Abstract
Background
Fetal alcohol spectrum disorder is an immense public health problem. In vitro studies support the hypothesis that L1 cell adhesion molecule (L1) is a target for ethanol developmental neurotoxicity. L1 is critical for the development of the central nervous system. It functions through signal transduction leading to phosphorylation and dephosphorylation of tyrosines on its cytoplasmic domain. The function of L1 is also dependent on trafficking through lipid rafts. Our hypothesis is that L1 is a target for ethanol neurotoxicity in vivo. Our objective is to demonstrate changes in L1 phosphorylation/dephosphorylation and lipid raft association in vivo.
Methods
Rat pups on postnatal day 6 are administered 4.5, 5.25 and 6 g/kg of ethanol divided into 2 doses 2 hours apart, then sacrificed. Cerebella are rapidly frozen for assay. Blood is analyzed for blood ethanol concentration. L1 tyrosine phosphorylation is determined by immunoprecipitation and dephosphorylation of tyrosine 1176 determined by immunoblot. Lipid rafts are isolated by sucrose density gradient and the distribution of L1 in lipid rafts is determined.
Results
Ethanol at all doses reduced the relative amount of Y1176 dephosphorylation as well as the relative amount of L1 phosphorylated on other tyrosines. The proportion of L1 present in lipid rafts is significantly increased in pups who received 6 g/kg ethanol compared to intubated controls.
Conclusions
L1 is a target for ethanol developmental neurotoxicity in vivo.
Keywords: Fetal alcohol syndrome, L1 cell adhesion molecule, ethanol, lipid raft, tyrosine phosphorylation
Introduction
The use of alcohol during pregnancy is a major public health problem. Every year, nearly 40,000 infants in the U.S.A are born with lifelong physical, mental and/or neurobehavioral deficits associated with alcohol consumption during pregnancy (Lupton, 2011). These birth defects are often referred to as fetal alcohol spectrum disorders (FASD), of which fetal alcohol syndrome (FAS) is the most devastating.
FAS is characterized by a wide range of abnormalities in brain morphology including hypoplasia of the corpus callosum, optic nerves and cerebellar vermis; the formation of neuronal heterotopias; microcephaly; and disorders in cortical lamination. The neuroanatomical pathologies and anomalies observed in patients with mutations in L1 cell adhesion molecule (L1) are remarkably similar to those found in patients with FAS, suggesting a possible role of L1 in FAS (Bearer et al., 1999; Charness et al., 1994; Ramanathan et al., 1996).
L1 is a transmembrane glycoprotein and a member of the immunoglobulin (Ig) superfamily (Moos et al., 1988), which has a critical role in the development of the CNS. L1 mediates cell-cell adhesion via a homophilic binding mechanism which generates intracellular signals and L1 trafficking (Kamiguchi and Lemmon, 1998; 2000; Kamiguchi et al., 1998; Kamiguchi and Yoshihara, 2001; Schaefer et al., 1999; 2002). The cytoplasmic domain of L1 contains 4 tyrosines whose phosphorylation status regulate L1 signaling and trafficking (Kamiguchi and Lemmon, 1998; 2000; Kamiguchi et al., 1998; Kamiguchi and Yoshihara, 2001; Schaefer et al., 1999; 2002). The tyrosine at position 1176 (Y1176) must be dephosphorylated to allow endocytosis of L1 and L1mediated neurite outgrowth to occur (Kamiguchi and Lemmon, 1998; Kamiguchi et al., 1998; Kamiguchi and Yoshihara, 2001; Schaefer et al., 1999). The tyrosine at position 1229 (Y1229) regulates ankyrin binding via phosphorylation (Garver et al., 1997; Tuvia et al., 1997). Ankyrin binding limits the lateral mobility of L1 and hence the endocytic recycling required for L1 mediated neurite outgrowth (Gil et al., 2003). A key part of L1 trafficking appears to be through a lipid raft compartment (Nakai and Kamiguchi, 2002; Tang et al., 2011). Disruption of lipid rafts using methyl-beta-cyclodextrin completely inhibits L1 mediated neurite outgrowth (Tang et al., 2011).
Multiple in vitro studies demonstrate the inhibitory effect of ethanol on L1 functions in mammalian cells. Ethanol inhibits both L1 homophilic binding and L1 mediated neurite outgrowth (Bearer et al., 1999; Charness et al., 1994; Ramanathan et al., 1996; Watanabe et al., 2004; Wilkemeyer and Charness, 1998; Wilkemeyer et al., 2000). In our previous work, we found that ethanol inhibits both the dephosphorylation of Y1176 and tyrosine phosphorylation of L1 following L1 activation (Yeaney et al., 2009). In addition, ethanol increases the proportion of L1 within the lipid raft compartment, indicating a disruption of L1 trafficking through the lipid raft (Tang et al., 2011).
To date, there is no direct in vivo evidence that ethanol targets L1. In the present study, we show that L1 is targeted by ethanol in vivo. Specifically, we demonstrate in vivo the effect of ethanol on: 1) the dephosphorylation of Y1176 of L1; 2) the tyrosine phosphorylation status of L1; and 3) the distribution of L1 in lipid rafts. Using a well-established rat pup model (Goodlett and Johnson, 1997; Light et al., 1998) that imitates human binge drinking patterns, we present evidence that ethanol at pharmacological concentrations inhibits the tyrosine dephosphorylation/phosphorylation of L1 and alters the distribution of L1 in lipid rafts. These results further confirm that L1 is a target for ethanol developmental neurotoxicity.
