (the Arc and lateral hypothalamus (LH)), the extended amygdala (the central amygdala (CeA) and bed nucleus of the stria terminalis (BNST)) as well as the paraventricular nucleus of the thalamus (PVT) of Sprague-Dawley rats (Navarro et al., 2008).
Because environmental factors have been shown to alter prohormone convertase activity, and in light of the observations that ethanol exposure can induce alterations of α-MSH and β-endorphin activity in the brain, an interesting possibility is that ethanol-induced alterations of α-MSH and β-endorphin expression are associated with ethanol-induced changes in PC1/3 and/or PC2 activity. To begin to address this possibility, we used immunohistochemistry procedures to assess PC1/3, PC2, β-endorphin, and POMC levels in the brains of rats with a history of ethanol exposure (we previously assessed α-MSH levels in this tissue in a recent study (Navarro et al., 2008)). Specifically, the present study determined if short-term (4 days) and/or long-term (18 days) exposure to an ethanol containing diet would alter the immunoreactivity (IR) of β-endorphin in similar brain regions that we have found ethanol-induced alterations of α-MSH IR in rat brain (Navarro et al., 2008).
POMC projections are found in most regions of the central nervous system, however, perikarya in the forebrain that express POMC mRNA are primarily located in the Arc, where prohormone convertase processing of POMC-derived neuropeptides occurs (Léger et al., 1990; Bronstein et al., 1992; Baubet et al., 1994; Givalois et al., 1999). Therefore, in the present study, POMC, PC1/3, and PC2 IR were evaluated only in the Arc.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles River, Raleigh, NC, USA) were obtained at 160–180g and were maintained at 22°C with a 12:12 light/dark cycle. All rats were individually housed in plastic rat cages with free access to water and food and all the procedures used were in compliance with the National Institute of Health Guideline and were approved by the University of North Carolina Institutional Animal Care and Use Committee (IACUC).
Ethanol and Control Diets
The diet was a lactalbumin/dextrose-based, nutritionally complete diet (Dyets, Inc., Bethlemen, PA). Dextrose calories in the control diet (CD) were equated with ethanol calories in the ethanol diet (ED). Rats were habituated to drink CD in the absence of rodent chow for 2 days (with the exception of the chow control group described below). During the study, all rats with ED were first habituated with 2 days access to a 4.5% (w/v) ED, followed by access to a 7% (w/v) ED for an additional 2 or 16 days. A modified pair-feeding design was used. To equate the caloric intake between groups, the rats maintained on the CD were give a volume of diet equivalent to the average volume consumed the previous day by the animals maintained on the ED. Rodent chow was removed from each rat cage during diets access and rats had access to a second bottle containing tap water all the times.
Following habituation, rats were distributed to 4 groups matches on body weight (n= 10/group) so that each group had approximately the same average weight at the beginning of the study. To control potential effects of diet on the IR, one group of rats was maintained on normal rodent chow (Chow) for the entire study. A second control group received CD in place of rodent chow for the duration of the study. A third group was given CD for 14 days, the 4.5% ED for 2 days, and the 7% ED for 2 days (ED4). A fourth group received the 4.5% ED for 2 days, followed by the 7% ED for 16 days (group ED18). Rats that experienced a similar protocol (15 days of access to a 7% ED) achieved blood ethanol concentrations ranging from 100 mg/dl to 200 mg/dl during the 15 days of access (Overstreet et al., 2002). Throughout the study, diet intake and body weight measures were recorded daily.
Perfusion, Brain preparation, and Immunohistochemistry (IHC)
Immunohistochemistry procedures are described in Navarro et al., (2008). Briefly, after 4 or 18 days of access to diet, at the end of the dark cycle, rats were perfused in pairs and the order was counterbalanced by diet condition. Rats remained in their cages and had access to diet up to the time of perfusions to avoid ethanol withdrawal in the ED groups. All perfusions were completed within a 5-hour window of time. The brains were collected and post-fixed in paraformaldehyde for 24 hours at 4°C, at which point they were transferred to PBS and cut using a vibrotome in to 40μm section for the IHC assay. Sections were evenly divided into four sets (every-other section) for processing with, β-endorphin, POMC, PC1/3 or PC2 antibodies. After rinsing in fresh PBS 4 times (10 minutes each), tissue sections were blocked in 10% goat serum and 0.1% triton-X-100 in PBS for 1 hour. Sections were then transferred to fresh PBS containing primary rabbit anti-PC1/3 or PC2 (Abcam Inc., Cambridge, MA; 1:1000), primary rabbit anti-β-endorphin (Chemicon International, Inc., Temecula, CA; 1:5000), or chicken anti-POMC (Chemicon International, Inc., Temecula, CA; 1:5000) for 3 days at 4°C (Navarro et al., 2008). As a control to determine if staining required the presence of the primary antibodies, some sections were run through the assay without primary antibody (PC1/3, PC2, β-endorphin or POMC). In each assay described below, tissue processed without the primary antibody failed to show staining that was evident in tissue processed with primary antibody. After 3 days of incubation, the sections were rinsed 4 times and then processed with Vectastain Elite kits (Vector Labs, Inc., Burlingame, CA) as per the manufacturer’s instructions for standard ABC/HRP/diaminobenzidine-based immunohistochemistry. The sections processed for PC1/3, PC2, β-endorphin or POMC were visualized by reacting the sections with a 3,3′-diamino-benzidine tetrahydrochloride (DAB, Polysciences, Inc., Warrington, PA) reaction solution containing 0.05% DAB, 0.0005% cobalt, 0.007% nickel ammonium sulfate, and 0.006% hydrogen peroxide. All sections were mounted on glass slides, air-dried overnight, and cover slipped for viewing.
