Abstract
Effective, low-cost, and safe treatments for sexually transmitted viral infections are urgently needed. Here, we show for the first time that intravaginal administration with nanoparticles of poly(lactic acid-co-glycolic acid) (PLGA) encapsulating short interfering RNA (siRNA) molecules is effective for prevention of genital HSV-2 infections in mice. PLGA nanoparticles (NPs) were designed to interfere with HSV-2 infection by siRNA-mediated knockdown of nectin, a host cell protein. NPs were characterized in vitro to determine the optimal formulation based on siRNA loading, controlled release profile, and mRNA knockdown. Mice inoculated intravaginally with a lethal dose of HSV-2, and treated with PLGA NPs, showed increased survival from ~9 days (in untreated mice) to > 28 days (in PLGA NP treated mice) - the longest survival ever observed with siRNA treatment in this mouse model. This work provides proof-of-concept that topical administration of NPs containing siRNA against a pathologically relevant host cell target can knockdown the gene in tissue and improve survival after HSV-2 infection. Furthermore, this system provides a safe delivery platform that employs materials that are already approved by the FDA and can be modified to enhance delivery of other microbicides.
Keywords: siRNA, HSV-2, Nanoparticle, Drug Delivery, Microbicide
INTRODUCTION
Sexually transmitted diseases (STDs) affect 340 million new people per year [1], with herpes simplex virus type 2 (HSV-2) infection impacting a startling ~17% of U.S. adults [2] and 536 million people worldwide [3], HSV-2 is a significant global health problem, as infection facilitates human immunodeficiency virus (HIV-1), Chlamydia, and syphilis coinfections [4-6]. Despite the use of antiviral drugs, the incidence of HSV has increased [7]. In addition to the societal burden of health care utilization, the disproportional effect on women, newborns, and immune compromised individuals is alarming. Current options for prevention and treatment of HSV-2 include antivirals and topically applied microbicides. Despite the identification of antigen-based targets, antiviral agents and vaccines have proven only moderately successful, primarily due to difficulties in delivery and a lack of understanding of the immune response [7-9]. Therefore, better HSV-2 prevention and treatment strategies are urgently needed.
Murine models of HSV-2 have been used by a number of groups to study vaginal infection [10-14], In comparison to infection in humans, vaginal HSV-2 infection in mice leads to subsequent infection of multiple neural and epithelial sites [15] and is ultimately lethal. It is believed that genital HSV-2 infection in mice spreads to the paracervical ganglia, resulting in urinary and rectal distention, hind-limb paralysis, and death [16]. While HSV-2 infection in mice is dependent on the estrous cycle [14, 16, 17], humans are susceptible to infection throughout the menstrual cycle.
Previous work in mice with genital HSV-2 infection has demonstrated that RNA interference (RNAi) - using targeted short interfering RNA (siRNA) molecules - can down-regulate proteins involved in HSV-2 infection and propagation [12, 13]. Specific proteins down-regulated include UL29.2 (a viral DNA binding protein affecting virus replication) and nectin-1 (a host cell receptor protein integral to initial virus binding and subsequent spread) [12, 13]. However, siRNA molecules are difficult to deliver intracellularly, and their effects can be short in duration. In previous studies, delivering siRNA against nectin-1, UL29.2, or both was more effective when siRNA was complexed with a transfection reagent such as Oligofectamine™ (OLF, lipoplexes), or conjugated to cholesterol [12, 13]. Yet even in the best cases, these studies extended survival against a lethal dose of HSV-2 from 9 days (untreated mice) to 14 days (with optimal lipoplex or cholesterol-conjugated treatment). Further, many vectors that promote intracellular delivery of siRNA - particularly those that are based on cationic lipids - are toxic to the sensitive mucosal epithelium of the female reproductive tract, and have been found to induce inflammation at high concentration [13, 18]. In this work we synthesized, characterized, and delivered intravaginally formulations of siRNA (against virus and host targets), encapsulated in poly(lactic co-glycolic acid) (PLGA) NPs to inhibit HSV-2 infection. To our knowledge, this is the first study that uses PLGA NPs [18], which we have previously shown to be safe for intravaginal administration, for delivery of siRNAs directed against HSV-2 host and viral targets and the first to evaluate survival after treatment in an HSV-2 murine model.
We previously demonstrated that intravaginal administration of PLGA NPs loaded with siRNA against EGFP provide RNAi throughout the mouse reproductive tract [18]. Here, we test the hypothesis that vaginal delivery of siRNA-containing NPs, specifically targeting genes necessary for HSV-2 infection and progression, extends the survival of mice administered a lethal dose of HSV-2 beyond that seen with other siRNA delivery vehicles.
METHODS
Formulation of siRNA Nanoparticles
We synthesized, characterized, and delivered intravaginally various formulations of PLGA NPs that encapsulate siRNA molecules targeted against either UL29.2 or nectin-1. The sequences for the siRNA were:
Nectin-1: sense: 5′-CCUGCAUUGUCAACUAUCAdTdT-3′ antisense: 5′-UGAUAGUUGACAAUGCAGGdTdT-3′, UL29.2: sense: 5′-CUUUCGCAAUCAAUUCCAA-3′, antisense 5′-UUGGAAUUGAUUGCGAAAG-3′.
siRNA NPs were synthesized using the double emulsion solvent evaporation technique as previously described [18], Briefly, 100-200 mg polymer (50:50 poly(DL-lactide co-glycolide) carboxylate end group, inherent viscosity range 0.55-0.75 dL/g, LACTEL®) was dissolved overnight in 1-2 mL dichloromethane (DCM). Complexes were formed between the siRNA and spermidine at molar ratios of the polyamine nitrogen to the nucleotide phosphate (N:P ratio) of 3:1, 8:1 or 14:1. These complexes were incubated at room temperature for 15 min prior to NP synthesis. One hundred nmol siRNA in Tris-EDTA buffer was added dropwise to 100 mg of polymer in solution while vortexing. This solution was sonicated and subsequently added to a 2.5% polyvinyl alcohol (PVA) solution to produce the second emulsion. NPs were hardened during solvent evaporation in 0.3% PVA for 3 hr. The hardened NPs were washed 3 times in deionized water to remove residual solvent, centrifuged at 4°C, lyophilized, and stored at −20°C until use.
Nanoparticle Characterization: Loading, Encapsulation Efficiency, and Size
To determine siRNA loading and encapsulation efficiency, three replicates of each NP formulation (3:1, 8:1, and 14:1) were incubated with DCM, and siRNA was extracted into aqueous buffer as described previously [18]. Briefly, 3-5 mg of siRNA NPs were dissolved in 0.5 mL of DCM for 30 min, and the siRNA was extracted into TE buffer (10 mM Tris-HCl, 1 mM EDTA). The quantity of extracted double-stranded siRNA was determined using the QuantIT™ PicoGreen™ assay (Invitrogen). Fluorescence was compared to a known siRNA standard. Encapsulation efficiency was determined by comparing the amount loaded into the NPs with theoretical loading (i.e. the 1 nmol siRNA/mg polymer that was introduced in the solvent evaporation process).