Materials and Methods
Antibodies
The mouse monoclonal antibody 74-5H7 which recognizes dephosphorylated Y1176 on the cytoplasmic tail of L1 is previously described (Schaefer et al., 2002). Monoclonal antibodies to phosphotyrosine ( PY-100) are from Cell Signaling (Danvers, MA). Rabbit polyclonal antibody against the cytoplasmic domain of L1 (L1CD) (Schaefer et al., 1999) is from Santa Cruz Biotechnology (Santa Cruz, CA). Horse radish peroxidase (HRP) conjugated cholera toxin B subunits (CTxB) is from Sigma-Aldrich (St. Louis, MO) and mouse monoclonal anti-transferrin receptor antibody is from Invitrogen (Carlsbad, CA). Mouse monoclonal anti N-cadherin antibody is from BD Transduction Laboratories (Sparks, MD). HRP-conjugated goat anti-mouse IgG (H + L) and HRP-conjugated donkey anti-goat IgG (H + L) secondary antibodies are from Jackson Immuno-Research Laboratories (West Grove, PA).
Study Design and Ethanol Dosing Protocol
The neonatal binge alcohol intubation model is used to investigate the effects of ethanol in vivo. This technique is well-described (Goodlett and Johnson, 1997). On postnatal day (PD) six, 10 (5 male, 5 female) Sprague Dawley rat pups from 1 litter are assigned randomly to one of five treatment groups (a male and female in each pair). One pair are suckle controls and left with the dam. The other pups are assigned to groups receiving an isocaloric formula containing 0, 4.5, 5.25 or 6 g/kg/day of ethanol. The pups are removed from the dam, placed on a heating pad at 37°C and weighed. Individual weights are used to calculate the individual volume of custom milk formula. Ethanol solutions are prepared with final concentrations of 10.2%, 11.9%, and 13.6%, and made isocaloric and isovolemic by adding maltose-dextrin solutions to the two lower concentrations. These concentrations and volumes result in a total ethanol dose of 4.5, 5.25, and 6.0 g/kg/day respectively. These concentrations of alcohol are used because they are known to cause a dose dependent loss of Purkinje cells within the cerebellum (Goodlett et al., 1998). Feeds are administered in two acute doses, two hours apart where each intubation uses a volume (ml) of 1/36th of the body weight (grams). For orogastric feeding of the pups PE-10 tubing (BD, NJ, USA) is coated with corn oil and attached to a 1 ml syringe containing the appropriate solution. Two hours after the second feeding, blood is collected from a tail clip using a heparinized hematocrit capillary tube. The pups are decapitated and the head is rapidly frozen in liquid nitrogen. Each experiment is repeated at least 3 times.
Blood alcohol concentrations (BAC) assays
Two hours after the second alcohol feeding, a 20 μl sample of blood is collected in heparinized capillary tubes. Samples are placed immediately in equivolume 6.25% trichloroacetic acid, sealed tightly, and stored at 4°C. The blood alcohol concentrations (BACs) are measured using an enzymatic spectrophotometric assay (Ethanol L3K kit, Diagnostic Chemicals Limited, Oxford, CT).
Tissue preparation
Frozen cerebella are dissected from whole brain, and homogenized in ice cold Tris-buffered saline containing 1% Triton X100, 10 mM sodium vanadate, 2μM aprotinin, 100 μM cypermethein, phosphatase inhibitor cocktail I (Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitor cocktail II (Sigma-Aldrich, St. Louis, MO). Homogenates are kept on ice for 30 min and then centrifuged at 13,000 × g for 10 min at 4°C and supernatants are collected. Protein was determined by Bradford Assay (Sigma-Aldrich, St. Louis, MO).
Immunoprecipitation
Supernatants containing equal amounts of protein are pre-cleared with 1 μg rabbit serum for 1 h, followed by a 1 h incubation with protein A/G agarose. The samples are centrifuged at 1000 × g for 4 min and supernatant is removed. 2 μg anti-L1CD antibody and 30 μg protein A/G-agarose beads are added to the supernatants, and the samples are incubated overnight at 4°C. The beads are washed 3 times with phosphate buffered saline (PBS), and boiled in 30μl 1× sample buffer for 5 min. The sample buffer containing the immunoprecipitates is collected by centrifugation of 1000 × g for 4 min and analyzed by immunoblotting.
Immunoblotting
Twenty μl of sample adjusted with tris buffered saline to contain equal amounts of protein are added to an equal amount of 2× sample buffer and boiled for 5 min. Boiled samples or the sample buffer containing immunoprecipitates are loaded onto SDS-PAGE 4-15% gradient gel and transferred to a PDVF membrane. The PDVF membrane is incubated with washing buffer (20 mM Tris, pH 7.4, 0.9% NaCl, and 0.1% Tween 20) containing 5% nonfat dry milk overnight to block nonspecific binding. Primary antibodies are diluted in washing buffer containing 5% nonfat dry milk and are applied to the PDVF membrane at room temperature. After washing, the blots are incubated with the appropriate HRP-conjugated secondary antibodies (diluted 1:1,000 or 1:2,000 in washing buffer with 5% milk) for 1 h at room temperature. For lipid raft identification, HRP conjugated CTxB is used in place of primary and secondary antibody. Blots are stripped to be reprobed with additional primary antibodies. Immunoreactive bands are visualized with the enhanced chemiluminescence system (Amersham Biosciences). Densitometric quantitation is carried out with KODAK ID image software. The 74-5H7 and PY-100 band densities are normalized against the band densities of L1CD protein in each lane.
Tissue preparation for lipid raft analysis
Frozen cerebella are dissected from whole brain, and homogenized in ice cold Tris-buffered saline containing 0.5% Triton X100, 10 mM sodium vanadate, 2μM aprotinin, 100 μM cypermethein, phosphatase inhibitor cocktail I and phosphatase inhibitor cocktail II. The tissue extracts are centrifuged at 13,000 × g for 10 min at 4°C and supernatants are collected.