Digital images of PC1/3, PC2, β-endorphin and POMC IR were obtained on a Nikon E400 microscope equipped with a Nikon Digital Sight DS-U1 digital camera run with Nikon-provided software. For analysis, great care was taken to match sections through the same region of brain and at the same level using anatomic landmarks with the aid of a rat stereotaxic atlas (Paxinos and Watson, 1986). We have shown previously that quantification by counting α-MSH positive cell bodies or by measuring the density of α-MSH using Image J software produced statistically similar results (Navarro et al., 2008). Thus, when specific cell bodies were evident in the present report we used the cell-body counting method. For cell counting, all visible cell bodies stained within the defined brain region were counted manually by an experimenter blinded to group condition. Data from each brain region in an animal were calculated by taking the average counts from 2 brain slices. Data from each slice were calculated by taking the average counts from the left and right side of the brain at the specific brain region of interest. For non-cell body localization of β-endorphin in a given brain region, densitometric procedures were used to assess protein levels. Flat-field corrected digital pictures (8-bit grayscale) were taken using the Digital Sight DS-U1 camera and density of staining was analyzed using Image J software (Image J, National Institute of Health, Bethesda, MD) by calculating the percent of the total area examined that showed signal (cell bodies and processes) relative to a subthreshold background. The size of the areas that were analyzed was the same between animals and groups. The subthreshold level for the images was set in such a way that any area without an experimenter defined level of staining (determined by terminal- and/or soma-positive regions) was given a value of zero (the same subthreshold level, determined by a condition-blinded experimenter, was used for all slices within a given region). Anatomically matched pictures of the left and right sides of the brain were used to produce an average density for each brain region from each slice. In all cases, quantification of IR data was conducted by an experimenter that was blinded to group identity.
Data Analyses
All data are presented as mean ± SEM and one-way analyses of variance (ANOVA) procedures were used for statistical assessment of group differences. Separate ANOVAs were performed for each brain region scored. If significant differences were found, post hoc analyses were conducted using the Tukey’s HSD test. P<0.05 (two-tailed) was used as the level of statistical significance. Because we obtained the same results with or without the inclusion of the chow-fed group, all groups were included in the analyses.
RESULTS
Body Weights
Analyses of body weight data have been described previously (Navarro et al., 2008). At the beginning of the study, no differences in body weight were found between the groups (Chow, 286.6±6.5g; CD, 287±5.7g; ED4, 287.7±4.4; ED18, 287.5±4.8g). While the group that ate chow for the duration of the study weighed more than groups of rats given access to liquid diet, importantly, there were no significant differences between the three diet groups at the end of the study (Chow, 396.5±9.4g; CD, 344.8±5.0g; ED4, 339.9±6.1g; ED18, 326.8±9.1g). Thus, differences in IR are unlikely related to differences related to energy balance between diet exposed groups.
