Abstract
Purpose
Cross-sectional studies have established the prevalence and functional impairment of somatic symptoms in cancer patients. The purpose of this study was to determine the trajectory and adverse consequences of such symptoms over time.
Methods
Secondary analysis of longitudinal data from 405 cancer patients enrolled in a telecare management trial for pain and/or depression. Somatic symptom burden was measured with a 22-item scale at baseline, 1, 3, 6, and 12 months. Outcomes included the SF-12 Physical Component Summary (PCS) and Mental Component Summary (MCS) scores, the Sheehan Disability Scale (SDS) score and self-reported total disability days (TDD). Mixed methods repeated measures (MMRM) analyses were conducted to determine whether antecedent change in somatic symptom burden predicted functional status and disability.
Results
Symptoms were highly prevalent at baseline, with 15 of the 22 symptoms endorsed by more than half of the patients. A rather constant cross-sectional prevalence over 12 months at the group level belied a quite different trajectory at the patient level where the median persistence, resolution and incidence rates for 14 of the most common symptoms were 39%, 37%, and 24%, respectively. A clinically significant (i.e., 5 points) reduction in somatic symptom burden predicted improvement in PCS, MCS, and SDI (all P < .001), as well as a lower likelihood of ≥ 14 disability days in the past 4 weeks (odds ratio, 0.84; 95% CI, 0.74 to 0.95).
Conclusions
Somatic symptoms remain burdensome in cancer patients over 12 months and symptom improvement predicts significantly better functional status and less disability.
Keywords: cancer, somatic symptoms, prognosis, disability, quality of life, functional status, symptom burden
Studies of symptom prevalence in cancer have often focused on patients with advanced cancer or with selected types of cancer seen at tertiary care centers.[1-11] A systematic review of 18 studies showed that common somatic symptoms include fatigue (62%), dry mouth (42%), insomnia (41%), pain (36%), anorexia (32%), numbness/tingling (29%), constipation (27%), dyspnea (26%), nausea (21%), and dizziness (20%).[12] Notably, 40% to 61% of patients experienced more than one symptom and 22% to 30% of patients experienced more than five concurrent symptoms. Somatic symptoms can have a substantial impact on functional status, quality of life, and even a desire for hastened death.[7,13-18]
The purpose of this report is to examine the longitudinal course of somatic symptoms in cancer patients and the impact of somatic symptoms on functional status and disability outcomes. Previous cross-sectional studies have established the point prevalence of symptoms but not their development or course over time, knowledge of which could guide decisions about the frequency of screening for somatic symptoms. Also, determining the functional consequences of somatic symptoms can help gauge their importance as a target for detection and treatment.
Data were derived from the baseline and follow-up interviews of patients enrolled in the Indiana Cancer Pain and Depression (INCPAD) trial. The specific questions addressed in this paper are:
What is the prevalence of specific somatic symptoms, and does the prevalence change over 12 months?
What is the trajectory of symptoms over 12 months, i.e., what proportion of symptoms resolve, persist, or develop de novo over 12 months?
Do changes in somatic symptom burden predict functional status and disability?
METHODS
Setting and Sample
The current paper uses baseline data from patients enrolled in the Indiana Cancer Pain and Depression (INCPAD) trial which is described in detail elsewhere.[19,20] Briefly, participants were recruited from 16 oncology outpatient sites throughout urban and rural areas of the state of Indiana to test the effectiveness of telecare management versus usual care for the treatment of depression and/or cancer-related pain. From March 2006 through August 2008, patients presenting to the oncology practices on selected days were screened for INCPAD study inclusion. Patients were potentially eligible if they had depression or pain of at least moderate severity (i.e., a Patient Health Questionnaire eight-item depression scale [PHQ-8] score of ≥ 10,[21,22] or worst pain in the past week of ≥ 6 on a 0 to 10 scale[19]). Additionally, pain had to be cancer-related and persistent despite the use of at least one analgesic.
Patients were excluded if they were non-English speaking, pregnant, or in hospice care; had moderately severe cognitive impairment, schizophrenia or other psychosis; or had a disability claim currently being adjudicated for pain.
Measures
Assessments were done at baseline, 1, 3, 6 and 12 months by research assistants blinded to treatment group. Somatic symptoms were measured by a 22-item somatic symptom burden scale. This scale consists of 14 symptoms from the PHQ-15 somatic scale (all items except sexual dysfunction)[23] plus 8 symptoms selected from the Memorial Symptom Assessment Scale[24] and the MD Anderson Symptom Inventory (MDASI).[8] Respondents are asked to rate on a 0 to 2 scale the degree to which each symptom has bothered them in the past 4 weeks from “not bothered at all” to “bothered a little” to “bothered a lot”. The scale had good internal reliability (Cronbach's alpha = 0.76). Increasing scores on this 0 to 44 point scale reflect a greater number and/or greater severity of symptoms; thus, higher scores reflect greater somatic symptom burden.