Particle size and morphology were determined using scanning electron microscopy (SEM). Average particle diameter and size distribution were determined from SEM images of at least 400 particles per batch using image analysis software (ImageJ, National Institutes of Health).
Controlled Release
In vitro controlled release for all NP formulations (3:1, 8:1, and 14:1) was measured in either phosphate buffered saline (PBS, pH 7.4) or citrate buffered saline with similar ionic strength as PBS (pH 5.0) to mimic physiological conditions relevant to delivery in the cervicovaginal tract. NPs (5-10 mg) were suspended in 1 mL of buffered solution, and incubated at 37°C with gentle agitation. At fixed time points (1, 2, 4, 6, 24, 48, 72, 96 hr, 1 wk, 2 wk, 3 wk, and 4 wk), samples were centrifuged at 12,100 rpm, and the supernatant was collected. The amount of siRNA in the supernatant at these time points was quantified using the PicoGreen™ assay. Burst release was defined as the total amount (pmol/mg) of siRNA released within the first 24 hr of incubation.
In Vitro Cytotoxicity
Cell viability was determined using the Cell Titer Blue Cell Viability Assay (Promega). HeLa cells were plated in 96-well tissue culture plates at a density of 5000 cells/well. Cells were incubated with a range of concentrations of 3:1 formulation ratio NPs (0-12.5 mg/mL NPs, corresponding to the range of siRNA delivered in lipoplexes 0-10 μM), lipoplexes (siRNA plus Lipofectamine™ transfection reagent), and cholesterol-conjugated siRNA. Medium alone (no treatment) was used as a control, as was the maximum NP dose (12.5 mg/mL) without cells. Treatment and control groups were incubated in 100 μL volumes, at 37°C, and allowed to proliferate 24 or 72 hr. On days 1 and 3, 20 μL of Cell Titer Blue was added to each well. Plates were gently shaken for 1 min and fluorescence was read every hour, up to 4 hr.
In Vitro mRNA Knockdown/Quantitative RT-PCR
In vitro mRNA knockdown experiments were conducted using HeLa cells to confirm siRNA bioactivity and to demonstrate a decrease in mRNA expression for the 3:1 NP formulation. Cells were plated in 6-well tissue culture plates at a density of 80,000 cells/well to obtain > 50% confluency after 24 hr. Ten nM and 100 nM NPs (n=5) were administered the day after plating (based on siRNA loading) and mRNA expression was evaluated 3 and 5 days post-treatment. Total RNA was extracted using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Extracted mRNA was quantified and reverse transcribed using iScript™ cDNA synthesis kit (Bio-Rad) to make cDNA. Real-time PCR was performed on 2 μL of cDNA mixed with iQ SyBr™ Green (Bio-Rad) to detect PCR products fluorescently. All PCR reactions were conducted in triplicate. mRNA expression was normalized for each sample relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. The following primers were used for RT-PCR:
GAPDH_Forward: 5′-TTC ACC ACC ATG GAG AAG GC-3′
GAPDH_reverse: 5′-GGC ATG GAC TGT GGT CAT GA-3′
Nectin-1 _Hu_forward: 5′-GGAC GTGAAGCTC ACCTGC AAAGC-3′
Nectin-l_Hu_reverse: 5′-TTGGTGG CCTCAC AGATGT AGGTC-3′
Nectin-l_Mu_forward: 5′-AGAT GTGAAGCTC ACGTGC AAAGC-3′
Nectin-l_Mu_reverse: 5′-TTGGTGG CCTCAC AGATGT AGGTT-3′In Vivo Delivery of siRNA Nanoparticles against HSV-2
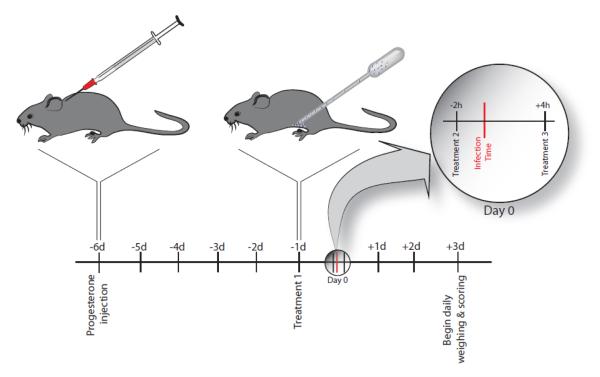
All animal procedures were approved by the Institutional Animal Care and Use Committee at Yale University. Female C57BL/6 mice (8-12 weeks) were obtained from the National Cancer Institute (NCI). To explore the efficacy of siRNA NPs against HSV-2 infection in vivo, we adapted the in vivo delivery regimen of Palliser et al. [12] and a schematic of delivery is shown in Figure 1. Five days prior to HSV-2 infection, mice were subcutaneously injected with 2mg Depo-Provera (medroxyprogesterone acetate, Pfizer) [17]. Prior to infection and treatment, the vagina of each mouse was swabbed with a calcium alginate tip (Fisher). The stage of estrous was confirmed by staining the vaginal swab by Papanicolau’s tri-chrome method, and observing cell staining and morphology. After confirming the mice were in diestrous, vaginal inoculation with a lethal dose of HSV-2 strain 186+ [19] (5000 PFU) was performed at time zero relative to NP or control treatments. In our adapted delivery scheme, 2 mg of either 3:1 or 14:1 formulation siRNA NPs in 10 μL PBS, were lavaged into the vaginal tract of 8-12 week old C57BL/6 mice on −1 day, −2 hr, and +4 hr relative to HSV-2 infection. The additional administration relative to the treatment schedule by Palliser et al. [12], −1 day relative to infection, was examined to account for the potentially longer time to release encapsulated siRNA from NPs compared to delivery of siRNA alone. Control mice receiving lipoplexes were treated using the same treatment schedule and one treatment consisted of transfection reagent + siRNA (Oligofectamine™ + 500 pmol si-UL29 or si-Nectin), or medium alone. In one study mice received a mixture of 1 nmol si-Nectin and 1 nmol si-UL29 conjugated to cholesterol (per treatment). For each study, five mice per treatment group were evaluated daily from 3 d to 4 wk post-infection for disease progression and survival as indicated by weight, hind limb paralysis, and vaginal inflammation. Disease severity and progression were evaluated daily according to a previously published five point scale [12]: 0, no signs of infection; 1, slight genital erythema and edema; 2, moderate genital inflammation; For all in vivo experiments, mice were euthanized by carbon dioxide asphyxiation when disease severity reached grade 4. The reproductive tracts were excised and then flash frozen to allow for confirmation of mRNA knockdown in vivo.
Figure 1.