β-Endorphin IR
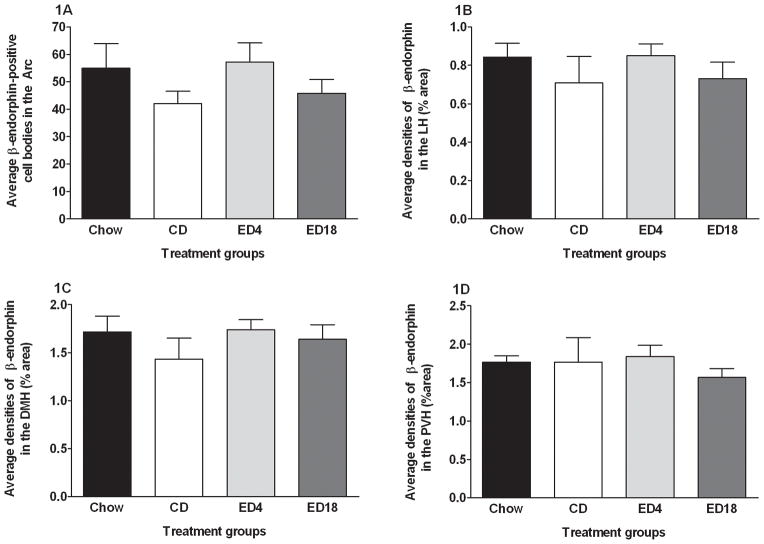
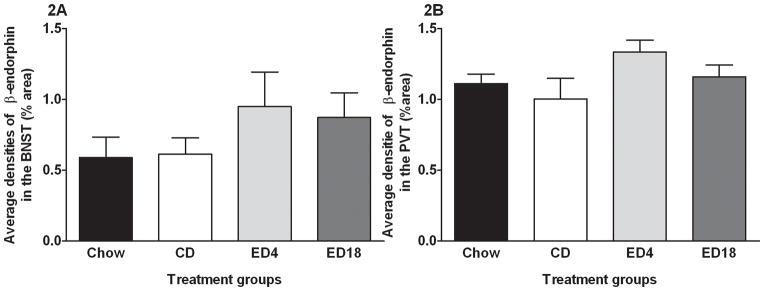
We observed β-endorphin IR in regions of the hypothalamus. Figure 1 shows average β-endorphin in the Arc (Fig. 1A), the LH (Fig. 1B), the dorsomedial hypothalamus (DMH; Fig. 1C), and the paraventricular nucleus of the hypothalamus (PVN; Fig. 1D). One-way ANOVAs performed on data from the Arc (F3, 36 = 1.195; p > 0.05), the LH (F3, 36 = 0.609; p > 0.05), the DMH (F3, 36 = 0.714; p > 0.05), and PVN (F3, 36 = 0.384; p > 0.05) failed to achieve statistical significance. We also observed β-endorphin IR in the BNST (Fig. 2A) and the PVT (Fig. 2B). One-way ANOVAs preformed data from the BNST (F3, 36 = 1.055; p > 0.05) and the PVT (F3, 36 = 1.915; p > 0.05) both failed to reach statistical significance.
Fig. 1.
Quantification of β-endorphin-positive cell bodies (A) in the arcuate nucleus of the hypothalamus (Arc), and β-endorphin immunoreactivity (% area) in the lateral nucleus of the hypothalamus (LH) (B), the dorsomedial nucleus of the hypothalamus (DMH) (C), and the paraventricular nucleus of the hypothalamus (PVN) (D). Groups were given 18 days of access to normal rodent chow (Chow) or an ethanol-free control diet (CD), or an ethanol diet for 4 (ED4) or 18 (ED18) days. Values are represented as mean ± SEM.
Fig. 2.
Quantification of β-endorphin immunoreactivity (% area) in the bed nucleus of the stria terminallis (BNST) (A) and paraventricular nucleus of the thalamus (PVT) (B).
POMC IR
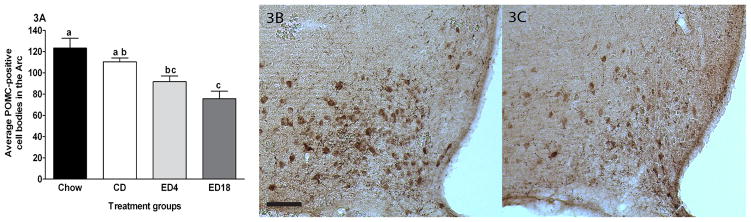
Data representing the average IR of POMC in the Arc are presented in Fig. 3A, and represented photomicrographs of POMC IR in the Arc are depicted in Fig. 3B–C. A one-way ANOVA performed on these data was significant (F3, 36 = 9.850; p < 0.001). Tukey’s HSD post hoc tests revealed that both groups ED4 and ED18 showed significant lower POMC IR relative to the Chow group. Relative to the CD group, only the ED18 group showed decreased POMC IR. We also ran a correlation between the total amount of ethanol consumed during the study and the amount of POMC IR. The correlation, r(20) = −0.298, p = 0.203, was not statistically significant, consistent with the observation that both the ED4 and ED18 groups showed reductions of POMC IR.
Fig. 3.