Functional status was assessed with the SF-12 Physical Component Summary (PCS) and Mental Component Summary (MCS) scores, each of which is scored 0 to 100 with 50 representing the normative value for the general population and lower scores representing greater functional impairment.[25] Disability was assessed with two measures. One was the 3-item Sheehan Disability Scale (SDS) which asks respondents to what extent on a 0 to 10 scale their health has interfered with their work, family life, and social life in the past month.[26] The SDS score is the mean of the 3 items and higher scores reflect greater disability. A second measure was total disability days (TDDs) in the past 4 weeks which was the number of days that respondents reported they either had to stay in bed or reduce their usual activities by at least 50% due to physical health or emotional problems.[27]
Depression severity was assessed with the 20-item Hopkins Symptom Checklist depression scale (HSCL-20), with higher scores on this 0 to 4 scale reflecting more severe depression.[28,29] Medical comorbidity was assessed by a checklist of 8 common categories of medical disorders, including heart disease, pulmonary disease, diabetes, hypertension, neurological conditions, arthritis, liver disease, and renal disease.[30] Sociodemographic variables included age, sex, race, education, employment, and income. The Socioeconomic Disadvantages (SED) index assigns one point each for low education (less than high school), unemployment, and low income (“not enough to make ends meet”).[31] Higher scores on this 0 to 3 scale represent worse socioeconomic conditions. Cancer type and phase were abstracted from the oncology records. Cancer phase was categorized as newly diagnosed, maintenance therapy only, disease-free, recurrent cancer, and progressive cancer.
Statistical Analysis
Study question 1
The prevalence of each of the 22 somatic symptoms was determined at baseline, 1 month, 3 months, 6 months, and 12 months.
Study question 2
For the study participants who completed a 12-month assessment, we determined the proportion reporting each symptom at both baseline and 12 months (a persistent symptom), at 12 months only (an incident symptom), and at baseline only (a resolved symptom).
Study question 3
Multivariable modeling using repeated measures (MMRM) analysis was conducted to determine whether antecedent change in somatic symptom burden predicted subsequent disability and functional status. This MMRM modeling approach [32,33] examined whether change from baseline to 1 month somatic symptom burden predicted 1 month disability and functional status; whether change in 1 month to 3 month somatic symptom burden predicted 3 month disability and functional status; and the same for 3 to 6 month change, and 6 to 12 month change. Separate models assessed 2 disability outcomes (SDS and TDD) and 2 functional status outcomes (PCS and MCS). TDD (the total number of disability days in the past 4 weeks) ranged from 0 to 28. However, the distribution of TDDs was bimodal (U shaped). We, therefore, recoded TDD as a binary variable (< 14 days = 0; ≥ 14 days =1).
For the disability outcomes (SDS and MDD), the predictor variable was somatic symptom severity change between each time point over 12 months (T0-T1: between baseline and 1 month; T1-T3: between 1 month and 3 months; T3-T6: between 3 months and 6 months; and T6-T12: between 6 months and 12 months). For the functional status outcomes (PCS and MCS), there were two somatic symptom change intervals (T0-T3 and T3-T12) since the SF-12 was assessed at only 3 time points (0, 3, and 12 months). Data from available participants at each time point were examined using linear mixed effects repeated measures analysis for the 3 continuous outcomes (SDS, PCS, and MCS), and generalized linear mixed effects repeated measures analysis for the binary outcome of TDD ≥ 14 days. The random subject effect was incorporated into the model to accommodate the potential correction among the repeatedly measured outcomes within the subject. Then we developed two adjusted models for each outcome. The first model adjusted for age, sex, race, socioeconomic disadvantage index, medical comorbidity, cancer type and phase, treatment group (intervention vs. control), time in months since baseline, and baseline value of the disability or functional status outcome being modeled. The second model adjusted for the same covariates plus the baseline HSCL-20 depression score. Since the predictor being modeled (the 22-item somatic symptom score change) already contained 5 pain symptoms, the model was not adjusted for the baseline Brief Pain Inventory score due to concerns about multicollinearity and overadjustment for pain. All analyses were performed using SAS Version 9.1 (SAS Institute, Cary, North Carolina).
RESULTS
Characteristics of Study Participants
Of the 405 participants enrolled, randomization resulted in intervention (n = 202) and control (n = 203) groups balanced in terms of baseline characteristics. The sample included 131 (32%) participants with depression only, 96 (24%) with pain only, and 178 (44%) with both depression and pain. Enrolled participants had a mean age of 58.8 (range, 23-96) years, 69% were women, 80% were white, and 49% were married. The type of cancer was breast in 118 (29%) of the participants, lung in 81 (20%), gastrointestinal in 70 (17%), lymphoma or hematological in 53 (13%), genitourinary in 41 (10%), and other in 42 (10%). The phase of cancer was new onset in 150 (37%), maintenance or disease-free in 172 (42%), and recurrent or progressive in 83 (21%). Additional characteristics of the INCPAD sample are detailed elsewhere.[20]
Prevalence of Symptoms Over 12 Months
The prevalence of each of the 22 somatic symptoms over time was determined for study participants who completed assessments at baseline (n = 405), 1 month (n = 354), 3 months (n = 335), 6 months (n =304), and 12 months (n = 269). The 12-month mortality rate was 21% (n = 85 participants), with death being the most common reason for non-assessment at each follow-up interview. Among participants still alive at each follow-up point, assessment rates were uniformly high, including 88.1% (354/402] at 1 month, 86.1% [335/389] at 3 months, 83.7% [304/363] at 6 months, and 84.1% [269/320] at 12 months.