Lipid raft isolation
Prior to the sacrifice of the animals, all equipment and buffers are cooled to 4°C. 1 ml of supernatant is mixed with an equal amount of 80% sucrose solution and then overlaid with 4 ml each of 32% and then 1% sucrose solutions. The gradient is centrifuged at 180,000 × g for 24 h at 4°C. Sequential 0.5 ml fractions are drawn off the top of the gradient. An aliquot from each fraction is analyzed by immunoblot analysis for the presence of both transferrin receptor and GM1 ganglioside. All GM1 ganglioside containing fractions are combined into a lipid raft (LR) containing pool. All remaining fractions are combined in a non-lipid raft pool (N) (Tang et al., 2011).
Statistical analysis
Means, standard deviations, ANOVAs and p values are calculated using Microsoft Excel software.
Results
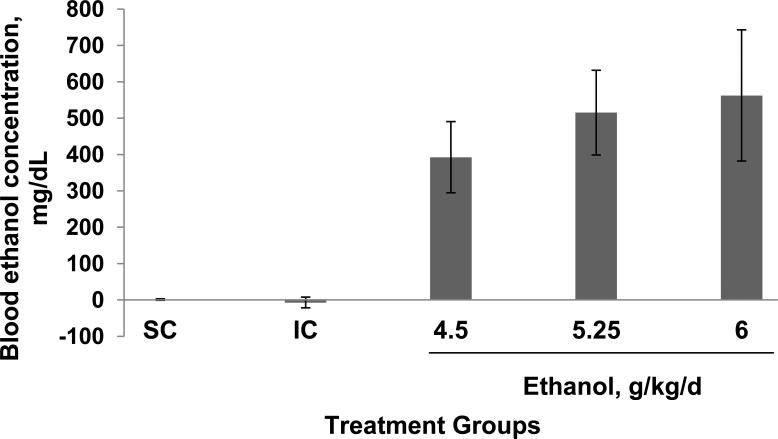
The mean ± SD BACs for the different groups, measured from samples taken 2 h after the second alcohol treatment, are 0, 0, 418 ± 97, 549 ± 116 and 577 ± 180 mg/dL in suckle controls and animal treated with 0, 4.5, 5.25 and 6 g/kd/day of alcohol respectively. The increasing dose of ethanol produces an increasing BAC (Fig 1). Blood alcohol levels of female pups were slightly higher than male pups, however these differences did not reach statistical significance (P=0.951) (results are not shown).
Figure 1.
Ethanol treatment produces increasing blood ethanol concentrations in postnatal day 6 rat pups. Animals are treated with 0, 4.5, 5.25 or 6.0 g/kg/d of ethanol, delivered in two feedings, 2 hours apart. Blood is obtained 2 hours after the second feeding from a tail clip. Results shown are from a male and female pair from each experiment averaged from 3 separate experiments. Blood ethanol concentrations are expressed as mg/dL. The bar indicates the mean values +/- SD of six pups. ANOVA single factor, p<10-10; *vs both SC and IC, p<0.0001, paired t-test.
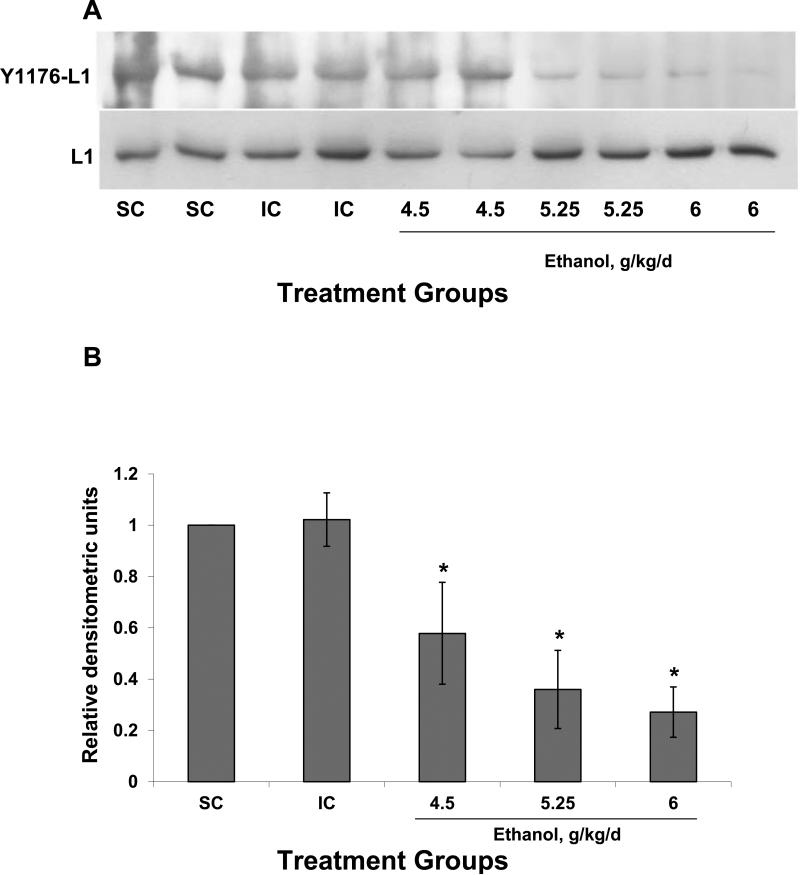
Ethanol treatment produces decreased amounts of dephosphorylated Y1176 (Y1176-L1) compared to intubated and suckle controls (Fig 2). The reduction in dephosphorylated Y1176 was dose dependent yielding a statistical significance between groups (P<0.001), whereas the group receiving 6 g/kg/day of ethanol showed the lowest amount of dephosphorylated Y1176 (Fig 2). Ethanol treatment did not affect the quantity of L1 compared to control animals (P=0.846) (Fig 2).
Figure 2.