Quantification of POMC-positive cell bodies in the arcuate nucleus (Arc) of the hypothalamus (A). Representative photomicroprahs of 40μm coronal sections showing POMC immmunoreactivity through the arcuate nucleus of the hypothalamus of rats give 18 days of exposure to the control diet (CD) (B) or the ethanol diet for 18 days (ED18) (C). Images were photographed and quantified at a magnitude of 10x. Scale bar = [200μm]. There are statistical differences between groups that do not share overlapping lettering (a, b or c p<0.05)
PC1/3 and PC2 IR
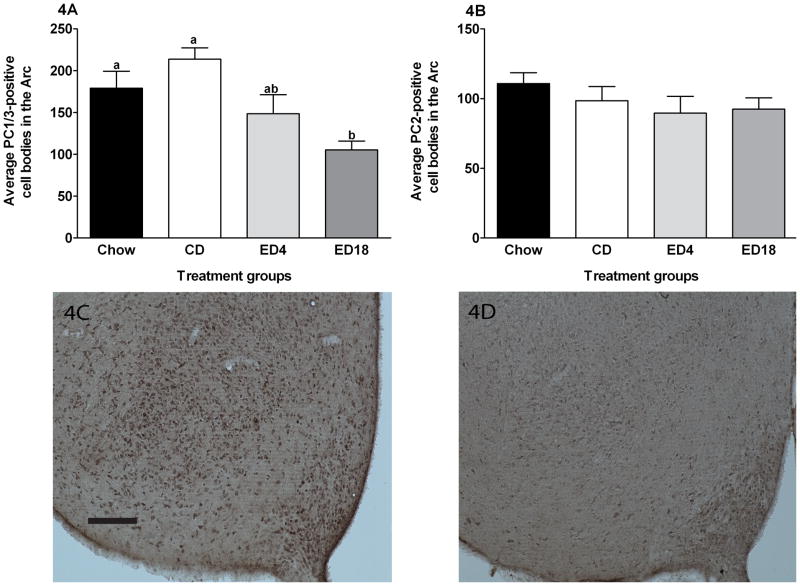
Data representing the average IR of PC1/3 and PC2 IR in the Arc are presented in Fig. 4A and B, respectively, and represented photomicrographs of PC1/3 IR in the Arc are depicted in Fig. 4C–D. A one-way ANOVA performed on PC1/3 IR data was significant (F3, 36 = 6.853; p < 0.001). Tukey’s HSD post hoc tests revealed that the ED18 group showed significant lower PC1/3 IR relative to the Chow and CD groups. No other group differences emerged. We ran a correlation between the total amount of ethanol consumed during the study and the amount of PC1/3 IR. The correlation, r(20) = −0.456, p = 0.043, was statistically significant, consistent with the observation that the ED18 group exhibited more robust reductions of PC1/3 than the ED4 group. Finally, a one-way ANOVA performed on PC2 data did not achieve significance (F3, 36 = 0.944; p > 0.05).
Fig. 4.
Quantification of PC1/3 (A) and PC2 (B) -positive cell bodies in the arcuate nucleus of the hypothalamus (Arc). Representative photomicroprahs of 40μm coronal sections showing PC1/3 immmunoreactivity through the arcuate nucleus of the hypothalamus of rats give 18 days of exposure to the control diet (CD) (C) or the ethanol diet for 18 days (ED18) (D). Images were photographed and quantified at a magnitude of 10x. Scale bar = [200μm]. There are statistical differences between groups that do not share overlapping lettering (a or b p<0.05)
DISCUSSION
The results obtained in the present study show that Sprague-Dawley rats exposed to an ethanol containing diet for 18 day (ED18) exhibit significant reductions of POMC and PC1/3 IR in the Arc relative to rats pair-fed a control diet (CD). On the other hand, rats exposed to an ethanol containing diet do not show any change of central β-endorphin or PC2 IR relative to rats pair-fed a CD, regardless of length of exposure. Importantly, ethanol-induced reduction of POMC and PC1/3 IR are consistent with ethanol-induced reduction of α-MSH IR that we have previously reported (Navarro et al., 2008).
There is a converging body of evidence implicating a role for the MC system in the modulation of neurobiological responses to ethanol, and our group and others have shown that ethanol exposure and withdrawal from chronic ethanol alter α-MSH IR in the brain (Rainero et al., 1990; Navarro et al., 2008; Kokare et al., 2008; 2010). The present report, to the best of our knowledge, provides the first evidence that chronic ethanol exposure promotes a significant reduction of prohormone convertase activity, specifically, a reduction of PC1/3. The lack of an influence of ethanol exposure on PC2 IR suggests that the effects of ethanol are specific to a subset of prohormone convertases. Since PC1/3 is necessary for the normal processing of α-MSH from the precursor POMC, it is interesting to speculate that ethanol-induced reduction of α-MSH IR stems for blunted processing of α-MSH from POMC. This possibility is supported by the high degree of co-expression of PC1/3 and POMC in the Arc (Helwig et al., 2006). Since ethanol exposure also reduces POMC IR, blunted α-MSH may also stem from low availability of POMC. Blunted POMC IR in the present report is consistent with previous findings showing that chronic ethanol exposure inhibits POMC mRNA in the Arc of rats (Rasmussen et al., 2002).