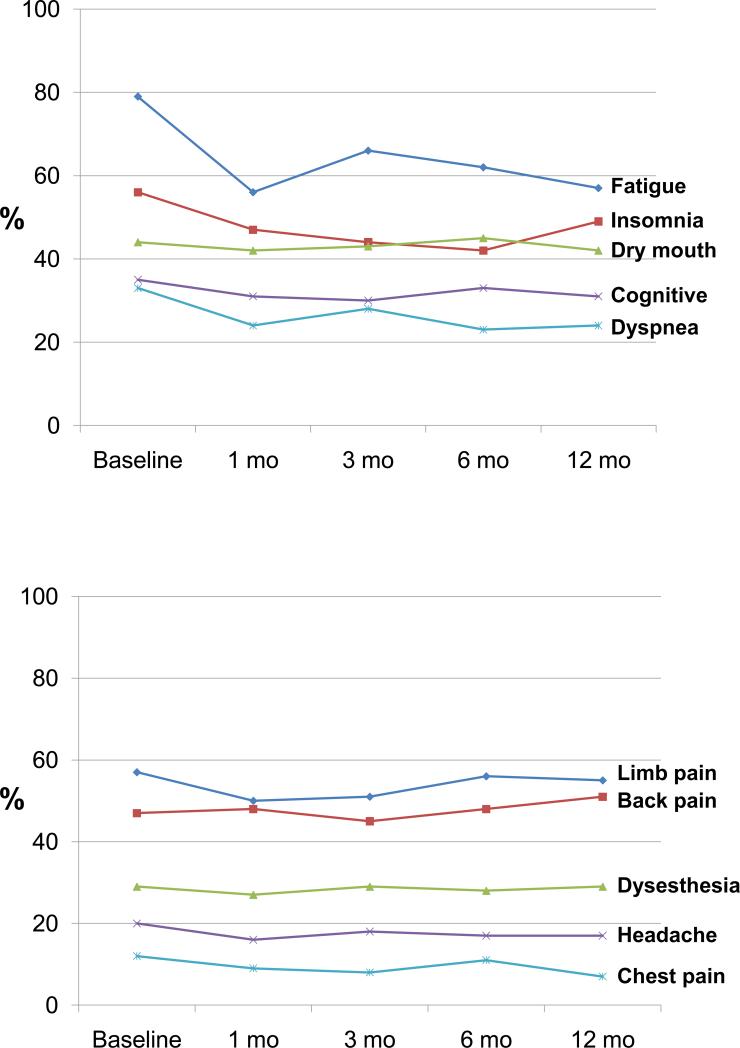
The proportion of patients reporting each symptom at the 5 assessment points is summarized in Table 1. Fatigue, insomnia, and pain complaints were the most common symptoms which is not surprising given the fact patients were enrolled because of depression and/or cancer-related pain. However, 15 of the 22 symptoms were endorsed by more than half of the patients at baseline, and 20 of the symptoms were endorsed by more than a quarter. When looking at only major symptoms (i.e., the subset which patients rated as being “bothered a lot” by), 13 of the 22 symptoms were endorsed by more than a quarter of patients at this more bothersome level of severity. Notably, the prevalence of most symptoms either remained unchanged or declined only modestly throughout the entire 12 months. Figure 1 illustrates the rather constant 12-month prevalence of selected general symptoms and pain symptoms.
Table 1.
Prevalence of Bothersomea Somatic Symptoms Over 12 Months in Cancer Patients with Pain and/or Depression*
| Somatic Symptom | Any Symptom (%) | Major Symptom (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 mo | 1 mo | 3 mo | 6 mo | 12 mo | 0 mo | 1 mo | 3 mo | 6 mo | 12 mo | |
| Feeling tired or having low energy | 97.5 | 92.7 | 89.8 | 91.5 | 90.0 | 78.8 | 69.5 | 65.7 | 62.2 | 56.9 |
| Trouble falling or staying asleep | 78.8 | 72.0 | 71.9 | 66.8 | 74.7 | 56.3 | 47.2 | 43.9 | 42.4 | 48.7 |
| Pain in arms, legs, or joints | 78.0 | 72.8 | 77.0 | 82.2 | 81.4 | 57.0 | 50.3 | 51.0 | 55.9 | 55.0 |
| Back pain | 74.8 | 74.3 | 73.4 | 78.0 | 78.8 | 47.4 | 48.3 | 45.1 | 47.7 | 50.9 |
| Problems with remembering things | 72.1 | 63.0 | 67.2 | 69.4 | 72.5 | 34.6 | 31.1 | 29.9 | 32.9 | 30.9 |
| Having a dry mouth | 69.9 | 66.9 | 69.9 | 67.4 | 67.7 | 44.0 | 42.1 | 43.3 | 44.7 | 41.6 |
| Shortness of breath | 64.7 | 56.8 | 60.0 | 57.6 | 60.6 | 32.8 | 23.7 | 28.4 | 22.7 | 23.8 |
| Gas or indigestion | 60.7 | 55.9 | 57.3 | 60.2 | 58.4 | 28.6 | 27.7 | 26.0 | 26.3 | 24.2 |
| Feeling drowsy/sleeping too much | 60.7 | 55.4 | 51.3 | 50.7 | 52.4 | 32.8 | 28.8 | 27.5 | 24.7 | 20.4 |
| Numbness or tingling | 59.8 | 57.9 | 52.8 | 57.6 | 58.7 | 29.4 | 27.4 | 29.3 | 28.0 | 29.0 |
| Lack of appetite | 56.5 | 51.1 | 48.1 | 41.5 | 41.3 | 30.4 | 26.0 | 20.0 | 16.1 | 19.0 |
| Headaches | 56.5 | 53.7 | 52.2 | 51.3 | 53.2 | 20.2 | 16.1 | 17.6 | 16.8 | 16.7 |
| Dizziness | 55.6 | 49.7 | 50.7 | 44.4 | 47.6 | 16.3 | 12.4 | 12.2 | 14.1 | 11.2 |
| Stomach pain | 54.6 | 52.8 | 49.0 | 49.3 | 50.2 | 24.0 | 20.9 | 22.1 | 23.7 | 16.7 |
| Nausea | 51.4 | 47.5 | 43.9 | 40.5 | 42.4 | 18.5 | 17.8 | 13.7 | 13.8 | 11.2 |
| Constipation | 47.4 | 47.5 | 48.4 | 44.4 | 42.0 | 23.7 | 24.3 | 18.2 | 21.1 | 16.0 |
| Feeling heart pound or race | 44.9 | 39.6 | 38.2 | 36.5 | 43.9 | 12.3 | 9.0 | 8.7 | 10.2 | 8.9 |
| Diarrhea or loose bowels | 43.2 | 39.8 | 39.7 | 41.8 | 42.4 | 18.8 | 14.7 | 14.9 | 13.8 | 15.6 |
| Chest pain | 34.1 | 31.9 | 33.7 | 29.0 | 31.6 | 12.1 | 9.3 | 8.4 | 10.5 | 7.1 |
| Vomiting | 26.7 | 27.7 | 23.6 | 21.1 | 26.4 | 8.1 | 8.2 | 6.6 | 5.9 | 5.2 |