Schematic of in vivo delivery regimen. Five days prior to treatment, mice were given progesterone to regulate the estrous cycle, and vaginal swabs were evaluated to ensure diestrous phase. Mice were treated with vaginal doses of NPs or relevant controls (lipoplexes or reverse siRNA sequence NPs) one day and 2 hr prior to, as well as +4 hr post-infection.
In Vivo mRNA Knockdown/Quantitative RT-PCR
Vaginal tissue was harvested 7 days after the last NP administration for extent of nectin-1 mRNA knockdown from five mice per treatment group. Tissue was stored in RNAlater overnight at 4°C, excess RNAlater was removed and the tissue was stored at −80°C. Each vaginal tissue slice was blotted dry with sterile gauze and placed in a homogenizer tube. Six hundred μL of RLT buffer from the RNeasy kit (Qiagen) was added and the tissue was homogenized for three 30 s durations. Homogenates were centrifuged at 12,000 rpm for 3 min and 500 μL of supernatant was transferred into a fresh sterile tube. Tissue was then processed as described in the in vitro protocol above using the RNeasy kit.
Necropsy and Histopathology
Mice were euthanized one day after NP administration by carbon dioxide asphyxiation. Vaginas and uteri were harvested and immersion fixed in 10% formalin, processed, embedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin (HE) by routine methods (Yale Mouse Research Pathology, Section of Comparative Medicine, Yale University School of Medicine). Tissues were examined for pathologic changes by a reviewer (CJB) blind to the experimental manipulation, on an Axiolmager.Al (Carl Zeiss, Micro Imaging, Inc. Thornwood, NY). Digital light microscopic images were taken on a Zeiss Axiolmager A1 microscope, AxoCam MRc5 camera and AxioVision 4.7.1 imaging software (Carl Zeiss Micro Imaging, Inc. Thornwood, NY) and images were optimized in Adobe® Photoshop® (Adobe Systems Incorporated, San Jose, California) of stained slides.
Statistical analysis
Sample replicates for experiments were conducted with a minimum sample size of n = 3 for each experiment. Data were analyzed using a two-sided Student’s t-test with a p-value of 0.05 or less defined as the threshold for statistical significance.
RESULTS
SEM
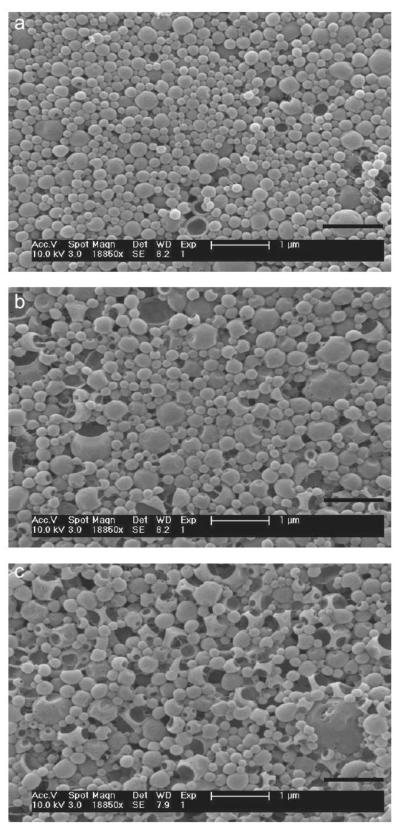
NPs loaded with siRNA against nectin-1 were synthesized at three different N:P ratios and examined by SEM (Fig 2) , as well as dynamic light scattering (DLS) and zeta potential analysis (Fig S1 and Table S1). As the spermidine polyamine:nucleic acid phosphate (N:P) ratio varied from 3:1 to 8:1 to 14:1, the particles displayed a more porous morphology (Figures A-C, respectively). However, the average particle diameter for each batch was comparable, showing similar size distributions with mean particle diameters of 161, 189, and 186 nm for particles prepared from 3:1, 8:1, and 14:1 N:P formulation ratios, respectively.
Figure 2.
SEM images of NP batches synthesized with (A) 3:1, (B) 8:1, and (C) 14:1 spermidine polyamine:siRNA phosphate ratio (N:P). Scale bars represent 1 μm.
Loading, Encapsulation Efficiency, and Controlled Release
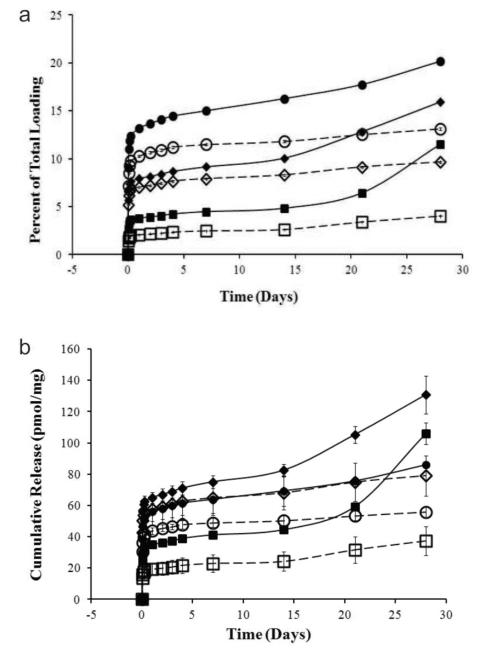
Loading of siRNA increased with decreasing N:P ratio: loadings of 920, 820, and 430 pmol/mg were obtained for 3:1, 8:1, and 14:1 formulations, respectively, with corresponding encapsulation efficiencies of 92%, 82%, and 43%. As observed previously [18], cumulative release of siRNA from each formulation in vitro was enhanced under slightly acidic conditions relative to neutral conditions (Fig 3). We found higher N:P ratios led to higher release as a fraction of total loading (Fig 3A). On a per mass basis, the 8:1 formulation provided the greatest amount of siRNA released over 28 days, followed by the 3:1 and 14:1 formulations at a pH of 5, representative of the intravaginal environment (Fig 3B). The 8:1 and 14:1 formulations provided greater initial burst release, relative to the 3:1 formulation. An increase in release was observed during the period 20 - 30 d for all formulations under acidic conditions.
Figure 3.
Cumulative release of siRNA from NPs shown as a function of: (A) percent total loading and (B) total mass released with respect to incubation time. Particles were incubated in slightly acidic (pH 5, solid line) or neutral (pH 7, dashed line) conditions. Squares, diamonds, and circles indicate an N:P ratio of 3, 8, and 14, respectively. Error bars represent standard deviation from the mean, and are very small in (A).