α-MSH containing cells are prominently seen in the Arc and are known to innervate other regions of the HT (DMN PVN, LH) as well as the extended amygdala (CeA, BNST) (Cone, 2005). Reductions of α-MSH IR after ethanol exposure were found in the Arc, LH, CeA and BNST (Navarro et al., 2008). The LH is critical for the expression of drug-seeking behaviours (Marchant et al., 2009; Di Leone et al., 2003). One of the neuropeptide systems present in the LH, orexin, has been established to play a role in drug seeking-behavior (Harris et al., 2005). Interestingly, there is an interaction between the MC and orexin system. For example, POMC deficient mice (POMC −/−) exhibit a marked increase in orexin mRNA levels in the LH and α-MSH appears to control the expression of orexins (López et al., 2007). Thus, it will be interesting in future research to determine if the decrease of POMC IR after chronic ethanol exposure is associated with changes in orexin levels, and if MC and orexin peptides interact in the modulation of ethanol consumption. The CeA was another area where reductions of α-MSH IR after ethanol exposure were observed (Navarro et al., 2008). Numerous studies have demonstrated the involvement of the extended amygdala in mediating the behavioral and physiological responses associated with anxiety (Koob, 2008; Davis et al., 2010). The MC system has been implicated in ethanol-induced anxiolysis and withdrawal-induced anxiety (Korare at al., 2006; 2008; 2010). Working with rats, Kokare et al. (2006), showed that the anxiolytic effect of ethanol was suppressed by i.c.v. administration of α-MSH. On the other hand, ethanol withdrawal-induced anxiety-like behavior was blocked by i.c.v. infusion of HS014 (a selective MC4R antagonist). Therefore, it is possible that ethanol-induced decreases of POMC IR in the Arc and α-MSH in the CeA could play a role in the anxiolytic action of ethanol. Additional research is needed to address these questions.
In the present report, while we noted positive β-endorphin IR in several brain regions, exposure to ethanol diet failed to alter β-endorphin IR. Thus, normal POMC and PC1/3 levels do not appear to be necessary to maintain normal levels of β-endorphin under the current conditions. These results are in accord with recent data where chronic exposure to ethanol did not change hypothalamic β-endorphin levels in rats (Leriche and Méndez, 2010), though previous studies have provided inconsistent results about how ethanol can alter β-endorphin levels in several regions of the brain. The response of β-endorphin to ethanol could differ depending of several variables, such as the rodent strain. It has been shown that in Sprague-Dawley rats, acute administration of ethanol (2.0/2.5 g/kg) increased β-endorphin levels in the hypothalamus 20 minutes and 1 hour after ethanol exposure (Schulz et al., 1980; Patel and Pohorecky, 1989). However in Wistar rats, similar doses (2.5g/kg) decreased β-endorphin content in the hypothalamus 1 hour after ethanol administration (Leriche and Méndez, 2010). Another variable that can modify the response of β-endorphin to ethanol is the route of administration. In the present study and others yielding similar results (Leriche and Mendez, 2010), animals self-administered ethanol and no changes in β-endorphin were found. However, other studies have reported an increase of β-endorphin in the hypothalamus after intra-gastric administration (Leriche and Méndez, 2010) or intraperitoneal injection of ethanol (Schulz et al., 1980; Patel and Pohorecky, 1988). When taken together, these data suggest that the response of the β-endorphin system to ethanol could differ depending on the rodent strain (Wistar versus Sprague Dawley), and/or the experimental protocol (the route of ethanol administration, length of ethanol exposure, time of testing, etc).
Central MC signaling modulates food intake and body weight (Fan et al., 1997; Huszar et al., 1997; Marsh et al., 1999; Chen et al., 2000; Schwartz and Wisse, 2000; Cone, 2005). As has been showed previously (Navarro et al., 2008), in the rats used in the present report there were no differences in body weight between the groups that received CD versus the groups that received ED at the end of the study. Additionally, caloric intake between diet groups was matched throughout the entire study. Thus, reduced PC1/3 and POMC IR cannot be explained by differences on caloric intake or body weight. These data suggest that reductions of PC1/3 and POMC in ED-treated groups stem directly from ethanol exposure.
In conclusion, we extend our previous findings by demonstrating that chronic ethanol exposure promotes decreases of POMC and PC1/3 IR in the Arc, which is consistent with our previous work showing that chronic ethanol exposure promotes reductions of central α-MSH IR. Since posttranslational production of α-MSH from POMC requires PC1/3, it is possible that ethanol-induced blunting of α-MSH IR stems from reduced α-MSH translation, though reduced availability of POMC is also a possible explanation. Finally, since MC agonists have been shown to blunt ethanol intake in rodents (Navarro et al., 2003; 2005; 2011), exogenous MC receptor agonists, as well as target that may increase the synthesis of endogenous α-MSH (e.g. PC1/3) may have therapeutic value for treating alcohol abuse disorders and alcoholism.