| Menstrual cramps or problemsb | 9.5 | 8.4 | 7.0 | 12.3 | 24.0 | 6.0 | 3.9 | 3.9 | 7.6 | 10.0 |
| Fainting spells | 6.9 | 5.4 | 6.3 | 6.3 | 5.9 | 2.2 | 2.3 | 1.5 | 1.6 | 1.1 |
Number of patients assessed at 0, 1, 3, 6, and 12 months was 405, 354, 335, 304, and 269, respectively. Any symptom includes reporting being “bothered a little” or “bothered a lot”, and major symptom includes only those reporting being “bothered a lot”
Menstrual symptoms were elicited only from women ≤ 50 y/o (n = 168, 155, 129, 106, and 50 at 0, 1, 3, 6, and 12 mo., respectively)
Figure 1.
Prevalence of selected general somatic symptoms (1A) and pain symptoms (1B) at 5 time points over 12 months.
Trajectory of Symptoms Over 12 Months
Table 2 shows the proportion of patients in whom symptoms were persistent, resolved, or incident over 12 months, derived from the sample of 269 patients in whom interviews were completed at both baseline and 12 months. This data on trajectory over 12 months complements in an important fashion the cross-sectional prevalence data for each time point. Though persistence is the most common category, resolution and new incidence constitute important subsets for most symptoms.
Table 2.
Trajectory of Highly Bothersomea Somatic Symptoms Over 12 Months in 269 patients with both Baseline and 12-Month Data)
| Somatic Symptom | Highly Bothersome Symptom at Baseline or 12 months | Highly Bothersomea Symptom Present at: | ||
|---|---|---|---|---|
| Baseline and 12 months Persistent | 12 months only Incident | Baseline only Resolved | ||
| N | Percent of Sample (n=269) | |||
| Feeling tired or having low energy | 225 | 52.4 | 4.9 | 27.0 |
| Pain in arms, legs, or joints | 199 | 41.0 | 14.2 | 19.0 |
| Trouble falling or staying asleep | 193 | 34.7 | 14.2 | 23.1 |
| Back pain | 174 | 34.5 | 16.9 | 13.9 |
| Having a dry mouth | 152 | 30.7 | 11.2 | 15.0 |
| Problems with remembering things | 130 | 19.5 | 11.6 | 17.6 |
| Shortness of breath | 98 | 17.2 | 6.7 | 12.7 |
| Numbness or tingling | 124 | 15.3 | 13.8 | 17.2 |
| Lack of appetite | 89 | 10.8 | 8.2 | 14.2 |
| Gas or indigestion | 112 | 9.3 | 14.9 | 17.5 |
| Headaches | 81 | 9.0 | 7.8 | 13.4 |
| Feeling drowsy or sleeping too much | 117 | 8.6 | 11.9 | 23.1 |
| Menstrual cramps or problemsb | 8 | 8.3 | 5.6 | 8.3 |
| Constipation | 82 | 7.5 | 8.6 | 14.6 |
| Diarrhea or loose bowels | 73 | 7.1 | 8.6 | 11.6 |
| Stomach pain | 80 | 6.7 | 10.0 | 13.0 |
| Nausea | 58 | 4.9 | 6.3 | 10.5 |
| Dizziness | 60 | 4.5 | 6.7 | 11.2 |
| Feeling your heart pound or race | 44 | 4.1 | 4.9 | 7.5 |
| Chest pain | 40 | 3.4 | 3.7 | 7.8 |
| Vomiting | 28 | 1.9 | 3.4 | 5.2 |
| Fainting spells | 9 | 0.0 | 1.1 | 2.2 |
Patient “bothered a lot” by the symptom in the past 4 weeks
Menstrual symptoms were asked about only in women ≤ 50 y/o (n = 50)
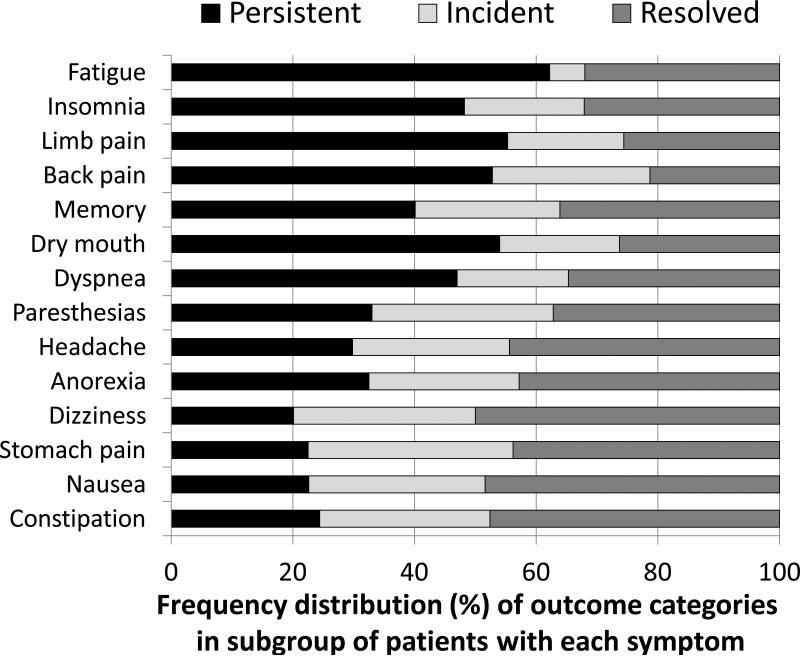