In Vitro Cytotoxicity
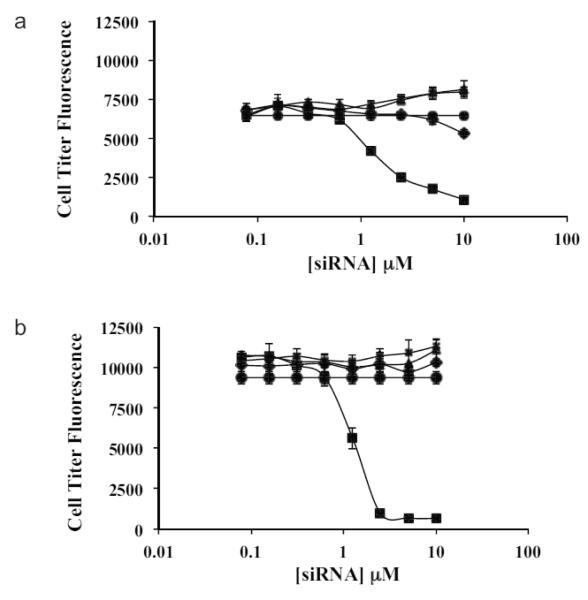
NPs synthesized with a 3:1 N:P formulation ratio, and cholesterol-conjugated siRNA demonstrated no cytotoxicity in HeLa cell cultures for the range of concentrations tested (0-10 μM); whereas lipoplexes were toxic at concentrations greater than 1 μM (Fig 4).
Figure 4.
PLGA NPs (3:1 formulation) encapsulating si-Nectin were evaluated for in vitro cytotoxicity in HeLa cervical carcinoma cells at (A) 1 day and (B) 3 days. Cell Titer Blue fluorescence intensity is plotted as a function of siRNA concentration for si-Nectin NPs (x); blank NPs (triangle); cholesterol-conjugated si-Nectin (diamond); si-Nectin lipoplexes (square); and no treatment (PBS, circle). Concentration of NPs was varied from 0-12.5 mg/mL to attain equal siRNA concentration as 0.1 μM to 10 μM siRNA lipoplexes. Error bars are shown as standard deviation from the mean.
In Vitro mRNA Knockdown/Quantitative RT-PCR
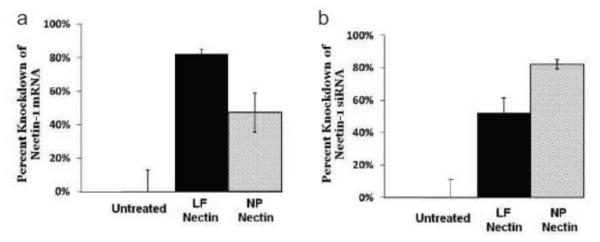
NPs encapsulating nectin-1 siRNA (si-Nectin) in a 3:1 N:P formulation ratio, provided in vitro knockdown of nectin in HeLa cells (Fig 5). After 3 d of incubation with 100 nM siRNA NPs, nectin-1 mRNA was reduced by 47% +/− 12% relative to the housekeeping gene, GAPDH, whereas knockdown achieved with si-Nectin lipoplexes was reduced by 82% +/− 3% (Fig 5A). Both 100 nM siRNA NPs and si-Nectin lipoplexes (10 nM) provided statistically significant knockdown (p < 0.05) relative to treatment with medium alone, NPs with reverse sequence siRNA, or 10 nM siRNA NPs. The effectiveness of siRNA delivery via NPs improved at longer incubation times. After 5 days incubation the NP knockdown efficacy was 82% +/− 3%, compared to 52% +/− 9% for lipoplexes (Fig 5B). Lipoplex and NP treatments were statistically significant relative to no treatment, at both days 3 and 5 (p < 0.05).
Figure 5.
In vitro nectin mRNA knockdown comparing si-Nectin lipoplexes and si-Nectin 3:1 formulation NPs (A) 3 and (B) 5 days after administration to HeLa cells. Error bars indicate standard error of the mean.
In Vivo Delivery of siRNA Nanoparticles against HSV-2
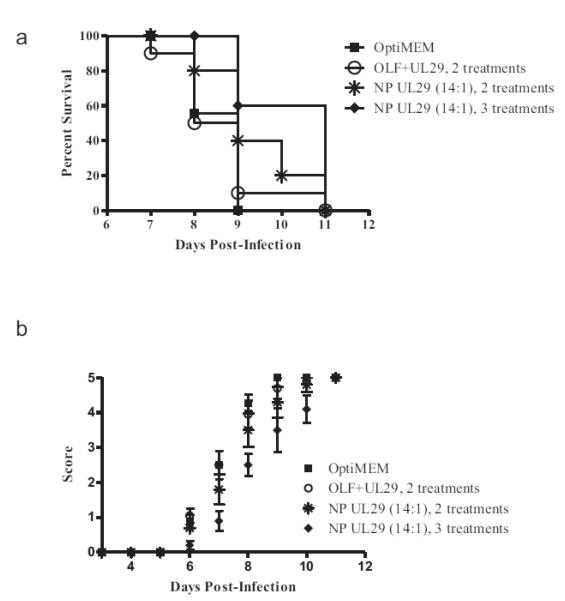
We delivered siRNA-loaded NPs by intravaginal administration to female mice infected with HSV-2, to test whether the NPs could protect against a lethal dose of virus. First, we defined the optimal treatment schedule (Fig 6), using siRNA directed against UL29.2 (si-UL29), and compared the efficacy of the 14:1 NP-siRNA formulation with lipoplexes. Mice received three separate doses of the 14:1 formulation NPs (−1 day, −2 hr, and +4 hr relative to infection) and showed both reduced HSV-2 symptoms and extended survival relative to mice receiving two doses (−2 hr and +4 hr relative to infection) of either NPs or lipoplexes. Both 14:1 NP formulation treatment groups, with 2 and 3 treatments, resulted in extended survival relative to lipoplexes (Fig 6).
Figure 6.
Animals receiving three separate doses of 14:1 formulation si-UL29 NPs showed reduced HSV-2 symptoms and extended survival relative to mice receiving two doses (−2 hr and 4 hr relative to infection) of either NPs, lipoplexes (OLF), or medium alone. Both NP treatment groups (2 or 3 treatments) resulted in extended survival relative to lipoplexes. (A) Percent survival of mice and (B) disease assessment score with respect to time. Disease severity and progression were evaluated daily according to a five point scale: 0, no signs of infection; 1, slight genital erythema and edema; 2, moderate genital inflammation; 3, purulent genital lesions; 4, hind limb paralysis; 5, death. Error bars represent standard error of the mean.