The amount of dephosphorylated Y1176 of L1 is decreased by ethanol in vivo. (A) Animals were suckled (SC) or intubated and treated with 0 (IC), 4.5, 5.25 or 6.0 g/kg of ethanol. Shown is a male:female pair in each group. Cerebella were harvested 2 hours after the second alcohol feeding and lyzed. Equal amounts of protein from each supernatant were run on SDS gel electrophoresis and immunoblotted for dephosphorylated Y1176-L1 using 74-5H7, a monoclonal antibody that specifically recognizes unphosphorylated Y1176 in the cytoplasmic domain of L1. Blots were stripped and reblotted for total L1 using a polyclonal antibody against the cytoplasmic domain of L1 as a loading control. (B) Densitometric quantification of dephosphorylated Y1176 corrected for total L1 is plotted for each treatment group. The values of 3 different experiments are shown. The bar indicates the mean of the values +/- S.D. All animals which received ethanol had significantly less dephosphorylated L1 than either the SC or the IC controls. ANOVA single factor, p<10-7; *vs both IC and SC, p< 0.05, paired t-test.
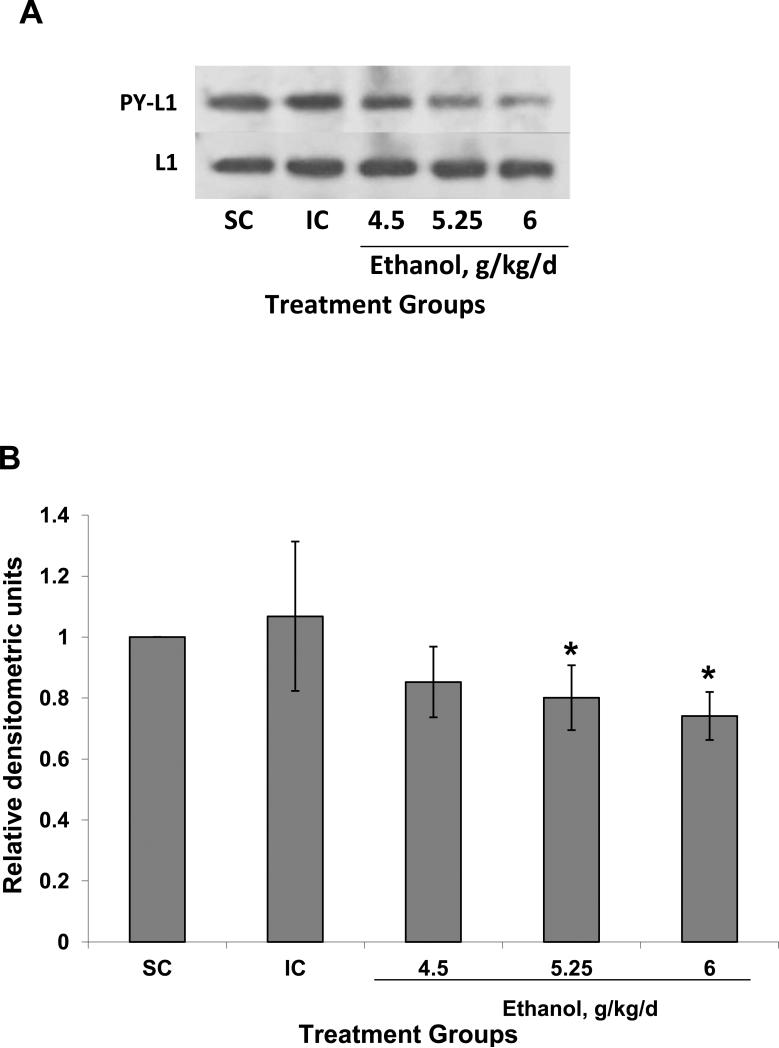
Ethanol treatment also decreased the level of L1 tyrosine phosphorylation (Fig. 3). Animals treated with ethanol showed a significant lower level of L1 tyrosine phosphorylation (P=0.03) (Fig 3) although the effect does not appear as dramatic as that of the reduction of the dephosphorylated form of L1 (Fig. 2). L1 quantity was not affected by ethanol treatment (Fig 3). We found a dose dependent effect where the higher ethanol dose (6 g/kd/d) caused the most significant inhibition (decrease of 26% compared to suckle control) of L1 phosphorylation (P<0.03) (Fig 3).
Figure 3.
Tyrosine phosphorylation of L1 is decreased by ethanol treatment in vivo. (A) Animals were treated and lysates prepared as described in Fig 2. Supernatants with equal amounts of protein were immunoprecipitated with antibodies to the cytoplasmic domain of L1, then immunoblotted for phosphotyrosine (PY-L1). Ethanol treatment decreased the amount of L1 which was phosphorylated on a tyrosine with the exception of Y1176 (Yeaney et al., 2009). Blots were stripped and reprobed for total L1 as a loading control (L1). (B) Densitometric quantification of tyrosine phosphorylation corrected for total L1 is plotted as relative densitometric units. The values of 3 different experiments are shown. The bar indicates the mean of the values +/- S.D. ANOVA single factor, p<0.07. Both 5.25 and 6.0 g/kg/d results in significantly reduce tyrosine phosphorylated L1 (*vs SC and IC, p<0.05, paired t-test). The 4.5 g/kg/d was nearing significance (vs SC, p<0.08; vs IC, p<0.06).