Figure 2 illustrates the frequency distribution of outcomes for the subset of patients who reported a particular symptom as highly bothersome at either the beginning or end of the study; data is shown for the 14 most common symptoms (i.e., those reported at either baseline or 12 months in 20% or more of the 269 patients). For the 14 symptoms overall, the mean (median) distribution of outcomes was 38.9% (36.6%) for symptom persistence, 23.8% (25.3%) for incidence, and 37.3% (36.7%) for resolution. Thus, what appears as a rather constant prevalence of symptoms over time when data is summarized at a group level (Table 1 and Figure 1) belies the fact that symptoms persist in some patients while resolving or developing de novo in others.
Figure 2.
Frequency distribution of outcome categories among subgroup of patients with a particular symptom who reported being “bothered a lot” by that symptom at both baseline and 12 months (persistent), at 12 months but not at baseline (incident), or at baseline but not at 12 months (resolved). The sample comprised the 269 patients who had completed interviews at both baseline and 12 months.
Somatic Symptoms Burden as a Predictor of Functional Status and Disability
The multivariable repeated measures modeling results summarized in Table 3 demonstrate that antecedent change in somatic symptom burden predicts subsequent functional status and disability. A reduction in somatic symptom burden predicted improvement in all 3 continuous outcomes: SDS, PCS, and MCS. A 5-point change in the somatic symptom burden score approximates a clinically significant change.[31] To convert this into a standardized effect size, the beta coefficient is multiplied by 5 and then divided by the baseline standard deviation (SD) for the outcome being modeled. The baseline SD for the SDS, PCS and MCS was 2.86, 8.83, and 12.43 respectively. Thus, the magnitude of improvement predicted, in fully adjusted models, by an antecedent 5-point reduction in somatic symptom burden is an effect size of .12 for SDI, .14 for PCS, and .10 for MCS.
Table 3.
Change in Somatic Symptom Burden as a Predictor of 12-Month Disability and Functional Status Outcomes
| 12-Month Functional Status Outcome | Parameter Estimate for Somatic Symptom Burden Change from Multivariable Modela | ||
|---|---|---|---|
| Beta | T | P | |
| Sheehan Disability Index (SDI) | |||
| Adjusted for covariates | .0686 | 7.13 | < .0001 |
| Adjusted for covariates including depression | .0699 | 7.28 | < .0001 |
| SF-12 Physical Component Summary score | |||
| Adjusted for covariates | .2455 | 4.68 | < .0001 |
| Adjusted for covariates including depression | .2471 | 4.71 | < .0001 |
| SF-12 Mental Component Summary score | |||
| Adjusted for covariates | .2343 | 3.26 | .0013 |
| Adjusted for covariates including depression | .2474 | 3.50 | .0005 |
Mixed effects repeated measures multivariable models examining preceding change in somatic symptom burden as a predictor of subsequent functional status. The SDI was assessed at 4 follow-up time points (1, 3, 6, and 12 months, while the SF-12 outcomes were assessed at 2 follow-up time points (3 and 12 months). A positive coefficient means that improvement in somatic symptom burden is associated with improvement in functional status.