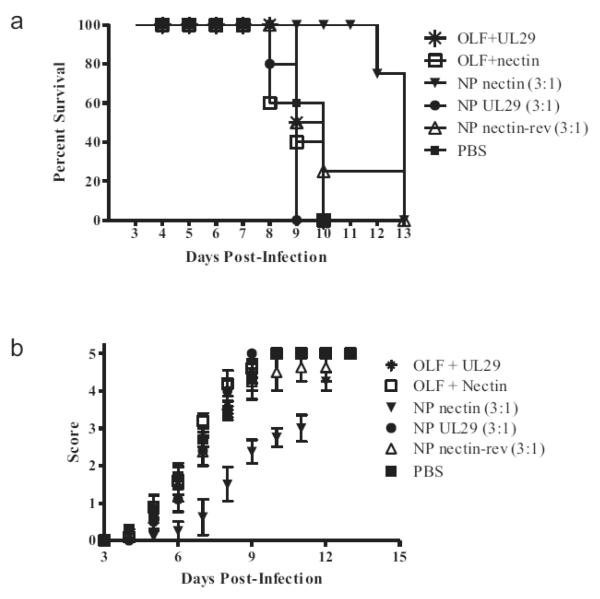
Based on the increased encapsulation efficiency demonstrated by the 3:1 formulation NPs relative to the 14:1 formulation NPs in in vitro studies, we next compared the effectiveness of si-Nectin and si-UL29 lipoplexes to the 3:1 formulation NPs encapsulating either si-Nectin, si-UL29, or a reverse sequence si-Nectin, using the three dose delivery regimen. Prolonged survival was observed in mice receiving NPs encapsulating si-Nectin, relative to control groups of lipoplexes, PBS alone, NPs encapsulating si-UL29, and NPs encapsulating the reverse nectin-1 siRNA sequence (Fig 7A). Animals receiving the 3:1 formulation si-Nectin NPs survived up to 13 days, whereas maximum survival in other treatment groups was 9 to 10 days, with the exception of one mouse who received reverse si-Nectin NPs, and survived to day 13. Mice receiving si-Nectin NPs also exhibited the lowest clinical disease score: this difference was statistically significant relative to all other lipoplex and NP treatments (Fig 7B).
Figure 7.
Efficacy of NP treatments compared to si-Nectin or si-UL29 lipoplexes, reverse sequence si-Nectin 3:1 NPs, or PBS, using the three dose delivery regimen. Prolonged survival and the lowest clinical disease score were observed in mice receiving NPs encapsulating si-Nectin, relative to all other groups. (A) Percent survival of mice and (B) disease assessment score with respect to time. Disease severity and progression were evaluated as previously described. Error bars represent standard error of the mean.
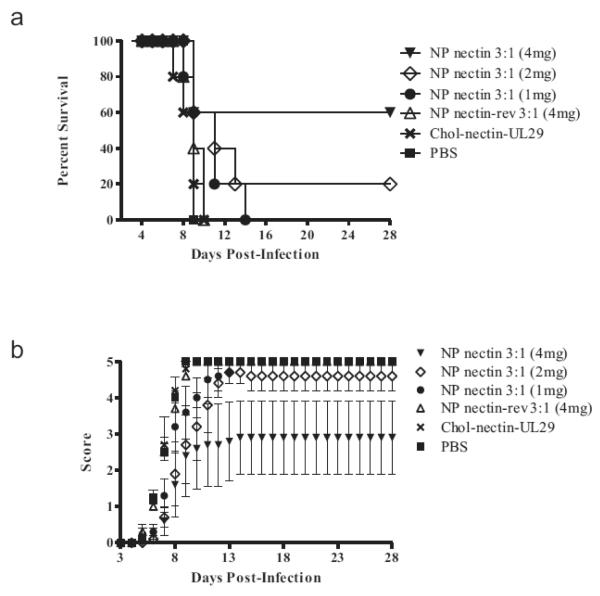
To determine the effect of NP dose on survival and disease progression, the dose of 3:1 formulation ratio si-Nectin NPs was varied from 1 to 4 mg/mouse. An increase in dose resulted in an increase in the number of mice surviving (Fig 8). Significantly, 20% and 60% of the mice receiving 2 or 4 mg of NPs containing si-Nectin, respectively, survived for 28 days. Mice receiving 1 mg of NPs survived 14 days, and all other mice (receiving a lower dose of NPs, cholesterol-conjugated si-Nectin and si-UL29, PBS, or NPs with reverse sequence siRNA) succumbed by day 10.
Figure 8.
In vivo dose response study for 3:1 formulation si-Nectin NPs. NP dose of 1 mg, 2 mg, or 4 mg/mouse was compared to no treatment (PBS) and reverse si-Nectin sequence NPs. An increase in dose resulted in an increase in the number of mice surviving. (A) Percent survival of mice and (B) disease assessment score with respect to time. Disease severity and progression were evaluated as previously described. Error bars represent standard error of the mean.
In Vivo mRNA Knockdown/Quantitative RT-PCR
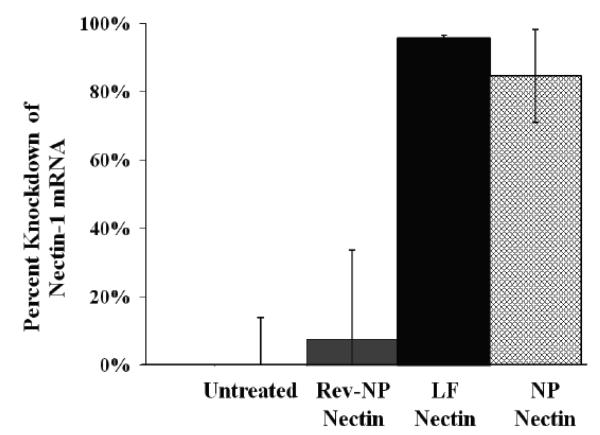
To confirm the mechanism of action of NPs in tissue, reproductive tracts from mice treated with 4 mg of 3:1 formulation si-Nectin NPs were evaluated for gene expression 7 d after the last treatment. NPs encapsulating si-Nectin provide in vivo knockdown of nectin in vaginal tissue (Fig 9). Seven days after treatment with 4 mg of 3:1 formulation siRNA NPs, nectin-1 mRNA was reduced by (85% +/− 14%) relative to the housekeeping gene, GAPDH, and was comparable to knockdown achieved with lipoplexes (95% +/− 1%). siRNA NPs and lipoplexes (10 nM) provided statistically significant (p < 0.05) knockdown relative to medium alone, and to NPs with reverse sequence siRNA.
Figure 9.
In vivo nectin mRNA knockdown from vaginal tissue 7 days after administration of 3:1 formulation si-Nectin NPs. Error bars represent standard error of the mean.