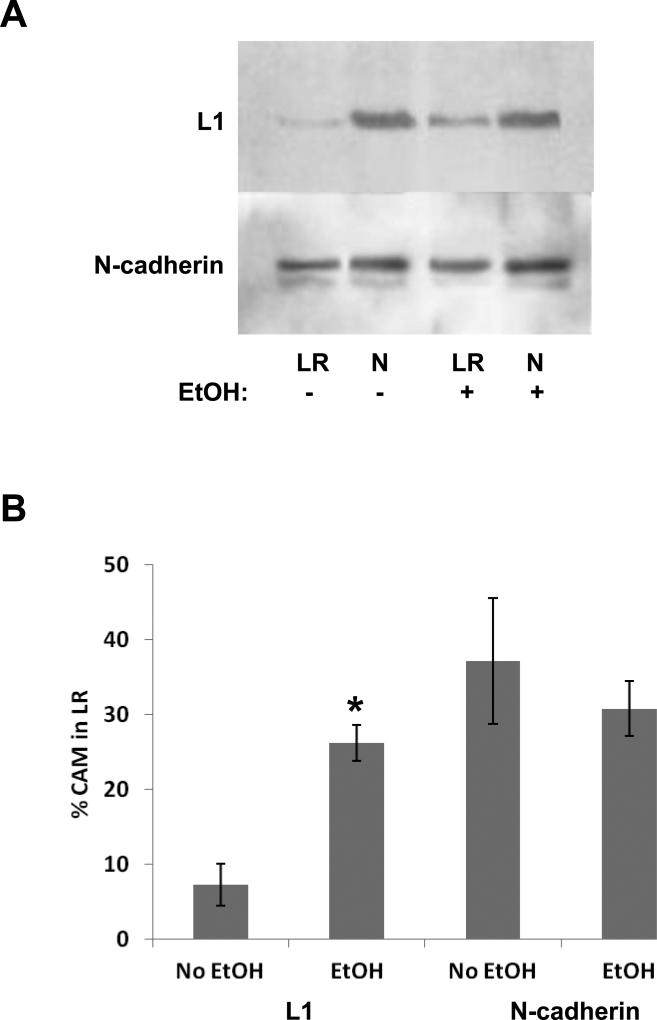
To determine the effect of ethanol exposure on the lipid raft distribution of L1, only the intubated control was compared to the ethanol treated animal at 6 g/kg/day due to the capacity of the ultracentrifuge needed to prepare the lipid rafts. In animals treated with ethanol (6g/kg/day), the percent of L1 in lipid rafts significantly increased to 26% of total L1, compared to 7% in the intubated control animals (P<0.001) (Fig. 4). As a control, we measured N-cadherin distribution in lipid rafts. Ethanol does not inhibit N-cadherin mediated neurite outgrowth (Bearer et al., 1999) nor does it alter the lipid raft distribution of N-cadherin in vitro (Tang et al., 2011). Ethanol did not significantly affect the distribution of N-cadherin in lipid rafts in vivo where 37% and 31% is in intubated control and ethanol treated animals respectively.
Figure 4.
Ethanol treatment in vivo shifts L1 into lipid rafts while not affecting N-cadherin distribution. Animals were treated with either 0 or 6.0 g/kg of ethanol. Cells were harvested 2 hours after the second intubation and separated into lipid raft (LR) and non-lipid raft (N) pools. Volumes representing equal fractions from each pool are loaded to represent equal fractions of the LR and N pools. The blots are immunoblotted for L1 then stripped and blotted for N-cadherin. A representative blot is shown in panel (A). (B) Densitometric analysis of blots (n=3). The total pixels in the L1 or N-cadherin bands in both the LR and N lanes are determined for both control and ethanol exposed animals. The % of cell adhesion molecules (CAM) in the LR is calculated by taking the pixels in the LR band and dividing by the sum of the pixels in the N and LR bands. The mean +/- SD is shown. *p<0.001, paired t-test.
Discussion
There is growing body of evidence in vitro supporting the hypothesis that ethanol effects on L1 cell adhesion molecule play a significant role in ethanol neurotoxicity (Bearer et al., 1999; Charness et al., 1994; Chen et al., 2001; Dou et al., 2011; Hoffman et al., 2008; Tang et al., 2006, 2011; Watanabe et al., 2004; Wilkemeyer et al., 2003; Yeaney et al., 2009). This study is the first report of a direct effect of ethanol exposure on L1 in vivo.
We studied the tyrosine dephosphorylation/phosphorylation of L1 and its lipid raft distribution based on data from in vitro studies. Dephosphorylation of tyrosine 1176 in L1 has been shown to be a critical regulatory point for L1 endocytosis and L1-cell mediated neurite outgrowth (Kamiguchi et al., 1998; Schaefer et al., 1999). Phosphorylation of tyrosine 1229 in the cytoplasmic domain of L1 regulates binding to ankyrin and hence to actin (Garver et al., 1997; Gil et al., 2003). This interaction is thought to allow the cytoskeleton to extend in neurite outgrowth. Thus, a decrease in tyrosine phosphorylation at 1229 promotes L1 binding to ankyrin and may reduce the ability of L1 to promote neurite outgrowth (Guan and Maness, 2010). Regulation of L1 binding to ezrin-radixin-moesin and ankyrin may also be regulated by phosphorylation/dephosphorylation of tyrosines 1151 and 1211, respectively (Cheng et al., 2005). Previously we have shown that ethanol inhibits L1 mediated dephosphorylation of Y1176 of L1 and inhibits the increase in tyrosine phosphorylation of L1 of cerebellar granule neurons in vitro (Tang et al., 2006; Yeaney et al., 2009). This study now demonstrates that ethanol treatment in vivo produces a significant and dose dependent reduction of both the dephosphorylation of Y1176 and tyrosine phosphorylation of L1. The increase in phosphorylated Y1176 L1 and reduction in global tyrosine phosphorylation may indicate an inability of L1 to dissociate from the cytoskeleton, and inability to be re-endocytosed. This result might suggest a slowing of the endocytic recycling pathway and reduction in rate of neurite outgrowth.