Covariates controlled for in models were age, sex, race, socioeconomic disadvantage index, medical comorbidity, cancer type and phase, treatment group (intervention vs. control), time in months since baseline, and baseline value of the functional status outcome being modeled. In second model, baseline HSCL-20 depression score was also added.
An antecedent change in somatic symptom burden also predicted total disability days. Specifically, a 5-point reduction in somatic symptom burden predicted a lower likelihood of reporting 14 or more disability days in the past 4 weeks, both in the model adjusting for covariates (odds ratio, 0.85; 95% CI, 0.75 to 0.96) as well as in the model adjusting for covariates and baseline depression severity (odds ratio, 0.84; 95% CI, 0.74 to 0.95).
DISCUSSION
Our 12-month longitudinal study of somatic symptoms in cancer patients with pain and/or depression has several important findings. First, the high prevalence of cancer symptoms previously demonstrated in multiple cross-sectional studies remains constant over time. Second, the presence or absence of specific symptoms at the level of the individual patient is not entirely a static phenomenon; rather, persistence in many patients is coupled with resolution or incidence in an important minority. Third, reduction in somatic symptom burden predicts better functional status and less disability.
All of the patients in our sample had depression and/or pain which could have inflated somatic symptom prevalence, especially since depression is known to be associated with increased somatic symptom reporting in non-cancer populations.[34] However, as summarized in Table 4, the cross-sectional prevalence of symptoms in our study is within the range reported in two recent literature syntheses: 18 studies totaling 3,227 patients with a wide range of cancer types and phases[12], and 44 studies totaling 25,074 patients with recurrent or progressive cancer.[10]
Table 4.
Prevalence of Somatic Symptoms in Current Study Compared to Two Literature Synthesesa
| Somatic Symptom | Current Study (n = 405) | Kim et al12 (n = 3,227)b | Teuuissen10 (n = 25,074)c |
|---|---|---|---|
| % of patients | |||
| Fatigue | 79 | 62 | 74 |
| Pain | 68 | 40 | 71 |
| Insomnia | 56 | 41 | 36 |
| Dry mouth | 44 | 42 | 40 |
| Memory/concentration difficulties | 35 | 25 | 28 |
| Drowsiness | 33 | 36 | 20 |
| Shortness of breath | 33 | 26 | 35 |
| Lack of appetite | 30 | 32 | 53 |
| Indigestion/dyspepsia/bloating | 29 | 29 | 29 |
| Numbness/tingling | 29 | 29 | -- |
| Constipation | 24 | 27 | 37 |
| Nausea | 19 | 21 | 31 |
| Diarrhea | 19 | 16 | 11 |
| Dizziness | 16 | 20 | 17 |
Prevalence rates represented mean pooled prevalence across all studies in which that symptom was assessed; not every symptom was assessed in each study. Current study included only those symptoms reported as major (i.e., “bothered a lot”) by patients.
Literature synthesis of 18 studies assessing multiple symptoms in patients with a range of cancer types and phases.
Literature synthesis of 44 studies assessing multiple symptoms in patients with recurrent or progressive cancer.
Although the effect size of a reduction in somatic symptom burden on improvements in disability and functional status is modest, it should be noted that our findings represent the independent effect after adjusting for multiple covariates, including patient age and other demographic characteristics, medical comorbidity, type and phase of cancer, intervention effects of the clinical trial, and depression. In addition to its positive effects on physical and mental functional status, a clinically significant reduction in somatic symptom burden predicted a 16% reduction in the likelihood of high disability (i.e., 2 or more weeks in the past month during which the patient had to limit his or her activities by at least 50%).
The majority of epidemiological studies of cancer-related symptoms have been cross-sectional rather than longitudinal. While demonstrating the point prevalence of symptoms, such studies can neither delineate the trajectory of symptoms over time nor determine the predictive impact of changes in somatic symptoms on subsequent disability and functional status. Longitudinal studies have been fewer and have principally focused on one or a few rather than multiple somatic symptoms and have assessed a single follow-up time point rather than multiple time points using repeated measures analysis.
Of the three studies most salient to our present paper, two were consistent with our findings that a majority of individuals with cancer have multiple concurrent symptoms that persist over time. Kjaer and colleagues assessed 18 symptoms multiple times over 12 months in 2,486 Danish cancer survivors participating in a rehabilitation program.[35] They found that 95.7% of patients reported having ≥ 1 symptom, and 62% of those rated the symptom as severe. Those with ≥ 1 severe symptom had significantly poorer quality of life and lowered physical, emotional, social, and cognitive functioning at baseline and at 12 months compared to those without a severe symptom. In a systematic review of 79 studies (72% were longitudinal), Harrington et al reported that a variety of symptoms are prevalent for 5 or more years following any type of primary treatment across multiple and diverse types of cancer.[36] In contrast, Yamagishi et al assessed 12 symptoms longitudinally (median of 6 assessments) among 462 Japanese cancer outpatients starting chemotherapy and, compared to our study, found a lower prevalence and persistence of somatic symptoms across all time points.[11] Of note, these authors found that higher psychological distress predicted greater somatic symptom burden at follow-up.