Histopathology
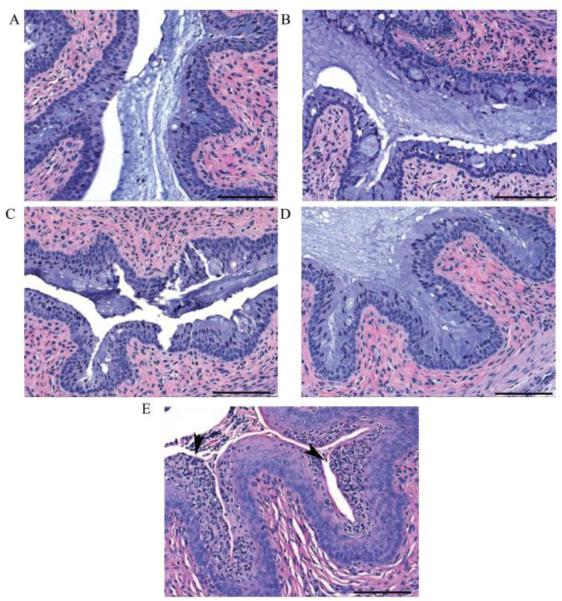
Grossly there were no signs of vaginal enlargement, inflammation, or other macroscopic changes in any of the treatment groups. Images of HE-stained sections of vaginal tissues from mice treated with either PBS (no virus, no treatment), virus only (no treatment), si-Nectin 3:1 formulation NPs, reverse si-Nectin 3:1 formulation NPs, or si-Nectin lipoplexes are shown in Figs. 10A-E respectively. All treatment groups, except for mice treated with lipoplexes, were morphologically similar to untreated control mice with no significant difference in numbers or types of inflammatory cells. In comparison, 2 out of 3 mice treated with lipoplexes showed significantly greater accumulations of polymorphonuclear neutrophils (PMN) in the vaginal mucosa (10E).
Figure 10.
Representative vaginal histopathology from mice treated with: (A) PBS (no virus, no treatment), (B) virus only (no treatment), (C) si-Nectin 3:1 NPs, (D) reverse si-Nectin 3:1 NPs, or reveal normal vaginal epithelium in mice in contrast mice treated with (E) si-Nectin lipoplexes have increased inflammation (arrow heads). HE, Scale bars = 100 μm.
DISCUSSION
Here we tested different siRNA NP systems to identify a formulation that would decrease disease severity and enhance the survival of mice after infection with a lethal dose of HSV-2. Previous work using intravaginal delivery of siRNA has employed complexation with lipids to form lipoplexes [12, 20, 21], conjugation to cholesterol [13] or conjugation to aptamers [22]. Thus far, lipoplexes and/or cholesterol-conjugated siRNA are the only modalities used to deliver siRNA intravaginally against HSV-2 targets. It is well-known that lipoplexes can induce cell toxicity and tissue inflammation at high concentration [23, 24]. For materials or agents applied to the female lower reproductive tract, lack of inflammation is particularly important, as mucosal irritation can increase susceptibility to virus infection [25, 26]. For example, the initial promise of nonoxynol-9, a detergent-based potential microbicide, was followed by disappointing failure, when it became clear that it caused inflammation that enhanced susceptibility to viral penetration [25, 27, 28]. Observation of inflammation and toxicity at high concentration prompted the development of a less toxic cholesterol-conjugated siRNA delivery system against HSV-2; however, both lipoplexes and cholesterol-conjugated formulations have only provided transient therapeutic benefit, with survival to 14 days after genital HSV-2 infection in mice [12, 13].
Microbicide developers seek more durable, low toxicity vehicles that provide acceptable vaginal retention [12, 13, 20, 29, 30]. An ideal delivery system would encapsulate agents at high density (to minimize the amount of vehicle that must be administered), protect the agents from enzymatic degradation, have the ability to target specific cell types, exhibit sustained release, and be maintained in the vaginal tissues for long time periods [13, 18, 20, 30-32]. All of these are integral considerations in the design of a successful microbicide. Thus far, the transient duration of survival imparted by current delivery systems (as observed in a mouse model) would require more frequent dosing, which is often associated with decreased user compliance [30-32]. Our goal was to design a delivery system to meet some of these needs.
We showed that by complexing siRNA with low molecular weight cationic agents such as spermidine, we could densely load PLGA NPs with siRNA [18]. When these were administered intravaginally, fluorescent NPs were detected throughout the reproductive tract and below the luminal surface for up to 7 days [18]. Efficient knockdown of genes with siRNA NPs was observed after intravaginal administration for up to 14 days in all regions of the reproductive tract in this earlier study. To build upon these data, here we initially developed new formulations of PLGA NPs loaded with siRNA that target either host or virus genes known to contribute to HSV-2 infection. The 3:1 NP formulation, containing siRNA against nectin-1, decreased the symptoms and improved survival duration in mice with lethal genital infections of HSV-2 from 9 days (control treatments) to 28 days (Fig 8). To our knowledge, this is the first study that uses polymer NPs as an intravaginal delivery system to protect against HSV-2 in vivo, and the first siRNA delivery system to extend survival against a lethal dose of HSV-2 beyond 14 days in this animal model.
The new formulations of siRNA PLGA NPs provided high encapsulation of siRNA. We discovered that varying the spermidine:siRNA complexation ratio (3:1, 8:1, or 14:1) allowed us to tune the loading and controlled release of siRNA from NPs (Fig 3). As the complexation ratio decreased from 14:1 to 3:1, the particles became less porous, had higher loading, higher encapsulation efficiency, and exhibited less fractional release (amount released relative to total loading) from the NPs (Fig 2 and 3). During the first 24 hr of incubation at pH 5-an environment representative of the conditions of the vaginal tract-the NPs with 3: 1 formulation ratio provided a modest burst release (~4% of siRNA), followed by sustained release of siRNA that persisted for at least 28 days (at which time ~11% of the encapsulated siRNA had been released). Of the 3:1 and 14:1 formulations tested in vivo, the 3:1 formulation released a greater total mass of 100 pmol siRNA per mg of NP at pH 5 (Fig 3), translating to 400 pmol siRNA released at the maximum dose (4 mg NPs/ treatment) administered in our mouse studies. For all formulations at pH 5, an increase in release was observed at 3 weeks. The first phase of release from PLGA is usually attributed to diffusion of agent near the particle surface and the second phase of release is attributed to release of agent entrapped in the polymer, which depends on the bulk degradation of the polymer [33, 34].
To ensure that the increases in survival observed in our experiments were due to gene-specific knockdown, we measured levels of nectin-1 mRNA in vitro and in tissues (Fig 5 and 9). In cell culture, NPs showed levels of knockdown comparable to lipoplexes at days 3 and 5: on day 5 the knockdown produced by NP was significantly greater than lipoplexes: 82% relative to 52% (Fig 5). Similarly when administered in vivo, our PLGA NP formulation showed comparable efficacy of knockdown on day 7, relative to lipoplexes (Fig 9). These results are similar to previous in vivo intravaginal delivery studies where nectin knockdown with lipoplexes was decreased by ~50% by days 3 and 7, and ~50% on days 3, 7, and 12 for cholesterol-conjugated siRNA [13]. Further, these results are comparable to previous work in our group in which PLGA si-EGFP NPs were applied intravaginally [18] and EGFP expression was reduced by 50-60% in vaginal tissue at 10 days and lipoplex delivery reduced EGFP expression by 50%. In aggregate, our work suggests that encapsulation of siRNA in NPs will induce equal if not greater knockdown than lipoplexes. Further, of the formulations we tested, the 3:1 formulation NPs appear to be the best, due to their high siRNA encapsulation efficiency and siRNA release, which correlates with their higher in vitro and in vivo transfection efficacies.