In the last decade, lipid rafts have become a target of extensive research aiming to identify their role in ethanol toxicity. Ethanol has been shown to affect essential upstream lipid raft-mediated TCR-dependent signaling events by interfering with colocalization of Lck, ZAP70, LAT, and PLCγ1 with plasma membrane lipid rafts (Ghare et al., 2011). Another study established that ethanol-induced inflammatory processes in the brain and glial cells involves recruitment of interleukin-1 beta receptor type I (IL-1RI) and toll-like receptor type 4 (TLR4) into the lipid rafts which trigger their endocytosis and downstream signaling stimulation (Blanco et al., 2008). Ethanol reportedly blocks the LPS redistribution of CD14 to a lower density fraction of the lipid rafts (Dai et al., 2005). Similarly, ethanol inhibits LPS-mediated redistribution of TLR4 to the lipid rafts but has no effect on the peptidoglycan-mediated redistribution of toll like receptor 2 (Dolganiuc et al., 2006). We recently reported the redistribution of L1 into lipid rafts in cerebellar granule neurons following a 1 h ethanol exposure (Tang et al., 2011). Our data reported here confirm that, in vivo, ethanol increases the distribution of L1 in lipid rafts.
Blood alcohol levels measured in our study were higher than other studies using similar models but different dosing techniques and times of sampling. Goodlett et al. reported blood alcohol levels approximating 225, 290 and 320 +/- 20 mg/dl in PD 4 rat pups intubated with ethanol doses of 4.5, 5.25 and 6 g/kd/day respectively in a vehicle of a milk composition 90 min after the second dose (Goodlett et al., 1998). Using a similar technique, Hsiao et al demonstrated blood alcohol levels of 302.5 +/- 6.3 mg/dl in animals treated with 4.9 g/kg/day ethanol mixed in a milk formula from PD 4 -6. Blood alcohol concentrations are measured after the second dose on PD6 (Hsiao et al., 2002). We also used an intralipid vehicle rather than a milk formula. The choice of vehicle has been shown to effect weight gain, which may indicate an effect on the blood alcohol level achieved after feedings. With the difference in number of doses prior to BAC measurement, difference in vehicle and difference in the timing of the BAC measurement (90 min versus 2 h after last dose), our BACs may be higher than in previous studies. Nevertheless, in our study we have shown a considerable effect of ethanol on tyrosine dephosphorylation/phosphorylation and redistribution of L1 in lipid rafts. Future studies will examine the dose response of lower ethanol doses.
The finding that higher doses of alcohol in vivo are used to find an effect on L1 is not surprising. The in vitro model can be manipulated to reduce background noise and cross talk. Our previous experiments have used serum starvation for 2 – 3 h prior to measurements of L1 phosphorylation state, pp60src and ERK1/2 activation to reduce background (Tang et al., 2006; Yeaney et al., 2009). The “triggering” of L1 endocytic recycling using the cross linked antibody, ASCS4, results in a timed course of phosphorylation and signal activation specific to L1 and thus a high signal to noise ratio. This manipulation cannot be done in vivo, where L1 may be undergoing endocytic recycling at the time of ethanol exposure. Reducing noise to signal ratio may be accomplished by using higher doses of ethanol. That the perturbation may occur at lower doses of ethanol may be difficult to detect but might still be present in the subset of L1 undergoing endocytic recycling at the time of ethanol exposure.
Our data support the hypothesis that ethanol disrupts L1 function in vivo. In our previous work we showed that both L1 tyrosine dephosphorylation and phosphorylation are downstream of pp60src kinase activation (Yeaney et al., 2009). Pp60src is a lipid raft associated protein (Tang et al., 2011). While an effect on apoptosis or cell proliferation may underlie phosphorylation changes in L1, the finding that L1 association with lipid rafts is altered in vivo suggests that the L1-lipid raft interaction may be the target for ethanol toxicity, and suggests a new target for intervention. There are reports of agents which cluster lipid rafts leading to apoptosis for use in chemotherapy of cancer cells (Gajate and Mollinedo, 2011). Nutritional modulation of lipid rafts with docosahexaenoic acid has been suggested to reduce inflammation associated with arteriosclerosis (Layne et al., 2011). Lipid raft manipulation has even been suggested as a treatment for peripheral nerve injury (Abrams and Widenfalk, 2005). Nutrition interventions for lipid raft function have been reported, and are particularly focused on dietary fats (Kim et al., 2009; Yaqoob and Shaikh, 2010). Nutrients, such as choline, GM1 ganglioside, and docosahexaenoic acid (DHA) known to participate in lipid raft function (Janich and Corbeil, 2007; Kuang et al., 2010; Langelier et al., 2010) have been shown to ameliorate ethanol's effects. Supplementation with choline (Thomas et al., 2004; Thomas and Tran, 2011), GM1 ganglioside (Chen et al., 1996), or DHA (Furuya et al., 2000) have been reported to improve outcomes of animals exposed prenatally to ethanol, decrease membrane-disordering effects of ethanol, and ameliorate the hyperactivity induced by in utero ethanol exposure in rat pups respectively. Nutritional interventions to reduce the morbidity of prenatal ethanol exposure are attractive as there are several successful nutritional interventions during pregnancy that reduce other morbidities, such as folic acid supplementation of breadstuffs to reduce neural tube defects (Obican et al., 2010). Future research is needed to assess the reduction in ethanol neurotoxicity possible with nutritional interventions.
Acknowledgements
This work was supported by NIH/NIAAA AA016398.