Acknowledgments
This work was supported by NIH grants AA013573, AA015148.
References
- Angelogianni P, Gianoulakis C. Chronic ethanol increases proopiomelanocortin gene expression in the rat hypothalamus. Neuroendocrinology. 1993;57:106–114. doi: 10.1159/000126348. [DOI] [PubMed] [Google Scholar]
- Alvaro JD, Tatro JB, Quillan JM, Fogliano M, Eisenhard M, Lerner MR, Nestler EJ, Duman RS. Morphine down-regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Mol Pharmacol. 1996;50:583–591. [PubMed] [Google Scholar]
- Baubet V, Fèvre-Montange M, Gay N, Debilly G, Bobillier P, Cespugio E. Effects of an acute immobilization stress upon proopiomelanocortin (POMC) mRNA levels in the mediobasal hypothalamus: a quantitative in situ hybridization study. Mol Brain Res. 1994;26:163–168. doi: 10.1016/0169-328x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Benjannet S, Rondeau N, Day R, Chretien M, Seidah NG. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci USA. 1991;88:3564–3568. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyadjieva N, Dokur M, Advis JP, Meadows GG, Sarkar DK. Chronic ethanol inhibits NK cell cytolytic activity: role of opioid peptide β-endorphin. J Immunol. 2001;167:5645–5652. doi: 10.4049/jimmunol.167.10.5645. [DOI] [PubMed] [Google Scholar]
- Bronstein DM, Schafer MKH, Watson SJ, Akil H. Evidence that β-endorphin is synthesized in cells in the nucleus tractus solitaries: detection of POMC mRNA. Brain Res. 1992;587:269–275. doi: 10.1016/0006-8993(92)91007-2. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs AF, Cao L, Metzger JM, Strack AM, Camacho E, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chan HY, Van der Ploeg LHT. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Gen. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdale in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–35. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73(6):759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Dores RM, Jain M, Akil H. Characterization of the forms of beta-endorphin and alpha MSH in the caudal medulla of the rat and guinea pig. Brain Res. 1986;377:251–260. doi: 10.1016/0006-8993(86)90866-8. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Li TK. Recent developments in alcoholism: opioid peptides. Recent Dev Alcohol. 1993;11:187–205. [PubMed] [Google Scholar]
- Gianoulakis C. Influence of the endogenous opioid system on high alcohol consumption and genetic predisposition to alcoholism. J Psychiatry Neurosci. 2001;26:304–318. [PMC free article] [PubMed] [Google Scholar]
- Givalois L, Li S, Pelletier G. Effects of ageing and dehydroepiandrosterone administration on pro-opiomelanocortin mRNA expression in the anterior and intermediate lobes of the pituitary. Neuroendocrinology. 1999;11:737–742. doi: 10.1046/j.1365-2826.1999.00392.x. [DOI] [PubMed] [Google Scholar]
- Hadley ME, Haskell-Luevano C. The proopiomelanocortin system. Ann NY Acad Sci. 1999;885:1–21. doi: 10.1111/j.1749-6632.1999.tb08662.x. [DOI] [PubMed] [Google Scholar]
- Hall FS, Sora I, Uhl GR. Ethanol consumption and reward are decreased in mu-opiate receptor knockout mice. Psychopharmacology. 2001;154:43–49. doi: 10.1007/s002130000622. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Helwig M, Khorooshi RHM, Tups A, Barret P, Archer ZA, Exner C, Rozman J, Braulke LJ, Mercer JG, Klingenspor M. PC1/3 and PC2 gene expression and post-translational endoproteolytic pro-opiomelanocortin processing is regulated by photoperiod in the seasonal Siberian hamster (Phodopus sungorus) J Neuroendocrinol. 2006;18:413–425. doi: 10.1111/j.1365-2826.2006.01431.x. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Roberts AJ, Schulteis G, Koob GF. Central administration of an opiate antagonist decreases oral ethanol self-administration in rats. Alcohol Clin Exp Res. 1991;23:1468–1476. [PubMed] [Google Scholar]