We assessed the toxicity of our delivery system in both cell culture and intravaginal tissue. We compared our NPs to lipoplexes formed from Lipofectamine™ and found that NP formulations were nontoxic to HeLa cells, in contrast to lipoplexes, which were toxic at concentrations greater than 1 μM (Fig 4). After delivery to the reproductive tract, lipoplexes induced a greater accumulation of polymorphonuclear neutrophils in the vaginal mucosa in 2 of 3 tissues samples, whereas no gross inflammation was observed after delivery of NP formulations (Fig 10).
The dosage regimen that we selected for in vivo studies was based on previous work [12, 13]. We chose to pursue the majority of our studies with siRNA NPs directed against nectin, due to the increased survival benefit observed (Fig 7). NP-mediated siRNA delivery required 4 or 12-fold higher doses (on an siRNA basis) to produce a similar therapeutic effect seen in previous studies using, respectively, lipoplexes or cholesterol-conjugated siRNA [12, 13]. However, because of the slow release properties of the NPs (Fig 3), we predict that, during the 28 day period of survival, the NP formulation (3:1) released amounts of siRNA nearly equivalent (400 pmol per treatment) to the 500 pmol per treatment administered in lipoplexes. Further, in contrast to previous work using cholesterol-conjugated siRNA in dose response studies, where mice treated with up to 5-fold higher doses of siRNA did not receive additional viral protection [13], an increase in survival was observed with increased doses of our NPs. While mice receiving less than 2 mg of NPs showed no survival benefit, higher doses led to a significant improvement in survival, with 20% and 60% of mice surviving to day 28 after administration of 2 mg and 4 mg of NPs, respectively.
In our hands we did not observe any survival advantage after administering lipoplex treatments (Fig 6 and 7) or a mixture of cholesterol-conjugated siRNAs (mixed si-UL29 and si-Nectin) that had previously provided some survival benefit in mice [13] (Fig 8). However, the timeframe of mortality induced by HSV-2 infection in our study is consistent with other HSV-2 mouse infection models, with survival in untreated mice typically spanning from 4 to 10 days [10-13]. We similarly observed death in untreated (and unsuccessfully treated) mice within this expected timeframe of 9-10 days across all studies. In fact, low doses of siRNA NPs did not exhibit a statistically significant difference in animal survival or score until 2 mg of NPs were administered.
As demonstrated previously [12, 13, 18] and confirmed by our data here, lipoplexes induce slight but significant inflammation. We believe that even the moderate inflammation observed with lipoplexes in tissue may have contributed to increased virus infection using our 3 dose regimen. Further, since the in vivo knockdown we observed at day 7 was efficacious and in agreement with intravaginal knockdown of ~50% at day 7 with nectin-1 in previous work [13], we believe that a slight variation in virus dosing may contribute to the lack of survival benefit we see when we administer the same lipoplex concentration used in previous work [12, 13]. Studies by Wu et al. [13] examined the effect of different viral doses at 2 LD50, 1 LD50, and 0.5 LD50 in mice administered OLF intravaginally −2 and +4 hr relative to HSV-2 infection. Survival was dependent on virus dose, with 100% of mice dead at day 8 or 9 for 2 LD50 and LD50 respectively, and 20% of mice living out to 10 days for 0.5 LD50. In fact, OLF treatment reduced the LD50 of HSV-2 infection by approximately 4-fold [13]. While the virus we administered was titrated and verified by plaque formation in culture, virus stocks may vary slightly between laboratories and strain, and likely has a strong effect on survival when administered in conjunction with lipoplexes. Furthermore, other researchers delivering fluorescently labeled siRNA lipoplexes intravaginally as a positive control [20], did not see persistence of lipoplexes in vaginal tissue as observed in previous work [12, 13].
After intravaginal administration of siRNA NPs complexed in a 3:1 formulation, 60% of mice both survived for at least 28 days. While a higher overall dose based on siRNA encapsulated in NPs may be needed to see efficacy relative to lipoplexes, the NPs released only a fraction of their total amount (similar to that delivered with lipoplexes), with an increase in release observed around day 20. Importantly, multiple treatments with the 3:1 formulation si-Nectin NPs did not result in overt damage to the epithelia (Fig 10C), indicating that compensatory mechanisms exist in preserving epithelial integrity upon knockdown of nectin long term. By providing a controlled release delivery vehicle for microbicide applications as demonstrated in this study, we show proof-of-concept in achieving efficacy against intravaginal virus infection. Subsequent to our prior work demonstrating that our NPs are small, have the ability to penetrate tissue, and can be loaded at high density with siRNA [18]; here we show for the first time, that our NPs formed from FDA-approved PLGA prevent lethal intravaginal infection of HSV-2 in mice. We also show that we can increase survival to 28 days, a full 2 weeks longer than prior reports of only 14 days survival post administration of a lethal dose of HSV-2 in mice. Our data support our conclusion that that our NP platform offers sustained delivery as demonstrated by controlled release, decreased toxicity due to the PLGA platform, and promising in vivo survival and symptomatic benefit against HSV-2 infection.
CONCLUSION
For the first time we demonstrate that intravaginal administration of PLGA NPs, encapsulating siRNA against a host cell target integral in HSV-2 infection, can provide knockdown and enhanced survival after HSV-2 infection. We synthesized, characterized, and intravaginally delivered various formulations of PLGA NPs that encapsulate siRNA molecules targeted against either UL29.2 or nectin-1. We demonstrate unprecedented in vivo efficacy of intravaginal administration of si-Nectin PLGA NPs in the HSV-2 mouse model. This work demonstrates the feasibility and applicability of PLGA siRNA NPs for HSV-2 treatment.