References
- Abrams M, Widenfalk J. Emerging strategies to promote improved functional outcome after peripheral nerve injury. Restor Neurol Neurosci. 2005;23:367–382. [PubMed] [Google Scholar]
- Bearer CF, Swick AR, O'Riordan MA, Cheng G. Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. J Biol Chem. 1999;274:13264–13270. doi: 10.1074/jbc.274.19.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco AM, Perez-Arago A, Fernandez-Lizarbe S, Guerri C. Ethanol mimics ligand-mediated activation and endocytosis of IL-1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. J Neurochem. 2008;106:625–639. doi: 10.1111/j.1471-4159.2008.05425.x. [DOI] [PubMed] [Google Scholar]
- Charness ME, Safran RM, Perides G. Ethanol inhibits neural cell-cell adhesion. Journal of Biological Chemistry. 1994;269:9304–9309. [PubMed] [Google Scholar]
- Chen S-, Wilkemeyer MF, Sulik KK, Charness ME. Octanol antagonism of ethanol teratogenesis. FASEB Journal. 2001;15:1649–1651. doi: 10.1096/fj.00-0862fje. [DOI] [PubMed] [Google Scholar]
- Chen SY, Yang B, Jacobson K, Sulik KK. The membrane disordering effect of ethanol on neural crest cells in vitro and the protective role of GM1 ganglioside. Alcohol. 1996;13:589–595. doi: 10.1016/s0741-8329(96)00073-0. [DOI] [PubMed] [Google Scholar]
- Cheng L, Itoh K, Lemmon V. L1-mediated branching is regulated by two ezrin-radixin-moesin (ERM)-binding sites, the RSLE region and a novel juxtamembrane ERM-binding region. J Neurosci. 2005;25:395–403. doi: 10.1523/JNEUROSCI.4097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Zhang J, Pruett SB. Ethanol alters cellular activation and CD14 partitioning in lipid rafts. Biochem Biophys Res Commun. 2005;332:37–42. doi: 10.1016/j.bbrc.2005.04.088. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Bakis G, Kodys K, Mandrekar P, Szabo G. Acute ethanol treatment modulates Toll-like receptor-4 association with lipid rafts. Alcohol Clin Exp Res. 2006;30:76–85. doi: 10.1111/j.1530-0277.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Dou X, Menkari CE, Shanmugasundararaj S, Miller KW, Charness ME. Two alcohol binding residues interact across a domain interface of the L1 neural cell adhesion molecule and regulate cell adhesion. J Biol Chem. 2011;286:16131–16139. doi: 10.1074/jbc.M110.209254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya H, Aikawa H, Yoshida T, Okazaki I. The use of docosahexaenoic acid supplementation to ameliorate the hyperactivity of rat pups induced by in utero ethanol exposure. Environ Health Prev Med. 2000;5:103–110. doi: 10.1265/ehpm.2000.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajate C, Mollinedo F. Lipid rafts and Fas/CD95 signaling in cancer chemotherapy. Recent Pat Anticancer Drug Discov. 2011;6:274–283. doi: 10.2174/157489211796957766. [DOI] [PubMed] [Google Scholar]
- Garver TD, Ren Q, Tuvia S, Bennett V. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. Journal of Cell Biology. 1997;137:703–714. doi: 10.1083/jcb.137.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghare S, Patil M, Hote P, Suttles J, McClain C, Barve S, Joshi-Barve S. Ethanol inhibits lipid raft-mediated TCR signaling and IL-2 expression: potential mechanism of alcohol-induced immune suppression. Alcohol Clin Exp Res. 2011;35:1435–1444. doi: 10.1111/j.1530-0277.2011.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil OD, Sakurai T, Bradley AE, Fink MY, Cassella MR, Kuo JA, Felsenfeld DP. Ankyrin binding mediates L1CAM interactions with static components of the cytoskeleton and inhibits retrograde movement of L1CAM on the cell surface. J Cell Biol. 2003;162:719–730. doi: 10.1083/jcb.200211011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett C, Pearlman AD, Lundahl KR. Binge neonatal alcohol intubations induce dose-dependent loss of purkinje cells. Neurotoxicology and Teratology. 1998;20:285–292. doi: 10.1016/s0892-0362(97)00102-5. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Guan H, Maness PF. Perisomatic GABAergic innervation in prefrontal cortex is regulated by ankyrin interaction with the L1 cell adhesion molecule. Cereb Cortex. 2010;20:2684–2693. doi: 10.1093/cercor/bhq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EJ, Mintz CD, Wang S, McNickle DG, Salton SR, Benson DL. Effects of ethanol on axon outgrowth and branching in developing rat cortical neurons. Neuroscience. 2008;157:556–565. doi: 10.1016/j.neuroscience.2008.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao SH, Parrish AR, Nahm SS, Abbott LC, McCool BA, Frye GD. Effects of early postnatal ethanol intubation on GABAergic synaptic proteins. Brain Res Dev Brain Res. 2002;138:177–185. doi: 10.1016/s0165-3806(02)00470-4. [DOI] [PubMed] [Google Scholar]
- Janich P, Corbeil D. GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett. 2007;581:1783–1787. doi: 10.1016/j.febslet.2007.03.065. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V. Recycling of the cell adhesion molecule L1 in axonal growth cones. Journal of Neuroscience. 2000;20:3676–3686. doi: 10.1523/JNEUROSCI.20-10-03676.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V. A neuronal form of the cell adhesion molecule L1 contains a tyrosine-based signal required for sorting to the axonal growth cone. J Neurosci. 1998;18:3749–3756. doi: 10.1523/JNEUROSCI.18-10-03749.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H, Long KE, Pendergast M, Schaefer AW, Rapoport I, Kirchhausen T, Lemmon V. The neural cell adhesion molecule L1 interacts with the AP-2 adaptor and is endocytosed via the clathrin-mediated pathway. J Neurosci. 1998;18:5311–5321. doi: 10.1523/JNEUROSCI.18-14-05311.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H, Yoshihara F. The role of endocytic L1 trafficking in polarized adhesion and migration of nerve growth cones. J Neurosci. 2001;21:9194–9203. doi: 10.1523/JNEUROSCI.21-23-09194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Chapkin RS, Barhoumi R, Ma DW. A novel role for nutrition in the alteration of functional microdomains on the cell surface. Methods Mol Biol. 2009;579:261–270. doi: 10.1007/978-1-60761-322-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang Y, Salem N, Corn DJ, Erokwu B, Tian H, Wang F, Lee Z. Transport and metabolism of radiolabeled choline in hepatocellular carcinoma. Mol Pharm. 2010;7:2077–2092. doi: 10.1021/mp1001922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelier B, Linard A, Bordat C, Lavialle M, Heberden C. Long chain-polyunsaturated fatty acids modulate membrane phospholipid composition and protein localization in lipid rafts of neural stem cell cultures. J Cell Biochem. 2010;110:1356–1364. doi: 10.1002/jcb.22652. [DOI] [PubMed] [Google Scholar]
- Layne J, Majkova Z, Smart EJ, Toborek M, Hennig B. Caveolae: a regulatory platform for nutritional modulation of inflammatory diseases. J Nutr Biochem. 2011;22:807–811. doi: 10.1016/j.jnutbio.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light KE, Kane CJM, Pierce DR, Jenkins D, Ge Y, Brown G, Yang H, Nyamweya N. Intragastric intubation: important aspects of the model for administration of ethanol to rat pups during the postnatal period. Alcohol Clin Exp Res. 1998;22:1600–1606. doi: 10.1111/j.1530-0277.1998.tb03954.x. [DOI] [PubMed] [Google Scholar]
- Lupton C. [9/2/11, 2011];The Financial Impact of Fetal Alcohol Syndrome. 2011 6/15/11. Available at: http://www.fasdcenter.samhsa.gov/publications/Cost.cfm.