- Hsu R, Taylor JR, Newton SS, Alvaro JD, Haile C, Han G, Hruby VJ, Nestler EJ, Duman RS. Blockade of melanocortin transmission inhibits cocaine reward. Eur J Neurosci. 2005;21:2233–2242. doi: 10.1111/j.1460-9568.2005.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Hyytiä P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Jacobowitz DM, O’Donohue TL. Alpha-melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. PNAS. 1978;75:6300–6304. doi: 10.1073/pnas.75.12.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Cummings R, Eiler WJ, II, Foster KL, McKay PF, Seyoum R, García M, McCane S, Grey C, Hawkins SE, Mason D. Central opioids receptors differentially regulate the nalmefene-induced suppression of ethanol-and saccharin-reinforced behaviors in alcohol-preferring (P) rats. Neuropsychopharmacology. 2004;29:285–299. doi: 10.1038/sj.npp.1300338. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Chopde CT, Subhedar NK. Participation of α-melanocyte stimulating hormone in ethanol-induced anxiolysis and withdrawal anxiety in rats. Neuropharmacology. 2006;51:536–545. doi: 10.1016/j.neuropharm.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Singru PS, Dandekar MP, Chopde CT, Subhedar NK. Involvement of alphamelanocyte stimulating hormone (alpha-MSH) in differential ethanol exposure and withdrawal related depression in rat: neuroanatomical-behavioral correlates. Brain Res. 2008;1216:53–67. doi: 10.1016/j.brainres.2008.03.064. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Dandekar MP, Singru PS, Gupta GL, Subhedar NK. Involvement of α-MSH in the social isolation anxiety-and depression-like behaviors in the rat. Neuropharmacology. 2010;58:1009–1018. doi: 10.1016/j.neuropharm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MP, Nurmi H, Rouvinen N, Kiianmaa K, Gianoulakis C. Effects of acute ethanol on β-endorphin release in the nucleus accumbens of selectively bred lines of alcohol-preferring AA and alcohol-avoiding ANA rats. Psychopharmacology. 2010;208:121–130. doi: 10.1007/s00213-009-1733-y. [DOI] [PubMed] [Google Scholar]
- Lam MP, Gianoulakis C. Effects of acute ethanol on corticotropin-releasing hormone and β-endorphin systems at the level of the rat central amygdala. Psychopharmacology. 2011;218:229–239. doi: 10.1007/s00213-011-2337-x. [DOI] [PubMed] [Google Scholar]
- Léger L, Lema F, Chastrette N, Charnay Y, Cespuglio R, Mazie JC, et al. A monoclonal antibody directed against CLIP (ACTH18–19). Anatomical distribution of immunoreactivity in the rat brain and hypophysis with quantification of the hypothalamic cell group. Chem Neuroanat. 1990;3:297–308. [PubMed] [Google Scholar]
- Leriche M, Méndez M. Ethanol exposure selectively alters β-endorphin content but not [3H]-DAMGO binding in discrete regions of the brain. Neuropeptides. 2010;44:9–16. doi: 10.1016/j.npep.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Opmane B, Mutulis F, Mutule I, Petrovska R, Klusa V, Bergstrom L, Wikberg JE. The MC4 receptor mediates alpha-MSH induced release of nucleus accumbens dopamine. NeuroReport. 2001;12:2155–2158. doi: 10.1097/00001756-200107200-00022. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Kask A, Hagg E, Harmark L, Bergstrom L, Wikberg J. Chronic infusion of melanocortin receptor agonist modulates dopamine receptor binding in the brain. Pharmacol Res. 2002;45:119–124. doi: 10.1006/phrs.2001.0913. [DOI] [PubMed] [Google Scholar]
- Liotta AS, Advis JP, Krause JE, McKelvy JF, Krieger DT. Demonstration of in vivo synthesis of pro-opiomelanocortin-beta-endorphin-, and alpha-melanotropin-like species in the adult rat brain. J Neurosci. 1984;4:956–965. doi: 10.1523/JNEUROSCI.04-04-00956.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López M, Lage R, Tung YC, Challis BG, Varela L, Virtue S, O’Rahilly S, Vidal-Puig A, Diéguez C, Coll AP. Orexin expression is regulated by α-Melanocyte-Stimulating hormone. J Neurosci. 2007;19:703–707. doi: 10.1111/j.1365-2826.2007.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, MxNallu GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29(5):1331–1342. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. Response of melanocortin-4 receptor-deficient mice to anoretic and orexigenic peptides. Nat Gen. 1999;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Knapp DJ, Thiele TE. MTII-induced reduction of voluntary ethanol drinking is blocked by pretreatment with AgRP-(83–132) Neuropeptides. 2003;37:338–344. doi: 10.1016/j.npep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Chen AS, Chen HY, Knapp DJ, Breese GR, Marsh DJ, Thiele TE. Effects of melanocortin receptor activation and blockade on ethanol intake: a possible role for the melanocortin-4 receptor. Alcohol Clin Exp Res. 2005;29:949–957. doi: 10.1097/01.ALC.0000167740.19702.8C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Knapp DJ, Breese GR, Thiele TE. Decreased immunoreactivity of the melanocortin neuropeptide α-melanocyte-stimulating hormone (α-MSH) after chronic ethanol exposure in Sprague-Dawley rats. Alcohol Clin Exp Res. 2008;32:266–276. doi: 10.1111/j.1530-0277.2007.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Lerma-Cabrera JM, Carvajal F, Lowery EG, Cubero I, Thiele TE. Assessment of voluntary ethanol consumption and the effects of a melanocortin (MC) receptor agonist on ethanol intake in mutant C57BL/6J mice lacking the MC-4 receptor. Alcohol Clin Exp Res. 2011;35:1058–1066. doi: 10.1111/j.1530-0277.2011.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donohue TL, Dorsa DM. The opiomelanotropinergic neuronal and endocrine systems. Peptides. 1982;3:353–395. doi: 10.1016/0196-9781(82)90098-5. [DOI] [PubMed] [Google Scholar]