Supplementary Material
ACKNOWLEDGMENTS
Funded by an NIH NIAID Postdoctoral Fellowship (F32AI093056-01A1) and Yale Proteomics and Genomics Training Fellowship (NIH T32-HG003198) to JMS and NIH R01 EB000487 to WMS. We thank Nha Duong for help with Figure 1 and Young-Eun Seo for technical assistance. We also thank Dr. Akiko Iwasaki for her generous donation of the HSV-2 virus stock.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rohan LC, Sassi AB. Vaginal drug delivery systems for HIV prevention. The AAPS journal. 2009;11(1):78–87. doi: 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. Jama. 2006;296(8):964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- [3].Looker KJ, Gamett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bulletin of the World Health Organization. 2008;86(10):805–812. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. Journal of acquired immune deficiency syndromes. 2004;35(5):435–445. doi: 10.1097/00126334-200404150-00001. (1999) [DOI] [PubMed] [Google Scholar]
- [5].Macmillan L, Ifere GO, He Q, Igietseme JU, Kellar KL, Okenu DM, Eko FO. A recombinant multivalent combination vaccine protects against Chlamydia and genital herpes. FEMS immunology and medical microbiology. 2007;49(1):46–55. doi: 10.1111/j.1574-695X.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- [6].Tobian AA, Quinn TC. Herpes simplex virus type 2 and syphilis infections with HIV: an evolving synergy in transmission and prevention. Current opinion in HIV and AIDS. 2009;4(4):294–299. doi: 10.1097/COH.0b013e32832c1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Koelle DM, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annual review of medicine. 2008;59:381–395. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- [8].Will there be an HIV vaccine in the next 10 years? Nature medicine. 13:518–519. [Google Scholar]
- [9].Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert review of vaccines. 2009;8(8):1023–1035. doi: 10.1586/erv.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fernandez-Romero JA, Abraham CJ, Rodriguez A, Kizima L, Jean-Pierre N, Menon R, Begay O, Seidor S, Ford BE, Gil PI, Peters J, Katz D, Robbiani M, Zydowsky TM. Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge. Antimicrob Agents Chemother. 56(1):358–368. doi: 10.1128/AAC.05461-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Overall JC, Jr., Kern ER, Schlitzer RL, Friedman SB, Glasgow LA. Genital herpesvirus hominis infection in mice. I. Development of an experimental model. Infect Immun. 1975;11(3):476–480. doi: 10.1128/iai.11.3.476-480.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Palliser D, Chowdhury D, Wang QY, Lee SJ, Bronson RT, Knipe DM, Lieberman J. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439(7072):89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- [13].Wu Y, Navarro F, Lai A, Basar E, Pandey RK, Manoharan M, Feng Y, Lee SJ, Lieberman J, Palliser D. Durable protection from Herpes Simplex Virus-2 transmission following intravaginal application of siRNAs targeting both a viral and host gene. Cell host & microbe. 2009;5(1) doi: 10.1016/j.chom.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. Journal of virology. 2003;77(8):4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Parr MB, Parr EL. Intravaginal administration of herpes simplex virus type 2 to mice leads to infection of several neural and extraneural sites. J Neurovirol. 2003;9(6):594–602. doi: 10.1080/13550280390246499. [DOI] [PubMed] [Google Scholar]
- [16].Yim KC, Carroll CJ, Tuyama A, Cheshenko N, Carlucci MJ, Porter DD, Prince GA, Herold BC. The cotton rat provides a novel model to study genital herpes infection and to evaluate preventive strategies. Journal of virology. 2005;79(23):14632–14639. doi: 10.1128/JVI.79.23.14632-14639.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Linehan MM, Richman S, Krummenacher C, Eisenberg RJ, Cohen GH, Iwasaki A. In vivo role of nectin-1 in entry of herpes simplex virus type 1 (HSV-1) and HSV-2 through the vaginal mucosa. Journal of virology. 2004;78(5):2530–2536. doi: 10.1128/JVI.78.5.2530-2536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nature materials. 2009;8(6):526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Spang AE, Godowski PJ, Knipe DM. Characterization of herpes simplex virus 2 temperature-sensitive mutants whose lesions map in or near the coding sequences for the major DNA-binding protein. Journal of virology. 1983;45(1):332–342. doi: 10.1128/jvi.45.1.332-342.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wu SY, Chang HI, Burgess M, McMillan NA. Vaginal delivery of siRNA using a novel PEGylated lipoplex-entrapped alginate scaffold system. J Control Release. 2011;155(3):418–426. doi: 10.1016/j.jconrel.2011.02.002. [DOI] [PubMed] [Google Scholar]
- [21].Zhang Y, Cristofaro P, Silbermann R, Pusch O, Boden D, Konkin T, Hovanesian V, Monfils PR, Resnick M, Moss SF, Ramratnam B. Engineering mucosal RNA interference in vivo. Mol Ther. 2006;14(3):336–342. doi: 10.1016/j.ymthe.2006.04.001. [DOI] [PubMed] [Google Scholar]
- [22].Wheeler LA, Trifonova R, Vrbanac V, Basar E, McKernan S, Xu Z, Seung E, Deruaz M, Dudek T, Einarsson JI, Yang L, Allen TM, Luster AD, Tager AM, Dykxhoorn DM, Lieberman J. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. J Clin Invest. 121(6):2401–2412. doi: 10.1172/JCI45876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Conwell CC, Liu F, Huang L. Several serum proteins significantly decrease inflammatory response to lipid-based non-viral vectors. Mol Ther. 2008;16(2):370–377. doi: 10.1038/sj.mt.6300356. [DOI] [PubMed] [Google Scholar]
- [24].Khazanov E, Simberg D, Barenholz Y. Lipoplexes prepared from cationic liposomes and mammalian DNA induce CpG-independent, direct cytotoxic effects in cell cultures and in mice. J Gene Med. 2006;8(8):998–1007. doi: 10.1002/jgm.933. [DOI] [PubMed] [Google Scholar]
- [25].Whaley KJ, Hanes J, Shattock R, Cone RA, Friend DR. Novel approaches to vaginal delivery and safety of microbicides: biopharmaceuticals, nanoparticles, and vaccines. Antiviral research. 88(Suppl 1):S55–66. doi: 10.1016/j.antiviral.2010.09.006. [DOI] [PubMed] [Google Scholar]
- [26].Buckheit RW, Jr., Watson KM, Morrow KM, Ham AS. Development of topical microbicides to prevent the sexual transmission of HIV. Antiviral research. 2010;85(1):142–158. doi: 10.1016/j.antiviral.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect Dis. 2008;8(11):685–697. doi: 10.1016/S1473-3099(08)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6(5):371–382. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- [29].Eszterhas SK, Ilonzo NO, Crozier JE, Celaj S, Howell AL. Nanoparticles containing siRNA to silence CD4 and CCR5 reduce expression of these receptors and inhibit HIV-1 infection in human female reproductive tract tissue explants. Infectious Disease Reports. 2011;3(11):52–61. doi: 10.4081/idr.2011.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mallipeddi R, Rohan LC. Nanoparticle-based vaginal drug delivery systems for HIV prevention. Expert Opin Drug Deliv. 7(1):37–48. doi: 10.1517/17425240903338055. [DOI] [PubMed] [Google Scholar]
- [31].Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Moms L, Taylor D. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science (New York, N.Y. 329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Katakowski JA, Palliser D. Optimizing siRNA delivery to the genital mucosa. Discov Med. 11(57):124–132. [PMC free article] [PubMed] [Google Scholar]
- [33].Corrigan OI, Li X. Quantifying drug release from PLGA nanoparticulates. Eur J Pharm Sci. 2009;37(3-4):477–485. doi: 10.1016/j.ejps.2009.04.004. [DOI] [PubMed] [Google Scholar]
- [34].Duvvuri S, Gaurav Janoria K, Mitra AK. Effect of polymer blending on the release of ganciclovir from PLGA microspheres. Pharm Res. 2006;23(1):215–223. doi: 10.1007/s11095-005-9042-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.