- Moos M, Tacke R, Schere H, Teplow D, Fruh K, Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988;334:701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Kamiguchi H. Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner. J Cell Biol. 2002;159:1097–1108. doi: 10.1083/jcb.200209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obican SG, Finnell RH, Mills JL, Shaw GM, Scialli AR. Folic acid in early pregnancy: a public health success story. FASEB J. 2010;24:4167–4174. doi: 10.1096/fj.10-165084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan R, Wilkemeyer M, Mittal B, Perides G, Charness M. Alcohol inhibits cell-cell adhesion mediated by human L1. J Cell Biol. 1996;133:381–390. doi: 10.1083/jcb.133.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AW, Kamei Y, Kamiguchi H, Wong EV, Rapoport I, Kirchhausen T, Beach CM, Landreth G, Lemmon SK, Lemmon V. L1 endocytosis is controlled by a phosphorylation-dephosphorylation cycle stimulated by outside-in signaling by L1. J Cell Biol. 2002;157:1223–1232. doi: 10.1083/jcb.200203024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AW, Kamiguchi H, Wong EV, Beach CM, Landreth G, Lemmon V. Activation of the MAPK signal cascade by the neural cell adhesion molecule L1 requires L1 internalization. J Biol Chem. 1999;274:37965–37967. doi: 10.1074/jbc.274.53.37965. [DOI] [PubMed] [Google Scholar]
- Tang N, Farah B, He M, Fox S, Malouf A, Littner Y, Bearer CF. Ethanol causes the redistribution of L1 cell adhesion molecule in lipid rafts. J Neurochem. 2011;119:859–867. doi: 10.1111/j.1471-4159.2011.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, He M, O'Riordan MA, Farkas C, Buck K, Lemmon L, Bearer CF. Ethanol inhibits L1 cell adhesion molcule activation of mitogen-activated protein kinases. J Neurochem. 2006;96:1480–1490. doi: 10.1111/j.1471-4159.2006.03649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2012;22:619–630. doi: 10.1002/hipo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvia S, Garver T, V B. The phosphorylation state of the FIGQY tyrosine of neurofascin determines ankyrin-binding activity and patterns of cell segregation. Proceedings of the National Academy of Science U S A. 1997;94:12957–12962. doi: 10.1073/pnas.94.24.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Ymazaki M, Miyazaki H, Arikawa C, Itoh K, Sasaki T, Maehama T, Frohman MA, Kanaho Y. Phospholipase D2 functions as a downstream signaling molecule of MAP kinase pathway in L1-stimulated neurite outgrowth of cerebellar granule neurons. J Neurochem. 2004;89:142–151. doi: 10.1111/j.1471-4159.2004.02308.x. [DOI] [PubMed] [Google Scholar]
- Wilkemeyer MF, Charness ME. Characterization of ethanol-sensitive and insensitive fibroblast cell lines expressing human L1. J Neurochem. 1998;71:2382–2391. doi: 10.1046/j.1471-4159.1998.71062382.x. [DOI] [PubMed] [Google Scholar]
- Wilkemeyer MF, Chen S-, Menkari CE, Brenneman DE, Sulik KK, Charness ME. Differential effects of ethanol antagonism and neuroprotection in peptide fragment NAPVSIPQ prevention of ethanol-induced developmental toxicity. Proc Natl Acad Sci U S A. 2003;100:8543–8548. doi: 10.1073/pnas.1331636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkemeyer MF, Sebastian AB, Smith SA, Charness ME. Antagonists of alcohol inhibition of cell adhesion. Proc Natl Acad Sci U S A. 2000;97:3690–3695. doi: 10.1073/pnas.050545697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqoob P, Shaikh SR. The nutritional and clinical significance of lipid rafts. Curr Opin Clin Nutr Metab Care. 2010;13:156–166. doi: 10.1097/MCO.0b013e328335725b. [DOI] [PubMed] [Google Scholar]
- Yeaney NK, He M, Tang N, Malouf AT, O'Riordan MA, Lemmon V, Bearer CF. Ethanol inhibits L1 cell adhesion molecule tyrosine phosphorylation and dephosphorylation and activation of pp60(src). J Neurochem. 2009;110:779–790. doi: 10.1111/j.1471-4159.2009.06143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]