- Oversteet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VA, Pohorecky LA. Acute and chronic ethanol treatment on beta-endorphin and catecholamine levels. Alcohol. 1989;6:59–63. doi: 10.1016/0741-8329(89)90074-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain stereotaxic coordinates. 2. Academic Press; San Diego, CA: 1986. [Google Scholar]
- Perello M, Stuart RC, Nillni EA. Differential effects of fasting and leptin on proopiomelanocortin peptides in the arcuate nucleus and in the nucleus of the solitary tract. Am J Physiol Endocrinol Metab. 2007;292:E1348–E1357. doi: 10.1152/ajpendo.00466.2006. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Kask A, Hyytia P, Schioth HB, Wikberg J, Nylander I. Effects of melanocortin receptor ligands on ethanol intake and opioid levels in alcohol-preferring AA rats. Brain Res Bull. 2002;59:97–104. doi: 10.1016/s0361-9230(02)00844-4. [DOI] [PubMed] [Google Scholar]
- Popp RL, Erickson CK. The effect of an acute ethanol exposure on the rat brain POMC opiopeptide system. Alcohol. 1998;16:139–148. doi: 10.1016/s0741-8329(98)00003-2. [DOI] [PubMed] [Google Scholar]
- Przewlocka B, Dziedzicka M, Lason W. The effects of ethanol treatment on endogenous opioid peptides level and analgesia in monoarthritic rats. Pol J Pharmacol Pharm. 1990;42:343–349. [PubMed] [Google Scholar]
- Rainero I, De Gennaro T, Visentin G, Brunetti E, Cerrato P, Torre E, Portaleone P, Pinessi L. Effects of chronic ethanol treatment on alpha-MSH concentration in rat brain and pituitariy. Neuropeptides. 1990;172:411–421. doi: 10.1016/0143-4179(90)90145-o. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Bryant CA, Boldt BM, Colasurdo EA, Levin N, Wilkinson CW. Acute ethanol effects on opiomelanocortinergic regulation. Alcohol Clin Exp Res. 1998;22:789–801. [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Wilkinson CW, Mitton DR. Chronic daily ethanol and withdrawal: 3. Forebrain pro-opiomelanocortin gene expression and implications for dependence, relapse, and deprivation effect. Alcohol Clin Exp Res. 2002;26:535–546. [PubMed] [Google Scholar]
- Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, et al. mu Opioid receptor knockout mice do not self-administer alcohol. J Pharm Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- Scanlon MN, Lazar-Wesley E, Grant KA, Kunos G. Proopiomelanocortin messenger RNA is decreased in the mediobasal hypothalamus of rats made dependent on ethanol. Alcohol Clin Exp Res. 1992;16:1147–1151. doi: 10.1111/j.1530-0277.1992.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Schulz R, Wüster M, Duka T, Herz A. Acute and chronic ethanol treatment changes endorphin levels in brain and pituitary. Psychopharmacology. 1980;68:221–227. doi: 10.1007/BF00428107. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Wisse BE. Role of melanocortins in control of obesity. Nat Gen. 2000;26:89. doi: 10.1016/S0140-6736(01)06037-8. [DOI] [PubMed] [Google Scholar]
- Seizinger BR, Bovermann K, Maysinger D, Hollt V, Herz A. Differential effects of acute and chronic ethanol treatment on particular opioid peptide systems in discrete regions of rats brain and pituitary. Pharmacol Biochem Behav. 1983;18:361–369. doi: 10.1016/0091-3057(83)90200-9. [DOI] [PubMed] [Google Scholar]
- Steiner DF. The proprotein convertases. Curr Opi Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- Thomas L, Leduc R, Thorne BA, Smeekens SP, Steiner DF, Thomas G. Kex2-like endoproteases PC2 and PC3 accurately cleave a model prohormone in mammalian cells: evidence for a common core of neuroendocrine processing enzymes. Proc Natl Acad Sci USA. 1991;88:5297–5301. doi: 10.1073/pnas.88.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Franck J, Spangler R, Maggos CE, Ho A, Kreek MJ. Reduced hypothalamic POMC and anterior pituitary CRF1 receptor mRNA levels after acute, but not chronic, daily “binge” intragastric alcohol administration. Alcohol Clin Exp Res. 2000;24:1575–1582. [PubMed] [Google Scholar]