Our study has several limitations. First, all of the patients in our sample had depression and/or pain. Although the cross-sectional prevalence of somatic symptoms was in the range reported in previous studies (Table 4), it is still possible that comorbid depression influenced somatic symptom trajectory over time as well as impact on functional status and disability. However, we did control for depression in our models. Also, since patients in the intervention arm of our study received aggressive treatment for their depression and pain, it is possible that the rather high persistence of somatic symptoms documented in our study may in fact be an underestimate. Still, the degree to which our findings apply to cancer patients without depression or pain needs further study. Second, we enrolled patients with a wide range of cancer types and phases which increases the generalizability of our findings but at the same time also decreases our ability to draw firm conclusions about any one type or phase of cancer. Again, however, we did control for the type and phase of cancer in our models, therefore demonstrating the independent effect of somatic symptom burden. Third, all measures, including disability, were self-report. Though other studies document the functional and work consequences of cancer, our findings would be further substantiated by independent measures of disability.
The potential clinical implications of our study should also be noted. One is the need for a multi-symptom approach to cancer care, in which all of the symptoms endorsed by a patient as problematic are identified and comprehensively managed. The promising work by Given and colleagues should catalyze much more clinical attention as well as research on multi-symptom management.[37-39] While some treatments are symptom-specific, others may be effective across more than one type of symptom (e.g., cognitive-behavioral therapy, antidepressants, exercise).[40] A second implication is the need for continuity of care for symptom management across the entire spectrum of cancer, since symptom prevalence was high across all types and phases of cancer. Frequently, symptom management may be the purview of the oncologist during active treatment of newly-diagnosed or progressive cancer, the primary care clinician in patients who are disease-free or on maintenance therapy, and the palliative care clinician during end-of-life care. This speaks not only to the need for training multiple types of clinicians in symptom management but also for effective communication among various members of the cancer care team so as to avoid gaps in symptom recognition and management. Third, new models for comprehensive management of cancer-related symptoms could be disseminated including collaborative care, telecare management, enhanced self-management, and expansion of palliative care services to include supportive care for symptoms across the continuum of cancer care.
Acknowledgments
Funding: This study was supported by a grant from the National Cancer Institute to Dr. Kroenke (R01 CA-115369)
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare with respect to the contents of this paper.
References
- 1.Funch DP. Predictors and consequences of symptom reporting behaviors in colorectal cancer patients. Med Care. 1988;26:1000–1008. doi: 10.1097/00005650-198810000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Curtis EB, Krech R, Walsh TD. Common symptoms in patients with advanced cancer. J Palliat Care. 1991;7:25–29. [PubMed] [Google Scholar]
- 3.Grond S, Zech D, Diefenbach C, Bischoff A. Prevalence and pattern of symptoms in patients with cancer pain: a prospective evaluation of 1635 cancer patients referred to a pain clinic. J Pain Symptom Manage. 1994;9:372–82. doi: 10.1016/0885-3924(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 4.Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Coyle N, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res. 1994;3:183–89. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly S, Walsh D, Rybicki L. The symptoms of advanced cancer: identification of clinical and research priorities by assessment of prevalence and severity. J Palliat Care. 1995;11:27–32. [PubMed] [Google Scholar]
- 6.Vainio A, Auvinen A. Prevalence of symptoms among patients with advanced cancer: an international collaborative study. Symptom Prevalence Group. J Pain Symptom Manage. 1996;12:3–10. doi: 10.1016/0885-3924(96)00042-5. [DOI] [PubMed] [Google Scholar]
- 7.Chang VT, Hwang SS, Feuerman M, Kasimis BS. Symptom and quality of life survey of medical oncology patients at a veterans affairs medical center: a role for symptom assessment. Cancer. 2000;88:1175–83. doi: 10.1002/(sici)1097-0142(20000301)88:5<1175::aid-cncr30>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Homsi J, Walsh D, Rivera N, Rybicki LA, Nelson KA, Legrand SB, et al. Symptom evaluation in palliative medicine: patient report vs systematic assessment. Support Care Cancer. 2006;14:444–53. doi: 10.1007/s00520-005-0009-2. [DOI] [PubMed] [Google Scholar]
- 10.Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de GA. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Yamagishi A, Morita T, Miyashita M, Kimura F. Symptom prevalence and longitudinal follow-up in cancer outpatients receiving chemotherapy. J Pain Symptom Manage. 2009;37:823–30. doi: 10.1016/j.jpainsymman.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Kim JE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37:715–36. doi: 10.1016/j.jpainsymman.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28:465–70. [PubMed] [Google Scholar]
- 14.Fox SW, Lyon DE. Symptom clusters and quality of life in survivors of lung cancer. Oncol Nurs Forum. 2006;33:931–36. doi: 10.1188/06.ONF.931-936. [DOI] [PubMed] [Google Scholar]
- 15.Given BA, Given CW, Sikorskii A, Hadar N. Symptom clusters and physical function for patients receiving chemotherapy. Semin Oncol Nurs. 2007;23:121–26. doi: 10.1016/j.soncn.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira KA, Kimura M, Teixeira MJ, Mendoza TR, da Nobrega JC, Graziani SR, et al. Impact of cancer-related symptom synergisms on health-related quality of life and performance status. J Pain Symptom Manage. 2008;35:604–16. doi: 10.1016/j.jpainsymman.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Hadi S, Fan G, Hird AE, Kirou-Mauro A, Filipczak LA, Chow E. Symptom clusters in patients with cancer with metastatic bone pain. J Palliat Med. 2008;11:591–600. doi: 10.1089/jpm.2007.0145. [DOI] [PubMed] [Google Scholar]
- 18.Foley KM. The relationship of pain and symptom management to patient requests for physician-assisted suicide. J Pain Symptom Manage. 1991;6:289–97. doi: 10.1016/0885-3924(91)90052-6. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Theobald D, Norton K, Sanders R, Schlundt S, McCalley S, et al. Indiana Cancer Pain and Depression (INCPAD) Trial: design of a telecare management intervention for cancer-related symptoms and baseline characteristics of enrolled participants. Gen Hosp Psychiatry. 2009;31:240–253. doi: 10.1016/j.genhosppsych.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroenke K, Theobald D, Wu J, Norton K, Morrison G, Carpenter J, et al. Effect of telecare management on pain and depression in patients with cancer: a randomized trial. JAMA. 2010;304:163–71. doi: 10.1001/jama.2010.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL. The PHQ-9: A new depression and diagnostic severity measure. Psychiatric Annals. 2002;32:509–21. [Google Scholar]
- 22.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–66. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Portenoy RK, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Sobel K, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326–36. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 25.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(Suppl 3):89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- 27.Rost K, Nutting P, Smith J, Werner J, Duan N. Improving depression outcomes in community primary care practice: a randomized trial of the quEST intervention. Quality Enhancement by Strategic Teaming. J Gen Intern Med. 2001;16:143–49. doi: 10.1111/j.1525-1497.2001.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroenke K, Bair MJ, Damush TM, Wu J, Hoke S, Sutherland J, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009;301:2099–110. doi: 10.1001/jama.2009.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unutzer J, Katon W, Callahan CM, Williams JW, Jr., Hunkeler E, Harpole L, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 30.Perkins AJ, Kroenke K, Unutzer J, Katon W, Williams JW, Hope C, et al. Common comorbidity scales were similar in their ability to predict health care costs and mortality. J Clin Epidemiol. 2004;57:1040–1048. doi: 10.1016/j.jclinepi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Kroenke K, Zhong X, Theobald D, Wu J, Tu W, Carpenter JS. Somatic symptoms in patients with cancer experiencing pain or depression: prevalence, disability, and health care use. Arch Intern Med. 2010;170:1686–94. doi: 10.1001/archinternmed.2010.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care 12:964-973. J Pain. 2011;12:964–73. doi: 10.1016/j.jpain.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H-L, Kroenke K, Wu J, Tu W, Theobald D, Rawl SM. Cancer-related pain and disability: a longitudinal study. J Pain Symptom Manage. 2011 doi: 10.1016/j.jpainsymman.2011.02.019. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroenke K. Patients presenting with somatic complaints: epidemiology, psychiatric comorbidity and management. Int J Methods Psychiatr Res. 2003;12:34–43. doi: 10.1002/mpr.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kjaer TK, Johansen C, Ibfelt E, Christensen J, Rottmann N, Hoybye MT, et al. Impact of symptom burden on health related quality of life of cancer survivors in a Danish cancer rehabilitation program: a longitudinal study. Acta Oncol. 2011;50:223–32. doi: 10.3109/0284186X.2010.530689. [DOI] [PubMed] [Google Scholar]
- 36.Harrington CB, Hansen JA, Moskowitz M, Todo BL, Feuerstein M. It's not over when it's over: long-term symptoms in cancer survivors -- a systematic review. Int J Psychiatry Med. 2010;40:163–81. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- 37.Given C, Given B, Rahbar M, Jeon S, McCorkle R, Cimprich B, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. J Clin Oncol. 2004;22:507–16. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- 38.Sherwood P, Given BA, Given CW, Champion VL, Doorenbos AZ, Azzouz F, et al. A cognitive behavioral intervention for symptom management in patients with advanced cancer. Oncol Nurs Forum. 2005;32:1190–1198. doi: 10.1188/05.ONF.1190-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sikorskii A, Given CW, Given B, Jeon S, Decker V, Decker D, et al. Symptom management for cancer patients: a trial comparing two multimodal interventions. J Pain Symptom Manage. 2007;34:253–64. doi: 10.1016/j.jpainsymman.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson JL, O'Malley PG, Kroenke K. Antidepressants and cognitive-behavioral therapy for symptom syndromes. CNS Spectr. 2006;11:212–22. doi: 10.1017/s1092852900014383. [DOI] [PubMed] [Google Scholar]